Abstract
Fragile X mental retardation protein (FMRP) is an RNA-binding protein important for the control of translation and synaptic function. The mutation or silencing of FMRP causes Fragile X syndrome (FXS), which leads to intellectual disability and social impairment. γ-aminobutyric acid (GABA) is the major inhibitory neurotransmitter of the mammalian central nervous system, and its metabotropic GABAB receptor has been implicated in various mental disorders. The GABAB receptor agonist baclofen has been shown to improve FXS symptoms in a mouse model and in human patients, but the signaling events linking the GABAB receptor and FMRP are unknown. In this study, we found that GABAB receptor activation upregulated cAMP response element binding protein-dependent Fmrp expression in cultured mouse cerebellar granule neurons via two distinct mechanisms: the transactivation of insulin-like growth factor-1 receptor and activation of protein kinase C. In addition, a positive allosteric modulator of the GABAB receptor, CGP7930, stimulated Fmrp expression in neurons. These results suggest a role for GABAB receptor in Fmrp regulation and a potential interest of GABAB receptor signaling in FXS improvement.
Fragile X mental retardation protein (FMRP) is an RNA-binding protein that controls translation and synaptic function1,2. FMRP mutation or silencing causes Fragile X syndrome (FXS), a common inherited disease associated with autism, intellectual disability, and social impairment3. Chemical compounds targeting metabotropic glutamate receptor 5 (mGluR5) and other neurotransmitter receptors such as γ-aminobutyric acid and serotonin receptors4,5 or downstream signaling pathways such as mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK)1/2 and phosphatidylinositol 3 kinase (PI3K)/glycogen synthase kinase 3β/Akt6 have been tested for their ability to improve FXS symptoms such as anxiety, seizure, and hyperactivity. Recent studies have demonstrated that the GABAB receptor agonist R-baclofen (STX209) can improve locomotor activity and motor coordination in patients with FXS and modify the pathophysiology induced by FMRP deficiency including the effects on protein synthesis, AMPA receptor turnover, and dendritic spine density7,8, suggesting a connection between GABAB receptor and FMRP regulation. However, the signaling events linking GABAB receptor activation to FMRP are not well understood.
The GABAB receptor is the metabotropic receptor of GABA, the main inhibitory neurotransmitter in the mammalian central nervous system9. The receptor is a seven transmembrane domain-containing protein belonging to class C G protein-coupled receptors (GPCRs)10 and is assembled as a heterodimer containing GABAB1 and GABAB2 subunits9. Only GABAB1 subunit can bind agonists, whereas GABAB2 subunit is responsible for G protein coupling11. Positive allosteric modulators bind within the GABAB2 transmembrane domain to potentiate the effect of agonists12. Presynaptic GABAB receptor activation inhibits neurotransmitter release through the depression of voltage-gated Ca2+ channels, whereas activated postsynaptic GABAB receptors open K+ channels to induce neuronal hyperpolarisation13. GABAB receptor activation induces the ERK1/2/cAMP response element-binding protein (CREB) signaling pathway, which is dependent on Gi/o protein14. GABAB receptor also activates PI3K/Akt signaling to decrease apoptosis in cerebellar granule neurons (CGNs)15,16 through transactivation of the insulin-like growth factor-1 receptor (IGF-1R).
This study investigated the link between GABAB receptor and Fmrp. The results show that activation of the GABAB receptor upregulated Fmr1 mRNA and protein expression via activation of CREB. IGF-1R- and protein kinase C (PKC)-dependent signaling pathways were found to be involved in CREB activation and Fmrp synthesis. In addition, we show that CGP7930, a positive allosteric modulators (PAMs) of GABAB receptor, also upregulated Fmrp expression.
Results
GABAB receptor activation upregulates Fmrp expression
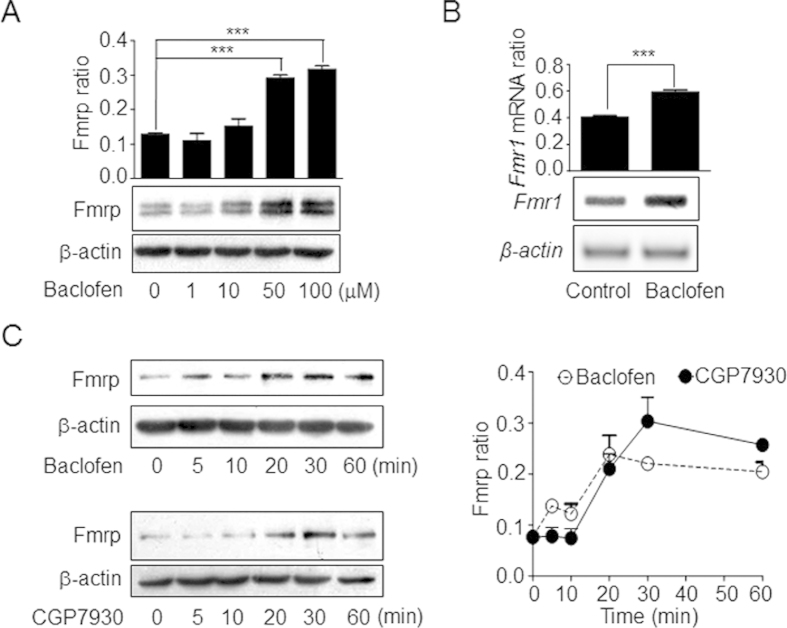
The link between GABAB receptor activation and Fmrp expression was investigated using the GABAB receptor agonist baclofen. Drug treatment increased Fmrp level in a dose-dependent manner in CGNs (Fig. 1A, Figure S1A), and increased Fmr1 mRNA expression as well as Fmrp protein synthesis in a time-dependent manner (Fig. 1B, C upper panel, Figure S1B, Figure S2) starting 20 min after drug application, with effects persisting for more than 60 min.
Figure 1. Activation of GABAB receptor upregulates Fmrp expression in CGNs.
(A) Fmrp expression in CGNs treated with indicated concentrations of baclofen. ***P < 0.001 vs. basal levels. (B) Fmr1 mRNA expression upon treatment with baclofen. (C) Time course of Fmrp expression induced by baclofen and CGP7930. Fmrp expression level was quantified based on three independent experiments (mean ± SEM). ***P < 0.001 vs. basal levels. Fmrp ratio and Fmr1 mRNA ratio were defined as the ratio between the density of each band and the sum of the densities of all the bands in a given blot. Full-size blots are shown in Supplementary Figure S1 and the band of interest is indicated by a red box.
PAMs potentiate the GABAB receptor activation by orthosteric agonists such as baclofen17; in the case of CGP7930, this is accomplished via binding to the transmembrane domain of GABAB2 subunit18,19,20. We recently showed that CGP7930 can directly activate the GABAB receptor in cultured cell lines and neurons in the absence of an agonist14,15. We found here that the kinetics of Fmrp expression after CGP7930 treatment were similar to those induced by baclofen (Fig. 1C, Figure S1C). These data demonstrate that GABAB receptor activation via the GABAB2 subunit increases Fmrp expression.
CREB is required for Fmrp upregulation induced by GABAB receptor
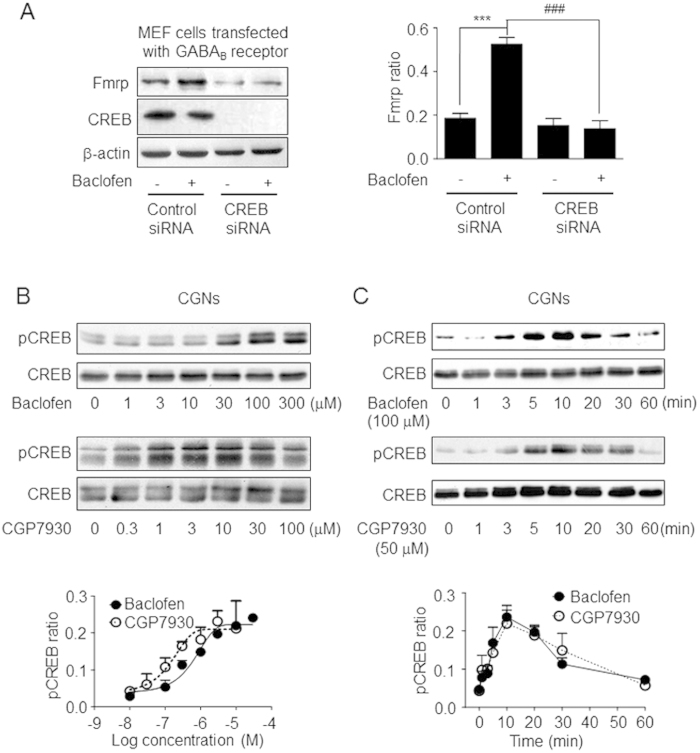
The Fmr1 gene promoter contains a CREB-binding site, and mGluR1 and 5 can regulate Fmrp expression through CREB21,22. Moreover, CREB itself is regulated by various receptors via downstream effectors such as PKA, PKC, ERK1/2, and Akt23,24,25. The role of CREB in GABAB receptor-mediated Fmrp upregulation was investigated by short interfering (si)RNA-knockdown of CREB in mouse embryonic fibroblasts (MEFs) co-transfected with GABAB1 and GABAB2. CREB depletion abolished the GABAB receptor-induced increase in Fmrp expression relative to the control (Fig. 2A, Figure S3A). These data indicate that GABAB receptor-induced CREB activity is required for Fmrp synthesis.
Figure 2. GABAB receptor-induced CREB signaling is required for Fmrp upregulation.
(A) Effect of siRNA-mediated knockdown of CREB on baclofen-induced Fmrp expression in MEFs co-transfected with GABAB1 and GABAB2. Representative western blots are shown. CREB phosphorylation level in control siRNA-transfected cells. Fmrp ratio was defined as in Fig. 1A. The level in baclofen-treated cells was quantified based on three independent experiments (mean ± SEM). ***P < 0.001 vs. basal with control siRNA. ###P < 0.001 vs. baclofen-treated cells transfected with control siRNA. (B) CREB phosphorylation in CGNs induced by indicated concentrations of baclofen or CGP7930. The data were quantified from three independent experiments (mean ± SEM). (C) Time course of CREB phosphorylation induced by baclofen and CGP7930. Protein level was quantified based on three independent experiments (mean ± SEM). pCREB ratio was defined as the ratio between the density of each band and the sum of the densities of all the bands in a given blot. Full-size blots are shown in Supplementary Figure S3 and the band of interest is indicated by a red box.
In CGNs, baclofen and CGP7930 treatment induced a concentration-dependent increase in the level of phosphorylated CREB without altering total CREB expression level (Fig. 2B, Figure S3B). The rapid and transient increase in CREB phosphorylation peaked at 10 min and decreased to the basal level at 60 min after drug application (Fig. 2C, Figure S3C). Interestingly, pre-treatment of CGNs with the competitive GABAB receptor antagonist CGP54626 blocked baclofen but not CGP7930-induced CREB phosphorylation (Figure S4). These results indicate that GABAB receptor activation can induce a transient increase in phosphorylation of CREB, a component of signaling pathway that important for Fmrp expression.
GABAB receptor-mediated transactivation of IGF-1R leads to CREB activation
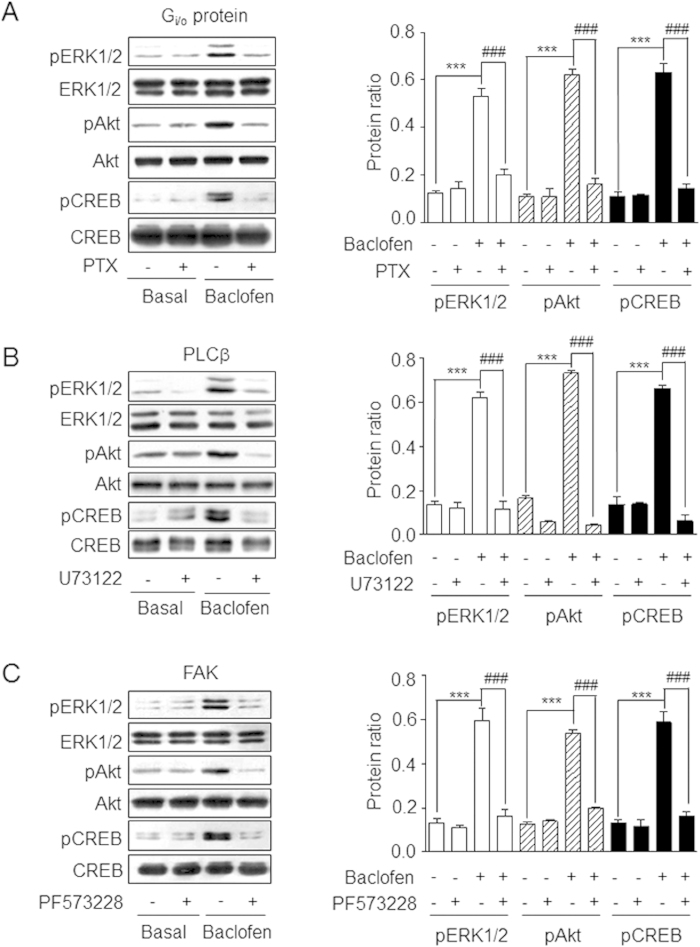
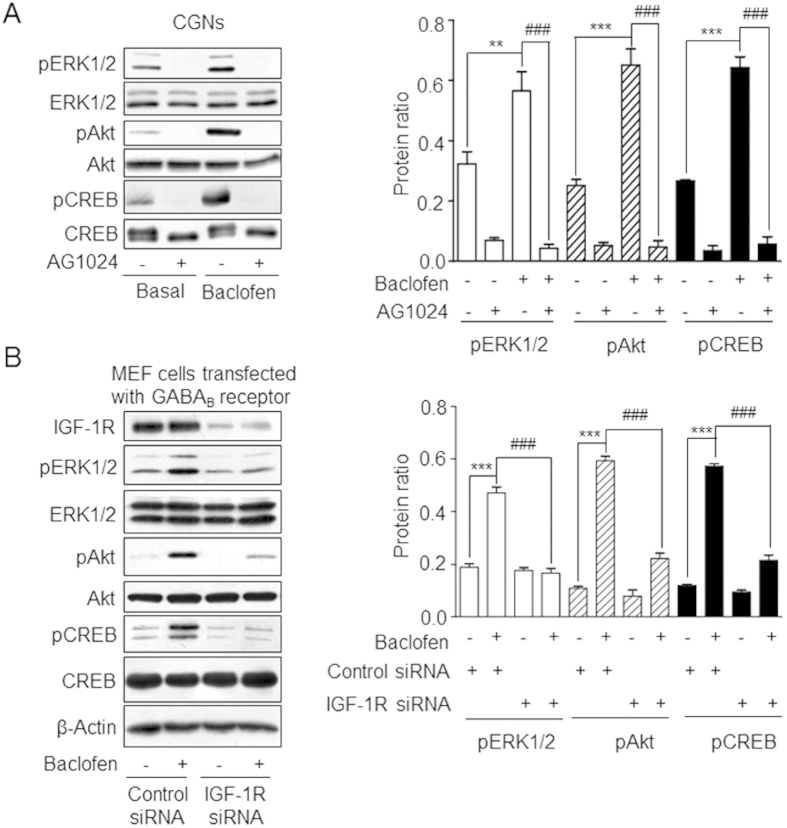
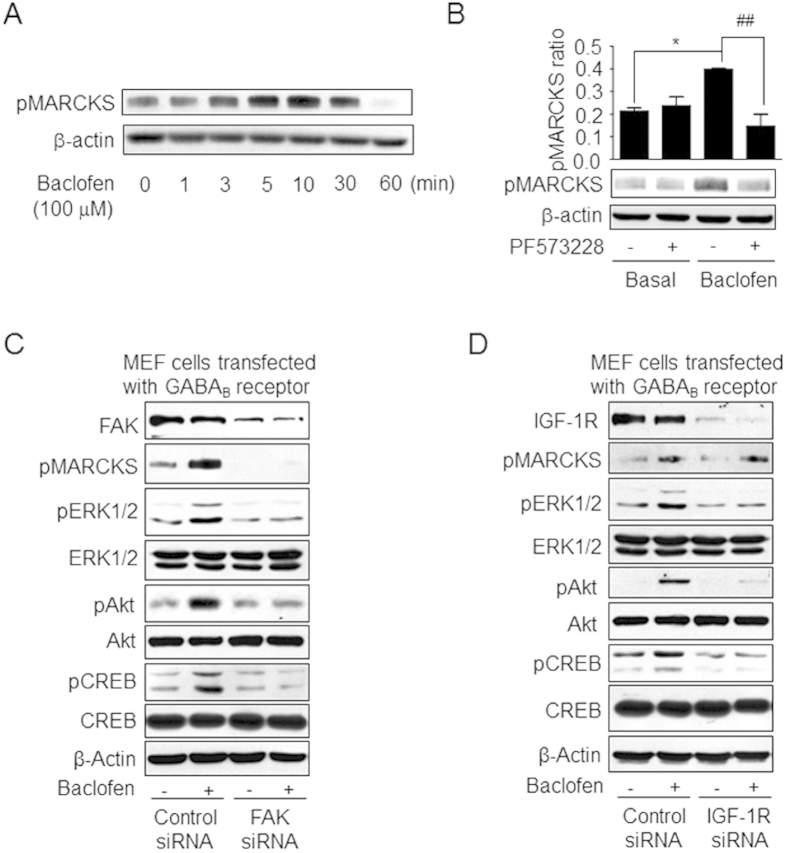
IGF-1R was reported to be transactivated by GABAB receptor through Gi/o protein, PLCβ and focal adhesion kinase (FAK), and then further induced MEK/ERK1/2 and PI3K/Akt activation15,16. Therefore, we investigated the role of the IGF-1R transactivation signaling pathway in the activation of CREB. ERK1/2, Akt, and CREB phosphorylation were blocked by treatment with pertussis toxin (PTX), which uncouples Gi/o proteins from GPCRs via ADP-ribosylation of Gαi/o subunits (Fig. 3A, Figure S5A), suggesting that GABAB receptor-mediated CREB activation in CGNs is Gi/o protein-dependent. Pre-treatment of CGNs with U73122 (PLCβ inhibitor) or PF573228 (FAK inhibitor) blocked baclofen-induced ERK1/2, Akt, and CREB phosphorylation (Fig. 3B, C, Figure S5B, C). Furthermore, the IGF-1R inhibitor AG1024 also blocked the baclofen-induced phosphorylation of CREB in CGNs, as well as that of ERK1/2 and Akt (Fig. 4A, Figure S6A). In MEFs expressing the recombinant GABAB receptor, siRNA knockdown of endogenous IGF-1R reduced baclofen-induced phosphorylation of CREB, ERK1/2, and Akt (Fig. 4B, Figure S6B). Similar results were obtained by short hairpin-mediated knockdown in MEFs using a shRNA targeting IGF-1R (Figure S7). Taken together, these results show that IGF-1R transactivation via Gi/o protein, PLCβ, and FAK is important for baclofen-induced CREB activation.
Figure 3. GABAB receptor-mediated CREB phosphorylation is dependent on Gi/o protein, PLCβ, and FAK.
CGNs were pretreated with PTX (A), U73122 (B), or PF573228 (C) before baclofen-stimulation. CREB, ERK1/2 and Akt phosphorylation was detected by western blotting. Protein ratio on the Y-axis was defined as the ratio between the density of each band and the sum of the densities of all the bands in a given blot. Data represent the mean ± SEM from three separate sets of immunoblots. ***P < 0.001 vs. basal level. ###P < 0.001 vs. baclofen-treated group. Full-size blots are shown in Supplementary Figure S5 and the band of interest is indicated by a red box.
Figure 4. GABAB receptor-mediated transactivation of IGF-1R is required for CREB phosphorylation.
(A) CGNs were pre-treated with the IGF-1R inhibitor AG1024 followed by baclofen. Expression levels of pERK1/2, pAkt and pCREB were quantified by western blotting. Data represent the mean ± SEM from at least three independent experiments. **P < 0.01, ***P < 0.001 vs. basal level. ###P < 0.001 vs. baclofen-treated group. (B) MEFs were transfected with GABAB1, GABAB2, and control or IGF-1R siRNA before treatment with baclofen. Phosphorylation of ERK1/2, Akt, and CREB was quantified by western blotting. ***P < 0.001 vs. basal level in control siRNA-transfected cells. ###P < 0.001 vs. baclofen-treated cells transfected with control siRNA. Protein ratio was defined as in Fig. 3. Full-size blots are shown in Supplementary Figure S6 and the band of interest is indicated by a red box.
PKC is required for GABAB receptor-induced CREB activation independent of IGF-1R signaling
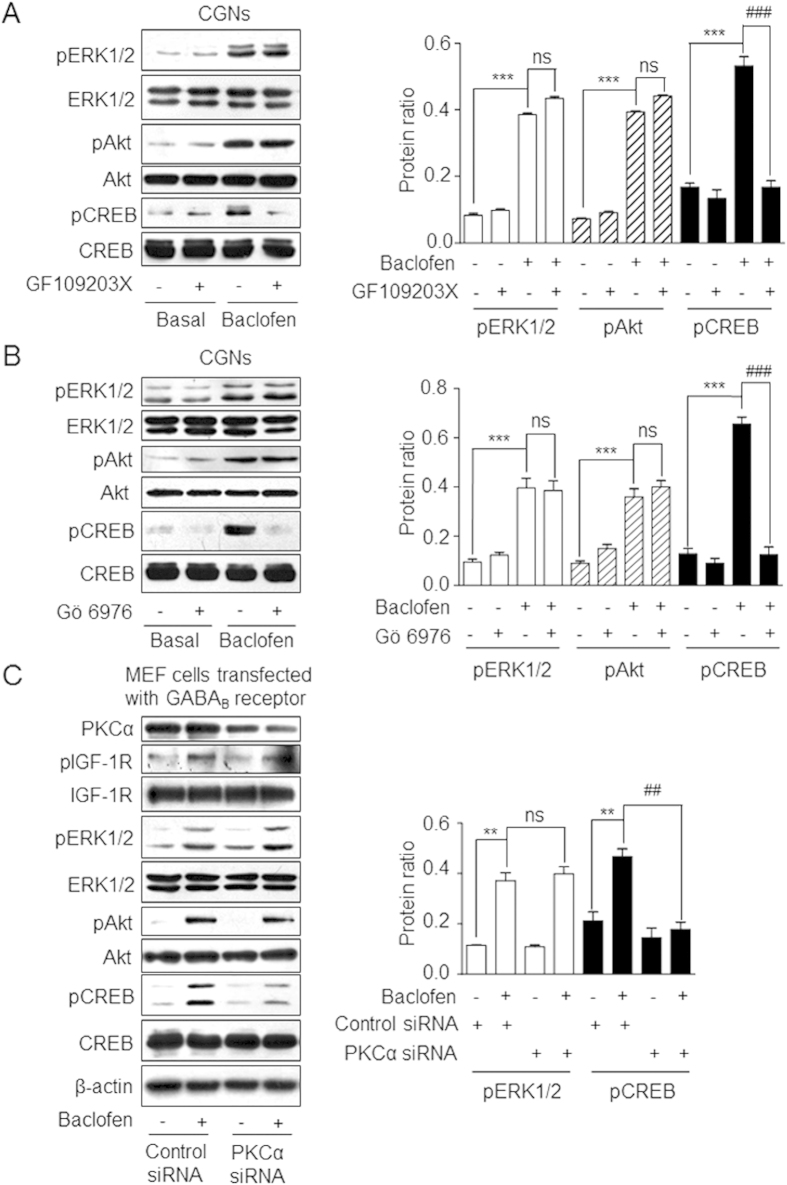
PKC was previously shown to be activated by baclofen treatment15. Phosphorylation of the PKC substrate MARCKS was increased in a time-dependent manner by baclofen treatment (Fig. 5A, Figure S8A). Phospho-MARCKS level was reduced by application of the FAK inhibitor PF573228 or by siRNA-mediated knockdown of FAK (Fig. 5B, C, Figure S8B, C), but not by IGF-1R knockdown (Fig. 5D, Figure S8D), suggesting that PKC acts downstream of FAK but independently of IGF-1R. Three PKC inhibitors (GF109203x, Gö-6983, and Gö-6976) were used to analyse the effect of PKC on CREB activation; GF109203x and Gö-6983 inhibit all PKC isozymes25,26, whereas Gö-6976 is selective for Ca2+ -sensitive PKC isotypes26. All three inhibitors blocked baclofen-induced CREB phosphorylation but had no effect on the phosphorylation of ERK1/2 and Akt (Fig. 6A, B, Figure S9A, B and Figure S10A). In addition, siRNA-mediated knockdown of PKCα or PKCβ in MEFs co-expressing GABAB1 and GABAB2 subunits of the GABAB receptor decreased baclofen-mediated CREB phosphorylation, whereas no changes in IGF-1R transactivation or ERK1/2 and Akt phosphorylation were observed (Fig. 6C, Figure S9C and Figure S10B). These results indicate that Ca2+ -sensitive PKCs are required for GABAB-induced CREB activation, but that this effect is independent of IGF-1R transactivation.
Figure 5. PKC activated by GABAB receptor acts downstream of FAK and is independent of IGF-1R signaling.
(A) Time course of phosphorylation of the PKC substrate MARCKS induced by baclofen in CGNs. (B) Effect of the FAK inhibitor PF573228 on MARCKS phosphorylation in CGNs. pMARCKS ratio was defined as the ratio between the density of each band and the sum of the densities of all the bands in a given blot. Values represent the mean ± SEM from three independent experiments. *P < 0.05 vs. basal level. ##P < 0.01 vs. baclofen-treated group. (C,D) Effect of siRNA-mediated knockdown of FAK or IGF-1R on baclofen-induced phosphorylation of MARCKS, ERK1/2, Akt, and CREB in MEFs co-transfected with GABAB1 and GABAB2. Full-size blots are shown in Supplementary Figure S8 and the band of interest is indicated by a red box.
Figure 6. PKC is required for GABAB receptor-induced CREB activation.
(A, B) CGNs were pre-treated with GF109203x (A) or Gö-6976 (B) followed by treatment with baclofen. ERK1/2, Akt, and CREB phosphorylation was quantified by western blotting and the protein ratio was defined as in Fig. 3. ***P < 0.001, vs. basal level. ###P < 0.001, ns, not significant vs. baclofen-treated group. (C) MEFs were co-transfected with GABAB1, GABAB2, and control or PKCα siRNA and then treated with baclofen. pERK1/2 and pCREB levels were quantified by western blotting and the protein ratio was defined as in panels A and B. Data represent the mean ± SEM from three independent experiments. **P < 0.01, vs. basal level in control siRNA-transfected cells. ##P < 0.01, ns, not significant vs. baclofen-treated cells transfected with control siRNA. Full-size blots are shown in Supplementary Figure S9 and the band of interest is indicated by a red box.
IGF-1R and PKC are required for GABAB receptor-induced upregulation of Fmrp expression
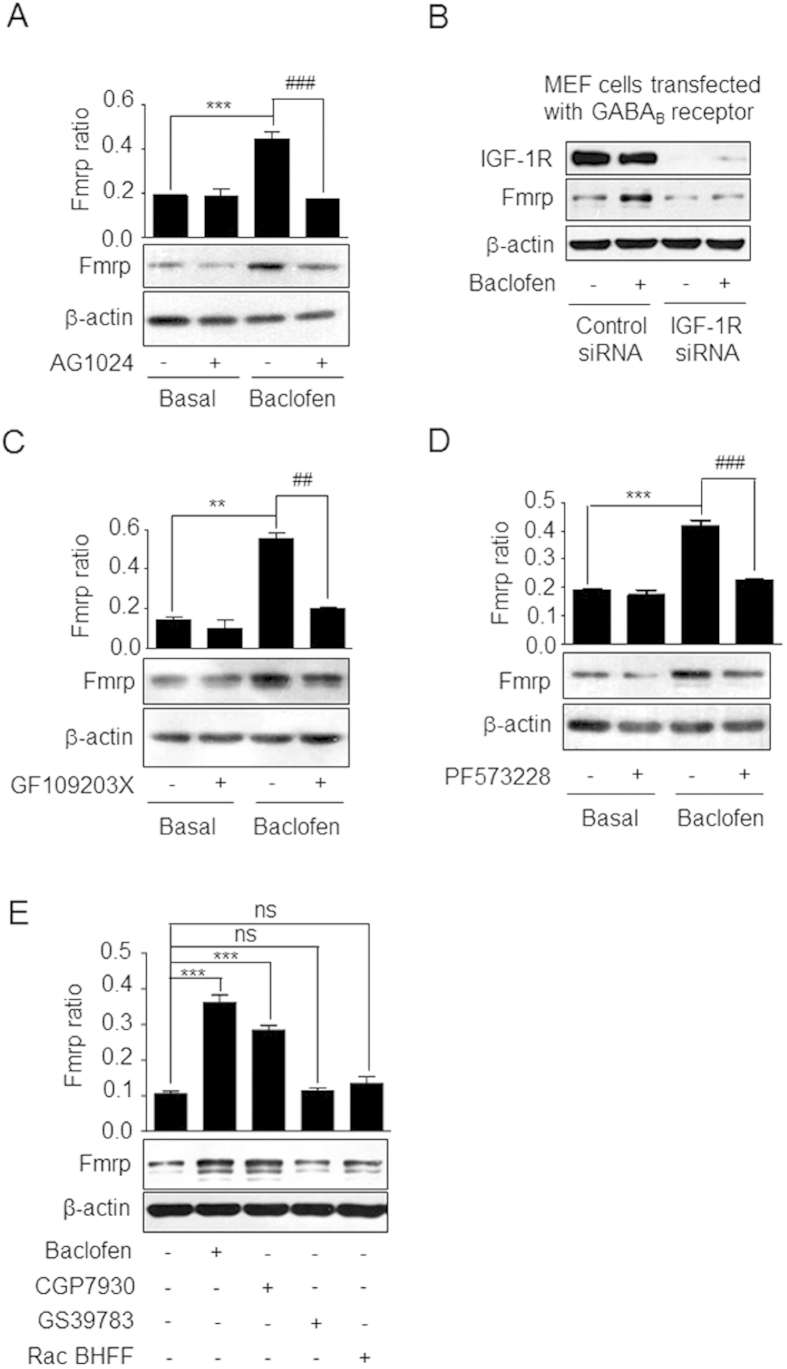
The role of IGF-1R and PKC in the GABAB receptor-induced expression of Fmrp was assessed. GABAB receptor-induced Fmrp synthesis was markedly reduced in CGNs by treatment with IGF-1R inhibitor (Fig. 7A, Figure S11A); siRNA-mediated IGF-1R knockdown abolished the baclofen-induced increase in Fmrp level in MEFs expressing the recombinant GABAB receptor (Fig. 7B, Figure S11B). PKC inhibitor also reduced baclofen-induced Fmrp expression (Fig. 7C, Figure S11C). FAK acts upstream of IGF-1R16 and PKC (Fig. 5B, C) in the GABAB receptor-mediated signaling pathway. Accordingly, pre-treatment with PF573228 decreased baclofen-induced Fmrp synthesis (Fig. 7D, Figure S11D). Taken together, these results indicate that both IGF-1R and PKC are critical for the upregulation of Fmrp expression induced by GABAB receptor activation.
Figure 7. IGF-1R and PKC are involved in GABAB receptor-mediated Fmrp synthesis.
(A) CGNs were pretreated with AG1024 followed by treatment with baclofen. Fmrp levels were detected by western blotting and Fmrp ratio was defined as in Fig. 1A. Data represent the mean ± SEM from three independent experiments. (B) MEFs were co-transfected with GABAB1, GABAB2, and control or IGF-1R siRNA and then treated with baclofen. Fmrp levels were quantified as in panel A. (C, D) CGNs were pretreated with GF109203x or PF573228, and then treated with baclofen. Fmrp levels were detected as in panel A. Data represent the mean ± SEM from three independent experiments. For results in A, C, D, **P < 0.01, ***P < 0.001 vs. basal level; ##P < 0.01, ###P < 0.001, vs. baclofen-treated group. (E) CGNs were treated with vehicle, baclofen, CGP7930, GS39783, or Rac BHFF and Fmrp expression level was quantified as in panel A. Data represent the mean ± SEM from three independent experiments. ***P < 0.001, ns, not significant vs. basal level. Full-size blots are shown in Supplementary Figure S11 and the band of interest is indicated by a red box.
GABAB receptor PAM increases Fmrp expression
PAMs bind to the transmembrane domain of the GABAB receptor at a location independent of the agonist-binding site, thereby potentiating the effect of the receptor agonist. Of the three commercially available GABAB receptor PAMs (CGP7930, GS39783, and Rac BHFF), CGP7930 and Rac BHFF but not GS39783 act as PAM agonists18,19,27,28,29. The PAMs were compared with respect to their effects on signaling events downstream of GABAB receptor activation. Interestingly, CGP7930 but not GS39783 or Rac BHFF induced the phosphorylation of Akt and CREB and increased the level of Fmrp in a manner similar to the agonist baclofen (Fig. 7E and Figure S12), confirming the role of GABAB receptor activation in the modulation of Fmrp expression.
Discussion
This study investigated the signaling events linking GABAB receptor activation to Fmrp expression. The results demonstrate that activation of the GABAB receptor by baclofen upregulates Fmrp synthesis via induction of CREB, which involves IGF-1R- and PKC-dependent signaling (Fig. 8). We also found that the GABAB receptor PAM CGP7930 upregulated Fmrp expression.
Figure 8. Schematic representation of the signaling pathway mediated by GABAB receptor leading to CREB activation and Fmrp upregulation in CGNs.
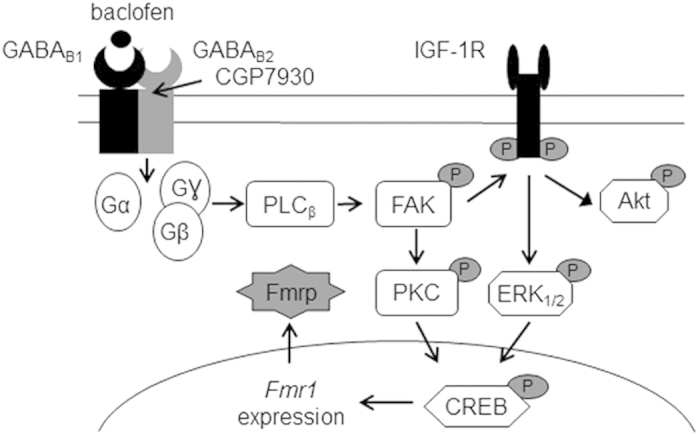
Agonist (baclofen) or PAM (CGP7930) activates the GABAB receptor, leading to Gi/o protein/PLCβ/FAK activation, which in turn transactivates the IGF-1R signaling pathway and induces PKC-mediated CREB phosphorylation, thereby upregulating Fmrp expression both at the mRNA and protein levels.
Our results clarify the signaling link between GABAB receptor and Fmrp expression. However, they cannot explain the beneficial effect of baclofen in FXS mouse model or in patients, where the Fmr1 gene is deleted or expression is blocked4,30. One possible explanation to reconcile these different findings may be through the activation of CREB by the GABAB receptor. CREB is a transcription factor involved in the activation of many genes25; CREB phosphorylation at serine 133 promotes its binding to the CRE site and leads to gene transcription25 and regulates learning and memory23,31. CREB-targeted genes may facilitate memory formation through the induction of long-term potentiation or long-term depression of synaptic plasticity32,33, the growth and formation of new synaptic spines and connections33,34, or new protein synthesis participating in memory reconstruction35 which might help to improve cognition in FXS.
Our study suggests a novel physiological role for Frmp in neurons. Indeed, Fmrp is wildly expressed in neurons and participates in a number of intracellular processes involving mRNAs metabolism related to synaptic function and maturation2,36. Several reports also implied that Fmrp played a role in neuronal survival and apoptosis37,38; the GABAB receptor was also found to transactivate IGF-1R and protect neurons against apoptosis15, suggesting a possible role of Fmrp in mediating the anti-apoptotic effects of GABAB receptor.
IGF-1R and PKC act independently in GABAB receptor/CREB/Fmrp regulation, but it is unclear how these two signaling pathways from membrane receptor and intracellular kinase integrated. We showed in our previous study that FAK serves as a platform for recruiting G protein, IGF-1R, and Akt to the activated GABAB receptor and further regulating GABAB receptor-induced neuroprotection16. FAK also has high affinity for PI3K and PLCγ39. PKC and its substrate MARCKS were reported to be activated after FAK phosphorylation40, supporting our finding that FAK acts upstream of PKC. However, as the phosphorylation profiles of FAK tyrosine and serine residues are important for distinct signal transduction cascades39,41, how FAK phosphorylation regulates IGF-1R and PKC is still under investigation. Meanwhile, given that PKC translocates from the cytosol to the membrane after GABAB receptor activation to modulate desensitization42, the PKC pathway may play a role in controlling CREB and Fmrp activity.
PAMs bind to the GABAB receptor at a site distinct from agonists such as R-baclofen (STX209)16,25,26, which was shown to improve FXS-associated symptoms in mice and humans7,8,43. Extensive data from preclinical studies on GABAB receptor PAMs indicate that their benefits are similar to those of agonists, but with superior side effect profiles44. Among them, GS39783 was used to treat FXS mice and showed no significant improvement in an audiogenic seizure test43, consistent with our observation that GS39783 had no effect on CREB activation and Fmrp expression. CGP7930 and Rac BHFF, both of which exhibit PAM agonist activity27, have not yet been tested in an FXS mouse model. In our study, only CGP7930 induced an upregulation in the level of Fmrp expression similar to baclofen. This is consistent with the finding of CGP7930 alone being able to activate ERK1/2 and Akt signaling14,15. These results suggest that CGP7930 is a promising candidate for the treatment of FXS symptoms and useful for the development of novel drugs.
Methods
Drugs
GABA was purchased from Sigma (St. Louis, MO, USA). (R)-Baclofen, CGP54626, CGP7930, GS39783, Rac BHFF, PTX, U73122, and PF573228 were purchased from Tocris (Fisher-Bioblock, Illkirch, France). AG1024 was purchased from Santa Cruz Biotechnology (Shanghai, China). Foetal bovine serum (FBS) and other solutions used for cell culture were from Invitrogen (Shanghai, China).
Antibodies
Primary antibodies against phospho-ERK1/2 (rabbit monoclonal), ERK1/2, phospho-Akt (Ser473) (193H12; rabbit monoclonal), Akt, phospho-CREB (rabbit), phospho-MARCKS, CREB, IGF-1Rβ, β-actin, and Fmrp, as well as horseradish peroxidase (HRP)-conjugated secondary antibodies were purchased from Cell Signaling Technology (Shanghai, China). Antibodies against PKCα and PKCβ were from Santa Cruz Biotechnology.
Primary culture of CGNs
All animal experiments were approved by the Animal Experimentation Ethics Committee of the School of Life Science and Technology at Huazhong University of Science and Technology and were carried out in accordance with the approved guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health (Bethesda, MD, USA). Primary CGN cultures were established as previously described14. Briefly, the cerebellum was dissected from 1-week-old KunMing mice of either sex obtained from Hubei Provincial Center for Disease Control and Prevention. Cells were maintained in a 1:1 mixture of Dulbecco’s Modified Eagle’s Medium (DMEM) with F-12 nutrients (Invitrogen) supplemented with 30 mM glucose, 2 mM glutamine, 3 mM sodium bicarbonate, 5 mM HEPES buffer, 30 mM KCl, and 10% FBS.
MEFs culture and transfection
MEFs were cultured in DMEM supplemented with 10% FBS. For RNA interference experiments, MEFs were transfected using Lipofectamine 2000 (Thermo Fisher Scientific, Shanghai, China) according to the manufacturer’s protocol, using siRNAs against IGF-1Rα/β (sc-35638), PKCα (sc-208), PKCβII (sc-210), and FAK (sc-35353) or control siRNA-A (sc-37007) from Santa Cruz Biotechnology. One day after transfection, cells were transfected with HA-GABAB1 and Flag-GABAB2 plasmids for another 24 h before drug treatment.
Drug treatments
Cultures were washed once with Ca2+ -free HEPES-buffered solution (HBS; 10 mM HEPES, 140 mM NaCl, 4 mM KCl, 2 mM MgSO4, 1 mM KH2PO4, pH 7.4) and pre-incubated at 37 °C in the same solution for 60 min. Drugs were freshly prepared in HBS with or without dimethyl sulfoxide (DMSO)/1 M NaOH. Inhibitor pre-treatment was as follows: AG1024 (0.1 μM, 1 h), PTX (200 ng/ml, 14–16 h), U73122 (5 μM, 1 h), PF573228 (10 μM, 1 h), GF109203x (10 μM, 1 h) and Gö-6976 (1 μM, 1 h). Baclofen (100 μM) and CGP7930 (50 μM) were applied in time-course experiments. Baclofen (100 μM) and CGP7930 (50 μM) were applied for 10 min to detect ERK1/2, Akt, CREB, IGF-1R, and MARCKS phosphorylation; baclofen (100 μM), CGP7930 (50 μM), GS39783 (50 μM), or Rac BHFF (50 μM) were applied for 30 min before measuring Fmrp expression. At the end of the treatment, cells were quickly washed with ice-cold phosphate-buffered saline (PBS; pH 7.4) before lysis buffer was added to the cells, which were immediately placed on ice. The cell monolayer was scraped into Eppendorf tubes. HBS containing the same concentration of DMSO or NaOH was used as the vehicle control.
Western blot analysis
Lysates from cultured cells were sonicated and protein concentrations were determined using the Bradford reagent (Bio-Rad Laboratories, Hertfordshire, UK). Equal amounts of protein (20 μg) were resolved by sodium dodecyl sulphate polyacrylamide gel electrophoresis. Proteins were transferred to nitrocellulose membranes (Millipore, Bedford, MA, USA), which were incubated in blocking buffer (5% non-fat dry milk in Tris-buffered saline and 0.1% Tween 20) for 1 h, followed by incubation with primary antibodies (1:1000) overnight at 4 °C and a 2 h incubation with HRP-conjugated secondary antibodies (1:20,000). Immunoreactivity was visualized on X-ray films using the enhanced chemiluminescence reagent (Pierce, Rockford, IL, USA). The density of the protein bands was measured using Image J software (National Institutes of Health, Bethesda, MD, USA) Protein ratio on the ordinates (Y) axis of the histograms was defined as the ratio between the density of each band and the sum of the densities of all the bands in a given blot.
Reverse transcription PCR
After drug treatment, total cellular RNA was isolated using TRIzol reagent, and reverse transcription was carried out according to the manufacturer’s protocol (Invitrogen). First-strand cDNA was generated from 4 μg total RNA using oligo-dT primer and M-MLV reverse transcriptase (Invitrogen). PCR analysis was performed using the following sense and antisense primers: Fmr1, 5′-CCG AAC AGA TAA TCG TCC ACG-3′ and 5′-ACG CTG TCT GGC TTT TCC TTC-3′ and β-actin, 5′-CCG CCC TAG GCA CCA GGG TG-3′ and 5′-GGC TGG GGT GTT GAA GGT CTC AAA-3′ (internal control). The mRNA ratio was defined as the ratio between the density of each band and the sum of the densities of all bands in a given gel.
Statistical analysis
Data are presented as mean ± SEM of at least three independent experiments. Data in Fig. 1B were analysed by the student’s t test and statistical analysis of other data was carried out with one-way ANOVA analysis.
Additional Information
How to cite this article: Zhang, W. et al. GABAB receptor upregulates fragile X mental retardation protein expression in neurons. Sci. Rep. 5, 10468; doi: 10.1038/srep10468 (2015).
Supplementary Material
Supplementary Figures 1-6
Acknowledgments
The authors thank Drs Jean-Philippe Pin (Institut de Génomique Fonctionnelle, Montpellier, France) and X.Z. Shawn Xu (University of Michigan, Ann Arbor, MI, USA) for critically reading an early version of the manuscript. This work was supported by the National Natural Science Foundation of China (NSFC grant nos. 31225011, 31130028, and 31420103909), the Ministry of Science and Technology (grant no. 2012CB518000), the Program of Introducing Talents of Discipline to the Universities of the Ministry of Education (grant no. B08029), the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT) [grant no. IRT13016], Natural Science Foundation of Hubei province [grant no. 2014CFA010], and the Mérieux Research Grants Program of Institut-Mérieux (to J.L.).
Footnotes
Author Contributions J.L. conceived the project; W.Z., C.X., H.T. and J.L. designed the experiments; W.Z., C.X., Y.W., Q.S., H.P. and Y.H. performed the experiments; W.Z., C.X., P.R. and J.L. analysed the results; and C.X. wrote the manuscript with J.L. and P.R.
References
- Bagni C. & Greenough W. T. From mRNP trafficking to spine dysmorphogenesis: the roots of fragile X syndrome. Nat Rev Neurosci 6, 376–87 (2005). [DOI] [PubMed] [Google Scholar]
- Pasciuto E. & Bagni C. SnapShot: FMRP mRNA targets and diseases. Cell 158, 1446–1446 e1 (2014). [DOI] [PubMed] [Google Scholar]
- Penagarikano O., Mulle J. G. & Warren S. T. The pathophysiology of fragile x syndrome. Annu Rev Genomics Hum Genet 8, 109–29 (2007). [DOI] [PubMed] [Google Scholar]
- Braat S. & Kooy R. F. Fragile X syndrome neurobiology translates into rational therapy. Drug Discov Today 19, 510–9 (2014). [DOI] [PubMed] [Google Scholar]
- Costa L. et al. Activation of 5-HT7 serotonin receptors reverses metabotropic glutamate receptor-mediated synaptic plasticity in wild-type and Fmr1 knockout mice, a model of Fragile X syndrome. Biol Psychiatry 72, 924–33 (2012). [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E. Mechanism-based treatments in neurodevelopmental disorders: fragile X syndrome. Pediatr Neurol 50, 297–302 (2014). [DOI] [PubMed] [Google Scholar]
- Henderson C. et al. Reversal of disease-related pathologies in the fragile X mouse model by selective activation of GABAB receptors with arbaclofen. Sci Transl Med 4, 152ra128 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry-Kravis E. M. et al. Effects of STX209 (arbaclofen) on neurobehavioral function in children and adults with fragile X syndrome: a randomized, controlled, phase 2 trial. Sci Transl Med 4, 152ra127 (2012). [DOI] [PubMed] [Google Scholar]
- Bettler B., Kaupmann K., Mosbacher J. & Gassmann M. Molecular structure and physiological functions of GABA(B) receptors. Physiol Rev 84, 835–67 (2004). [DOI] [PubMed] [Google Scholar]
- Pin J. P. et al. Activation mechanism of the heterodimeric GABA(B) receptor. Biochem Pharmacol 68, 1565–72 (2004). [DOI] [PubMed] [Google Scholar]
- Liu J. et al. Molecular determinants involved in the allosteric control of agonist affinity in the GABAB receptor by the GABAB2 subunit. J Biol Chem 279, 15824–30 (2004). [DOI] [PubMed] [Google Scholar]
- Pin J. P. et al. Allosteric functioning of dimeric class C G-protein-coupled receptors. FEBS J 272, 2947–55 (2005). [DOI] [PubMed] [Google Scholar]
- Chalifoux J. R. & Carter A. G. GABAB receptor modulation of synaptic function. Curr Opin Neurobiol 21, 339–44 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu H. et al. Dominant role of GABAB2 and Gbetagamma for GABAB receptor-mediated-ERK1/2/CREB pathway in cerebellar neurons. Cell Signal 19, 1996–2002 (2007). [DOI] [PubMed] [Google Scholar]
- Tu H. et al. GABAB receptor activation protects neurons from apoptosis via IGF-1 receptor transactivation. J Neurosci 30, 749–59 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X. et al. An activity-based probe reveals dynamic protein-protein interactions mediating IGF-1R transactivation by the GABA(B) receptor. Biochem J 443, 627–34 (2012). [DOI] [PubMed] [Google Scholar]
- Pin J. P. & Prezeau L. Allosteric modulators of GABA(B) receptors: mechanism of action and therapeutic perspective. Curr Neuropharmacol 5, 195–201 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binet V. et al. The heptahelical domain of GABA(B2) is activated directly by CGP7930, a positive allosteric modulator of the GABA(B) receptor. J Biol Chem 279, 29085–91 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urwyler S. et al. Positive allosteric modulation of native and recombinant gamma-aminobutyric acid(B) receptors by 2,6-Di-tert-butyl-4-(3-hydroxy-2,2-dimethyl-propyl)-phenol (CGP7930) and its aldehyde analog CGP13501. Mol Pharmacol 60, 963–71 (2001). [PubMed] [Google Scholar]
- Onali P., Mascia F. M. & Olianas M. C. Positive regulation of GABA(B) receptors dually coupled to cyclic AMP by the allosteric agent CGP7930. Eur J Pharmacol 471, 77–84 (2003). [DOI] [PubMed] [Google Scholar]
- Wang H., Wu L. J., Zhang F. & Zhuo M. Roles of calcium-stimulated adenylyl cyclase and calmodulin-dependent protein kinase IV in the regulation of FMRP by group I metabotropic glutamate receptors. J Neurosci 28, 4385–97 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. et al. Roles of CREB in the regulation of FMRP by group I metabotropic glutamate receptors in cingulate cortex. Mol Brain 5, 27 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon W. A. Jr., Duman R. S. & Nestler E. J. The many faces of CREB. Trends Neurosci 28, 436–45 (2005). [DOI] [PubMed] [Google Scholar]
- Henderson C. et al. Reversal of disease-related pathologies in the fragile X mouse model by selective activation of GABA(B) receptors with arbaclofen. Sci Transl Med 4, 152ra128 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannessen M., Delghandi M. P. & Moens U. What turns CREB on? Cell Signal 16, 1211–27 (2004). [DOI] [PubMed] [Google Scholar]
- Martiny-Baron G. et al. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J Biol Chem 268, 9194–7 (1993). [PubMed] [Google Scholar]
- Malherbe P. et al. Characterization of (R,S)-5,7-di-tert-butyl-3-hydroxy-3-trifluoromethyl-3H- benzofuran-2-one as a positive allosteric modulator of GABAB receptors. Br J Pharmacol 154, 797–811 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urwyler S. et al. N,N’-Dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4,6-diamine (GS39783) and structurally related compounds: novel allosteric enhancers of gamma-aminobutyric acidB receptor function. J Pharmacol Exp Ther 307, 322–30 (2003). [DOI] [PubMed] [Google Scholar]
- Urwyler S., Gjoni T., Koljatic J. & Dupuis D. S. Mechanisms of allosteric modulation at GABAB receptors by CGP7930 and GS39783: effects on affinities and efficacies of orthosteric ligands with distinct intrinsic properties. Neuropharmacology 48, 343–53 (2005). [DOI] [PubMed] [Google Scholar]
- Braat S. & Kooy R. F. Insights into GABAAergic system deficits in fragile X syndrome lead to clinical trials. Neuropharmacology 88, 48–54 (2015). [DOI] [PubMed] [Google Scholar]
- Kandel E. R. The molecular biology of memory: cAMP, PKA, CRE, CREB-1, CREB-2, and CPEB. Mol Brain 5, 14 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha-Molstad H., Keller D. M., Yochum G. S., Impey S. & Goodman R. H. Cell-type-specific binding of the transcription factor CREB to the cAMP-response element. Proc Natl Acad Sci USA 101, 13572–7 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie H., Morishita W., Yu X., Calakos N. & Malenka R. C. Generation of silent synapses by acute in vivo expression of CaMKIV and CREB. Neuron 45, 741–52 (2005). [DOI] [PubMed] [Google Scholar]
- Martin K. C. et al. Synapse-specific, long-term facilitation of aplysia sensory to motor synapses: a function for local protein synthesis in memory storage. Cell 91, 927–38 (1997). [DOI] [PubMed] [Google Scholar]
- Kida S. et al. CREB required for the stability of new and reactivated fear memories. Nat Neurosci 5, 348–55 (2002). [DOI] [PubMed] [Google Scholar]
- Pasciuto E. & Bagni C. SnapShot: FMRP interacting proteins. Cell 159, 218–218 e1 (2014). [DOI] [PubMed] [Google Scholar]
- Jeon S. J. et al. Cellular stress-induced up-regulation of FMRP promotes cell survival by modulating PI3K-Akt phosphorylation cascades. J Biomed Sci 18, 17 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon S. J. et al. Positive feedback regulation of Akt-FMRP pathway protects neurons from cell death. J Neurochem 123, 226–38 (2012). [DOI] [PubMed] [Google Scholar]
- Parsons J. T. Focal adhesion kinase: the first ten years. J Cell Sci 116, 1409–16 (2003). [DOI] [PubMed] [Google Scholar]
- Garrett A. M., Schreiner D., Lobas M. A. & Weiner J. A. gamma-protocadherins control cortical dendrite arborization by regulating the activity of a FAK/PKC/MARCKS signaling pathway. Neuron 74, 269–76 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Sinnett-Smith J. & Rozengurt E. Differential FAK phosphorylation at Ser-910, Ser-843 and Tyr-397 induced by angiotensin II, LPA and EGF in intestinal epithelial cells. Cell Signal 19, 1000–10 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontier S. M. et al. Coordinated action of NSF and PKC regulates GABAB receptor signaling efficacy. EMBO J 25, 2698–709 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacey L. K., Tharmalingam S. & Hampson D. R. Subchronic administration and combination metabotropic glutamate and GABAB receptor drug therapy in fragile X syndrome. J Pharmacol Exp Ther 338, 897–905 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip M. et al. GABAB receptors as a therapeutic strategy in substance use disorders: focus on positive allosteric modulators. Neuropharmacology 88, 36–47 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-6