Abstract
The development of small animal models that elicit human immune responses to dengue virus (DENV) is important since prior immunity is a major risk factor for developing severe dengue disease. This study evaluated anti-DENV human antibody (hAb) responses generated from immortalized B cells after DENV-2 infection in NOD-scid IL2rγnull mice that were co-transplanted with human fetal thymus and liver tissues (BLT-NSG mice). DENV-specific human antibodies predominantly of the IgM isotype were isolated during acute infection and in convalescence. We found that while a few hAbs recognized the envelope protein produced as a soluble recombinant, a number of hAbs only recognized epitopes on intact virions. The majority of the hAbs isolated during acute infection and in immune mice were serotype-cross-reactive and poorly neutralizing. Viral titers in immune BLT-NSG mice were significantly decreased after challenge with a clinical strain of dengue. DENV-specific hAbs generated in BLT-NSG mice share some of the characteristics of Abs isolated in humans with natural infection. Humanized BLT-NSG mice provide an attractive preclinical platform to assess the immunogenicity of candidate dengue vaccines.
Keywords: B cells, viral, human, transgenic mice, dengue
Introduction
Dengue virus, the causative agent of dengue fever (DF), is one of four closely related viruses known as dengue serotypes 1–4. Primary (1°) infection with one serotype provides life-long immunity to that serotype but does not protect against the other three serotypes.1 Secondary (2°) infection with a heterologous serotype puts people at greater risk for developing severe forms of dengue disease, dengue hemorrhagic fever (DHF), and dengue shock syndrome (DSS).2,3 Dengue virus (DENV)-specific immune responses are hypothesized to contribute to the immunopathology seen during secondary infection.4 Most patients who present to the hospital with dengue infections live in endemic areas and are experiencing a secondary infection. The serotype of the previous DENV infection is difficult to determine since antibodies with a broader pattern of neutralization to all four serotypes are elevated during and after a second infection.5 Adoptive transfer of immune sera in mice and prospective cohort studies in humans provide evidence for antibodies in protection from severe disease.6–8 Weakly neutralizing antibodies from the first infection, however, have the potential to bind to the second serotype and enhance infection of FcγR bearing myeloid cells such as monocytes and macrophages by a process known as antibody-dependent enhancement (ADE).9–11
During acute dengue infection, there is rapid activation and expansion of dengue-specific plasmablasts.12–15 Several groups have generated and characterized human monoclonal antibodies isolated from B cells in DENV-immune donors.16–21 Cross-reactive antibodies specific for the envelope (E), premembrane (prM) protein and non-structural protein-1 (NS1) with poor, moderate, or potent neutralizing activity have been isolated. A number of hmAbs from DENV-immune donors bind quaternary structures and conformation-sensitive epitopes detected only on mature virions and not on E proteins produced as a soluble recombinant (rE).22 Given the potential for DENV-specific antibodies to protect from or enhance severe disease, human studies and animal models are essential to determine how B-cell responses and Abs generated in response to DENV infection differ in primary versus secondary cases or mild versus severe disease.
Humanized mice have been used recently to evaluate human immune responses to dengue infection and dengue viral insect transmission.23–27 We recently demonstrated heightened DENV-specific antibody responses in the sera of humanized BLT-NSG mice compared to cord blood hematopoietic stem cell (HSC) engrafted mice.24 Immune sera from BLT-NSG mice were able to neutralize DENV infection in vitro. Studies with human monoclonal anti-DENV antibodies (hmAbs) have highlighted both similarities and major differences between the behavior of sera from convalescent DENV patients and purified hmAbs.16–18,20,28,29 Therefore, similar studies need to be performed in all animal models that seek to be useful in understanding aspects of human immunity to dengue.
In this manuscript, we performed a detailed analysis of the human B cell compartment following infection with DENV in BLT-NSG mice. Furthermore, we isolated and compared the functional characteristics of hAbs generated from immortalized B cells during acute DENV infection and in convalescence. Our data indicate that antigen-specific human IgM Abs are the predominant immunoglobulin isotype secreted by B lymphocytes in DENV-infected BLT-NSG mice. Some of the functional characteristics of the hAbs generated in BLT-NSG mice resemble characteristics of DENV-specific Abs isolated in humans with natural infection. Finally, we provide evidence that mice immunized with a candidate vaccine strain of DENV had decreased viral loads compared to naïve mice when challenged with a clinical strain of DENV.
Methods
Ethics statement
All experiments were performed in accordance with guidelines of the Institutional Review Board and the Institutional Animal Care and Use Committee of the University of Massachusetts Medical School. Animal use conforms with the recommendations in the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Research Council, National Academy of Sciences, 1996).
Generation of BLT-NSG mice
NOD.Cg-PrkdcscidIl2rgtm1Wjll/SzJmice (NSG) and NOD.Cg-PrkdcscidIl2rgtm1Wjll Ifnar1tm1Agt/SzJ mice (NSG-Type 1 IFNR KO) mice were bred at The Jackson Laboratory and subsequently maintained in the animal facilities at the University of Massachusetts Medical School. NSG mice at 6–8 weeks of age were irradiated (200 cGy) and surgically implanted together under the same kidney capsule with 1 mm3 fragments of human fetal thymus and liver on the day as the tissues were received as detailed in our recent report.30 Tissues were purchased from Advanced Bioscience Resources (Alameda, CA). On the same day as the tissue transplant, CD3-depleted hematopoietic cells derived from autologous fetal liver were injected by the intravenous route into the mice to achieve 1 to 5 × 105 CD34 + cells, as a source of HSC. Human cells were allowed to engraft and to generate an immune system in recipient mice for at least 12 weeks, at which time human hematolymphoid engraftment was validated by flow cytometry on peripheral blood. Successfully engrafted mice (BLT-NSG) were then randomized based on engraftment levels for use.
Infection of BLT-NSG mice with DENV
Dengue virus serotype-2 strain S16803 was initially provided by Dr Robert Putnak at Walter Reed Army Institute of Research and propagated at the University of Massachusetts Medical School. Groups of BLT-NSG mice were inoculated with a live attenuated candidate vaccine strain DENV-2 S16803 (108 PFU) by the subcutaneous (s.c.) route. In our previous studies, immunization by the s.c. route yielded better responses than immunization by the i.p. route.24,25 Dengue virus serotype-2 C0576/94 strain was provided by Dr Alan Rothman at the University of Rhode Island. For challenge studies, mice were inoculated with a low-passaged clinical DENV-2 strain, C0576/94 (∼106 PFU) by the intravenous route. Splenocytes were depleted of RBCs using RBC lysis buffer (SIGMA, St. Louis, MO) and processed to make single cell suspensions for B cell assays. Aliquots of sera were immediately frozen at −80℃ for RNA analysis and antibody titers.
Quantification of viral RNA
Serum viral RNA was extracted and purified using the QIAamp Viral RNA Mini Kit (Qiagen, Valencia, CA). Viral RNA copy numbers in sera were measured by using the quantitative real-time RT-PCR-based TaqMan system (Applied Biosystems, Foster City, CA). The RNA was subjected to reverse transcription and amplification using a TaqMan One-Step RT-PCR Master Mix Reagents Kit with DENV-2 consensus primers (forward, 5′AAGGTGAGATGAAGCTGTAGTCTC-3′, and reverse, 5′CATTCCATTTTCTGGCGTTCT-3′) and DENV-2 consensus TaqMan probe (6FAM-5′CTGTCTCCTCAGCATCATTCCAGGCA-3′-TAMRA). Probed products were quantitatively monitored by their fluorescence intensity with the ABI 7300 real-time PCR system (Applied Biosystems). DENV-2 viral RNA was used as control RNA for quantification. Viral RNA in sera was calculated based on the standard curve of control RNA. All assays were carried out in triplicate.
Generation of bulk cultures
Splenocytes (5 × 106) from DENV-infected BLT-NSG mice were immortalized with Epstein-Barr virus (EBV) in the presence of 2.5 µg/mL CpG (Operon Technologies, Alameda, CA, USA), 1000 U/mL rhIL-2, and 30 µg/mL holo-transferrin (Sigma Biochemicals, St. Louis, MO, USA), as previously described.31 After two weeks, cells were counted and were plated at 100 cells/well in 96-well plates, together with 1 × 105 allogeneic irradiated PBMC and maintained thereafter with bi-weekly stimulation with CpG, rhIL-2, and transferrin. Supernatants were collected every two weeks and tested for antibody secretion.
Sequencing and cloning of antibody genes from bulk cultures
RNA was extracted from ∼1 × 106 EBV transformed and cultured cells using an RNAqueous RNA isolation kit (Life Technologies, Grand Island, NY, USA). One microliter of purified RNA was used for the reverse-transcriptase (RT-PCR) reaction. In brief, RNA was converted to cDNA using a One-Step RT-PCR kit (Qiagen). Individual reactions were performed for the human heavy chain (five primers), kappa light chain (four primers), and lambda light chain (eight primers), as detailed for single cell reactions in Smith et al.32 The reaction mix was then run on an agarose gel to confirm the presence of a properly sized band and lack of contamination in the buffer controls. Positive samples were then sequenced to obtain V and J gene usage. Finally, another round of PCR prepared the VH(D)J/VLJ for cloning into a transient expression vector. Details of this vector and expression conditions can be found in our previously published manuscript.32 Nucleotide alignments with germline genes were performed using the sequence analysis tools IgBLAST and IMGT/V-QUEST.
Flow cytometry
Splenocytes were washed and rested in RPMI/10% FBS at 37℃ for 2 h. Cells were washed with FACS buffer (PBS/2% FBS/0.1% sodium azide) and incubated with a cocktail of monoclonal antibodies: hCD45 Alexa700, mCD45 Pacific Orange, CD19 PeCy7, CD20 PerCPCy5.5, CD38 APC, CD27 APC Cy7 (all from BD BioSciences, San Diego, CA), CD10 PECy5 (Beckman Coulter, Brea, CA), IgM PE, and IgD FITC (Dako, Carpinteria, CA, USA). Titrated amounts of antibodies were added to cells from the bone marrow and spleen and incubated at 4℃ for an additional 30 min. Cells were washed and fixed with BD Stabilizing Fixative™ (BD Biosciences). Data were collected on a BD FACSAria™ equipped with Diva v7.0 and CS&T v2.0 software (BD Biosciences) and analyzed using FlowJo version 10.
Detection of DENV specific antibody responses
DENV-2 recombinant envelope (rE) protein was purchased from Hawaii Biotech/Merck. The DENV-1-4 antigens were prepared from infected Vero cell monolayers, as previously described.24 To detect intact virions, 96-well plates were coated with concanavalin A (ConA;Vector Laboratories, Burlingame, CA, USA) at 25 µg/mL in 0.01 M HEPES (Gibco) in a total volume of 100 µL/well for 1 h. The wells were washed and 50 µL serum-free DENV preparations was added for 18 h. Then, 96-well microplates were coated overnight with 100 ng/well of DENV-2 E protein (Hawaii Biotech) or 1:40 dilution of DENV-2 infected vero cell lysate or 50 µL Con A immobilized virions. The plates were blocked with 1% bovine serum albumin for 90 min. A 1:10 dilution of sera or different dilutions of supernatants as indicated from bulk lines and B cell lines were added to the wells for 1 h. Supernatants from B cell lines that secreted hAbs were tested for binding to uninfected vero cell lysates or Con A alone to confirm specificity of binding. In every assay, wells which contained media that B cell lines were grown in were added as negative controls. Positive controls included known bulk supernatants from splenocytes of immune BLT-NSG mice. All assays were carried out in duplicate. The plates were washed with PBS containing 0.1% Tween-20. Horseradish peroxidase-labeled goat anti-human IgM (Bethyl Laboratories INC. Montgomery, TX, USA) was added as the secondary antibody. TMB solution (SIGMA-ALDRICH Inc., St. Louis, MO) was used as the substrate. The enzyme reaction was stopped by addition of 1 M HCL and the plates were read at 450 nm.
Avidity assay
Two sets of 96-well microplates were coated overnight with a 1:40 dilution of DENV inactivated lysate or 50 µL of Con A immobilized DENV. Plates were blocked with 1% BSA for 90 min and incubated with 100 µL serially diluted supernatants from hAbs in duplicate for 1 h at 37℃. Plates were washed with PBS/0.1% Tween-20. One set of plates was incubated with 8 M urea for 10 min at 37℃. Both sets of plates were washed and incubated with anti-human IgM-HRP (A80-104P; Bethyl Laboratories) for 1 h at 37℃. TMB (3,3′,5,5′-Tetramethylbenzidine) solution was then added, and the enzyme reaction was stopped by addition of 1 M hydrochloric acid. Absorbance values greater than two-fold above background were considered positive. Avidity indices were calculated as the ratio of the optical density (O.D.) with urea to the O.D. without urea.
Flow cytometry-based neutralization assay
Supernatants of B cells were tested for DENV neutralization activity. The culture supernatants were serially diluted in MEM medium containing1% bovine albumin supplemented with penicillin and streptomycin. DENV-2 was added to the diluted supernatant and incubated at 4℃ for 1 h. The virus and supernatant mixture was added to the U937 cells expressing DC-SIGN. Each dilution was assayed in duplicate. Cells were permeabilized using Cytofix/Cytoperm and stained with 1:100 dilution of DENV-specific antibody 2H2 (Millipore) followed by 1:200 dilution of FITC-conjugated anti-mouse IgG as a secondary antibody (Sigma). The percent neutralization was calculated for each dilution using formula 100-[(frequency of infected cells in the presence of antibody × 100)/frequency of infected cells in the absence of antibody].
Passive transfer studies with immune sera in NSG-Type 1 IFNR KO mice
Polyclonal anti-DENV immune sera were obtained from BLT-NSG mice infected 4–8 weeks previously with DENV-2 S16803. To prepare polyclonal serum, blood was collected via cardiac puncture, allowed to clot, and then serum was removed and pooled. Initial validation studies were performed in NSG-Type 1 IFNR KO mice using the DENV-2 strain 16681. Approximately 100 PFU of DENV-2 16681 was pre-incubated with 100 µL of 3H5-antibody (1 mg/mL), 100 µL immune sera, immune sera diluted 1:4 or 100 µL PBS for 1 h at 37℃. Groups of NSG-Type 1 IFNR KO mice were then inoculated with 100 µL per mouse of each mixture via subcutaneous injection. Serum was collected at days 5 and 10 post-infection.
Statistical analysis
All statistical calculations were performed using Graph Pad Prism version 5. Mann–Whitney U tests (two-tailed) were performed to determine statistically significant differences between median values of each data set. P < 0.05 was considered significant.
Results
B cell engraftment in BLT-NSG mice
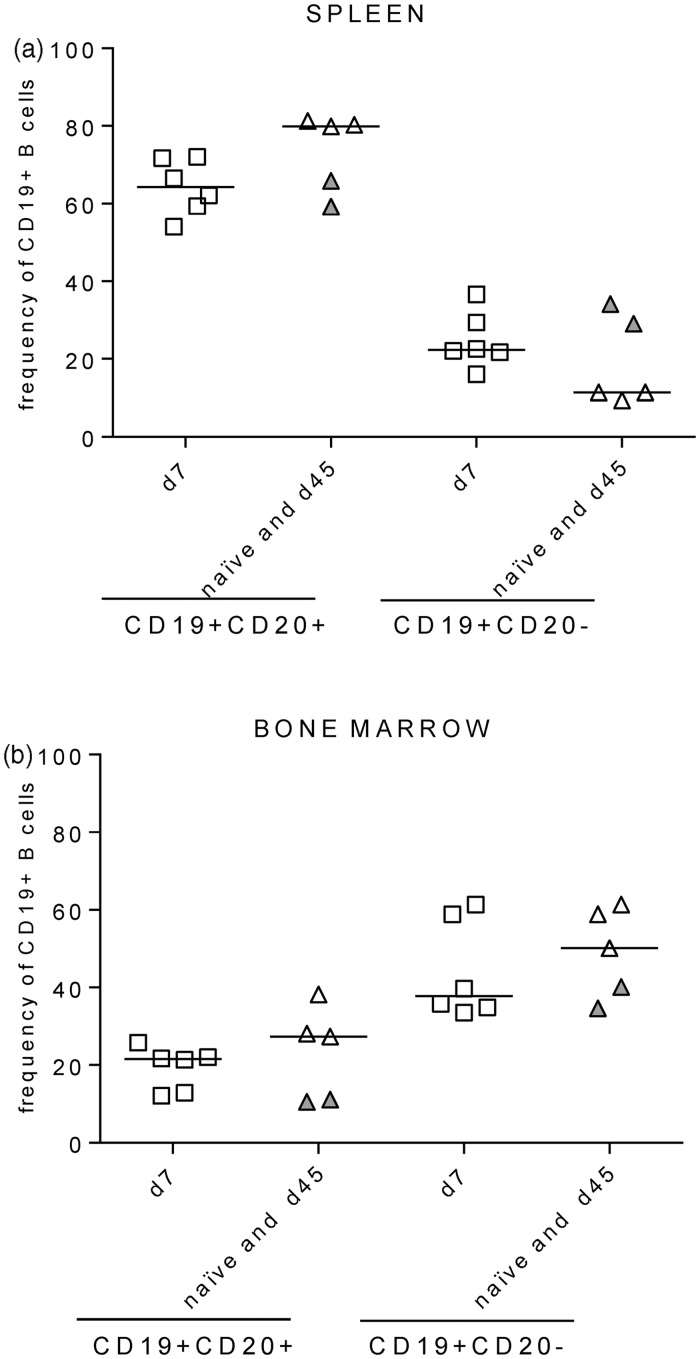
We recently demonstrated that BLT-NSG mice implanted with HLA-A2-positive or negative human fetal thymus and liver had high-level engraftment of multiple human T and B cell populations in their bone marrow and spleen.24 In our previous study, we analyzed the overall T cell and antibody responses in the sera of immune BLT-NSG mice. We wanted to substantially extend our previous studies and determine whether infection with DENV altered the frequency, phenotype of B cells and hAbs secreted by B cells in BLT-NSG mice. BLT-NSG mice were infected by the subcutaneous (s.c.) infection with 108 PFU of a parent strain of a candidate vaccine DENV-2 S16803. We first compared the frequency and phenotype of human B cells by multiparametric flow cytometry analyses in the spleen and bone marrow of acutely infected (d7 post-DENV infection) mice to naïve and immune (d45 post-DENV infection) mice using the gating strategy shown in Supplementary Figure 1(a). Frequencies of CD19 + CD20 + and CD19 + CD20 − B cells were similar in the spleen and bone marrow of acutely infected (open squares), naïve (open triangles), and immune BLT-NSG mice (closed triangles) (Figure 1(a) and (b)).
Figure 1.
B cell engraftment in BLT-NSG mice. B cells were identified using the phenotypic markers CD19 and CD20 on the CD3 negative population within the human CD45+ lymphocyte gate. Values on the Y-axis represent frequencies of hCD19+CD20+ and CD19+CD20− cells within the human CD45+ lymphocyte gate in the (a) spleen and in the (b) bone marrow of naïve (open triangles; n = 3), acutely infected (open squares; n = 6), and immune mice (filled triangles; n = 2)
CD10 expression on B cells during acute DENV infection
We next wanted to directly phenotype human B cell subsets in the spleen (peripheral lymphoid organ) and bone marrow (the site of B cell development) in acutely infected and immune BLT-NSG mice. Biswas et al. reported that B cells in the periphery of BLT-NSG mice express high levels of CD5 and CD10.33 Antibodies against CD19, CD20, CD38, CD27, IgM and IgD typically used to phenotype human B cells and antibodies against CD10 were used to further phenotype B cells from BLT NSG mice. In humans, CD5 is a marker associated with a subset of B cells called B-1 B cells predominantly of the IgM subclass34 and CD10 expression is a marker associated with immature B cells.35 We found significant expression of CD10 on CD19 + CD20 + and CD19 + CD20-B cells isolated “ex vivo” from the spleens of BLT-NSG mice. We compared the intensity of CD10 expression (hi, lo and negative) on major B cell populations in the spleen and bone marrow cells of naïve, acutely infected, and immune BLT-NSG mice. Our results indicate that there was a significant increase in the percentage of cells that were CD10hi on CD19 + CD20 + and CD19 + CD20 − B cells in splenocytes from acutely infected (d7) mice compared to naïve or immune mice (Figure 2(a) and (b)). The overall frequency of CD10hi cells was higher in the bone marrow compared to the spleen as expected; the difference in the frequency of CD10hi cells between acutely infected and naïve mice was also apparent in the bone marrow (Figure 2(c) and (d)). Representative flow plots of the intensity of CD10 expression on splenocytes and bone marrow cells are shown in Supplementary Figure 1(b) to (e) and (f) to (i), respectively.
Figure 2.
CD10 expression on CD19+CD20+ and CD19+CD20- B cells. (a, b) Frequencies of CD10hi, CD10lo, and CD10neg cells on CD19+CD20+ and CD19 + CD20 − B cells in the spleen. (c, d) Frequencies of CD10hi, CD10lo, and CD10neg cells on CD19+CD20+ and CD19 + CD20 − B cells in the bone marrow. Symbols represent individual mice that were naïve (open triangles; n = 3), infected 7 days (acute; open squares; n = 6) or 45 days prior (immune; filled triangles; n = 2) with 108 PFU DENV-2 S16803, respectively. Data shown are from three separate experiments (n = 2/experiment) for acutely infected mice. P values, as determined by Mann–Whitney U test, are shown for statistically significant comparisons
We further characterized the surface expression of CD38, CD27, IgM, and IgD on B lymphocytes with varying intensities of CD10 expression in the spleens of acutely infected mice. The majority of the CD10hi cells were IgM + IgD-CD27-CD38hi while CD10lo and CD10neg cells were >60%IgM + IgD + with intermediate CD38 expression (Supplementary Figure 2). Our data indicate an increased frequency of B-cells expressing CD10 in the spleen and bone marrow during acute DENV infection of BLT-NSG mice.
Isolation of DENV-specific hAbs from B cells in BLT-NSG mice
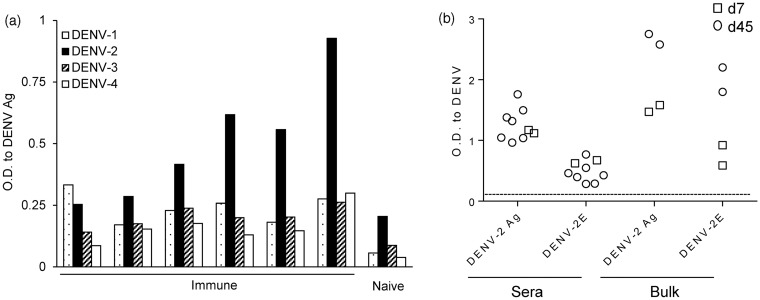
We assessed the serotype-specificity of sera from immune BLT-NSG mice by using inactivated lysates from all four serotypes of DENV. We found that the sera from immune BLT-NSG mice (Figure 3a) bound inactivated lysates of DENV-2 best with moderate binding to lysates of the other three serotypes.
Figure 3.
Serotype cross reactivity of sera and bulk culture antibody profile in BLT NSG mice. (a) Inactivated DENV lysates from all four serotypes of dengue were immobilized on ELISA plates. A 1:10 dilution of sera from immune or naïve BLT NSG mice was tested for cross-reactivity to DENV lysates. Splenocytes from mice infected 7 or 45 days prior with 108 PFU DENV-2 S16803 by the s.c. route were stimulated with CpG + IL-2 and EBV. Culture supernatants obtained 14 days later were used as a source of antibodies to perform an ELISA. (b) IgM responses to DENV-2 antigen and DENV-2 rE protein were tested using undiluted bulk supernatants from splenocytes of acutely infected (open squares) and immune (open circles) mice and a 1:10 dilution of sera
We wanted to isolate hAbs from BLT-NSG mice to determine whether they have characteristics similar to Ab responses detected in humans after natural dengue infection. Splenocytes from mice obtained 7 (acute) or 45 (immune) days post-subcutaneous (s.c.) infection with 108 PFU DENV-2 S16803 were immortalized with EBV and stimulated with CpG as previously described.31 We used vero cell-derived DENV lysates which contain multiple structural and non-structural proteins as well as purified recombinant E protein to assess the breadth of DENV responses in the sera and bulk supernatants from infected mice. IgM Abs specific for DENV-2 antigens and rE protein were detectable in the bulk supernatants from stimulated splenocytes and in the sera from both acutely infected and immune mice (Figure 3b). DENV-specific IgG antibodies were not detected in the bulk supernatants or immune sera (data not shown). Bulk supernatants from stimulated naïve BLT-NSG splenocytes did not bind DENV-2 Ag and rE protein (data not shown).
Splenocytes from the bulk cultures were seeded at 100 cells/well in 96-well plates, and the immortalized B cell culture supernatants were screened four weeks later for Abs that bound an inactivated DENV-2 lysate. DENV-specific IgM Abs were detected in 132/960 (13%) wells from acutely infected mice and 43/960 (4.4%) wells in immune mice (data not shown). We selected 16 wells from acutely infected mice and 8 wells from immune mice with O.D. values ≥5 times above background for further analysis. Cells were expanded and supernatants were collected every two weeks and tested by enzyme linked immunosorbent assay (ELISA) to define the major antigens they recognized.
hAbs are serotype cross-reactive and recognize epitopes on the envelope protein and intact virions
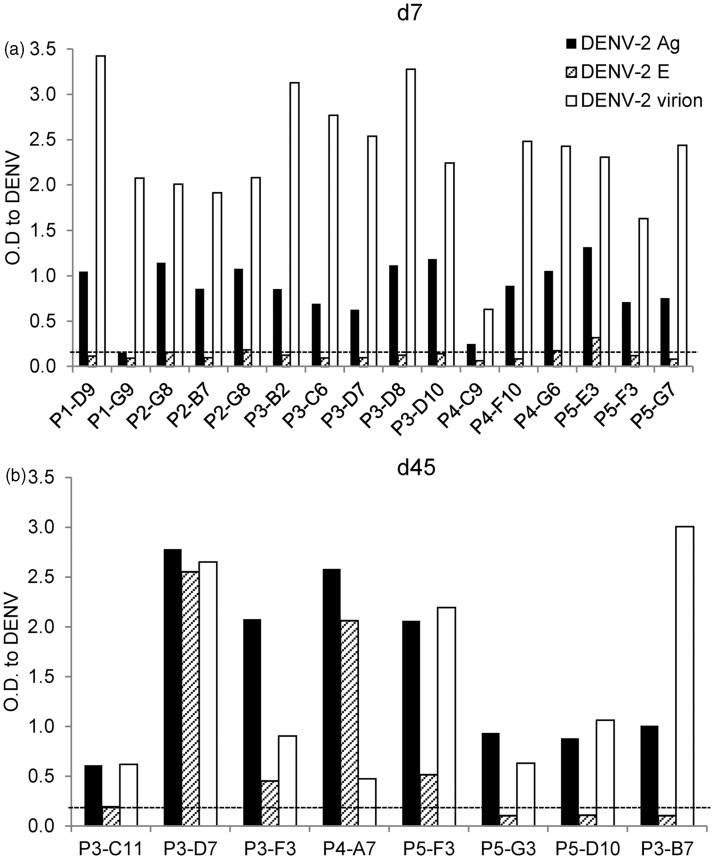
We performed an in-depth characterization of the antigens recognized by hAbs, serotype-specificity, and neutralization potency of hAbs produced by immortalized B cell lines generated from DENV-infected BLT-NSG mice. We used vero cell-derived DENV lysates, soluble recombinant E protein, and Con A immobilized intact virions in ELISA assays to identify the antigens recognized by hAbs. We first confirmed that B cells that were positive initially in screening continued to secrete Abs that bound the DENV-2 inactivated lysates (Figure 4(a) and (b)). Since the inactivated cell lysates contain multiple structural and nonstructural proteins of DENV, we next tested the supernatants for recognition of DENV envelope protein, the major target of anti-DENV antibodies using a soluble rE protein. While the majority (14/16) of Abs secreted by B cells obtained from acutely infected mice bound inactivated DENV lysates, none of the Abs bound rE (Figure 4a). In contrast 4/8 hAbs isolated from B lymphocytes in immune mice bound the rE protein (Figure 4b). To determine whether the hAbs generated in BLT-NSG mice were able to bind mature virions, we used a modified ELISA previously tested in our human studies.36 We used concanavalin A (ConA) to affinity-immobilize viral glycoproteins,18,37 and we detected substantial binding of all hAbs to Con A immobilized DENV-2 (Figure 4(a) and (b)). A number of hAbs isolated during acute infection were not able to bind rE soluble protein but bound epitopes exposed on intact virions and on inactivated cell lysates. Supernatants were tested for binding to uninfected vero cell lysates, media, and Con A to confirm specificity of binding. ELISA results from representative supernatants of hAbs P3-D7 and P3-C11 are shown (Supplementary Figure 3a) and indicate that binding of hAbs was directed against DENV components.
Figure 4.
hAbs isolated during acute DENV infection in BLT-NSG mice bind intact virions. Twenty-four hAbs were tested for their ability to bind inactivated DENV-2 lysates (DENV-2 Ag), soluble rE protein, and intact DENV virions. Recognition of DENV determinants by hAbs isolated from splenocytes at (a) d7or (b) d45 post-DENV infection. The majority of hAbs isolated at d7 and d45 recognize DENV-2 inactivated lysates and intact DENV virions. One of two representative experiments is shown
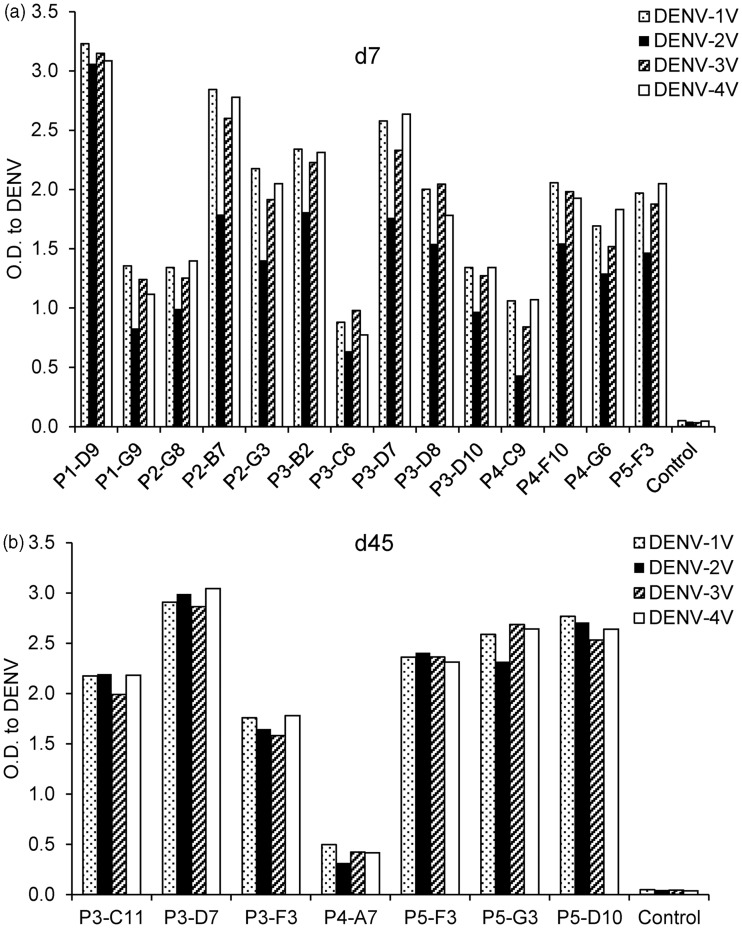
All hAbs generated in BLT-NSG mice were tested for cross-reactivity to inactivated cell lysates and intact virions from all four serotypes of DENV. They were found to be cross-reactive and able to bind more than one serotype of intact DENV virions and inactivated DENV lysates (Figure 5(a) and (b) and data not shown).
Figure 5.
hAbs isolated during acute infection and in immune mice. Intact virions were immobilized on ELISA plates using a modified ELISA. Supernatants from hAbs isolated from splenocytes at (a) d7 or (b) d45 post-DENV infection were tested by ELISA for cross-reactivity to multiple serotypes of Con A immobilized intact DENV virions. One of three representative experiments is shown
hAbs generated in BLT-NSG mice have poor neutralizing activity
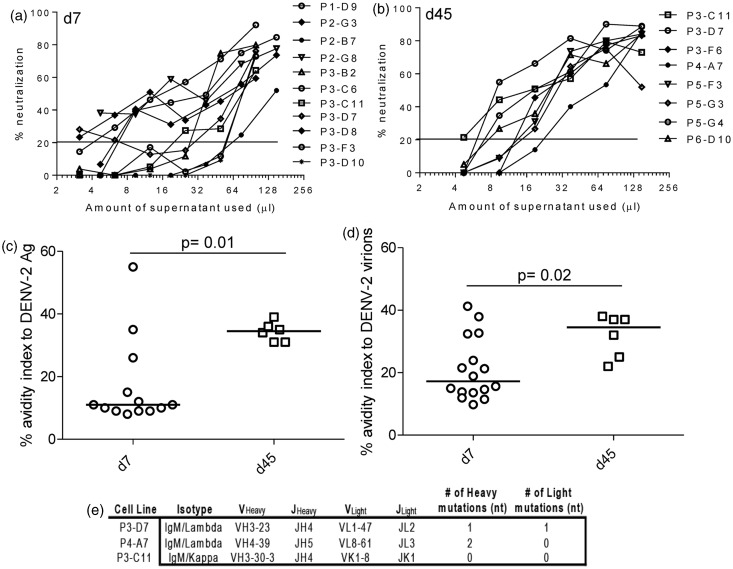
We tested the neutralizing activity of hAbs using a flow-based neutralization assay with U937 DC SIGN cells.38 Dengue immune sera and human AB sera were used as controls to establish the assay (Supplementary Figure 3b). Neutralization reached 100% only at high concentrations of hAbs used from immunized BLT-NSG mice. Shown are graphs depicting neutralization percentage in the presence of different amounts of supernatants collected from B cells obtained during acute infection and in immune mice (Figure 6(a) and (b)). While all hAbs had poor neutralizing activity in vitro, the hAbs isolated in immune mice were able to neutralize DENV at higher dilutions compared to hAbs isolated during acute infection.
Figure 6.
Neutralization activity and antibody avidity and of hAbs isolated from DENV-infected BLT-NSG mice. Neutralization activity of representative hAbs isolated at (a) d7 or (b) d45 using a flow-based neutralization assay. Percent neutralization of serially diluted supernatants from stimulated B cells that secrete hAbs was calculated. Avidity indices of hAbs to (c) DENV-2 inactivated lysates and (d) Con A-immobilized DENV-2. The avidity index was calculated as the ratio of the O.D. with urea to the O.D. without urea multiplied by 100. P values, as determined by Mann–Whitney U test, are shown for statistically significant comparisons. (e) RNA was isolated from three of the B cell lines, and the antibody mRNA was converted to cDNA and sequenced
hAbs generated in immune mice have higher avidity index compared to hAbs obtained during acute infection
We wanted to determine whether hAbs generated in immune mice had a higher avidity compared to hAbs isolated during acute infection reflecting affinity maturation of the antibodies over time. We used avidity assays previously established on samples from dengue-immune donors and our clinical studies.36,39 We first assessed the avidity index of bulk supernatants generated from stimulated splenocytes of acutely infected and immune mice. We found a similar avidity index of bulk supernatants from acutely infected and immune mice to all four serotypes of DENV lysates (Supplementary Figure 3c). We next compared the avidity index between hAbs generated during acute infection and in immune mice with inactivated DENV lysate and Con A immobilized intact virions. Our data indicate the hAbs generated in immune mice had a higher avidity index to DENV antigens (Figure 6c) and whole virions (Figure 6d) compared to hAbs isolated during acute infection.
This increase in avidity between day 7 and day 45 may indicate affinity maturation of the antibodies at day 45. To determine whether the antibodies have undergone somatic hypermutation (SHM), the antibody genes of several day 45 cultures were sequenced. Sequences from three of these antibodies were identical to germline sequences, indicating that no SHM had taken place, nor was it responsible for the avidity increase (Figure 6e) in antibodies detected at day 45. Therefore, the increase in avidity detected on hAbs at day 45 did not represent affinity maturation of the Abs tested through somatic hypermutation.
DENV-immunized BLT-NSG mice have decreased viral titers
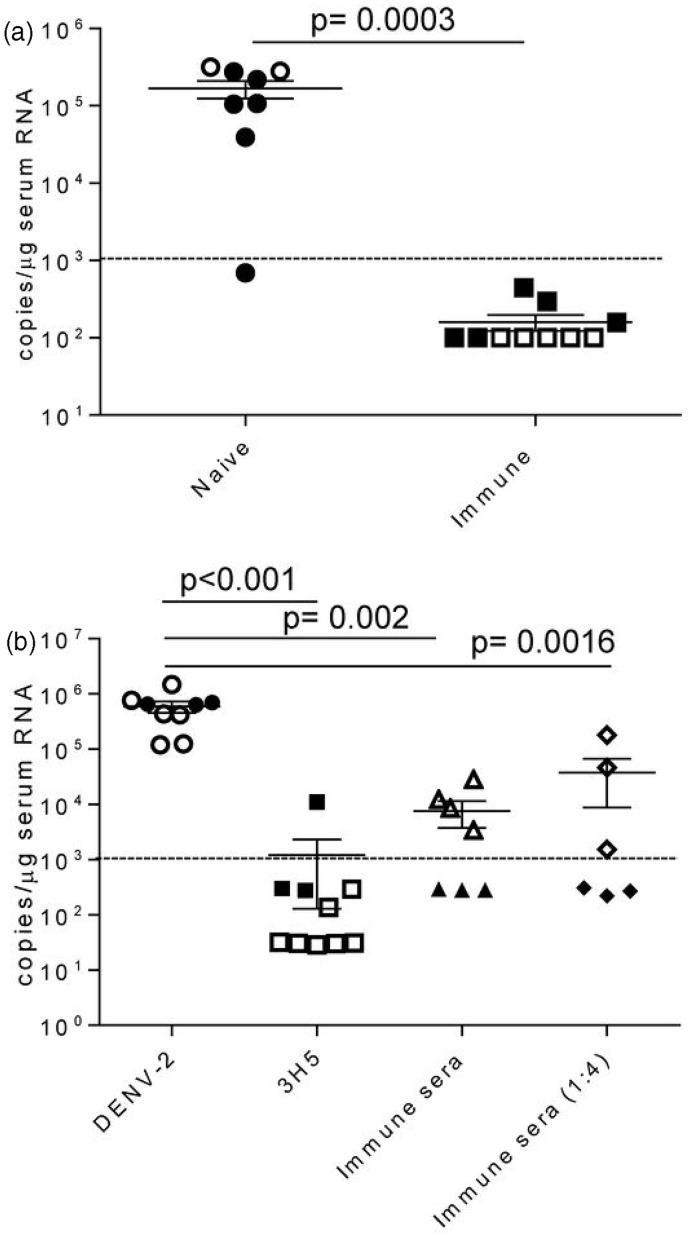
We next determined whether BLT-NSG mice immunized with a candidate vaccine strain DENV-2 S16803 were able to reduce viral replication when challenged with a clinical strain of DENV. Naïve or immunized (administered approximately 108 PFU of DENV-2 S16803 3-9 weeks prior) BLT-NSG mice were inoculated with a clinical strain of DENV by the intravenous route. Sera were obtained seven days later and assessed for the presence of DENV-2. By qRT-PCR with a detection limit of 1000 copies per reaction, viral titers were only detected in the sera of naive mice challenged with a clinical DENV-2 strain (Figure 7a).
Figure 7.
Decreased viral titers in immune BLT-NSG mice. (a) Mice were immunized three (closed squares) or nine (open squares) weeks prior with 1 × 108 PFU or 6 × 107 PFU of DENV-2 S16803, respectively, by the s.c. route. Matched naïve (closed and open circles) or immunized BLT-NSG mice were inoculated with ∼1 × 106 PFU of a clinical strain DENV-2 C0576/94 by the i.v. route. Mice were bled at day 7and RNA was isolated from serum and subjected to one-step RT-PCR and quantitative PCR. Values represent copy number of viral RNA present per µg serum RNA detected by quantitative RT-PCR. Two separate experiments were performed (open and closed symbols) and were pooled together for analysis; n = 8 naïve BLT NSG and n = 10 immune BLT NSG mice. (b) DENV-2 16681 (1 × 103 PFU/mL) was incubated with 100 µL PBS (DENV-2), 100 µL 3H5 antibody (3H5), 100 µL BLT-NSG immune serum, and 100 µL diluted (1:4) BLT NSG immune serum for 1 h at 37℃. NSG-Type 1 IFNR KO mice were injected s.c. with 100 µL of a 1:1 mixture and mice were bled at day 5. RNA was isolated from serum and subjected to one-step RT-PCR and quantitative PCR. Values represent copy number of viral RNA present per µg serum RNA detected by quantitative RT-PCR. Two separate experiments were performed (open and closed symbols) and were pooled together for analysis. P values, as determined by Mann Whitney U test, are shown for statistically significant comparisons. Error bars represent the mean with SEM. Dashed line represents the limit of detection
We used NSG-Type 1 IFNR KO mice where we see consistent viral replication with very low doses of DENV (unpublished observations), to determine whether immune sera from BLT-NSG mice were able to confer protection. We pre-incubated pooled immune sera from BLT-NSG mice with DENV-2 and were able to show a significant decrease in viral replication in the presence of immune sera five days post-infection in two separate experiments (Figure 7b). The suppression of viral replication was weaker in one of the two experiments performed when diluted immune serum from BLT-NSG mice was used. When DENV-2 was pre-incubated with a well characterized DENV mAb 3H5, there was a significant decrease in viral titers in infected mice at day 5 by qRT-PCR. Together, our data indicate that DENV-immunized BLT-NSG mice have decreased viral titers when challenged with a clinical strain of DENV. Furthermore, immune sera from BLT-NSG mice were able to effectively decrease viral replication.
Discussion
We demonstrate in this manuscript that IgM hAbs generated in humanized BLT-NSG mice have some of the functional characteristics of mAbs isolated from patients with natural dengue infection. hAbs isolated during acute infection and in immune BLT-NSG mice were highly cross-reactive, poorly neutralizing, and many recognized determinants on intact virions but not rE protein.16,18–20,36 Furthermore, immunized BLT-NSG mice were able to effectively control viral replication when challenged with a clinical strain of DENV. However, as shown by other groups, BLT NSG mice have compromised B cell differentiation with an elevated number of immature B cells in the periphery33,40,41 and lack of production of DENV-specific IgG. These findings indicate that BLT-NSG mice require further optimization before they can be used as an appropriate animal model to assess human Ab and B cell responses after controlled primary and secondary homologous and heterologous DENV infections in vivo.
The vast majority of patients with dengue infection live in endemic areas and are experiencing a second infection. The mechanism(s) responsible for the development of more severe disease remain to be clearly defined but Abs are proposed to prevent or enhance disease. Ab responses are markedly different during 1° and 2° DENV infections in humans.42,43 Following a 1° DENV infection, humans develop Abs that cross-react with all four serotypes, but mainly neutralize the homologous serotype responsible for the infection. Following a 2° infection with DENV, Ab responses are characterized by a much higher titer with a broader pattern of neutralization to all four serotypes. Due to the broad pattern of Ab reactivity, it is difficult to identify the specific serotype of the first DENV infection. We have shown that human B cell responses and hAb responses are detected in BLT-NSG mice after a 1° infection. The ability to control the dose, serotype, and route of infection for a second infection is an important advantage of this model necessary to delineate the contributions of prior humoral immunity to a second infection with DENV.
As has been reported by other groups, a large number of B-cells in the periphery of BLT-NSG mice are immature.33,40,41 Lang et al. recently reported that a more mature population of B-cells in BALB/c-Rag2nullIL2rγnullmice was detected which was associated with antigen-specific IgG responses in mice maintained over 16 weeks.44 We found B-cells at all major stages of the developmental pathway (early immature, immature and mature naïve) in BLT-NSG mice, but cells expressing CD27, associated with a memory phenotype (either IgM or IgG), were not present. During acute infection, we found an increase in the frequency of B-cells expressing high levels of CD10 in the spleen. The CD10hi cells expressed markers mostly associated with immature cells (CD38hiIgM + IgD-) which lead us to conclude that immature cells were recruited into the periphery during acute infection. This observation in BLT NSG mice adds two complications to the studies. First, malaria and HIV are known to cause a flux of CD10hi cells in the periphery.45,46 It is unclear, however, whether DENV also induces CD10hi B cells to be recruited into the periphery or whether blunted B cell differentiation in humanized models results in the expansion of CD10hi (presumably immature) cells. Furthermore, although they are likely to be antigen-inexperienced, it is unknown whether these cells are able to secrete Abs.
Our data suggest that DENV can activate IgM-secreting B-cells in humanized BLT-NSG mice. The Abs that only recognize intact virions may recognize glycosylated epitopes present on the intact virion but not on the recombinant E protein or it is possible that they recognize conformation-dependent epitopes present on intact virions. Abs that recognize intact virions may represent a more dominant proportion in BLT-NSG mice compared to responses in humans. Natural antibodies secreted by B cells are predominantly of the IgM subclass. These Abs have been characterized extensively in mice and are present during early human life and have been shown to be polyreactive.47 In addition to displaying low affinity interactions with self-antigens, these Abs have also been found to play a major role in early protective responses against pathogens.34 Recently, cord blood HSC-engrafted NSG mice were used to demonstrate that the resolution of the primary episode of bacteremia was concurrent with the generation of Borrelia hermsii-specific IgM Abs.48 When three of the IgM hAbs in our study were sequenced, cloned, and expressed recombinantly with a human IgG1 Fc, we saw significant level of poly-reactivity in all three clones (data not shown). Whether the poly-reactivity was a reflection of the Abs being “natural” is unknown and requires further examination.
We previously showed that IgM levels in the sera of immunized BLT-NSG mice did not decline significantly even at eight weeks after immunization.24 Our findings that hAbs isolated in immune BLT-NSG mice have a higher avidity and better neutralization capacity compared to Abs isolated during acute dengue infection are interesting. The data suggest that B cells present after resolution of infection in BLT-NSG mice may be functionally more mature and perhaps have undergone some form of affinity maturation. However, the sequences of Abs characterized at day 45 were not mutated and were similar to germline Abs. It is possible that repeated exposure to virus, either by conventional germinal center reactions or by TLR stimulation, may have caused cycles of selection, eventually allowing only the B cells with the higher affinity receptors to predominate.
Our findings that immune BLT-NSG mice had lower viral titers compared to naïve mice administered with a clinical strain of DENV suggested that DENV-specific IgM Abs or T cells maintained for a long term in BLT-NSG mice were important for viral control. We transferred immune or naïve sera from BLT NSG mice or a well-characterized dengue monoclonal Ab 3H5 into naive cord blood engrafted or BLT NSG mice, and challenged the mice with a clinical strain of DENV. In several experiments, we were unable to show clear differences in viral titers between mice that received PBS, immune sera, naïve sera, or even the mAb 3H5 (data not shown). Although we have no definitive proof, we speculate that insufficient amounts of immune sera and variable replication of DENV in humanized mice contributed to these inconclusive results. We therefore used NSG-Type 1 IFNR KO mice where we see consistent replication even with very low doses of DENV to determine whether immune sera from BLT-NSG mice can reduce viral titers. In this model, we have shown that transfer of immune sera from BLT-NSG mice resulted in a significant decrease in viral titers. Further studies will need to be performed to conclusively determine whether individual hAbs or T cells isolated from BLT-NSG mice can also decrease viral titers.
There are a number of limitations with generating antigen-specific Ab responses in current models of immunodeficient mice engrafted with human HSC. The poor ability of Abs to class switch has been attributed to the presence of B cells at all stages of the development pathway in the periphery, the lack of HLA restricted T cell help, inadequate germinal center formation, and perhaps limited T cell helper activity.49 Watanabe et al. were able to show that mature IgM + IgD + B cells in NOG mice (that have a truncated IL2rγ gene) could class switch in vitro and in vivo thereby suggesting that there was no intrinsic defect in B cells generated in NOG mice engrafted with HSC but rather an impairment in T cell help.41 In our hands, DENV-specific B cells from BLT-NSG mice were not able to class switch in vitro when provided with key cytokines known to enhance class switching (data not shown). The absence of DENV-specific IgG is a significant limitation of this model and it will be challenging to evaluate ADE in current models of humanized mice.
In conclusion, we have demonstrated that DENV-specific IgM Abs generated in BLT-NSG mice have some of the functional characteristics of human Abs. We are actively looking at novel humanized models with improved engraftment and increased expression of cytokines critical for B and T cell maturation and function. Given the role that prior immunity plays in protection and/or pathogenesis during secondary DENV infections, improvement of these animal models is likely to provide key insights into the pathogenesis of DENV infection that would be challenging to perform in humans.
ACKNOWLEDGEMENTS
This study was supported by a pilot project from grant U19 AI57319, discretionary fund project U19 AI57234, R21AI103650 from the NIAID, NIH grants CA34196 and 046629, and NIH Diabetes Endocrinology Research Center (DERC) grant DK52530.
Author contributions
SJ, AR, KS, MW, and PP performed the experiments. KS performed the antibody sequencing and cloning. SJ, MW, MAB, and AM were involved in the design of the experiments. SJ and AM wrote the manuscript. LDS, DLG, and MAB provided engrafted humanized mice, reviewed the manuscript, and were involved with multiple aspects of the design and conception of the study.
References
- 1.Sabin AB. Research on dengue during World War II. Am J Tropic Med Hygiene 1952; 1: 30–50. [DOI] [PubMed] [Google Scholar]
- 2.Burke DS, Nisalak A, Johnson DE, Scott RM. A prospective study of dengue infections in Bangkok. Am J Tropic Med Hygiene 1988; 38: 172–80. [DOI] [PubMed] [Google Scholar]
- 3.Thein S, Aung MM, Shwe TN, Aye M, Zaw A, Aye K, Aye KM, Aaskov J. Risk factors in dengue shock syndrome. Am J Tropic Med Hygiene 1997; 56: 566–72. [DOI] [PubMed] [Google Scholar]
- 4.Rothman AL. Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms. Nat Rev 2011; 11: 532–43. [DOI] [PubMed] [Google Scholar]
- 5.Murphy BR, Whitehead SS. Immune response to dengue virus and prospects for a vaccine. Ann Rev Immunol 2011; 29: 587–619. [DOI] [PubMed] [Google Scholar]
- 6.Kaufman BM, Summers PL, Dubois DR, Eckels KH. Monoclonal antibodies against dengue 2 virus E-glycoprotein protect mice against lethal dengue infection. Am J Tropic Med Hygiene 1987; 36: 427–34. [DOI] [PubMed] [Google Scholar]
- 7.Sukupolvi-Petty S, Austin SK, Engle M, Brien JD, Dowd KA, Williams KL, Johnson S, Rico-Hesse R, Harris E, Pierson TC, Fremont DH, Diamond MS. Structure and function analysis of therapeutic monoclonal antibodies against dengue virus type 2. J Virol 2010; 84: 9227–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Endy TP, Nisalak A, Chunsuttitwat S, Vaughn DW, Green S, Ennis FA, Rothman AL, Libraty DH. Relationship of pre-existing dengue virus (DV) neutralizing antibody levels to viremia and disease severity in a prospective cohort study of DV infection in Thailand. J infect Dis 2004; 189: 990–1000. [DOI] [PubMed] [Google Scholar]
- 9.Goncalvez AP, Engle RE, St Claire M, Purcell RH, Lai CJ. Monoclonal antibody-mediated enhancement of dengue virus infection in vitro and in vivo and strategies for prevention. In: Proceedings of the National Academy of Sciences of the United States of America, 2007;104:9422–7. [DOI] [PMC free article] [PubMed]
- 10.Kliks SC, Nimmanitya S, Nisalak A, Burke DS. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am J Tropic Med Hygiene 1988; 38: 411–9. [DOI] [PubMed] [Google Scholar]
- 11.Kliks SC, Nisalak A, Brandt WE, Wahl L, Burke DS. Antibody-dependent enhancement of dengue virus growth in human monocytes as a risk factor for dengue hemorrhagic fever. Am J Tropic Med Hygiene 1989; 40: 444–51. [DOI] [PubMed] [Google Scholar]
- 12.Wrammert J, Onlamoon N, Akondy RS, Perng GC, Polsrila K, Chandele A, Kwissa M, Pulendran B, Wilson PC, Wittawatmongkol O, Yoksan S, Angkasekwinai N, Pattanapanyasat K, Chokephaibulkit K, Ahmed R. Rapid and massive virus-specific plasmablast responses during acute dengue virus infection in humans. J Virol 2012; 86: 2911–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu M, Hadinoto V, Appanna R, Joensson K, Toh YX, Balakrishnan T, Ong SH, Warter L, Leo YS, Wang CI, Fink K. Plasmablasts generated during repeated dengue infection are virus glycoprotein-specific and bind to multiple virus serotypes. J Immunol 2012; 189: 5877–85. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Bates TM, Cordeiro MT, Nascimento EJ, Smith AP, Soares de Melo KM, McBurney SP, Evans JD, Marques ET, Jr, Barratt-Boyes SM. Association between magnitude of the virus-specific plasmablast response and disease severity in dengue patients. J Immunol 2012; 190: 80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zompi S, Montoya M, Pohl MO, Balmaseda A, Harris E. Dominant cross-reactive B cell response during secondary acute dengue virus infection in humans. PLoS Neglect Tropic Dis 2012; 6: e1568–e1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beltramello M, Williams KL, Simmons CP, Macagno A, Simonelli L, Quyen NT, Sukupolvi-Petty S, Navarro-Sanchez E, Young PR, de Silva AM, Rey FA, Varani L, Whitehead SS, Diamond MS, Harris E, Lanzavecchia A, Sallusto F. The human immune response to Dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activity. Cell Host Microbe 2010; 8: 271–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dejnirattisai W, Jumnainsong A, Onsirisakul N, Fitton P, Vasanawathana S, Limpitikul W, Puttikhunt C, Edwards C, Duangchinda T, Supasa S, Chawansuntati K, Malasit P, Mongkolsapaya J, Screaton G. Cross-reacting antibodies enhance dengue virus infection in humans. Science 2010; 328: 745–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schieffelin JS, Costin JM, Nicholson CO, Orgeron NM, Fontaine KA, Isern S, Michael SF, Robinson JE. Neutralizing and non-neutralizing monoclonal antibodies against dengue virus E protein derived from a naturally infected patient. Virol J 2010; 7: 28–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Alwis R, Beltramello M, Messer WB, Sukupolvi-Petty S, Wahala WM, Kraus A, Olivarez NP, Pham Q, Brian J, Tsai WY, Wang WK, Halstead S, Kliks S, Diamond MS, Baric R, Lanzavecchia A, Sallusto F, de Silva AM. In-depth analysis of the antibody response of individuals exposed to primary dengue virus infection. PLoS Neglect Tropic Dis 2010; 5: e1188–e1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith SA, Zhou Y, Olivarez NP, Broadwater AH, de Silva AM, Crowe JE., Jr Persistence of circulating B memory cell clones with potential for dengue virus disease enhancement for decades following infection. J Virol 2011. doi:10.1128/JVI.06335-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Setthapramote C, Sasaki T, Puiprom O, Limkittikul K, Pitaksajjakul P, Pipattanaboon C, Sasayama M, Leuangwutiwong P, Phumratanaprapin W, Chamnachanan S, Kusolsuk T, Jittmittraphap A, Asai A, Arias JF, Hirai I, Kuhara M, Okuno Y, Kurosu T, Ramasoota P, Ikuta K. Human monoclonal antibodies to neutralize all dengue virus serotypes using lymphocytes from patients at acute phase of the secondary infection. Biochem Biophys Res Commun 2012; 423: 867–72. [DOI] [PubMed] [Google Scholar]
- 22.Wahala WM, Silva AM. The human antibody response to dengue virus infection. Viruses 2011; 3: 2374–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cox J, Mota J, Sukupolvi-Petty S, Diamond MS, Rico-Hesse R. Mosquito bite delivery of dengue virus enhances immunogenicity and pathogenesis in humanized mice. J Virol 2012; 86: 7637–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaiswal S, Pazoles P, Woda M, Shultz LD, Greiner DL, Brehm MA, Mathew A. Enhanced humoral and HLA-A2-restricted dengue virus-specific T-cell responses in humanized BLT NSG mice. Immunology 2012; 136: 334–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaiswal S, Pearson T, Friberg H, Shultz LD, Greiner DL, Rothman AL, Mathew A. Dengue virus infection and virus-specific HLA-A2 restricted immune responses in humanized NOD-scid IL2rgammanull mice. PloS One 2009; 4: e7251–e7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuruvilla JG, Troyer RM, Devi S, Akkina R. Dengue virus infection and immune response in humanized RAG2(-/-)gamma(c)(-/-) (RAG-hu) mice. Virology 2007; 369: 143–52. [DOI] [PubMed] [Google Scholar]
- 27.Mota J, Rico-Hesse R. Humanized mice show clinical signs of dengue fever according to infecting virus genotype. J Virol 2009; 83: 8638–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costin JM, Zaitseva E, Kahle KM, Nicholson CO, Rowe DK, Graham AS, Bazzone LE, Hogancamp G, Figueroa Sierra M, Fong RH, Yang ST, Lin L, Robinson JE, Doranz BJ, Chernomordik LV, Michael SF, Schieffelin JS, Isern S. Mechanistic study of broadly neutralizing human monoclonal antibodies against dengue virus that target the fusion loop. J Virol 2013; 87: 52–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Alwis R, Smith SA, Olivarez NP, Messer WB, Huynh JP, Wahala WM, White LJ, Diamond MS, Baric RS, Crowe JE, Jr., de Silva AM. Identification of human neutralizing antibodies that bind to complex epitopes on dengue virions. In: Proceedings of the National Academy of Sciences of the United States of America, 2012;109:7439–44. [DOI] [PMC free article] [PubMed]
- 30.Covassin L, Jangalwe S, Jouvet N, Laning J, Burzenski L, Shultz LD, Brehm MA. Human immune system development and survival of non-obese diabetic (NOD)-scid IL2rgamma(null) (NSG) mice engrafted with human thymus and autologous haematopoietic stem cells. Clin Experiment Immunol 2013; 174: 372–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Traggiai E, Becker S, Subbarao K, Kolesnikova L, Uematsu Y, Gismondo MR, Murphy BR, Rappuoli R, Lanzavecchia A. An efficient method to make human monoclonal antibodies from memory B cells: potent neutralization of SARS coronavirus. Nat Med 2004; 10: 871–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith K, Garman L, Wrammert J, Zheng NY, Capra JD, Ahmed R, Wilson PC. Rapid generation of fully human monoclonal antibodies specific to a vaccinating antigen. Nat Protocol 2009; 4: 372–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biswas S, Chang H, Sarkis PT, Fikrig E, Zhu Q, Marasco WA. Humoral immune responses in humanized BLT mice immunized with West Nile virus and HIV-1 envelope proteins are largely mediated via human CD5+ B cells. Immunology 2011; 134: 419–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaveri SV, Silverman GJ, Bayry J. Natural IgM in immune equilibrium and harnessing their therapeutic potential. J Immunol 2012; 188: 939–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sims GP, Ettinger R, Shirota Y, Yarboro CH, Illei GG, Lipsky PE. Identification and characterization of circulating human transitional B cells. Blood 2005; 105: 4390–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friberg H, Jaiswal S, West K, O'Ketch M, Rothman AL, Mathew A. Analysis of human monoclonal antibodies generated by dengue virus-specific memory B cells. Viral Immunol 2012; 25: 348–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robinson JE, Holton D, Liu J, McMurdo H, Murciano A, Gohd R. A novel enzyme-linked immunosorbent assay (ELISA) for the detection of antibodies to HIV-1 envelope glycoproteins based on immobilization of viral glycoproteins in microtiter wells coated with concanavalin A. J Immunol Method 1990; 132: 63–71. [DOI] [PubMed] [Google Scholar]
- 38.Kraus AA, Messer W, Haymore LB, de Silva AM. Comparison of plaque- and flow cytometry-based methods for measuring dengue virus neutralization. J Clin Microbiol 2007; 45: 3777–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mathew A, West K, Kalayanarooj S, Gibbons RV, Srikiatkhachorn A, Green S, Libraty D, Jaiswal S, Rothman AL. B-cell responses during primary and secondary dengue virus infections in humans. J Infect Dis 2011; 204: 1514–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brainard DM, Seung E, Frahm N, Cariappa A, Bailey CC, Hart WK, Shin HS, Brooks SF, Knight HL, Eichbaum Q, Yang YG, Sykes M, Walker BD, Freeman GJ, Pillai S, Westmoreland SV, Brander C, Luster AD, Tager AM. Induction of robust cellular and humoral virus-specific adaptive immune responses in human immunodeficiency virus-infected humanized BLT mice. J Virol 2009; 83: 7305–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watanabe Y, Takahashi T, Okajima A, Shiokawa M, Ishii N, Katano I, Ito R, Ito M, Minegishi M, Minegishi N, Tsuchiya S, Sugamura K. The analysis of the functions of human B and T cells in humanized NOD/shi-scid/gammac(null) (NOG) mice (hu-HSC NOG mice). Int Immunol 2009; 21: 843–58. [DOI] [PubMed] [Google Scholar]
- 42.Calisher CH, Karabatsos N, Dalrymple JM, Shope RE, Porterfield JS, Westaway EG, Brandt WE. Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. J Gen Virol 1989; 70: 37–43. [DOI] [PubMed] [Google Scholar]
- 43.Innis B. Antibody responses to dengue virus infection. In: Gubler DJ, Kuno G. (eds). Antibody responses to dengue virus infection; Dengue and dengue hemorrhagic fever, Cambridge, UK: CAB International, 1997, pp. 221–43. [Google Scholar]
- 44.Lang J, Kelly M, Freed BM, McCarter MD, Kedl RM, Torres RM, Pelanda R. Studies of lymphocyte reconstitution in a humanized mouse model reveal a requirement of T cells for human B cell maturation. J Immunol 2013; 190: 2090–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Asito AS, Moormann AM, Kiprotich C, Ng'ang'a ZW, Ploutz-Snyder R, Rochford R. Alterations on peripheral B cell subsets following an acute uncomplicated clinical malaria infection in children. Malaria J 2008; 7: 238–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malaspina A, Moir S, Ho J, Wang W, Howell ML, O'Shea MA, Roby GA, Rehm CA, Mican JM, Chun TW, Fauci AS. Appearance of immature/transitional B cells in HIV-infected individuals with advanced disease: correlation with increased IL-7. Proc Natl Acad Sci USA 2006; 103: 2262–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jones DD, DeIulio GA, Winslow GM. Antigen-driven induction of polyreactive IgM during intracellular bacterial infection. J Immunol 2012; 189: 1440–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vuyyuru R, Liu H, Manser T, Alugupalli KR. Characteristics of Borrelia hermsii infection in human hematopoietic stem cell-engrafted mice mirror those of human relapsing fever. Proc Natl Acad Sci USA 2012; 108: 20707–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shultz LD, Brehm MA, Garcia-Martinez JV, Greiner DL. Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev 2012; 12: 786–98. [DOI] [PMC free article] [PubMed] [Google Scholar]