Abstract
Aim
After pulmonary vein isolation (PVI), dormant conduction (DC) is present in at least one vein in a substantial number of patients. The present study seeks to determine whether there is a relationship between poor contact forces (CF) and the presence of DC after PVI.
Methods and results
This prospective, operator-blinded, non-randomized dual-centre trial enrolled 34 consecutive patients with paroxysmal atrial fibrillation who were candidates for PVI. Radiofrequency (RF) energy was delivered by using an irrigated-tip force-sensing ablation catheter (Tacticath®, St Jude Medical) at pre-defined target power. The operators were blinded to the CF data at all times. A total of 1476 RF applications were delivered in 743 pre-defined PV segments. For each application, the precise location of the catheter was registered and the following data were extracted from the Tacisys® unit: application duration, minimum contact force, maximum contact force, average contact force (CF), and force–time integral (FTI). Sixty minutes after PVI, spontaneous early recovery (ER) of the left atrium (LA) to PV conduction was evaluated. In the absence of ER, the presence of a DC was evaluated by using intravenous adenosine (ATP). In the 34 patients recruited (23 males; mean age: 62 ± 9 years), all PVs were successfully isolated. At the end of the 60 min waiting period, 22 patients demonstrated at least one spontaneous ER or DC under ATP. The mean CF and FTI per PV segment differed significantly among the different veins but the sites of ER and DC were evenly distributed. However, both the minimum, the first and the mean CF and FTI per PV segment were significantly lower in the PV segments presenting either ER or DC as compared with those without ER or DC (mean CF: 4.9 ± 4.8 vs. 12.2 ± 1.65 g and mean FTI: 297 ± 291 vs. 860 ± 81 g s, P < 0.001 for both). Using multivariate analysis, both the mean CF and the FTI per lesion remained significantly associated with the risk of ER or DC. Moreover, a CF < 5 g per PV segment predicted ER+ and DC+ with a sensitivity of 71% and specificity of 82%. In contrast, ER and DC were very unlikely if RF application was performed with a mean CF > 10 g (negative predictive value: 98.7%).
Conclusion
Both a low CF and a low FTI are associated with the ER of the PVI and DC after PVI.
Keywords: Atrial fibrillation, Radiofrequency ablation, Pulmonary vein isolation, Contact force, Tacticath, Adenosine, Dormant conduction
What's new?
Early recovery and dormant conduction revealed by intravenous adenosine 60 min after pulmonary vein isolation can be predicted by the contact force (CF) profile of each radiofrequency (RF) lesion.
Early recovery and dormant conduction are very unlikely if RF application is performed with a mean CF > 10 g (NPV: 98.7%). The interest of systematically testing for dormant conduction in this setting is therefore mollified.
Introduction
Despite acute procedural success, recurrence of atrial arrhythmia after pulmonary vein isolation (PVI) is frequently associated with recovery of conduction between the pulmonary veins (PVs) and the left atrium (LA).1,2 This phenomenon of acute electrical disconnection and later recovery of the PVs is related to a state of functional block called ‘dormant conduction’ (DC). Typically, DC progresses to permanent conduction recovery during the healing process occurring in the weeks and months following ablation.3 A number of strategies have been proposed to reduce the chances of PV conduction recovery, including prolonged waiting period, intravenous boluses of adenosine, and high output pacing from the ablation line.4–6 It has been proven that the presence of DC is associated with higher risk of recurrence after PVI and that its elimination may contribute to obtain better long-term results.4,7 In the present paper, we hypothesize that low contact force (CF) during ablation potentiates oedema formation rather than deeper, permanent lesions and that areas of low CF will demonstrate either a recovery or a positive adenosine response after PVI. The aim of the present study was therefore to evaluate, in a non-randomized fashion, the relationship between CF and DC during catheter ablation for paroxysmal atrial fibrillation (AF).
Methods
Consecutive paroxysmal AF patients were enrolled simultaneously at the Cliniques Universitaires Saint-Luc (Brussels, Belgium) and at AF Ablation Clinic, Hollywood Private Hospital (Perth, Australia). The study was cleared by ethics committees at each institution. All patients gave written inform consent before the procedure. Pulmonary vein isolation was performed on patients effectively anticoagulated and after a transoesophageal echocardiogram has excluded left atrial thrombus. Antiarrhythmic agents were discontinued before the procedure, allowing a washout period of five half-lives (except for amiodarone), while AV blocking agents were permitted in highly symptomatic patients. Patients were requested to fast the night before the procedure and all procedures were performed under conscious sedation or general anaesthesia according to the patient preference.
Pulmonary vein isolation procedure
Ablation strategy consisted of circumferential PV antrum ablation with PV electrical isolation as the procedural end point. After central venous access was obtained, a multipolar catheter was placed in the coronary sinus (CS). One or two transseptal punctures were performed for access into the LA. After transseptal access was obtained, boluses of intravenous heparin were given followed by a continuous infusion to maintain an activated clotting time of >300 s. Through one transseptal access, a decapolar, circular catheter was advanced into the LA via a non-steerable sheath (Fast-Cath SL-0, St Jude Medical). Directly besides this first access (one transseptal puncture) or via a second transseptal puncture (two transseptal punctures), the Tacticath (Tacticath, St Jude Medical), a 7 french, 3.5 mm open irrigated-tip ablation catheter allowing for the measurement of both the magnitude and the direction of the CF was placed in the LA. The study protocol did not allow the use of a long sheath for the Tacticath catheter. Importantly, in Brussels, only medium curves (65 cm) were used, while at Hollywood Private Hospital the Tacticath 75 cm curve catheter was used. Electroanatomic mapping (EnSite NavX, St Jude Medical) with or without image integration was used in Brussels, while computed tomography fluoroscopic overlay (Siemens iPilot) was used in Perth. Using the Tacticath, circumferential continuous point-by-point ablation lesions set was placed 1–2 cm from the PV ostia to encircle and electrically isolate the PVs. Radiofrequency energy was delivered at a pre-defined target power of 25–35 W (25 W for all posterior segments and 35 W for anterior segments) with a catheter irrigation set at 17 mL/min with 0.9% NaCl. The duration of each RF application was according to operator discretion; however, a minimum of 60 s was advised if the catheter position was stable. As the PVs were encircled, the circular catheter was used to confirm electrical isolation of each vein from the LA. Circumferential lesions around the veins were considered complete when the circular catheter no longer recorded PV potentials. The site of each RF application was recorded relative to eight pre-defined circumferential positions around the PV (superior, antero-superior, anterior, antero-inferior, inferior, postero-inferior, posterior, postero-superior) (Figure 1). For each RF application, a tag was placed on the LA shell and the following data were extracted from the Tacisys® unit: application duration, average CF, and force–time integral (FTI). Per position, the total number of RF applications, application duration, minimum CF, maximum CF, average CF and FTI, and the CF and FTI during the first application was entered into a dedicated database. In addition, the stability of the catheter (estimated by the jumping index: number of untouched segments between two consecutive RF applications), the total of application per vein, the average CF and FTI per vein, and the number of un-ablated segments per vein were also recorded. Importantly, the operators were blinded to the contact force data at all times. Patients in AF at the conclusion of the procedure were cardioverted back to sinus rhythm electrically to allow remapping of all PVs to confirm PV isolation in sinus rhythm.
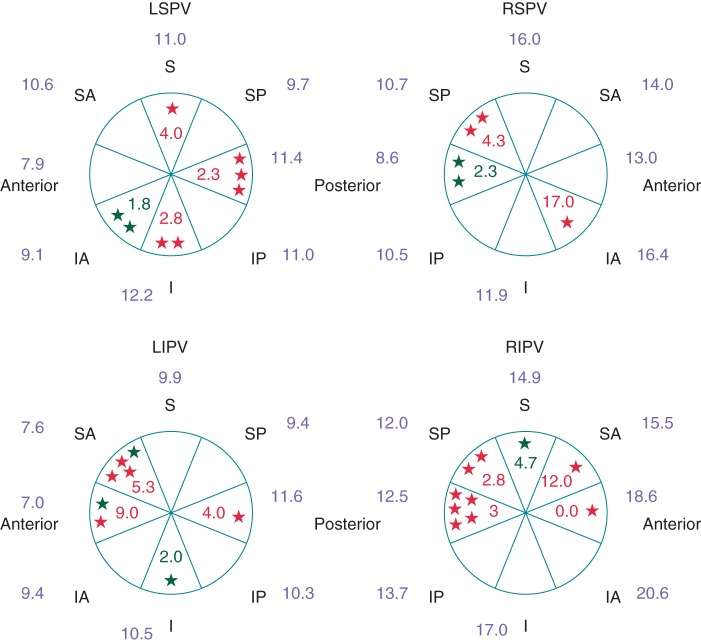
Figure 1.
Average CF (g) per PV segment according to the presence or absence of ER or DC at 60 min after PVI. ER+: red stars and values; DC+: green stars and values; ER−/DC−: values in blue. LSPV, left superior pulmonary vein; RSPV, right superior pulmonary vein; LIPV, left inferior pulmonary vein; RIPV, right inferior pulmonary vein.
Evaluation of dormant conduction
Sixty minutes after the assessment of the PV isolation, remapping of all PVs was performed to detect early recovery (ER) of the PV to LA conduction. In the absence of ER, the presence of a DC was assessed in each vein by injecting intravenously a bolus of 12 mg or more of adenosine to obtain a minimum of one beat of complete AV block or a pause of at least 3 s. Dormant conduction was defined by reappearance of PV conduction as demonstrated by associated PV spikes recorded by the circular catheter for ≥1 beat. If DC was unmasked by adenosine, the precise position of the PV–LA breakthrough was recorded and further ablation was undertaken to abolish this conduction. The position where RF application resulted in a change of sequence or in abolition of the DC was also recorded. Abolition of DC was assessed by repeated injections of adenosine using the same doses previously used to reveal DC.
Statistical analysis
Data were analysed with the SPSS 17.0 software (SPSS Inc.). Data are reported as mean ± one standard deviation (SD). Clinical and procedural data were compared with unpaired t-test. All tests were two-sided and a P-value < 0.05 was considered indicative of a statistical significance. The initial projected sample size for this study was 35 patients. This sample size was determined assuming that ER or DC revealed by intravenous adenosine will be present in at least one vein in 70% of our population. With an alpha error at 0.05 and a statistical power of 90%, we calculated that we should find at least 30 ER+/DC+ ablated positions. Data of recovered (ER+/DC+) PV segment were compared with that of the non-recovered positions (ER−/DC−) by using the Mann–Whitney–Wilcoxon rank test and χ2 test when appropriate. All clinical and electrophysiological data that correlate with ER+/DC+ with univariate analysis were subsequently submitted to a multistepwise multivariate analysis. The predictive values of different thresholds of CF and FTI for ER and/or DC were assessed using the sensitivity (Se), specificity (Sp), and the receiving operator characteristic curves analysis.
Results
Three experienced operators treated 34 patients (23 males; age 62 ± 9 years) at the two sites. Patient characteristics are presented in Table 1. All targeted PV (n = 132) were successfully isolated at the end of the procedure. A total of 1476 RF applications were delivered in 743 PV segments. Both the minimum and the first CF and FTI applied per segment (range from 0 to 60 g and 0 to 3221 g s, respectively) were very close to the mean CF and FTI per segment (range from 0 to 60 g and 0 to 4666 g s, respectively). At the end of the 60 min waiting period, a total of 31 lesion sites presented either an ER or a DC. Early recovery occurred in 23 PV segments in 18 different patients. Dormant conduction was revealed by adenosine infusion in eight additional PV segments and four different patients. Three patients presented both ER and DC. Among the 34 patients enrolled in the present study, 64% presented at least one ER or DC revealed by adenosine infusion 1 h after the validation of the PV isolation.
Table 1.
Population characteristic
| Overall (n = 34) | Range or percentage | |
|---|---|---|
| Age (years) | 62 ± 9 | 36–77 |
| Gender (n, % male) | 23 | 68% |
| Body mass index | 27 ± 5 | 22–35 |
| Mean blood pressure (mmHg) | 101 ± 12 | 85–135 |
| Antiarrhythmic | ||
| Amiodarone | 6 | 18% |
| Class I | 14 | 41% |
| Sotalol | 8 | 23% |
| β-Blockers | 12 | 35% |
| Decreased LV ejection fraction | 1 | 3% |
| Time since first AF diagnosis (months) | 42 | 2–144 |
| CHA2DS2VASC | ||
| 0 | 9 | 27% |
| 1 | 11 | 32% |
| ≥2 | 14 | 41% |
| LA volume (mL) | 107 ± 24 | 69–141 |
Contact force and force–time integral characteristics in recovered vs. non-recovered pulmonary veins
Procedural characteristics of the lesions for both the ER+/DC+ and the ER−/DC− groups are presented in Table 2. Overall, the mean CF and FTI values were significantly more important in the right PVs as compared with the left PVs (CF: 10.3 ± 5.6 vs. 13.7 ± 6.3; P = 0.002) and at the posterior aspect of the left PV when compared with the anterior (CF: 10.2 ± 7.4 vs. 8.6 ± 6.1; P = 0.05). However, ER and DC were evenly distributed around the PV (see Figures 1 and 2). Except for the left superior pulmonary vein (LSPV), no significant differences in CF or FTI were observed between the veins with DC and those without. In the LSPV, the average CF per vein was significantly lower in the presence of ER or DC (7.2 ± 4.5 vs. 11.2 ± 4.4; P = 0.03).
Table 2.
Procedural characteristics of RF delivery per PV segments and per PVs according to the presence or absence of ER and DC
| ER+/DC+ (N = 31) | ER−/DC− (N = 731) | P-value or χ2 | |
|---|---|---|---|
| Ablation in AF | 2/31 | 86/731 | 0.57 |
| Sedation (% general anaesthesia) | 35% | 48% | 0.13 |
| Analysis per PV segment | |||
| Number of applications | 1.8 ± 1.4 | 1.6 ± 1.3 | 0.23 |
| RF duration (s) | 66 ± 63 | 77 ± 63 | 0.34 |
| Average CF | 4.9 ± 4.7 | 12.2 ± 1.6 | <0.001 |
| Average FTI | 297 ± 291 | 860 ± 751 | <0.001 |
| Min CF | 4.9 ± 4.9 | 10.7 ± 8.7 | <0.001 |
| Min FTI | 186 ± 218 | 458 ± 456 | <0.001 |
| CF at first RF delivery | 5.3 ± 4.7 | 12.0 ± 9.1 | <0.001 |
| FTI at first RF delivery | 192 ± 216 | 529 ± 481 | <0.001 |
| Analysis per PV | |||
| Common ostium | 1 | 2 | na |
| Number of applications per vein | 13.5 ± 9.5 | 10.5 ± 8.8 | 0.11 |
| Average CF | 9.9 ± 4.9 | 12.6 ± 6.4 | 0.03 |
| Average FTI | 886 ± 499 | 726 ± 416 | 0.12 |
| Jumping index | 6.0 ± 5.0 | 4.5 ± 4 | 0.09 |
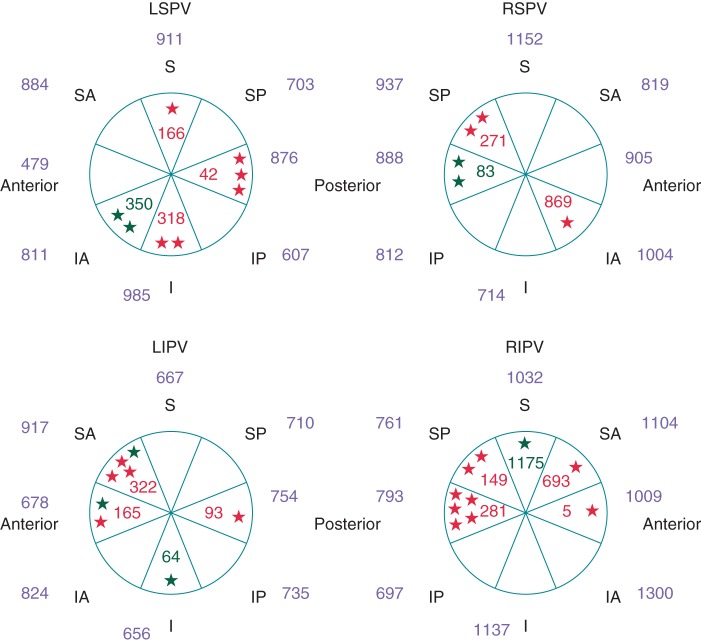
Figure 2.
Average FTI (g s) per PV segment according to the presence or the absence of ER or DC at 60 min after PVI. ER+: red stars and values; DC+: green stars and values; ER−/DC−: values in blue (legend as in Figure 1).
Contact force and force–time integral characteristics in recovered vs. non-recovered PV segment
Both the mean CF and FTI were significantly lower in ER+/DC+ PV segments as compared with the ER−/DC− PV segments (4.9 ± 4.7 vs. 12.1 ± 4.2 g; P < 0.001 and 297 ± 290 vs. 860 ± 254 g s; P < 0.001). The minimum CF and FTI and the CF and FTI during the first RF application were also significantly lower in this group (cf. Table 2). No significant differences were noted between the ER+ and DC+ lesions in terms of mean, minimum, or first CF and FTI (mean CF: 5.4 ± 4.9 vs. 3.7 ± 4.4 g; P = 0.40 and mean FTI 269 ± 224 vs. 376 ± 442 g s; P = 0.38). With the univariate analysis, the minimum, first, and the mean CF and FTI per lesion correlated with ER and DC (P < 0.001 for all). Using multivariate analysis, the mean CF and the mean FTI per segment remained predictive of ER or DC (cf. Table 3).
Table 3.
Multivariate analysis
| HR [95% SD] | P-value | |
|---|---|---|
| Average force | 0.74 [0.64;0.85] | <0.001 |
| Average FTI | 0.80 [0.65;0.99] | 0.037 |
Prediction of early recovery and dormant conduction after pulmonary vein isolation
Receiving operator characteristic curves were drawn for the both the mean CF and the FTI. The mean CF per PV segment had the best predictive value for ER and DC with an area under the curve of 0.80 (vs. 0.79 for FTI). Using the Youden index, we found that a CF < 5 g had the best sensitivity (71%) and specificity (82%) compromise for predicting ER+ and DC+ (cf. Figure 3). Indeed, 14% of the PV segments ablated with a CF < 5 g presented an ER or DC at 60 min. If the mean CF was >10 g, this probability was only 1.2% (cf. Figure 4). For the lesions performed with < 5 g or >10 g, no statistically significant additional predictive value was brought by the RF application time or the FTI. Although among the group of lesions with a mean CF > 10 g, ER or DC occurred in 6% (2 of 33) of the lesions if the FTI was <400 g s vs. 0.8% (3 of 359) if the FTI was >400 g s. Additionally, if the mean CF was between 5 and 10 g, a low FTI (<850 g) was highly predictive of ER or DC (sensitivity: 100% and specificity: 21%).
Figure 3.
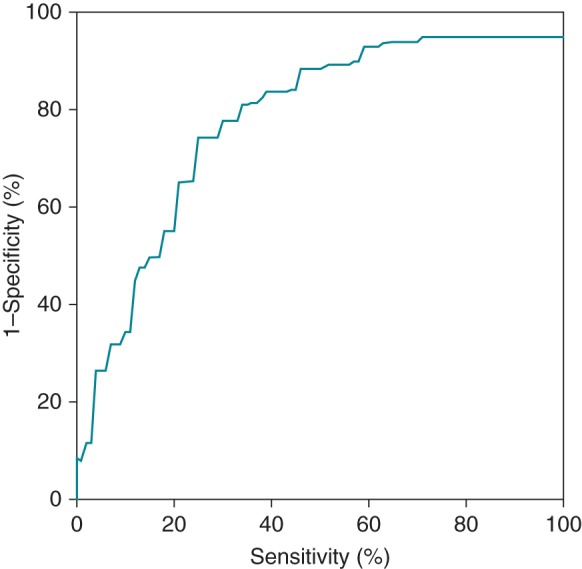
Receiving operator curve for ER/DC predictability. The mean CF had the best predictive value with an area under the curve of 0.80. The best compromise between the sensitivity and specificity was found at 5 g (Se: 71%; Sp: 81%; PPV: 14%).
Figure 4.
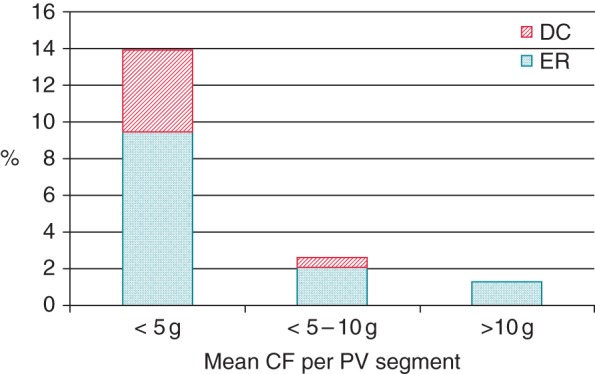
Rate of ER and DC revealed by adenosine infusion 60 min after validation of pulmonary vein isolation according to the mean contact force per pulmonary vein segment.
Discussion
Acute PV isolation is usually easily achieved with RF ablation. However, acute PV reconnection frequently occurs and is associated with unfavourable long-term clinical results.1–4 Elimination of DC identified by adenosine markedly improves the arrhythmia-free survival of the patient but significantly prolongs the procedural time.5,7 In the present study, we hypothesize that DC can be anticipated by the CF characteristics of each RF lesion.
Our results demonstrate a clear relationship between the absence of CF during PVI and the occurrence of ER and DC revealed by adenosine infusion 60 min after the procedure. Using a fixed target power of 25/35 W, a mean CF < 5 g was highly predictive of ER or DC (Se: 71% and Sp: 81%). In contrast, the incidence of ER or DC was very low in the group of lesions created with a mean CF > 10 g (negative predictive value: 98.7%). Surprisingly, in our series, for both of these groups of lesion (mean CF < 5 g or >10 g), the RF application time and the subsequent FTI did not add significant information over the CF for the prediction of ER or DC. In the absence of CF (<5 g), this could be easily explained by the fact that RF applications resulted in non-transmural lesion and oedema. Hence, oedema may reduce thermal convection and the delivery of the RF energy to the surrounding myocardium. Increasing the application time in such situation might just not improve the lesion transmurality.8–10 For the group of lesion created with a CF > 10 g, the relatively homogeneous application time, the high FTI values (92% of these applications presented an FTI > 400 g s), and the small number of ER/DC (1.2%) in this group might explain why the application time and the FTI did not overpass the predictive value of the CF. However, for the lesions created with a CF between 5 and 10 g, we identified a grey zone with an intermediate probability of ER and DC. For this subgroup of lesions, we found that a low FTI (<850 g s) predicted the outcome with a very high sensitivity. These results first support the hypothesis that DC relates to incomplete, non-transmural lesions and possible oedema formation due to insufficient CF. They are thus in agreement with previous clinical studies who correlated low CF and FTI values and the absence of transmurality identified either by the change in the bipolar electrogram amplitude or by DE-cMRI following AF ablation.11,12 They also fit with the clinical results reported in the TOCCATA study where clinical recurrence after PVI occurred in 75% of the patients treated with CF < 10 g.13 Similarly, the EFFICACE I study demonstrated that 65% of the patients undergoing a PVI had at least one recovered PV segment 3 months after the procedure.14,15 Interestingly, the authors found that PV segments with gaps presented a significantly lower minimum CF and FTI at the index procedure. Accordingly, they recommended targeting a CF of 20 g and a FTI of 400 g s for each new lesion. The legitimacy of these values was validated in the EFFICAS II study, which demonstrated that best clinical and electrophysiological results at 3 months after the procedures are obtained by creating continuous PV ablation line with a stable CF > 10 g and FTI > 400 g s.15,16 These target values were missing in 28 of the 31 recovered PV segments in our population. Thus, our results confirm these recommendations. Although in line with the EFFICAS studies, our results showed that the mean CF per PV segment was more relevant than the minimum per segment (whereas the opposite was previously demonstrated). The explanation simply relates to the definition of PV segment: in the present study, each vein was sub-divided into eight different segments while in EFFICAS I, for example, there was eight PV segments per pair of ipsilateral veins. Therefore in our study, the minimum CF and mean CF strongly correlated.
Clinical implications
This study provides a number of unique insights that confirm the critical importance of CF for the electrophysiologist:
Dormant conduction identification and elimination has been shown to improve long-term clinical results after PVI. Our results suggest, however, that searching for DC (by respecting a prolonged waiting period and injecting boluses of adenosine) might be of little interest if appropriate CF (>10 g) and FTI > 400 g s are reached.
In the absence of CF (CF < 5 g), our results suggest that it is certainly better not to deliver RF but first try to improve the contact (by using a different angle of approach or a deflectable sheath). Importantly, if no better contact is possible (>10 g), higher FTI (>850 g s) are required to reduce the probability of ER and DC.
Limitations
This study presents several limitations that should be acknowledged. First, the only Tacticath catheter model available in Europe at the time of this study was the medium curve. Although PVI was successfully achieved in all included patients without the help of a deflectable sheath (not allowed per protocol), we feel that this curve was not always optimal for PVI and that it might have negatively impacted the CF and FTI in a significant number of patients in our study. Secondly, RF ablation was performed with the multipolar circular mapping catheter positioned at the PV ostium. Although great care was taken to avoid contact between the Tacticath and the lasso catheter during RF delivery, we cannot formally exclude that this happened for some RF applications. Removing the lasso catheter from the PV during the ablation might have reduced this risk but is not possible for electro-guided procedures. Usually, contact between the lasso catheter and the Tacticath can be suspected in the presence of very high transient CF or in the presence of electrical artefacts on the PV recoding channels, and confirmed by multiple angle fluoroscopy. Also, not only the CF and the application time, but also the delivered power is important to determine the lesion size. In EFFICAS II, the lesion size index (LSI) was the best predictor of PV reconnection 3 months after PVI.17 Although we used a fixed target power of 25–35 W, the energy delivered to the tissue varied, rendering thus post hoc calculations of possibly more sensitive indices, such as the LSI or the force–power–time index,18 impossible. Therefore, the predictive value of these indices was not evaluated. Finally, being blind to all CF data during the procedure, the impact of increasing the power to 30 or 40 W in case of intermediate CF was not evaluated. Regarding this aspect, it is important to remark that our results are applicable to the power setting used in this study. Moreover, it is possible that power around 40 W or higher could still achieve permanent lesions even with a contact force of <10 g.
Conclusion
Both low mean CF and FTI are associated with DC after PVI. Below 5 g, DC is frequently encountered, and better contact should be obtained before applying RF current. In contrast, if RF lesions are created with a CF > 10 g, DC occurs very rarely and the search for DC might be of little clinical interest.
Funding
During the last 2 years, J.B.L.P.D.W. received non-significant compensations for teaching and proctoring purposes from Medtronic, Boston Scientific, St Jude Medical and Biotronic. J.B.L.P.D.W. and C.S. report research grant from Medtronic. Funding to pay the Open Access publication charges for this article was provided by non-profit organization ENRYTHME.
Acknowledgments
The authors would like to thank Ulla Walser, Patrick Bassereau and Marc Voskamp for their help in collecting the data.
Conflict of interest: none declared.
References
- 1.Macle L, Jais P, Weerasooriya R, Hocini M, Shah DC, Choi KJ, et al. Irrigated-tip catheter ablation of pulmonary veins for treatment of atrial fibrillation. J Cardiovasc Electrophysiol 2002;13:1067–73. [DOI] [PubMed] [Google Scholar]
- 2.Jais P, Cauchemez B, Macle L, Daoud E, Khairy P, Subbiah R, et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation 2008;118:2498–505. [DOI] [PubMed] [Google Scholar]
- 3.Arentz T, Macle L, Kalusche D, Hocini M, Jais P, Shah D, et al. “Dormant” pulmonary vein conduction revealed by adenosine after ostial radiofrequency catheter ablation. J Cardiovasc Electrophysiol 2004;15:1041–7. [DOI] [PubMed] [Google Scholar]
- 4.Matsuo S, Yamane T, Date T, Inada K, Kanzaki Y, Tokuda M, et al. Reduction of AF recurrence after pulmonary vein isolation by eliminating ATP-induced transient venous re-conduction. J Cardiovasc Electrophysiol 2007;18:704–8. [DOI] [PubMed] [Google Scholar]
- 5.Andrade JG, Pollak SJ, Monir G, Khairy P, Dubuc M, Roy D, et al. Pulmonary vein isolation using a pace-capture-guided versus an adenosine-guided approach: effect on dormant conduction and long-term freedom from recurrent atrial fibrillation: a prospective study. Circ Arrhythm Electrophysiol 2013;6:1103–8. [DOI] [PubMed] [Google Scholar]
- 6.De Potter TJ, Eisenberger M, McCann C, Peytchev P, Geelen P. Adenosine plus dipyridamole: a novel strategy to enhance adenosine-induced conduction recovery after pulmonary vein isolation. Europace 2012;14:1567–71. [DOI] [PubMed] [Google Scholar]
- 7.Macle L, Khairy P, Weerasooriya R, Novak PG, Verma A, Willems S, et al. Guided pulmonary vein isolation for the treatment of atrial fibrillation: results of the prospective multicenter randomized ADVICE trial. HRS Congress, Late-Breaking Clinical Trial I, 8 May 2014. [Google Scholar]
- 8.Yokoyama K, Nakagawa H, Shah DC, Lambert H, Leo G, Aeby N, et al. Novel contact force sensor incorporated in irrigated radiofrequency ablation catheter predicts lesion size and incidence of steam pop and thrombus. Circ Arrhythm Electrophysiol 2008;1:354–62. [DOI] [PubMed] [Google Scholar]
- 9.Thiagalingam A, D'Avila A, Foley L, Guerrero JL, Lambert H, Leo G, et al. Importance of catheter contact force during irrigated radiofrequency ablation: evaluation in a porcine ex vivo model using a force-sensing catheter. J Cardiovasc Electrophysiol 2010;21:806–11. [DOI] [PubMed] [Google Scholar]
- 10.Swale MJ, Gard JJ, Madhavan M, Johnson SB, Parker KD, Packer DL. Critical impact of contact force on lesion size: an in vivo validation. Boston, MA: Heart Rhythm Society 2012. PO1-132. Heart Rhythm 2012;9:S138. [Google Scholar]
- 11.Squara F, Latcu DG, Massaad Y, Mahjoub M, Bun S, Saoudi N. Contact force and force–time integral in atrial radiofrequency ablation predict transmurality of lesions. Europace 2014;16:660–7. [DOI] [PubMed] [Google Scholar]
- 12.Karim R, Gao G, Harrison J, Arujuna A, Wright M, Linton N, et al. Magnetic resonance imaging analysis of tissue-contact force following catheter ablation for paroxysmal atrial fibrillation. San Francisco, CA: Heart Rhythm Society; 2011. AB15-1. [Google Scholar]
- 13.Reddy VY, Shah D, Kautzner J, Schmidt B, Saoudi N, Herrera C, et al. The relationship between contact force and clinical outcome during radiofrequency catheter ablation of atrial fibrillation in the TOCCATA study. Heart Rhythm 2012;9:1789–95. [DOI] [PubMed] [Google Scholar]
- 14.Neuzil P, Reddy VY, Kautzner J, Petru J, Wichterle D, Shah D, et al. Electrical reconnection following PVI is contingent on contact force during initial treatment—results from the EFFICAS I Study. Circ Arrhythm Electrophysiol 2013;6:327–33. [DOI] [PubMed] [Google Scholar]
- 15.Wissner E, Schoonderwoerd B, Metzner A, Petru J, Neuzil P, Peichl P, et al. The true incidence of conduction gaps 3 months following pulmonary vein isolation in relation to quality of life and recurrent symptoms: lessons from the prospective, multicenter EFFICAS Studies. Boston, MA: Heart Rhythm Society; 2012. PO06-65. Heart Rhythm 2012;9:S462. [Google Scholar]
- 16.Kautzner J, Neuzil P, Peichl P, Petru J, Cihak R, Skoda J, et al. Contact force, force time integral and lesion continuity are critical to improve durable PV isolation: EFFICAS II Results. Boston, MA: Heart Rhythm Society 2012. AB12-05. Heart Rhythm 2012;9:S28. [Google Scholar]
- 17.Neuzil P, Kuck KH, Nakagawa H, Kautzner J, Shah DC, Fremont O, et al. Lesion size index for prediction of reconnection risk following RF ablation for PVI. Heart Rhythm 2012;9:S492. [Google Scholar]
- 18.Nakagawa H, Ikeda A, Constantine G, Govari A, Sharma T, Pitha JV, et al. Controlling lesion size and incidence of steam pop by controlling contact force, radiofrequency power and application time (force-power-time index) in canine beating heart. Heart Rhythm 2012;9:S210. [Google Scholar]