Abstract
PURPOSE
To evaluate differences in T2 values in femoral and tibial cartilage at magnetic resonance (MR) imaging in patients with varying degrees of osteoarthritis (OA) compared with healthy subjects and to develop a mapping and display method based on calculation of T2 z scores for visual grading and assessment of cartilage heterogeneity in patients with OA.
MATERIALS AND METHODS
Knee cartilage was evaluated in 55 subjects who were categorized with radiography as healthy (n=7) or as having mild OA (n=20) or severe OA (n=28). Cartilage regions were determined with manual segmentation of an MR image acquired with spoiled gradients and fat suppression. The segmentation was applied to a map of T2 relaxation time and was analyzed in four knee cartilage compartments (ie, the medial and lateral tibia and femur). Differences between cartilage compartment T2 values and subject groups were analyzed with analysis of covariance. Correlations of cartilage T2 values with clinically reported symptoms and cartilage thickness and volume were examined. Cartilage T2 values were converted to z scores per voxel on the basis of normal population values in the same cartilage compartment to better interpret cartilage heterogeneity and variation from normal.
RESULTS
Healthy subjects had mean T2 values of 32.1–35.0 msec, while patients with mild and severe OA had mean T2 values of 34.4–41.0 msec. All cartilage compartments except the lateral tibia showed significant (P< .05) increases in T2 relaxation time between healthy and diseased knees; however, no significant difference was found between patients with mild and severe OA. Correlation of T2 values with clinical symptoms and cartilage morphology was found predominantly in medial compartments.
CONCLUSION
Femoral and medial tibial cartilage T2 values increase with the severity of OA.
Index terms: Cartilage, MR, 452.121411, Knee, arthritis, 452.7, Knee, MR, 452.121411, Magnetic resonance (MR), tissue characterization, 452.121411
Osteoarthritis (OA) is an important health concern, as joint disease is the single largest cause of disability in elderly persons (1). Between 13% and 17% of Americans aged 55–74 years have pain and functional problems related to knee OA (2), and estimated economic costs were $12.2 billion dollars in 1994 (3).
Quantitative measures of T2 relaxation times may be useful in the characterization and long-term tracking of OA. Previous reports have demonstrated spatial variation of T2 relaxation times in cartilage explants (4), healthy subjects (5), and substantial changes with age (6), but we are unaware of reports that present T2 relaxation time variation with OA in humans. Cartilage tissue analysis has shown increased water content in tissue that has been degraded through OA processes (7) and decreased glycosaminoglycan concentration and proteoglycan size in diseased tissue (8,9). These findings support those of previous studies (10) that early cartilage degeneration due to collagen damage and changes in collagen content and arrangement will increase water mobility in the tissue, thus increasing T2 relaxation time. Thus, the purpose of our study was to evaluate differences in femoral and tibial cartilage T2 relaxation times in healthy subjects and patients with varying degrees of OA and to develop a mapping and display method based on calculating T2 relaxation time z scores for visual grading and assessment of cartilage heterogeneity in patients with OA.
MATERIALS AND METHODS
Patients and Status
Unilateral knee magnetic resonance (MR) imaging was performed from mid-1999 to late 2001 in 59 patients in whom OA was clinically suspected. Of these patients, four had gross motion between the MR imaging series and were unable to be analyzed, which left 55 patients with a mean age of 61 years. After being told about the procedure, which was approved by our institutional review board, all participants granted informed consent. Patients were evaluated at the orthopedic clinic. MR images and standard radiographs of patients were obtained in conjunction with another study that involved the same patients (11). The clinically evaluated knee was selected for imaging.
Two radiologists (one with 10 years experience, the other with 20 years experience) worked in consensus and used the Kellgren-Lawrence scoring system (12,13) to grade OA with standing anteroposterior radiographs. The radiologists were blinded to patient information during evaluation. The Kellgren-Lawrence scoring system has five categories. A score of 0 (none) is given if no osteoarthritic features are present. A score of 1 (doubtful) indicates minute osteophytes of doubtful importance. A score of 2 (minimal) indicates definite osteophytes without reduction of the joint space. A score of 3 (moderate) is given when the joint space has diminished. A score of 4 (severe) is given for greatly reduced joint space and sclerosis of the subchondral bone.
In our study, subjects with a score of 0 were grouped in the healthy population, while those with a score of 1 or 2 were categorized as having mild OA. Subjects with a score of 3 or 4 were classified as having severe OA. Seven subjects were graded as healthy (mean age, 38 years; range, 22–70 years) and included three women (mean age, 34 years; range, 22–47 years) and four men (mean age, 41 years; range, 23–70 years). Twenty patients were classified as having mild OA (mean age, 63 years; range, 46–81 years) and included 13 women (mean age, 65 years; range, 50–78 years) and seven men (mean age, 56 years; range, 46–81 years). Twenty-eight patients were classified as having severe OA (mean age, 67 years; range, 43–88 years) and included 14 women (mean age, 70 years; range, 46–88 years) and 14 men (mean age, 64 years; range, 43–78 years).
We used the Western Ontario and Mc-Master University (WOMAC) osteoarthrosis index to determine function, quality of life, and joint pain (14). This arthrosis impact scale has been used in studies of patients with knee OA to determine both the effect of different therapies for treatment of pain and the relationship between disease severity and clinical importance of symptoms. Extensive validation of the WOMAC index has been performed with regard to the measures of function and quality of life in patients with OA (14).
An orthopedic surgeon (M.D.R.) interviewed all participants about the amount of knee pain and stiffness they were experiencing. Patients were also asked questions in an effort to assess their ability to complete everyday tasks such as ascending stairs, standing, walking, rising from bed, or going shopping. The responses were used to determine the overall WOMAC score on a scale that ranges from 0 (no pain or stiffness) to 500 (extreme pain, stiffness, and impaired function).
Imaging and Evaluation
Imaging was performed with a Signa 1.5-T echo-speed system (GE Medical Systems, Milwaukee, Wis) with a dual phasedarray coil (USA Instruments, Cleveland, Ohio). A custom leg holder, with the knee in approximately 20° of flexion, was used to minimize motion and position the coil. A two-dimensional dual-echo spin-echo sequence was used (repetition time msec/echo times msec, 1,500/10 and 45; voxel size, 0.468 × 0.468 × 4 mm; examination time, 5 minutes 24 seconds; field of view, 12 cm; matrix, 256 × 256) to generate a sagittal T2 relaxation time map with custom software (IDL; Research Systems, Boulder, Colo), assuming a single exponential decay component. Spoiled gradient-echo images with fat suppression were acquired by using the spectral inversion at lipids, or SPECIAL, technique (15) (repetition time msec/echo time msec/inversion time msec, 30/3.3/8; flip angle, 40°; voxel size, 0.234 × 0.234 × 2 mm; examination time, 9 minutes 31 seconds, field of view, 12 cm; matrix, 512 × 512) along with the T2 relaxation time maps.
Because of the inhomogeneous reception of the surface coils, the signal intensity throughout the sections is inhomogeneous. Thus, all images are subjected to a preprocessing step with a three-dimensional low-pass filter-based correction algorithm that will normalize voxel intensity through the knee volume but will not affect the calculation of T2 values, as verified by the results of experiments that are not presented herein. In those experiments, the coefficient of variation was found to be less than 2% when comparing T2 values from regions of interest with and without the low-pass filter correction. The cartilage was manually segmented from the spoiled gradient-echo images by using custom graphic user interface programs that were developed with Matlab (Mathworks, Natick, Mass). Two research staff scientists at our institution, both with 2 years of experience, placed points on the image that were an average of 8–10 pixels (approximately 2 mm) apart and served as knots for a two-dimensional spline that defined the outline of the cartilage boundary, as shown in Figure 1. The boundary defined the outer limits of a binary mask, a matrix in which elements corresponding to cartilage have a value of one and otherwise have a value of zero. The mask was down-sampled in two dimensions by a factor of two, which halved the number of in-plane pixels in the image in both dimensions. Also, the union of adjacent sections was created. In this way, the voxel sizes of the cartilage map and the T2 map were made equal. The mask was multiplied by the corresponding matrix element with the T2 map. Thickness measurements were made with custom software routines (Matlab; Mathworks) on sagittal cartilage images by using the diameters of the largest circle possible that could fit inside the cartilage mask with the center of the circles along the midline axis (16). The diameters ranged in size from 2 to 30 pixels (approximately 0.4–6.0 mm). The mean cartilage thickness for each section was the mean value of the circle diameters, while the thickness for each compartment was the average thickness of all sections. Cartilage volumes for the femur and tibia were computed from the segmented cartilage compartments. The analysis focused on the weight-bearing surfaces; therefore, patellar cartilage was not segmented for analysis.
Figure 1.
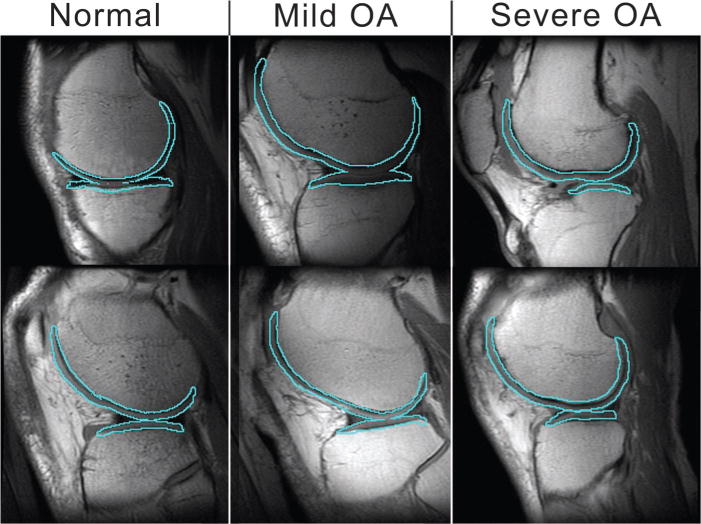
Example of registration between the T2-weighted MR image and the cartilage mask in six subjects (ie, two from each group). Sagittal view of femoral and tibial compartments outlined over the first echo of the dual two-dimensional spin-echo MR image (1,500/10 and 45; voxel size, 0.468 × 0.468 × 4 mm; examination time, 5 minutes 24 seconds; field of view, 12 cm; matrix, 256 × 256). Note that these are only single sections of the entire knee volume and may not reflect specific regions of disease that account for OA grade.
The cartilage compartments were determined on the basis of anatomic landmarks and were classified as the medial and lateral tibial and medial and lateral femoral compartments. The tibial cartilage compartments are separated by a gap of noncartilaginous material in the medial to lateral direction where the cruciate ligaments attach. Since cartilage is continuous over the end of the femur, the interior aspects of the tibial compartments were used to define the interior aspects of the two femoral cartilage compartments. Thus, the central region of the femoral cartilage that articulates with the patella is not considered in this analysis. Two research staff scientists (with 2 and 3 years experience) at our institution, including one author (T.C.D.), wrote the software and conducted the image processing.
Statistical Analysis
All statistical processing was performed with SAS version 8.2 software (SAS Institute, Cary, NC). First, we summarized and compared patient characteristics in the three groups. We used the analysis of variance to test for significant age differences and the Fisher exact test to compare the sex difference.
Several authors (T.C.D., Y.L., H.J., S.M.) analyzed in consensus the distribution of T2 values in each cartilage compartment of each subject. T2 values of more than 200 msec were considered outliers, occurred infrequently (usually <1% of voxels in the compartment), and were excluded from analysis because they were more than double the highest expected mean T2 value of healthy cartilage (5,17). The differences in mean T2 values for each disease group for each cartilage compartment were compared with the analysis of covariance. To account for the small differences in standard error of the estimated mean for each group, we used the weight of inverse variance of the estimated mean (1 divided by the squared standard error) for each patient and performed a weighted analysis of covariance.
Because age was significantly different among groups, we adjusted for this factor in our analysis of covariance models. Tukey-Kramer tests were used to compare pairwise differences in mean T2 values between groups. Residuals of the analysis of covariance models were also examined for normal distribution by using the Kolmogorov-Smirov test, and none were significantly different from normal values. Least-squares means and standard errors were used as summary statistics for each group.
The relations between mean T2 of cartilage with WOMAC scores, cartilage thickness, and cartilage volume were evaluated with the Spearman correlation coefficient. In addition to the relation of the T2 value to volume and thickness of each compartment, cartilage volume and thickness relations were evaluated for the opposing compartment. For example, the medial femoral cartilage is the opposing volume of the medial tibial cartilage.
Per-pixel and per-compartment z scores were generated by using the mean and standard deviation of T2 values from the healthy subjects in each compartment with the following equation:
where Voxeli is the T2 value in the voxel of interest, Meanhealthy is the mean T2 value for all voxels of healthy knees in the same compartment (medial or lateral tibia or femur), and SDhealthy is the standard deviation of the same T2 distribution. Image generation and data reduction were performed with custom software developed with Matlab (Mathworks). The z score conversion normalizes the results on the basis of the difference from normal values based on the size of the variation of the healthy group. In this way, variations between cartilage compartments can be removed and compared with a common standard.
RESULTS
A summary of the population of the study groups by age and sex is presented in Table 1. Age was significantly different between groups but sex was not; therefore, we only adjusted for age differences in our analysis of covariance.
TABLE 1.
Summary of the Population by Age and Sex
| Characteristic | Healthy Subjects | Patients with OA
|
P Value | |
|---|---|---|---|---|
| Mild OA | Severe OA | |||
| No. of patients | 7 | 20 | 28 | NA* |
| Age (y)† | 37.9 ± 4.5 | 62.9 ± 2.6 | 66.7 ± 2.3 | <.001‡ |
| Male sex§ | 4 (57) | 7 (33) | 14 (50) | 0.481‖ |
NA = not applicable.
Data are mean ± standard deviation.
Calculated with analysis of variance.
Data in parentheses are percentages.
Calculated with the Fisher exact test.
An example distribution of T2 values in the medial femur compartment in a 76-year-old man with severe OA is shown in Figure 2. The mean value of the distribution is used to represent the T2 value in the compartment.
Figure 2.
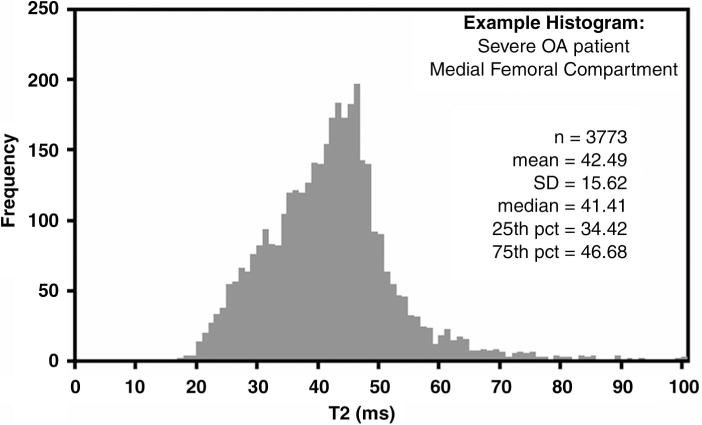
Graph shows distribution of T2 values in the medial femur cartilage compartment in 76-year-old man with severe OA. pct percentile.
The T2 values of cartilage were found to increase significantly (P < .05) between the healthy subjects and the patients with OA for all cartilage compartments except that of the lateral tibia; however, no significant difference was found between the patients with mild OA and those with severe OA (Table 2). In healthy subjects, the least squares mean T2 values ± 1 standard error were 32.1 msec ± 1.4, 34.9 msec ± 1.8, 34.9 msec ± 1.0, and 35.0 msec ± 1.1 in the medial tibial, lateral tibial, medial femoral, and lateral femoral cartilage compartments, respectively. These values increased significantly (P < .05) in the patients with mild OA and those with severe OA for all compartments except the lateral tibia. In patients with mild OA, the least squares mean T2 values ± 1 standard error were 34.7 msec ± 0.9, 34.4 msec ± 0.8, 39.2 msec ± 0.7, and 39.7 msec ± 0.7, for the medial tibial, lateral tibial, medial femoral, and lateral femoral cartilage compartments, respectively. In patients with severe OA, the least squares mean T2 values ± 1 standard error were 36.0 msec ± 0.7, 34.5 msec ± 0.8, 41.0 msec ± 0.7, and 39.1 msec ± 0.6 in the medial tibial, lateral tibial, medial femoral, and lateral femoral cartilage compartments, respectively. The differences between the least squares mean T2 values of patients with mild OA and those with severe OA were not significant for any cartilage compartment. Table 3 shows that in patients with severe OA, the per-compartment z scores are greater than 1.3 in all cartilage compartments, with the exception of the lateral tibial cartilage compartment.
TABLE 2.
Comparison of Cartilage Compartment T2 Values*
| A: Comparison of Cartilage Compartment T2 Values*
| ||||
|---|---|---|---|---|
| OA Group | Lateral Femur | Lateral Tibia | Medial Femur | Medial Tibia |
| Healthy subjects | 35.0 ± 1.1 | 34.9 ± 1.8 | 34.9 ± 1.0 | 32.1 ± 1.4 |
| Patients with mild OA | 39.7 ± 0.7 | 34.4 ± 0.8 | 39.2 ± 0.7 | 34.7 ± 0.9 |
| Patients with severe OA | 39.1 ± 0.6 | 34.5 ± 0.8 | 41.0 ± 0.7 | 36.0 ± 0.7 |
| B: Comparison of Cartilage Compartment P Values†
| ||||
|---|---|---|---|---|
| Comparison | Lateral Femur | Lateral Tibia | Medial Femur | Medial Tibia |
| Healthy subjects and patients with mild OA | .002 | .97 | .003 | .25 |
| Healthy subjects and patients with severe OA | .004 | .99 | <.001 | .037 |
| Patients with mild OA and patients with severe OA | .73 | .99 | .18 | .48 |
| Overall | .002 | .97 | <.001 | .044 |
Data are the mean ± standard error in milliseconds.
Data are P values.
TABLE 3.
Mean z Score Values for Cartilage Compartments
| OA Group | Lateral Femur | Lateral Tibia | Medial Femur | Medial Tibia |
|---|---|---|---|---|
| Healthy subjects | 0.00 | 0.00 | 0.00 | 0.00 |
| Patients with mild OA | 1.63 | −0.19 | 1.34 | 0.76 |
| Patients with severe OA | 1.62 | 0.17 | 1.88 | 1.33 |
A significant (P < .05) positive correlation was found between mean cartilage T2 values and WOMAC function assessment for all compartments except the lateral tibia (Table 4). There was a significant (P < .05) positive correlation between the medial cartilage compartments and WOMAC pain scores, but no significant correlation was found between mean T2 values and WOMAC stiffness scores.
TABLE 4.
Correlation between Cartilage T2 and WOMAC Score
| Cartilage Compartment | WOMAC Score
|
||||||
|---|---|---|---|---|---|---|---|
| Function | Pain | Stiffness | Cartilage Volume | Cartilage Thickness | Opposing Volume* | Opposing Thickness† | |
| Medial femur | |||||||
| R value | 0.35 | 0.32 | NA | −0.30 | −0.39 | −0.33 | −0.36 |
| P value | .013 | .027 | >.05 | .027 | .004 | .013 | .007 |
| Medial tibia | |||||||
| R value | 0.29 | 0.40 | NA | −0.38 | −0.53 | −0.45 | −0.48 |
| P value | .042 | .005 | >.05 | .004 | >.001 | >.001 | >.001 |
| Lateral femur | |||||||
| R value | 0.31 | NA | NA | NA | NA | NA | 0.35 |
| P value | .028 | >.05 | >.05 | >.05 | >.05 | >.05 | .009 |
| Lateral tibia | |||||||
| R value | NA | NA | NA | NA | −0.43 | NA | NA |
| P value | >.05 | >.05 | >.05 | >.05 | .001 | >.05 | >.05 |
Note.—NA not applicable.
Opposing volume is the volume of tibial cartilage for femoral cartilage and the volume of femoral cartilage for tibial cartilage.
Opposing thickness is the volume of tibial cartilage for femoral cartilage and the thickness of femoral cartilage for tibial cartilage.
The mean cartilage T2 value was found to have a significant negative correlation with cartilage thickness and volume in the medial cartilage compartments. The Spearman correlation values between T2 values and cartilage volume and thickness were all less than −0.30, and the P value was less than .027. The correlation results for the lateral compartments were neither consistent nor significant in all but two relationships; these two relationships were the thickness of the lateral tibia with T2 values of the lateral tibia and femur.
Figure 3 shows cartilage viewed as a z score image. Each cartilage compartment is referenced to the normal values for the compartment in the conversion; areas of yellow indicate increases in T2 value.
Figure 3.
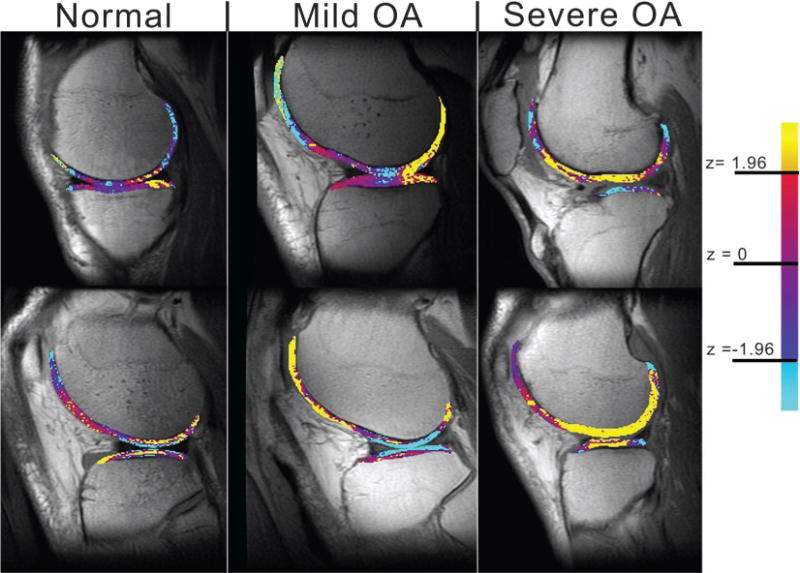
Representative z score conversion sagittal MR images obtained in six subjects (ie, two from each group) (1,500/10 and 45; voxel size, 0.468 × 0.468 × 4 mm; examination time, 5 minutes 24 seconds; field of view, 12 cm; matrix, 256 × 256). Note the increase in area of regions of high z scores (indicated by yellow areas) in patients with mild and severe OA. Note that these are only single sections of the entire knee volume and may not reflect specific regions of disease that account for OA grade.
DISCUSSION
Articular cartilage consists of a porous matrix swollen by water (18), with the bulk of the matrix consisting of type II collagen and proteoglycans. The collagen has a specific arrangement in the tissue, beginning at the surface as a zone of tangentially aligned fibril sheets. Below this is a transitional zone of randomly aligned fibrils, followed by a deep radial zone with fibrils aligned perpendicular to the articular surface. The proteoglycans have many glycosaminoglycan side chains, with a high negative charge that provides the driving gradient to swell the cartilage with water. Previous reports have documented the decrease of proteoglycan size and glycosaminoglycan content with aging and disease (8), which in turn increases the water content and mobility in the cartilage.
The complex structure of cartilage provides its unique mechanical and imaging properties. The presence of a high concentration of proteoglycans that are immobilized in the collagen network of cartilage creates a large osmotic pressure that draws water into the tissue, which provides resistance to compressive loads (1). Previous descriptions of MR images of damaged cartilage have alluded to the apparent increase in T2 values because of an increase in the relative water content and a decrease of T2-shortening effects of the matrix on the water (19). Moreover, these effects could be seen at the earliest stages of degradation, before any thinning of the matrix occurred (19).
It is possible that the underlying cartilage changes that lead to a higher T2 value similarly occur early in the development of the disease and do not progress as much as mechanical degradation of the cartilage. Thus, defining disease groups on the basis of the Kellegren-Lawrence grading system, which is heavily influenced by bulk cartilage changes, may not be sensitive to cartilage changes that affect T2 relaxation time; hence, very little difference is seen between patients with mild OA and those with severe OA. Furthermore, the Kellgren-Lawrence grading system has some recognized limitations (2); in particular, an emphasis is placed on the presence of osteophytes, which we would not expect to alter the mean T2 value in the cartilage compartment.
Buckwalter and Mankin (20) have reviewed the mechanism of cartilage degradation in detail, and they describe the initial stage of OA changes to increase water mobility in the cartilage. The longer T2 values found with OA agree with this mechanism. Furthermore, Lusse et al (10) have demonstrated a positive correlation between increases in T2 relaxation time and cartilage water content in ex vivo cartilage from patients with OA who are undergoing total knee replacement surgery.
To our knowledge, previous studies of cartilage T2 values in patients with OA have not been performed; however, Mosher et al (6) have shown a significant increase in T2 values with aging. Unfortunately, in our study, age matching was not achieved between healthy subjects, patients with mild OA, and patients with severe OA. Thus, age-related changes in cartilage T2 values may confound OA-related changes in cartilage T2 values. We accounted for this possibility by using the analysis of covariance, with age as a confounding factor.
The changes in cartilage T2 values with OA severity are supported by significant negative correlation with cartilage compartment volume and thickness in the medial region. Thus, the cartilage degradation is reflected in higher T2 values. In our study, however, we evaluated two-dimensional thickness in the sagittal plane, and we did not correct for out-of-plane curvature of cartilage and bone. Clinical indicators of disease progression, as evaluated with WOMAC function and pain assessments, appear to correlate with increased cartilage T2 values, particularly in the medial compartments.
The correlation of bone marrow edema and pain or disease severity has been evaluated (11). In contrast to the present finding of a correlation of WOMAC function and pain scores with increased T2 relaxation times, bone marrow edema did not correlate with any of the WOMAC indexes.
Care needs to be taken when interpreting T2 values of articular cartilage. Good-win and Dunn (21) have described how T2 values will vary with depth from the articular surface because of collagen fibril orientation to the constant magnetic induction field. Imaging of the femoral chondyles can be problematic, because the bulk curvature of the cartilage alters the depth-dependent fibril orientation. A comprehensive in vivo study, however, has shown that the magic angle effect may not be the major determinant of T2 heterogeneity in high-curvature articular cartilage (17). The effect of curvature on the T2 value in cartilage is an area of ongoing research; therefore, we recognize that the location of focal defects could alter the mean T2 value determined with our method and lead to greater uncertainty in the determination of T2 values. Further characterization of T2 values around cartilage lesions is an area of future work.
While we found significant differences between T2 values in healthy and OA cartilage in three of the four cartilage compartments analyzed, we did not find significant differences between the patients with mild OA and those with severe OA. We did note trends of increased T2 values in the severe OA group compared to the mild OA group in the medial compartments, which typically experience higher weight bearing and are more likely to develop OA (22); however, the number of patients in these groups may not have been large enough to allow us to detect a significant difference.
Previous investigators have used more than two echoes to determine the T2 value; however, we chose this technique because of a number of limiting factors. The current study was part of a longitudinal study (11); therefore, imaging duration was an important limitation, because multiple imaging series were performed, and the entire examination was limited to 1½ hours. Furthermore, due to the variable and focal nature of OA, complete coverage of the knee compartment was a primary requirement. Finally, the original examinations were performed in 1999, when the MR imaging hardware was limited in gradient speed and power; thus, obtaining a T2-weighted image with more than two echoes would increase the imaging time by at least a factor of two if complete knee coverage was to be maintained. Over the course of the study, updates to the MR imaging hardware were made that allowed for faster acquisition of multiple echo time images, but changes were not made to the acquisition parameters to maintain consistency for all examinations. Had hardware and imaging duration not prevented the implementation of a sequence with multiple echo times for this imaging, we may have had better precision in the measured T2 values, and differences between healthy and OA cartilage may have differed in magnitude. The model developed by MacFall et al (23) supports the decrease in variance of the measured T2 relaxation time with an increased number of echoes.
It is proposed that viewing T2 values normalized to the expected normal values could aid in the assessment of tissue heterogeneity and tracking of OA progression. Further quantification of the variation of T2 values through the cartilage volume could enhance the information available to assess disease progression. For example, Mosher et al (6) and Dardzinski et al (5) have shown the utility of line profiles—viewing intensity values as a function of distance along a specific line—of T2 values from the subchondral region to the articulating surface. MR signal heterogeneity across the thickness of cartilage through the major histologic zones (tangential, transitional, and deep radial) has been shown previously (24). In the present study, we attempted to use a z score conversion per pixel to allow direct visualization of the T2 heterogeneity present in the cartilage by highlighting differences from T2 values of healthy cartilage.
Future work could lead to use of this technique for better visualization of variation through the cartilage thickness and in weight-bearing regions. Distinct depiction of the chondral layers, however, was difficult and inconsistent in the present study. This was likely due to partial volume effects from insufficient spatial resolution that obscured the chondral layers. As opposed to the study of Frank et al (25), no custom imaging hardware (ie, a local gradient coil) was used to enhance the signal-to-noise ratio to allow clear demonstration of the cartilage layering due to collagen fibril orientation. Analyzing T2 relaxation time in weight-bearing regions on the basis of margins of the meniscus could prove useful; however, this was not attempted in the present study because we were unable to define the meniscus in several patients with severe meniscal damage.
The z score images of T2 values based on normal values for each cartilage compartment may be a useful indicator of disease, particularly for focal areas of T2 changes. High z scores were observed both in patients with mild OA and in patients with severe OA in the medial and lateral femoral compartments, which are areas of maximal mechanical loading, indicating that T2 relaxation time changes at early stages of OA. Viewing a z score conversion of the T2 values could enable better differentiation from healthy tissue when tracking or diagnosing OA. Studies of the longitudinal progression of OA are recommended, and T2 values and z scores may provide a measure of temporal disease development.
Acknowledgments
The authors acknowledge Srinka Ghosh, PhD, Katherine Hall, MS, Colleen Lindsey, BS, Thomas M. Link, MD, Anita Narasimhan, MS, and Lynne Steinbach, MD, for their substantial contribution to the material discussed in this article.
Supported by NIH grant R01 AR46905.
Abbreviations
- OA
osteoarthritis
- WOMAC
Western Ontario and McMaster University
Footnotes
Author contributions:
Guarantors of integrity of entire study, S.M., T.C.D.; study concepts and design, S.M., T.C.D.; literature research, T.C.D.; experimental studies, S.M.; data acquisition, S.M.; data analysis/interpretation, all authors; statistical analysis, Y.L., H.J.; manuscript preparation, T.C.D.; manuscript definition of intellectual content, S.M., T.C.D.; manuscript editing, T.C.D.; manuscript revision/review and final version approval, all authors
References
- 1.Hardingham T, Bayliss M. Proteoglycans of articular cartilage: changes in aging and in joint disease. Semin Arthritis Rheum. 1990;20:12–33. doi: 10.1016/0049-0172(90)90044-g. [DOI] [PubMed] [Google Scholar]
- 2.Sharma L. Epidemiology of osteoarthritis. In: Moskowitz RW, Howell DS, Altman RD, Buckwalter JA, Goldberg VM, editors. Osteoarthritis: diagnosis and medical/surgical management. 3. Philadelphia, Pa: Saunders; 2001. pp. 3–28. [Google Scholar]
- 3.Yelin E. The economics of osteoarthritis. In: Brandt KD, Doherty KD, Lohmander LS, editors. Osteoarthritis. New York, NY: Oxford University Press; 1998. pp. 23–30. [Google Scholar]
- 4.Xia Y, Farquhar T, Burton-Wurster N, Ray E, Jelinski LW. Diffusion and relaxation mapping of cartilage-bone plugs and excised disks using microscopic magnetic resonance imaging. Magn Reson Med. 1994;31:273–282. doi: 10.1002/mrm.1910310306. [DOI] [PubMed] [Google Scholar]
- 5.Dardzinski BJ, Mosher TJ, Li S, Van Slyke MA, Smith MB. Spatial variation of T2 in human articular cartilage. Radiology. 1997;205:546–550. doi: 10.1148/radiology.205.2.9356643. [DOI] [PubMed] [Google Scholar]
- 6.Mosher TJ, Dardzinski BJ, Smith MB. Human articular cartilage: influence of aging and early symptomatic degeneration on the spatial variation of T2—preliminary findings at 3 T. Radiology. 2000;214:259–266. doi: 10.1148/radiology.214.1.r00ja15259. [DOI] [PubMed] [Google Scholar]
- 7.Sweet MB, Thonar EJ, Immelman AR, Solomon L. Biochemical changes in progressive osteoarthrosis. Ann Rheum Dis. 1977;36:387–398. doi: 10.1136/ard.36.5.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuettner K, Thonar E, Aydelotte M. Articular cartilage: structure and chondrocyte metabolism. In: Muir HM, Hirohata K, Shichikawa K, editors. Mechanisms of articular cartilage damage and repair in osteoarthritis. Toronto, Canada: Hogrefe & Huber; 1990. pp. 11–30. [Google Scholar]
- 9.Bashir A, Gray ML, Burstein D. Gd-DTPA2- as a measure of cartilage degradation. Magn Reson Med. 1996;36:665–673. doi: 10.1002/mrm.1910360504. [DOI] [PubMed] [Google Scholar]
- 10.Lusse S, Claassen H, Gehrke T, et al. Evaluation of water content by spatially resolved transverse relaxation times of human articular cartilage. Magn Reson Imaging. 2000;18:423–430. doi: 10.1016/s0730-725x(99)00144-7. [DOI] [PubMed] [Google Scholar]
- 11.Link TM, Steinbach LS, Ghosh S, et al. Osteoarthritis: MR imaging findings in different stages of disease and correlation with clinical findings. Radiology. 2003;226:373–381. doi: 10.1148/radiol.2262012190. [DOI] [PubMed] [Google Scholar]
- 12.Kellgren J, Lawrence J. Radiological assessment of osteoarthritis. Ann Rheum Dis. 1957;16:494–501. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hart DJ, Spector TD. Osteoarthritis. Oxford, England: Oxford University Press; 1998. Assessment of changes in joint tissues in patients treated with a disease-modifying osteoarthritis drug (DMOAD): monitoring outcomes; pp. 450–458. [Google Scholar]
- 14.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 15.Ghosh S, Majumdar S. Proceedings of the Ninth Meeting of the International Society for Magnetic Resonance in Medicine. Vol. 2001. Berkeley, Calif: International Society for Magnetic Resonance in Medicine; Application of spectro-spatial pulses for contrast in cartilage imaging (abstr) p. 916. [Google Scholar]
- 16.Ghosh S. Thesis. University of California San Francisco, San Francisco, Calif. 2001. Magnetic resonance imaging based evaluation of articular cartilage degeneration in osteoarthritis. [Google Scholar]
- 17.Mosher TJ, Smith H, Dardzinski BJ, Schmithorst VJ, Smith MB. MR imaging and T2 mapping of femoral cartilage: in vivo determination of the magic angle effect. AJR Am J Roentgenol. 2001;177:665–669. doi: 10.2214/ajr.177.3.1770665. [DOI] [PubMed] [Google Scholar]
- 18.Mow VC, Holmes MH, Lai WM. Fluid transport and mechanical properties of articular cartilage: a review. J Biomech. 1984;17:377–394. doi: 10.1016/0021-9290(84)90031-9. [DOI] [PubMed] [Google Scholar]
- 19.Peterfy CG. Magnetic resonance imaging. In: Brandt KD, Doherty KD, Lohmander LS, editors. Osteoarthritis. New York, NY: Oxford University Press; 1998. pp. 473–494. [Google Scholar]
- 20.Buckwalter JA, Mankin HJ. Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect. 1998;47:487–504. [PubMed] [Google Scholar]
- 21.Goodwin DW, Dunn JF. High-resolution magnetic resonance imaging of articular cartilage: correlation with histology and pathology. Top Magn Reson Imaging. 1998;9:337–347. [PubMed] [Google Scholar]
- 22.Riegger-Krugh C, Gerhart TN, Powers WR, Hayes WC. Tibiofemoral contact pressures in degenerative joint disease. Clin Orthop. 1998;348:233–245. [PubMed] [Google Scholar]
- 23.MacFall JR, Riederer SJ, Wang HZ. An analysis of noise propagation in computed T2, pseudodensity, and synthetic spin-echo images. Med Phys. 1986;13:285–292. doi: 10.1118/1.595956. [DOI] [PubMed] [Google Scholar]
- 24.Modl JM, Sether LA, Haughton VM, Kneeland JB. Articular cartilage: correlation of histologic zones with signal intensity at MR imaging. Radiology. 1991;181:853–855. doi: 10.1148/radiology.181.3.1947110. [DOI] [PubMed] [Google Scholar]
- 25.Frank LR, Wong EC, Luh WM, Ahn JM, Resnick D. Articular cartilage in the knee: mapping of the physiologic parameters at MR imaging with a local gradient coil—preliminary results. Radiology. 1999;210:241–246. doi: 10.1148/radiology.210.1.r99ja01241. [DOI] [PubMed] [Google Scholar]


