Abstract
Purposes
To determine the distribution and glutamate-mediated activation of NFκB members in the retina and pan-purified retinal ganglion cells (RGCs), and to characterize steps in the signal transduction events which lead to NFκB activation.
Methods
Expression patterns in the retina and RGCs were evaluated for five NFκB proteins with the aid of immunohistochemistry. Retinal explants or RGCs were treated with glutamate with or without the presence of the NDMA receptor antagonist, memantine, the calcium chelator, EGTA, or a specific inhibitor for CaMKII. Characterizations of NFκB activation were performed with the aid of electrophoretic mobility shift assays, and super shift assays.
Results
All five NFκB proteins were present in the retina and in the pan-purified RGCs. In response to a glutamate stimulus, all NFκB proteins except c-Rel were activated. P65 was unique in that it was not constitutively active but showed a glutamate-inducible activation in the retina and in the cultured RGCs. Memantine, or EGTA, or AIP inhibited NFκB activation in the retina. Furthermore, AIP significantly reduced the level of glutamate-induced degradation of IκBs.
Conclusions
These data indicate that glutamate activates distinct NFκB proteins in the retina. P65 activation may be especially important with regard to RGC responses to glutamate, given that its activity is induced by conditions which are known to lead to death of these cells. The NMDA receptor-Ca2+-CaMKII signaling pathway is involved in glutamate-induced NFκB activation. Since AIP blocks the degradation of IκB, its regulation is clearly downstream of CaMKII.
The nuclear factor-κB (NFκB), a ubiquitously expressed transcription factor, is a critical regulator of many genes involved in inflammatory processes, cell differentiation, and apoptosis. The factor has been implicated in mechanisms which mediate both cell survival and cell death1. In mammals, the NFκB family comprises five members, p65 (RelA), RelB, c-Rel, p50/p105 (NFκB1) and p52/p100 (NFκB 2), which share an N-terminal Rel homology domain allowing dimerization, nuclear localization and DNA binding. These proteins form homo- or hetero-dimers and are retained inactive in the cytoplasm through interaction with inhibitory molecules, called IκBs, which mask the NFκB nuclear localization and DNA-binding domains.2 Activation of NFκB can be induced by multiple stimuli including inflammation, infection, injury and stress. Upon stimulation, IκB protein subunits are phosphorylated by IκB kinases (IKK) followed by polyubiquitination and subsequent, rapid degradation through the proteasome. This phosphorylation leads to the release of NFκB, which is then translocated to the nucleus, where it binds to DNA and activates the transcription of target genes3. Both pro- and anti-apoptotic properties have been attributed to NFκB in neurons3–5 and the balance between cell death and survival in response to external stimuli may rely on the activation of distinct NFκB proteins5, a complete characterization of which has not yet been demonstrated for any of the cells in the retina.
Retinal Ischemia is a common clinical entity and has been widely studied because of its proposed relationship to, for example, anterior ischemic optic neuropathy, retinal and choroidal vessal occlusion, glaucoma, diabetic retinopathy, retinopathy of prematurity and traumatic optic neuropathy.6 All of these diseases/disorders have been shown to lead to injury or loss of the retinal ganglion cells (RGCs) leading to blindness. The mechanisms mediating RGC death are still not well understood, and multiple pathogenic mechanisms have been proposed. Glutamate excitotoxicity is one of the most studied models for inducing death of the RGCs. This model is supported by a large body of literature showing that the level of glutamate is elevated in retinal ischemia and that excess glutamate plays a role in the pathogenesis of ischemic retinopathy.6–20
Ischemic and excitotoxic stressors are some of the known initiators that activate NFκB in neurons.21–27 For example, NFκB is activated in the RGCs in several model paradigms, including NMDA-induced retinal neurotoxicity (p65)28, 29, retinal ischemia and reperfusion injury (p65)30, diabetic retinopathy (p50 and p65)31 and optic nerve transaction (p50 and p65).32, 33 However, the mechanisms underlying NFκB protein activation and the cell death/survival signal transduction pathways following these types of injuries remain unclear or controversial.
Studies have shown that glutamate stimulation can activate NFκB in a Ca2+-dependent manner.34, 35 CaMKII (calcium/calmodulin-dependent protein kinase-II), an essential kinase mediating the Ca2+ message, has also been implicated in regulating NFκB activation35–37. This enzyme is downstream of glutamate receptor and responds to increases in intracellular Ca2+ resulting from stimulation of NMDA receptors. Several studies over the last decade have implicated CaMKII in regulating cell death/survival responses in a variety of cell systems.38–41 Inhibition of CaMKII activity with a specific inhibitor, AIP (autocamtide-2-related inhibitory peptide) protects retinal neurons from NMDA-induced retinal neurotoxicity.42 Taken together, we postulate that the NFκB machinery is a prospective target for CaMKII.
Since the pro- or anti-apoptotic properties of NFκB may rely on activation of distinct NFκB proteins, the focus of the present study is to investigate which NFκB members are present and which are activated in response to excitotoxic stress in the retina, specifically in the RGCs. Subsequently, we investigated whether the NMDA-receptor, Ca2+, CaMKII pathway is indeed involved in regulating the activation of NFκB.
MATERIALS AND METHODS
All animals were handled in accordance with policies and procedures recommended by the Institutional Animal Care and Use Committee at the University of Louisville, and all procedures adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Retinal Explant Culture
Retinal organ cultures were performed according to previously described protocols with some modification43, 44. Briefly, Sprague-Dawley (SD) rats were sacrificed at postnatal day 14 (P14) and their eyes were enucleated. The anterior segment, vitreous body, and sclera were removed, and the retina was mounted immediately on Millicell-CM inserts (0.4uM, Millipore, Billerica, MA) with the photoreceptor side down. Retinal explants were cultured in 1.1 ml Neuroabasal-A (Invitrogen Corporation, Carlsbad, CA) supplemented with 2% B27, 2% FBS, 1mM glutamine, and antibiotics. Considering the possible affects of ex vivo culture conditions on NFκB activation that may interfere with glutamate-induced response, pilot experiments with the aid of EMSA (see below) were performed to compare NFκB binding activation in retinas without glutamate treatment at 0, 2, 4, 6, and 20 hours in culture. Since dissection only took 1–2 minutes, retinal explants immediately after dissection (0 hour in culture) for protein extraction should represent the basal level of NFkB in the in vivo condition. No significant change in NFκB activity was observed until 4 hours later in culture (data not shown). Therefore, retinal explants were treated immediately after dissection, with or without glutamate (2, or 5 mM) for 2–4 hours, in the presence or absence of CaMKII inhibitor AIP (20uM) (Calbiochem, La Jolla, CA), or Ca2+ chelator EGTA (2mM), or NMDA-receptor antagonist memantine (20–100uM) (Tocris Cookson Inc., Ellisville, MO), or APMA-KA receptor antagonist DNQX (50uM) (Tocris Cookson Inc., Ellisville, MO). During treatment, retinal explants were maintained at 37°C in a humidified environment of 5 % CO2 and 95% air. The concentrations of glutamate were selected based on a review of the literature45, 46 and our pilot data (not shown) in order to over-stimulate glutamate receptors. Six retinas were used at each time point for each condition. At the indicated time points, retinal explants were either fixed for sectioning and immunohistochemistry, or processed on ice for nuclear and cytoplasmic protein extraction.
RGC culture
RGCs isolated from postnatal SD rat retinas were pan-purified as previously described by Barres et al.47, 48 Briefly, eyes were enucleated from SD rats (P6–8) and rinsed with Dulbecco’s phosphate-buffered saline (Invitrogen Corporation, Carlsbad, CA). Retinas were dissected under a microscope and dissociated with the aid of a Papain Dissociation System kit (Worthington Biochemicals, Lakewood, NJ) at 37° for 40 minutes to create a single-cell suspension. RGCs were isolated from this suspension using a sequential immunopanning protocol.47 The purified RGCs were seeded on poly-D-lysine/ laminin-coated 12 mm glass coverslips at a density of 2×104 per coverslip. Cells were maintained in B27-supplemented Neurobasal medium (Invitrogen, Corporation, Carlsbad, CA), containing bovine serum albumin (100 µg/mL), progesterone (60 ng/mL), insulin (5 µg/mL), pyruvate (1 mM), glutamine (1 mM), putrescine (16 µg/mL), sodium selenite (40 ng/mL), transferrin (100 µg/mL), triiodothyronine (30 ng/mL), brain-derived neurotrophic factor (BDNF; 50 ng/mL), ciliary neurotrophic factor (CNTF; 20 ng/mL), bFGF (10 ng/mL), forskolin (5 µM), inosine (100 µM), and antibiotics (Sigma-Aldrich, St. Louis, MO). RGCs were identified by expression of cell markers including Thy-1 and by their characteristic cell morphology. The purity of RGCs isolated by this sequential immunopanning is usually >99%. Cultures were maintained at 37°C in a humidified environment of 10 % CO2 and 90% air. Cells in culture for 1 week were treated with 100 µM glutamate49 for 1–2 hours and then processed for immunocytochemistry.
Immunohistochemstry
The expression patterns of the NFκB proteins were assessed in both retina and purified RGCs with the aid of double immunofluorescence labeling using specific antibodies against distinct NFκB members and thy-1, a marker for the RGCs. Whole eyes (P60), and/or retinal explants (P14) from SD rats, were fixed with 4% paraformaldehyde for 2 hours at room temperature, followed by cryoprotection in 30% sucrose at 4°C overnight and sectioning (10uM). Frozen sections were permeabilized using 0.2% Triton-X-100 (Sigma). Purified RGCs plated on poly-L-lysine/laminin coated cover slips were fixed with cold acetone:methanol (1:1) at −20°C for 10 min. Following blocking of non-specific binding sites, tissue sections or cultured RGCs were incubated with primary antibodies overnight at 4°C. NFκB antibodies used were anti-p50 (H-119), -p52 (447), -p65(C-20), RelB(C-19), and c-Rel (N-466) polyclonal antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Anti-thy-1 was a monoclonal antibody (Chemicon, International, Temecula, CA). The primary antibodies were visualized with Cy3-conjugated goat anti-mouse secondary antibody (Chemicon, International, Temecula, CA) or with Alexa 488-conjugated goat anti-rabbit secondary antibody (Molecular Probes, Eugene, OR). The slides were mounted with anti-fade mounting medium (Vector Laboratories, Burlingame, CA) and viewed with the aid of a fluorescence microscope. Images were recorded with equal exposure conditions for each specific antibody.
Electrophoretic Mobility Shift Assays (EMSA)
Nuclear proteins were extracted from retinal explants using the NE-PER Nuclear and Cytoplasmic Extraction Reagent kit (Pierce Biotechnology, Rockford, IL), following the manufacturer’s protocol. The concentrations of all protein samples were determined by the Coomassie Plus Protein Assay (Pierce Biotechnology, Rockford, IL). Equal amounts of nuclear protein extracts were analyzed for NFκB binding activity with the aid of LightShift® Chemiluminescent EMSA kit (Pierce Biotechnology, Rockford, IL) and a Biotin-labeled κB oligonucleotide probe (5’-AGTTGAGGGGACTTTCCCAGGC-3’, NFκB target underlined) (Panomics, Redwood city, CA). Briefly, 5 µg nuclear protein was combined with 20 fmol biotin-labeled κB probe in reaction buffer (1x binding buffer, 2.5% glycerol, 50ng/µl poly (dI•dC), 1%NP-40, 2.5 mM dithiothreitol, and 0.5mM EDTA) in a total volume of 20 µl for 20 min at room temperature. Competition with a 200-fold excess of unlabeled NFκB DNA probe was used to demonstrate the specificity of protein-DNA interactions. DNA–protein complexes were resolved on a 6% DNA retardation gel (Invitrogen Corporation, Carlsbad, CA), transferred to Nylon membrane (Pierce Biotechnology, Rockford, IL) and cross-linked using 254nm UV. Biotin-labeled DNA was detected using the Chemiluminescent Nucleic Acid Detection Module (Pierce Biotechnology, Rockford, IL). The relative intensities of the DNA–protein complex bands were estimated quantitatively with the aid of a computerized image analysis system (Alpha Innotech, CA) as integrated density values.
In supershift experiments, antibodies specific for different members of the NFκB family were selected for their ability to interfere with DNA binding activity. Nuclear proteins were incubated with antibodies (3 µg) against different NFκB subunits overnight at 4°C before the addition of the other components of the reaction mixture. Incubation proceeded for an additional 20 min. Polyclonal anti-p50, anti-p52, anti-p65, anti-RelB, and anti-c-Rel antibodies were the same as used for immunohistochemistry.
Western Blots
Samples containing equal amounts of cytoplasmic-protein were obtained from retinal explants and separated on 10% SDS-PAGE gels, and then transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA). The blots were blocked overnight at 4°C in 0.1% Tween-20 Tris-buffered saline solution containing 5% nonfat dry milk and then incubated with anti-IκB-α or anti-IκB-β (Cell Signaling Technology, Inc., Danvers, MA). The antibody binding was detected with horseradish peroxidase-conjugated anti–rabbit (Chemicon International Inc. Temecula, CA) secondary antibodies and ECL Western blotting detection reagents (Amersham Life Science, Buckinghamshire, England). For quantitative assays, the density of the immunolabeled bands from three independent experiments were calculated with a computerized image analysis system (Alpha Innotech, CA) as integrated density values, normalized to that of β-actin and compared with the controls, whose expression level was taken as 1.
Statistical Analysis
All quantitative data from blots were expressed as means ± S.E.M. A minimum of three independent experiments with 3–6 determinates for each condition were performed. The student’s T-test was used for two group comparisons. ANOVA was used for multiple comparisons followed by Newman-Keuls paired comparison. A P value < 0.05 significance cut off was used.
RESULTS
Expression of NFκB members in retina and RGCs
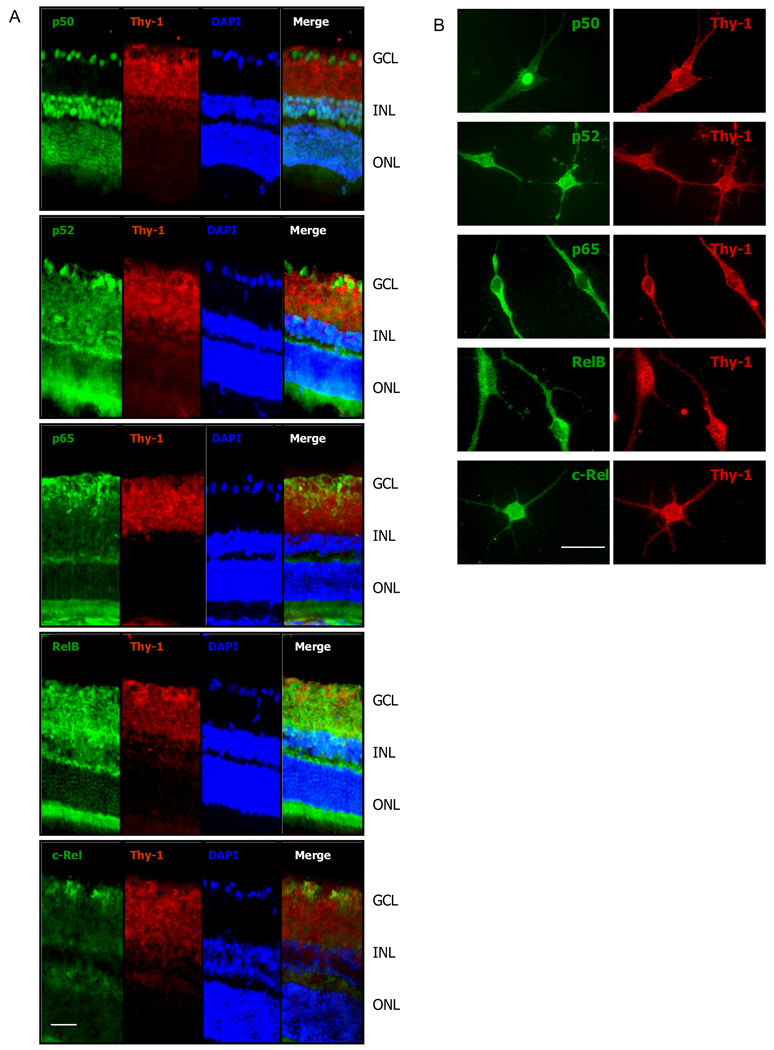
Expression patterns of NFκB proteins were investigated in both retina and pan-purified RGCs, with the aid of immunofluorescence labeling using antibodies which are specific for distinct members of the NFκB family. As shown (Figure 1A), all five members of the mammalian NFκB family were detected in the retina from sections taken from whole eyes. The expression patterns of individual members varied in the retina. While p65 and c-Rel had the most restricted expressions, largely confined to the ganglion cell layer (GCL), the p50, p52 and RelB members were expressed more widely in the other retinal layers in addition to the GCL.
Figure 1.
A. Double immunofluorescence labeling for NFκB (green) and Thy-1(red) in fixed tissue sections of retina. NFκB presents a labeling pattern of cytoplasm or nuclei or both. Co-localization (yellow) of NFκB and Thy-1 is present in the retinal ganglion cell (RGC) layer. While all the five NFκB proteins are present in the retina and the RGC layer, the expression pattern varies, with p65 and c-Rel mostly restricted to the GCL, and the other three members existed in additional layers. NFκB p50 exhibited significant constitutive nuclear localization. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. B. RGCs were purified from postnatal rat eyes (P6–8) using the two step immuno-panning method. The cells were cultured for a week before immunostaining for NFκB proteins. RGCs were identified by positive Thy-1 staining. All the five NFκB members are present in RGCs. The labeling patterns for each NFκB protein RGCs in vitro are similar to that in vivo. Scale bar, A. 50 µm; B. 25 µm
Double immunofluorescence labeling for NFκB and Thy-1 revealed some co-localization with various members of NFκB in the retinal ganglion cell layer (GCL). These results were clarified through an examination of NFκB members in pan-purified RGCs. For example, the NFκB member p50 exhibited an apparent constitutive or nuclear localization in the GCL and INL of the retina (Figure 1A) and its nuclear localization in the GCL was confirmed with the aid of purified RGCs (Figure 1B). Thus the RGCs contained a constitutively active NFκB-p50. The p52 expression appeared to be present in the nuclei and/or cytoplasm of some cells in the GCL of the retina whereas its presence in the purified RGCs appeared more peri-nuclear. The peri-nuclear labeling appeared to co-label with thy-1 in merged double labeled cells in the retinal sections suggesting also a RGC cytoplasmic presence. Rel-B labeling was evident in a diffuse pattern throughout the GCL, the IPL, the INL and the OPL. The most intense labeling was observed in the inner part of the INL. It appeared that some labeling was nuclear, but there was also much cytoplasmic labeling. A peri-nuclear labeling was evident in the purified RGCs. P65 was detected mainly in GCL and IPL, showing cytopalsmic labeling that co-localized with thy-1 staining. c-Rel showed a predominant expression pattern in GCL, with some faint labeling in OPL. The expression patterns in the retina for NFκB members are presented in Table 1.
Table 1.
Expression of NFκB proteins in retina.
| p50 | p52 | p65 | Rel-B | c-Rel | |
|---|---|---|---|---|---|
| GCL | + | + | + | + | + |
| IPL | _ | + | + | + | _ |
| INL | + | + | _ | + | _ |
| OPL | _ | + | +/_ | + | _ |
| ONL | + | _ | _ | _ | _ |
| IS/OS | _ | + | _ | + | _ |
Immunofluorescence labeling indicating presence (+) or absence (_) in retinal layers. GCL= ganglion cell layer; IPL=inner plexiform layer; INL=inner nuclear layer; OPl=outer plexiform layer; ONL=outer nuclear layer; IS/OS=inner segment and/or outer segment layer.
There were no differences in NFκB expression patterns between sections taken from the whole eye and sections taken from retinal explants (less than 4 hours in culture), or between retinas from P14 and adult animals (data not shown). In summary, these immunofluorescence data demonstrated the presence of all five NFκB proteins in the retina and in the pan-purified RGCs. Moreover, a significant constitutive presence of p50 was demonstrated in nuclei within the retina GCL and INL as well as in the purified RGCs. While both nuclear and peri-nuclear distributions of p52 and Rel-B were observed throughout the retina, p65 and c-Rel mainly showed cytoplasmic presence in GCL.
Activation of NFκB in retina in response to glutamate treatment
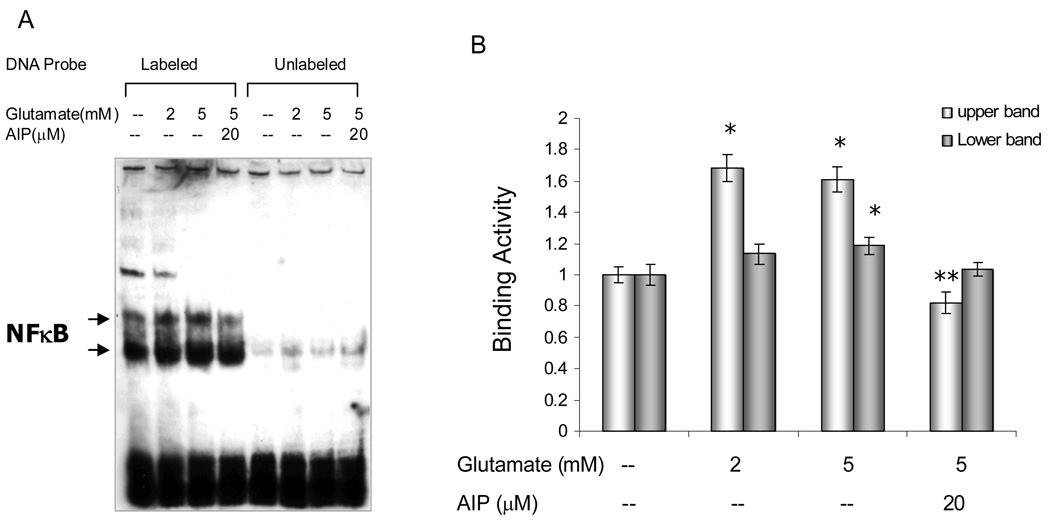
To determine steps in the signal transduction pathway for activation of NFκB, retinal explants were treated with or without glutamate (2 and 5 mM), in the presences or absence of AIP (20µM) for 4 hours, a time point when the ex vivo culture conditions caused no significant change in the level of NFκB activity, when compared to retinal explants at 0 hour (data not shown). Nuclear protein extracts were obtained and their NFκB binding activities were assayed by EMSA. As shown (Figure 2A), the specific protein-DNA interactions (labeled lanes) were demonstrated by competition with a 200-fold excess of the unlabeled NFκB probe (unlabeled lanes). The two bands (upper and lower) that changed in response to glutamate treatment reportedly represent different NFκB dimers.35 A basic level of constitutive NFκB binding activity was detected with a pan-NFkB probe in control retinal explants (lane 1; 4 hrs without glutamate), which confirmed the immunolabeling data (nuclear labeling in fixed tissue). Glutamate at concentrations of 2 or 5 mM induced a significant increase in the level of NFκB-probe binding activity (Fig 2A, lanes 2,3; Fig. 2B). Application of the CaMKII inhibitor, AIP, significantly reduced this glutamate-elicited NFκB activation (Fig 2A, lane 4; Fig 2B), which indicated an involvement of CaMKII in the activation of NFκB in some part of the retina.
Figure 2.
Retinal explants were treated with or without glutamate (2 and 5mM) for 4hrs, in the presence or absence of CaMKII inhibitor AIP (20µM). A. Nuclear extracts from the retinas were analyzed by electrophoretic mobility shift assay (EMSA), with the aid of Biotin-labeled κB oligonucleotide probe (5’-AGTTGAGGGGACTTTTCCCAGGC-3’, NFκB target underlined). Competition with a 200-fold excess of unlabeled NFκB DNA probe demonstrated the specific protein:DNA interaction. The two bands (upper and lower) may represent different NFκB dimers. B. Densitometric analyses of NFκB activation from EMSA results, showing a significant increase in NFκB binding activity in retina in response to glutamate stimulation. AIP inhibited glutamate-induced NFκB activation. The values of binding activity were expressed as a fold change of control values (without glutamate treatment), which were taken as 1. Data are means ± S.E.M from at least three independent experiments carried out in different retinal explants. * p<0.05 versus controls, **p<0.05 versus glutamate-treated groups (ANOVA).
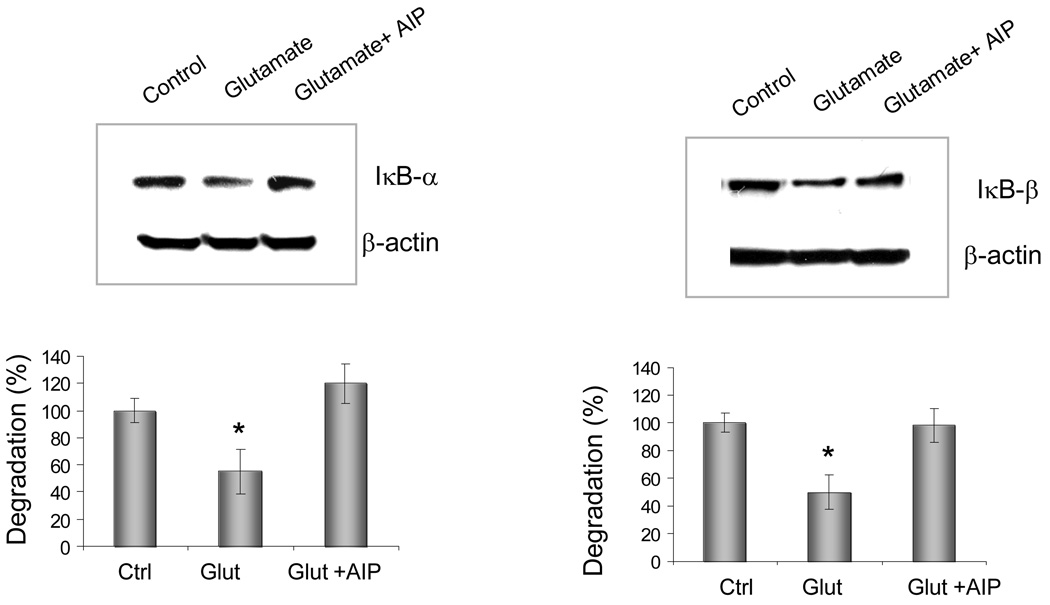
Degradation of IκB in response to glutamate treatment
To confirm the involvement of CaMKII in the activation of NFκB, cytoplasimc extracts were prepared from retinal explants 1–2 hours after glutamate exposure with or without the presence of AIP (20µM). Western blots were treated with specific antibodies for IκB-α or IκB-β. The blots were analyzed with a densitometer. Glutamate-mediated activation of NFκB was associated with reduced levels of IκBα and IκBβ after 1–2 hours exposure (Figure 3). In contrast, in the presence of AIP, these reductions in the levels of IκBα and IκBβ were not evident. These results indicated that the glutamate-induced degradation of IκB was downstream of CaMKII, and in the CaMKII-containing cells of the INL and GCL, this enzyme has an important role in the regulation of NFκB activity.
Figure 3.
The effects of glutamate and the CaMKII inhibitor, AIP, on IκB degradation in retinas. Cytoplasimc extracts were prepared from retinal explants 2 hours after glutamate exposure with or without the presence of AIP (20µM), and immunoblotted with specific antibody against IκB-α (A) or IκB-β (B). For quantitative assays, the density of the immunolabeled bands from three independent experiments were calculated with a computerized image analysis system (Alpha Innotech, CA) as the integrated density value, normalized to that of β-actin and compared with the controls, whose expression level was taken as 100%. * p<0.01 versus control or AIP-treated retinas (ANOVA)
Characterization of NFκB activation elicited by glutamate in retina
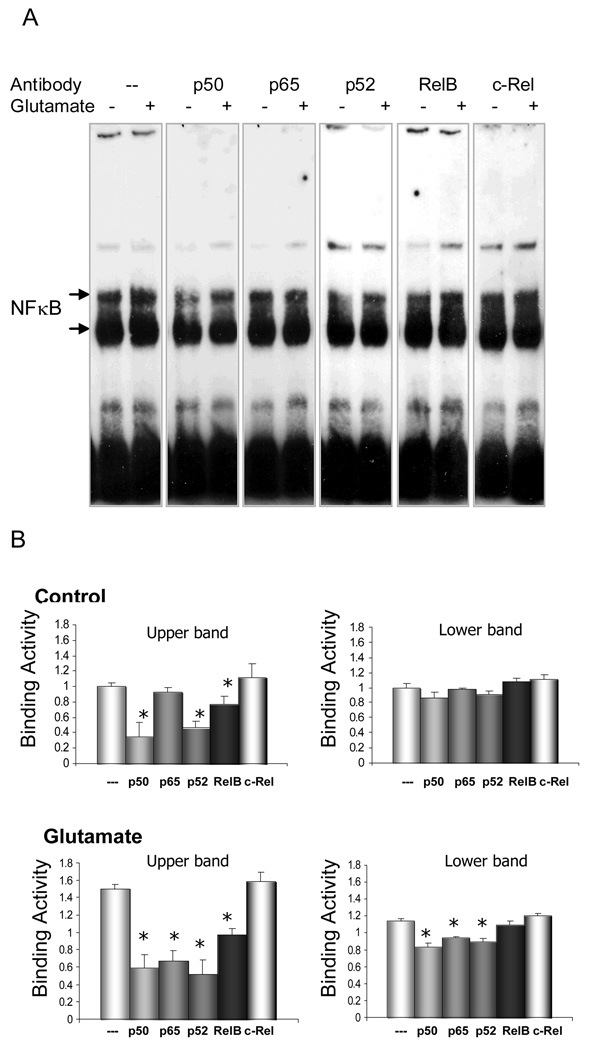
To investigate which distinct NFκB proteins are important components of the signaling machinery subsequent to glutamate stimulation, the molecular composition of NFκB complexes activated by glutamate was assessed with the aid of supershift assays. Antibodies specific for different members of the NFκB family were selected for their ability to interfere with NFκB-probe binding activity. Nuclear extracts obtained from control and glutamate-treated retinal explants were first incubated with specific antibodies against p50, p52, p65, RelB or c-Rel, and then followed by EMSA. The p50, p52 and RelB antiserum inhibited the formation of NFκB complex from the control retinal explants, but p65 and c-Rel antibodies did not modify the binding activity of NFκB (Fig 4A, lanes (−) and 4B, Control panel). These data indicate that p50, p52 and RelB were involved in NFκB constitutive activity in the ‘resting’ state. In the glutamate-stimulated retinal explants, antibodies to either p65, p50, p52 or RelB reduced NFκB binding activity, whereas antibodies to c-Rel did not show any modification to NFκB binding (Fig 4A, lanes (+) and 4B, Glutamate panel). The result indicates that c-Rel was not involved in the glutamate-elicited NFκB activation. In contrast, p50, p52 and RelB, revealed both constitutive and inducible activation. Of particular interest, p65 showed evidence of inducible activity only, implicating an important role for this member of the NFκB family in the signaling response to glutamate. In summary, c-rel was not implicated in constitutive or induced activation although it was present in the retina. The p65 member was implicated in inducible activity only, and the p50, p52 and RelB members were constitutively active but also showed additional inducible activation in response to a glutamate stimulus.
Figure 4.
A. EMSA and supershift analyses were performed in retinal explants with or without glutamate treatment (2mM, 4hrs). The molecular composition of the NFκB complexes was investigated by incubating nuclear extracts in the presence of antibodies against p50, p65, p52, RelB, and c-Rel. B. Densitometric analyses of NFκB activation from EMSA results. The values of binding activity were expressed as a fold change of control values (without glutamate treatment), which were taken as 1. Data are means ± S.E.M from three independent experiments carried out in different retinal explants. * p<;0.05 versus corresponding binding values obtained in the absence of an antibody (ANOVA). While p50, p52 and RelB were implicated in constitutive NFκB activity in control retinal explants, they also showed inducible activation in response to glutamate treatment. In contrast to p50, p52 and RelB, p65 only showed inducible activity. c-Rel was not involved in glutamate stimulation in retinal explants.
Activation of NFκB, especially p65, in RGCs in response to glutamate treatment
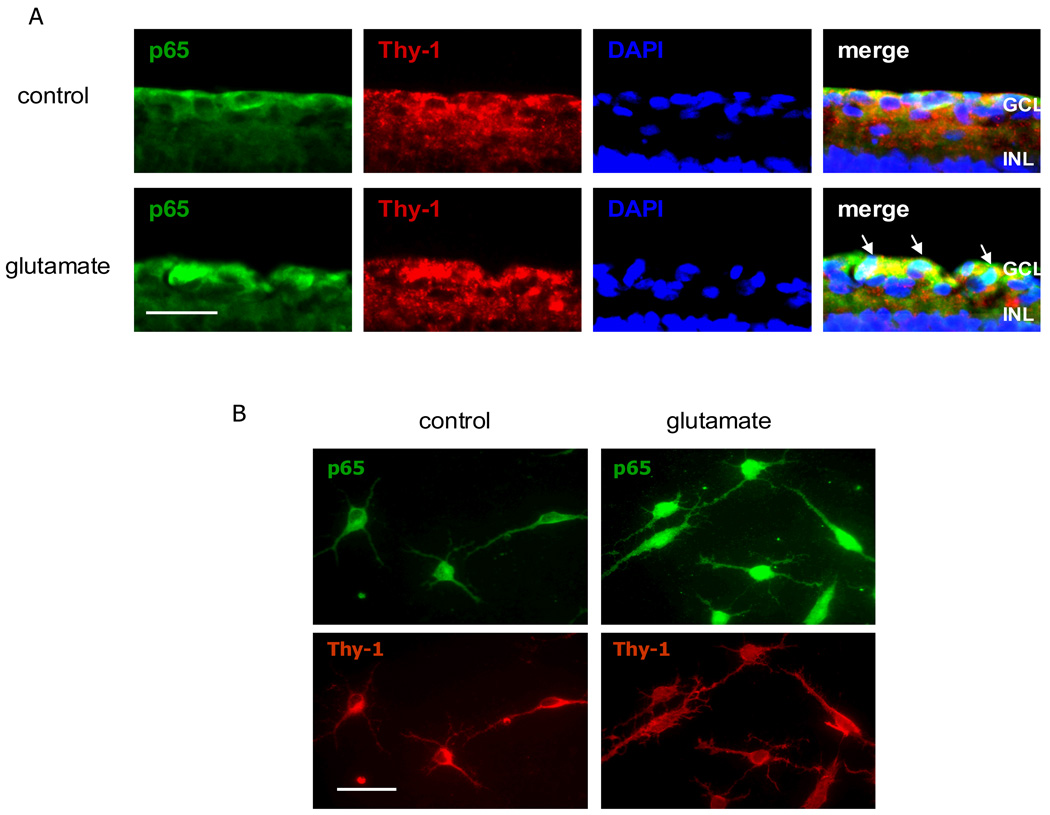
To investigate if NFκB is activated in response to glutamate stimulation specifically in RGCs, we used immunolabeling with retinal explants and pan-purified RGCs to determine if p65 was inducible in these cells. Retinal explants and purified RGCs were treated with or without glutamate for 2–4 hours, and the activation of NFκB was assayed by double immunolabeling with antibodies to p65 and Thy-1. Glutamate treatment resulted in a translocation of the p65 from the cytoplasm to the nucleus of cells in the GCL of the retinal explants (Figure 5A). This glutamate-induced translocation also occurred in the pan-purified RGCs (Figure 5B).
Figure 5.
A. Retinal explants were treated with or without glutamate (2mM, 4 hrs). Double immunofluorescence labeling for NFκB and Thy-1 plus nuclei (DAPI) staining in retina sections showed that glutamate treatment caused increased immunoreactivity and nuclear localization of p65. Co-localization of p65 and DAPI was shown in retinal ganglion cell layer in glutamate-treated retina (arrowhead). B. Purified RGCs were treated with glutamate (100µM, 2 hrs). Glutamate treatment caused the nuclear localization of p65 proteins. Scale bar, A. 50 µm; B. 25 µm
The NMDA-receptor and Ca2+signaling are involved in the activation of NFκB
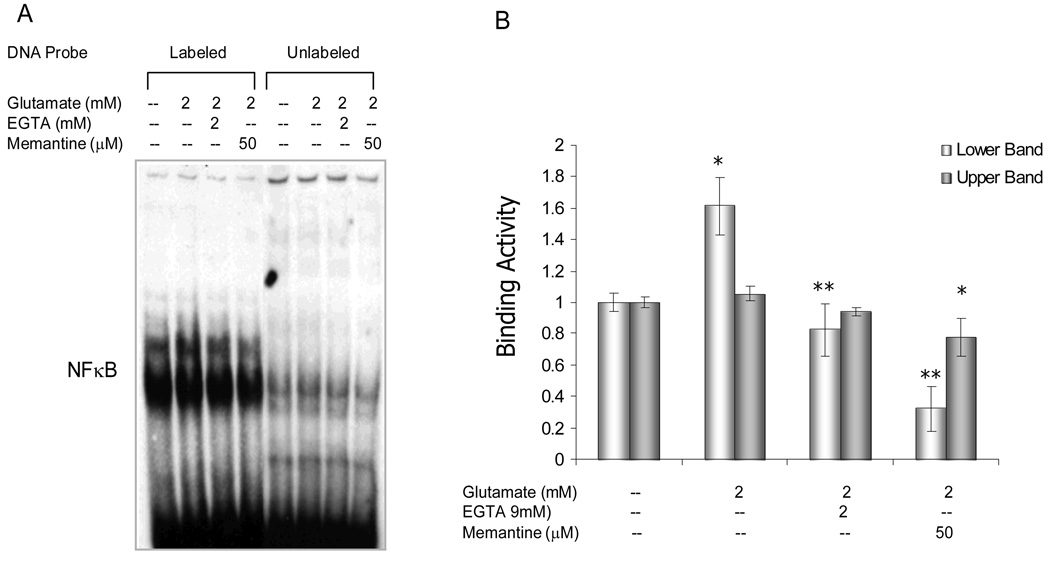
The finding that the specific CaMKII inhibitor, AIP, reduced NFκB activation in response to glutamate stimulation (Fig 2) led us to further investigate whether this NFκB activation was NMDA-receptor- and Ca2+-mediated. Retinal explants were treated with glutamate in the presence or absence of the non-competitive NMDA receptor antagonist, memantine, or the AMPA-KA receptor antagonist, DNQX, or the Ca2+ chelator, EGTA. NFκB binding activity was assessed by EMSA using retinal nuclear extracts. Blocking the NMDA receptor with memantine significantly inhibited NFκB activation (Fig 6), DNQX did not change NFκB binding activity (data not shown), suggesting that glutamate-induced NFκB activation was effected through stimulation of the NMDA receptor. Furthermore, chelation of extracelluar Ca2+ also inhibited NFκB activation (Figure 6). Together, these data indicated that the NMDA receptor-Ca2+-CaMKII signaling pathway was involved in glutamate-induced activation of NFκB.
Figure 6.
Retinal explants were treated with or without glutamate (2mM) for 4hrs, in the presence or absence of Ca2+ chelator, EGTA(2mM), or NMDA antagonist, memantine (50µM). A. Nuclear extracts from the retinal explants were analyzed by electrophoretic mobility shift assay (EMSA). B. Densitometric analyses of NFκB activation from EMSA results, showing a significant reduction in glutamate-induced NFκB binding activity by EGTA or memantine. The values of binding activity were expressed as a fold change of control values (without glutamate treatment), which were taken as 1. Data are means ± S.E.M from three independent experiments carried out in different retinal explants. * p<0.05 versus controls, **p<0.05 versus glutamate-treated retinas (ANOVA).
DISCUSSION
The present study demonstrates the presence of all five NFκB proteins in the retina, although different patterns of expression for each protein are observed. While NFκB p65 and c-Rel are mostly restricted to the GCL, p50, p52 and RelB exist in additional layers. The different expression patterns may reflect distinctive cellular phenotypes present in the retina. The presence of all five NFκB proteins, specifically in the RGCs, is also demonstrated with the aid of pan-purified RGCs.
We have used more than one technique to confirm that constitutive activity of NFκB is observed. Thus p50, p52 and RelB show basal activity with EMSA and also can be seen in the nuclei of cells within the retina. P50 is the best example of this phenomenon because it is readily identified in nuclei of cells in the GCL of the retina as well as in the nuclei of pan-purified RGCs. In contrast, c-Rel and p65 do not show evidence of constitutive activity. The finding of constitutive activity of NFκB in the retina is consistent with prior indications in neurons of the hippocampus and cerebral cortex.50 It has been suggested that the constitutive NFκB activity is the result of ongoing synaptic activity.22, 50, 51 However, the demonstration that p65 does not show constitutive activity in the retina is not consistent with other studies which indicate that p50/p65 is the major NFκB dimer functioning in synaptic transmission.21, 22, 35, 50 Whether the discrepancy is due to the cell- or tissue-type specificity is unknown. It has also been shown that constitutive activity of NFκB is required for neuronal survival in other CNS locations,52 but further studies are required to demonstrate such a role in retinal neurons.
The results demonstrating that Rel-B and p52, in particular, are constitutively active in the retina are novel. Rel-B is unique in that it does not homodimerize, and further, is unable to heterodimerize with c-Rel or p65.2 Rel-B forms heterodimers with p100, p52, and p50, and Rel-B/p52 or Rel-B/p50 heterodimers have been previously implicated in constitutive activity in multiple tissues.2, 53–55 and therefore these results in the retina are consistent.
Prior studies have shown that the loss of Rel-B results in increased inflammatory infiltration in multiple organs and this phenotype is exaggerated in the p50 knockout mouse. This indicates that Rel-B and p50 cooperate in the regulation of genes that limit inflammation.56, 57 This could be one of the mechanisms underlying “immunoprivilege” in the CNS, including in the retina, since high level of RelB/p50 constitutive activation as shown here may endow the retina with an “inflammation- or immuno-suppressive” microenvironment.58, 59 This is an area which could be explored further in the retina. As for homodimers of p52 or p50, which lack a transactivation domain (TAD), they have no intrinsic ability to drive transcription. In fact, binding of p52 or p50 homodimers to κB sites of resting cells leads to repression of gene expression.2 If this occurs and under what conditions in the retina and its RGCs need to be further studied.
Retinal ischemia, in particular, has been associated with increased levels of retinal glutamate and ultimately cell death. In the models used here, glutamate treatment eventually leads to the death of the RGCs.45, 46, 49 In response to glutamate treatment, p65, p50, RelB and p52 are activated. It is to be especially noted that, among glutamate activated NFκB proteins, p65 shows only inducible activity. This is further confirmed in purified RGC cultures. Indeed, previous studies have shown that expression and activity of NFκB p65 increases in RGCs and inner nuclear layers in retinal ischemia-reperfusion30 and NMDA-induced retinal neurotoxicity models.28, 29 Furthermore, studies on other neurons from CNS also reveal that ischemic and glutamate stimuli mainly activate p65 and/or p50.5, 25, 60, 61 Together, these studies may imply a specific and important but prospective role for p65 with respect to the death of RGCs. Based on the data presented here that p50 and p52 also exhibit inducible activity, it is possible that p65/p50 and/or p65/p52 are relevant complexes for further investigation in RGCs. In addition, our data indicate that the glutamate-activated dimmers of RelB and p52, or RelB and p50 may also exist, although their roles remain to be further identified. Since NFκB protein dimers are retained inactive in the cytoplasm by interaction with inhibitory molecules, IκBs, it is not surprising to demonstrate that the activation of NFκB mediated by glutamate correlates with a degradation of both IκBα and IκBβ in retina.
It has been reported that glutamate-induced NFκB is activated in a Ca2+-dependent manner34, 35, and that glutamate receptors (NMDA, AMPA and kainate subtypes)4, 22, 34, 62 may be involved. As an essential kinase mediating the Ca2+ message, CaMKII has also recently been shown to play an important role in mediating NFκB activation in other neurons.35 63 In the present study, we have shown an involvement of the NMDA receptor-Ca2+-CaMKII signaling pathway in NFκB activation in the retina. In addition, we have demonstrated that, the inhibition of NFκB activity through treatment with AIP significantly reduces the level of glutamate-induced IκBα and IκBβ degradation. This indicates that IκB could either be a direct substrate for CaMKII, or that some other substrate such as IKKα or IKKβ is downstream of CaMKII. This is supported by other studies showing that IKKα and IKKβ are phosphorylated by CaMKII.64, 65 To our knowledge, the results reported here provide the first evidence for an involvement of CaMKII in promoting IκB degradation, and therefore, regulation of NFκB activation in the retina in response to an excitotoxic stimulus. This part of the signaling pathway is present within the cell cytoplasm and therefore cytoplasmic-CaMKII is seen as a key control point in the glutamate-induced activation of NFκB in retina, including RGCs, given that CaMKII is known to be present in cells of the INL and GCL.
The regulation of neuronal survival or death by NFκB may depend on activation of a distinct combination of subunits, resulting in the differential regulation of target genes and the induction of diverse genetic programs that dictate the fate of cells within the retina. For example, excitotoxic stimulation-induced activation of NFκB p65/p50 may switch on expression of those κB-responsive genes involved in the control of neuronal cell death, including various pro-apoptotic genes such as p53, c-Myc, or the Fas ligand and its receptor (FAS/CD95) which could mediate a cell death response as reported elsewhere.25, 66, 67 However, the inclusion of c-Rel as part of NFκB dimers can reportedly provide a neuroprotective effect. In this case, anti-apoptotic genes such as manganese superoxide dismutase, Bcl-XL and Bfl-1 are direct transcriptional targets of c-Rel protein.68–72
Although in the present study, we have not investigated the role of a specific NFκB protein and its target gene(s) that control the cell death/survival pathways, our study may provide with some insight into the mechanism underlying NFkB activation and neuronal death/survival response. It has been shown that activation of distinct NFkB subunit (s) and the pro-apoptosis/survival properties may be stimuli-specific.5, 73, 74
Whereas some studies suggest that NFkB activation is pro-survival for RGCs, these studies were conducted using non-excitotoxic stimuli such as optic nerve transaction32 and serum deprivation.75 On the other hand, it is well documented that excitotoxic simulation induces NFkB (p65 and p50) activation and neuronal death, 25, 66, 67 including RGCs in retina.28–30 Our findings reveal that p65 and p50 are activated but c-Rel is not involved in the response to glutamate stimulation, suggesting that glutamate induces pro-apoptotic NFkB subunit(s) activation. Taken together with the finding that AIP, an inhibitor of CaMKII and a neuroprotectant against NMDA-induced retinal excitotoxicity,42 inhibits glutamate-induced NFkB activation, this could be indicative that the neurotoxic-glutamate-induced NFκB activation plays a role in mediating neuronal cell death in the retina. However, this needs to be determined through further assays. As for Rel-B and p52, which are also shown to be activated in retina subjected to glutamate stimulus, their role in regulating retinal neuronal death/survival pathways is completely unknown. Further studies, with the aid of conditional knockouts or siRNA-knockdowns of specific NFκB proteins are needed to identify the particular roles of the distinctive NFκB protein in the regulation of death and survival pathways. Thus, future studies should seek to show how these distinct NFκB proteins, and combinations thereof, can affect pro-apoptotic, anti-apoptotic or pro-survival gene-cascades.
Acknowledgments
Supported in part by NIH grants: R01EY017594; P20RR016481; P30ESO14443
References
- 1.Mattson MP, Camandola S. NF-{{kappa}}B in neuronal plasticity and neurodegenerative disorders. J Clin Invest. 2001;107:247–254. doi: 10.1172/JCI11916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayden MS, Ghosh S. Signaling to NF-{kappa}B. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 3.Mattson MP, Culmsee C, Yu Z, Camandola S. Roles of nuclear factor kappaB in neuronal survival and plasticity. J Neurochem. 2000;74:443–456. doi: 10.1046/j.1471-4159.2000.740443.x. [DOI] [PubMed] [Google Scholar]
- 4.de Erausquin GA, Hyrc K, Dorsey DA, et al. Nuclear Translocation of Nuclear Transcription Factor-kappa B by alpha -Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid Receptors Leads to Transcription of p53 and Cell Death in Dopaminergic Neurons. Mol Pharmacol. 2003;63:784–790. doi: 10.1124/mol.63.4.784. [DOI] [PubMed] [Google Scholar]
- 5.Pizzi M, Goffi F, Boroni F, et al. Opposing Roles for NF-kappa B/Rel Factors p65 and c-Rel in the Modulation of Neuron Survival Elicited by Glutamate and Interleukin-1beta. J Biol Chem. 2002;277:20717–20723. doi: 10.1074/jbc.M201014200. [DOI] [PubMed] [Google Scholar]
- 6.Osborne NN, Casson RJ, Wood JPM, Chidlow G, Graham M, Melena J. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Progress in Retinal and Eye Research. 2004;23:91–147. doi: 10.1016/j.preteyeres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Otori Y, Wei JY, Barnstable CJ. Neurotoxic effects of low doses of glutamate on purified rat retinal ganglion cells. Invest Ophthalmol Vis Sci. 1998;39:972–981. [PubMed] [Google Scholar]
- 8.Sucher NJ, Lipton SA, Dreyer EB. Molecular basis of glutamate toxicity in retinal ganglion cells. Vision Res. 1997;37:3483–3493. doi: 10.1016/S0042-6989(97)00047-3. [DOI] [PubMed] [Google Scholar]
- 9.Low HC, Gionfriddo JR, Madl JE. Assessment of glutamate loss from the ganglion cell layer of young DBA/2J mice with glaucoma. Am J Vet Res. 2006;67:302–309. doi: 10.2460/ajvr.67.2.302. [DOI] [PubMed] [Google Scholar]
- 10.Hirooka K, Miyamoto O, Jinming P, et al. Neuroprotective effects of D-allose against retinal ischemia-reperfusion injury. Invest Ophthalmol Vis Sci. 2006;47:1653–1657. doi: 10.1167/iovs.05-1018. [DOI] [PubMed] [Google Scholar]
- 11.Okuno T, Oku H, Sugiyama T, Ikeda T. Glutamate level in optic nerve head is increased by artificial elevation of intraocular pressure in rabbits. Exp Eye Res. 2006;82:465–470. doi: 10.1016/j.exer.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Wakabayashi Y, Yagihashi T, Kezuka J, Muramatsu D, Usui M, Iwasaki T. Glutamate levels in aqueous humor of patients with retinal artery occlusion. Retina. 2006;26:432–436. doi: 10.1097/00006982-200604000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Uckermann O, Vargova L, Ulbricht E, et al. Glutamate-evoked alterations of glial and neuronal cell morphology in the guinea pig retina. J Neurosci. 2004;24:10149–10158. doi: 10.1523/JNEUROSCI.3203-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harada T, Harada C, Watanabe M, et al. Functions of the two glutamate transporters GLAST and GLT-1 in the retina. Proc Natl Acad Sci U S A. 1998;95:4663–4666. doi: 10.1073/pnas.95.8.4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lagreze WA, Knorle R, Bach M, Feuerstein TJ. Memantine is neuroprotective in a rat model of pressure-induced retinal ischemia. Invest Ophthalmol Vis Sci. 1998;39:1063–1066. [PubMed] [Google Scholar]
- 16.Romano C, Price MT, Almli T, Olney JW. Excitotoxic neurodegeneration induced by deprivation of oxygen and glucose in isolated retina. Invest Ophthalmol Vis Sci. 1998;39:416–423. [PubMed] [Google Scholar]
- 17.Gorovits R, Avidan N, Avisar N, Shaked I, Vardimon L. Glutamine synthetase protects against neuronal degeneration in injured retinal tissue. Proc Natl Acad Sci U S A. 1997;94:7024–7029. doi: 10.1073/pnas.94.13.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Louzada-Junior P, Dias JJ, Santos WF, Lachat JJ, Bradford HF, Coutinho-Netto J. Glutamate release in experimental ischaemia of the retina: an approach using microdialysis. J Neurochem. 1992;59:358–363. doi: 10.1111/j.1471-4159.1992.tb08912.x. [DOI] [PubMed] [Google Scholar]
- 19.Neal MJ, Cunningham JR, Hutson PH, Hogg J. Effects of ischaemia on neurotransmitter release from the isolated retina. J Neurochem. 1994;62:1025–1033. doi: 10.1046/j.1471-4159.1994.62031025.x. [DOI] [PubMed] [Google Scholar]
- 20.Joo CK, Choi JS, Ko HW, et al. Necrosis and apoptosis after retinal ischemia: involvement of NMDA-mediated excitotoxicity and p53. Invest Ophthalmol Vis Sci. 1999;40:713–720. [PubMed] [Google Scholar]
- 21.Guerrini L, Blasi F, Denis-Donini S. Synaptic activation of NF-kappa B by glutamate in cerebellar granule neurons in vitro. Proc Natl Acad Sci U S A. 1995;92:9077–9081. doi: 10.1073/pnas.92.20.9077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaltschmidt C, Kaltschmidt B, Baeuerle PA. Stimulation of ionotropic glutamate receptors activates transcription factor NF-kappa B in primary neurons 72. Proc Natl Acad Sci U S A. 1995;92:9618–9622. doi: 10.1073/pnas.92.21.9618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scholzke MN, Potrovita I, Subramaniam S, Prinz S, Schwaninger M. Glutamate activates NF-kappaB through calpain in neurons. Eur J Neurosci. 2003;18:3305–3310. doi: 10.1111/j.1460-9568.2003.03079.x. [DOI] [PubMed] [Google Scholar]
- 24.Kaltschmidt B, Widera D, Kaltschmidt C. Signaling via NF-[kappa]B in the nervous system. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2005 doi: 10.1016/j.bbamcr.2005.05.009. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- 25.Schneider A, Martin-Villalba A, Weih F, Vogel J, Wirth T, Schwaninger M. NF-kappaB is activated and promotes cell death in focal cerebral ischemia. Nat Med. 1999;5:554–559. doi: 10.1038/8432. [DOI] [PubMed] [Google Scholar]
- 26.Schwaninger M, Inta I, Herrmann O. NF-kappaB signalling in cerebral ischaemia. Biochem Soc Trans. 2006;34:1291–1294. doi: 10.1042/BST0341291. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Jiang S, Kwong JM, Sanchez RN, Sadun AA, Lam TT. Nuclear factor-kappaB p65 and upregulation of interleukin-6 in retinal ischemia/reperfusion injury in rats. Brain Res. 2006 doi: 10.1016/j.brainres.2006.01.077. [DOI] [PubMed] [Google Scholar]
- 28.Kitaoka Y, Kumai T, Kitaoka Y, et al. Nuclear factor-kappa B p65 in NMDA-induced retinal neurotoxicity. Molecular Brain Research. 2004;131:8–16. doi: 10.1016/j.molbrainres.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 29.Kitaoka Y, Munemasa Y, Nakazawa T, Ueno S. NMDA-induced interleukin-1beta expression is mediated by nuclear factor-kappa B p65 in the retina. Brain Res. 2007 doi: 10.1016/j.brainres.2007.01.097. [DOI] [PubMed] [Google Scholar]
- 30.Chen YG, Zhang C, Chiang SK, Wu T, Tso MO. Increased nuclear factor-kappa B p65 immunoreactivity following retinal ischemia and reperfusion injury in mice. J Neurosci Res. 2003;72:125–131. doi: 10.1002/jnr.10548. [DOI] [PubMed] [Google Scholar]
- 31.Zheng L, Howell SJ, Hatala DA, Huang K, Kern TS. Salicylate-based anti-inflammatory drugs inhibit the early lesion of diabetic retinopathy. Diabetes. 2007;56:337–345. doi: 10.2337/db06-0789. [DOI] [PubMed] [Google Scholar]
- 32.Choi JS, Kim J, Kim DH, et al. Failure to activate NF-[kappa]B promotes apoptosis of retinal ganglion cells following optic nerve transection. Brain Res. 2000;883:60–68. doi: 10.1016/s0006-8993(00)02886-9. [DOI] [PubMed] [Google Scholar]
- 33.Choi JS, Sungjoo KY, Joo CK. NF-kappa B activation following optic nerve transection. Korean J Ophthalmol. 1998;12:19–24. doi: 10.3341/kjo.1998.12.1.19. [DOI] [PubMed] [Google Scholar]
- 34.Ko HW, Park KY, Kim H, et al. Ca2+-mediated activation of c-Jun N-terminal kinase and nuclear factor kappa B by NMDA in cortical cell cultures. J Neurochem. 1998;71:1390–1395. doi: 10.1046/j.1471-4159.1998.71041390.x. [DOI] [PubMed] [Google Scholar]
- 35.Meffert MK, Chang JM, Wiltgen BJ, Fanselow MS, Baltimore D. NF-kappa B functions in synaptic signaling and behavior 73. Nat Neurosci. 2003;6:1072–1078. doi: 10.1038/nn1110. [DOI] [PubMed] [Google Scholar]
- 36.Cuschieri J, Bulger E, Garcia I, Jelacic S, Maier RV. Calcium/calmodulin-dependent kinase II is required for platelet-activating factor priming. Shock. 2005;23:99–106. doi: 10.1097/01.shk.0000148075.19190.db. [DOI] [PubMed] [Google Scholar]
- 37.Mishra S, Mishra JP, Gee K, McManus DC, LaCasse EC, Kumar A. Distinct role of calmodulin and calmodulin-dependent protein kinase-II in lipopolysaccharide and tumor necrosis factor-alpha-mediated suppression of apoptosis and antiapoptotic c-IAP2 gene expression in human monocytic cells. J Biol Chem. 2005;280:37536–37546. doi: 10.1074/jbc.M504971200. [DOI] [PubMed] [Google Scholar]
- 38.Fladmark KE, Brustugun OT, Mellgren G, et al. Ca2+/Calmodulin-dependent Protein Kinase II Is Required for Microcystin-induced Apoptosis. J Biol Chem. 2002;277:2804–2811. doi: 10.1074/jbc.M109049200. [DOI] [PubMed] [Google Scholar]
- 39.Hajimohammadreza I, Probert AW, Coughenour LL, et al. A specific inhibitor of calcium/calmodulin-dependent protein kinase-II provides neuroprotection against NMDA- and hypoxia/hypoglycemia-induced cell death. J Neurosci. 1995;15:4093–4101. doi: 10.1523/JNEUROSCI.15-05-04093.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takano H, Fukushi H, Morishima Y, Shirasaki Y. Calmodulin and calmodulin-dependent kinase II mediate neuronal cell death induced by depolarization. Brain Res. 2003;962:41–47. doi: 10.1016/s0006-8993(02)03932-x. [DOI] [PubMed] [Google Scholar]
- 41.Wright SC, Schellenberger U, Ji L, Wang H, Larrick JW. Calmodulin-dependent protein kinase II mediates signal transduction in apoptosis. FASEB J. 1997;11:843–849. doi: 10.1096/fasebj.11.11.9285482. [DOI] [PubMed] [Google Scholar]
- 42.Laabich A, Cooper NG. Neuroprotective effect of AIP on N-methyl-D-aspartate-induced cell death in retinal neurons. Brain Res Mol Brain Res. 2000;85:32–40. doi: 10.1016/s0169-328x(00)00226-6. [DOI] [PubMed] [Google Scholar]
- 43.Caffe AR, Visser H, Jansen HG, Sanyal S. Histotypic differentiation of neonatal mouse retina in organ culture. Curr Eye Res. 1989;8:1083–1092. doi: 10.3109/02713688908997401. [DOI] [PubMed] [Google Scholar]
- 44.McKernan DP, Caplis C, Donovan M, O'brien CJ, Cotter TG. Age-dependent susceptibility of the retinal ganglion cell layer to cell death. Invest Ophthalmol Vis Sci. 2006;47:807–814. doi: 10.1167/iovs.05-0520. [DOI] [PubMed] [Google Scholar]
- 45.Chen TA, Yang F, Cole GM, Chan SO. Inhibition of caspase-3-like activity reduces glutamate induced cell death in adult rat retina. Brain Res. 2001;904:177–188. doi: 10.1016/s0006-8993(01)02485-4. [DOI] [PubMed] [Google Scholar]
- 46.Rocha M, Martins RAP, Linden R. Activation of NMDA receptors protects against glutamate neurotoxicity in the retina: evidence for the involvement of neurotrophins. Brain Res. 1999;827:79–92. doi: 10.1016/s0006-8993(99)01307-4. [DOI] [PubMed] [Google Scholar]
- 47.Barres BA, Silverstein BE, Corey DP, Chun LL. Immunological, morphological, and electrophysiological variation among retinal ganglion cells purified by panning 9. Neuron. 1988;1:791–803. doi: 10.1016/0896-6273(88)90127-4. [DOI] [PubMed] [Google Scholar]
- 48.Meyer-Franke A, Kaplan MR, Pfrieger FW, Barres BA. Characterization of the signaling interactions that promote the survival and growth of developing retinal ganglion cells in culture. Neuron. 1995;15:805–819. doi: 10.1016/0896-6273(95)90172-8. [DOI] [PubMed] [Google Scholar]
- 49.Fan W, Li X. Cooper NGF. CaMKII{alpha}B Mediates a Survival Response in Retinal Ganglion Cells Subjected to a Glutamate Stimulus. Invest Ophthalmol Vis Sci. 2007;48:3854–3863. doi: 10.1167/iovs.06-1382. [DOI] [PubMed] [Google Scholar]
- 50.Kaltschmidt C, Kaltschmidt B, Neumann H, Wekerle H, Baeuerle PA. Constitutive NF-kappa B activity in neurons. Mol Cell Biol. 1994;14:3981–3992. doi: 10.1128/mcb.14.6.3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O'Neill LA, Kaltschmidt C. NF-kappa B: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci. 1997;20:252–258. doi: 10.1016/s0166-2236(96)01035-1. [DOI] [PubMed] [Google Scholar]
- 52.Bhakar AL, Tannis LL, Zeindler C, et al. Constitutive Nuclear Factor-kappa B Activity Is Required for Central Neuron Survival. J Neurosci. 2002;22:8466–8475. doi: 10.1523/JNEUROSCI.22-19-08466.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dobrzanski P, Ryseck RP, Bravo R. Differential interactions of Rel-NF-kappa B complexes with I kappa B alpha determine pools of constitutive and inducible NF-kappa B activity. EMBO J. 1994;13:4608–4616. doi: 10.1002/j.1460-2075.1994.tb06782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lernbecher T, Kistler B, Wirth T. Two distinct mechanisms contribute to the constitutive activation of RelB in lymphoid cells. EMBO J. 1994;13:4060–4069. doi: 10.1002/j.1460-2075.1994.tb06723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lernbecher T, Muller U, Wirth T. Distinct NF-kappa B/Rel transcription factors are responsible for tissue-specific and inducible gene activation. Nature. 1993;365:767–770. doi: 10.1038/365767a0. [DOI] [PubMed] [Google Scholar]
- 56.Weih F, Carrasco D, Durham SK, et al. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-[kappa]B/Rel family. Cell. 1995;80:331–340. doi: 10.1016/0092-8674(95)90416-6. [DOI] [PubMed] [Google Scholar]
- 57.Weih F, Durham SK, Barton DS, Sha WC, Baltimore D, Bravo R. p50-NF-kappa B Complexes Partially Compensate for the Absence of RelB: Severely Increased Pathology in p50-/-relB-/- Double-knockout Mice. J Exp Med. 1997;185:1359–1370. doi: 10.1084/jem.185.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Massa PT, Aleyasin H, Park DS, Mao X, Barger SW. NFkappaB in neurons? The uncertainty principle in neurobiology. J Neurochem. 2006;97:607–618. doi: 10.1111/j.1471-4159.2006.03810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Streilein JW. Immune privilege as the result of local tissue barriers and immunosuppressive microenvironments. Curr Opin Immunol. 1993;5:428–432. doi: 10.1016/0952-7915(93)90064-y. [DOI] [PubMed] [Google Scholar]
- 60.Shen W, Zhang C, Zhang G. Nuclear factor kappaB activation is mediated by NMDA and non-NMDA receptor and L-type voltage-gated Ca(2+) channel following severe global ischemia in rat hippocampus. Brain Res. 2002;933:23–30. doi: 10.1016/s0006-8993(02)02291-6. [DOI] [PubMed] [Google Scholar]
- 61.Ishige K, Tanaka M, Arakawa M, Saito H, Ito Y. Distinct nuclear factor-kappaB/Rel proteins have opposing modulatory effects in glutamate-induced cell death in HT22 cells. Neurochem Int. 2005;47:545–555. doi: 10.1016/j.neuint.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 62.Cruise L, Ho LK, Veitch K, Fuller G, Morris BJ. Kainate receptors activate NF-kappaB via MAP kinase in striatal neurones. Neuroreport. 2000;11:395–398. doi: 10.1097/00001756-200002070-00034. [DOI] [PubMed] [Google Scholar]
- 63.Lilienbaum A, Israel A. From Calcium to NF-{kappa}B Signaling Pathways in Neurons. Mol Cell Biol. 2003;23:2680–2698. doi: 10.1128/MCB.23.8.2680-2698.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hughes K, Edin S, Antonsson A, Grundstrom T. Calmodulin-dependent kinase II mediates T cell receptor/CD3- and phorbol ester-induced activation of IkappaB kinase. J Biol Chem. 2001;276:36008–36013. doi: 10.1074/jbc.M106125200. [DOI] [PubMed] [Google Scholar]
- 65.Liu AM, Wong YH. Activation of nuclear factor {kappa}B by somatostatin type 2 receptor in pancreatic acinar AR42J cells involves G{alpha}14 and multiple signaling components: a mechanism requiring protein kinase C, calmodulin-dependent kinase II, ERK, and c-Src. J Biol Chem. 2005;280:34617–34625. doi: 10.1074/jbc.M504264200. [DOI] [PubMed] [Google Scholar]
- 66.Grilli M, Memo M. Possible role of NF-kappaB and p53 in the glutamate-induced pro-apoptotic neuronal pathway. Cell Death Differ. 1999;6:22–27. doi: 10.1038/sj.cdd.4400463. [DOI] [PubMed] [Google Scholar]
- 67.Qin ZH, Chen RW, Wang Y, Nakai M, Chuang DM, Chase TN. Nuclear Factor kappa B Nuclear Translocation Upregulates c-Myc and p53 Expression during NMDA Receptor-Mediated Apoptosis in Rat Striatum. J Neurosci. 1999;19:4023–4033. doi: 10.1523/JNEUROSCI.19-10-04023.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen C, Edelstein LC, Gelinas C. The Rel/NF-kappa B Family Directly Activates Expression of the Apoptosis Inhibitor Bcl-xL. Mol Cell Biol. 2000;20:2687–2695. doi: 10.1128/mcb.20.8.2687-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grumont RJ, Rourke IJ, Gerondakis S. Rel-dependent induction of A1 transcription is required to protect B cells from antigen receptor ligation-induced apoptosis. Genes Dev. 1999;13:400–411. doi: 10.1101/gad.13.4.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zong WX, Edelstein LC, Chen C, Bash J, Gelinas C. The prosurvival Bcl-2 homolog Bfl-1/A1 is a direct transcriptional target of NF-kappa B that blocks TNFalpha -induced apoptosis. Genes Dev. 1999;13:382–387. doi: 10.1101/gad.13.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bernard D, Quatannens B, Begue A, Vandenbunder B, Abbadie C. Antiproliferative and Antiapoptotic Effects of cRel May Occur within the Same Cells via the Up-Regulation of Manganese Superoxide Dismutase. Cancer Res. 2001;61:2656–2664. [PubMed] [Google Scholar]
- 72.Qiu J, Grafe MR, Schmura SM, et al. Differential NF-kappa B regulation of bcl-x gene expression in hippocampus and basal forebrain in response to hypoxia. J Neurosci Res. 2001;64:223–234. doi: 10.1002/jnr.1070. [DOI] [PubMed] [Google Scholar]
- 73.Pizzi M, Sarnico I, Boroni F, Benetti A, Benarese M, Spano PF. Inhibition of IkappaBalpha phosphorylation prevents glutamate-induced NF-kappaB activation and neuronal cell death. Acta Neurochir Suppl. 2005;93:59–63. doi: 10.1007/3-211-27577-0_8. [DOI] [PubMed] [Google Scholar]
- 74.Pizzi M, Spano P. Distinct roles of diverse nuclear factor-[kappa]B complexes in neuropathological mechanisms. European Journal of Pharmacology. 2006;545:22–28. doi: 10.1016/j.ejphar.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 75.Charles I, Khalyfa A, Kumar DM, et al. Serum deprivation induces apoptotic cell death of transformed rat retinal ganglion cells via mitochondrial signaling pathways. Invest Ophthalmol Vis Sci. 2005;46:1330–1338. doi: 10.1167/iovs.04-0363. [DOI] [PubMed] [Google Scholar]