Abstract
Given its profound analgesic nature, neuraxial opioids are frequently used for pain management. Unfortunately, the high incident rate of itch/pruritus after spinal administration of opioid analgesics reported in postoperative and obstetric patients greatly diminishes patient satisfaction and thus the value of the analgesics. Many endeavors to solve the mystery behind neuraxial opioid-induced itch had not been successful, as the pharmacological antagonism other than the blockade of mu opioid receptors remains elusive. Nevertheless, as the characteristics of all opioid receptor subtypes have become more understood, more studies have shed light on the potential effective treatments. This review discusses the mechanisms underlying neuraxial opioid-induced itch and compares pharmacological evidence in nonhuman primates with clinical findings across diverse drugs. Both nonhuman primate and human studies corroborate that mixed mu/kappa opioid partial agonists seem to be the most effective drugs in ameliorating neuraxial opioid-induced itch while retaining neuraxial opioid-induced analgesia.
Keywords: Agonist, Analgesics, Antagonist, Antipruritics, Epidural, Intrathecal, Itch, Monkey, Mouse, Opioid receptor, Pain, Pruritus, Rat, Spinal cord
1 Neuraxial Opioids
1.1 Clinical Applications of Neuraxial Opioids
Neuraxial administration of drugs offers the techniques that deliver drugs in close proximity to the spinal cord, i.e., intrathecally into the cerebrospinal fluid or epidurally into the fatty tissues surrounding the dura. Neuraxial administration of opioids provides effective analgesia before and after a surgical procedure. The modern era of spinal opioid administration began with a report by Yaksh and Rudy in 1976, demonstrating analgesia in rats by intrathecal administration of morphine (Yaksh and Rudy 1976). In 1979, Wang et al. published the first controlled clinical study of intrathecally administered opioid in humans conducted in a double-blind placebo setting. Eight cancer patients were selected based on severe pain in the back and legs and failure to respond to systemic analgesics when given at reasonable dose levels and frequencies. Each patient received both morphine at either 0.5 mg or 1.0 mg dosage and the placebo saline. Injections were administered at the second or third lumbar interspace at various intervals ranging from 4 to 48 h, depending on each patient’s response to the treatment. In the end, 17 injections of morphine and 12 injections of saline were administered in total. Three quarters of the patients reported long-lasting pain relief after being treated with intrathecal morphine, suggesting that there is a clear distinction between the analgesic effects of morphine and placebo saline (Wang et al. 1979). No sign of central nervous system depression was reported in this study; hence, it was concluded that intrathecally administered opioids were advantageous for relieving pain and free from compromising sensory and motor functions (Wang et al. 1979).
These findings encouraged further studies of intrathecally administered opioids to explore the possibilities in obstetrics and postoperative pain treatment. Later studies concluded that because of its selective blockade in pain conduction, i.e., an absence of sympathetic blockade, spinal opioid allows patients’ motor functions to remain intact upon receiving treatment, rendering spinal opioid therapeutically advantageous over local anesthetics (Cousins and Mather 1984).
1.2 Side Effects of Neuraxial Opioids
The use of neuraxial opioids to relieve pain is not without its side effects, however. Itch/pruritus, nausea, vomiting, urinary retention, and respiratory depression are the prominent side effects. This review focuses on the discussion of itch/pruritus because it can sometimes become a more irritating problem than pain itself. Spinal opioid-induced pruritus is an unwanted itch sensation often seen in obstetric and postoperative patients, with an incidence of 20–100 % (Ganesh and Maxwell 2007; Krajnik and Zylicz 2001; Szarvas et al. 2003). The onset of pruritus begins shortly after analgesia, differing in severity and duration depending on different classes of opioids and the dosage used. This unpleasant sensation, causing a reflex or desire to scratch, may warrant the use of antipruritic drugs, which may in turn create hormonal changes in obstetric patients (Ganesh and Maxwell 2007; Szarvas et al. 2003).
The itch sensation caused by neuraxial opioid is not only disturbing to and inconvenient for patients but also a self-limiting factor as it reduces the efficacy of neuraxial opioids for pain relief (Ballantyne et al. 1988; Cousins and Mather 1984; Ganesh and Maxwell 2007; Szarvas et al. 2003). This long-standing troublesome problem associated with neuraxial opioid-induced itch has prompted many scientists and physicians to target studies on potential treatment options (Dominguez and Habib 2013; Ganesh and Maxwell 2007; Kumar and Singh 2013; Waxler et al. 2005). In an attempt to prevent or treat neuraxial opioid-induced itch, a wide variety of pharmacological agents have been evaluated in both animals and humans. However, there is not yet a widely accepted non-opioid drug for treating neuraxial opioid-induced itch. From the perspective of receptor mechanisms underlying opioid-induced itch, the purpose of this review is to discuss the treatment of neuraxial opioid-induced itch and its pharmacological antagonistic mechanisms through examining the evidence provided by preclinical and clinical studies from available literature to date.
2 Mechanisms of Neuraxial Opioid-Induced Itch
2.1 The Molecular Basis
The molecular mechanism of neuraxial opioid-induced itch has been somewhat unveiled by a recent study (Liu et al. 2011). Liu et al. conducted a series of elegant experiments, demonstrating the uncoupling of morphine-induced itch and morphine-induced analgesia in the mouse spinal cord. The mu opioid receptor (MOP) isoform MOP1D is required for intrathecal morphine-induced itch. MOP1D heterodimerizes with gastrin-releasing peptide receptor (GRPR) in the spinal cord, together relaying itch neurotransmission. In particular, MOP agonist-induced scratching responses were nearly abolished in GRPR knockout mice and intrathecal morphine-induced scratching was inhibited by coadministration with a GRPR antagonist (Liu et al. 2011). However, the presence of MOP1D in the rat spinal cord has been questioned (Oldfield et al. 2008). A recent study in monkeys showed that the GRPR antagonist could not attenuate scratching responses elicited by intrathecal administration of an MOP-preferring ligand, β-endorphin, but the same GRPR antagonist significantly attenuated intrathecal gastrin-releasing peptide-induced scratching (Ko 2013). Although the identification of MOP1D in mice implicates that there may be a possible groundbreaking analgesic treatment without causing the pruritic side effect (Liu et al. 2011), future studies are warranted to investigate whether these exciting findings can be translated to other species and advanced to the clinical setting.
As promising as the separation of morphine-induced itch and morphine-induced analgesia implies, rodents subjected to intrathecal morphine did not display profound scratching activities. It is worth noting the dramatic differences in the scratching activities elicited by intrathecal morphine between rodents and primates. Both the magnitude and duration of intrathecal morphine-induced scratching in mice are very mild, i.e., 15 scratching bouts as peak activity 10 min after injection and the increased scratching activity only lasted for 10–15 min (Liu et al. 2011). As compared to scratching responses elicited by intrathecal vehicle or saline, mice display a similar profile of scratching activity which made other researchers conclude that intrathecal morphine failed to elicit scratching responses in mice (Sukhtankar and Ko 2013). Intrathecal morphine over a wide dose range also failed to elicit scratching responses in rats (Lee et al. 2003). Nevertheless, intrathecal morphine elicited profound scratching responses in nonhuman primates, i.e., approximately 600 scratches within a 15-min bin/time sampling and such profound scratching lasted for several hours (Ko and Naughton 2000; Ko et al. 2004). Such dramatic species differences in intrathecal morphine-induced scratching may affect the interpretations of the pharmacological and neurobiological findings.
2.2 The Cellular Basis
The cellular mechanisms of neuraxial opioid-induced itch have been elucidated in depth by pharmacological studies in nonhuman primates. First, microinjection of a kappa opioid receptor (KOP) agonist, U-50488H, or a delta opioid receptor (DOP) agonist, DPDPE, into the medullary dorsal horn did not evoke facial scratching in monkeys (Thomas et al. 1992). Second, intrathecal administration of U-50488H and a DOP agonist, SNC80, produced moderate antinociception, but both ligands did not produce scratching in monkeys (Ko et al. 2003a). Third, intrathecal administration of a nociceptin/orphanin FQ peptide receptor (NOP) agonist produced full antinociceptive effects without eliciting scratching (Ko et al. 2006). Fourth, the antagonist potency of nalmefene, an MOP-preferring antagonist, validates that MOP mainly mediates intrathecal morphine-induced itch scratching (Ko and Naughton 2000). Last, pretreatment with an MOP antagonist (clocinnamox), rather than a DOP antagonist (naltrindole) or a KOP antagonist (nor-binaltorphimine), blocked intrathecal morphine-induced scratching (Ko et al. 2004). Taken together, these findings clearly demonstrate that the MOP, but not other opioid receptor subtypes, mainly mediates neuraxial opioid-induced itch in primates.
There is a well-known theory for opioid-induced itch. As pain inhibits itch, opioid analgesics elicit itch sensation by providing pain relief, i.e., removal of pain unmasks itch sensation (Ikoma et al. 2006; McMahon and Koltzenburg 1992). However, functional evidence from pharmacological studies does not support this notion. By using receptor-selective ligands, pharmacological approaches allow researchers to elucidate the function of each opioid receptor subtype in modulating pain and itch sensations. The DOP, KOP, and NOP agonists produce analgesic properties across diverse pain modalities following intrathecal and systemic administration (Brandt et al. 2001; Butelman and Kreek 2013; Butelman et al. 1993; Hu et al. 2010; Ko et al. 2009; Sukhtankar et al. 2014). Interestingly, these three types of opioid receptor agonists do not elicit scratching responses over a wide antinociceptive dose range (Ko et al. 2004, 2009; Sukhtankar et al. 2014). These findings clearly demonstrate that only MOP agonists produce analgesic effects accompanied by itch scratching responses. Other opioid receptor subtypes, DOP, KOP, and NOP, do not mediate neuraxial opioid-induced itch. It is important to further investigate physiological properties of sensory neurons expressing MOP and/or other opioid receptor subtypes in the spinal cord. More importantly, opioid-induced analgesia and itch can be distinguished at the receptor level. From the perspective of developing novel neuraxial opioids, it is promising to reveal that spinal administration of NOP agonists produces morphine-comparable analgesic effects without evoking itch in nonhuman primates (Hu et al. 2010; Ko and Naughton 2009; Ko et al. 2006). Such important findings will facilitate future advances of spinal analgesics (Lin and Ko 2013; Molinari et al. 2013; Schröder et al. 2014).
2.3 Animal Models with Translational Values
By using intrathecal administration, animal studies have shown that intrathecal morphine elicited scratching responses of different magnitudes and temporal patterns between rodents and nonhuman primates (Ko and Naughton 2000; Kuraishi et al. 2000; Lee et al. 2003; Liu et al. 2011; Sukhtankar and Ko 2013). Perhaps, the most important characteristic of intrathecal morphine is that it simultaneously provides pain relief and elicits itch sensation in patients. To the best of our knowledge, the nonhuman primate model can simulate this therapeutic profile very well. Intrathecal morphine over a wide dose range (10–320 µg) produced antinociceptive effects and it also produced profound scratching responses for several hours in rhesus monkeys (Ko and Naughton 2000; Ko et al. 2004). These observations closely parallel the behavioral effects and physiological relevance of spinal morphine in humans (Bailey et al. 1993; Palmer et al. 1999; Waxler et al. 2005). Accordingly, nonhuman primates can serve as a translational bridge to explore and validate potential drugs that may be effective in treating neuraxial opioid-induced itch in humans.
Recently, several studies using in vivo electrophysiology have shed light on regulation of pain and itch sensations by sensory neurons. Moser and Giesler (2013) have identified trigeminothalamic tract (VTT) neurons in anesthetized rats that are differentially affected by morphine. Briefly, intrathecal morphine increased the ongoing activity of pruriceptive VTT neurons but inhibited the ongoing activity and responses to noxious stimuli in nociceptive VTT neurons. In addition, the spinothalamic tract (STT) responds to pruritogens with activation that reflects the time course of histamine-induced itch sensation in humans (Andrew and Craig 2001; Davidson et al. 2007; Simone et al. 2004). More interestingly, the responses of STT neurons to histamine were inhibited by scratching the skin, indicating a neural correlate of scratching-induced relief and the importance of spinal processing in controlling itch neurotransmission (Davidson et al. 2009). These observations pinpoint pruriceptive STT neurons being positioned within a plastic circuitry that can provide a locus for pharmacological management of itch (Davidson et al. 2009, 2012). Future research integrating both pharmacological and electrophysiological approaches in both rodents and nonhuman primates will advance our understanding of how the spinal neural circuits regulate MOP-mediated itch and analgesia. Based on the current literature, several pharmacological studies in nonhuman primates have been conducted to evaluate the effectiveness of diverse ligands in treating intrathecal morphine-induced scratching in adult rhesus monkeys (Table 1). These summarized findings in nonhuman primates are discussed and compared in the sections below (pharmacological antagonism) in terms of effectiveness of opioid- and non-opioid-related ligands in treating neuraxial opioid-induced itch in adult patients.
Table 1.
Summary of preclinical studies evaluating the effectiveness of diverse ligands in managing neuraxial opioid-induced scratching in adult rhesus monkeys
| Neuraxial opioids |
Treatment drug and doses | Outcomes and conclusion |
References |
|---|---|---|---|
| Intrathecal morphine 10–320 µg | Intravenous nalmefene (MOP antagonist) 10–32 µg/kg | Effective Scratching responses: ↓ | Ko and Naughton (2000) |
| Intrathecal morphine 32 µg | Intramuscular clocinnamox (MOP antagonist) 0.1 mg/kg | Effective Scratching responses: ↓ | Ko et al. (2004) |
| Intrathecal morphine 32 µg | Subcutaneous butorphanol (mixed KOP/MOP partial agonist) 10–32 µg/kg | Effective Scratching responses: ↓ | Lee et al. (2007) |
| Intrathecal morphine 10–32 µg | Subcutaneous U-50488H (KOP agonist) 0.1–0.32 mg/kg | Effective Scratching responses: ↓ | Ko et al. (2003b) |
| Intrathecal morphine 32 µg | Intramuscular nalfurafine (KOP agonist) 0.3–1 µg/kg | Effective Scratching responses: ↓ | Ko and Husbands (2009) |
| Intrathecal morphine 32 µg | Intramuscular naltrindole (DOP antagonist) 1 mg/kg | Ineffective No change in scratching responses | Ko et al. (2004) |
| Intrathecal morphine 50 nmol | Intrathecal N/OFQ (NOP agonist) 10–100 nmol | Ineffective No change in scratching responses | Ko and Naughton (2009) |
| Intrathecal morphine 32 µg | Intravenous ondansetron (5HT3 antagonist) 0.1–3.2 mg/kg | Ineffective No change in scratching responses | Fig. 1 |
| Intrathecal morphine 32 µg | Intramuscular diphenhydramine (antihistamine) 0.32–3.2 mg/kg | Ineffective No change in scratching responses | Ko et al. (2004) |
| Intrathecal morphine 32 µg | Intravenous ketorolac (NSAID) 1–10 mg/kg | Ineffective No change in scratching responses | Fig. 2 |
Note: ↓ = decrease/ inhibition, MOP mu opioid receptor, KOP kappa opioid receptor, NOP nociceptin/orphanin FQ peptide receptor, DOP delta opioid receptor, NSAID non-steroidal anti-inflammatory drug
3 Pharmacological Antagonism by Opioid-Related Ligands
3.1 Mu Opioid Receptor Antagonists
As most opioid analgesics used in the clinics are MOP agonists, it is expected that MOP antagonists are effective in treating neuraxial opioid-induced itch in patients (Dominguez and Habib 2013; Ganesh and Maxwell 2007; Kumar and Singh 2013; Waxler et al. 2005). A systematic review of randomized trials involving obstetric patients indicated that intravenous naloxone (0.25–2.4 µg/kg/h) was effective in managing opioid-induced itch (Kjellberg and Tramer 2001). However, MOP antagonists are not widely useful in patients receiving neuraxial opioids for pain relief because MOP antagonists reverse or shorten neuraxial opioid-induced analgesia (Abboud et al. 1990; Cohen et al. 1992; Rawal et al. 1986; Wang et al. 1998).
Antagonist studies in nonhuman primates demonstrate that pretreatment with a single dose of nalmefene (32 µg/kg) was equally potent to block intrathecal morphine-induced itch scratching and antinociception (Ko and Naughton 2000). In this study, the in vivo pKB analysis was used to verify functional receptor populations underlying the actions of intrathecal morphine. The same dose of nalmefene produced approximately tenfold rightward shifts in each subject’s dose–response curves of intrathecal morphine for scratching and antinociception. Accordingly, nalmefene pKB values were similar for both endpoints, indicating that intrathecal morphine-induced scratching and antinociception are mediated by the same MOP population in primates (Ko and Naughton 2000). These findings indicate a narrow window between reversal of itch and analgesia by MOP antagonists and support the clinical findings that MOP antagonists such as naloxone and nalmefene may not be ideal drugs for treating pruritus in obstetric patients. Nevertheless, the MOP antagonist is one of the treatment options for ameliorating cholestatic pruritus, which may be caused by elevated levels of endogenous opioid peptides (Bergasa 2008; Jones and Bergasa 1992).
3.2 Opioid Receptor Partial Agonists
Both nalbuphine and butorphanol are opioid receptor partial agonists that have been used clinically as analgesics with limited abuse liability (Preston and Jasinski 1991). The radioligand binding assay suggests that both drugs have reasonable binding affinity for both MOP and KOP sites in monkey brain membranes, although nalbuphine has a higher selectivity for MOP over KOP (Butelman et al. 1998). In the cell lines expressing MOP or KOP, both drugs displayed low-mid efficacy as measured by the stimulation of [35S]GTPγS binding, i.e., low-mid intrinsic activity (Emmerson et al. 1996; Remmers et al. 1999; Zhu et al. 1997). Interestingly, due to its low efficacy, nalbuphine displays partial MOP agonist actions with its context-dependent agonist/antagonist effects in nonhuman primate behavioral assays (Gerak et al. 1994; Gerak and France 1996). By contrast, butorphanol is characterized as a partial agonist acting at both KOP and MOP sites by diverse in vivo assays in nonhuman primates (Butelman et al. 1995; Lee et al. 2007; Vivian et al. 1999).
Both nalbuphine and butorphanol are effective in alleviating neuraxial opioid-induced itch (Table 2). In particular, systemic nalbuphine between 3 and 10 mg seems effective in decreasing the incidence of pruritus in most of the clinical studies. However, with a high dose of nalbuphine (20 mg), Morgan et al. (1991) did not find pruritus relief by nalbuphine. Butorphanol seems less popular than nalbuphine for treating opioid-induced itch probably due to potential drowsiness following systemic administration. Nevertheless, several studies have shown a decreased incidence of pruritus without other side effects when butorphanol was administered with morphine epidurally in pediatric patients (Bailey et al. 1994; Gunter et al. 2000; Lawhorn et al. 1995; Lawhorn and Brown 1994). A recent systematic review also indicates the potential benefits of using butorphanol to prevent neuraxial morphine-induced itch and decrease pain intensity and postoperative nausea and vomiting without increasing other side effects (Du et al. 2013). Importantly, a pharmacological study demonstrates that butorphanol’s partial agonist actions at both MOP and KOP sites contribute to its antipruritic actions, i.e., low-efficacy ligands antagonize high-efficacy ligand’s action in producing itch sensation (Lee et al. 2007). Compared with MOP antagonists, opioid receptor partial agonists seem to have an advantage for ameliorating itch while retaining analgesia (Dominguez and Habib 2013; Ganesh and Maxwell 2007; Kumar and Singh 2013; Waxler et al. 2005). These observations are in line with preclinical studies demonstrating that butorphanol is effective in alleviating MOP agonist-induced itch without reversing analgesia in nonhuman primates (Lee et al. 2007). Due to butorphanol’s unique pharmacological profile, i.e., partial agonist actions at both MOP and KOP sites, dermatologists are very interested in developing a transdermal formulation of butorphanol for the treatment of chronic itch (Dawn and Yosipovitch 2006; Lim et al. 2008).
Table 2.
Summary of clinical studies evaluating the effectiveness of opioid receptor partial agonists in managing neuraxial opioid-induced pruritus in adult patients
| Neuraxial opioids |
Treatment drugs and doses | Outcomes and conclusion | References |
|---|---|---|---|
| Epidural morphine 0.1 mg/kg | Intravenous nalbuphine 0.1 mg/kg | Effective Pruritus score: ↓ | Penning et al. (1988) |
| Epidural morphine 5 mg | Intravenous nalbuphine 20 mg | Ineffective No change in the degree of pruritus | Morgan et al. (1991) |
| Epidural morphine 5 mg | Intravenous nalbuphine 5 mg | Effective Severity of pruritus: ↓ | Cohen et al. (1992) |
| Epidural morphine 5 mg | Intravenous nalbuphine 2.5 mg/h | Effective Pruritus score: ↓ | Kendrick et al. (1996) |
| Epidural morphine 3 mg/12 h | Intravenous nalbuphine 60 µg/kg/h | Effective Incidence of pruritus (13 %): ↓ | Wang et al. (1998) |
| Epidural morphine 1.5 mg/12 h | Intramuscular nalbuphine 10 mg | Effective Incidence of pruritus (44 %): ↓ Severity of pruritus: ↓ | Liao et al. (2011) |
| Intrathecal morphine 200 µg | Nalbuphine (no specified delivery route) 5–10–10 mg, stepwise | Effective VAS score of zero (83 %) | Alhashemi et al. (1997) |
| Intrathecal morphine 200 µg | Intravenous nalbuphine 3 mg | Effective Treatment success rate (83 %): ↑ | Charuluxananan et al. (2001) |
| Intrathecal morphine 200 µg | Intravenous nalbuphine 4 mg | Effective Pruritus score: ↓ Request for pruritus treatment: ↓ | Charuluxananan et al. (2003) |
| Intrathecal morphine 150 µg | Intravenous nalbuphine 2–3 mg | Effective % of successful treatment (87–97 %): ↑ | Somrat et al. (1999) |
| Intrathecal fentanyl 50 µg | Intravenous nalbuphine 4 mg | Partially effective Incidence of pruritus (61 %) | Ben-David et al. (2002) |
| Epidural morphine 4 mg | Epidural butorphanol 3 mg | Effective % patients treated for pruritus (0 %): ↓ | Lawhorn et al. (1991) |
| Epidural morphine 4 mg | Epidural butorphanol 3 mg | Effective Incidence of pruritus (20 %): ↓ | Wittels et al. (1993) |
| Epidural morphine 3 mg | Epidural butorphanol 3 mg | Ineffective No change in VAS for pruritus | Gambling et al. (1994) |
| Epidural morphine 60 µg/kg | Epidural butorphanol 30 µg/kg | Effective Severity of pruritus: ↓ | Bailey et al. (1994) |
| Intrathecal morphine 150 µg | Intravenous butorphanol 2 mg | Ineffective No change in the intensity of pruritus | Sakai et al. (2001) |
| Intrathecal morphine 100 µg | Intravenous butorphanol Bolus 1 mg with 0.2 mg/h | Effective Incidence of pruritus (13 %): ↓ | Wu et al. (2012) |
Note: VAS visual analog scale, ↓ = decrease/inhibition, ↑ = increase
3.3 Kappa Opioid Receptor Agonists
Numerous preclinical and clinical studies have indicated that KOP is a viable therapeutic target for potential antipruritics (Cowan and Gmerek 1986; Ko et al. 2003b; Kumagai et al. 2010, 2012). Original studies in rodents showed that systemic administration of KOP agonists inhibited scratching activity evoked by pruritogens such as bombesin-related peptides (Gmerek and Cowan 1983, 1984). In particular, KOP agonists inhibited scratching behavior without interfering with locomotor activity in rodents (Inan et al. 2009; Togashi et al. 2002; Wang et al. 2005). Recent studies have identified a subset of inhibitory interneurons regulating itch in the dorsal horn of mouse spinal cord (Ross et al. 2010). It will be important to investigate the role of KOP modulating these inhibitory interneurons. Furthermore, pharmacological studies in nonhuman primates have demonstrated that KOP agonists, at nonsedating doses, can attenuate intrathecal morphine-induced scratching without affecting antinociception (Ko and Husbands 2009; Ko et al. 2003b). These findings facilitated the development of a KOP agonist, nalfurafine, as an antipruritic. To date, two clinical trials have reported that nalfurafine is a safe and effective antipruritic in hemodialysis patients suffering from uremic pruritus (Kumagai et al. 2010, 2012).
KOP agonists produce several effects opposite to those of MOP agonists in primates. For example, MOP agonists produce euphoria, whereas KOP agonists produce dysphoria (Kumor et al. 1986; Walsh et al. 2001); MOP agonists produce antidiuretic effects, while KOP agonists produce diuresis (Peters et al. 1987; Weiskopf et al. 1987). Although there is no selective KOP agonist approved for treating neuraxial opioid-induced itch, it seems promising to develop KOP-related ligands, especially mixed KOP/MOP agonists for this purpose or as spinal analgesics. Clinically used mixed KOP/MOP agonists such as butorphanol and pentazocine have a low incidence of pruritus and are effective in treating spinal morphine-induced itch (Abboud et al. 1989; Ackerman et al. 1989; Lawhorn et al. 1991; Tamdee et al. 2009). In addition, butorphanol produces neither euphoria nor dysphoria in humans and it does not cause diuresis (Butelman et al. 1995; Dershwitz et al. 1991). These findings strengthen the notion that mixed KOP/MOP agonists may have a therapeutic advantage over selective MOP agonists. It will be important to further develop novel opioid agonists with dual actions at both KOP and MOP sites with different degrees of intrinsic efficacy and advance the medicine of neuraxial opioids.
4 Pharmacological Antagonism by Non-Opioid Ligands
4.1 Serotonin 5-HT3 Receptor Antagonists
The effectiveness of a 5-HT3 receptor antagonist, ondansetron, in treating neuraxial opioid-induced itch varies across different clinical studies (Table 3). Several studies showed that intravenous ondansetron (4–8 mg) was effective in decreasing the incidence of pruritus in patients receiving either epidural or intrathecal morphine, fentanyl, or combination of MOP agonists. However, several other studies concluded that ondansetron was ineffective in treating itch in most of the patients receiving intrathecal fentanyl or combination of MOP agonists (Bonnet et al. 2008). It will be important to investigate whether fentanyl, sufentanil, or a combination of MOP agonists elicits a higher intensity of itch as both fentanyl and sufentanil have been characterized in the agonist stimulation of [35S]GTPγS binding as full MOP agonists with higher intrinsic activity as compared to morphine (Emmerson et al. 1996).
Table 3.
Summary of clinical studies evaluating the effectiveness of a 5-HT3 receptor antagonist, ondansetron, in managing neuraxial opioid-induced pruritus in adult patients
| Neuraxial opioids | Treatment drug and doses |
Outcomes and conclusion | References |
|---|---|---|---|
| Epidural morphine, 2 mg Intrathecal morphine, 0.2 mg | Intravenous ondansetron 8 mg | Effective Success rate (70 %): ↑ | Borgeat and Stirnemann (1999) |
| Epidural morphine 3 mg | Intravenous ondansetron 4 mg | Effective Incidence of pruritus (28 %): ↓ | Tzeng et al. (2003) |
| Intrathecal sufentanil 2.5 µg and morphine 100 µg | Intravenous ondansetron 8 mg | Ineffective No change in the frequency and severity of pruritus | Yazigi et al. (2002) |
| Intrathecal morphine 160 µg and fentanyl 15 µg | Intravenous ondansetron 8 mg | Ineffective No change in the incidence of pruritus | Sarvela et al. (2006) |
| Intrathecal morphine 250 µg | Intravenous ondansetron 4 mg | Effective Incidence of pruritus (34 %): ↓ | Iatrou et al. (2005) |
| Intrathecal morphine 200 µg | Intravenous ondansetron 4–8 mg | Effective Request for pruritus treatment: ↓ | Charuluxananan et al. (2003) |
| Intrathecal morphine 200 µg | Intravenous ondansetron 4 mg | Effective Treatment success rate (80 %): ↑ | Charuluxananan et al. (2000) |
| Intrathecal morphine 200 µg | Intravenous ondansetron 4 mg Orally disintegrating tablets 8 mg | Effective Incidence of pruritus (56–66 %): ↓ | Pirat et al. (2005) |
| Intrathecal morphine 150 µg | Intravenous ondansetron 0.1 mg/kg | Effective Incidence of pruritus (25 %): ↓ | Yeh et al. (2000) |
| Intrathecal fentanyl 25 µg | Intravenous ondansetron 4–8 mg | Ineffective No change in the incidence and severity of pruritus | Wells et al. (2004) |
| Intrathecal fentanyl 25 µg | Intravenous ondansetron 8 mg | Effective Incidence of pruritus (39 %): ↓ | Gurkan and Toker (2002) |
| Intrathecal fentanyl 25 µg | Intravenous ondansetron 8 mg | Effective Incidence of pruritus (6 %): ↓ | Gulhas et al. (2007) |
| Intrathecal fentanyl 15 µg | Intravenous ondansetron 8 mg | Ineffective No change in the incidence of pruritus | Browning et al. (2013) |
| Intrathecal fentanyl 10 µg | Intravenous ondansetron 4–8 mg | Ineffective No change in the incidence and severity of pruritus | Korhonen et al. (2003) |
| Intrathecal sufentanil 10 µg | Intravenous ondansetron 8 mg | Ineffective No change in the incidence and severity of pruritus | Waxler et al. (2004) |
Note: VAS visual analog scale, ↓ = decrease/inhibition, ↑ = increase
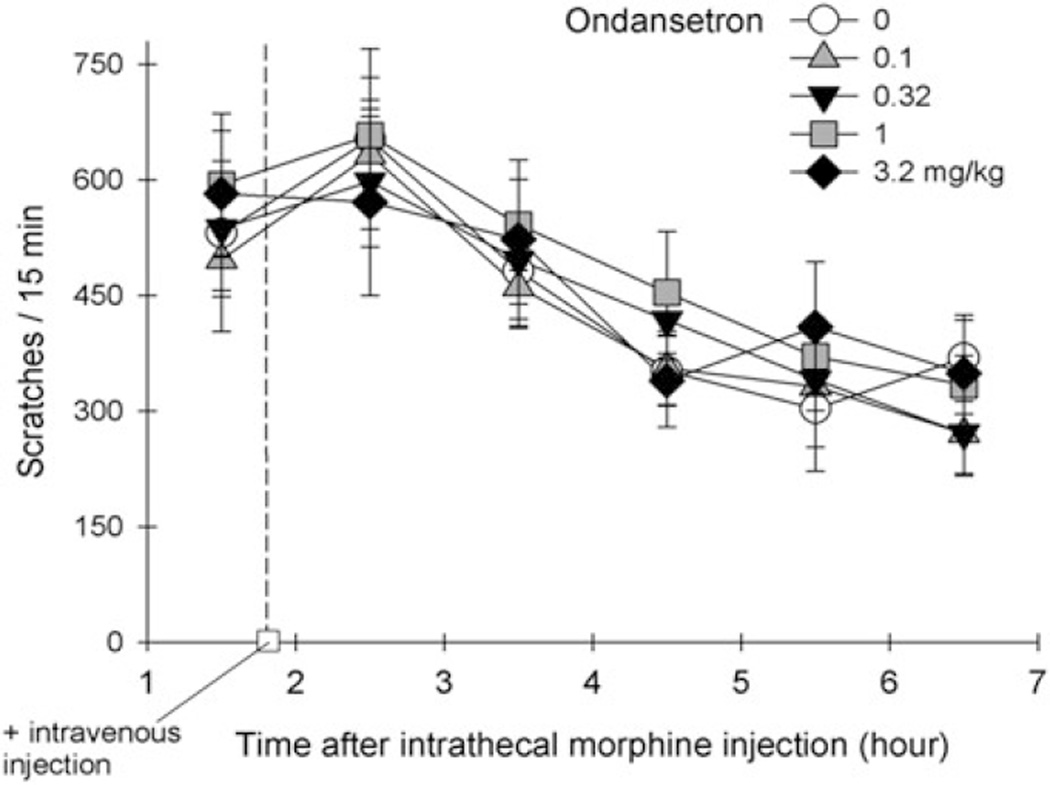
The exact mechanism for ondansetron to alleviate itch is unknown. Although the 5-HT3 receptors can be identified in the spinal cord of rodents and primates (Laporte et al. 1996; Waeber et al. 1988), there is no anatomical evidence for the co-localization of the 5-HT3 receptor with MOP in the spinal cord or functional evidence for the interaction between the 5-HT3 receptor and MOP in any animal models. Since patients with cholestatic pruritus have elevated levels of endogenous opioids, there were several randomized controlled trials exploring the effects of ondansetron (Jones et al. 2007). It was concluded that ondansetron has negligible effect on cholestatic or uremic pruritus based on a recent systematic review (To et al. 2012). Figure 1 illustrates the effects of ondansetron on intrathecal morphine-induced scratching in monkeys. Intrathecal administration of morphine (32 µg) elicited profound scratching responses (i.e., ~600 scratches within a 15-min bin/time sampling) in rhesus monkeys (n = 8) (unpublished data from the Ko lab). Intravenous ondansetron (0.1–3.2 mg/kg) was given approximately 2 h after subjects received intrathecal morphine. Within these doses tested herein, ondansetron was ineffective in attenuating intrathecal morphine-induced scratching. A higher dose of ondansetron (10 mg/kg) caused extrapyramidal reactions in monkeys (i.e., involuntary head jerking, both legs were rigid and were in extensor spasm) which led to the termination of experiments.
Fig. 1.
Effects of intravenous ondansetron on intrathecal morphine-induced itch scratching responses in rhesus monkeys. Each value represents means +/− S.E.M. (n = 8)
4.2 Histamine H1 Receptor Antagonists
Although morphine can trigger the release of histamine from mast cells, clinical studies have indicated that antihistamines are not effective in relieving neuraxial opioid-induced itch (Dunteman et al. 1996; Horta et al. 2006). Pharmacological studies in nonhuman primates also found that an antihistamine, diphenhydramine, over a wide dose range could not attenuate intrathecal morphine-induced scratching (Ko et al. 2004). Moreover, other MOP agonists such as fentanyl and alfentanil do not stimulate histamine release (Hermens et al. 1985; Rosow et al. 1982), whereas they evoke itch/scratching in humans and nonhuman primates (Ellis et al. 1990; Ko et al. 2004). As tachyphylaxis develops quickly in response to histamine-induced itch, the role of histamine is minimal in both neuraxial opioid-induced itch and chronic itch. Nevertheless, the sedative effects of antihistamines may be helpful by providing needed sleep and interrupting the itch–scratch cycle while being barely effective in decreasing the severity of itch (Krajnik and Zylicz 2001; Szarvas et al. 2003).
4.3 Nonsteroidal Anti-Inflammatory Drugs
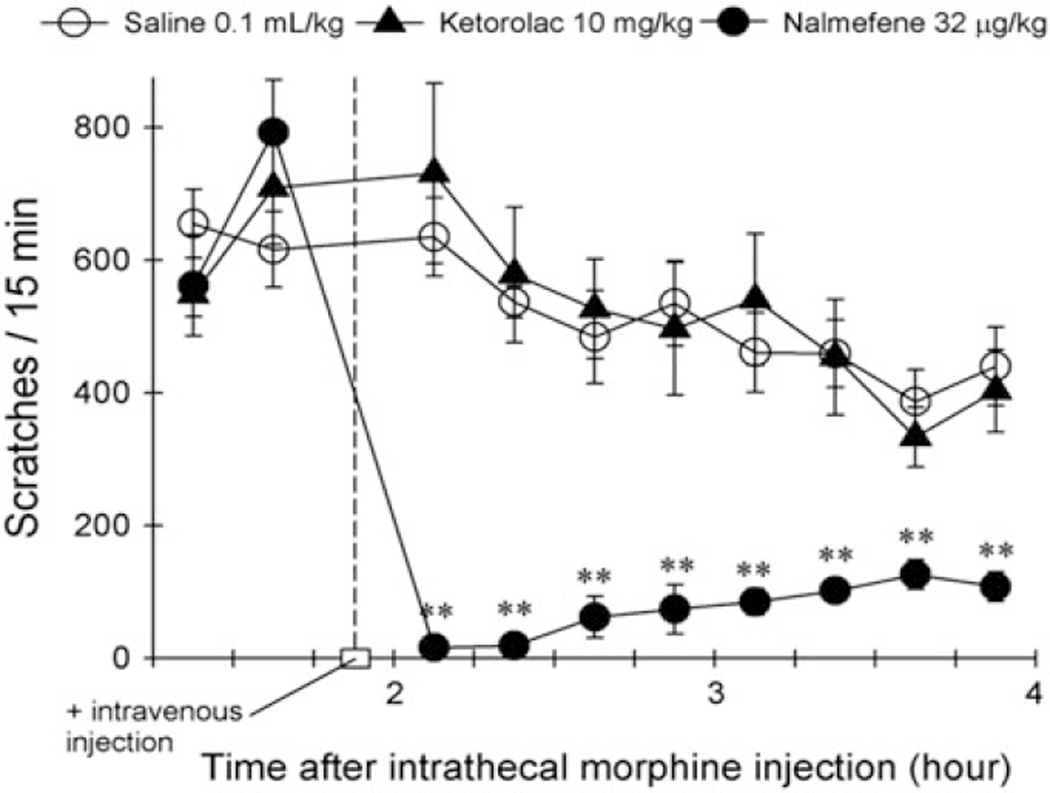
Nonsteroidal anti-inflammatory drugs (NSAIDs) attenuate inflammatory pain by inhibiting cyclooxygenases and decreasing prostaglandin levels. Intravenous tenoxicam and rectal diclofenac have been reported to attenuate neuraxial opioid-induced itch (Colbert et al. 1999a, b). However, other studies using celecoxib and lornoxicam found no changes in the severity of pruritus (Gulhas et al. 2007; Lee et al. 2004). In the nonhuman primate inflammatory pain model, systemic administration of ketorolac (0.3–10 mg/kg) dose-dependently attenuated carrageenan-induced thermal allodynia/hyperalgesia (Sukhtankar et al. 2014). However, the same dose range of intravenous ketorolac did not attenuate scratching responses elicited by intrathecal morphine (32 µg) (unpublished data from the Ko lab). Figure 2 compares the effects of ketorolac and nalmefene on intrathecal morphine-induced scratching in the same rhesus monkeys (n = 5). Either ketorolac (10 mg/kg) or nalmefene (32 µg/kg) was administered intravenously approximately 2 h after subjects received intrathecal morphine (32 µg). In this experimental setting, intravenous nalmefene, but not ketorolac, significantly attenuated scratching responses. Based on these results, NSAIDs may not be useful therapeutic agents to treat neuraxial opioid-induced itch. It seems unlikely that prostaglandins play a significant role as itch mediators associated with neuraxial opioids.
Fig. 2.
Effects of intravenous ketorolac and nalmefene on intrathecal morphine-induced itch scratching responses in rhesus monkeys. Each value represents mean +/− S.E.M. (n = 5). The asterisks represent significant differences from the saline condition
5 Conclusion
A variety of drugs have been evaluated in treating neuraxial opioid-induced itch. These diverse drugs, including gabapentin, dopamine D2 receptor antagonists, propofol, mirtazapine, and dexamethasone, have been discussed in recent review articles, but all have mixed results from a very limited number of clinical studies (Dominguez and Habib 2013; Ganesh and Maxwell 2007; Kumar and Singh 2013). As these drugs have not been extensively studied in nonhuman primates, there is no further discussion on the potential pharmacological antagonism of these drugs on neuraxial opioid-induced itch. Most importantly, accumulated pharmacological evidence in nonhuman primates (Table 1, Figs. 1 and 2) supports that (1) MOP antagonists and mixed KOP/MOP partial agonists are the most effective treatment options for managing neuraxial opioid-induced itch (Table 2) and (2) non-opioid ligands, including the 5-HT3 antagonist ondansetron, antihistamines, and NSAIDs, are not effective in treating neuraxial opioid-induced itch (Table 3). Collectively, these pharmacological studies indicate that rhesus monkeys may serve as a surrogate species for humans in preclinical studies to identify effective treatments for neuraxial opioid-induced itch.
Acknowledgment
Funding from the National Institutes of Health in the United States (DA-013685, AR-059193, and AR-064456) to support the research of neuraxial opioid-induced itch is gratefully acknowledged.
Abbreviations
- DOP
Delta opioid receptor
- GRPR
Gastrin-releasing peptide receptor
- KOP
Kappa opioid receptor
- MOP
Mu opioid receptor
- NOP
Nociceptin/orphanin FQ peptide receptor
References
- Abboud TK, Afrasiabi A, Zhu J, Mantilla M, Reyes A, D’Onofrio L, Khoo N, Mosaad P, Richardson M, Kalra M, et al. Epidural morphine or butorphanol augments bupivacaine analgesia during labor. Reg Anesth. 1989;14:115–120. [PubMed] [Google Scholar]
- Abboud TK, Lee K, Zhu J, Reyes A, Afrasiabi A, Mantilla M, Steffens Z, Chai M. Prophylactic oral naltrexone with intrathecal morphine for cesarean section: effects on adverse reactions and analgesia. Anesth Analg. 1990;71:367–370. doi: 10.1213/00000539-199010000-00008. [DOI] [PubMed] [Google Scholar]
- Ackerman WE, Juneja MM, Kaczorowski DM, Colclough GW. A comparison of the incidence of pruritus following epidural opioid administration in the parturient. Can J Anaesth. 1989;36:388–391. doi: 10.1007/BF03005335. [DOI] [PubMed] [Google Scholar]
- Alhashemi JA, Crosby ET, Grodecki W, Duffy PJ, Hull KA, Gallant C. Treatment of intrathecal morphine-induced pruritus following caesarean section. Can J Anaesth. 1997;44:1060–1065. doi: 10.1007/BF03019227. [DOI] [PubMed] [Google Scholar]
- Andrew D, Craig AD. Spinothalamic lamina I neurons selectively sensitive to histamine: a central neural pathway for itch. Nat Neurosci. 2001;4:72–77. doi: 10.1038/82924. [DOI] [PubMed] [Google Scholar]
- Bailey PL, Rhondeau S, Schafer PG, Lu JK, Timmins BS, Foster W, Pace NL, Stanley TH. Dose–response pharmacology of intrathecal morphine in human volunteers. Anesthesiology. 1993;79:49–59. doi: 10.1097/00000542-199307000-00010. discussion 25A. [DOI] [PubMed] [Google Scholar]
- Bailey AG, Valley RD, Freid EB, Calhoun P. Epidural morphine combined with epidural or intravenous butorphanol for postoperative analgesia in pediatric patients. Anesth Analg. 1994;79:340–344. doi: 10.1213/00000539-199408000-00025. [DOI] [PubMed] [Google Scholar]
- Ballantyne JC, Loach AB, Carr DB. Itching after epidural and spinal opiates. Pain. 1988;33:149–160. doi: 10.1016/0304-3959(88)90085-1. [DOI] [PubMed] [Google Scholar]
- Ben-David B, DeMeo PJ, Lucyk C, Solosko D. Minidose lidocaine-fentanyl spinal anesthesia in ambulatory surgery: prophylactic nalbuphine versus nalbuphine plus droperidol. Anesth Analg. 2002;95:1596–1600. doi: 10.1097/00000539-200212000-00023. table of contents. [DOI] [PubMed] [Google Scholar]
- Bergasa NV. Pruritus in primary biliary cirrhosis: pathogenesis and therapy. Clin Liver Dis. 2008;12:385–406. doi: 10.1016/j.cld.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Bonnet MP, Marret E, Josserand J, Mercier FJ. Effect of prophylactic 5-HT3 receptor antagonists on pruritus induced by neuraxial opioids: a quantitative systematic review. Br J Anaesth. 2008;101:311–319. doi: 10.1093/bja/aen202. [DOI] [PubMed] [Google Scholar]
- Borgeat A, Stirnemann HR. Ondansetron is effective to treat spinal or epidural morphine-induced pruritus. Anesthesiology. 1999;90:432–436. doi: 10.1097/00000542-199902000-00017. [DOI] [PubMed] [Google Scholar]
- Brandt MR, Furness MS, Mello NK, Rice KC, Negus SS. Antinociceptive effects of delta-opioid agonists in Rhesus monkeys: effects on chemically induced thermal hypersensitivity. J Pharmacol Exp Ther. 2001;296:939–946. [PubMed] [Google Scholar]
- Browning RM, Fellingham WH, O’Loughlin EJ, Brown NA, Paech MJ. Prophylactic ondansetron does not prevent shivering or decrease shivering severity during cesarean delivery under combined spinal epidural anesthesia: a randomized trial. Reg Anesth Pain Med. 2013;38:39–43. doi: 10.1097/AAP.0b013e31827049c6. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Kreek MJ. Kappa opioids: problems and opportunities in analgesia. In: Ko MC, Husbands SM, editors. Research and development of opioid-related ligands. Washington DC: American Chemical Society; 2013. pp. 245–255. [Google Scholar]
- Butelman ER, Negus SS, Ai Y, de Costa BR, Woods JH. Kappa opioid antagonist effects of systemically administered nor-binaltorphimine in a thermal antinociception assay in rhesus monkeys. J Pharmacol Exp Ther. 1993;267:1269–1276. [PubMed] [Google Scholar]
- Butelman ER, Winger G, Zernig G, Woods JH. Butorphanol: characterization of agonist and antagonist effects in rhesus monkeys. J Pharmacol Exp Ther. 1995;272:845–853. [PubMed] [Google Scholar]
- Butelman ER, Ko MC, Sobczyk-Kojiro K, Mosberg HI, Van Bemmel B, Zernig G, Woods JH. kappa-Opioid receptor binding populations in rhesus monkey brain: relationship to an assay of thermal antinociception. J Pharmacol Exp Ther. 1998;285:595–601. [PubMed] [Google Scholar]
- Charuluxananan S, Somboonviboon W, Kyokong O, Nimcharoendee K. Ondansetron for treatment of intrathecal morphine-induced pruritus after cesarean delivery. Reg Anesth Pain Med. 2000;25:535–539. doi: 10.1053/rapm.2000.7809. [DOI] [PubMed] [Google Scholar]
- Charuluxananan S, Kyokong O, Somboonviboon W, Lertmaharit S, Ngamprasertwong P, Nimcharoendee K. Nalbuphine versus propofol for treatment of intrathecal morphine-induced pruritus after cesarean delivery. Anesth Analg. 2001;93:162–165. doi: 10.1097/00000539-200107000-00032. [DOI] [PubMed] [Google Scholar]
- Charuluxananan S, Kyokong O, Somboonviboon W, Narasethakamol A, Promlok P. Nalbuphine versus ondansetron for prevention of intrathecal morphine-induced pruritus after cesarean delivery. Anesth Analg. 2003;96:1789–1793. doi: 10.1213/01.ANE.0000066015.21364.7D. table of contents. [DOI] [PubMed] [Google Scholar]
- Cohen SE, Ratner EF, Kreitzman TR, Archer JH, Mignano LR. Nalbuphine is better than naloxone for treatment of side effects after epidural morphine. Anesth Analg. 1992;75:747–752. [PubMed] [Google Scholar]
- Colbert S, O’Hanlon DM, Chambers F, Moriarty DC. The effect of intravenous tenoxicam on pruritus in patients receiving epidural fentanyl. Anaesthesia. 1999a;54:76–80. doi: 10.1046/j.1365-2044.1999.00610.x. [DOI] [PubMed] [Google Scholar]
- Colbert S, O’Hanlon DM, Galvin S, Chambers F, Moriarty DC. The effect of rectal diclofenac on pruritus in patients receiving intrathecal morphine. Anaesthesia. 1999b;54:948–952. doi: 10.1046/j.1365-2044.1999.01066.x. [DOI] [PubMed] [Google Scholar]
- Cousins MJ, Mather LE. Intrathecal and epidural administration of opioids. Anesthesiology. 1984;61:276–310. [PubMed] [Google Scholar]
- Cowan A, Gmerek DE. In-vivo studies on kappa opioid receptors. Trends Pharmacol Sci. 1986;7:69–72. [Google Scholar]
- Davidson S, Zhang X, Yoon CH, Khasabov SG, Simone DA, Giesler GJ., Jr The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci. 2007;27:10007–10014. doi: 10.1523/JNEUROSCI.2862-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S, Zhang X, Khasabov SG, Simone DA, Giesler GJ., Jr Relief of itch by scratching: state-dependent inhibition of primate spinothalamic tract neurons. Nat Neurosci. 2009;12:544–546. doi: 10.1038/nn.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S, Zhang X, Khasabov SG, Moser HR, Honda CN, Simone DA, Giesler GJ., Jr Pruriceptive spinothalamic tract neurons: physiological properties and projection targets in the primate. J Neurophysiol. 2012;108:1711–1723. doi: 10.1152/jn.00206.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawn AG, Yosipovitch G. Butorphanol for treatment of intractable pruritus. J Am Acad Dermatol. 2006;54:527–531. doi: 10.1016/j.jaad.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Dershwitz M, Rosow CE, DiBiase PM, Zaslavsky A. Comparison of the sedative effects of butorphanol and midazolam. Anesthesiology. 1991;74:717–724. doi: 10.1097/00000542-199104000-00016. [DOI] [PubMed] [Google Scholar]
- Dominguez JE, Habib AS. Prophylaxis and treatment of the side-effects of neuraxial morphine analgesia following cesarean delivery. Curr Opin Anaesthesiol. 2013;26:288–295. doi: 10.1097/ACO.0b013e328360b086. [DOI] [PubMed] [Google Scholar]
- Du BX, Song ZM, Wang K, Zhang H, Xu FY, Zou Z, Shi XY. Butorphanol prevents morphine-induced pruritus without increasing pain and other side effects: a systematic review of randomized controlled trials. Can J Anaesth. 2013;60:907–917. doi: 10.1007/s12630-013-9989-4. [DOI] [PubMed] [Google Scholar]
- Dunteman E, Karanikolas M, Filos KS. Transnasal butorphanol for the treatment of opioid-induced pruritus unresponsive to antihistamines. J Pain Symptom Manage. 1996;12:255–260. doi: 10.1016/0885-3924(96)00154-6. [DOI] [PubMed] [Google Scholar]
- Ellis DJ, Millar WL, Reisner LS. A randomized double-blind comparison of epidural versus intravenous fentanyl infusion for analgesia after cesarean section. Anesthesiology. 1990;72:981–986. doi: 10.1097/00000542-199006000-00006. [DOI] [PubMed] [Google Scholar]
- Emmerson PJ, Clark MJ, Mansour A, Akil H, Woods JH, Medzihradsky F. Characterization of opioid agonist efficacy in a C6 glioma cell line expressing the mu opioid receptor. J Pharmacol Exp Ther. 1996;278:1121–1127. [PubMed] [Google Scholar]
- Gambling DR, Howell P, Huber C, Kozak S. Epidural butorphanol does not reduce side effects from epidural morphine after cesarean birth. Anesth Analg. 1994;78:1099–1104. doi: 10.1213/00000539-199406000-00011. [DOI] [PubMed] [Google Scholar]
- Ganesh A, Maxwell LG. Pathophysiology and management of opioid-induced pruritus. Drugs. 2007;67:2323–2333. doi: 10.2165/00003495-200767160-00003. [DOI] [PubMed] [Google Scholar]
- Gerak LR, France CP. Discriminative stimulus effects of nalbuphine in rhesus monkeys. J Pharmacol Exp Ther. 1996;276:523–531. [PubMed] [Google Scholar]
- Gerak LR, Butelman ER, Woods JH, France CP. Antinociceptive and respiratory effects of nalbuphine in rhesus monkeys. J Pharmacol Exp Ther. 1994;271:993–999. [PubMed] [Google Scholar]
- Gmerek DE, Cowan A. An animal model for preclinical screening of systemic antipruritic agents. J Pharmacol Methods. 1983;10:107–112. doi: 10.1016/0160-5402(83)90073-6. [DOI] [PubMed] [Google Scholar]
- Gmerek DE, Cowan A. In vivo evidence for benzomorphan-selective receptors in rats. J Pharmacol Exp Ther. 1984;230:110–115. [PubMed] [Google Scholar]
- Gulhas N, Erdil FA, Sagir O, Gedik E, Togal T, Begec Z, Ersoy MO. Lornoxicam and ondansetron for the prevention of intrathecal fentanyl-induced pruritus. J Anesth. 2007;21:159–163. doi: 10.1007/s00540-007-0503-4. [DOI] [PubMed] [Google Scholar]
- Gunter JB, McAuliffe J, Gregg T, Weidner N, Varughese AM, Sweeney DM. Continuous epidural butorphanol relieves pruritus associated with epidural morphine infusions in children. Paediatr Anaesth. 2000;10:167–172. doi: 10.1046/j.1460-9592.2000.00447.x. [DOI] [PubMed] [Google Scholar]
- Gurkan Y, Toker K. Prophylactic ondansetron reduces the incidence of intrathecal fentanyl-induced pruritus. Anesth Analg. 2002;95:1763–1766. doi: 10.1097/00000539-200212000-00054. table of contents. [DOI] [PubMed] [Google Scholar]
- Hermens JM, Ebertz JM, Hanifin JM, Hirshman CA. Comparison of histamine release in human skin mast cells induced by morphine, fentanyl, and oxymorphone. Anesthesiology. 1985;62:124–129. doi: 10.1097/00000542-198502000-00005. [DOI] [PubMed] [Google Scholar]
- Horta ML, Morejon LC, da Cruz AW, Dos Santos GR, Welling LC, Terhorst L, Costa RC, Alam RU. Study of the prophylactic effect of droperidol, alizapride, propofol and promethazine on spinal morphine-induced pruritus. Br J Anaesth. 2006;96:796–800. doi: 10.1093/bja/ael072. [DOI] [PubMed] [Google Scholar]
- Hu E, Calo G, Guerrini R, Ko MC. Long-lasting antinociceptive spinal effects in primates of the novel nociceptin/orphanin FQ receptor agonist UFP-112. Pain. 2010;148:107–113. doi: 10.1016/j.pain.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iatrou CA, Dragoumanis CK, Vogiatzaki TD, Vretzakis GI, Simopoulos CE, Dimitriou VK. Prophylactic intravenous ondansetron and dolasetron in intrathecal morphine-induced pruritus: a randomized, double-blinded, placebo-controlled study. Anesth Analg. 2005;101:1516–1520. doi: 10.1213/01.ANE.0000181338.35454.6A. [DOI] [PubMed] [Google Scholar]
- Ikoma A, Steinhoff M, Stander S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nat Rev Neurosci. 2006;7:535–547. doi: 10.1038/nrn1950. [DOI] [PubMed] [Google Scholar]
- Inan S, Dun NJ, Cowan A. Nalfurafine prevents 5′-guanidinonaltrindole- and compound 48/80-induced spinal c-fos expression and attenuates 5′-guanidinonaltrindole-elicited scratching behavior in mice. Neuroscience. 2009;163:23–33. doi: 10.1016/j.neuroscience.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EA, Bergasa NV. The pruritus of cholestasis and the opioid system. JAMA. 1992;268:3359–3362. [PubMed] [Google Scholar]
- Jones EA, Molenaar HA, Oosting J. Ondansetron and pruritus in chronic liver disease: a controlled study. Hepatogastroenterology. 2007;54:1196–1199. [PubMed] [Google Scholar]
- Kendrick WD, Woods AM, Daly MY, Birch RF, DiFazio C. Naloxone versus nalbuphine infusion for prophylaxis of epidural morphine-induced pruritus. Anesth Analg. 1996;82:641–647. doi: 10.1097/00000539-199603000-00037. [DOI] [PubMed] [Google Scholar]
- Kjellberg F, Tramer MR. Pharmacological control of opioid-induced pruritus: a quantitative systematic review of randomized trials. Eur J Anaesthesiol. 2001;18:346–357. doi: 10.1046/j.0265-0215.2000.00826.x. [DOI] [PubMed] [Google Scholar]
- Ko MC. Inhibitory effects of dynorphin-A on spinally administered beta-endorphin- and GRP-induced itch scratching in nonhuman primates. The proceedings of the 7th World Congress on Itch; Boston, MA, USA. 2013. [Google Scholar]
- Ko MC, Husbands SM. Effects of atypical kappa-opioid receptor agonists on intrathecal morphine-induced itch and analgesia in primates. J Pharmacol Exp Ther. 2009;328:193–200. doi: 10.1124/jpet.108.143925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Naughton NN. An experimental itch model in monkeys: characterization of intrathecal morphine-induced scratching and antinociception. Anesthesiology. 2000;92:795–805. doi: 10.1097/00000542-200003000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Naughton NN. Antinociceptive effects of nociceptin/orphanin FQ administered intrathecally in monkeys. J Pain. 2009;10:509–516. doi: 10.1016/j.jpain.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Lee H, Harrison C, Clark MJ, Song HF, Naughton NN, Woods JH, Traynor JR. Studies of micro-, kappa-, and delta-opioid receptor density and G protein activation in the cortex and thalamus of monkeys. J Pharmacol Exp Ther. 2003a;306:179–186. doi: 10.1124/jpet.103.050625. [DOI] [PubMed] [Google Scholar]
- Ko MC, Lee H, Song MS, Sobczyk-Kojiro K, Mosberg HI, Kishioka S, Woods JH, Naughton NN. Activation of kappa-opioid receptors inhibits pruritus evoked by subcutaneous or intrathecal administration of morphine in monkeys. J Pharmacol Exp Ther. 2003b;305:173–179. doi: 10.1124/jpet.102.044909. [DOI] [PubMed] [Google Scholar]
- Ko MC, Song MS, Edwards T, Lee H, Naughton NN. The role of central mu opioid receptors in opioid-induced itch in primates. J Pharmacol Exp Ther. 2004;310:169–176. doi: 10.1124/jpet.103.061101. [DOI] [PubMed] [Google Scholar]
- Ko MC, Wei H, Woods JH, Kennedy RT. Effects of intrathecally administered nociceptin/orphanin FQ in monkeys: behavioral and mass spectrometric studies. J Pharmacol Exp Ther. 2006;318:1257–1264. doi: 10.1124/jpet.106.106120. [DOI] [PubMed] [Google Scholar]
- Ko MC, Woods JH, Fantegrossi WE, Galuska CM, Wichmann J, Prinssen EP. Behavioral effects of a synthetic agonist selective for nociceptin/orphanin FQ peptide receptors in monkeys. Neuropsychopharmacology. 2009;34:2088–2096. doi: 10.1038/npp.2009.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen AM, Valanne JV, Jokela RM, Ravaska P, Korttila K. Ondansetron does not prevent pruritus induced by low-dose intrathecal fentanyl. Acta Anaesthesiol Scand. 2003;47:1292–1297. doi: 10.1046/j.1399-6576.2003.00206.x. [DOI] [PubMed] [Google Scholar]
- Krajnik M, Zylicz Z. Understanding pruritus in systemic disease. J Pain Symptom Manage. 2001;21:151–168. doi: 10.1016/s0885-3924(00)00256-6. [DOI] [PubMed] [Google Scholar]
- Kumagai H, Ebata T, Takamori K, Muramatsu T, Nakamoto H, Suzuki H. Effect of a novel kappa-receptor agonist, nalfurafine hydrochloride, on severe itch in 337 haemodialysis patients: a Phase III, randomized, double-blind, placebo-controlled study. Nephrol Dial Transplant. 2010;25:1251–1257. doi: 10.1093/ndt/gfp588. [DOI] [PubMed] [Google Scholar]
- Kumagai H, Ebata T, Takamori K, Miyasato K, Muramatsu T, Nakamoto H, Kurihara M, Yanagita T, Suzuki H. Efficacy and safety of a novel k-agonist for managing intractable pruritus in dialysis patients. Am J Nephrol. 2012;36:175–183. doi: 10.1159/000341268. [DOI] [PubMed] [Google Scholar]
- Kumar K, Singh SI. Neuraxial opioid-induced pruritus: an update. J Anaesthesiol Clin Pharmacol. 2013;29:303–307. doi: 10.4103/0970-9185.117045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumor KM, Haertzen CA, Johnson RE, Kocher T, Jasinski D. Human psychopharmacology of ketocyclazocine as compared with cyclazocine, morphine and placebo. J Pharmacol Exp Ther. 1986;238:960–968. [PubMed] [Google Scholar]
- Kuraishi Y, Yamaguchi T, Miyamoto T. Itch-scratch responses induced by opioids through central mu opioid receptors in mice. J Biomed Sci. 2000;7:248–252. doi: 10.1007/BF02255473. [DOI] [PubMed] [Google Scholar]
- Laporte AM, Doyen C, Nevo IT, Chauveau J, Hauw JJ, Hamon M. Autoradiographic mapping of serotonin 5-HT1A, 5-HT1D, 5-HT2A and 5-HT3 receptors in the aged human spinal cord. J Chem Neuroanat. 1996;11:67–75. doi: 10.1016/0891-0618(96)00130-5. [DOI] [PubMed] [Google Scholar]
- Lawhorn CD, Brown RE., Jr Epidural morphine with butorphanol in pediatric patients. J Clin Anesth. 1994;6:91–94. doi: 10.1016/0952-8180(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Lawhorn CD, McNitt JD, Fibuch EE, Joyce JT, Leadley RJ., Jr Epidural morphine with butorphanol for postoperative analgesia after cesarean delivery. Anesth Analg. 1991;72:53–57. doi: 10.1213/00000539-199101000-00009. [DOI] [PubMed] [Google Scholar]
- Lawhorn CD, Boop FA, Brown RE, Jr, Andelman PD, Schmitz ML, Kymer PJ, Shirey R. Continuous epidural morphine/butorphanol infusion following selective dorsal rhizotomy in children. Childs Nerv Syst. 1995;11:621–624. doi: 10.1007/BF00300716. [DOI] [PubMed] [Google Scholar]
- Lee H, Naughton NN, Woods JH, Ko MC. Characterization of scratching responses in rats following centrally administered morphine or bombesin. Behav Pharmacol. 2003;14:501–508. doi: 10.1097/01.fbp.0000095082.80017.0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LH, Irwin MG, Lim J, Wong CK. The effect of celecoxib on intrathecal morphine-induced pruritus in patients undergoing caesarean section. Anaesthesia. 2004;59:876–880. doi: 10.1111/j.1365-2044.2004.03797.x. [DOI] [PubMed] [Google Scholar]
- Lee H, Naughton NN, Woods JH, Ko MC. Effects of butorphanol on morphine-induced itch and analgesia in primates. Anesthesiology. 2007;107:478–485. doi: 10.1097/01.anes.0000278876.20263.a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao CC, Chang CS, Tseng CH, Sheen MJ, Tsai SC, Chang YL, Wong SY. Efficacy of intramuscular nalbuphine versus diphenhydramine for the prevention of epidural morphine-induced pruritus after cesarean delivery. Chang Gung Med J. 2011;34:172–178. [PubMed] [Google Scholar]
- Lim GJ, Ishiuji Y, Dawn A, Harrison B, Kim do W, Atala A, Yosipovitch G. In vitro and in vivo characterization of a novel liposomal butorphanol formulation for treatment of pruritus. Acta Derm Venereol. 2008;88:327–330. doi: 10.2340/00015555-0480. [DOI] [PubMed] [Google Scholar]
- Lin AP, Ko MC. The therapeutic potential of nociceptin/orphanin FQ receptor agonists as analgesics without abuse liability. ACS Chem Neurosci. 2013;4:214–224. doi: 10.1021/cn300124f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XY, Liu ZC, Sun YG, Ross M, Kim S, Tsai FF, Li QF, Jeffry J, Kim JY, Loh HH, Chen ZF. Unidirectional cross-activation of GRPR by MOR1D uncouples itch and analgesia induced by opioids. Cell. 2011;147:447–458. doi: 10.1016/j.cell.2011.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon SB, Koltzenburg M. Itching for an explanation. Trends Neurosci. 1992;15:497–501. doi: 10.1016/0166-2236(92)90102-e. [DOI] [PubMed] [Google Scholar]
- Molinari S, Camarda V, Rizzi A, Marzola G, Salvadori S, Marzola E, Molinari P, McDonald J, Ko MC, Lambert DG, Calo G, Guerrini R. [Dmt1]N/OFQ(1–13)-NH2: a potent nociceptin/orphanin FQ and opioid receptor universal agonist. Br J Pharmacol. 2013;168:151–162. doi: 10.1111/j.1476-5381.2012.02115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan PJ, Mehta S, Kapala DM. Nalbuphine pretreatment in cesarean section patients receiving epidural morphine. Reg Anesth. 1991;16:84–88. [PubMed] [Google Scholar]
- Moser HR, Giesler GJ., Jr Itch and analgesia resulting from intrathecal application of morphine: contrasting effects on different populations of trigeminothalamic tract neurons. J Neurosci. 2013;33:6093–6101. doi: 10.1523/JNEUROSCI.0216-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield S, Braksator E, Rodriguez-Martin I, Bailey CP, Donaldson LF, Henderson G, Kelly E. C-terminal splice variants of the mu-opioid receptor: existence, distribution and functional characteristics. J Neurochem. 2008;104:937–945. doi: 10.1111/j.1471-4159.2007.05057.x. [DOI] [PubMed] [Google Scholar]
- Palmer CM, Emerson S, Volgoropolous D, Alves D. Dose–response relationship of intrathecal morphine for postcesarean analgesia. Anesthesiology. 1999;90:437–444. doi: 10.1097/00000542-199902000-00018. [DOI] [PubMed] [Google Scholar]
- Penning JP, Samson B, Baxter AD. Reversal of epidural morphine-induced respiratory depression and pruritus with nalbuphine. Can J Anaesth. 1988;35:599–604. doi: 10.1007/BF03020347. [DOI] [PubMed] [Google Scholar]
- Peters GR, Ward NJ, Antal EG, Lai PY, deMaar EW. Diuretic actions in man of a selective kappa opioid agonist: U-62,066E. J Pharmacol Exp Ther. 1987;240:128–131. [PubMed] [Google Scholar]
- Pirat A, Tuncay SF, Torgay A, Candan S, Arslan G. Ondansetron, orally disintegrating tablets versus intravenous injection for prevention of intrathecal morphine-induced nausea, vomiting, and pruritus in young males. Anesth Analg. 2005;101:1330–1336. doi: 10.1213/01.ANE.0000180830.12355.D9. [DOI] [PubMed] [Google Scholar]
- Preston KL, Jasinski DR. Abuse liability studies of opioid agonist-antagonists in humans. Drug Alcohol Depend. 1991;28:49–82. doi: 10.1016/0376-8716(91)90053-2. [DOI] [PubMed] [Google Scholar]
- Rawal N, Schott U, Dahlstrom B, Inturrisi CE, Tandon B, Sjostrand U, Wennhager M. Influence of naloxone infusion on analgesia and respiratory depression following epidural morphine. Anesthesiology. 1986;64:194–201. doi: 10.1097/00000542-198602000-00011. [DOI] [PubMed] [Google Scholar]
- Remmers AE, Clark MJ, Mansour A, Akil H, Woods JH, Medzihradsky F. Opioid efficacy in a C6 glioma cell line stably expressing the human kappa opioid receptor. J Pharmacol Exp Ther. 1999;288:827–833. [PubMed] [Google Scholar]
- Rosow CE, Moss J, Philbin DM, Savarese JJ. Histamine release during morphine and fentanyl anesthesia. Anesthesiology. 1982;56:93–96. doi: 10.1097/00000542-198202000-00003. [DOI] [PubMed] [Google Scholar]
- Ross SE, Mardinly AR, McCord AE, Zurawski J, Cohen S, Jung C, Hu L, Mok SI, Shah A, Savner EM, Tolias C, Corfas R, Chen S, Inquimbert P, Xu Y, McInnes RR, Rice FL, Corfas G, Ma Q, Woolf CJ, Greenberg ME. Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron. 2010;65:886–898. doi: 10.1016/j.neuron.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Fukano T, Sumikawa K. IV butorphanol reduces analgesia but not pruritus or nausea associated with intrathecal morphine. Can J Anaesth. 2001;48:831–832. doi: 10.1007/BF03016714. [DOI] [PubMed] [Google Scholar]
- Sarvela PJ, Halonen PM, Soikkeli AI, Kainu JP, Korttila KT. Ondansetron and tropisetron do not prevent intraspinal morphine- and fentanyl-induced pruritus in elective cesarean delivery. Acta Anaesthesiol Scand. 2006;50:239–244. doi: 10.1111/j.1399-6576.2006.00934.x. [DOI] [PubMed] [Google Scholar]
- Schröder W, Lambert DG, Ko MC, Koch T. Functional plasticity of the N/OFQ-NOP receptor system determines analgesic properties of NOP receptor agonists. Br J Pharmacol. 2014;171:3777–3800. doi: 10.1111/bph.12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone DA, Zhang X, Li J, Zhang JM, Honda CN, LaMotte RH, Giesler GJ., Jr Comparison of responses of primate spinothalamic tract neurons to pruritic and algogenic stimuli. J Neurophysiol. 2004;91:213–222. doi: 10.1152/jn.00527.2003. [DOI] [PubMed] [Google Scholar]
- Somrat C, Oranuch K, Ketchada U, Siriprapa S, Thipawan R. Optimal dose of nalbuphine for treatment of intrathecal-morphine induced pruritus after caesarean section. J Obstet Gynaecol Res. 1999;25:209–213. doi: 10.1111/j.1447-0756.1999.tb01149.x. [DOI] [PubMed] [Google Scholar]
- Sukhtankar DD, Ko MC. Physiological function of gastrin-releasing peptide and neuromedin B receptors in regulating itch scratching behavior in the spinal cord of mice. PLoS One. 2013;8:e67422. doi: 10.1371/journal.pone.0067422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhtankar DD, Lee H, Rice KC, Ko MC. Differential effects of opioid-related ligands and NSAIDs in nonhuman primate models of acute and inflammatory pain. Psychopharmacology. 2014;231:1377–1387. doi: 10.1007/s00213-013-3341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szarvas S, Harmon D, Murphy D. Neuraxial opioid-induced pruritus: a review. J Clin Anesth. 2003;15:234–239. doi: 10.1016/s0952-8180(02)00501-9. [DOI] [PubMed] [Google Scholar]
- Tamdee D, Charuluxananan S, Punjasawadwong Y, Tawichasri C, Patumanond J, Sriprajittichai P. A randomized controlled trial of pentazocine versus ondansetron for the treatment of intrathecal morphine-induced pruritus in patients undergoing cesarean delivery. Anesth Analg. 2009;109:1606–1611. doi: 10.1213/ANE.0b013e3181b72e93. [DOI] [PubMed] [Google Scholar]
- Thomas DA, Williams GM, Iwata K, Kenshalo DR, Jr, Dubner R. Effects of central administration of opioids on facial scratching in monkeys. Brain Res. 1992;585:315–317. doi: 10.1016/0006-8993(92)91227-6. [DOI] [PubMed] [Google Scholar]
- To TH, Clark K, Lam L, Shelby-James T, Currow DC. The role of ondansetron in the management of cholestatic or uremic pruritus–a systematic review. J Pain Symptom Manage. 2012;44:725–730. doi: 10.1016/j.jpainsymman.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Togashi Y, Umeuchi H, Okano K, Ando N, Yoshizawa Y, Honda T, Kawamura K, Endoh T, Utsumi J, Kamei J, Tanaka T, Nagase H. Antipruritic activity of the kappa-opioid receptor agonist, TRK-820. Eur J Pharmacol. 2002;435:259–264. doi: 10.1016/s0014-2999(01)01588-6. [DOI] [PubMed] [Google Scholar]
- Tzeng JI, Chu KS, Ho ST, Cheng KI, Liu KS, Wang JJ. Prophylactic iv ondansetron reduces nausea, vomiting and pruritus following epidural morphine for postoperative pain control. Can J Anaesth. 2003;50:1023–1026. doi: 10.1007/BF03018366. [DOI] [PubMed] [Google Scholar]
- Vivian JA, DeYoung MB, Sumpter TL, Traynor JR, Lewis JW, Woods JH. kappa-Opioid receptor effects of butorphanol in rhesus monkeys. J Pharmacol Exp Ther. 1999;290:259–265. [PubMed] [Google Scholar]
- Waeber C, Dixon K, Hoyer D, Palacios JM. Localisation by autoradiography of neuronal 5-HT3 receptors in the mouse CNS. Eur J Pharmacol. 1988;151:351–352. doi: 10.1016/0014-2999(88)90825-4. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Strain EC, Abreu ME, Bigelow GE. Enadoline, a selective kappa opioid agonist: comparison with butorphanol and hydromorphone in humans. Psychopharmacology (Berl) 2001;157:151–162. doi: 10.1007/s002130100788. [DOI] [PubMed] [Google Scholar]
- Wang JK, Nauss LA, Thomas JE. Pain relief by intrathecally applied morphine in man. Anesthesiology. 1979;50:149–151. doi: 10.1097/00000542-197902000-00013. [DOI] [PubMed] [Google Scholar]
- Wang JJ, Ho ST, Tzeng JI. Comparison of intravenous nalbuphine infusion versus naloxone in the prevention of epidural morphine-related side effects. Reg Anesth Pain Med. 1998;23:479–484. doi: 10.1016/s1098-7339(98)90031-1. [DOI] [PubMed] [Google Scholar]
- Wang Y, Tang K, Inan S, Siebert D, Holzgrabe U, Lee DY, Huang P, Li JG, Cowan A, Liu-Chen LY. Comparison of pharmacological activities of three distinct kappa ligands (Salvinorin A, TRK-820 and 3FLB) on kappa opioid receptors in vitro and their antipruritic and antinociceptive activities in vivo. J Pharmacol Exp Ther. 2005;312:220–230. doi: 10.1124/jpet.104.073668. [DOI] [PubMed] [Google Scholar]
- Waxler B, Mondragon SA, Patel SN, Nedumgottil K. Prophylactic ondansetron does not reduce the incidence of itching induced by intrathecal sufentanil. Can J Anaesth. 2004;51:685–689. doi: 10.1007/BF03018426. [DOI] [PubMed] [Google Scholar]
- Waxler B, Dadabhoy ZP, Stojiljkovic L, Rabito SF. Primer of postoperative pruritus for anesthesiologists. Anesthesiology. 2005;103:168–178. doi: 10.1097/00000542-200507000-00025. [DOI] [PubMed] [Google Scholar]
- Weiskopf RB, Reid IA, Fisher DM, Holmes MA, Rosen JI, Keil LC. Effects of fentanyl on vasopressin secretion in human subjects. J Pharmacol Exp Ther. 1987;242:970–973. [PubMed] [Google Scholar]
- Wells J, Paech MJ, Evans SF. Intrathecal fentanyl-induced pruritus during labour: the effect of prophylactic ondansetron. Int J Obstet Anesth. 2004;13:35–39. doi: 10.1016/j.ijoa.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Wittels B, Glosten B, Faure EA, Moawad AH, Ismail M, Hibbard J, Amundsen L, Binstock W, Senal JA, Cox SM, et al. Opioid antagonist adjuncts to epidural morphine for postcesarean analgesia: maternal outcomes. Anesth Analg. 1993;77:925–932. doi: 10.1213/00000539-199311000-00009. [DOI] [PubMed] [Google Scholar]
- Wu Z, Kong M, Wang N, Finlayson RJ, De Tran QH. Intravenous butorphanol administration reduces intrathecal morphine-induced pruritus after cesarean delivery: a randomized, double-blind, placebo-controlled study. J Anesth. 2012;26:752–757. doi: 10.1007/s00540-012-1421-7. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA. Analgesia mediated by a direct spinal action of narcotics. Science. 1976;192:1357–1358. doi: 10.1126/science.1273597. [DOI] [PubMed] [Google Scholar]
- Yazigi A, Chalhoub V, Madi-Jebara S, Haddad F, Hayek G. Prophylactic ondansetron is effective in the treatment of nausea and vomiting but not on pruritus after cesarean delivery with intrathecal sufentanil-morphine. J Clin Anesth. 2002;14:183–186. doi: 10.1016/s0952-8180(01)00381-6. [DOI] [PubMed] [Google Scholar]
- Yeh HM, Chen LK, Lin CJ, Chan WH, Chen YP, Lin CS, Sun WZ, Wang MJ, Tsai SK. Prophylactic intravenous ondansetron reduces the incidence of intrathecal morphine-induced pruritus in patients undergoing cesarean delivery. Anesth Analg. 2000;91:172–175. doi: 10.1097/00000539-200007000-00032. [DOI] [PubMed] [Google Scholar]
- Zhu J, Luo LY, Li JG, Chen C, Liu-Chen LY. Activation of the cloned human kappa opioid receptor by agonists enhances [35S]GTPgammaS binding to membranes: determination of potencies and efficacies of ligands. J Pharmacol Exp Ther. 1997;282:676–684. [PubMed] [Google Scholar]