Abstract
BirA is a biotin ligase from Escherichia coli that specifically biotinylates a lysine side-chain within a 15-amino acid acceptor peptide (also known as Avi-tag). We developed a protocol for producing recombinant BirA ligase in E. coli for in vitro biotinylation (Li and Sousa, Prot Expr Purif, 82:162–167, 2012) in which the target protein was expressed as both thioredoxin and MBP fusions, and was released by TEV protease-mediated cleavage. The liberated ligase and the fusion proteins were enzymatically active. Based on that observation, we have now developed a novel system for in vivo biotinylation by co-expressing the Avi-tagged target protein with the MBP–BirA fusion. The effectiveness of this system was demonstrated by the successful in vivo labeling of antimicrobial protein, scygonadin. This new system shows improved efficiency compared with pre-existing one and this is likely attributed to the high expression level and solubility of the co-expressed MBP–BirA.
Keywords: Biotinylation, BirA, Co-expression, Fusion protein, Thrombin cleavage, Thioredoxin
Introduction
Biotin binds avidin and streptavidin with high affinity (Weber et al. 1989). Proteins can be covalently labeled with biotin through chemical or enzymatic means. The extremely tight binding between biotin and avidin or streptavidin makes biotin-labeling a useful tool for many applications such as detection, immobilization, and purification (Bayer and Wilchek 1990). The biotin ligase, BirA, from E. coli can catalyze the covalent attachment of biotin to the lysine side chain within a 15-amino acid peptide termed the Avi-tag (Barker and Campbell 1981; Schatz 1993; Beckett et al. 1999; Cull and Schatz 2000) and many proteins have been labeled with biotin via the Avi-tag for a variety of purposes (Crawford et al. 1998; Duffy et al. 1998; Chen et al. 2005; Howarth and Ting 2008).
Although BirA-mediated biotinylation can be carried out in vitro and in vivo, the latter approach has the advantage of avoiding purification of the ligase. In general, in vivo biotinylation is achieved by co-expression of Avi (or other biotin acceptor peptide) tagged protein of interest with the bacterial biotin ligase, BirA. This strategy has been used for in vivo labeling of target protein in bacteria (Smith et al. 1998; Verhaegen and Christopoulos 2002), yeasts (Scholler et al. 2006), insects (Yang et al. 2004; Strübbe et al. 2011) and mammalian cells (Predonzani et al. 2008; Viens et al. 2008). Applying the same strategy, avidity (Aurora, Colorado) developed a commercial system for in vivo biotinylation in E. coli. The key component of this system is an E. coli B strain (AVB101) that contains pBirAcm, an engineered pACYC184 plasmid with an inducible birA gene. When transformed with plasmid encoding the Avi-tagged target protein, strain AVB101 co-expresses BirA and the protein of interest, allowing in vivo biotinylation of the latter. However, while using this system we found that only a portion of the target protein was biotinylated in vivo and the efficiency varied from batch to batch. Others have experienced the same problems (personal communication with Dr. Claes von Wachenfeldt, Lund University, Sweden). We suspect that the incomplete and unstable biotin incorporation is caused by the poor expression and solubility of the BirA ligase encoded by pBirAcm (see results).
We previously developed a protocol for producing recombinant BirA ligase in E. coli for in vitro biotinylation (Li and Sousa 2012). In that approach, the target protein was expressed as both thioredoxin- and maltose-binding protein (MBP) fusions, and was released by tobacco etch virus (TEV) protease-mediated cleavage. Not only the liberated ligase but also the fusion proteins were highly active in catalyzing in vitro biotinylation. In this study, we have developed a novel system for in vivo biotinylation based on this observation. The effectiveness of this newly developed system was demonstrated by the successful in vivo labeling of scygonadin, an 11 kDa antimicrobial protein recently isolated from the mud crab (Huang et al. 2006; Wang et al. 2007).
Materials and methods
Materials
One-shot BL21(DE3) competent cell and dNTP mix were obtained from Invitrogen. E. coli B strain AVB101 harboring pBirAcm (a pACYC-184 plasmid that contains the birA gene) was purchased from avidity LLC (Aurora, CO). Plasmid pET-32a was obtained from Novagen. Genes encoding Avi-tag (plus a His-tag and a thrombin cleavage site) and scygonadin were synthesized by GenScript. Oligonucleotide primers were synthesized at the Nucleic Acids Core Facility at the University of Texas Health Science Center at San Antonio. Vent DNA polymerase, restriction enzymes, calf intestinal alkaline phosphatase (CIP), quick ligation kit and DNA markers were obtained from New England Biolabs. QIAquick PCR purification kit, QIAquick gel extraction kit, QIAprep spin miniprep kit, and Ni–NTA agarose were purchased from Qiagen. Other materials were from established commercial suppliers.
Vector modification and construction
We modified the commercial vector pET-32a by replacing the coding sequence for the 15-amino acid S tag with that encoding the same-sized Avi-tag (Supplementary Fig. 1a, b). This was achieved by amplifying the sequence encoding His tag and Avi-tag, which are connected by a thrombin cleavage site, with primers 1 and 2 (Supplementary Table 1) using a synthetic gene as template and inserting the MscI and KpnI doubly digested PCR product into similarly digested pET-32a. The modified vector was named pET-32a−Avi. The scygonadin coding sequence was amplified by PCR with primers 3 and 4 (Supplementary Table 1) also using a synthetic gene as template. The forward and reverse primers contain KpnI and NcoI sites, respectively. The forward primer also contains the sequence encoding a TEV protease cleavage site. The PCR amplified scygonadin coding sequence was digested with KpnI and NcoI, and ligated into the modified pET-32a plasmid doubly digested with the same enzymes. The resultant recombinant plasmid was named scygonadin/pET-32a−Avi (Supplementary Fig. 1c). The presence and identity of Avi-tag and scygonadin coding sequences in scygonadin/pET-32a−Avi was verified by diagnostic restriction digestion and DNA sequencing.
We previously constructed a vector, MBP–BirA/pET-28a (Supplementary Fig. 2a), for producing BirA expressed as a MBP fusion (Li and Sousa 2012). This vector was modified by deleting the coding sequence for a thrombin cleavage site existing in the original pET-28a vector. This was achieved by amplifying the corresponding DNA fragment without the thrombin site coding sequence with primers 5 and 6 (Supplementary Table 1) using pET-28a as template and inserting the BglII and NdeI doubly digested PCR product into similarly digested MBP–BirA/pET-28a. The resultant plasmid was named MBP–BirA/pET-28a (Supplementary Fig. 2b).
Production of biotinylated scygonadin
E. coli BL21(DE3) cells co-transformed with recombinant vectors scygonadin/pET-32a−Avi and MBP–BirA/pET-28a were grown overnight in 100 ml LB broth (with 100 µg ampicillin/ml and 50 µg kanamycin/ml) at 37 °C. This overnight culture was used to inoculate 1 l LB medium and cells were grown at 37 °C with shaking at 250 rpm. When the culture OD600 reached 0.6, protein expression was induced by adding IPTG at 1 mM. Biotin was also added at this point at 200 µM. The culture temperature was reduced to 30 °C following induction. After additional 6 h cultivation, cells were harvested by centrifugation at ~6,000×g for 10 min. The bacterial pellet (~4.7 g) was resuspended in 30 ml cell lysis buffer (25 mM Tris/HCl, 200 mM NaCl, pH 8.0) containing 10 % glycerol and cells were lysed by sonication. The cell lysate was then shaken at 4 °C for 15 min followed by centrifugation at ~30,000×g for 40 min. The supernatant was combined with 5 ml Ni–NTA resin suspension and shaken at 4 °C for 3 h, centrifuged (~100 g) for 5 min and the supernatant, containing unbound proteins, was discarded. After being washed twice with 35 ml lysis buffer containing 20 mM imidazole at 4 °C for 30 min, the resin was resuspended in 15 ml of cell lysis buffer and treated with 0.4 mg thrombin. The cleavage reaction was carried out with gentle shaking at room temperature for 16 h. Avi-tagged scygonadin released by on-resin cleavage was further purified by running through a HiLoad 16/60 Superdex 75 column equilibrated with cell lysis buffer.
Production of non-biotinylated scygonadin
E. coli BL21(DE3) harboring recombinant vector scygonadin/pET-32a−Avi was grown at 37 °C until OD600 reached 0.6, at which point IPTG was added at 1 mM. After 4 h, bacteria were harvested and resuspended in cell lysis buffer. Cells were lysed by sonication and the cell lysate supernatant was incubated with Ni–NTA resin for 3 h with shaking at 4 °C. After washing twice with 25 mM imidazole, the protein was eluted with cell lysis buffer containing 300 mM imidazole. After being further purified by running through a HiLoad 16/60 Superdex 75 column, the fusion protein was treated with thrombin protease. Avi-tagged scygonadin released by the cleavage was separated from the carrier portion using a HisTrap HP column (5 ml).
Confirmation of biotinylation
In vivo biotinylation was evaluated by incubating the labeled and unlabeled scygonadin with streptavidin-sepharose, which should capture the biotinylated version of the target protein.
Results
Untagged BirA is insoluble when expressed and does not drive efficient in vivo biotinylation
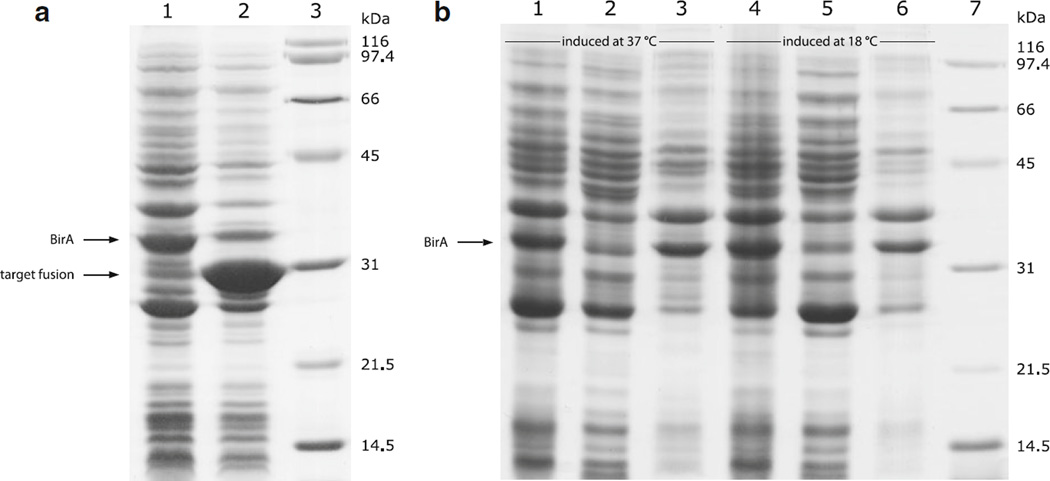
We previously used avidity’s system to produce a biotinylated protein, whose coding sequence was inserted into the T7 RNAP driven expression vector pET-32a (Li and Sousa 2012). Since the AVB101 strain, which contains the BirA encoding vector pBirAcm, does not have a genomic gene expressing T7 RNAP and will therefore not express genes from the T7 promoter-driven pET-32a vector, we isolated pBirAcm and co-transformed it with pET-32a harboring the target gene into E. coli strain BL21(DE3). BirA was co-expressed with the Avi-tagged target protein. However, only a portion of the target protein was biotinylated in vivo in this way, and the biotinylation efficiency varied from batch to batch. The expression level of BirA also dropped significantly when it was co-expressed with the Avi-tagged target protein (Fig. 1a). In addition, untagged BirA was largely insoluble when protein expression was induced at 37 °C and reducing the temperature to 18 °C did not help (Fig. 1b). We suspect that the low and unstable biotinylation efficiency of this system is caused by the poor expression and solubility of the BirA ligase in the absence of any solubility tags.
Fig. 1.
Expression level and solubility of untagged BirA. a Changes in BirA expression level as following by SDS-PAGE (12 %). Lane 1, BirA expressed by itself; Lane 2, BirA co-expressed with thioredoxin-scygonadin fusion; Lane 3, protein standards. b Analysis of BirA solubility when protein expression was induced at 37 °C (lane 1–3) and 18 °C (lane 4–6). The untagged BirA was expressed by itself without any substrate. Lanes 1 and 4, whole cell lysate; Lanes 2 and 5, cell lysate supernatant; Lanes 3 and 6, cell lysate pellet (resuspended in 8 M urea); Lane 7, protein standards
Production of biotinylated scygonadin
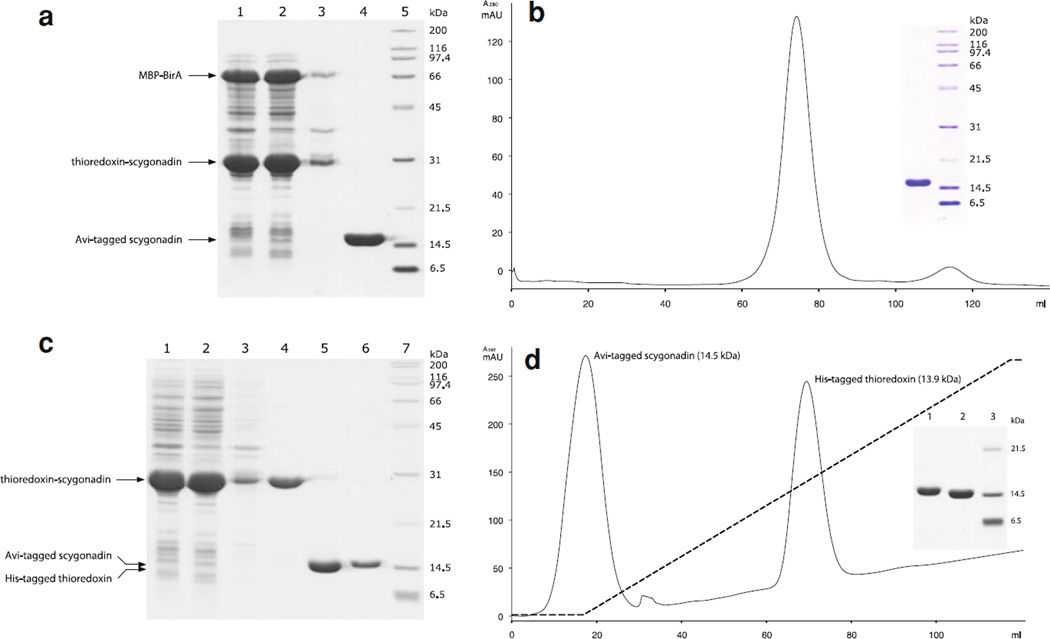
Our new system was based on the co-expression of Avi-tagged target protein (as a thioredoxin fusion) and MBP–BirA, the schematic representations of which are shown in Supplementary Fig. 3. These two fusion proteins were well expressed and mainly found in the soluble portion when protein expression was induced at 30 °C (Fig. 2a, Lane 1–3). They both contain His-tags and bind to the Ni–NTA resin. On-resin thrombin cleavage allowed the Avi-tagged scygonadin to be released (Fig. 2a, Lane 4). The final product was obtained in high quality after being further purified by size-exclusion chromatography (Fig. 2b).
Fig. 2.
Production of biotinylated and non-biotinylated scygonadin. a Co-expression of thioredoxin-scygonadin with MBP–BirA and target protein release as followed by SDS-PAGE (12 %). Lane 1, whole cell lysate; Lane 2, cell lysate supernatant; Lane 3, cell lysate pellet (resuspended in 8 M urea); Lane 4, scygonadin released from the thioredoxin fusion by on-resin thrombin cleavage; Lane 5, protein standards. b Size-exclusion chromatography elution profile of the Avi-tagged scygonadin released from on-resin thrombin cleavage. Inset shows SDS-PAGE analysis of the major peak component. c Thioredoxin-scygonadin fusion expression, purification, cleavage and target protein isolation as followed by SDS-PAGE (12 %). Lane 1, whole cell lysate; Lane 2, cell lysate supernatant; Lane 3, cell lysate pellet (resuspended in 8 M urea); Lane 4, affinity and size-exclusion chromatography purified fusion protein; Lane 5, reaction mixture after TEV protease cleavage (the two fragments resulted from the cleavage, which are very similar in size, run as one band on the gel); Lane 6, purified Avi-tagged scygonadin (not labeled); Lane 7, protein standards. d Imidazole gradient (indicated by a dashed line) elution of thrombin protease cleaved thioredoxin-scygonadin fusion from a HisTrap HP column. Inset shows SDS-PAGE analysis of the fractions covering the two peaks
Production of non-biotinylated scygonadin
Thioredoxin-scygonadin fusion protein was well expressed and highly soluble (Fig. 2c, Lane 1–3). The fusion can be purified to a high degree using affinity and size-exclusion chromatography (Fig. 2c, Lane 4). The thrombin cleavage reaction, which released the target protein, was complete in 16 h at room temperature when 10 µg thrombin per mg fusion protein was used (Fig. 2c, Lane 5). The released scygonadin can be readily separated from the carrier portion using a HisTrap HP column (Fig. 2c, Lane 6d).
Confirmation of biotinylaton
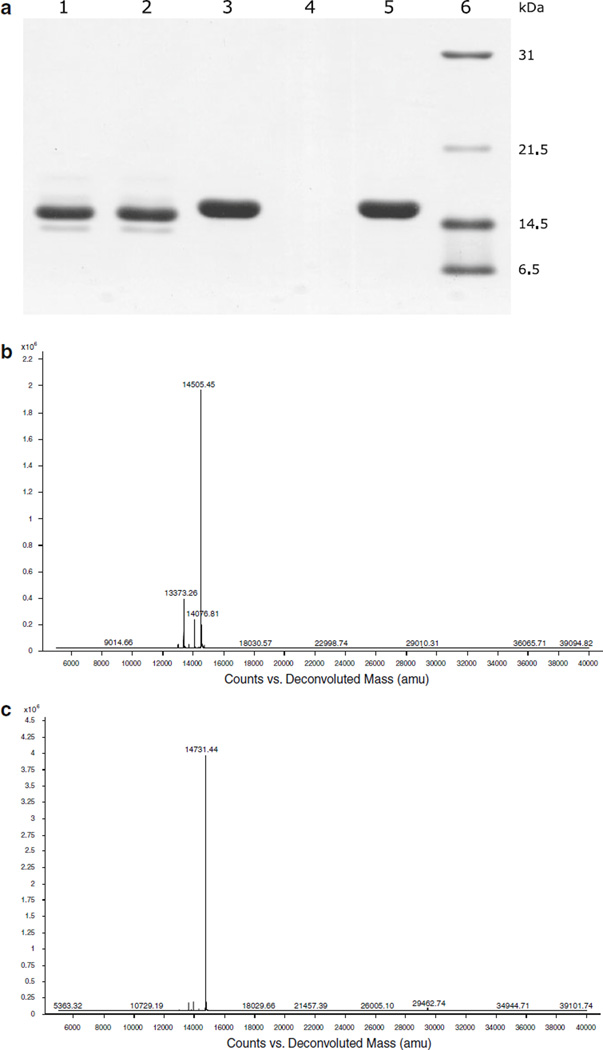
After incubation with streptavidin-sepharose, Avi-tagged scygonadin from the control reaction (non-biotinylated form) remained in the solution (Fig. 3a, Lane 2) whereas the in vivo labeled substrate was captured and completely disappeared (Fig. 3a, Lane 4). This result indicated not only that the scygonadin co-expressed with MBP–BirA was successfully labeled but also that the labeling was complete. The captured substrate can be recovered by applying biotin to the resin (Fig. 3a, Lane 5). In addition, similar to the observation made with biotinylated thioredoxin (Li and Sousa 2012), labeled scygonadin migrates slightly slower than its unlabeled counterpart. Finally, biotinylation was further confirmed by mass spectrometry analysis (Fig. 3b, c). The measured molecular weight for scygonadin labeled with biotin (14731.44) perfectly matches the expected value (14731.54).
Fig. 3.
Confirmation of biotinylation. a Capture of biotin-labeled scygonadin by streptavidin-sepharose. Lane 1, unlabeled scygonadin; Lane 2, solution of unlabeled scygonadin after incubation with streptavidin-sepharose; Lane 3, in vivo labeled scygonadin; Lane 4, solution of biotin-labeled scygonadin after incubation with streptavidin-sepharose; Lane 5, biotin eluate that recovers the labeled scygonadin captured by the resin; Lane 6, protein standards. b, c Mass spectra of unlabeled and in vivo biotin labeled scygonadin, respectively. The determined molecular weights (14505.45 and 14731.44, respectively) matched the corresponding theoretical values (14505.24 and 14731.54, respectively)
Discussion
We previously developed a protocol for producing recombinant BirA ligase in E. coli (Li and Sousa 2012). The enzyme was expressed as thioredoxin and MBP fusions, both of which proved to be active in catalyzing in vitro biotinylation. In this study, we co-expressed the Avi-tag bearing target protein with MBP–BirA. Unlike the untagged BirA encoded by pBirAcm, MBP–BirA fusion was highly overexpressed together with the target protein. While producing the recombinant BirA ligase we found that MBP–BirA fusion was largely insoluble when protein expression was induced at 37 °C and switched culture temperature to 18 °C after adding IPTG to obtain soluble protein (Li and Sousa 2012). In this study co-expression of Avi-tagged target and MBP–BirA was induced at 30 °C, which increased the efficiency of in vivo biotinylation but still resulted in both fusion proteins being found mainly in the soluble portion (Fig. 2a, Lane 1–3). The Avi-tagged target protein, scygonadin, was efficiently biotinylated in vivo, which was unambiguously demonstrated by the substrate’s ability to interact with streptavidin, its different migration rate on SDS-gel compared with the unlabeled form and mass spectrometry analysis (Fig. 3). The high expression level and solubility of MBP–BirA likely contributes to the improved efficiency of this newly developed in vivo biotinylation system.
In addition to in vivo biotinylation of Avi-tagged substrate, the system also allowed production of a significant amount of MBP–BirA fusion, which was co-expressed with the target protein. After scygonadin was released by on-resin thrombin cleavage, the MBP–BirA fusion bound to the resin can be eluted with imidazole and separated from the co-eluted thioredoxin (left from thrombin cleavage) using a gel filtration column (Supplementary Fig. 4). We previously learned that labeled proteins may gradually lose their biotin during storage and the purified MBP–BirA fusion can be used to relabel these substrates in vitro (Li and Sousa 2012).
In summary, we have developed a novel system for in vivo biotinylation, which is based on co-expression of Avi-tagged target protein with MBP–BirA fusion. The efficiency of this system was demonstrated by the successful in vivo labeling of the antimicrobial protein scygonadin.
Supplementary Material
Acknowledgments
This work was supported by departmental funding dedicated to the protein production core facility and NIH-GM52522 (to R.S.). The authors would like to thank Sammy Pardofor and Dr. Susan Weintraub for mass spectrometry analysis.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10529-012-0942-3) contains supplementary material, which is available to authorized users.
References
- Barker DF, Campbell AM. The BirA gene of Escherichia coli encodes a biotin holoenzyme synthetase. J Mol Biol. 1981;146:451–467. doi: 10.1016/0022-2836(81)90042-5. [DOI] [PubMed] [Google Scholar]
- Bayer EA, Wilchek M. Protein biotinylation. Methods Enzymol. 1990;184:138–160. doi: 10.1016/0076-6879(90)84268-l. [DOI] [PubMed] [Google Scholar]
- Beckett D, Kovaleva E, Schatz PJ. A minimal peptide substrate in biotin holoenzyme synthetase-catalyzed biotinylation. Protein Sci. 1999;8:921–929. doi: 10.1110/ps.8.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I, Howarth M, Lin W, Ting AY. Site-specific labeling of cell surface proteins with biophysical probes using biotin ligase. Nat Methods. 2005;2:99–104. doi: 10.1038/nmeth735. [DOI] [PubMed] [Google Scholar]
- Crawford F, Kozono H, White J, Marrack P, Kappler J. Detection of antigen-specific T cells with multivalent soluble class II MHC covalent peptide complexes. Immunity. 1998;8:675–682. doi: 10.1016/s1074-7613(00)80572-5. [DOI] [PubMed] [Google Scholar]
- Cull MG, Schatz PJ. Biotinylation of proteins in vivo and in vitro using small peptide tags. Methods Enzymol. 2000;326:430–440. doi: 10.1016/s0076-6879(00)26068-0. [DOI] [PubMed] [Google Scholar]
- Duffy S, Tsao KL, Waugh DS. Site-specific, enzymatic biotinylation of recombinant proteins in Spodoptera frugiperda cells using biotin acceptor peptides. Anal Biochem. 1998;262:122–128. doi: 10.1006/abio.1998.2770. [DOI] [PubMed] [Google Scholar]
- Howarth M, Ting AY. Imaging proteins in live mammalian cells with biotin ligase and monovalent streptavidin. Nat Protoc. 2008;3:534–545. doi: 10.1038/nprot.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WS, Wang KJ, Yang M, Cai JJ, Li SJ, Wang GZ. Purification and part characterization of a novel antibacterial protein scygonadin, isolated from the seminal plasma of mud crab, Scylla serrata (Forskål 1775) J Exp Mar Biol Ecol. 2006;339:37–42. [Google Scholar]
- Li Y, Sousa R. Expression and purification of E. coli BirA biotin ligase for in vitro biotinylation. Protein Expr Purif. 2012;82:162–167. doi: 10.1016/j.pep.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Predonzani A, Arnoldi F, López-Requena A, Burrone OR. In vivo site-specific biotinylation of proteins within the secretory pathway using a single vector system. BMC Biotechnol. 2008;8:41. doi: 10.1186/1472-6750-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz PJ. Use of peptide libraries to map the substrate specificity of a peptide-modifying enzyme: a 13 residue consensus peptide specifies biotinylation in Escherichia coli. Biotechnology (NY) 1993;11:1138–1143. doi: 10.1038/nbt1093-1138. [DOI] [PubMed] [Google Scholar]
- Scholler N, Garvik B, Quarles T, Jiang S, Urban N. Method for generation of in vivo biotinylated recombinant antibodies by yeast mating. J Immunol Methods. 2006;317:132–143. doi: 10.1016/j.jim.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PA, Tripp BC, DiBlasio-Smith EA, Lu Z, LaVallie ER, McCoy JM. A plasmid expression system for quantitative in vivo biotinylation of thioredoxin fusion proteins in Escherichia coli. Nucleic Acids Res. 1998;26:1414– 1420. doi: 10.1093/nar/26.6.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strübbe G, Popp C, Schmidt A, Pauli A, Ringrose L, Beisel C, Paro R. Polycomb purification by in vivo biotinylation tagging reveals cohesin and Trithorax group proteins as interaction partners. Proc Natl Acad Sci USA. 2011;108:5572– 5577. doi: 10.1073/pnas.1007916108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaegen M, Christopoulos TK. Bacterial expression of in vivo-biotinylated aequorin for direct application to bioluminometric hybridization assays. Anal Biochem. 2002;306:314–322. doi: 10.1006/abio.2002.5724. [DOI] [PubMed] [Google Scholar]
- Viens A, Harper F, Pichard E, Comisso M, Pierron G, Ogryzko V. Use of protein biotinylation in vivo for immunoelectron microscopic localization of a specific protein isoform. J Histochem Cytochem. 2008;56:911–919. doi: 10.1369/jhc.2008.951624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KJ, Huang WS, Yang M, Chen HY, Bo J, Li SJ, Wang GZ. A male-specific expression gene, encodes a novel anionic antimicrobial peptide, scygonadin, in Scylla serrate. Mol Immunol. 2007;44:1961–1968. doi: 10.1016/j.molimm.2006.09.036. [DOI] [PubMed] [Google Scholar]
- Weber PC, Ohlendorf DD, Wendoloski JJ, Salemme FR. Structural origins of high-affinity biotin binding to streptavidin. Science. 1989;243:85–88. doi: 10.1126/science.2911722. [DOI] [PubMed] [Google Scholar]
- Yang J, Jaramillo A, Shi R, Kwok WW, Mohanakumar T. In vivo biotinylation of the major histocompatibility complex (MHC) class II/peptide complex by coexpression of BirA enzyme for the generation of MHC class II/tetramers. Hum Immunol. 2004;65:692–699. doi: 10.1016/j.humimm.2004.04.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.