Summary
Recent structural data have revealed two distinct conformations of the ribosome during initiation. We employed single-molecule fluorescence methods to probe the dynamic relation of these ribosomal conformations in real time. In the absence of initiation factors, the ribosome assembles in two distinct conformations. The initiation factors discriminate between these two conformations, guiding assembly of the conformation that can enter the elongation cycle. In particular, IF2 both accelerates the rate of subunit joining and actively promotes the transition to the elongation-competent conformation. Blocking GTP hydrolysis by IF2 results in 70S complexes formed in the conformation unable to enter elongation. We observe that rapid GTP hydrolysis by IF2 drives the transition to the elongation-competent conformation, thus committing the ribosome to enter the elongation cycle.
Introduction
Initiation of translation by the ribosome establishes reading frame on an mRNA and regulates progression to the elongation cycle. Protein synthesis is initiated on a ribosome positioned at an AUG start codon and loaded with an initiator tRNA in the peptidyl site (P site). (Gualerzi and Pon, 1990; Laursen et al., 2005). Prokaryotic initiation occurs through a stepwise process. First, mRNA and initiator tRNA are selected and positioned on the small (30S) ribosomal subunit, forming a pre-initiation complex (PIC) (Lockwood et al., 1971; Blumberg et al., 1979). The large (50S) ribosomal subunit then joins the 30S PIC to form the 70S initiation complex, which is poised to select tRNA encoded by the subsequent mRNA codon and form the first peptide bond. The assembly of elongation-competent ribosome complexes is highly regulated to avoid improper levels of gene expression and synthesis of aberrant polypeptides due to out-of-frame mRNA decoding (Hartz et al., 1989; Gold and Stormo, 1990).
Three translation initiation factors, IF1, IF2 and IF3, control the initiation process in prokaryotes (Laursen et al., 2005; Marintchev and Wagner, 2004). IF1 and IF3 work in concert to prevent premature association of 50S subunits and discriminate between elongator and initiator tRNAs in the P-site. (Grunberg-Manago et al., 1975; Dottavio-Martin et al., 1979; Dallas and Noller, 2001; Antoun et al., 2004; Antoun et al., 2006b). IF2, a 100 kDa ribosomal GTPase, has binding sites on both the 30S and 50S subunits. (Bollen et al., 1975; Heimark et al., 1976; La Teana et al., 2001; Marzi et al., 2003; Caserta et al., 2006). IF2 binds 30S subunits during initiator tRNA selection and recognizes the formyl group of fMet-tRNAfMet, enhancing its affinity for the 30S subunit (Wintermeyer and Gualerzi, 1983; Canonaco et al., 1986; Guenneugues et al., 2000; Antoun et al. 2006a).
IF2 accelerates subunit joining – the decisive event in translation initiation. Docking of the 50S subunit at a correctly formed 30S PIC triggers events that prepare the ribosome for entry into the elongation cycle: hydrolysis of GTP by IF2, dissociation of each initiation factor from the 30S PIC, and binding of the first elongator tRNA. Subunit joining stimulates the GTPase function of IF2; GTP hydrolysis occurs within milliseconds of subunit joining (Rodnina et al., 2000; Tomsic et al., 2000; Antoun et al., 2003). Disruption of IF2 GTPase function by mutations in its highly conserved G-domain (domain II/III) or with non-hydrolyzable GTP analogs prevents release of IF2 from 70S ribosomes and the transition of these complexes into elongation (Luchin et al., 1999; Tomsic et al., 2000; Antoun et al., 2003). However, the precise functional role of GTP hydrolysis in translation initiation remains unclear.
Structural biology has recently provided snapshots of translation initiation intermediates. Cryoelectron microscopy (cryo EM) of the 30S PIC reveals initiator tRNAfMet in the P site, with IF2 positioned across the intersubunit interface of the 30S particle (Simonetti et al., 2008). The C-terminal domain IV of IF2 contacts the exposed CCA-end of initiator tRNA, whereas the IF2 GTPase domain is located along the body of the 30S subunit, near sites that form intersubunit contacts with a joining 50S subunit; initiator tRNA is rotated such that its 3′end would be directed into the E-site on a joined 50S subunit. This resembles a hybrid-state (P/E) conformation of a tRNA post peptide-bond formation within a 70S elongation complex (Valle et al., 2003; Agirrezabala et al., 2008; Julían et al., 2008). The cryo EM structure of IF2-bound 70S initiation complex has been solved using non-hydrolyzable GTP analogs to trap this intermediate state (Allen et al., 2005; Myasnikov et al., 2005). This structure shows the three initiation factors on the ribosome, with the G-domain of IF2 (domain II/III) now in contact with the GTPase activating center of the 50S subunit. Additional rearrangements of IF2 and tRNA are observed compared to the 30S initiation complex. GTP hydrolysis leads to the GDP form of IF2-bound 70S initiation complex, which shows further rearrangement of IF2 within the 70S particle (Myasnikov et al., 2005). The major difference between the 70S complex stalled with non-hydrolyzable GTP and the post-hydrolysis complex is a rotation of the small subunit with respect to the large subunit and accommodation of the 3′end of initiator tRNA into the peptidyl transferase center P site.
30S rotation with respect to the 50S subunit has been implicated in initiation, elongation, and termination of translation. Structural studies have identified two intersubunit conformations, differing by a ~5° counterclockwise rotation of the 30S subunit with respect to the 50S subunit (Frank and Agrawal, 2000). Here, we refer to these as the non-rotated and rotated states, respectively. In elongation, the two conformations have been correlated to the conformation of tRNAs on the ribosome (Cornish et al., 2008; Marshall et al., 2008). Prior to peptide bond formation, tRNAs occupy a classical configuration within the P and A sites and the 30S subunit is in the non-rotated state. Cryo EM studies of 70S complexes post peptide-bond formation show movement of tRNAs into a P/E and A/P hybrid conformation, with correlated rotation of the small subunit (Agirrezabala et al., 2008; Julían et al., 2008). EF-G binding and GTP hydrolysis return the ribosome to the non-rotated state. Evidence for GTP hydrolysis-mediated subunit rotation is also observed in 70S initiation complexes: pre-GTP hydrolysis, 70S complexes resemble the rotated conformation, whereas post-GTP hydrolysis, they resemble the classic conformation (Myasnikov et al., 2005). GTP hydrolysis by IF2 may drive dynamic rearrangements of the initiator tRNA, IF2, and ribosomal subunits, committing the ribosome to elongation.
Pre-steady state kinetic studies and static structures of stalled-initiation complexes have not probed the conformational pathway by which ribosomes assemble and how intersubunit motion might prepare the ribosome to enter elongation. We have previously used single-molecule fluorescence techniques to show that irreversible chemical steps couple conformational dynamics of ribosomal subunits to the elongation phase of translation (Marshall et al., 2008).
We apply here these single-molecule techniques to investigate the role of intersubunit dynamics in the late phases of initiation. We directly observe subunit joining and the transition to elongation in real time. 70S initiation complexes formed in both the classic and rotated states are stable on the minutes timescale. In agreement with prior studies, we show that the initiation factors, in particular IF2, facilitate subunit joining and guide assembly of elongation-competent 70S initiation complexes. IF2-catalyzed GTP hydrolysis drives rotation of the 30S subunit from the rotated to the classical state, committing the ribosome to elongation.
Results
Real-time observation of subunit joining by smFRET
Fluorescence resonance energy transfer (FRET) between site-specifically labeled Cy3-30S and Cy5-50S ribosomal subunits directly reports on ribosome conformation. (Dorywalska et al., 2005; Marshall et al., 2008). These dye-labeled ribosomes permit the unambiguous detection of conformational changes as small as 5Å, and are active in subunit joining, peptide bond formation, translocation, and full protein synthesis (Marshall et al., 2008; Uemura et al., 2007). These labeling sites are distant from the binding sites of IF1, IF2, and IF3, the ribosomal sites responsible for GTPase stimulation, and dynamic domains such as the 30S head, and 50S L1 and L7/L12 stalk regions (Figure 1) (Carter et al., 2001; Allen et al., 2005; Myasnikov et al., 2005; Dallas and Noller, 2001; Pioletti et al., 2001; Schuwirth et al., 2005; Agrawal et al., 1999b; Stark et al., 2000; Ogle et al., 2001; Harms et al., 2001).
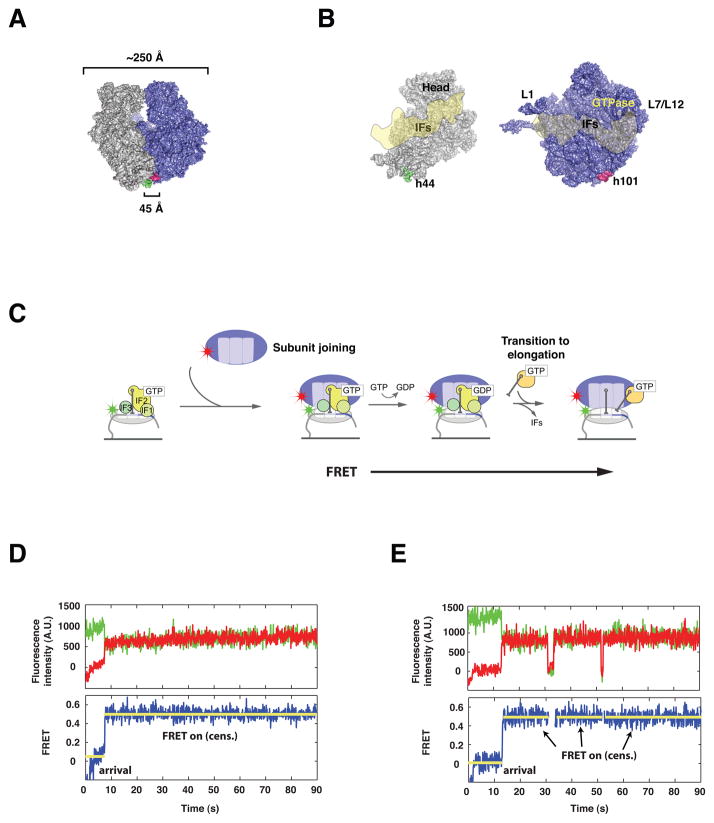
Figure 1. Intersubunit FRET between specifically labeled ribosomal subunits reports on intersubunit dynamics without interfering with ribosome function.
(A) Surface representation of the E. coli 70S ribosome, showing the location of Cy3 (green) and Cy5 (red) labels. The 30S subunit (grey) is labeled at helix 44, whereas the 50S subunit (blue) is labeled at helix 101. (B) View of the 30S (grey) and 50S (blue) subunits from the subunit interface, with an overlay of the estimated binding footprint for the initiation factors (IFs), modeled from structural studies. (C) Surface-immobilization of Cy3-labeled 30S pre-initiation complexes containing 30S subunits (grey), initiation factors (green), tRNA and mRNA, followed by delivery of Cy5-labeled 50S (blue) results in formation of a 70S initiation complex and establishment of a FRET signal sensitive to intersubunit conformation. (D, E) Representative fluorescence vs. time trajectories obtained from single 70S complexes. Raw fluorescence from Cy3 (green) and Cy5 (red) are used to calculate FRET (blue). Upon stop-flow delivery of cy5-50S, an initial dwell time is observed, followed by a burst of FRET. This FRET signal is stable and often censored by the end of observation (D) or less frequently by photophysical events (E).
Subunit joining and subsequent intersubunit dynamics were observed for hundreds of single 70S initiation complexes via total internal reflection microscopy. Cy3-labeled 30S PICs containing a 57-nt 5′ biotinylated mRNA, all three IFs, and fMet-tRNAfMet were formed in vitro and immobilized on a derivatized quartz microscope slide as previously described (Marshall et al., 2008). Stopped-flow delivery of Cy5-labeled 50S subunits resulted in subunit joining, as evidenced by anti-correlated fluctuations in Cy3 and Cy5 fluorescence, or FRET (FRET = ICy5 / [ICy3 + ICy5], where ICy5 represents Cy5 fluorescence corrected to remove Cy3 bleedthrough). We observed FRET for 53% of immobilized single 30S PICs (data not shown). The population Cy3-30S PICs in which FRET did not occur likely includes defective ribosome particles, inaccessible subunits, PICs that bind unlabeled 50S subunits, and 30S PICs in which Cy3 photobleaches prior to 50S arrival. Each trace provides information on the rate of subunit joining, stability of 70S complexes, and dynamics of ribosomal subunits during and after subunit joining (Figure 1).
Surface-immobilized 30S PICs effectively bind 50S subunits to form stable 70S initiation complexes. The apparent first-order rate for the arrival of FRET upon stopped-flow delivery of 20nM Cy5-50S subunits is 0.056 ± 0.007 s−1, consistent with the bimolecular rate for subunit joining determined in bulk (Antoun et al., 2004 and 2006a). The rate of subunit joining is sensitive to 50S concentration, consistent with a bimolecular process. Once formed, surface-initiated ribosomes are stable on the minutes timescale, with a mean FRET lifetime of 115.3 s. In fact, FRET lifetimes for 70S complexes are limited largely by dye stability; calculated lifetimes likely underestimate those measured in bulk. However, the comparison of FRET lifetimes reports on the relative stability of different 70S complexes.
Rarely, multiple fluctuations between FRET and zero-FRET states (12%) occur on the time scale of our measurements (supplementary figure 1) In this subset of molecules, FRET events occur on average 2.4 times during a single trace, and their lifetime is 45.7 s, over two-fold less than that of the subset of molecules that show a single event. Zero-FRET states, which represent the dwell times between FRET events, occur on average 1.8 times per trace, with an average lifetime of 29.0 s. This dwell lifetime approaches that of the FRET arrival time for subunit joining, suggesting that they may arise from the bimolecular association of subunits. Decreasing the concentration of 50S subunits ten-fold reduces the frequency of these FRET fluctuations to approximate 6% of observed molecules. However, these events are also sensitive to illumination power; 0.5 kW/cm illumination decreases the population of fluctuating molecules again to ~ 6%. Together these results suggest that FRET fluctuations arise from a combination of 50S association and dissociation events and Cy5 blinking phenomena (Table 1). Recent bulk measurements suggest that 50S subunits transiently sample 30S PICs prior to GTP hydrolysis by IF2 (Grigoriadou et al., 2007a). Under observation at 20 ms frame rates, we similarly observe short intersubunit FRET events (supplementary figure 2).
Table 1.
Kinetic parameters for subunit joining with all initiation factors.
| # Molecules | Intersubunit FRET events | Dwell Events | |||||
|---|---|---|---|---|---|---|---|
| Total # | Uncensored (%) | Mean lifetime (s) | Total # | Uncensored (%) | Mean lifetime (s) | ||
| 20 nM Cy5 50S – 1 kW/cm2 | 417 | 577 | 39 | 115.3 | 330 | 22 | 136.5 |
| Molecules with FRET on to FRET off fluctuations | 48 | 117 | 57 | 45.7 | 86 | 83 | 29.0 |
| Molecules without FRET fluctuations | 369 | 460 | 35 | 119.4 | 244 | 0 | N/A |
| 20 nM Cy5 50S – 0.5 kW/cm2 | 171 | 207 | 25 | 287.3 | 65 | 17 | 275.4 |
| Molecules with FRET on to FRET off fluctuations | 13 | 38 | 42 | 112.4 | 21 | 52 | 23.8 |
| Molecules without FRET fluctuations | 158 | 169 | 21 | 238.8 | 44 | 0 | N/A |
| 2 nM Cy5 50S – 1 kW/cm2 | 148 | 200 | 28 | 174.2 | 87 | 10 | 413.7 |
| Molecules with FRET on to FRET off fluctuations | 10 | 21 | 48 | 79.7 | 14 | 64 | 48.3 |
| Molecules without FRET fluctuations | 138 | 179 | 25 | 195.2 | 73 | 0 | N/A |
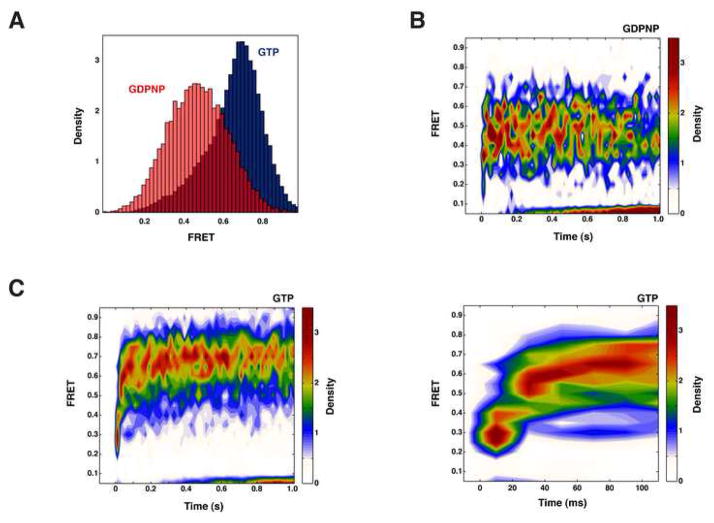
The distribution of FRET efficiencies from the ensemble of surface-initiated 70S initiation complexes is bi-modal, and is well approximated by a mixture of two gaussian distributions centered at 0.44 (σ = 0.07, 85% density) and 0.30 (σ = 0.05, 15% density)(Figure 2). The FRET value of 0.44 (~50 Å inter-dye distance) is consistent with the non-rotated conformation of the ribosome observed by cryo-EM and in previous bulk and smFRET studies (Frank and Agrawal, 2000; Ermolenko et al., 2007; Cornish et al., 2008; Marshall et al., 2008), whereas the 0.30 FRET value (~56 Å inter-dye distance) is consistent with the rotated conformation of the ribosome. We do not observe spontaneous inter-conversion of these two conformations, consistent with a significant energy barrier to intersubunit rotation proposed previously (Figure 1D,E).
Figure 2. Initiation factors guide the assembly of elongation-competent 70S complexes.
(A, B) FRET intensity histograms for 70S complexes formed in the absence (A) and presence (B) of initiation factors. In the absence of initiation factors, 70S complexes form equally in both the rotated (green curve, low FRET) and non-rotated (red curve, high FRET) conformations, whereas initiation factors preferentially select the non-rotated (high FRET) conformation. (C) Two dimensional histogram of FRET trajectories obtained in the presence of ternary complex (TC), post-synchronized to the arrival of FRET, demonstrating the high FRET state to be elongation competent.
Effects of Mg2+ions on subunit joining
The rate of subunit joining is sensitive to the presence of divalent Mg2+ ions, yet the dissociation rate and conformation of 70S initiation complexes are relatively insensitive to Mg2+ ions (Table 2). Increasing Mg2+ concentration from 5 to 15 mM accelerates subunit joining three-fold (Table 2), consistent with recent bulk studies (Antoun et al., 2004). In contrast, only a modest decrease (< 15 %) in the dissociation rate of 70S initiation complexes is observed under the same conditions. Increased Mg2+ concentrations have no effect on the FRET distribution or the conformational dynamics of ribosomal subunits following 70S formation (supplementary Figure 3). Removal of polyamines from the reaction buffer decrease 30S PIC complex stability, and lead to no observable subunit joining in the presence of IF2 and GTP. Electrostatic repulsion is a key contribution to the activation barrier to subunit association, and the orientation and stability of the intersubunit interactions following subunit joining are only subtly impacted by electrostatics.
Table 2.
Kinetic parameters for subunit joining in the presence of GTP and varied factor composition.
| Condition | # Molecules | Mean arrival time (s) | Mean intersubunit FRET lifetime (s) |
|---|---|---|---|
| All IFs | 417 | 17.8 ± 2.0 | 115.3 |
| All IFs – 15 mM Mg2+ | 236 | 5.6 ± 0.9 | 143.8 |
| No IFs | 70 | 54.0 ± 8.0 | 254.8 |
| No IFs – 15 mM Mg2+ | 55 | 31.3 ± 7.3 | 286.7 |
| IF2 only | 270 | 12.2 ± 1.4 | 160.1 |
| IF2 and IF1 | 192 | 14.3 ± 2.3 | 129.9 |
| IF2 and IF3 | 143 | 15.1 ± 3.1 | 155.7 |
Initiation factors guide subunit joining
Initiation factors are required to accelerate 70S initiation complex formation, whereas their absence enhances the kinetic stability of 70S complexes (Table 2). In the absence of initiation factors, subunit joining slows approximately 3-fold (0.018 ± 0.008 s−1), consistent with results obtained in bulk (Antoun et al., 2006a). This highlights the critical role that the initiation factors play in lowering the activation barrier to 70S complex formation. This rate enhancement is further increased at higher Mg2+ concentrations, suggesting that initiation factors and Mg2+ may work in concert to enhance subunit joining. 70S initiation complexes formed in the absence of factors have a mean lifetime of over 4 minutes (t = 254.8 s). The increased stability of 70S initiation complexes may result from the absence of IF1 and IF3, which act as anti-association factors during translation initiation, and increase the dissociation rate of 70S complexes (Grunberg-Manago et al., 1975; Dottavio-Martin et al., 1979; Antoun et al., 2004 and 2006a).
In the absence of factors, surface-initiated 70S initiation complexes display increased conformational heterogeneity: 70S initiation complexes form in the classic and rotated conformations with roughly equal likelihood (Figure 2). We have previously shown that only the classic conformation (0.44 FRET) is competent to enter the elongation cycle (Marshall et al., 2008). In the presence of ternary complex, subunit joining, as evidenced by the appearance of FRET, is followed by an irreversible transition to lower FRET, consistent with the rotated intersubunit conformation. This transition signals entry to the elongation cycle (supplementary figure 4). 70S complexes trapped in the rotated (low FRET) conformation are unaffected by the addition of ternary complex. Thus initiation factors guide formation of the elongation-competent ribosome.
Of those 70S complexes formed in the presence of ternary complex, 31% display this transition from high to low FRET, setting the efficiency of ternary complex delivery in the presence of all initiation factors. In the presence of IF2 alone, this efficiency is unchanged. Initiation factors extend the time 70S complexes wait in the non-rotated (high FRET) conformation prior to the transition to low FRET. The mean lifetime of the high FRET state – an indirect measure of the time between subunit joining and formation of the first peptide bond – is nearly 30% shorter in the absence of IF1 and IF3 (19.5 ± 1.9 s vs. 27.3 ± 4.0 s for all initiation factors. In the absence of all initiation factors, the high FRET dwell time is shortened even further (8.0 ± 1.8 s vs 23.5 ± 2.5 s). Initiation factors may impede the delivery of the first ternary complex, and factor dissociation may serve as a final checkpoint in the discrimination between properly and improperly formed 70S initiation complex prior to elongation.
IF2 is necessary and sufficient to guide rapid formation of stable 70S initiation complexes (Table 2). The rate of subunit joining in the presence of IF2 alone is 0.082 ± 0.011 s−1, ~ 50% faster than subunit joining in the presence of all initiation factors and nearly five-fold faster than in the absence of initiation factors. A similar trend has been observed in recent bulk studies (Antoun et al., 2006a). Following subunit joining, 70S initiation complexes formed in the presence of IF2 have a mean lifetime of 160.1 s, slightly longer than that of 70S initiation complexes formed with all initiation factors.
IF1 and IF3 likely act in a concerted fashion to tune the rate of subunit joining yet have minimal effect on the orientation and stability of 70S initiation complexes. In the presence of either IF1 and IF2, or IF3 and IF2, the rate of subunit joining (0.066 ± 0.016 s−1 and 0.070 ± 0.013 s−1, respectively) is between that observed in the presence of IF2 only and in the presence of all initiation factors (Table 2). The lifetime of 70S initiation complexes in the presence of IF1 and IF2 or IF3 and IF2 is comparable to that seen with IF2 only or all initiation factors, suggesting that the composition of 70S initiation complexes following subunit joining is independent of the factor composition present during its formation, or that the factor composition of the 70S initiation complexes does not effect its stability. The FRET distribution for these 70S initiation complexes is indistinguishable from that observed with IF2 only or all initiation factors (supplementary Figure 5). These data support the proposed synergistic, anti-association role of IF1 and IF3 during subunit joining whereas IF2 likely directs both recruitment and orientation of the ribosomal subunits during initiation.
Effects of GTP analogs
To dissect the role of GTP hydrolysis by IF2 in 70S initiation complex formation, we substituted GDPNP and GTPγS for GTP in single-molecule subunit joining assays, in order to stall or slow GTP hydrolysis by IF2. The rate of 70S formation in the presence GDPNP is 0.056 ± 0.009 s−1, which is equivalent to the rate observed for subunit joining with GTP (Table 3). This agrees with numerous studies that have shown initiation factor-guided subunit joining to be uninhibited by non-hydrolyzable GTP analogs (Tomsic et al., 2000; Antoun et al., 2003). In contrast, 50S delivery in the presence of GDP does not result in significant subunit joining, likely because the GDP-bound conformation of IF2 has decreased affinity for the ribosomal subunits (data not shown). 70S complexes formed with GDPNP are nearly 3-fold less stable than those formed in the presence of GTP (45.3 s vs 115.3 s). Additionally, fluctuations between FRET-on and zero FRET occur nearly 3-fold more frequently in the presence of GDPNP −11.5% to 27.6%. The decreased stability of 70S complexes and increase in fluctuations between FRET-on and zero FRET likely represent repeated 50S binding and dissociation events because GDPNP does not have a significant effect on Cy5 photophysics.
Table 3.
Kinetic parameters for subunit joining with all initiation factors in the presence of non-hydrolyzable GTP analogs.
| # Molecules | Intersubunit FRET events | ||||
|---|---|---|---|---|---|
| Total # | Uncensored (%) | Mean lifetime (s) | Mean arrival time (s) | ||
| All IFs - GTP | 417 | 577 | 39 | 115.3 | 17.8 ± 2.0 |
| Molecules with FRET on to FRET off fluctuations | 48 | 117 | 57 | 45.7 | 12.6 ± 4.6 |
| Molecules without FRET fluctuations | 369 | 460 | 35 | 119.4 | 18.4 ± 2.1 |
| All IFs - GDPNP | 261 | 404 | 56 | 45.9 | 18.0 ± 2.5 |
| Molecules with FRET on to FRET off fluctuations | 72 | 182 | 67 | 27.8 | 17.7 ± 5.3 |
| Molecules without FRET fluctuations | 189 | 222 | 48 | 66.1 | 18.1 ± 3.0 |
| All IFs - GTPγS | 145 | 225 | 44 | 104.2 | 16.7 ± 3.4 |
| Molecules with FRET on to FRET off fluctuations | 22 | 59 | 56 | 54.7 | 11.0 ± 7.4 |
| Molecules without FRET fluctuations | 123 | 166 | 40 | 144.7 | 17.2 ± 3.9 |
In contrast to 70S complexes formed with GTP or in the absence of factors, only a single 70S conformer is formed during initiation in the presence of GDPNP. The distribution of FRET efficiency values obtained from the ensemble of 70S complexes is centered at 0.37 (σ =0.06) FRET, consistent with the rotated conformation of the ribosome, as well as the minor population of 70S complexes formed upon initiation with GTP (supplementary figure 6) (Marshall et al., 2008). Like 70S initiation complexes formed with GTP, 70S complexes formed in the presence of GDPNP do not interconvert between high and low FRET, consistent with a significant energy barrier to intersubunit movement during initiation. GTP hydrolysis and the related ribosomal conformational change signal the transition from semi-stable IF2-GTP bound form of the ribosome to the more stable post-GTP hydrolysis form of the ribosome.
The GTP analog, GTPγS, in which one of the three chemically-equivalent oxygen atoms on the γ-phosphate of GTP is replaced by a sulfur atom, moderately slows GTP hydrolysis by EF-Tu (Thompson and Karim, 1982). We employed saturating concentrations of GTPγS in single-molecule subunit joining assays to slow GTP hydrolysis by IF2 and to observe the transition from low to high FRET (Table 3). However, we observe no additional transitions in the presence of GTPγS. Like GDPNP, GTPγS has little effect on the rate of subunit joining (0.060 ± 0.015 s−1) and shifts the predominant FRET distribution for the ensemble of 70S initiation complexes from high to low FRET (supplementary figure 6). The lifetime of intersubunit FRET is 104.2 s, between that observed in the presence of GTP and GDPNP. It is apparent that the tolerance of IF2 for GTPγS as a substrate is not like that of prokaryotic elongation factor, EF-Tu (Thompson and Karim, 1982).
Fast frame-rate observation reveals a transient low FRET intermediate
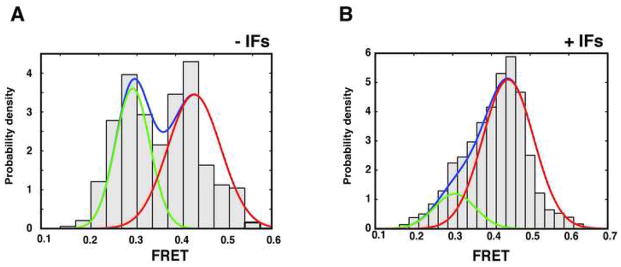
Upon subunit joining, structural models suggest that the ribosome undergoes a ~5° clockwise rotation of the 30S subunit relative to the 50S subunit (viewed from solvent side of 30S subunit) upon GTP hydrolysis by IF2. However, we do not detect any transitions from the rotated (low FRET) to classic (high FRET) state in single-molecule trajectories collected at 100 ms frame rates. To detect this event, we repeated 50S delivery experiments in the presence of IF2 at a frame rate of 20 ms. To improve our ability to discriminate between the rotated and classic conformations under reduced signal to noise conditions, we replaced the Cy3 fluorophore used to label 30S subunits with Cy3B, a rigidified version of Cy3 with vastly increased quantum yield. FRET distributions for 70S subunits obtained under these conditions are identical in nature to those obtained with Cy3-labeled 30S subunits, except that their medians are shifted to higher FRET values (Figure 3A).
Figure 3. IF2-catalyzed GTP hydrolysis drives subunit rotation and commits the ribosome to elongation.
(A) FRET intensity histograms comparing 70S complexes formed in the presence of GTP (blue) with 70S complexes formed in the presence of GDPNP (red). (B-D) Two dimensional FRET histograms postsynchronized to the arrival of FRET reveal a transient low FRET state in the progression to high FRET in the presence of GTP (C), whereas 70S complexes formed in the presence of GDPNP (B) proceed directly to low FRET. Magnification of the GTP histogram (D) shows a low FRET state with an approximate lifetime of 20–30 ms.
To reveal the presence of a transient low FRET intermediate, we superimposed single-molecule FRET time traces postsynchronized to the arrival of FRET (Figure 3B,C). This representation has been used to resolve potential intermediate states during the process of tRNA selection (Blanchard et al., 2004). In the presence of GDPNP, 50S subunits dock and rapidly form 70S complexes in the rotated conformation (low FRET). In the presence of GTP, however, 70S complexes ultimately assemble in the classic conformation (high FRET). In addition, postsynchronization reveals initial FRET density at low FRET upon subunit joining. Magnification of the two-dimensional histogram shows a low FRET event lasting ~20–30 ms prior to progression to the high FRET state, in agreement with the timescale of GTP hydrolysis determined previously in bulk (Tomsic et al., 2000). 50S subunits initially dock with the 30S PIC to form 70S complexes in the rotated conformation. IF2-catalyzed GTP hydrolysis drives rotation of the 30S subunit to form the classic conformation, committing the ribosome to elongation.
Discussion
The single-molecule approach presented here allows real-time monitoring of translation initiation. We have focused on the late steps of initiation, during which a 50S subunit joins with an already-formed 30S preinitiation complex. Subunit joining during initiation commits the ribosome to enter the repetitive elongation cycle. This event is regulated to ensure that only 30S subunits positioned at the appropriate start site and primed with initiator tRNA proceed to elongation. By placing fluorescent probes at appropriate positions on the 30S and 50S ribosomal subunits, we directly monitored subunit joining and subsequent intersubunit conformational changes.
Our single-molecule translation initiation system reproduces the behavior of bulk systems. 50S subunits join 30S PICs rapidly and stably in the presence of Mg2+ and initiation factors. The rate of subunit joining increases with increasing 50S subunit concentration; rates derived from single-event lifetime distributions yield association rates similar to those measured previously by bulk biophysical methods. In the presence of all initiation factors and GTP, the initial interaction of subunits, as observed by intersubunit FRET, results in formation of stable 70S subunits for most observed events. 70S complexes formed in the presence of factors are 3-fold less stable than those formed in the absence of factors, consistent with the known antiassociation activity of IF3.
Our experiments confirm a significant electrostatic barrier to subunit joining. Increasing Mg2+ concentration increases the rate of subunit joining, as does the presence of polyamines. Structural models of the 70S ribosome complex shows that the nearly the entire ~6000 Å2 dimerization interface of the 30S and 50S subunits is composed of negatively charged rRNA (Allen et al., 2005). Twelve intersubunit bridges, 80% of which are comprised of RNA-RNA interactions, and rRNA-tRNA interactions on both the 30S and 50S subunits are created to stabilize and orient the 70S complex, and overcome the negative contribution of electrostatics in subunit joining (Yusupov et al., 2001; Schuwirth et al., 2005). Mg2+ allows subunit assembly to overcome the electrostatic barrier to forming the RNA-RNA contacts that are central to 70S particle assembly. In contrast, millimolar concentrations of Mg2+ have only slight effects on the dissociation rates of formed 70S complexes, consistent with previous measurements.
Our results show similarly strong dependence of subunit association times on the divalent ion concentration in either the presence or absence of IFs. Initiation factors, particularly IF2, work in concert with divalent ions to lower this electrostatic barrier to subunit association. In the absence of initiation factors, formation of intersubunit bridges is slow and highly dependent on the concentration of Mg2+ ions in solution (Hennelly et al., 2005).
IF2 likely chaperones subunit joining during initiation, burying significant portions of the negatively charged 30S and 50S subunit interfaces and thereby decreasing the barrier to 50S docking at 30S PICs. Cryo-EM models show that initiation factors, most notably IF2, bury nearly half of the 30S–50S dimerization interface, ~2600 Å2 and make direct contacts with rRNA and ribosomal proteins on both subunits in 70S initiation complexes stalled with GDPNP (Allen et al., 2005). This interaction may contribute ~ 10 kcal/mol to reduce unfavorable interaction energies (Brooijmans et al., 2002). IF2 effectively replaces the slow forming intersubunit bridges that are required to stabilize the 70S complex with quickly forming rRNA-protein and protein-protein bridges between the ribosome and IF2 (Bollen et al., 1975; Heimark et al., 1976; La Teana et al., 2001; Marzi et al., 2003; Caserta et al., 2006). Comparison of the structures of 30S and 70S initiation complexes shows that IF2 rearranges conformation upon subunit joining. Acceleration of the subunit joining rate by IF2 does not require GTP hydrolysis, as confirmed by the similarity of GDPNP and GTP joining rates.
IF2-catalyzed GTP hydrolysis acts as a checkpoint during subunit joining; in the absence of GTP hydrolysis, 70S complexes do not proceed to elongation (Luchin et al., 1999; Tomsic et al., 2000; Antoun et al., 2003). GTP hydrolysis is linked to rotational movement of ribosomal subunits, which may prepare 70S initiation complexes to accept aminoacyl tRNA in the A-site (Allen et al. 2005; Myasnikov et al., 2005). Our approach has probed these structural states in real time, and highlights the key importance of GTP hydrolysis by IF2 during initiation.
GTP hydrolysis by IF2 controls the orientation of subunits during translation initiation (Figure 4). In the presence of GTP and IF2, 70S complexes form primarily in a conformation with a FRET value of 0.45. This high FRET value is consistent with a non-rotated conformation of the subunits. The GTP analogs GDPNP and GDPγS stall the process of subunit joining, resulting in stably-associated ribosomes with a conformation represented by 0.37 FRET. This low FRET value is consistent with a rotated subunit conformation. The magnitude of the FRET transition (equivalent to ~5 Å distance change) agrees with the intersubunit rotation upon GTP hydrolysis by IF2 suggested by cryo EM studies (Myasnikov et al., 2005).
Figure 4. A model for translation initiation, highlighting the role of IF2.
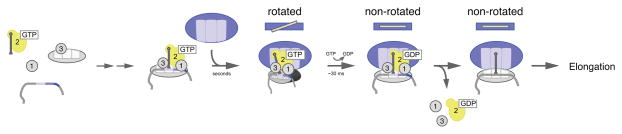
mRNA, tRNA and the initiation factors assemble on the 30S subunit (grey) to form the 30S pre-initiation complex, followed by 50S arrival to form the 70S initiation complex. The ribosomal subunits are initially assembled in the rotated conformation, with the peptidyl stem of the initiator tRNA held near the E-site by IF2 (yellow). GTP hydrolysis by IF2 occurs rapidly, driving the alignment of the subunits into the non-rotated conformation, and moving the peptidyl stem of initiator tRNA into the P-site, in preparation for peptidyl transfer. Following dissociation of the initiation factors, the non-rotated 70S is committed to enter the elongation cycle.
Our results suggest a pathway for translation initiation that is guided by IF2 and GTP hydrolysis. Initial 50S subunit joining with IF2-bound PICs occurs in the rotated low FRET conformation that is observed prior to GTP hydrolysis. GTP hydrolysis serves as a checkpoint prior to entry into the elongation cycle. This regulatory role of GTP hydrolysis is similar to that observed for eIF5B (Shin et al., 2002). Data acquired at our highest sensitivity and time resolution (20 ms) show that subunit joining proceeds rapidly from the rotated low FRET state to the classical high FRET conformation in the presence of IF2 and GTP. These data, which show a 20–30 ms dwell in the rotated state are consistent with the measured rates of IF2-mediated GTP hydrolysis. Brief intersubunit sampling events were observed primarily in the rotated conformation, consistent with this proposed pathway of subunit joining and rotation to the classical conformation.
Intersubunit rotation requires the breaking and reforming of interactions between the 30S and 50S subunits, suggesting a significant activation barrier between states. Consistent with this, fluctuations between the two rotational states are rarely observed in these single-molecule experiments. GTP hydrolysis by IF2 lowers the energy barrier to intersubunit rotation. Our prior single-molecule experiments have shown that these two conformational states do not interconvert during elongation, except upon completion of irreversible chemical steps such as peptide bond formation or GTP hydrolysis by EF-G. Thus, ribosomal rotation is carefully controlled by an external free energy source (Marshall et al., 2008).
Prior to GTP hydrolysis, initiation factors may occlude up to 5 of the 12 intersubunit bridges when bound to 70S initiation complexes (Allen et al., 2005). High-resolution structures of aIF5B, the archaeal homolog of IF2 and eIF5B, demonstrate that GTP hydrolysis causes a subtle conformational change in the G, II, and III domains, which correspond to the G1, G2, and C1 domains of IF2 (Roll-Mecak et al., 2000). Cryo EM shows that these conformational changes in IF2 may expose all but two of the intersubunit bridge sites (Myasnikov et al., 2005). GTP hydrolysis may drive a conformational change in IF2, which exposes intersubunit bridge sites and promotes intersubunit rotation.
GTP hydrolysis and intersubunit rotation may enhance the fidelity of initiation. In the presence of initiation factors, the rotated conformation is either transiently accessed along the pathway to stably associated 70S complexes at high FRET (supplementary figure 5) or rarely (~15% of the time), remains stalled at this low FRET state for an extended period of time (Figure 2). This population of stably associated 70S complexes at low FRET may represent 30S PICs that are unable activate the GTPase function of IF2 and 70S initiation complexes that are incorrectly formed and are kept from entering elongation.
GTP hydrolysis stabilizes 70S complexes. In the presence of GTP, 70S initiation complexes remain stably associated for over 100 s, a measure that is likely limited by the lifetime of the fluorophores used in these experiments. In contrast, complexes formed with GDPNP are between 3- and 16-fold less stable than those formed with GTP depending on the composition of initiation factors used in the experiment. It is possible that the formation of additional intersubunit bridges upon GTP hydrolysis and intersubunit rotation provides this enhanced stability.
Subunit rotation is coupled to loading of initiator tRNA into the 70S P site (Simonetti et al., 2008; Myasnikov et al., 2005). We propose that upon GTP hydrolysis by IF2, subunits spontaneously transition from the rotated conformation (low FRET) to the non-rotated, conformation (high FRET). This would correlate structurally with movement of the 3′end of initiator tRNA near the E-site, where it is held by IF2, to the P-site where it can participate in peptidyl transfer. The conformation of the ribosome consistent with low FRET represents the initial binding conformation of the 50S subunit on 30S PICs, and roughly correspond to the rotated state of ribosomes in elongation that has a P/E hybrid conformation for the P-site tRNA. As such, initiation would be a reversal of normal A to P to E site movement of the tRNA, and would explain the rotated to classical progression of subunits during initiation.
Following GTP hydrolysis by IF2, 70S initiation complexes are competent to enter elongation, but may require ternary complex stimulated dissociation of initiation factors prior to binding of the first elongator tRNA in the A-site. FRET transitions are observed that represent subunit joining and peptide bond formation, respectively (supplementary figure 4). In the absence of initiation factors, the lag between the subunit joining and peptide bond formation is shorter than that observed in their presence. Concomitant factor release and ternary complex binding may control the transition from initiation to elongation (Myasnikov et al., 2005; Grigoriadou et al., 2007a). Structural and biochemical approaches have shown that the binding of ribosomal GTPases is mutually exclusive and that IF2-GDP is stably associated with 70S initiation complexes; these results and data presented here are also consistent with this proposal.
The ribosome adopts at least two rotational states during both initiation and elongation. This does not preclude the existence of additional states undetectable by our single-molecule approach. Our results, coupled with prior structural and biochemical data, show how these states regulate progression of the ribosome through steps of initiation and elongation. Chemical steps control the interconversion of these states, which may couple to directional movement of ribosomes along an mRNA. Modulation of these two states by irreversible chemical events may represent a general mechanism of ribosome function. However, further work is required to probe the general role of rotational movements throughout elongation, to correlate intersubunit and tRNA movements, and to delineate the interplay of factor binding and ribosome conformation.
Experimental Procedures
Fluorescently-labeled 30S and 50S subunits, translation factors, S1, mRNA, and tRNA were prepared as described previously (Blanchard et al., 2004; Dorywalska et al., 2005; Marshall et al., 2008). 30S PICs were formed by mixing 0.25 μM Cy3-30S pre-incubated with stoichiometric S1, 0.5 μM IF1, 0.5 μM IF2, 0.5 μM IF3, 1 μM fMet-tRNAfMet, 1 μM biotinylated mRNA, and 4 mM GTP (GDPNP or GTPγS where noted) in a previously-described Tris-based polymix buffer system without reducing agents (Marshall et al., 2008), followed by incubation at 37 °C for 5 minutes. The Mg2+ concentration for all buffers was 5 mM unless otherwise mentioned.
PICs were then diluted in buffer containing 0.5 μM IF1, 0.5μM IF2, 0.5 μM IF3, 1 μM fMet-tRNAfMet, and 4 mM GTP (GDPNP or GTPγS where noted), and immobilized on a neutravidin-derivatized quartz slide (Marshall et al., 2008). Immobilized PICs were washed with buffer containing 0.5 μM IF1, 0.5μM IF2, 0.5 μM IF3, 1 μM fMet-tRNAfMet, 4 mM GTP (GDPNP or GTPγS where noted), 1 mM Trolox, 2.5 mM 3,4 dihydroxybenzoic acid, and 250 nM protocatechuate dioxygenase (Aitken et al., 2008). Subunit joining was initiated by stopped-flow delivery of 20 nM Cy5-50S in wash buffer. Delivery of Cy5-50S and ternary complex was performed as previously described (Marshall et al., 2008).
All single-molecule fluorescence experiments were performed using a prism-based total internal reflection instrument described previously (Aitken et al., 2008). Cy3-PICs were illuminated with a solid-state diode-pumped 532 nm laser at intensities of either 0.5 or 1 kW/cm2. Image acquisition and initial analysis were performed using Metamorph imaging software (Molecular Devices). Subsequent analysis of fluorescence trajectories was performed with MATLAB. FRET states were assigned using a hidden Markov Model approach as previously described (Aitken et al., 2008). To correct for photophysical and experimental censoring of event lifetimes, we calculated maximum-likelihood estimations of event lifetimes. To do this we classify the lifetime data from FRET on and FRET off states into two subsets: uncensored events T = (t1, t2, …, tn), and censored events . Then, we can maximize the hybrid likelihood function for the lifetime data:
ξi = 1/λi, where λi is the mean lifetime of this state. Here, the likelihood function for an uncensored data point ti is the exponential probability density function with parameter ξ, whereas for a censored data point tj we use the probability that the sampled time is greater than tj.
This function has a global maximum whenever n > 0, at
Supplementary Material
Acknowledgments
The authors would like to thank N. Heredia and D. Maar for cloning of IF2α, S.
Bacallado, and A. Petrov for their assistance with data processing tools, and M. Margaris for her support. C.E.A is supported by an NIH minority fellowship. This work was made possible by grants from NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agirrezabala X, Lei J, Brunelle JL, Ortiz-Meoz RF, Green R, Frank J. Visualization of the hybrid state of tRNA binding promoted by spontaneous ratcheting of the ribosome. Mol Cell. 2008;32:190–7. doi: 10.1016/j.molcel.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal RK, Lata RK, Frank J. Conformational variability in Escherichia coli 70S ribosome as revealed by 3D cryo-electron microscopy. Int J Biochem Cell Biol. 1999;31:243–54. doi: 10.1016/s1357-2725(98)00149-6. [DOI] [PubMed] [Google Scholar]
- Aitken CE, Marshall RA, Puglisi JD. An oxygen scavenging system for improvement of dye stability in single-molecule fluorescence experiments. Biophys J. 2008;94:1826–35. doi: 10.1529/biophysj.107.117689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen GS, Zavialov A, Gursky R, Ehrenberg M, Frank J. The cryo-EM structure of a translation initiation complex from Escherichia coli. Cell. 2005;121:703–12. doi: 10.1016/j.cell.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Antoun A, Pavlov MY, Andersson K, Tenson T, Ehrenberg M. The roles of initiation factor 2 and guanosine triphosphate in initiation of protein synthesis. EMBO J. 2003;22:5593–601. doi: 10.1093/emboj/cdg525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoun A, Pavlov MY, Tenson T, Ehrenberg M. Ribosome formation from subunits studied by stopped-flow Rayleigh light scattering. Biol Proced Online. 2004;6:35–54. doi: 10.1251/bpo71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoun A, Pavlov MY, Lovmar M, Ehrenberg M. How initiation factors tune the rate of initiation of protein synthesis in bacteria. EMBO J. 2006;25:2539–50. doi: 10.1038/sj.emboj.7601140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoun A, Pavlov MY, Lovmar M, Ehrenberg M. How initiation factors maximize the accuracy of tRNA selection in initiation of bacterial protein synthesis. Mol Cell. 2006;23:183–93. doi: 10.1016/j.molcel.2006.05.030. [DOI] [PubMed] [Google Scholar]
- Blanchard SC, Kim HD, Gonzalez RL, Jr, Puglisi JD, Chu S. tRNA dynamics on the ribosome during translation. Proc Natl Acad Sci USA. 2004;101:12893–8. doi: 10.1073/pnas.0403884101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg BM, Nakamoto T, Kezdy FJ. Kinetics of initiation of bacterial protein synthesis. Proc Natl Acad Sci USA. 1979;76:251–5. doi: 10.1073/pnas.76.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen A, Heimark RL, Cozzone A, Traut RR, Hershey JW. Cross-linking of initiation factor IF-2 to Escherichia coli 30 S ribosomal proteins with dimethylsuberimidate. J Biol Chem. 1975;250:4310–4. [PubMed] [Google Scholar]
- Brooijmans N, Sharp KA, Kuntz ID. Stability of macromolecular complexes. Proteins. 2002;48:645–53. doi: 10.1002/prot.10139. [DOI] [PubMed] [Google Scholar]
- Canonaco MA, Calogero RA, Gualerzi CO. Mechanism of translational initiation in prokaryotes – Evidence for a direct effect of IF2 on the activity of the 30-S ribosomal subunit. FEBS Letters. 1986;207:198–204. doi: 10.1016/0014-5793(86)81488-0. [DOI] [PubMed] [Google Scholar]
- Carter AP, Clemons WM, Jr, Brodersen DE, Morgan-Warren RJ, Hartsch T, Wimberly BT, Ramakrishnan V. Crystal structure of an initiation factor bound to the 30S ribosomal subunit. Science. 2001;291:498–501. doi: 10.1126/science.1057766. [DOI] [PubMed] [Google Scholar]
- Caserta E, Tomsic J, Spurio R, La Teana A, Pon CL, Gualerzi CO. Translation initiation factor IF2 interacts with the 30 S ribosomal subunit via two separate binding sites. J Mol Biol. 2006;362:787–99. doi: 10.1016/j.jmb.2006.07.043. [DOI] [PubMed] [Google Scholar]
- Cornish PV, Ermolenko DN, Noller HF, Ha T. Spontaneous intersubunit rotation in single ribosomes. Mol Cell. 2008;30:578–88. doi: 10.1016/j.molcel.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallas A, Noller HF. Interaction of translation initiation factor 3 with the 30S ribosomal subunit. Mol Cell. 2001;8:855–64. doi: 10.1016/s1097-2765(01)00356-2. [DOI] [PubMed] [Google Scholar]
- Dorywalska M, Blanchard SC, Gonzalez RL, Jr, Kim HD, Chu S, Puglisi JD. Site-specific labeling of the ribosome for single-molecule spectroscopy. Nuc Acid Res. 2005;33:182–89. doi: 10.1093/nar/gki151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dottavio-Martin D, Suttle DP, Ravel JM. The effects of initiation factors IF-1 and IF-3 on the dissociation of Escherichia coli 70 S ribosomes. FEBS Lett. 1979;97:105–10. doi: 10.1016/0014-5793(79)80062-9. [DOI] [PubMed] [Google Scholar]
- Ermolenko DN, Majumdar ZK, Hickerson RP, Spiegel PC, Clegg RM, Noller HF. Observation of intersubunit movement of the ribosome in solution using FRET. J Mol Biol. 2007;370:530–40. doi: 10.1016/j.jmb.2007.04.042. [DOI] [PubMed] [Google Scholar]
- Frank J, Agrawal RK. A ratchet-like intersubunit reorganization of the ribosome during translocation. Nature. 2000;406:318–22. doi: 10.1038/35018597. [DOI] [PubMed] [Google Scholar]
- Gold L, Stormo GD. High-level translation initiation. Methods Enzymol. 1990;185:89–93. doi: 10.1016/0076-6879(90)85009-d. [DOI] [PubMed] [Google Scholar]
- Grigoriadou C, Marzi S, Kirillov S, Gualerzi CO, Cooperman BS. A quantitative kinetic scheme for 70 S translation initiation complex formation. J Mol Biol. 2007;373:562–72. doi: 10.1016/j.jmb.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunberg-Manago M, Dessen P, Pantaloni D, Godefroy-Colburn T, Wolfe AD, Dondon J. Light-scattering studies showing the effect of initiation factors on the reversible dissociation of Escherichia coli ribosomes. J Mol Biol. 1975;94:461–78. doi: 10.1016/0022-2836(75)90215-6. [DOI] [PubMed] [Google Scholar]
- Gualerzi CO, Pon CL. Initiation of mRNA translation in prokaryotes. Biochemistry. 1990;29:5881–89. doi: 10.1021/bi00477a001. [DOI] [PubMed] [Google Scholar]
- Guenneugues M, Caserta E, Brandi L, Spurio R, Meunier S, Pon CL, Boelens R, Gualerzi CO. Mapping the fMet-tRNAfMet binding site of initiation factor IF2. EMBO J. 2000;19:5233–40. doi: 10.1093/emboj/19.19.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms J, Schluenzen F, Zarivach R, Bashan A, Gat S, Agmon I, Bartels H, Franceschi F, Yonath A. High Resolution Structure of the Large Ribosomal Subunit from a Mesophilic Eubacterium. Cell. 2001;107:679–88. doi: 10.1016/s0092-8674(01)00546-3. [DOI] [PubMed] [Google Scholar]
- Hartz D, McPheeters DS, Gold L. Selection of the initiator tRNA by Escherichia coli initiation factors. Genes & Dev. 1989;3:1899–912. doi: 10.1101/gad.3.12a.1899. [DOI] [PubMed] [Google Scholar]
- Heimark RL, Hersehy JW, Traut RR. Cross-linking of initiation factor IF2 to proteins L7/L12 in 70 S ribosomes of Escherichia coli. J Biol Chem. 1976;251:779–84. [PubMed] [Google Scholar]
- Hennelly SP, Antoun A, Ehrenberg M, Gualerzi CO, Knight W, Lodmell JS, Hill WE. A time-resolved investigation of ribosomal subunit association. J Mol Biol. 2005;346:1243–58. doi: 10.1016/j.jmb.2004.12.054. [DOI] [PubMed] [Google Scholar]
- Howe JG, Hershey JW. Initiation factor and ribosome levels are coordinately controlled in Escherichia coli growing at different rates. J Biol Chem. 1983;258:1954–9. [PubMed] [Google Scholar]
- Julián P, Konevega AL, Scheres SH, Lázaro M, Gil D, Wintermeyer W, Rodnina MV, Valle M. Structure of ratcheted ribosome with tRNA in hybrid states. Proc Natl Acad Sci USA. 2008;105:16924–7. doi: 10.1073/pnas.0809587105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Teana A, Gualerzi CO, Dahlberg AE. Initiation factor IF 2 binds to the alpha-sarcin loop and helix 89 of Escherichia coli 23S ribosomal RNA. RNA. 2001;7:1173–9. doi: 10.1017/s1355838201010366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen BS, Sorensen HP, Mortensen KK, Sperling-Petersen HU. Initiation of protein synthesis in bacteria. Microbiol Mol Biol Rev. 2005;69:101–23. doi: 10.1128/MMBR.69.1.101-123.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood AH, Chakraborty PR, Maitra U. A complex between initiation factor IF2, guanosine triphosphate, and fMet-tRNA: an intermediate in initiation complex formation. Proc Natl Acad Sci USA. 1971;68:3122–6. doi: 10.1073/pnas.68.12.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchin S, Putzer H, Hershey JWB, Cenatiempo Y, Grunberg-Manago M, Laalami S. In vitro study of two dominant inhibitory GTPase mutants of Escherichia coli translation initiation factor IF2: Direct evidence that GTP hydrolysis is necessary for factor recycling. J Biol Chem. 1999;274:6074–79. doi: 10.1074/jbc.274.10.6074. [DOI] [PubMed] [Google Scholar]
- Marintchev A, Wagner G. Translation initiation: structures, mechanisms and evolution. Q Rev Biophys. 2004;37:197–284. doi: 10.1017/S0033583505004026. [DOI] [PubMed] [Google Scholar]
- Marshall RA, Dorywlaska M, Puglisi JD. Irreversible chemical steps control intersubunit rotation during translation. Proc Natl Acad Sci USA. 2008;105:15364–9. doi: 10.1073/pnas.0805299105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzi S, Knight W, Brandi L, Caserta E, Soboleva N, Hill WE, Gualerzi CO, Lodmell JS. Ribosomal localization of translation initiation factor IF2. RNA. 2003;9:958–69. doi: 10.1261/rna.2116303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myasnikov AG, Marzi S, Simonetti A, Giuliodori AM, Gualerzi CO, Yusupova G, Yusupov M, Klaholz BP. Conformational transition of initiation factor 2 from the GTP- to GDP-bound state visualized on the ribosome. Nat Struct Mol Biol. 2005;12:1145–9. doi: 10.1038/nsmb1012. [DOI] [PubMed] [Google Scholar]
- Ogle JM, Brodersen DE, Clemons WM, Tarry MJ, Carter AP, Ramakrishnan V. Recognition of cognate transfer RNA by the 30S ribosomal subunit. Science. 2001;292:897–902. doi: 10.1126/science.1060612. [DOI] [PubMed] [Google Scholar]
- Pioletti M, Schlunzen F, Harms J, Zarivach R, Gluhmann M, Avila H, Bashan A, Bartels H, Auerbach T, Jacobi C, Hartsch T, Yonath A, Franceschi F. Crystal structures of complexes of the small ribosomal subunit with tetracycline, edeine and IF3. EMBO J. 2001;20:1829–39. doi: 10.1093/emboj/20.8.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodnina MV, Stark H, Savelsbergh A, Wieden HJ, Mohr D, Matassova NB, Peske F, Daviter T, Gualerzi CO, Wintermeyer W. GTPases mechanisms and functions of translation factors on the ribosome. Biol Chem. 2000;381:377–87. doi: 10.1515/BC.2000.050. [DOI] [PubMed] [Google Scholar]
- Roll-Mecak A, Cao C, Dever TE, Burley SK. X-Ray structures of the universal translation initiation factor IF2/eIF5B: conformational changes on GDP and GTP binding. Cell. 2000;103:781–92. doi: 10.1016/s0092-8674(00)00181-1. [DOI] [PubMed] [Google Scholar]
- Schuwirth BS, Borovinskaya MA, Hau CW, Zhang W, Vila-Sanjurjo A, Holton JM, Cate JH. Structures of the bacterial ribosome at 3.5 Å resolution. Science. 2005;310:827–34. doi: 10.1126/science.1117230. [DOI] [PubMed] [Google Scholar]
- Shin BS, Maag D, Roll-Mecak A, Arefin MS, Burley SK, Lorsch JR, Dever TE. Uncoupling of initiation factor eIF5B/IF2 GTPase and translational activities by mutations that lower ribosome affinity. Cell. 2002;111:1015–1025. doi: 10.1016/s0092-8674(02)01171-6. [DOI] [PubMed] [Google Scholar]
- Simonetti A, Marzi S, Myasnikov AG, Fabbretti A, Yusupov M, Gualerzi CO, Klaholz BP. Structure of the 30S translation initiation complex. Nature. 2008;455:416–20. doi: 10.1038/nature07192. [DOI] [PubMed] [Google Scholar]
- Stark H, Rodnina MV, Wieden HJ, van Heel M, Wintermeyer W. Large scale movement of elongation factor G and extensive conformational change of the ribosome dring translocation. Cell. 2000;100:301–309. doi: 10.1016/s0092-8674(00)80666-2. [DOI] [PubMed] [Google Scholar]
- Thompson RC, Karim AM. The accuracy of protein biosynthesis is limited by its speed: high fidelity selection by ribosomes of aminoacyl-tRNA ternary complexes containing GTP[gamma S] Proc Natl Acad Sci USA. 1982;79:4922–6. doi: 10.1073/pnas.79.16.4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomsic J, Vitali LA, Daviter T, Savelsbergh A, Spurio R, Striebeck P, Wintermeyer W, Rodnina MV, Gualerzi CO. Late events of translation initiation in bacteria: A kinetic analysis. EMBO J. 2000;19:2127–36. doi: 10.1093/emboj/19.9.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura S, Dorywalska M, Lee T, Kim HD, Puglisis JD, Chu S. Peptide bond formation destabilizes Shine-Dalgarno interaction on the ribosome. Nature. 2007;446:454–457. doi: 10.1038/nature05625. [DOI] [PubMed] [Google Scholar]
- Valle M, Zavialov A, Sengupta J, Rawat U, Ehrenberg M, Frank J. Locking and unlocking of ribosomal motions. Cell. 2003;114:123–34. doi: 10.1016/s0092-8674(03)00476-8. [DOI] [PubMed] [Google Scholar]
- Wintermeyer W, Gualerzi CO. Effect of Escherichia-coli initiation factors on the kinetics of N-acetylphenylalanyl transfer RNA binding to 30S ribosomal subunits: A fluorescence stopped-flow study. Biochemistry. 1983;22:690–94. doi: 10.1021/bi00272a025. [DOI] [PubMed] [Google Scholar]
- Yusupov MM, Yusupova GZ, Baucom A, Lieberman K, Earnest TN, Cate JHD, Noller HF. Crystal structure of the ribosome at 5.5 Å resolution. Science. 2001;292:883–96. doi: 10.1126/science.1060089. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.