Abstract
Purpose
Improved therapeutic approaches are needed for the treatment of pancreatic ductal adenocarcinoma (PDAC). As dual MEK and PI3K inhibition is presently being employed in clinical trials for PDAC patients, we sought to test the efficacy of combined targeting of these pathways in PDAC using both in vitro drug screens and genetically engineered mouse models (GEMMs).
Experimental Design
We performed high-throughput screening of >500 human cancer cell lines (including 46 PDAC lines), for sensitivity to 50 clinically-relevant compounds, including MEK and PI3K inhibitors. We tested the top hit in the screen, the MEK1/2 inhibitor, AZD-6244, for efficacy alone or in combination with the PI3K inhibitors, BKM-120 or GDC-0941, in a KRASG12D-driven GEMM that recapitulates the histopathogenesis of human PDAC.
Results
In vitro screens revealed that PDAC cell lines are relatively resistant to single-agent therapies. The response profile to the MEK1/2 inhibitor, AZD-6244, was an outlier, showing the highest selective efficacy in PDAC. While MEK inhibition alone was mainly cytostatic, apoptosis was induced when combined with PI3K inhibitors (BKM-120 or GDC-0941). When tested in a PDAC GEMM and compared to the single agents or vehicle controls, the combination delayed tumor formation in the setting of prevention and extended survival when used to treat advanced tumors, although no durable responses were observed.
Conclusions
Our studies point to important contributions of MEK and PI3K signaling to PDAC pathogenesis and suggest that dual targeting of these pathways may provide benefit in some PDAC patients.
Keywords: Pancreatic cancer, MEK, PI3K, KRAS, high-throughput screens, Genetically engineered mouse models
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is the fourth most common cause of cancer-related death in the United States and carries a median survival of less than six months (1). A small proportion of cases can be treated with potentially curative surgical resection, whereas most are locally advanced or metastatic at diagnosis (2). Chemotherapy for advanced disease can range from single agent gemcitabine, with a modest extension in survival and relatively few side effects, to more effective combinations such as gemcitabine and nabpaclitaxel or 5-fluorouracil, leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX), though coming with a cost of increased toxicity (3). A series of molecularly targeted therapies have failed to show benefit in clinical trials, and notably, unlike a number of other types of solid tumor types, genetically defined subsets of PDAC have yet to show acute response to specific inhibitors (2). While the EGFR inhibitor erlotinib has been approved for PDAC in combination with gemcitabine, the survival benefit compared to gemcitabine alone is less than one month (4).
A number of factors likely conspire to produce poor therapeutic response of PDAC, including frequent diagnosis at advanced disease stage, high levels of genome instability with genetic variability across tumors in the same patient, and dense fibroblastic stroma and poor perfusion that may limit drug delivery (5). An additional key element for the lack of response may be the high rate of activating KRAS mutations in these cancers (present in >90% of cases) (6, 7). Multiple lines of investigation point to a central role for activated KRAS in PDAC initiation as well as in tumor maintenance (8-11). Unfortunately, direct inhibitors of the common mutant KRAS alleles have yet to be developed, and effective targeted therapy strategies for treating KRAS mutant cancers have remained elusive.
There is currently considerable interest in defining the critical KRAS effectors required for tumor maintenance since such factors may provide surrogate drug targets to extinguish the biological actions of KRAS. In this regard, the combined use of MEK and PI3K pathway inhibitors has been shown to be effective in KRAS-driven mouse models of lung cancer as well as in other RAS mutant cancer models (12, 13). Overall, the specific pathways activated by KRAS and the contributions of such pathways to tumor maintenance are likely to depend on tissue type and on the set of coincident genetic and epigenetic alterations. For instance, PI3K/Pdk1 signaling is selectively required for tumor initiation in Kras-driven PDAC, but not NSCLC, models (14). In addition, recent studies have shown that, due to the existence of feedback control circuits in the pathway, inhibition of specific RAS signaling components can lead to unanticipated augmentation of oncogenic signaling outputs (15). Thus, as strategies to target various potential RAS effector pathways are being considered for future clinical trials, it is essential to apply relevant preclinical models as a guide to support a given therapeutic approach. More broadly, it will be important to define additional signaling dependencies in PDAC tumorigenesis.
Here, we sought to examine systematically the effect of a panel of known anti-cancer drugs on PDAC, and to then provide in vivo support for their therapeutic potential. To this end, we employed a large-scale screen of a set of well-characterized chemical inhibitors for their efficacy against a panel of more than 500 cell lines derived from a series of solid tumor types. Among the 50 compounds analyzed, this screen identified the MEK1/2 inhibitor, AZD-6244 (ARRY-142886) (16, 17), as the most effective drug against PDAC cell lines. The capacity of AZD-6244 to promote apoptosis was significantly enhanced when combined with class I PI3K inhibitors. Moreover, this drug combination showed efficacy in a PDAC GEMM driven by mutations that define human PDAC, both delaying tumor onset when administered prior to tumor formation, and extending survival when used to treat established cancers. However, the effects were transient in both settings. While the promising results seen upon MEK and PI3K inhibition in other preclinical models have prompted clinical trials of this regimen in KRAS mutant tumors, our results indicate that only limited benefit may be provided in the context of PDAC.
MATERIALS AND METHODS
Cell Lines
PDAC cell lines were grown in DMEM/F12 (GIBCO) with 10% FBS and assayed in DMEM/F12 with 5% FBS and were obtained from the MGH Center for Molecular Therapeutics (CMT), which performs routine cell line authentication testing by SNP and STR analysis.
High-throughput cell viability assay
Compounds were obtained from commercial sources or provided by AstraZeneca (Supplementary Table 1). Small molecule inhibitors were used at 3 concentrations 10 fold apart (see Supplementary Table 2). Cell viability was determined as previously described28. Briefly, cells were seeded in medium containing 5% FBS at density insuring cell growth throughout drug treatment (~15% for most cell lines). Drug treatment was started 24h post seeding and continued for 72 hours. Cell were fixed and stained using Syto60 (Invitrogen) a red fluorescent DNA stain. Relative cell number was calculated by taking the ratio of the relative fluorescence intensity from drug treated wells over untreated wells after background subtraction (no cells seeded). Values are average from triplicate wells.
Annexin V Apoptosis Assays
Cells were seeded at ~30 to 40% confluence in 6 cm plates. After overnight incubation, media was aspirated and replaced with media with or without various concentrations of indicated drugs. After 72 hrs, media was collected. Cells were washed with PBS and trypsinized. PBS washed and trypsinized cells were added to the collected media in a single tube. Cells were pelleted, washed once with PBS and resuspended in Annexin binding buffer (BD Biosciences) at ~1 × 106 cells/mL. Cells were stained with propidium iodide (BD Biosciences) and Annexin V Cy5 (Biovision) according to the manufacturer's protocol and assayed on a LSRII flow cytometer (BD Biosciences).
Statistical Analysis
Relative efficacy of the compounds tested against PDAC cell lines was evaluated by comparing the viability of PDAC lines and non-PDAC lines for each compound. A Fisher exact test was used to determine statistical significance. For each compound the three concentrations tested were evaluated separately. The statistical test was iteratively run using a threshold of sensitivity corresponding to a cell viability of 10 to 80% by increment of 10% (the first test was done by classifying cell lines with viability of 10% or under as sensitive and cell lines with viability >10% as resistant). The minimum P value (one-tailed) obtained for a given compound across all concentrations and viability thresholds (24 tests per compound) was used to compare relative sensitivity of all compounds towards PDAC lines. All results of the Fisher exact test (two-tailed values) are in Supplementary table 2. Combination index to measure combined activity was analyzed with Compusyn (ComboSyn Inc.). To test tissue specific activity of AZD6244 a Fisher exact test was used to determine the statistical significance of the activity of AZD6244 at 2 μM against cell lines of different origin. For each tissue of origin viability of the cell lines was compared to viability of all lines from other tissue of origin. A threshold of 60% viability was used, other viability thresholds tested led to similar results. For each Tissue we compute the effect: Effect = Ln (Mean viability of All Other Lines / Mean viability of Tissue Lines). For the survival studies, statistical analysis was performed using Prism statistical software version 4.0a May 11, 2003. Survival was determined using the Kaplan-Meir method and comparisons between treatment groups were determined using the Logrank test. Animals that displayed signs of illness and were found to have advanced cancers on necropsy were included as events. Animals that died for reasons other than advanced cancer were censored.
Mouse Strains and Histologic Analysis
The Pdx1-Cre;LSL-KRASG12D;p53Lox/+ mouse PDAC model has been previously described (18). Mice were housed in the pathogen-free environment maintained by the Center for Comparative Medicine (CCM) at the Massachusetts General Hospital. Mice were handled in strict accordance with good animal practice, as defined by the Subcommittee on Research Animal Care (SRAC) at Massachusetts General Hospital, and all mouse work was done with SRAC approval (protocol 2005N000148).
Chemical inhibitors
We purchased the MEK inhibitor ARRY-142886 (AZD6244) and GDC-0941 from commercial sources (Otava Chemicals). The PI3K inhibitor, BKM-120, and the dual PI3K-mTOR inhibitor, NVP-BEZ235-AN, were obtained from Novartis Institutes for Biomedical Research. We reconstituted BKM-120 and AZD-6244 in one volume of N-methyl-2-pyrrolidone (69118, Fluka) and then added nine volumes of PEG300 (81160, Fluka), and administered these drugs by oral gavage daily at 50 mg/kg and 25 mg/kg, respectively. GDC-0941 was dissolved in 0.5% methylcellulose with 0.2% Tween-80 and administered by oral gavage at 75mg/kg daily. NVP-BEZ235-AN was reconstituted in 0.5% methyl cellulose (Fluka) and 0.4% polysorbate (Tween 80; Fluka) and administered daily by oral gave at a dosage of 25 mg/kg.
Western Blot analysis
Western blotting was performed using standard methods. Cells were washed with cold PBS and lysed in the following lysis buffer: 20 mM Tris pH 7.4, 150 mM NaCl, 1% Nonidet P-40, 10% glycerol, 1 mM EDTA, 1 mM EGTA, 5 mM sodium pyrophosphate, 50 mM NaF, 10 nM β–glycerophosphate, 1 mM sodium vanadate, 0.5 mM DTT, 4 mg/mL leupeptin, 4 mg/mL pepstatin, 4 mg/mL aprotinin, 1 mM phenylmethylsulfonyl fluoride. Lysates were centrifuged at 16,000 x g for 5 min at 4°C. Pancreatic tissues (100-200mg) were minced using a homogenizer, but otherwise processed as above. Protein concentrations were determined by BCA assay (Thermo Scientific). Proteins were resolved by SDS-PAGE and transferred to a polyvinylidene difluoride membrane (Hybond-P, Amersham). Immunoblotting was performed per antibody manufacturer's specifications.
Immunofluorescence and Immunohistochemistry
Samples were fixed in 10% formalin and embedded in paraffin. After deparaffinization, slides were washed with 9.83% NaCl for 3 min followed by a PBS wash and a wash in distilled water for 5 min. Heat induced antigen retrieval was performed with pressure cooker (2100 Retriever, PickCell Laboratories B.V.) and R-Buffer A (pH6.0, Electron Microscopy Sciences). Sections were incubated with 2% H2O2 in Methanol for 15 minutes for endogenous peroxidase quenching and washed and blocked for non-specific binding in 1% BSA in PBS-Triton 0.3% v/v (PBST) for 1 hour. Subsequently, sections were sequentially incubated with primary antibody at 1:100 dilution for 1 hour, secondary peroxidase goat anti-rabbit IgG antibody (Vector) at 1:250 dilution for 1 hour and Tyramide Signal Amplification (TSA) Fluorescein System (Perkin Elmer, Cat.: NEL701A) according to kit manual. Slides were mounted with Vectashield mounting medium with DAPI (Vector), photographed with Nikon C2 Confocal Microscope system and subsequently stained with Hematoxylin and Eosin. p-AKT (Thr308) p-ERK (Thr202/Tyr204), p-S6 (Ser235/236), and CC3 (Asp 175) were obtained from Cell Signaling, Inc. Ki67 staining was performed as previously described (19). Quantification of Ki67 staining was performed by scoring the nuclei of cells from each lesion type found in a minimum of ten high-powered fields.
Organotypic Tissue Cultures
Organotypic tissue cultures were prepared from primary PDAC using previously described methods (20). In brief a Vibratome VT1200 (Leica Microsystems) was used to cut thin (300–500 μm) slices from fresh tissue. Tissue slices were cultured on organotypic inserts (Teflon membranes with 0.4-μm pores) for up to 120 h (two slices per insert; Millipore). Tissue culture was performed at 37 °C in a 5% CO2 humidified incubator using 1 ml of Ham F-12 media supplemented with 20% inactivated FBS (GIBCO), 100 U/mL penicillin (Invitrogen), 100 μg/mL streptomycin (GIBCO), 2.5 μg/mL amphoterycin B, and 100 μg/mL of kanamycin (Sigma Aldrich). Medium was changed every 2 days. Tissue slices were harvested at baseline time (T0) and thereafter, at 24-h intervals; slices was formalin-fixed and paraffin-embedded for morphological (H&E) and IHC evaluation.
Magnetic Resonance
MRI measurements were performed using a 4.7 T Bruker Avance horizontal bore system equipped with a 200 mm inner diameter gradient set capable of 30G/cm gradient strength. The mice were anesthetized with 1% isoflurane in an oxygen/air mixture. The animals’ respiratory and cardiac rates were monitored using Biotrig Software. The animals were imaged with T2 weighted turbo spin echo (RARE) sequence (TR = 2000 ms, TE effect = 36 ms, echo train length =4, number of averages =8) in the coronal planes with a 0.9mm slice thickness and with the number of slices sufficient to cover the entire abdomen, and with a matrix size of 256 × 256, field of view (FOV) of 4 × 5.5 cm2, resulting in an in-plane resolution of .25 × .12 mm. With the same geometry as described above, the animals were also imaged with a T1-weighted RARE sequence (TR=900ms, TE effective = 14msec.) and parameters equivalent to the T2 weighted sequence described above with respiratory and cardiac gating, in both the coronal and axial planes prior to and following the intravenous administration of 0.3 mmol/kg of Gd-DTPA (Magnevist; Schering, Berlin, Germany). Using the post-contrast sequence scans, volume measurements of the tumors were performed using in-house customized software on an Osirix ® (Lausanne, Switzerland). In specific, the longest diameter in each plane (anterior posterior, right-left, and cranialcaudal) were measured and multiplied as a product of the perpendicular diameters (PPD).
RESULTS
Screening of a panel of anti-cancer drugs identifies MEK inhibitors as compounds with highest efficacy in PDAC cell lines
In order to identify drugs that show selective efficacy in PDAC, we conducted a high-throughput cell line screen that examined the responsiveness of human cancer cell lines to a panel of 50 clinically relevant small molecule compounds (consisting mainly of rationally designed agents, Supplementary Table 1). The screen incorporated >500 human cancer cell lines, including 46 PDAC lines. Statistical analysis of the sensitivity of PDAC lines relative to all other lines shows that for the majority of compounds, PDAC cell lines were significantly less sensitive than non-PDAC cells (Figure 1A, red dots compared to green dots). Moreover, we did not observe subsets of PDAC cell lines that showed acute sensitivity to any of the compounds (data not shown). Thus, the general therapeutic resistance of PDAC appears to be recapitulated in cultured cell lines.
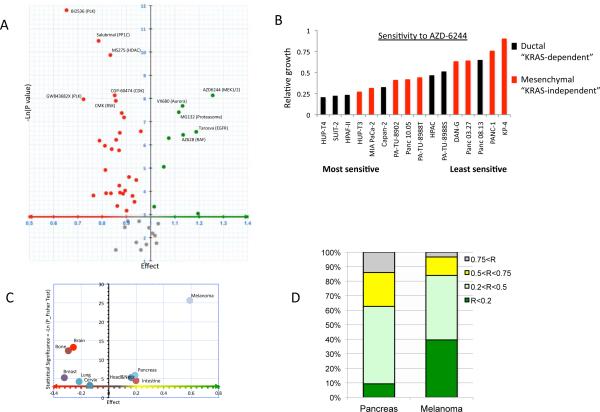
Figure 1. High-throughput screening identifies the MEK1/2 inhibitor, AZD6244 as the most active compound against PDAC cell lines.
A) Volcano plot representing the responsiveness of PDAC cell lines (N=46) compared to non-PDAC cancer cell lines (N=500) to a series of 50 potential anti-cancer drugs. X axis: relative sensitivity (Effect > 1: PDAC cells are on average more sensitive than non-PDAC cells), Y axis: statistical significance (Gray dots: compounds with p>0.05; Green dots: compounds preferentially targeting PDAC; Red dots: compounds for which PDAC cell lines are more resistant than other types of cell lines).
B) Relative sensitivity to AZD-6244 of PDAC cell lines identified as “KRAS-dependent” and “–independent”(8, 9). C) Organ specificity profile of AZD-6244. Cell lines from cancer types were iteratively compared to the rest of the cell collection to determine the specific targeting by tissue of origin. X axis: relative sensitivity (Effect: average survival of cell lines from a given organ/survival of the other lines). Y axis: statistical significance of the organ enrichment (Fisher exact test). D) Relative sensitivity of PDAC and melanoma lines to 2 μM AZD-6244. Bar color indicates relative cell number (R) compared to control. Bar height represents the percentage of cell lines of each tumor type showing the indicated degree of growth inhibition.
In spite of the overall resistance of the PDAC cell lines, we did observe that a small number of compounds showed selective activity in this cancer type (Figure 1A, green dots). Erlotinib, the only approved targeted therapy for PDAC (approved in combination with gemcitabine (4)), was among the top hits, supporting that this approach can accurately identify compounds with clinical activity in PDAC. Among these compounds, the MEK kinase inhibitor, AZD-6244, clearly stood out as having the greatest relative efficacy in PDAC (see Figure 1A showing that AZD-6244 has highest effect and p value of the PDAC-selective compounds). Notably, >90% of the PDAC cell lines have activating KRAS mutations, and MEK inhibitors were found to be the most effective compounds against KRAS mutant cancers across all tumor types examined. Previous studies have shown that KRAS mutant PDAC cell lines can be divided into subsets that have either a high or a low dependency on KRAS activity for proliferation as assessed by KRAS knockdown (8, 9). The cell lines in either group showed overlapping sensitivity profiles to AZD-6244 (Figure 1B), consistent with MEK serving as an important mediator of PDAC growth across molecularly distinct subsets of PDAC.
Although AZD-6244 was the most effective compound in PDAC cell lines, the overall magnitude of the responses was weak as compared to that of melanoma lines, a majority of which harbor sensitizing BRAF mutations (Figure 1C and D). Thus, these in vitro studies support the further evaluation of MEK inhibitors in PDAC but suggest that their activity as a single-agent therapies may be limited.
Combinatorial effects of dual MEK and PI3K inhibition on apoptosis in PDAC cell lines
For many drugs, the capacity to induce apoptosis in vitro is a better predictor of in vivo efficacy than cell cycle arrest(21, 22). Since our high-throughput assay does not distinguish between growth arrest and induction of cell death, we examined directly the impact of AZD-6244 on apoptosis in a panel of PDAC cell lines. This compound induced modest levels of apoptosis relative to vehicle in most cell lines, despite effectively downregulating phospho-ERK levels (Figure 2A, B, and Supplementary Figure 1A). Since prior studies have shown that inhibitors of the PI3K survival pathway can enhance the efficacy of MEK inhibition in other KRAS mutant cancers (12, 13), we also studied the apoptotic potential of the PI3K inhibitors, BKM-120 and GDC-0941. Both compounds caused small increases in apoptosis as single agents in PDAC cell lines but, when combined with MEK inhibitors, acted either additively or synergistically to significantly augment levels of apoptosis in most PDAC lines (Figure 2A, B, and Supplementary Figure 1A), supporting the combined use of these compounds.
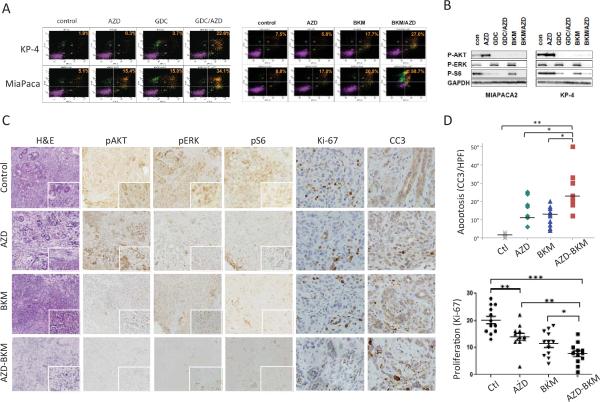
Figure 2. Analysis of cell lines and ex vivo organotypic cultures supports the combined targeting of MEK and PI3K in PDAC.
A, B) Treatment of PDAC cell lines with vehicle control or with the indicated drugs (AZD: AZD-6244; BKM: BKM-120; GDC: GDC-0941) used at 1 μ#. A) FACS plots showing PI/AnnexinV staining. The % of apoptotic cells is indicated. B) Western blot showing effect of the inhibitors on p-AKT (Thr308) and p-ERK (Thr202/Tyr204) levels.
C-D) Analysis of therapeutic responses of ex vivo organotypic cultures of primary PDAC. C) Freshly derived organotypic cultures were treated with the indicated compounds (each at 1 μM) for 24 hours and then processed for staining with H&E or with antibodies to p-ERK (Thr202/Tyr204), p-AKT (Thr308), p-S6 (Ser235/236), Ki-67, and cleaved Caspase 3. D) Quantification of apoptosis at 24 hrs (cleaved caspase-3 staining) and of proliferation (Ki-67 staining). Statistical significance is indicated; p<0.01 (*), p<0.0001 (**).
Efficacy of dual MEK and PI3K inhibition in ex vivo organotypic models derived from primary PDAC
We next sought to test the impact of single or dual MEK/PI3K inhibition in primary tumors. Consistent with previous studies25-28, IHC analysis of human PDAC showed staining for both phospho-ERK and phospho-AKT in the tumor epithelium, indicating ongoing activation of these pathways (Supplementary Figure 1B). We first tested the efficacy of AZD-6244 and BKM-120 in an ex vivo organotypic model (20). This model, which employs thin (300–500 μm) sections of primary tumors obtained using a vibratome (see Materials and Methods), enables evaluation of therapeutic efficacy in the context of preserved tumor-stroma interactions and 3-D architecture. Treatment of sections of the same tumor with AZD-6244 or BKM-120 extinguished p-ERK staining and p-AKT staining, respectively, and the combination caused loss of both signals (Figure 2C). Although these compounds induced apoptosis (cleaved-caspase-3 staining) and reduced proliferation (Ki-67 staining) when used as single-agent, their combination resulted in significantly more pronounced effects (Figure 2C; data are quantified in Figure 2D). These alterations were most notable in the tumor epithelium rather than the stroma.
Dual MEK/PI3K inhibition delays PDAC initiation and progression in the KRAS-p53 mouse model
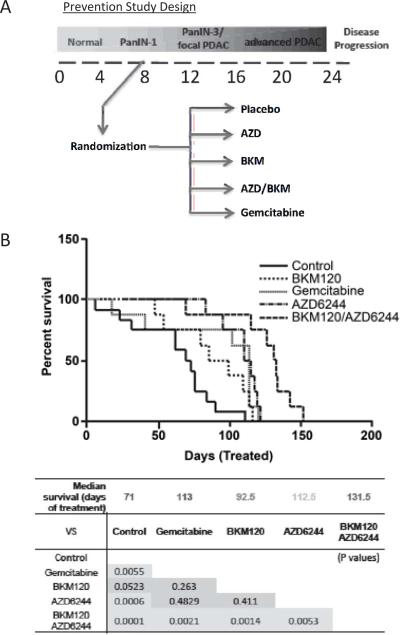
We next sought to establish the impact of MEK/PI3K inhibition on primary PDAC in vivo. We used a genetically engineered mouse model (GEMM) of PDAC driven by activation of KRAS and inactivation of p53 (Pdx1-Cre; LSL- KRASG12D; p53Lox/+, designated KRAS-p53 mice) that recapitulates the genetics and histopathologic progression of the human disease in a synchronous and predictable manner (18). First, we investigated the potential of AZD-6244 and BKM-120 to limit the initiation and progression of PDAC when administered prior to the onset of frank tumors. As shown in the study design schematic in Figure 3A, mice were treated with these compounds starting at 8 weeks of age, a time point when only pre-invasive pancreatic ductal lesions (pancreatic intraepithelial neoplasias; PanINs) are present (18). The impact of these drugs was compared with vehicle control and with gemcitabine. The drugs were well-tolerated as single agents and in combination, consistent with reports using other MEK and PI3K inhibitors (12). In this setting, the combination of AZD-6244 and BKM-120 gave the strongest protective effect, nearly doubling the length of survival compared to controls (median 131.5 days versus 71 days)(Figure 3B). Single agent treatment with AZD-6244, BKM-120, or gemcitabine produced an intermediate extension in survival (Figure 3B). As shown in Supplementary Figure 2, we also observed a significant extension of survival in a prevention study using AZD-6244 combined with the dual specificity PI3K/mTOR inhibitor, BEZ-235 (85.2 days compared to 44.5 days for controls, p<0.001)—in these studies, treatment was initiated at 10 weeks when disease is slightly more advanced, consisting of higher grade PanINs or early PDAC. Despite the extension in lifespan conferred by the inhibitors, all mice eventually developed pancreatic tumors. Histological examination showed that the tumors arising in all groups had comparable pathological histological features, consisting of invasive, well-differentiated to poorly-differentiated PDAC (data not shown). Thus, dual MEK/PI3K inhibition significantly delays the initiation and malignant progression of PDAC while not strongly altering the intrinsic malignant phenotype of the tumors that eventually arise.
Figure 3. Combined targeting of MEK and PI3K delays the progression of PDAC from PanIN lesions in the KRAS-p53 mouse model.
A) Schematic of experimental design for prevention studies. Mice were treated prior to the onset of PDAC and monitored for evidence of tumor progression.
B) Survival curve (Kaplan-Meier Analysis) calculated as length of time between start of treatment and sacrifice. Chart shows statistical analysis of survival data.
Dual MEK/PI3K inhibition induces response in advanced PDAC
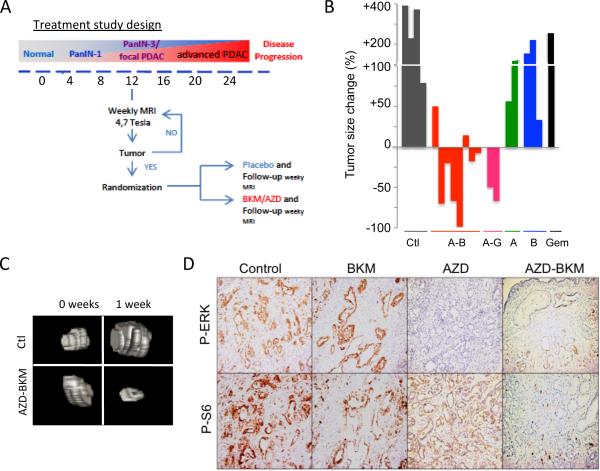
We conducted therapeutic studies in the more clinically relevant setting of advanced PDAC. Mice were examined for the presence of established tumors using MRI beginning at the age of 12 weeks and then randomized into treatment groups (control, AZD-6244, PI3K inhibitor (either BKM-120 or GDC-0941)). Serial MRI was used to monitor changes in tumor volume (the study design is shown in Figure 4A). Whereas progressive disease was observed in all mice treated with vehicle, or the single MEK and PI3K inhibitors, combined MEK/PI3K inhibition for 7 days resulted in initial reduction in tumor size in 8/10 mice (Figure 4B; representative three-dimensional renderings of MRI scans are shown in Figure 4C). Immunohistochemical analysis showed that p-AKT, p-ERK , and p-S6 levels in the tumors were effectively reduced upon administration of AZD-6244 and BKM-120, respectively, as compared to vehicle treated controls, indicating robust targeting of the MEK and PI3K pathways (Figure 4D and Supplementary Figure 3A). Of note, suppression of TORC1 signaling, as evidenced by p-S6, required concomitant inhibition of both PI3K and MEK, consistent with our earlier findings in a lung cancer GEMM (Figure 4D)(12).
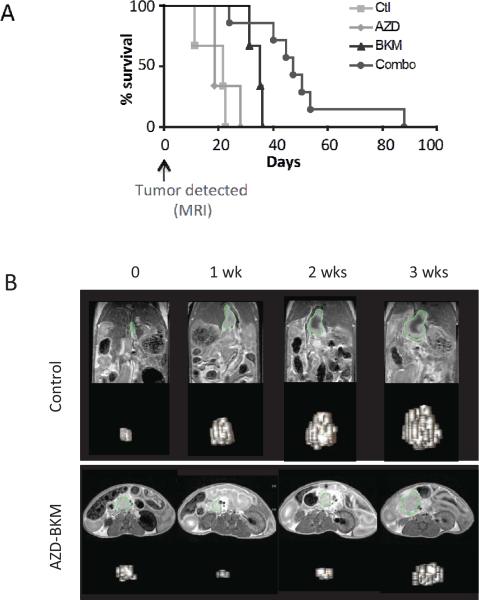
Figure 4. Advanced PDAC in the KRAS-p53 mouse model are responsive to dual MEK/PI3K inhibition.
A) Schematic of experimental design for treatment of advanced PDAC. Mice were monitored for the presence of tumors by MRI. Upon detection of PDAC 3-10 mm in size, mice were randomized into the treatment and control groups.
B) Waterfall plot showing efficacy of AZD-6244 (A) combined with either BKM-120 (B) or GDC-0941 (G) in promoting PDAC regression. No responses were seen with the single agents or with gemcitabine (Gem). Statistical significance: combination versus control (p<0.0001), versus AZD-6244 (p<0.01), versus BKM-120 (p<0.001).
C) Representative three-dimensional reconstructions of MRI scans prior to treatment, or after 1 week in the indicated treatment groups.
D) Immunohistochemical staining for p-ERK (Thr202/Tyr204) and p-S6 (Ser235/236), in PDAC isolated from KRAS-p53 mice treated for 1 week with the indicated compounds.
The responses seen by MRI in the mice treated with the dual inhibitor regimen translated into a temporally sustained control of the disease burden increase in survival after diagnosis. Whereas the control group had a median survival of 27 days after tumor detection, mice treated with the combination lived a median of 59 days (Figure 5A). By comparison, AZD-6244 provided no survival benefit, and BKM-120 had an intermediate effect. Serial MRI showed that the effects of the combination were transient, with most tumors showing increasing size within 2-3 weeks of treatment (representative data shown in Figure 5B). On histology, we found that tumors, in which MRI response was identified, show marked surrounding fibrosis and reduced parenchymal invasion when compared to controls (Supplementary Figure 3B). Thus, combined targeting of MEK and PI3K provides clinically significant responses in a genetically and histologically faithful GEMM of PDAC, although the regimen is not curative and does not produce a durable response.
Figure 5. Clinical benefit of dual MEK/PI3K inhibition in the KRAS-p53 PDAC models.
A) Survival curve (Kaplan-Meier analysis) of KRAS-53 mice after MRI detection of PDAC and administration of the indicated treatments. The differences between the AZD-6244/BKM-120 combination and the other groups are statically significant (compared to control, p=0.0008; compared to AZD, p=0.0061; compared to BKM, p=0.022).
B) Representative MRI scans of KRAS-p53 mice and 3D reconstructions at sequential time points in the indicated treatment groups. The vehicle-treated mouse show rapid tumor progression (upper panel). Treatment with AZD-6244 and BKM-120 results in partial tumor regression in some mice, although the effects are transient.
DISCUSSION
Together with the findings presented by Junttila et al. (in press), the present data suggest that the combination therapy may not offer marked improvement for PDA patients. Although we cannot rule out that the moderate responses were due to limited drug delivery to the tumors, our signaling studies indicated that the target pathways were effectively suppressed. Additionally, our experiments in the prevention setting where there is initially limited stroma revealed only a limited delay in tumor development. These findings are in contrast with KRAS-driven murine lung cancers which show pronounced and sustained responses to dual MEK/PI3K inhibition. Notably, genetic studies in mouse models of KRAS mutant lung cancer, colon cancer, and PDAC show that there are multiple differences in their requirements for pathways downstream or intersecting with KRAS for tumorigenicity, suggesting that alternatives therapeutic strategies will be required (14, 23).
The biggest advances in treatment have come from retooled versions of long-standing cancer drugs — abraxane and gemcitabine or combinations of drugs commonly used in other diseases (3). These new effective chemotherapy choices have had a significant positive impact on patients but further incremental improvements using similar approaches are likely to be limited by the increasing toxicity and side effects of multi-layered cytotoxic chemotherapy. One of the biggest question clinical investigators face is how to choose drugs to test clinically from among the many new agents available and gaining traction in other cancers. In light of the increasingly limited federal resources this is a significant problem and being able to demonstrate in preclinical models agents with limited effectiveness may help to direct these resources more effectively. Just how studies, using these preclinical GEM models, should be used to guide clinical development remains an open question, and the ongoing evaluation of preclinical models in light of clinical trial results will be important to understand how to best use these models.
TRANSLATIONAL RELEVANCE.
Our results support the potential efficacy of combined MEK/PI3K inhibition in PDAC. MEK inhibitors were the most selectively active agents against PDAC cell lines in a high-throughput screen of 50 clinically relevant drugs in >500 human cancer cell lines. In combination with PI3K inhibitors, AZD-6244 promoted apoptosis in PDAC lines, and acted combinatorially with PI3K inhibitors in suppressing tumor initiation and progression in a genetically engineered mouse model of PDAC. Although the combination did not produce durable responses, our findings suggest that combined targeting of MEK and PI3K may be beneficial for a subset of PDAC patients.
ACKNOWLEDGEMENTS
N.B. is a member of the Andrew L. Warshaw Institute for Pancreatic Cancer Research and hold the Gallagher Chair in Gastrointestinal Cancer Research at Massachusetts General Hospital.
Grant Support:
This work was supported by grants to N.B. from the AACR/Pancreatic Cancer Action Network, the NIH (NCI 2P01CA117969-06), and Dana-Farber/Harvard Cancer Center Gastrointestinal Cancer SPORE grant P50 CA127003 (to N.B. and J.A.E.). R.B.C was supported by NIH training grant T32 CA071345. G.C. was supported by Fondazione Umberto Veronesi, Associazione Italiana per la ricerca sul Cancro and American Italian Cancer Foundation.
Footnotes
Conflicts of interest:
J.A. Engelman has a commercial research grant from Novartis, Sanofi-Aventis, and AstraZeneca; and is a consultant/advisory board member of Novartis, Sanofi-Aventis, Chugai, and AstraZeneca. The other others have no conflicts to declare.
REFERENCES
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–17. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 3.Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 4.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–6. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 5.Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:4266–76. doi: 10.1158/1078-0432.CCR-11-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–6. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491:399–405. doi: 10.1038/nature11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh A, Greninger P, Rhodes D, Koopman L, Violette S, Bardeesy N, et al. A gene expression signature associated with “K-Ras addiction” reveals regulators of EMT and tumor cell survival. Cancer Cell. 2009;15:489–500. doi: 10.1016/j.ccr.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collisson EA, Sadanandam A, Olson P, Gibb WJ, Truitt M, Gu S, et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med. 2011;17:500–3. doi: 10.1038/nm.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins MA, Brisset JC, Zhang Y, Bednar F, Pierre J, Heist KA, et al. Metastatic pancreatic cancer is dependent on oncogenic Kras in mice. PloS one. 2012;7:e49707. doi: 10.1371/journal.pone.0049707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ying H, Kimmelman AC, Lyssiotis CA, Hua S, Chu GC, Fletcher-Sananikone E, et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell. 2012;149:656–70. doi: 10.1016/j.cell.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engelman JA, Chen L, Tan X, Crosby K, Guimaraes AR, Upadhyay R, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14:1351–6. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts PJ, Usary JE, Darr DB, Dillon PM, Pfefferle AD, Whittle MC, et al. Combined PI3K/mTOR and MEK inhibition provides broad antitumor activity in faithful murine cancer models. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:5290–303. doi: 10.1158/1078-0432.CCR-12-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eser S, Reiff N, Messer M, Seidler B, Gottschalk K, Dobler M, et al. Selective requirement of PI3K/PDK1 signaling for Kras oncogene-driven pancreatic cell plasticity and cancer. Cancer Cell. 2013;23:406–20. doi: 10.1016/j.ccr.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 15.Cox AD, Der CJ. The raf inhibitor paradox: unexpected consequences of targeted drugs. Cancer Cell. 2010;17:221–3. doi: 10.1016/j.ccr.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 16.Yeh TC, Marsh V, Bernat BA, Ballard J, Colwell H, Evans RJ, et al. Biological characterization of ARRY-142886 (AZD6244), a potent, highly selective mitogenactivated protein kinase kinase 1/2 inhibitor. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:1576–83. doi: 10.1158/1078-0432.CCR-06-1150. [DOI] [PubMed] [Google Scholar]
- 17.Adjei AA, Cohen RB, Franklin W, Morris C, Wilson D, Molina JR, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral, small-molecule mitogenactivated protein kinase kinase 1/2 inhibitor AZD6244 (ARRY-142886) in patients with advanced cancers. J Clin Oncol. 2008;26:2139–46. doi: 10.1200/JCO.2007.14.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bardeesy N, Aguirre AJ, Chu GC, Cheng KH, Lopez LV, Hezel AF, et al. Both p16(Ink4a) and the p19(Arf)-p53 pathway constrain progression of pancreatic adenocarcinoma in the mouse. Proc Natl Acad Sci U S A. 2006;103:5947–52. doi: 10.1073/pnas.0601273103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou D, Conrad C, Xia F, Park JS, Payer B, Yin Y, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16:425–38. doi: 10.1016/j.ccr.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaira V, Fedele G, Pyne S, Fasoli E, Zadra G, Bailey D, et al. Preclinical model of organotypic culture for pharmacodynamic profiling of human tumors. Proc Natl Acad Sci U S A. 2010;107:8352–6. doi: 10.1073/pnas.0907676107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faber AC, Li D, Song Y, Liang MC, Yeap BY, Bronson RT, et al. Differential induction of apoptosis in HER2 and EGFR addicted cancers following PI3K inhibition. Proc Natl Acad Sci U S A. 2009;106:19503–8. doi: 10.1073/pnas.0905056106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faber AC, Corcoran RB, Ebi H, Sequist LV, Waltman BA, Chung E, et al. BIM Expression in Treatment-Naïve Cancers Predicts Responsiveness to Kinase Inhibitors Cancer Discovery. 2011 doi: 10.1158/2159-8290.CD-11-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navas C, Hernandez-Porras I, Schuhmacher AJ, Sibilia M, Guerra C, Barbacid M. EGF receptor signaling is essential for k-ras oncogene-driven pancreatic ductal adenocarcinoma. Cancer Cell. 2012;22:318–30. doi: 10.1016/j.ccr.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]