Abstract
BACKGROUND
Predicting life expectancy (LE) in patients with metastatic cancer who are receiving palliative therapies is a difficult task. The purpose of the current study was to develop a LE prediction model among patients receiving palliative radiotherapy (RT) that identifies those patients with short (<3 months) and long (>1 year) LEs.
METHODS
The records of 862 patients with metastatic cancer receiving palliative RT at the Dana-Farber/Brigham and Women’s Cancer Center between June 2008 and July 2011 were retrospectively reviewed. Cox proportional hazards models were used to evaluate established and potential clinical predictors of LE to construct a model predicting LE of <3 months and >1 year.
RESULTS
The median survival was 5.6 months. On multivariate analysis, factors found to be significantly associated with a shorter LE were cancer type (lung and other vs breast and prostate), older age (>60 years vs ≤60 years), liver metastases, Eastern Cooperative Oncology Group performance status (2–4 vs 0–1), hospitalizations within 3 months before palliative RT (0 vs ≥1), and prior palliative chemotherapy courses (≥2 vs 0–1). Patients were divided into 3 groups with distinct median survivals: group A (those with 0–1 risk factors), 19.9 months (95% confidence interval [95% CI, 13.9 months–31.1 months]); group B (those with 2–4 risk factors), 5.0 months (95% CI, 4.3 months–5.6 months); and group C (those with 5–6 risk factors), 1.7 months (95% CI, 1.2 months–2.1 months).
CONCLUSIONS
The TEACHH model (type of cancer, Eastern Cooperative Oncology Group performance status, age, prior palliative chemotherapy, prior hospitalizations, and hepatic metastases) divides patients receiving palliative RT into 3 distinct LE groups at clinically informative extremes of the LE spectrum. It holds promise to assist radiation oncologists in tailoring palliative therapies to a patient’s LE.
Keywords: life expectancy, metastatic, palliative, prediction, model
INTRODUCTION
Oncologists often face the difficult task of estimating prognosis in patients with metastatic disease whose life expectancies (LEs) can vary from days to years. A physician’s ability to predict prognosis accurately has important clinical implications, including determining the potential benefit of palliative cancer therapies and initiating end-of-life conversations and planning. Radiotherapy (RT) is frequently used to provide palliation of symptomatic metastases in patients with advanced disease. In 2010, Gripp et al published a study of 216 patients referred for consideration of palliative RT between December 2003 and July 2004.1 The study demonstrated that of 30 patients who received RT within the last month of life, 27% were undergoing treatment for the last 81% to 100% of their lives. In a Surveillance, Epidemiology, and End Results (SEER) study performed by Guadagnolo et al in 2013, among 15,287 patients who received RT in the last month of life, 17.8 % received >10 days of treatment.2 These data point to the need for improved tailoring of palliative RT to a patient’s LE, with regard to both decisions for its use and dose fractionation schedules.
Although physicians use LE to inform clinical decision-making, their predictions tend to be inaccurate.3–5 Improving the ability of radiation oncologists to tailor palliative RT to each patient’s illness trajectory is a key element of quality care for advanced cancer as highlighted within the American Society of Clinical Oncology 2011 statement entitled “Toward Individualized Care for Patients With Advanced Cancer.”6 Critical to achieving this goal is a tool that improves a physician’s ability to estimate survival in patients receiving palliative RT and is sufficiently informative to impact decision-making. More specifically, a prognostic tool should 1) provide accurate predictions of survival together with confidence intervals (CIs) to characterize expected ranges of LE and 2) predict LEs at clinically significant time points. For example, a LE <6 months is critical to hospice eligibility and for revisiting goals of care and end-of-life planning within the context of a limited prognosis.7–9 Important LE thresholds to palliative RT decision-making include ≤3 months (eg, eligibility for operative management of spinal cord compressions10,11) and >1 year (eg, potential use of dose escalation due to a high rate of disease recurrence after conventional palliative RT doses12).
Chow et al created and validated a prognostic model based on a cohort of patients who received palliative RT at 2 centers in Toronto, Ontario, Canada.13 This model categorized palliative cancer patients into 1 of 3 prognostic groups using 3 clinical factors: cancer type (breast vs nonbreast), Karnofsky performance status (PS) (<70 vs ≥70), and metastasis location (bone only vs other). The median survivals of the 3 groups were 13.8 months in group A (95% CI, 8.6 months–16.3 months), 6.0 months in group B (95% CI, 4.7 months–7.2 months), and 2.1 months in group C (95% CI, 1.4 months–2.6 months). The model has also been validated among a cohort of patients with breast and prostate cancer who were receiving palliative RT for bone metastases.14
The objectives of the current study were to 1) evaluate the model developed by Chow et al13 and 2) build on this model to create a model that predicts median survivals and identifies patients at the extremes of the prognostic spectrum, namely those with LE ranges of ≤3 months and >1 year.
MATERIALS AND METHODS
Data from all patients treated with palliative-intent RT from July 2008 to June 2010 at Brigham and Women’s Hospital and Dana-Farber Cancer Institute were analyzed. Patients were excluded if they were aged <18 years, had hematologic malignancies, were classified incorrectly as receiving palliative care, or had nonmetastatic disease. The charts of all patients were assessed for disease, treatment, and patient factors that could influence patient LE, including those previously reported as well as other potential factors based on related literature: age at treatment,15 primary tumor origin,13,16 location of all metastases present at the time of radiation consult,13,17 Eastern Cooperative Oncology Group (ECOG) PS,13,16–23 hospitalizations within the 3 months before the radiation consult,24 time from primary cancer diagnosis to diagnosis of metastatic disease,19 time from diagnosis of metastatic disease to radiation consult,19 and number of prior palliative chemotherapy and RT courses.25 A total of 249 patients had undergone >1 course of RT during the time period analyzed. To eliminate interdependence of courses, we analyzed only each patient’s first course.
The primary outcome was overall survival (OS), defined as the time from the first day of RT to the date of death or last follow-up. Dates of death were obtained via the publicly available Social Security Death Index. Patients with no date of death listed in the Social Security Death Index were censored at the date of last follow-up.
Statistical Analysis
Cox proportional hazards models were used to evaluate the association between each of the 3 factors used in the model of Chow et al13 with patient LE. Using the significant prognostic factors identified on multivariable analysis (MVA), patients were classified into 3 risk groups based on the number of risk factors (NRF) method as described by Chow et al.13 Each patient’s NRF score was based on the sum of those predictors present. Median survivals and 95% CIs were calculated for each group and compared with those in the model of Chow et al.13
To create a new prognostic model identifying patients at the extremes of the LE spectrum (median survivals and 95% CIs of <3 months and >1 year), we used Cox regression analysis to evaluate the associations between LE and the factors included in the model by Chow et al13 as well as the other aforementioned factors potentially predictive of LE. The Collett model selection method26 was used with a level of statistical significance of .20 for the univariate screening and stay and entry criteria of .05 for the MVA. Using the statistically significant prognostic factors identified on MVA, patients were classified into 3 risk groups based on the partial score method (PSM) and NRF method analogous to those performed by Chow et al.13
For the PSM, we created a survival prediction score for each patient by assigning a partial score to each factor found to be significant on MVA. The partial score was derived by dividing the value of each statistically significant regression coefficient by the smallest statistically significant regression coefficient in the full model, and the results were rounded to the nearest half-integer. Each regression coefficient was then multiplied by 2 to obtain a whole number. Partial scores for each variable were then summed to calculate a total PSM score for each patient. Each patient’s NRF score was based on the sum of those predictors present.
PSM and NRF scores were then used to classify patients into 3 groups with the goal of identifying those patients with the worst (≤3 months) and best (>1 year) LEs. Survival curves according to the PSM and NRF methods were calculated using the Kaplan-Meier method. To evaluate the adequacy of the risk prediction of the models, the C-statistic proposed by Uno et al was used.27 The C-statistic is the probability that the predicted and observed outcomes are concordant for a randomly selected pair of patients. A value of 0.5 implies no predictive discrimination and a value of 1.0 indicates perfect separation of patients with good outcomes from those with bad outcomes.27
All analyses were performed using SAS (version 9.2; SAS Institute Inc, Cary, NC) and R (version 2.10.0; R Foundation for Statistical Computing, Vienna, Austria) statistical software and 2-sided P values <.05 were considered significant.
RESULTS
Between July 2008 and June 2010, 1077 patients were treated with palliative-intent RT at the Brigham and Women’s Hospital and Dana-Farber Cancer Institute. A total of 215 patients were excluded because: 1) the intent of treatment was listed incorrectly as palliative (i.e, the patient’s cancer diagnosis and treatment were consistent with curative management); 2) the patients had hematologic malignancies; and/or 3) the patients did not have metastatic disease. The remaining 862 patients were included in the current analysis. Patient characteristics are listed in Table 1. The lung was the most common primary tumor site (42%) and the majority of patients (60%) had bone metastases at the time of radiation consult. The median OS survival was 5.6 months.
TABLE 1.
Patient Characteristics (N = 862)
| Characteristic | No. | % |
|---|---|---|
| Sex | ||
| Male | 420 | 48.7% |
| Female | 442 | 51.3% |
| Type of cancer | ||
| Breast | 86 | 10.0% |
| Prostate | 63 | 7.3% |
| Lung | 365 | 42.3% |
| Othera | 348 | 40.4% |
| Location of metastasis | ||
| Bone | 514 | 59.6% |
| Lung | 177 | 20.5% |
| Liver | 203 | 23.6% |
| CNS | 354 | 41.1% |
| Other | 205 | 23.8% |
| Metastasis burden | ||
| Bone only | 227 | 26.3% |
| Non-bone only | 635 | 73.7% |
| KPS | ||
| ≤60 | 225 | 26.1% |
| >60 | 637 | 73.9% |
| ECOG PS | ||
| 0, 1 | 514 | 59.6% |
| 2 | 216 | 25.1% |
| 3, 4 | 132 | 15.3% |
| Prior hospitalizations within the last 3 mo | ||
| No | 385 | 44.7% |
| Yes | 477 | 55.3% |
| Age at treatment, y | ||
| ≤60 | 384 | 44.5% |
| >60 | 478 | 55.5% |
| Median | 62 | |
| Range | 22–98 | |
| No. of prior palliative chemotherapy courses | ||
| 0 | 466 | 54.1% |
| 1–2 | 249 | 28.9% |
| >2 | 147 | 17.1% |
| Median | 0 | |
| Range | 0–12 | |
| No. of prior palliative RT courses | ||
| No | 756 | 87.7% |
| Yes | 106 | 12.3% |
| Median | 0 | |
| Range | 0–4 | |
| Time from primary diagnosis to metastasis diagnosis, mo | ||
| Median | 0.3 | |
| Range | 0–380.0 | |
| Time from metastasis diagnosis to RT consult, mo | ||
| Median | 1.5 | |
| Range | 0–273.6 | |
Abbreviations: CNS, central nervous system; ECOG PS, Eastern Cooperative Oncology Group performance status; KPS, Karnofsky performance status; RT, radiotherapy.
Composition of “Other” category: colorectal, 4.2%; pancreas, 1.7%; stomach, 0.35%; esophageal, 3.1%; gynecological, 4.9%; lymphoma, 2.1%; melanoma, 5.3%; renal, 5.2%; head and neck, 1.4%; CNS primary, 0.1%; sarcoma, 2.6%; and other, 9.4%.
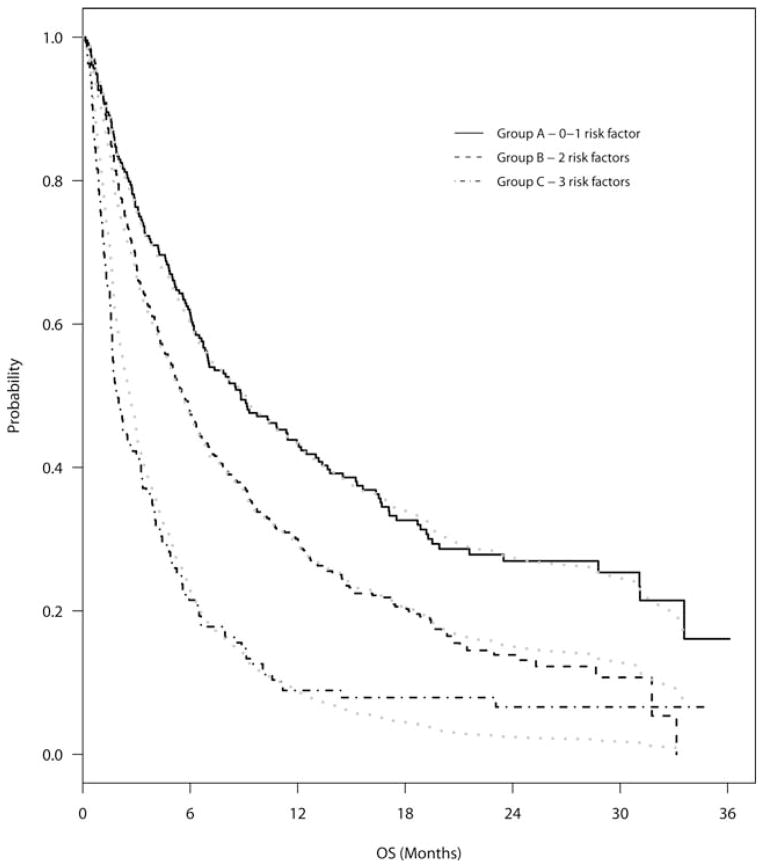
All 3 risk factors identified in the model by Chow et al13 were found to be statistically significantly associated with OS in our data set. However, although the hazards ratio associated with prostate cancer in the model by Chow et al was 1.93 and significantly increased compared with breast cancer,13 it was not found to be significantly increased in our model. The 3 factors used in the model by Chow et al (cancer histology, bone-only metastases vs nonbone-only metastases, and Karnofsky PS) divided our patients into 3 distinct survival groups using the NRF method. The median survivals in our cohort were 8.8 months in group A (those with 0–1 risk factors) (95% CI, 6.7 months–12.0 months), 5.7 months in group B (those with 2 risk factors) (95% CI, 4.9 months–6.5 months), and 2.0 months in group C (those with 3 risk factors) (95% CI, 1.6 months–3.0 months). These groups are shown in Figure 1.13
Figure 1.
Overall survival (OS) is shown for the survival groups using the number of risk factors model by Chow et al.13 Survival estimated using the Cox regression method (gray line) and actual survival calculated by the Kaplan-Meier method (black line) are shown.
Results of univariate analyses examining the factors of Chow et al13 and the other potential predictors of patient LE are shown in Table 2. On MVA, 6 factors (for which we used the acronym TEACHH) were found to be significant predictors of OS: type of cancer (“T”) (breast, prostate, lung, or other); ECOG PS (“E”) (0–1, 2, and 3–4); 3) age at treatment (“A”) (<60 years vs ≥60 years); 4) prior palliative chemotherapy (“C”) (0–2 or >2); prior hospitalizations within the last 3 months (“H”) (0 or ≥1); and hepatic metastases (“H”). Hazards ratios and partial scores for each of these variables are shown in Table 3.
TABLE 2.
Univariate and Multivariate Analyses of Potential Predictors of Overall Survival in Patients With Advanced Cancer
| Variable | Univariate Analysis
|
Multivariate Analysis
|
|||||
|---|---|---|---|---|---|---|---|
| HR | P | HR | P | Parameter Estimate | Partial Scorea | NRF Scoreb | |
| Type of cancer | .0001 | ||||||
| Breast | 1.00 | — | 1.00 | — | 0.00 | 0.0 × 2 = 0 | 0 |
| Prostate | 1.20 | .38 | 1.29 | .2182 | 0.26 | 0.0 × 2 = 0 | 0 |
| Lung | 1.77 | .0001 | 2.04 | <.0001 | 0.71 | 2.5 × 2 = 5 | 1 |
| Other | 1.52 | .0046 | 1.43 | .0175 | 0.36 | 1.0 × 2 = 2 | 1 |
| ECOG PS | <.0001 | ||||||
| 0, 1 | 1.00 | — | 1.00 | — | 0.00 | 0.0 × 2 = 0 | 0 |
| 2 | 2.00 | <.0001 | 1.77 | <.0001 | 0.57 | 2.0 × 2 = 4 | 1 |
| 3, 4 | 2.68 | <.0001 | 2.42 | <.0001 | 0.88 | 3.0 × 2 = 6 | 1 |
| Location of metastasis in liver | |||||||
| No | 1.00 | — | 1.00 | — | 0.00 | 0.0 × 2 = 0 | 0 |
| Yes | 1.63 | <.0001 | 1.78 | <.0001 | 0.58 | 2.0 × 2 = 4 | 1 |
| No. of prior palliative chemotherapy courses | |||||||
| 0–2 | 1.00 | — | 1.00 | — | 0.00 | 0.0 × 2 = 0 | 0 |
| >2 | 1.41 | .0006 | 1.70 | <.0001 | 0.53 | 1.5 × 2 = 3 | 1 |
| Age at treatment, y | |||||||
| ≤60 | 1.00 | — | 1.00 | — | 0.00 | 0.0 × 2 = 0 | 0 |
| >60 | 1.32 | .0005 | 1.37 | .0001 | 0.31 | 1.0 × 2 = 2 | 1 |
| Prior hospitalizations in the last 3 mo | |||||||
| No | 1.00 | — | 1.00 | — | 0.00 | 0.0 × 2 = 0 | 0 |
| Yes | 1.60 | <.0001 | 1.38 | .0002 | 0.32 | 1.0 × 2 = 2 | 1 |
| Location of metastasis: bone | |||||||
| No | 1.00 | — | |||||
| Yes | 1.05 | .55 | |||||
| Location of metastasis: lung | |||||||
| No | 1.00 | — | |||||
| Yes | 1.02 | .83 | |||||
| Location of metastasis: CNS | |||||||
| No | 1.00 | — | |||||
| Yes | 1.02 | .79 | |||||
| Location of metastasis: other | |||||||
| No | 1.00 | — | |||||
| Yes | 1.39 | .0003c | |||||
| Prior RT courses | |||||||
| No | 1.00 | — | |||||
| Yes | 1.08 | .50 | |||||
| Log time from primary diagnosis to metastasis diagnosis | 0.99 | .0882 | |||||
| Log time from metastasis diagnosis to RT consult | 0.98 | .11 | |||||
Abbreviations: CNS, central nervous system; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazards ratio; NRF, number of risk factors; RT, radiotherapy.
Partial score method: a survival prediction score was created for each patient by assigning a partial score to each factor found to be significant on multivariate analysis. The partial score was derived by dividing the value of each statistically significant regression coefficient (parameter estimate) by the smallest statistically significant regression coefficient in the full model, and the results were rounded to the nearest half-integer. Each regression coefficient was then multiplied by 2 to obtain a whole number. For example, the regression coefficient for >2 courses of palliative chemotherapy was 0.53. This was divided by 0.31, the smallest statistically significant regression coefficient (age >60 y), to give 1.71. This was rounded to 1.5 and multiplied by 2 to give a partial score of 3.0.
NRF method: each patient’s NRF score was based on the sum of those predictors present.
Dropped out of the model on multivariate analysis.
TABLE 3.
Median Survival by TEACHH Model Survival Group
| Group (Score) | Total | No. of Deaths | Censored, % | Median OS, Months | 95% CI, Months | ||
|---|---|---|---|---|---|---|---|
| TEACHH model | NRF | A (0–1) | 119 | 59 | 50.4 | 19.9 | 13.9–31.1 |
| B (2–4) | 694 | 566 | 18.4 | 5.0 | 4.3–5.6 | ||
| C (5–6) | 49 | 48 | 2.0 | 1.7 | 1.2–2.1 | ||
| PSM | A (0–4) | 149 | 77 | 48.3 | 17.5 | 13.4–28.8 | |
| B (5–15) | 680 | 564 | 17.1 | 4.8 | 4.2–5.3 | ||
| C (16–20) | 33 | 32 | 3.0 | 1.6 | 1.0–2.3 |
Abbreviations: 95% CI, 95% confidence interval; NRF, number of risk factors; OS, overall survival; PSM, partial score method; TEACHH, type of cancer, Eastern Cooperative Oncology Group performance status, age, prior palliative chemotherapy, prior hospitalizations, and hepatic metastases.
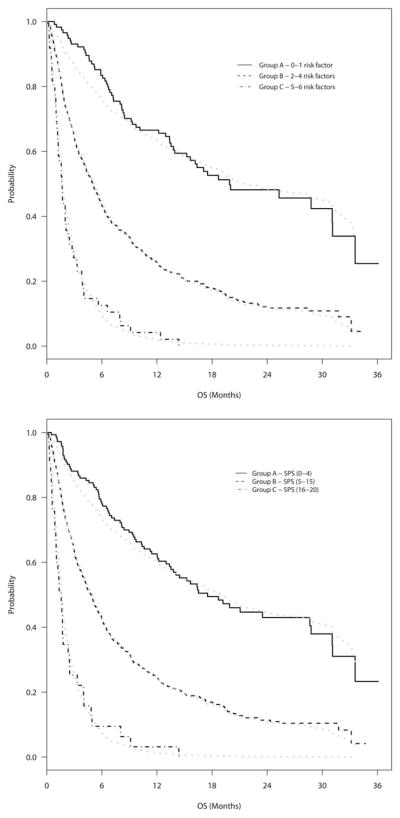
Using both the PSM and NRF methods, patients were split into 3 distinct survival groups. The most favorable prognostic group had a predicted survival of >1 year, whereas the least favorable prognostic group had a predicted survival of <3 months (Fig. 2). Based on the PSM, 149 patients (17%) were included in group A (those with a partial score of 0–4), 680 patients (79%) were included in group B,5–15 and 33 patients (4%) were included in group C.16–20 Based on the NRF method, 119 patients (14%) were included in group A (those with 0–1 risk factors), 694 patients (81%) were included in group B (those with 2–4 risk factors), and 49 patients (6%) were included in group C (those with 5–6 risk factors). Table 3 shows median survivals and 95% CIs for groups A, B, and C using both the PSM and NRF methods. Risk groups had substantial separation with both the PSM and NRF methods, with no overlapping of 95% CIs. The C-statistic was 0.59 using both the PSM and NRF methods. Given their equivalent accuracy and similar group findings, the NRF method was nonetheless considered the superior model because of its greater ease of use.
Figure 2.
Overall survival (OS) is shown for each TEACHH (type of cancer, Eastern Cooperative Oncology Group performance status, age, prior palliative chemotherapy, prior hospitalizations, and hepatic metastases) survival group. Survival estimated by the Cox regression method (gray line) and actual survival calculated using the Kaplan-Meier method (black line) are shown. (Top) Estimated survival is shown based on the number of risk factors method. (Bottom) Estimated survival is shown based on the partial score method. SPS indicates summated partial score.
DISCUSSION
The TEACHH model (type of cancer, ECOG PS, age, prior palliative chemotherapy, prior hospitalizations, and hepatic metastases) is an easily calculated prognostic tool for patients with metastatic disease who present for palliative RT, and who comprise approximately 20% to 40% of patients treated in radiation oncology departments.28–31 Using clinical factors that are readily available from medical chart review, it identifies 3 distinct prognostic groupings for patients seen for palliative RT.
The TEACHH model was developed with the goal of identifying patients at the extremes of the prognostic spectrum (LEs of <3 months and >1 year), who represented 20% of those patients seen for palliative RT in the current study sample. The LE grouping of <3 months is critical to palliative care decision-making, such as operative management of cord compression before RT.32 It is also the time frame in which, if RT is indicated, hypofractionated regimens should be used to avoid protracted courses of RT near death.1,2 Furthermore, an LE of <3 months is also the time frame in which the management of brain metastases should favor hypofractionated RT and include consideration of monitoring and steroids alone, particularly for patients with asymptomatic brain metastases.33 In contrast, the durability of treatment response is an important consideration for patients with an LE of >1 year because recurrences after palliative RT are likely to occur within this time frame.12,34 These patients may be candidates for dose escalation, which has been associated with improved local control for certain palliative disease sites.12,35 Finally, the LE groupings in the TEACHH model assist in both identifying those patients who are eligible for hospice care7 and guiding end-of-life discussions and planning by oncologists with their patients with advanced cancer in accordance with national guidelines.8,9
The TEACHH model is distinct in 3 notable ways. First, the model includes 3 novel clinical predictive variables. To the best of our knowledge, the number of prior palliative chemotherapy courses and prior hospitalizations have not been previously reported as factors predictive of LE, but are clinically consistent findings given that they are markers for progressive disease and/or increasing symptom burden. Furthermore, although Chow et al defined the location of metastatic disease broadly as bone versus nonbone,13 the TEACHH model may further refine which nonbone metastatic site (the liver) is largely responsible for a decrease in survival. Hepatic metastases have not to our knowledge been previously identified as a factor predicting LE in this patient population, although visceral metastases more generally have been shown to be predictive.36,37 Hepatic metastases may herald the terminal stage of cancer progression and/or they may be directly responsible for a patient’s clinical deterioration (eg, liver dysfunction causing metabolic derangements and/or precluding anticancer chemotherapies). Age, although found to be predictive of survival in other disease-specific advanced cancer settings,15 has not yet been found to be a significant factor in prognostic models of general patient populations with advanced cancer. As noted in the disease-specific setting,15 patient age may be a hallmark of both treatment tolerance and comorbid conditions, both of which are likely to impact survival. Cancer type (lung vs other types) and PS, the remaining significant predictors of survival, have been shown in numerous studies to be significant predictors of survival.13,16–23 Second, our model uses ECOG PS as opposed to Karnofsky PS.13,21 The broader performance categories used in the ECOG scale are arguably easier for clinicians to use and it has largely become the standard internationally for evaluating the PS of patients with cancer.38 Finally, as previously discussed, our model identifies LE groups at meaningful prognostic time points for patients with cancer and provides nonoverlapping 95% CIs for median survivals of each group to optimize clinical usefulness and applicability.
The TEACHH model has important limitations. First, it did not examine the prognostic usefulness of laboratory values (eg, albumin) or symptoms (eg, fatigue, delirium), which have been included in other models.17,19,21,23 Such factors were not included in the current analysis because of the study objective to include only those clinical factors that can be reliably assessed for any patient referred for palliative RT and a desire to avoid including subjective measures that may have low interrater reliability. Next, the TEACHH model was developed specifically in patients receiving palliative RT and therefore may not be generalizable to other patient populations with metastatic cancer. In addition, to eliminate any interdependence of courses, only patients’ first courses of RT were used in the model development, which could lead to a selection bias toward patients with longer survival times. To examine this bias, we reran our analysis to include all RT courses received by a patient, and the results were unchanged. In addition, although the TEACHH model provides nonoverlapping CIs for median survivals, ideally it would also provide clinicians with an estimated range of possible survivals for each patient, for which further studies in larger patient populations are required. Finally, the TEACHH model requires external validation to assess its accuracy in disparate settings of patients with advanced cancer presenting for palliative RT.
TEACHH is a prognostic model that was developed among patients with metastatic cancer being treated with palliative RT. The model discriminates 3 groups of patients with median LEs relevant to RT clinical decision-making. By providing LE estimates, this model may help clinicians provide quality palliative care to their patients with advanced cancer and their families.
Acknowledgments
FUNDING SUPPORT
This research was supported by an American Society of Clinical Oncology Conquer Cancer Foundation Career Development Award to Tracy A. Balboni MD, MPH.
Footnotes
Presented as an oral presentation at the 54th Annual Meeting of the American Society for Radiation Oncology; October 28–31, 2012; Boston, MA.
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
References
- 1.Gripp S, Mjartan S, Boelke E, et al. Palliative radiotherapy tailored to life expectancy in end-stage cancer patients: reality or myth? Cancer. 2010;116:3251–3256. doi: 10.1002/cncr.25112. [DOI] [PubMed] [Google Scholar]
- 2.Guadagnolo BA, Liao KP, Elting L, et al. Use of radiation therapy in the last 30 days of life among a large population-based cohort of elderly patients in the United States. J Clin Oncol. 2013;31:80–87. doi: 10.1200/JCO.2012.45.0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chow E, Harth T, Hruby G, et al. How accurate are physicians’ clinical predictions of survival and the available prognostic tools in estimating survival times in terminally ill cancer patients? A systematic review. Clin Oncol (R Coll Radiol) 2001;13:209–218. doi: 10.1053/clon.2001.9256. [DOI] [PubMed] [Google Scholar]
- 4.Christakis NA, Lamont EB. Extent and determinants of error in physicians’ prognoses in terminally ill patients: prospective cohort study. West J Med. 2000;172:310–313. doi: 10.1136/ewjm.172.5.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mackillop WJ, Quirt CF. Measuring the accuracy of prognostic judgments in oncology. J Clin Epidemiol. 1997;50:21–29. doi: 10.1016/s0895-4356(96)00316-2. [DOI] [PubMed] [Google Scholar]
- 6.Peppercorn JM, Smith TJ, Helft PR, et al. American Society of Clinical Oncology. American society of clinical oncology statement: toward individualized care for patients with advanced cancer. J Clin Oncol. 2011;29:755–760. doi: 10.1200/JCO.2010.33.1744. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Medicare and Medicaid Services. Medicare Hospice Benefits. Baltimore, MD: Centers for Medicare and Medicaid Services, US Department of Health and Human Services; 2010. [Google Scholar]
- 8.National Comprehensive Cancer Network. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology: Palliative Care. Fort Washington, PA: National Comprehensive Cancer Network; 2011. [Google Scholar]
- 9.Dahlin C, editor. Clinical Practice Guidelines for Quality Palliative Care. 3. Pittsburgh, PA: National Consensus Project; 2013. National Consensus Project for Quality Palliative Care. [Google Scholar]
- 10.Tokuhashi Y, Matsuzaki H, Oda H, et al. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976) 2005;30:2186–2191. doi: 10.1097/01.brs.0000180401.06919.a5. [DOI] [PubMed] [Google Scholar]
- 11.Tomita K, Kawahara N, Kobayashi T, et al. Surgical strategy for spinal metastases. Spine (Phila Pa 1976) 2001;26:298–306. doi: 10.1097/00007632-200102010-00016. [DOI] [PubMed] [Google Scholar]
- 12.Rades D, Panzner A, Rudat V, et al. Dose escalation of radiotherapy for metastatic spinal cord compression (MSCC) in patients with relatively favorable survival prognosis. Strahlenther Onkol. 2011;187:729–735. doi: 10.1007/s00066-011-2266-y. [DOI] [PubMed] [Google Scholar]
- 13.Chow E, Abdolell M, Panzarella T, et al. Predictive model for survival in patients with advanced cancer. J Clin Oncol. 2008;26:5863–5869. doi: 10.1200/JCO.2008.17.1363. [DOI] [PubMed] [Google Scholar]
- 14.Chow E, James JL, Hartsell W, et al. Validation of a predictive model for survival in patients with advanced cancer: secondary analysis of RTOG 9714. World J Oncol. 2011;2:181–190. doi: 10.4021/wjon325w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–751. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 16.Martin L, Watanabe S, Fainsinger R, et al. Prognostic factors in patients with advanced cancer: use of the patient-generated subjective global assessment in survival prediction. J Clin Oncol. 2010;28:4376–4383. doi: 10.1200/JCO.2009.27.1916. [DOI] [PubMed] [Google Scholar]
- 17.Gwilliam B, Keeley V, Todd C, et al. Development of prognosis in palliative care study (PiPS) predictor models to improve prognostication in advanced cancer: prospective cohort study. BMJ. 2011;343:d4920. doi: 10.1136/bmj.d4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiang JK, Cheng YH, Koo M, et al. A computer-assisted model for predicting probability of dying within 7 days of hospice admission in patients with terminal cancer. Jpn J Clin Oncol. 2010;40:449–455. doi: 10.1093/jjco/hyp188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feliu J, Jimenez-Gordo AM, Madero R, et al. Development and validation of a prognostic nomogram for terminally ill cancer patients. J Natl Cancer Inst. 2011;103:1613–1620. doi: 10.1093/jnci/djr388. [DOI] [PubMed] [Google Scholar]
- 20.Lingjun Z, Jing C, Jian L, et al. Prediction of survival time in advanced cancer: a prognostic scale for Chinese patients. J Pain Symptom Manage. 2009;38:578–586. doi: 10.1016/j.jpainsymman.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Pirovano M, Maltoni M, Nanni O, et al. A new palliative prognostic score: a first step for the staging of terminally ill cancer patients. Italian Multicenter and Study Group on Palliative Care. J Pain Symptom Manage. 1999;17:231–239. doi: 10.1016/s0885-3924(98)00145-6. [DOI] [PubMed] [Google Scholar]
- 22.Suh SY, Choi YS, Shim JY, et al. Construction of a new, objective prognostic score for terminally ill cancer patients: a multicenter study. Support Care Cancer. 2010;18:151–157. doi: 10.1007/s00520-009-0639-x. [DOI] [PubMed] [Google Scholar]
- 23.Tredan O, Ray-Coquard I, Chvetzoff G, et al. Validation of prognostic scores for survival in cancer patients beyond first-line therapy. BMC Cancer. 2011;11:95. doi: 10.1186/1471-2407-11-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan EY, Wu HY, Chan YH. Revisiting the Palliative Performance Scale: change in scores during disease trajectory predicts survival. Palliat Med. 2013;27:367–374. doi: 10.1177/0269216312451613. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez-Munoz A, Perez-Ruiz E, Saez MI, et al. Limited impact of palliative chemotherapy on survival in advanced solid tumours in patients with poor performance status. Clin Transl Oncol. 2011;13:426–429. doi: 10.1007/s12094-011-0677-y. [DOI] [PubMed] [Google Scholar]
- 26.Collett D. Modelling Survival Data in Medical Research. London: Chapman & Hall; 1994. [Google Scholar]
- 27.Uno H, Cai T, Pencina MJ, D’Agostino RB, Wei LJ. On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat Med. 2011;30:1105–1117. doi: 10.1002/sim.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coia LR, Hanks GE, Martz K, et al. Practice patterns of palliative care for the United States 1984–1985. Int J Radiat Oncol Biol Phys. 1988;14:1261–1269. doi: 10.1016/0360-3016(88)90405-1. [DOI] [PubMed] [Google Scholar]
- 29.Huang J, Zhou S, Groome P, et al. Factors affecting the use of palliative radiotherapy in Ontario. J Clin Oncol. 2001;19:137–144. doi: 10.1200/JCO.2001.19.1.137. [DOI] [PubMed] [Google Scholar]
- 30.Janjan NA. An emerging respect for palliative care in radiation oncology. J Palliat Med. 1998;1:83–88. doi: 10.1089/jpm.1998.1.83. [DOI] [PubMed] [Google Scholar]
- 31.McCloskey SA, Tao ML, Rose CM, et al. National survey of perspectives of palliative radiation therapy: role, barriers, and needs. Cancer J. 2007;13:130–137. doi: 10.1097/PPO.0b013e31804675d4. [DOI] [PubMed] [Google Scholar]
- 32.Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366:643–648. doi: 10.1016/S0140-6736(05)66954-1. [DOI] [PubMed] [Google Scholar]
- 33.Expert Panel on Radiation Oncology-Brain Metastases. Videtic GM, Gaspar LE, Aref AM, et al. American College of Radiology appropriateness criteria on multiple brain metastases. Int J Radiat Oncol Biol Phys. 2009;75:961–965. doi: 10.1016/j.ijrobp.2009.07.1720. [DOI] [PubMed] [Google Scholar]
- 34.Howell DD, James JL, Hartsell WF, et al. Single-fraction radiotherapy versus multifraction radiotherapy for palliation of painful vertebral bone metastases-equivalent efficacy, less toxicity, more convenient: a subset analysis of Radiation Therapy Oncology Group trial 97–14. Cancer. 2013;119:888–896. doi: 10.1002/cncr.27616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rades D, Panzner A, Dziggel L, Haatanen T, Lohynska R, Schild SE. Dose-escalation of whole-brain radiotherapy for brain metastasis in patients with a favorable survival prognosis. Cancer. 2012;118:3852–3859. doi: 10.1002/cncr.26680. [DOI] [PubMed] [Google Scholar]
- 36.Douglas S, Huttenlocher S, Bajrovic A, Rudat V, Schild SE, Rades D. Prognostic factors for different outcomes in patients with metastatic spinal cord compression from cancer of unknown primary. BMC Cancer. 2012;12:261. doi: 10.1186/1471-2407-12-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rades D, Douglas S, Huttenlocher S, et al. Prognostic factors and a survival score for patients with metastatic spinal cord compression from colorectal cancer. Strahlenther Onkol. 2012;188:1114–1118. doi: 10.1007/s00066-012-0141-0. [DOI] [PubMed] [Google Scholar]
- 38.Buccheri G, Ferrigno D, Tamburini M. Karnofsky and ECOG performance status scoring in lung cancer: a prospective, longitudinal study of 536 patients from a single institution. Eur J Cancer. 1996;32A:1135–1141. doi: 10.1016/0959-8049(95)00664-8. [DOI] [PubMed] [Google Scholar]