Abstract
The Unfolded Protein Response (UPR) is a cytoprotective response aimed at restoring cellular homeostasis following physiological stress exerted on the endoplasmic reticulum (ER) that also invokes innate immune signaling in response to invading microorganisms. While the UPR is modulated by various viruses, recent evidence indicates that it also plays multiple roles during bacterial infections. In this Review, we describe how bacteria adapt to live in the ER and discuss the intricacies of bacterial interactions with the UPR, including how UPR subversion promotes the proliferation of intracellular bacterial pathogens and how the UPR contributes to innate immune responses against invading bacteria.
Bacterial pathogens with an intracellular life cycle have devised various strategies to subvert specific compartments within host cells and generate niches that ensure their survival, persistence and proliferation. Bacterial entry into eukaryotic cells generally results in bacteria residing within phagosomes, which are intracellular compartments dedicated to innate immune detection and degradation of incoming microorganisms, leading to antigen presentation and development of adaptive immunity. Despite these immune processes, bacteria entrapped within phagosomes can achieve intracellular survival by various means, including interference with phagosomal maturation to impair fusion with lysosomes, phagosomal disruption and release into the cytosol. Bacteria can also transform the original phagosome into an idiosyncratic vacuole that acquires functional properties of less antimicrobial intracellular compartments. For example, bacteria can modulate the phagosome to interact with the endoplasmic reticulum (ER), a large membrane-bound organelle that ensures biosynthesis of proteins, carbohydrates and lipids, and orchestrates their transport along the secretory pathway. The ER delivers these components to their destination compartments, which include the ER itself, the Golgi apparatus, the plasma membrane, the extracellular milieu, or the endocytic and autophagic pathways. Given its biosynthetic functions and role along the secretory pathway, the ER stands as a nutrient-rich intracellular location that is presumably devoid of bactericidal functions, such as antimicrobial peptides or hydrolytic enzymes, intuitively making it a suitable niche for the intracellular survival, persistence and proliferation of intracellular bacteria.
The ER plays crucial roles in cellular homeostasis by controlling processing and folding of secretory and membrane proteins. When protein folding requirements exceed the ER processing capacity, unfolded proteins accumulate, induce ER stress and trigger the unfolded protein response (UPR), an evolutionarily conserved cytoprotective signaling pathway. By inhibiting mRNA translation, increasing the ER protein folding capacity and ER-associated degradation (ERAD), the UPR serves to relieve physiological stress on the ER and maintain cellular homeostasis1. Failure to restore ER functions results in programmed cell death. In addition, the UPR triggers signal transduction events associated with innate immunity and host defense, linking this physiological response to detection of intracellular pathogens2.
Viral infections have been long known to exert stress on the ER and induce the UPR due to their demand on protein synthesis, and several viruses modulate the UPR to ensure viral protein production, replication and cell survival3 (Box 1). Similarly, bacterial proliferation in the ER likely causes physiological strain on this compartment that can result in ER stress and the induction of the UPR. In agreement with this scenario, recent evidence indicates important roles of the UPR in either promoting or counteracting intracellular proliferation of bacterial pathogens that subvert ER functions, and in sensing effects of bacterial protein delivery into cells. Here we will present and discuss recent findings that support the UPR as a key component of crosstalk between the ER, intracellular bacteria and their pathogenic activities, and how it may contribute to inflammatory and immune responses to intracellular bacteria.
Box 1. Viruses and the UPR.
Viral replication co-opts ER functions for production of viral glycoproteins, leading to induction of the UPR109. Since downstream effects of UPR activation including translational attenuation, ERAD and cell death can inhibit viral protein production, viruses express mechanisms of manipulation and avoidance of the UPR to replicate successfully. While the exact mechanism for this is unknown for most viruses, some of the viral proteins involved have been identified. For example, cytomegaloviruses (CMV) can both induce and modulate the UPR110–112. Induction of the UPR by human CMV protein US11 has been proposed to lead to degradation of MHC Class I, a mechanism that may promote chronic infection112. Further, HCMV protein UL50 and its murine CMV homolog M50, can both downregulate IRE1 to suppress the UPR111. Hepatitis C virus also downregulates the IRE1-XBP1 pathway to promote viral replication113,114. Rotavirus can modulate the UPR via sequestration of UPR components115,116. Since bacteria that replicate within the ER face a similar environment, they may share some of these strategies for modulation of the UPR with viral pathogens, however additional studies are needed to test this idea. Further, similar to what has been proposed for Brucella abortus infection62, stimulation of the UPR during viral infection may serve as a pathogen-associated pattern that activates antiviral innate immunity117.
The UPR: components and functions
Nearly one third of the eukaryotic proteome is synthesized in the endoplasmic reticulum (ER) and enters the secretory pathway. The ER is also the site for biosynthesis of membrane lipids and cholesterol. In the lumen of the ER, proteins fold into their correct conformation with the assistance of the protein glycosylation machinery and dedicated chaperones, such as BiP (also known as GRP78), calnexin and calreticulin4. Under certain stress conditions, such as elevated synthesis of secretory proteins, perturbations in calcium homeostasis and redox balance, the abundance of misfolded proteins exceeds the capacity of the protein folding machinery. In response to these perturbations, three sensors located in the ER membrane, inositol-requiring enzyme 1 (IRE1), double-stranded RNA-dependent protein kinase (PKR)-like ER kinase (PERK) and activating transcription factor 6 (ATF6), direct induction of the UPR1,4,5 (Figure 1). Each of these proteins has an ER-targeting transmembrane domain that links a cytosolic effector domain and a domain localized to the lumen of the ER. While the precise molecular mechanisms leading to activation of these ER sensors are still unclear, experimental evidence supports two different inputs to this process. The first is direct binding of unfolded proteins to the lumenal domain. This interaction has been demonstrated for yeast Ire1, for which the dimerized protein was shown to form a polypeptide binding groove6, and a similar structural feature has been identified in PERK1. A second input is binding of the ER-lumenal domains of IRE1, PERK and ATF6 by the ER chaperone BiP. As a result of ER stress conditions that increase the load of protein misfolding or misassembly of protein complexes, the cell’s capacity for protein folding is exceeded, and BiP is recruited away from IRE1, PERK and ATF6 to assist in refolding proteins within the ER, which correlates with activation of IRE1, PERK and ATF6. Additional signals emanating from the ER membrane, as well as the cytosol, have also been proposed to activate the UPR1. Activation of IRE1, PERK and ATF6 initiates signaling pathways of the UPR to restore homeostasis in the ER, via increasing the production of chaperones to assist in protein folding, arresting translation of proteins not involved in resolving ER stress, and degradation of terminally misfolded proteins via the ER-associated degradation (ERAD) pathway, which orchestrates their retrotranslocation through the ER membrane, polyubiquitination and targeting to the proteasome7.
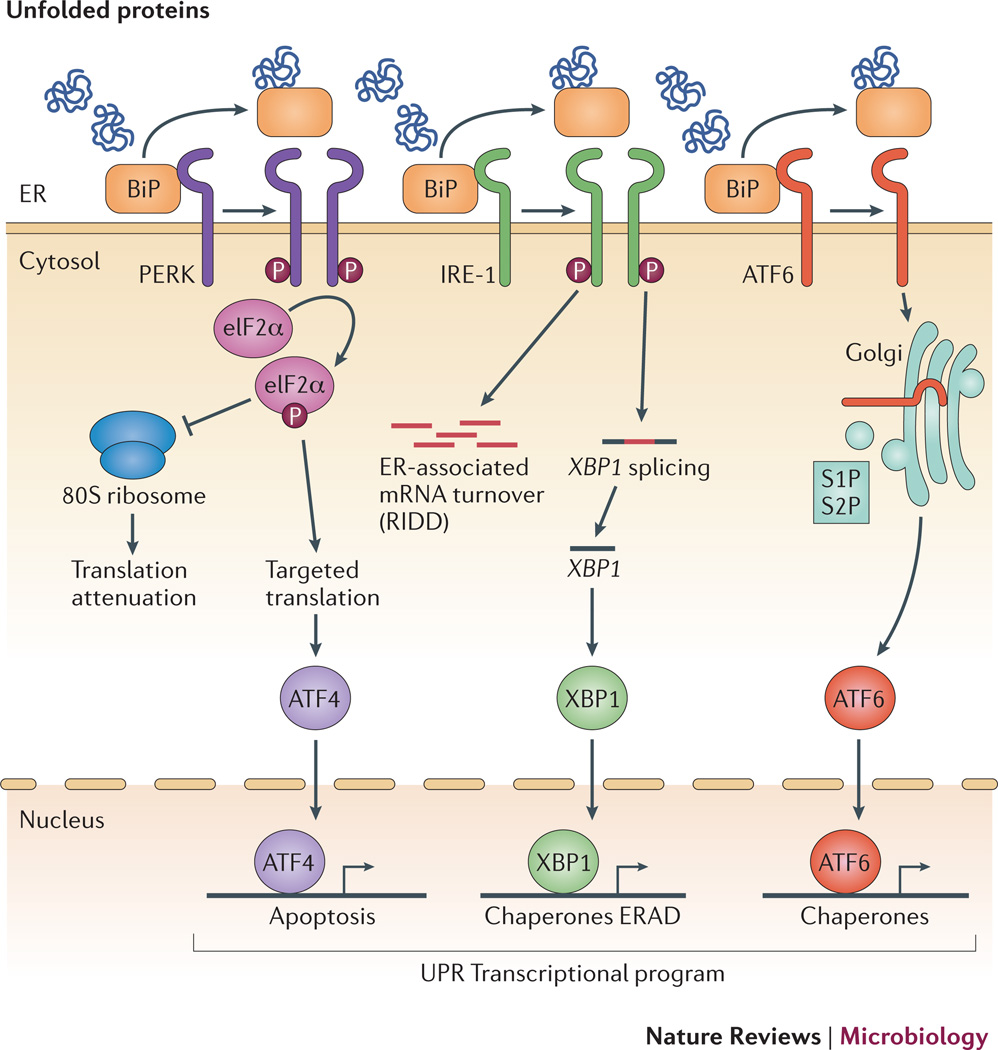
Figure 1. Cellular responses to endoplasmic reticulum stress.
In response to increased in unfolded proteins in the ER lumen, three sensors located in the ER membrane, PERK, IRE1 and ATF6 activate the unfolded protein response. Under homeostatic conditions, PERK, IRE1 and ATF6 are bound by BiP, which suppresses their activity. In response to ER stress, BiP is recruited away from PERK, IRE1 and ATF6 to promote protein folding, which leads to activation of these ER stress sensors. PERK is a kinase, that auto-activates, dimerizes and phosphorylates its target eIF2, thereby preventing assembly of functional 80S ribosomes. Further, eIF2 directs selective translation of the transcription factor ATF4, which activates transcription of genes involved in the unfolded protein response. IRE1 is a bifunctional enzyme with kinase and endonuclease activity. In response to ER stress, IRE1 auto-phosphorylates via its kinase domain, which leads to dimerization and activation of its endonuclease activity. The endonuclease function of IRE1 degrades mRNA at the ER to decrease protein biosynthesis and also splices the transcript encoding XBP1, a second transcription factor directing expression of genes involved in restoring cellular homeostasis. ATF6 is a transcription factor that is anchored in the ER membrane, but in response to ER stress, it translocates to the Golgi, where the transcription factor domain is released from the membrane by sequential action of Site 1 (S1) and Site 2 (S2) proteases, allowing its translocation to the nuclease to activate transcription of UPR target genes. ATF4, XBP1 and ATF6 direct a transcriptional program that upregulates chaperones, components of the ERAD pathway, and factors involved in autophagy and apoptosis that act to restore cellular homeostasis or if the disruption to ER function cannot be resolved, initiate programmed cell death.
IRE1 is a multifunctional protein that possesses kinase and endonuclease activities. The activity of IRE1 is downmodulated by binding of BiP8–10 and is stimulated by direct binding of unfolded proteins2,6. Upon activation, IRE1 aggregates and autophosphorylates, activating its endonuclease activity11. IRE1’s site-specific endonuclease activity splices the mRNA encoding the transcription factor XBP1, which results in translation of an active protein12. Production of XBP1 directs the expression of ER-resident molecular chaperones and protein folding enzymes13. In addition to splicing the XBP1 transcript, IRE1 has a nonspecific endonuclease activity that is responsible for rapid degradation of ER membrane-associated mRNA by a process known as regulated IRE1-dependent decay (RIDD)14,15. This response reduces the load of protein entering the secretory pathway.
Like IRE1, PERK is also an ER stress-activated kinase. Upon activation by ER stress, PERK homodimerizes and transphosphorylates, which is accompanied by dissociation of BiP. Activated PERK can then phosphorylate its target, the translation initiation factor eIF2α8,16. Phosphorylation of eIF2α inhibits synthesis of secretory proteins by inhibiting the assembly of the 80S ribosome, thereby promoting cellular survival during ER stress17. At the same time, phosphorylated eIF2α directs translation of the mRNA encoding the transcription factor ATF4, which induces expression of UPR target genes involved in amino acid transport and oxidative stress resistance17. Under conditions of prolonged or severe ER stress that the UPR cannot resolve, ATF4 also increases the levels of the transcription factor CHOP, which upregulates genes involved in the induction of apoptotic cell death18.
The third arm of the UPR is initiated by ATF6. Activation of ATF6 results in its translocation to the Golgi, where it is cleaved by the Golgi-resident proteases site-1 protease (S1P) and S2P19. The active amino terminus of ATF6 is translocated to the nucleus, where it binds the ER stress response element upstream of a subset of UPR genes to activate their transcription20. The set of ATF6-dependent genes overlaps partially with genes induced by IRE1α and PERK and includes genes with functions in protein folding, protein transport and lipid biosynthesis20,21. In addition, ATF6 can homodimerize with XBP1 to activate genes involved in ERAD22.
One of the cellular responses downstream of the UPR is the induction of autophagy. Induction of the UPR increases membrane lipid biogenesis and triggers ER expansion via the XBP1 and ATF6 pathways, as a means to enhance ER function23–25. Selective autophagy of the ER (so-called reticulophagy or ER-phagy) aims to regulate ER expansion and promote restoration of cellular homeostasis upon resolution of ER stress. While induction of autophagy has been shown to promote survival of cancer cells26, the effect of ER stress-induced autophagy on cell survival appears to be context-dependent, as ER stress-induced autophagy led to death of primary cells27. Under conditions of strong or sustained ER stress, a failure of the cell to restore homeostasis triggers apoptotic cell death. While the precise pathways of apoptosis induced by ER stress are not known, the PERK-eIF2α-ATF4-CHOP pathway plays an important role by reversing translational arrest, increasing generation of reactive oxygen species and promoting calcium efflux from the ER. Together, these signals lead to cytochrome c release from mitochondria and loss of membrane potential, resulting in apoptosis18.
In addition to autophagy, UPR signaling also interacts with innate immune signaling pathways leading to inflammation, as activation of IRE1 by ER stress can activate the MAP kinase JNK28, a key player in the response to inflammatory stimuli28(see below). Therefore, although residing in the ER might benefit the survival of intracellular bacteria, it is possible that UPR induction might serve as an innate immune mechanism against invading bacteria.
The ER, a safe niche for intracellular bacteria
Although the ER presumably embodies a hospitable, nutrient-rich organelle devoid of antimicrobial functions, only a handful of bacterial pathogens take advantage of its nature and functions to ensure their intracellular survival and proliferation. Additionally, a few other intracellular bacteria have developed tight interactions between their vacuole and this compartment. The first evidence of bacterial interactions with the ER came from the observations that Legionella pneumophila and Brucella spp. occupy ribosome-studded intracellular vacuoles29–31, which were confirmed to be derived from the ER at both ultrastructural and functional levels32–36. Since these seminal demonstrations, Legionella longbeachae37, Chlamydia trachomatis38,39 and the Chlamydia-related bacterium Simkania negevensis40 have also been shown to survive and replicate in organelles that closely interact with the ER.
Bacterial trafficking to the ER
The trafficking events of bacterial vacuoles leading to the biogenesis of L. pneumophila and Brucella spp. ER-derived organelles have been well characterized and both invoke subversion of early secretory vesicles, yet via distinct mechanisms (Figure 2). Upon phagocytosis by macrophages, plasma membrane-derived Legionella-containing vacuoles (LCV) avoid phagosomal maturation along the endocytic pathway. They instead intercept early secretory vesicles trafficking between the ER and the Golgi apparatus, which cover and subsequently fuse with LCVs, transforming them into vacuoles with functional features of early secretory compartments34,36,41. These events eventually allow LCVs to fuse with ER membranes34,36,41 and become a replication-permissive organelle that expands upon bacterial proliferation. Similarly, Brucella spp. reside within a Brucella-containing vacuole (BCV) following phagocytic uptake or entry within non-professional phagocytes. Unlike Legionella, however, BCVs first undergo maturation along the endocytic pathway to become endosomal BCVs (eBCVs)42, and partially acquire phagolysosomal properties33,43–45. This trafficking step is followed by interactions of BCVs with ER exit sites (ERES)43,45, a sub-compartment of the ER dedicated to formation and budding of secretory vesicles prior to their transport to the Golgi apparatus. Sustained BCV-ERES interactions lead to biogenesis of ER-derived vacuoles – named replicative BCVs (rBCVs) – through progressive exchange of endocytic membranes for ER-derived membranes43. rBCVs support bacterial replication, which is accompanied by further ER membrane accretion and a dramatic reorganization of the ER into rBCVs when bacterial proliferation is extensive35.
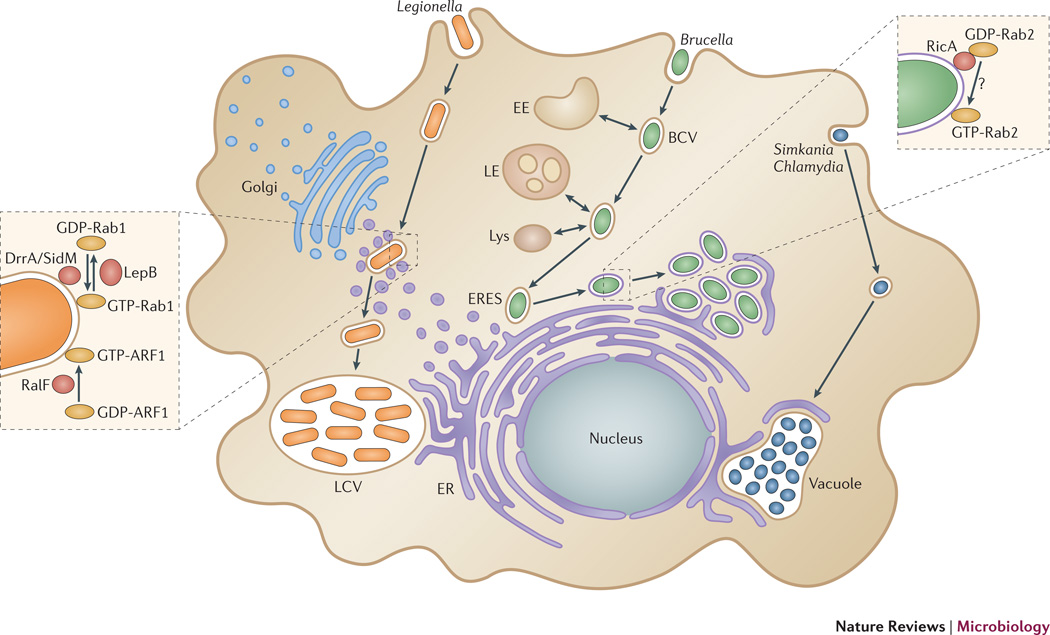
Figure 2. Trafficking and biogenesis of ER-associated replicative organelles by bacterial pathogens.
Legionella pneumophila recruits early secretory vesicles to its plasma membrane-derived phagosome via delivery of the Dot/Icm Type IV secretion system effectors DrrA/SidM, LepB and RalF. These effectors modulate activities of the small GTPases Rab1 and ARF1 on the Legionella phagosome (see inset) to mediate bypass of the endocytic pathway and promote fusion of the Legionella phagosome with the ER and biogenesis of an ER-derived replicative vacuole. Brucella spp. reside within a vacuole (BCV) that traffics along the endocytic pathway, then is redirected to ER exit sites (ERES) and fuses with the ER via the action of VirB Type IV effector proteins and the small GTPases Sar1 and Rab2. The Brucella effector RicA binds GDP-bound Rab2 in an unknown manner and is required for accumulation of activated, GTP-bound Rab2 on BCVs (see inset). Chlamydia spp. and Simkania control the traffic and maturation of their original vacuole into a large inclusion that physically interacts with the ER at specific contact points (synapses).
The obligate intracellular bacteria Chlamydia spp. reside within a large vacuole, the Chlamydia inclusion, which intercepts exocytic traffic between the Golgi apparatus and the plasma membrane to acquire sphingolipids necessary for inclusion biogenesis and bacterial growth46,47. Recently, interactions between the Chlamydia inclusion and the ER have been revealed in the form of points of contact, or “synapses”, between these compartments38,39. These synapses may deliver ER-derived material to the inclusion39 and are important for inclusion biogenesis and bacterial growth38,39. Similarly, the Chlamydia-like organism S. negevensis generates a large networked vacuole that also harbors contact sites with the ER40, suggesting a common strategy of ER subversion among Chlamydiales.
Mechanisms of biogenesis of ER-derived bacterial vacuoles
While the mechanisms underlying Chlamydia inclusion interactions with the ER remain to be characterized, extensive studies of the biogenesis of L. pneumophila and Brucella abortus ER-derived replicative organelles have provided useful insight into how these pathogens reach the secretory compartment and establish residency in the ER. Both pathogens express a Type IV secretion system (T4SS), the Legionella Dot/Icm and Brucella VirB T4SS (Box 2), which direct the intracellular fate of the bacteria by delivering effector proteins into infected cells that modulate host cell pathways. Concomitant with uptake, L. pneumophila delivers the Dot/Icm effector proteins DrrA/SidM, LepB and RalF, which coordinately act to mediate LCV targeting to the ER via the modulation of small GTPases that normally control early secretory vesicle transport (Figure 2). DrrA/SidM acts as a GDP/GTP exchange factor (GEF) that recruits and activates Rab1 on plasma membrane-derived LCVs to promote recruitment of early secretory vesicles48,49. This process is negatively regulated by LepB, a GTPase activating protein (GAP) that deactivates Rab150. Fusion of secretory vesicles with LCVs involves DrrA-dependent unusual pairing of plasma membrane and ER-derived vesicle SNARE proteins51,52, which allows direct fusions between a plasma membrane-derived vacuole and early secretory vesicles, thereby mediating LCV bypass of the endocytic pathway. Subsequent to LCV fusion with secretory vesicles, RalF acts as a GEF for ARF1 and promotes its recruitment to LCVs and fusion with ER membranes to generate an ER-derived Legionella replication-permissive organelle41,53,54. Hence, this pathogen uses an array of effector proteins that co-opt membrane trafficking processes between secretory compartments to promote its residence within an ER-derived organelle.
Box 2. Type IV secretion systems in pathogenic bacteria.
Type IV secretion systems (T4SS) are multi-protein complexes organized in ATP-powered, membrane spanning machineries that deliver proteins, protein-protein or nucleoprotein complexes into either prokaryotic or eukaryotic cells. Expressed in both Gram-negative and Gram-positive bacteria, they fulfill a range of functions including DNA transfer and effector protein delivery into target cells, therefore playing important roles in transfer of antibiotic resistance traits via bacterial conjugation, or in virulence mechanisms of pathogenic bacteria118. Based on phylogenetic relationships with T4SS harbored by the canonical conjugative plasmids, T4SS have been classified in subfamilies (Type IVA and Type IVB) that are represented in pathogenic bacteria by the VirB and Dot/Icm systems, respectively (reviewed in119). Many pathogenic bacteria express T4SS to mediate their interactions with host cells. These include Agrobacterium tumefaciens, Helicobacter pylori, Bordetella pertussis, Rickettsia spp., Brucella spp. and Bartonella spp., which encode Type IVA VirB T4SSs that deliver DNA-protein, multi-subunit protein toxin and proteins into eukaryotic cells, and the intracellular pathogens Legionella pneumophila and Coxiella burnetii, which express Type IVB Dot/Icm T4SSs that deliver effector protein into target cells120. T4SSs such as the Brucella VirB apparatus121 and the Legionella and Coxiella Dot/Icm systems122 are essential to the survival and proliferation of these intracellular pathogens, by translocating into various host cell compartments arrays of effector molecules that take control of host functions associated with the biogenesis and trafficking of the bacterial vacuole. As such, they constitute key virulence factors of many pathogenic bacteria.
The role of the Brucella VirB T4SS in BCV conversion into an ER-derived organelle has been established based on the inability of virB mutants to undergo sustained interactions with ERES and fuse with the ER, which results in failure to replicate and killing within eBCVs35,43,55. Through acidification and presumably additional intravacuolar cues, eBCVs signal intracellular Brucella to induce expression of the VirB apparatus44,45,56. Unlike Legionella, very little is known about the effector proteins that the Brucella VirB T4SS delivers during infection. The recent identification of 14 Brucella proteins delivered into host cells by the VirB apparatus57–61 is an important step towards a molecular understanding of VirB functions, but it has yet to yield information on whether these effectors mediate rBCV biogenesis. Several VirB effectors, such as VceC, BspA, BspB, BspC, BspD and BspF, target compartments of the secretory pathway when expressed ectopically in mammalian cells60,62, suggesting they may modulate specific functions associated with secretory transport. Accordingly, expression of BspA, BspB and BspF impairs secretory trafficking during Brucella infection60, indicating that the bacterium employs Type IV secretion to modulate functions of the secretory pathway. Indeed, Brucella requires functional ERES and the small GTPase Sar1 – which controls COPII-dependent vesicular budding from ERES63,64 – to generate rBCVs43. rBCV biogenesis also requires the small GTPase Rab2, which the Brucella effector RicA binds58,65. RicA specifically interacts with the GDP-bound form of Rab2 but does not exhibit any guanine nucleotide exchange factor (GEF) activity58, so how this interaction modulates Rab2 function remains unclear. Unlike Legionella, ARF1-dependent vesicular trafficking between ERES and the Golgi apparatus is not required for rBCV biogenesis43, highlighting mechanistic differences in how both pathogens reach the ER.
Bacteria and the UPR
While many of the mechanistic underpinnings of bacterial interactions with the ER remain to be understood, Legionella and Brucella spp. stand as model organisms to study bacterial exploitation of this compartment and its consequences. The rare utilization of the ER by bacterial pathogens for survival and replication raises the question of its actual permissiveness to infection. Much like many viruses need to modulate the UPR to ensure their replication while overwhelming the biosynthetic capacity of the ER (Box 1) and protozoan parasites modulate UPR pathways to promote their pathogenesis (Box 3), bacteria must be able to deal with the consequences of their proliferation within the ER. Although these consequences are unclear, bacterial replication within the ER may elicit the UPR, which could trigger sensing of bacterial pathogenic activities.
Box 3. Toxoplasma gondii and the UPR.
The Apicomplexan protozoan parasite Toxoplasma gondii occupies a parasitophorous vacuole that displays close interactions with the ER123,124, suggesting it may influence functions of this compartment. Consistently, recent evidence indicates that apoptosis of placental trophoblasts and neural stem cells infected with T. gondii involves activation of ER stress pathways125,126, via induction of CHOP, caspase 12 and the JNK pathway. Trophoblast apoptosis is initiated by oxidative stress following reactive oxygen species (ROS) production, while cell death stimuli in neural stem cells are unknown, yet these findings highlight a UPR response to T. gondii infection. Whether the UPR is actively modulated by T. gondii or an indirect response to cellular effects caused by the parasite needs further clarification. Yet, the demonstration that the T. gondii rhoptry protein ROP18 destabilizes the UPR transducer ATF6β to promote its pathogenesis127 strongly suggests that this pathogen also manipulates UPR signaling to its benefit.
Bacterial induction of the UPR
Several examples support the notion that bacterial infections trigger ER stress and can be sensed via the UPR. The UPR is induced in macrophage-rich granulomatous lesions of mouse lungs infected with virulent Mycobacterium tuberculosis, where apoptotic events are detectable66 (Table 1). UPR induction has also been correlated with Helicobacter-induced gastric carcinogenesis67 and occurs in gastric epithelial cells via the action of the vacuolating cytotoxin VacA68. VacA intoxication activates PERK and IEF2α, resulting in CHOP induction, mitochondrial dysfunction and apoptosis68 (Table 1). This highlights the role of ER stress in responses to infections and toxin activities.
Table 1.
Bacteria and bacterial products that modulate the UPR.
| Bacteria | Toxin or effector | Effect | Mode of action | Reference |
|---|---|---|---|---|
| Mycobacterium tuberculosis | induces ER stress in macrophages | unknown | 66 | |
| Helicobacter pylori | VacA | induces the UPR | PERK activation | 67,68 |
| Listeria monocytogenes | Listeriolysin O (LLO) | intracellular Ca2+ imbalance? | 70 | |
| Brucella melitensis | TcpB | induces the UPR | unknown | 69 |
| Brucella abortus | VceC | induces the UPR | BiP binding | 62 |
| BspC, G, H, I, K | induces the UPR | unknown | 60 | |
| Simkanianegevensis | inhibits the UPR | unknown | 40 | |
| Escherichia coli (STEC) | SubtilaseSubAB | activates IRE1, ATF6, PERK | degradesBiP | 75–77 |
| Shiga toxin 1 (Stx1) | induces the UPR | Ca2+ efflux from ER | 103 | |
| Vibrio cholerae | Cholera Toxin (CT) | activates IRE1 | IRE1 binding | 2 |
| various | Pore Forming Toxins (PFT) | induces the UPR | p38 MAPK pathway activation | 74 |
Similarly, in vitro cellular models of infection have revealed UPR induction in macrophages and epithelial cells infected with either Brucella melitensis and B. abortus62,69 or Listeria monocytogenes70 (Table 1). The first demonstration of a role of components of the UPR in the Brucella intracellular cycle was the discovery that IRE1α is necessary for B. abortus intracellular growth, through an RNAi screen for ER-associated factors required for bacterial replication in Drosophila melanogaster S2 cells71. Confirmed in mammalian cells, IRE1α dependency of Brucella replication suggests that the UPR is beneficial to the infection. However, depletion of neither ATF6 nor PERK reproduced the effect of IRE1α depletion71, which made a role for a canonical UPR in Brucella replication less substantiated.
Recently, direct evidence that B. melitensis in vitro and in vivo infection of murine macrophages leads to induction of the three major signaling pathways of the UPR established that Brucella infection induces physiological ER stress69. Whether bacterial residence and proliferation in the ER is required for induction of the UPR was however not addressed in this study, and the fact that a VirB-deficient mutant (which does not reach the ER or replicate) also induced UPR signaling69 suggests that this response may not be related to bacterial replication in the ER. It will also be important to address whether the three main UPR regulators are required for Brucella-induced UPR, or whether only IRE1α-dependent signaling contribute to bacterial replication. Counteracting the UPR response with the pharmacological chaperone tauroursodeoxycholic acid (TUDCA) reduces B. melitensis intracellular growth, suggesting that the UPR promotes bacterial intracellular growth69. Yet, obtaining more direct evidence will be important to further substantiate this beneficial role of the UPR.
L. monocytogenes induction of the UPR occurs via extracellular secretion of its cytolysin Listeriolysin O (LLO), which induces all arms of the UPR and leads to ER stress-specific apoptosis70 (Table 1). The molecular mechanisms by which this toxin acts remain unclear, but may be related to LLO’s ability to alter intracellular Ca2+ homeostasis via its pore-forming activity72, as Ca2+ imbalance causes ER stress. Nonetheless, evidence that LLO treatment of mammalian cells itself triggers the UPR indicates that the UPR may be induced via external stimuli and may not be restricted to ER-dwelling pathogens.
Bacterial subversion of the UPR
While the occurrence of UPR induction upon bacterial infection is now supported by several examples, indications that bacteria can modulate this response are rather sparse. L. pneumophila recruits components of the ERAD on its vacuole to mediate turnover of bacterial effectors on the vacuolar surface54, and the proteasome to generate amino-acids necessary for its intracellular growth73, but it is undocumented whether Legionella modulates the UPR. By contrast, S. negevensis generates a replicative vacuole associated with induction of ER stress, which the bacterium subsequently down-regulates40. Additionally, S. negevensis prevents ER stress chemically-induced using tunicamycin or thapsigargin, as judged by decreased induction of BiP, impaired nuclear translocation of CHOP and reduced activation of eIF2α40. Both compounds induce the UPR by perturbing distinct ER functions - tunicamycin inhibits N-linked glycosylation in the ER and affects protein folding, while thapsigargin blocks the sarco-endoplasmic reticulum calcium ATPases (SERCA), leading to depletion of ER calcium stores - indicating that S. negevensis likely interfere with common UPR signaling pathways via mechanisms that are yet to be determined. Interestingly, high concentrations of these UPR inducers can overcome S. negevensis-mediated inhibition of UPR and affect bacterial growth40, suggesting that the bacterium actively modulates this response to benefit its intracellular proliferation.
Additional evidence that bacteria might modulate the UPR originates from the study of bacterial toxins (Table 1). Similar to the effect of LLO during Listeria infections70, pore-forming toxin (PFT) intoxication of cells leads to induction of the UPR via the p38 MAP kinase pathway. Pore formation likely triggers p38 MAPK activation, which in turn induces the UPR via the IRE1-XBP-1 and ATF6, but not PERK, pathways, arguing that the UPR response to PFTs is specific and distinct from that induced by unfolded proteins74. UPR induction is protective against PFT74, indicating that the UPR acts as a defense mechanism against cellular injury caused by bacterial toxins. Studies of the mode of action of the AB5 cytotoxin family subtilase SubAB from Shiga-toxigenic Escherichia coli (STEC) have revealed that SubAB specifically cleaves the ER chaperone BiP75,76, due to unique structural features of its active site. BiP cleavage triggers the IRE1, PERK and ATF6 signaling arms of the UPR to induce transient ER stress and causes cell cycle arrest75,77. Cell cycle arrest at the G0/G1 phase is thought to be a consequence of Cyclin D1 decrease via both inhibition of translation and proteasomal degradation75. This exemplifies the ability of pathogenic bacteria to induce the UPR via delivery of specific proteins that exhibit enzymatic activities directed towards host cell factors.
The observation that UPR induction following Brucella infection promotes intracellular growth also suggests that the UPR might be targeted by specific bacterial mechanisms to promote survival. UPR induction by B. melitensis depends upon the protein TcpB (Toll/Interleukin-1-like receptor domain-containing protein) – also called BtpA (Brucella TIR protein A)61 – since a mutant lacking tcpB failed to induce the UPR and purified TcpB was sufficient to cause UPR induction69. TcpB has been shown to antagonize TLR2 and TLR4 signaling, via its interaction with MyD88 and the adapter protein Mal/TIRAP and inhibition of NF-κB activation78–83, and also stabilizes microtubules80, potentially affecting ER morphology. It will therefore be interesting to examine whether TcpB-mediated UPR induction results from any of these activities, or whether its UPR-inducing effect reflects yet another function. The recent demonstration that BtpA is translocated into host cells by B. abortus, possibly in a VirB-dependent manner61, emphasizes the concept that bacterial pathogens may trigger ER stress via delivery of effectors or toxins, but also raises the question of VirB dependency of UPR induction by Brucella: if TcpB/BtpA is a VirB effector, one would expect that a VirB-deficient strain also fails to induce the UPR. Hence, further studies are needed to clarify these aspects of Brucella induction of the UPR.
UPR induction by Brucella may however result from more complex interactions with host cells involving several effectors. For example, the VirB T4SS effector VceC induces IRE1α-dependent signaling, leading to induction of the proinflammatory cytokines TNFα and interleukin (IL)-662. VceC-mediated stimulation of UPR signaling possibly results from its interactions with and possible sequestration of the ER chaperone BiP, which could alter its ability to down-modulate IRE1α activation9,10 (Figure 3). Additionally, individual expression of various Brucella effectors (BspC, BspG, BspH, BspI, BspK) also leads to induction of ER stress in HeLa cells, regardless of whether these proteins target the secretory compartment60. Interestingly, all these effectors induced BiP overexpression, but not CHOP60, suggesting that they modulate the IRE1 and ATF6, but not the PERK, pathways, potentially avoiding inhibition of translation, cell cycle arrest and cell death. Hence, our current knowledge of Brucella induction of the UPR suggests a multifactorial process that allows a finely tuned modulation of this pathway to the bacterium’s benefit.
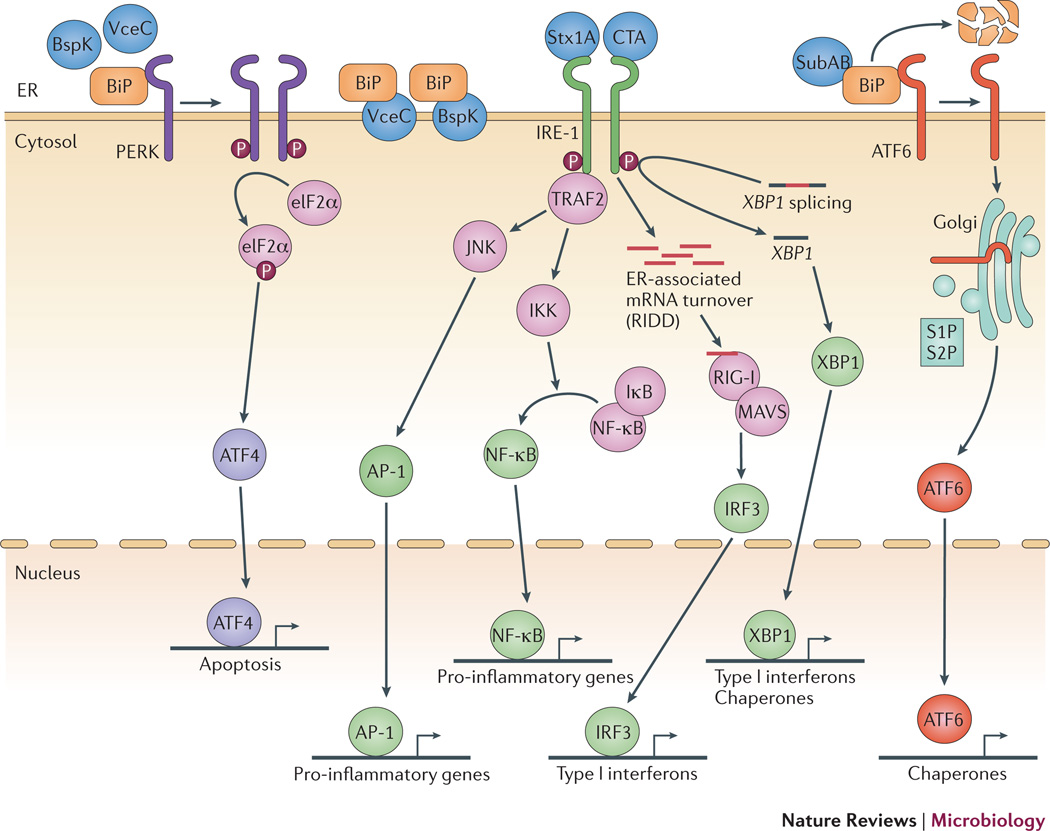
Figure 3. Induction of the UPR and inflammation by bacterial virulence factors.
Both AB-type toxins and T4SS effectors have been implicated in activation of the UPR and subsequent inflammation. The A subunit of CTX binds IRE1α and activates its endonuclease activity, leading to activation of Type I interferon and NF-κB, and Shiga toxin A subunit appears to elicit the same pathway2. Subtilase cytotoxin also localizes to the ER and elicits the UPR by cleavage of the chaperone BiP76, leading to activation of the UPR pathways and to NF-κB-dependent proinflammatory cytokine production. Two B. abortus T4SS effectors, VceC and BspK, target the ER, where VceC has been shown to bind to BiP. This binding may inhibit the interaction of BiP with PERK, IRE1α and ATF6, thereby promoting their activation. IRE1α interacts with TRAF2, leading to NF-κB activation62.60,69,70
UPR and innate immune signaling
The co-occurrence of ER stress and inflammation in many chronic pathologies, including neurodegenerative disease, diabetes, obesity and inflammatory bowel disease, suggests interactions between the UPR and inflammation84. Indeed, proinflammatory cytokines can promote ER stress signaling via oxidative stress, which can alter redox homeostasis in the ER and lead to protein misfolding. In addition, nitric oxide (NO) produced during inflammation can perturb ER function via S-nitrosylation of protein disulfide isomerase (PDI), inhibiting its ability to catalyze disulfide bond formation during protein folding85. Further, enhancement of proinflammatory cytokine responses by the IRE1-XBP1 pathway in response to TLR2 and TLR4 ligands suggests additional points of intersection between innate immune and ER stress signaling pathways86.
Conversely, ER stress can activate proinflammatory signaling pathways via multiple mechanisms (Figure 3). Overexpression of viral proteins in the ER has long been known to activate NF-κB and AP-1 transcription factors, which induce expression of proinflammatory cytokines such as IL-687,88. One of the pathways linking ER stress to proinflammatory cytokine expression is dependent on IRE1’s kinase activity. Activated IRE1 forms a complex with TRAF2, that activates JNK28, an upstream signaling molecule leading to AP-1 activation28. In the TNF receptor signaling pathway, activated TRAF2 can also recruit IκB kinases, leading to their activation, which suggests that recruitment of TRAF2 to the ER membrane by IRE1 activation during the UPR may also promote activation of NF-κB. IRE1-dependent mRNA decay (RIDD)15 can also activate innate immune responses, via generation of immunostimulatory mRNA fragments in the cytosol2. Similar to fragments generated by the antiviral endoribonuclease RNase L89,90, these mRNA fragments generated by RIDD can be detected by the innate immune receptor RIG-I to activate NF-κB and IRF-3, driving expression of proinflammatory cytokines and interferon beta (IFNβ)2,89,91. IRE1’s endonuclease activity also activates XBP-1, which has been shown to promote transcription of IFNβ via binding of an upstream enhancer, as well as promoting transcription of IL-6 and TNFα86,92. In addition to the NF-κB pathway, under conditions of irremediable ER stress, IRE1 can also activate NLRP3 inflammasomes in response to redox stress via thioredoxin-interacting protein (TXNIP), leading to IL-1β activation and pyroptotic cell death93–95.
The PERK-eIF2-ATF4-CHOP arm of the UPR, which mediates inhibition of translation, interacts with innate immune signaling via activation of NF-κB. Since IκB has a short half-life, translational inhibition by this pathway results in more rapid turnover of IκB and, consequently, activation of NF-κB (Figure 3). ATF6 also plays a role in activation of NF-κB in the context of cellular intoxication by Subtilase cytotoxin, however the precise mechanism for this is not known96. An ATF6-like transcription factor, CREBH, is activated by ER stress in a manner similar to ATF6 requiring cleavage by S1P and S2P, and plays a role in innate immunity by activating hepcidin expression, thereby limiting iron availability to infecting bacteria97,98. It is therefore likely that depending on the cellular and tissue context of infection, different combinations of these pathways may play a role in induction of innate immune responses by ER stress.
Studies of viral infection have revealed that hijacking of ER functions for production of viral proteins can trigger innate immune responses, however several viruses are able to exploit the resulting unfolded protein response to promote their replication (Box 1)3. Therefore, it is conceivable that subversion of ER function for intracellular replication of bacterial pathogens such as Legionella, Brucella, Simkania or Chlamydia may also activate a subset of these surveillance pathways.
Brucella spp. are an excellent model to study the intersection of the UPR and innate immunity, because they signal only weakly through TLRs that detect bacteria, such as TLR4 and TLR299,100. Further, in a mouse model of infection, innate immune responses to Brucella are highly dependent on function of its VirB T4SS101. In infected macrophages, the IRE1 arm of the UPR appears to play a key role in the innate immune response to the T4SS, since silencing of IRE1 dampened production of inflammatory cytokines by infected cells. VceC, a T4SS effector protein that localizes to the ER, can activate IRE1-dependent secretion of IL-6 and TNFα62. As described above, the newly-identified T4SS substrates BspC, BspG, BspH, BspI, and BspK, can also activate the IRE1 pathway in HeLa cells; therefore it will be interesting to determine whether they synergize with VceC in inducing expression of proinflammatory cytokines during infection60. Further, it is not known how BtpA, recently shown to induce all three pathways of the UPR, influences UPR-induced inflammation69. However, induction of UPR-dependent inflammation during Brucella infection may underline some similarities in the host response between Brucella and viral infections with regard to their gene expression profiles in vivo101,102.
Bacterial toxins can also induce UPR-dependent inflammation (Table 1). Some toxins, such as Shiga toxin and cholera toxin, reach their cytosolic targets via cellular uptake and retrotranslocation to the ER, where they fold before reaching their targets in the cell cytosol. Localization of an enzymatically inactive A subunit of cholera toxin (CTA) to the ER was recently found to activate NF-κB via an IRE1-dependent pathway in intestinal epithelial cells, via binding of unfolded CTA to IRE12 (Figure 3). Interestingly, the pathway leading to NF-κB activation by CTA involves activation of RIDD and sensing of the resulting mRNA fragments via a RIG-I/MAVS-dependent pathway. The A subunit of Shiga toxin, which triggers death of intoxicated cells via the UPR103, is also able to activate IRE1 and induce NF-kB activation, likely via a mechanism similar to that identified for CTA2. Activation of immune cells, particularly T cells, by Shiga toxins has been postulated to play a role in disease progression of hemolytic-uremic syndrome caused by Shiga toxin-producing E. coli (STEC), raising the possibility of a connection between UPR activation in intoxicated cells and systemic inflammatory pathology105.
As mentioned above, cleavage of BiP by Subtilase cytotoxin of STEC leads to induction of the three branches of the UPR, and consequently to a transient activation of NF-κB96,106. However, at subcytotoxic doses, pre-stimulation of cells with subtilase cytotoxin was shown to blunt NF-κB activation induced by heterologous stimuli such as TLR4 agonists107. Therefore, the effect of this toxin on inflammatory responses during an infection may depend on the toxin dose in the context of an infection. The above examples illustrate that disruption of ER integrity can serve as a pathogenic pattern of infection that can be induced both by pathogens that target the ER directly to promote infection and pathogens that, via disruption of other cellular processes, perturb ER function. Further, in the context of additional infection-related signals, including TLR ligands86 and proinflammatory cytokines5, cellular surveillance of ER function is heightened to generate a more rapid innate immune response to ER stress.
The UPR – friend or foe?
While a number of bacterial pathogens can induce the UPR during infection, it is not clear in each case whether this response benefits the host or the pathogen. Modulation of ER function during infection by intracellular bacteria can promote bacterial infection by providing a replicative niche, but at the same time the resulting disruption of the secretory pathway can provide a pattern of pathogenesis that aids the innate immune system in recognizing intracellular infection and in mounting an appropriate defense. However, considering the more rapid evolution of bacterial pathogens compared to their hosts, it is likely that bacteria have evolved to modulate the UPR to their advantage during infection. Given the multitude of virulence genes expressed by bacterial pathogens that replicate in ER-associated cellular compartments, future work is likely to reveal to what extent viruses and bacteria use shared strategies to exploit this intracellular niche to promote their replication, as well as which novel mechanisms are employed by bacteria to manipulate signaling pathways emanating from the ER. Further, since the UPR has been targeted therapeutically in inflammatory disease and cancer108, it would be intriguing to explore this avenue as an adjunct for treatment of intracellular bacterial infection, such as tuberculosis and brucellosis, which currently require long antibiotic treatment regimens. Given the central importance of the UPR in a multitude of disease processes, insights from this area of study are likely to lead to an improved understanding of the links between ER stress signaling pathways and pathology, not only in the context of infection, but also of neurodegenerative disease, diabetes, obesity, inflammatory bowel disease, and cancer.
References
- 1. Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. An excellent current review of the unfolded protein response
- 2. Cho JA, et al. The unfolded protein response element IRE1alpha senses bacterial proteins invading the ER to activate RIG-I and innate immune signaling. Cell host & microbe. 2013;13:558–569. doi: 10.1016/j.chom.2013.03.011. This paper shows that generation of RNA fragments by IRE1 in response to toxin translocation to the ER activates innate immune responses.
- 3.He B. Viruses, endoplasmic reticulum stress, and interferon responses. Cell Death Differ. 2006;13:393–403. doi: 10.1038/sj.cdd.4401833. [DOI] [PubMed] [Google Scholar]
- 4.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annual review of biochemistry. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 5. Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. An excellent synthesis of the links between ER stress and inflammation.
- 6.Gardner BM, Walter P. Unfolded proteins are Ire1-activating ligands that directly induce the unfolded protein response. Science. 2011;333:1891–1894. doi: 10.1126/science.1209126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olzmann JA, Kopito RR, Christianson JC. The mammalian endoplasmic reticulum-associated degradation system. Cold Spring Harbor perspectives in biology. 2013;5 doi: 10.1101/cshperspect.a013185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 9.Kimata Y, Oikawa D, Shimizu Y, Ishiwata-Kimata Y, Kohno K. A role for BiP as an adjustor for the endoplasmic reticulum stress-sensing protein Ire1. The Journal of cell biology. 2004;167:445–456. doi: 10.1083/jcb.200405153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pincus D, et al. BiP binding to the ER-stress sensor Ire1 tunes the homeostatic behavior of the unfolded protein response. PLoS biology. 2010;8:e1000415. doi: 10.1371/journal.pbio.1000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gardner BM, Pincus D, Gotthardt K, Gallagher CM, Walter P. Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harbor perspectives in biology. 2013;5:a013169. doi: 10.1101/cshperspect.a013169. An excellent current review of how the UPR is initiated.
- 12.Lee K, et al. IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes & development. 2002;16:452–466. doi: 10.1101/gad.964702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Molecular and cellular biology. 2003;23:7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hollien J, et al. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. The Journal of cell biology. 2009;186:323–331. doi: 10.1083/jcb.200903014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hollien J, Weissman JS. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science. 2006;313:104–107. doi: 10.1126/science.1129631. [DOI] [PubMed] [Google Scholar]
- 16.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 17.Harding HP, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Molecular cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 18.Urra H, Dufey E, Lisbona F, Rojas-Rivera D, Hetz C. When ER stress reaches a dead end. Biochimica et biophysica acta. 2013;1833:3507–3517. doi: 10.1016/j.bbamcr.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 19.Haze K, Yoshida H, Yanagi H, Yura T, Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Molecular biology of the cell. 1999;10:3787–3799. doi: 10.1091/mbc.10.11.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adachi Y, et al. ATF6 is a transcription factor specializing in the regulation of quality control proteins in the endoplasmic reticulum. Cell structure and function. 2008;33:75–89. doi: 10.1247/csf.07044. [DOI] [PubMed] [Google Scholar]
- 21.Wu J, et al. ATF6alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev Cell. 2007;13:351–364. doi: 10.1016/j.devcel.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto K, et al. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev Cell. 2007;13:365–376. doi: 10.1016/j.devcel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 23.Bernales S, McDonald KL, Walter P. Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol. 2006;4:e423. doi: 10.1371/journal.pbio.0040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bommiasamy H, et al. ATF6alpha induces XBP1-independent expansion of the endoplasmic reticulum. Journal of cell science. 2009;122:1626–1636. doi: 10.1242/jcs.045625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuck S, Prinz WA, Thorn KS, Voss C, Walter P. Membrane expansion alleviates endoplasmic reticulum stress independently of the unfolded protein response. The Journal of cell biology. 2009;187:525–536. doi: 10.1083/jcb.200907074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogata M, et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Molecular and cellular biology. 2006;26:9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding WX, et al. Differential effects of endoplasmic reticulum stress-induced autophagy on cell survival. J Biol Chem. 2007;282:4702–4710. doi: 10.1074/jbc.M609267200. [DOI] [PubMed] [Google Scholar]
- 28. Urano F, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. This paper shows that IRE1 links ER stress to JNK, an activator of AP-1.
- 29.Detilleux PG, Deyoe BL, Cheville NF. Entry and Intracellular Localization of Brucella spp. in Vero Cells: Fluorescence and Electron Microscopy. Veterinary Pathology. 1990;27:317–328. doi: 10.1177/030098589002700503. [DOI] [PubMed] [Google Scholar]
- 30.Detilleux PG, Deyoe BL, Cheville NF. Penetration and intracellular growth of Brucella abortus in nonphagocytic cells in vitro. Infection and immunity. 1990 doi: 10.1128/iai.58.7.2320-2328.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horwitz MA. Formation of a novel phagosome by the Legionnaires' disease bacterium (Legionella pneumophila) in human monocytes. Journal of Experimental Medicine. 1983;158:1319–1331. doi: 10.1084/jem.158.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pizarro-Cerdá J, et al. Brucella abortus transits through the autophagic pathway and replicates in the endoplasmic reticulum of nonprofessional phagocytes. Infection and immunity. 1998;66:5711–5724. doi: 10.1128/iai.66.12.5711-5724.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pizarro-Cerdá J, Moreno E, Sanguedolce V, Mege JL, Gorvel JP. Virulent Brucella abortus prevents lysosome fusion and is distributed within autophagosome-like compartments. Infection and immunity. 1998;66:2387–2392. doi: 10.1128/iai.66.5.2387-2392.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tilney LG, Harb OS, Connelly PS, Robinson CG, Roy CR. the parasitic bacterium Legionella pneumophila modifies its phagosome and transforms it into rough ER: implications for conversion of plasma membrane to the ER. 2001 doi: 10.1242/jcs.114.24.4637. [DOI] [PubMed] [Google Scholar]
- 35. Celli J, et al. Brucella evades macrophage killing via VirB-dependent sustained interactions with the endoplasmic reticulum. The Journal of experimental medicine. 2003;198:545–556. doi: 10.1084/jem.20030088. This paper provide evidence that Brucellaexploits the endoplasmic reticulum via its VirB Type IV secretion system to evade killing by macrophages.
- 36.Robinson CG, Roy CR. Attachment and fusion of endoplasmic reticulum with vacuoles containing Legionella pneumophila. Cellular microbiology. 2006;8:793–805. doi: 10.1111/j.1462-5822.2005.00666.x. [DOI] [PubMed] [Google Scholar]
- 37.Asare R, Abu Kwaik Y. Early trafficking and intracellular replication of Legionella longbeachaeae within an ER-derived late endosome-like phagosome. Cellular microbiology. 2007;9:1571–1587. doi: 10.1111/j.1462-5822.2007.00894.x. [DOI] [PubMed] [Google Scholar]
- 38.Derré I, Swiss R, Agaisse H. The Lipid Transfer Protein CERT Interacts with the Chlamydia Inclusion Protein IncD and Participates to ER-Chlamydia Inclusion Membrane Contact Sites. PLoS pathogens. 2011;7:e1002092. doi: 10.1371/journal.ppat.1002092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dumoux M, Clare DK, Saibil HR, Hayward RD. Chlamydiae Assemble a Pathogen Synapse to Hijack the Host Endoplasmic Reticulum. Traffic (Copenhagen, Denmark) 2012;13:1612–1627. doi: 10.1111/tra.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mehlitz A, et al. The Chlamydial Organism Simkania negevensis Forms ER Vacuole Contact Sites and Inhibits ER-stress. Cellular microbiology. 2014 doi: 10.1111/cmi.12278. Identifies a new bacterial pathogen that associates with the endoplasmic reticulum.
- 41. Kagan JC, Roy CR. Legionella phagosomes intercept vesicular traffic from endoplasmic reticulum exit sites. Nature cell biology. 2002;4:945–954. doi: 10.1038/ncb883. Showed that Legionella recruits early secretory vesicles after internalization by phagocytic cells.
- 42.Starr T, et al. Selective subversion of autophagy complexes facilitates completion of the Brucella intracellular cycle. Cell Host & Microbe. 2012;11:33–45. doi: 10.1016/j.chom.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Celli J, Salcedo SP, Gorvel J-P. Brucella coopts the small GTPase Sar1 for intracellular replication. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:1673–1678. doi: 10.1073/pnas.0406873102. This paper provided evidence that Brucella abortus intercepts traffic from ER exit sites to promote its intracellular replication.
- 44.Porte F, Liautard JP, Köhler S. Early Acidification of Phagosomes Containing Brucella suis Is Essential for Intracellular Survival in Murine Macrophages. 1999 doi: 10.1128/iai.67.8.4041-4047.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Starr T, Ng TW, Wehrly TD, Knodler LA, Celli J. Brucella intracellular replication requires trafficking through the late endosomal/lysosomal compartment. Traffic (Copenhagen, Denmark) 2008;9:678–694. doi: 10.1111/j.1600-0854.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- 46.Robertson DK, Gu L, Rowe RK, Beatty WL. Inclusion Biogenesis and Reactivation of Persistent Chlamydia trachomatis Requires Host Cell Sphingolipid Biosynthesis. PLoS pathogens. 2009;5:e1000664. doi: 10.1371/journal.ppat.1000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Ooij C, et al. Host cell-derived sphingolipids are required for the intracellular growth of Chlamydia trachomatis. Cellular microbiology. 2000;2:627–637. doi: 10.1046/j.1462-5822.2000.00077.x. [DOI] [PubMed] [Google Scholar]
- 48.Machner MP, Isberg RR. Targeting of Host Rab GTPase Function by the Intravacuolar Pathogen Legionella pneumophila . Developmental cell. 2006;11:47–56. doi: 10.1016/j.devcel.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 49. Murata T, et al. The Legionella pneumophila effector protein DrrA is a Rab1 guanine nucleotide-exchange factor. Nature cell biology. 2006;8:971–977. doi: 10.1038/ncb1463. These two papers show that Legionella translocates a guanosine nucleotide exchange factor into infected cells to manipulate ER-to-Golgi traffic.
- 50.Ingmundson A, Delprato A, Lambright DG, Roy CR. Legionella pneumophila proteins that regulate Rab1 membrane cycling. Nature. 2007;450:365–369. doi: 10.1038/nature06336. [DOI] [PubMed] [Google Scholar]
- 51.Arasaki K, Roy CR. Legionella pneumophila promotes functional interactions between plasma membrane syntaxins and Sec22b. Traffic. 2010;11:587–600. doi: 10.1111/j.1600-0854.2010.01050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arasaki K, Toomre DK, Roy CR. The Legionella pneumophila effector DrrA is sufficient to stimulate SNARE-dependent membrane fusion. Cell host & microbe. 2012;11:46–57. doi: 10.1016/j.chom.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nagai H. A Bacterial Guanine Nucleotide Exchange Factor Activates ARF on Legionella Phagosomes. Science. 2002;295:679–682. doi: 10.1126/science.1067025. [DOI] [PubMed] [Google Scholar]
- 54.Dorer MS, Kirton D, Bader JS, Isberg RR. RNA Interference Analysis of Legionella in Drosophila Cells: Exploitation of Early Secretory Apparatus Dynamics. PLoS pathogens. 2006;2:e34. doi: 10.1371/journal.ppat.0020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Comerci DJ, Lorenzo MM, Sieira R, Gorvel J-P, Ugalde RA. Essential role of the VirB machinery in the maturation of the Brucella abortus-containing vacuole. Cellular microbiology. 2001;3:159–168. doi: 10.1046/j.1462-5822.2001.00102.x. [DOI] [PubMed] [Google Scholar]
- 56.Boschiroli ML. The Brucella suis virB operon is induced intracellularly in macrophages. Proceedings of the National Academy of Sciences. 2002;99:1544–1549. doi: 10.1073/pnas.032514299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Jong MF, Sun YH, den Hartigh AB, van Dijl JM, Tsolis RM. Identification of VceA and VceC, two members of the VjbR regulon that are translocated into macrophages by the Brucella type IV secretion system. Mol Microbiol. 2008;70:1378–1396. doi: 10.1111/j.1365-2958.2008.06487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Barsy M, et al. Identification of a Brucella spp. secreted effector specifically interacting with human small GTPase Rab2. Cellular microbiology. 2011;13:1044–1058. doi: 10.1111/j.1462-5822.2011.01601.x. [DOI] [PubMed] [Google Scholar]
- 59.Marchesini MI, Herrmann CK, Salcedo SP, Gorvel J-P, Comerci DJ. In search of Brucella abortus type IV secretion substrates: screening and identification of four proteins translocated into host cells through VirB system. Cellular microbiology. 2011;13:1261–1274. doi: 10.1111/j.1462-5822.2011.01618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Myeni S, et al. Brucella Modulates Secretory Trafficking via Multiple Type IV Secretion Effector Proteins. PLoS pathogens. 2013;9:e1003556. doi: 10.1371/journal.ppat.1003556. Identified several novel T4SS effectors as well as a potential role for these effectors in induction of the UPR.
- 61.Salcedo SP, et al. BtpB, a novel Brucella TIR-containing effector protein with immune modulatory functions. Frontiers in Cellular and Infection Microbiology. 2013;3 doi: 10.3389/fcimb.2013.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. de Jong MF, et al. Sensing of bacterial type IV secretion via the unfolded protein response. mBio. 2013;4:e00418–e00412. doi: 10.1128/mBio.00418-12. Showed that elements of the UPR sense localization of a Brucella effector protein to the ER.
- 63.Barlowe C, et al. COPII: A membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 64.Kuge O, et al. Sar1 promotes vesicle budding from the endoplasmic reticulum but not Golgi compartments. The Journal of cell biology. 1994;125:51–65. doi: 10.1083/jcb.125.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fugier E, et al. The glyceraldehyde-3-phosphate dehydrogenase and the small GTPase Rab 2 are crucial for Brucella replication. PLoS Pathog. 2009;5:e1000487. doi: 10.1371/journal.ppat.1000487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seimon TA, et al. Induction of ER Stress in Macrophages of Tuberculosis Granulomas. PloS one. 2010;5:e12772. doi: 10.1371/journal.pone.0012772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baird M, et al. The unfolded protein response is activated in Helicobacter-induced gastric carcinogenesis in a non-cell autonomous manner. Laboratory Investigation. 2012;93:112–122. doi: 10.1038/labinvest.2012.131. [DOI] [PubMed] [Google Scholar]
- 68.Akazawa Y, et al. Endoplasmic Reticulum Stress Contributes to Helicobacter pylori VacA-Induced Apoptosis. PloS one. 2013;8:e82322. doi: 10.1371/journal.pone.0082322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Smith JA, et al. Brucella Induces an Unfolded Protein Response via TcpB That Supports Intracellular Replication in Macrophages. PLoS pathogens. 2013;9:e1003785. doi: 10.1371/journal.ppat.1003785. Implicates a bacterial TIR domain-containing protein in induction of the UPR in B. abortus-infected macrophages.
- 70.Pillich H, Loose M, Zimmer K-P, Chakraborty T. Activation of the unfolded protein response by Listeria monocytogenes . Cellular microbiology. 2012;14:949–964. doi: 10.1111/j.1462-5822.2012.01769.x. [DOI] [PubMed] [Google Scholar]
- 71. Qin QM, et al. RNAi Screen of Endoplasmic Reticulum–Associated Host Factors Reveals a Role for IRE1α in Supporting Brucella Replication. PLoS pathogens. 2008;4:e1000110. doi: 10.1371/journal.ppat.1000110. This paper reports the first implication of a UPR-associated factor in promoting bacterial replication.
- 72.Gekara NO, Groebe L, Viegas N, Weiss S. Listeria monocytogenes desensitizes immune cells to subsequent Ca2+ signaling via listeriolysin O-induced depletion of intracellular Ca2+ stores. Infect Immun. 2008;76:857–862. doi: 10.1128/IAI.00622-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Price CTD, Al-Quadan T, Santic M, Rosenshine I, Abu Kwaik Y. Host Proteasomal Degradation Generates Amino Acids Essential for Intracellular Bacterial Growth. Science. 2011;334:1553–1557. doi: 10.1126/science.1212868. [DOI] [PubMed] [Google Scholar]
- 74. Bischof LJ, et al. Activation of the unfolded protein response is required for defenses against bacterial pore-forming toxin in vivo. PLoS Pathog. 2008;4:e1000176. doi: 10.1371/journal.ppat.1000176. This paper showed that the UPR acts as a defense mechanism against the action of bacterial pore-forming toxins.
- 75.Morinaga N, Yahiro K, Matsuura G, Moss J, Noda M. Subtilase cytotoxin, produced by Shiga-toxigenic Escherichia coli, transiently inhibits protein synthesis of Vero cells via degradation of BiP and induces cell cycle arrest at G1 by downregulation of cyclin D1. Cellular microbiology. 2008;10:921–929. doi: 10.1111/j.1462-5822.2007.01094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Paton AW, et al. AB5 subtilase cytotoxin inactivates the endoplasmic reticulum chaperone BiP. Nature. 2006;443:548–552. doi: 10.1038/nature05124. Shows that a toxin of enterohemorrhagic E. colican cleave BiP.
- 77.Wolfson JJ, et al. Subtilase cytotoxin activates PERK, IRE1 and ATF6 endoplasmic reticulum stress-signalling pathways. Cellular microbiology. 2008;10:1775–1786. doi: 10.1111/j.1462-5822.2008.01164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chaudhary A, et al. The Brucella TIR-like protein TcpB interacts with the death domain of MyD88. Biochemical and biophysical research communications. 2012;417:299–304. doi: 10.1016/j.bbrc.2011.11.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cirl C, et al. Subversion of Toll-like receptor signaling by a unique family of bacterial Toll/interleukin-1 receptor domain-containing proteins. Nature medicine. 2008;14:399–406. doi: 10.1038/nm1734. [DOI] [PubMed] [Google Scholar]
- 80.Radhakrishnan GK, Harms JS, Splitter GA. Modulation of microtubule dynamics by a TIR domain protein from the intracellular pathogen Brucella melitensis . Biochemical Journal. 2011;439:79–83. doi: 10.1042/BJ20110577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sengupta D, et al. Subversion of innate immune responses by Brucella through the targeted degradation of the TLR signaling adapter, MAL. J Immunol. 2010;184:956–964. doi: 10.4049/jimmunol.0902008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Snyder GA, et al. Crystal structures of the Toll/Interleukin-1 receptor (TIR) domains from the Brucella protein TcpB and host adaptor TIRAP reveal mechanisms of molecular mimicry. J Biol Chem. 2014;289:669–679. doi: 10.1074/jbc.M113.523407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Salcedo SP, et al. Brucella control of dendritic cell maturation is dependent on the TIR-Containing protein btp1. PLoS pathogens. 2008;4:e21. doi: 10.1371/journal.ppat.0040021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hasnain SZ, Lourie R, Das I, Chen AC, McGuckin MA. The interplay between endoplasmic reticulum stress and inflammation. Immunology and cell biology. 2012;90:260–270. doi: 10.1038/icb.2011.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Uehara T, et al. S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature. 2006;441:513–517. doi: 10.1038/nature04782. [DOI] [PubMed] [Google Scholar]
- 86.Martinon F, Chen X, Lee AH, Glimcher LH. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nature immunology. 2010;11:411–418. doi: 10.1038/ni.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Pahl HL, Baeuerle PA. A novel signal transduction pathway from the endoplasmic reticulum to the nucleus is mediated by transcription factor NF-kappa B. Embo J. 1995;14:2580–2588. doi: 10.1002/j.1460-2075.1995.tb07256.x. Provided the first evidence of a link between ER stress and NF-kB signaling.
- 88.Pahl HL, Baeuerle PA. Expression of influenza virus hemagglutinin activates transcription factor NF-kappa B. Journal of virology. 1995;69:1480–1484. doi: 10.1128/jvi.69.3.1480-1484.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Malathi K, Dong B, Gale M, Jr, Silverman RH. Small self-RNA generated by RNase L amplifies antiviral innate immunity. Nature. 2007;448:816–819. doi: 10.1038/nature06042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Malathi K, et al. RNase L releases a small RNA from HCV RNA that refolds into a potent PAMP. Rna. 2010;16:2108–2119. doi: 10.1261/rna.2244210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yoneyama M, et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nature immunology. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 92.Zeng L, et al. XBP-1 couples endoplasmic reticulum stress to augmented IFN-beta induction via a cis-acting enhancer in macrophages. J Immunol. 2010;185:2324–2330. doi: 10.4049/jimmunol.0903052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lerner AG, et al. IRE1alpha induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell metabolism. 2012;16:250–264. doi: 10.1016/j.cmet.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Menu P, et al. ER stress activates the NLRP3 inflammasome via an UPR-independent pathway. Cell death & disease. 2012;3:e261. doi: 10.1038/cddis.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Oslowski CM, et al. Thioredoxin-interacting protein mediates ER stress-induced beta cell death through initiation of the inflammasome. Cell metabolism. 2012;16:265–273. doi: 10.1016/j.cmet.2012.07.005. These three papers demonstrate a link between ER stress and activation of the NLRP3 inflammasome.
- 96.Yamazaki H, et al. Activation of the Akt-NF-kappaB pathway by subtilase cytotoxin through the ATF6 branch of the unfolded protein response. J Immunol. 2009;183:1480–1487. doi: 10.4049/jimmunol.0900017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vecchi C, et al. ER stress controls iron metabolism through induction of hepcidin. Science. 2009;325:877–880. doi: 10.1126/science.1176639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang K, et al. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell. 2006;124:587–599. doi: 10.1016/j.cell.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 99.Barquero-Calvo E, et al. Brucella abortus uses a stealthy strategy to avoid activation of the innate immune system during the onset of infection. PloS one. 2007;2:e631. doi: 10.1371/journal.pone.0000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lapaque N, et al. Differential inductions of TNF-alpha and IGTP, IIGP by structurally diverse classic and non-classic lipopolysaccharides. Cell Microbiol. 2006;8:401–413. doi: 10.1111/j.1462-5822.2005.00629.x. [DOI] [PubMed] [Google Scholar]
- 101.Roux CM, et al. Brucella requires a functional Type IV secretion system to elicit innate immune responses in mice. Cell Microbiol. 2007;9:1851–1869. doi: 10.1111/j.1462-5822.2007.00922.x. [DOI] [PubMed] [Google Scholar]
- 102.Chang WL, et al. Influenza virus infection causes global respiratory tract B cell response modulation via innate immune signals. J Immunol. 2007;178:1457–1467. doi: 10.4049/jimmunol.178.3.1457. [DOI] [PubMed] [Google Scholar]
- 103.Lee SY, Lee MS, Cherla RP, Tesh VL. Shiga toxin 1 induces apoptosis through the endoplasmic reticulum stress response in human monocytic cells. Cell Microbiol. 2008;10:770–780. doi: 10.1111/j.1462-5822.2007.01083.x. [DOI] [PubMed] [Google Scholar]
- 104.Lee MS, Kim MH, Tesh VL. Shiga toxins expressed by human pathogenic bacteria induce immune responses in host cells. J Microbiol. 2013;51:724–730. doi: 10.1007/s12275-013-3429-6. [DOI] [PubMed] [Google Scholar]
- 105.Heyderman RS, Soriani M, Hirst TR. Is immune cell activation the missing link in the pathogenesis of post-diarrhoeal HUS? Trends in microbiology. 2001;9:262–266. doi: 10.1016/s0966-842x(01)02045-5. [DOI] [PubMed] [Google Scholar]
- 106.Zhao Y, et al. Subtilase cytotoxin activates MAP kinases through PERK and IRE1 branches of the unfolded protein response. Toxicological sciences : an official journal of the Society of Toxicology. 2011;120:79–86. doi: 10.1093/toxsci/kfq368. [DOI] [PubMed] [Google Scholar]
- 107.Harama D, et al. A subcytotoxic dose of subtilase cytotoxin prevents lipopolysaccharide-induced inflammatory responses, depending on its capacity to induce the unfolded protein response. J Immunol. 2009;183:1368–1374. doi: 10.4049/jimmunol.0804066. [DOI] [PubMed] [Google Scholar]
- 108.Mimura N, et al. Blockade of XBP1 splicing by inhibition of IRE1alpha is a promising therapeutic option in multiple myeloma. Blood. 2012;119:5772–5781. doi: 10.1182/blood-2011-07-366633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Watowich SS, Morimoto RI, Lamb RA. Flux of the paramyxovirus hemagglutinin-neuraminidase glycoprotein through the endoplasmic reticulum activates transcription of the GRP78-BiP gene. Journal of virology. 1991;65:3590–3597. doi: 10.1128/jvi.65.7.3590-3597.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Isler JA, Skalet AH, Alwine JC. Human cytomegalovirus infection activates and regulates the unfolded protein response. Journal of virology. 2005;79:6890–6899. doi: 10.1128/JVI.79.11.6890-6899.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stahl S, et al. Cytomegalovirus downregulates IRE1 to repress the unfolded protein response. PLoS Pathog. 2013;9:e1003544. doi: 10.1371/journal.ppat.1003544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tirosh B, et al. Human cytomegalovirus protein US11 provokes an unfolded protein response that may facilitate the degradation of class I major histocompatibility complex products. Journal of virology. 2005;79:2768–2779. doi: 10.1128/JVI.79.5.2768-2779.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tardif KD, Mori K, Kaufman RJ, Siddiqui A. Hepatitis C virus suppresses the IRE1-XBP1 pathway of the unfolded protein response. J Biol Chem. 2004;279:17158–17164. doi: 10.1074/jbc.M312144200. [DOI] [PubMed] [Google Scholar]
- 114.Chan SW. Unfolded protein response in hepatitis C virus infection. Frontiers in microbiology. 2014;5:233. doi: 10.3389/fmicb.2014.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Trujillo-Alonso V, Maruri-Avidal L, Arias CF, Lopez S. Rotavirus infection induces the unfolded protein response of the cell and controls it through the nonstructural protein NSP3. Journal of virology. 2011;85:12594–12604. doi: 10.1128/JVI.05620-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zambrano JL, et al. Rotavirus infection activates the UPR but modulates its activity. Virology journal. 2011;8:359. doi: 10.1186/1743-422X-8-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Smith JA. A new paradigm: innate immune sensing of viruses via the unfolded protein response. Frontiers in microbiology. 2014;5:222. doi: 10.3389/fmicb.2014.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fronzes R, Christie PJ, Waksman G. The structural biology of type IV secretion systems. Nature reviews. Microbiology. 2009;7:703–714. doi: 10.1038/nrmicro2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Juhas M, Crook DW, Hood DW. Type IV secretion systems: tools of bacterial horizontal gene transfer and virulence. Cell Microbiol. 2008;10:2377–2386. doi: 10.1111/j.1462-5822.2008.01187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nagai H, Kubori T. Type IVB Secretion Systems of Legionella and Other Gram-Negative Bacteria. Frontiers in microbiology. 2011;2:136. doi: 10.3389/fmicb.2011.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.de Jong MF, Tsolis RM. Brucellosis and type IV secretion. Future microbiology. 2012;7:47–58. doi: 10.2217/fmb.11.136. [DOI] [PubMed] [Google Scholar]
- 122.Voth DE, Broederdorf LJ, Graham JG. Bacterial Type IV secretion systems: versatile virulence machines. Future microbiology. 2012;7:241–257. doi: 10.2217/fmb.11.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Goldszmid RS, et al. Host ER-parasitophorous vacuole interaction provides a route of entry for antigen cross-presentation in Toxoplasma gondii-infected dendritic cells. J Exp Med. 2009;206:399–410. doi: 10.1084/jem.20082108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sinai AP, Webster P, Joiner KA. Association of host cell endoplasmic reticulum and mitochondria with the Toxoplasma gondii parasitophorous vacuole membrane: a high affinity interaction. Journal of cell science. 1997;110(Pt 17):2117–2128. doi: 10.1242/jcs.110.17.2117. [DOI] [PubMed] [Google Scholar]
- 125.Wang T, et al. Toxoplasma gondii induce apoptosis of neural stem cells via endoplasmic reticulum stress pathway. Parasitology. 2014;141:988–995. doi: 10.1017/S0031182014000183. [DOI] [PubMed] [Google Scholar]
- 126.Xu X, et al. Reactive oxygen species-triggered trophoblast apoptosis is initiated by endoplasmic reticulum stress via activation of caspase-12, CHOP, and the JNK pathway in Toxoplasma gondii infection in mice. Infect Immun. 2012;80:2121–2132. doi: 10.1128/IAI.06295-11. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 127.Yamamoto M, et al. ATF6beta is a host cellular target of the Toxoplasma gondii virulence factor ROP18. J Exp Med. 2011;208:1533–1546. doi: 10.1084/jem.20101660. [DOI] [PMC free article] [PubMed] [Google Scholar]