Abstract
Objectives
Researchers have proposed biological (inflammation) and psychological (depression) factors as potential mechanisms for poorer outcomes and readmissions in heart failure (HF) patients. However, studies investigating the link between inflammation and depressive symptoms in these patients are few. We examined the relationships between levels of the inflammatory markers C-reactive protein (CRP), interleukin (IL)-6, and soluble tumor necrosis factor receptor 2 (sTNR2) and depressive symptoms in HF outpatients.
Method
55 patients (74.5% men; 60% Whites; mean age 71.6 ± 11.3 years) with New York Heart Association Class II, III, or IV HF (49%, 47%, and 4%, respectively) and mean ejection fraction (EF) 29.9 ± 7.1% completed the Patient Health Questionnaire (PHQ)-9 as a measure of depressive symptoms. We also obtained height, weight, and CRP, IL-6, and sTNFR2 levels. We used multivariate regressions to assess the predictive value of PHQ-9 scores on each inflammatory marker.
Results
22 (40%) participants reported depressive symptoms (PHQ-9 score ≥ 5). After controlling for age, gender, body mass index, HF etiology, EF, and statin use, we found significant relationships between levels of both sTNFR2 (β = .35, p = .01) and IL-6 (β = .30, p = .04), but not CRP (β = −.96, p = .52), and depression scores.
Conclusion
Our findings add to a growing body of evidence supporting the proposition that heightened inflammation explains the effect depression has on HF. Health care providers should screen for depression in HF patients, as they may be at higher risk of augmented inflammation and poor outcomes.
Keywords: heart failure, inflammation, depressive symptoms, TNF-α, TNF-Receptors, IL-6, CRP
Despite significant advances in treatment and management, chronic heart failure (HF) continues to be a public health concern in the United States. HF currently affects approximately 5 million Americans, with a projected 550,000 new cases each year, and is associated with repeated hospitalizations, high mortality rates, and increased medical expenditures (Roger et al., 2011). Researchers have proposed both biological and psychological factors as potential mechanisms to explain these vulnerabilities.
Some experts have described HF as an inflammatory state in which inflammatory markers and inflammatory cytokines are elevated (Deswal et al., 2001). Pro-inflammatory cytokines, which are compensatory at first, become deleterious and predictive of poor outcomes when they are chronically elevated. Besides their correlation with disease severity, increased plasma levels of interleukin (IL)-6 and tumor necrosis factor (TNF)-α and its soluble receptors correlate with an increased risk of adverse outcomes (Bozkurt, Mann, & Deswal, 2010; Deswal et al., 2001; Ferrari et al., 1995). Cytokines, particularly IL-6, enhance the production of acute-phase proteins from the liver. Researchers have found, in fact, a positive relationship between elevated C-reactive protein (CRP) levels and increased levels of IL-6 (Sato et al., 1999; Villacorta, Masetto, & Mesquita, 2007). Although the role of CRP in the progression of HF is not fully understood, CRP levels do predict HF onset and severity, hospitalization, and death (Alonso-Martinez et al., 2002;Vasan et al., 2003).
Similarly, depression, which is common in patients with HF, is a predictor of cardiovascular readmissions, worsening cardiac function, mortality, and increased health care utilization (Jiang et al., 2001; Sherwood et al., 2007; Sullivan, Simon, Spertus, & Russo, 2002; Vaccarino, Kasl, Abramson, & Krumholz, 2001). One line of evidence suggests that depression may exert its adverse effects on HF through behavioral and biological processes. Some of these include nonadherence, inadequate self-care management, activation of the sympathetic nervous system, hypercoagulability, heart rate variability, and immune–inflammatory and neuroendocrine dysregulation. The role that inflammation plays as a modifiable risk factor for both HF and depression has generated particular interest and has led to postulations that inflammation represents a plausible pathway for poor outcomes in depressed patients with chronic HF (Pasic, Levy, & Sullivan, 2003).
To date, the few studies that have examined the relationship between depression and inflammation in HF patients have yielded inconsistent results. Some researchers have observed dysregulation in the production of anti- and pro-inflammatory markers in HF patients with depressive symptoms, as evidenced by low IL-10 levels and high TNF-α levels and IL-6/IL-10 ratio (Ferketich, Ferguson, & Binkley, 2005; Parissis et al., 2004, 2009; Redwine et al., 2007). The relationships between IL-6 and CRP and depression, however, have shown more variation among studies, with some finding higher levels of IL-6 and/or CRP and others lower in HF patients with depression (Ferketich et al., 2005; Johansson et al., 2011; Parissis et al., 2004, 2009; Redwine et al., 2007; Wirtz et al., 2010).
The existing evidence about the relationship between depression and inflammation in HF patients is thus not sufficient to draw firm conclusions. To address this gap, we undertook the present study to examine the relationships between the inflammatory markers CRP, IL-6, and soluble TNF receptor 2 (sTNR2) and depressive symptoms in patients with chronic HF.
Materials and Methods
Subjects and Design
We used a cross-sectional descriptive design for this study. It conformed to the principles outlined in the Declaration of Helsinki, and we obtained approval from the Institutional Review Board. We recruited a convenience sample from three community outpatient cardiology clinics located within neighboring cities in Los Angeles County via referral from cardiologists or nurse practitioners. We obtained written informed consent from potential participants and screened them for eligibility according to the following inclusion criteria: history of HF for at least 6 months, New York Heart Association (NYHA) Class II to IV (mild to severe) HF, and presence of left ventricular (LV) dysfunction with LV ejection fraction (EF) of 40% or less (documented by echocardiography, cardiac stress test, or ventriculography). Patients with conditions, medications, or behaviors that affect the immune system (i.e., autoimmune diseases, malignancy, human immunodeficiency virus [HIV], cancer, corticosteroids, smoking, excessive alcohol intake) were excluded from the study.
Measures
We obtained sociodemographic data (i.e., age, gender, ethnicity, level of education, marital status) and clinical information (NYHA class, EF, etiology of HF, current medications, comorbidities) through self-report questionnaires and medical chart abstraction. We measured body weight and height to calculate body mass index (BMI: weight in kilograms divided by height in square meters). For weight, we used a calibrated professional scale to take two consecutive readings, recorded each reading to the nearest 0.1 kg, and calculated the mean of the two. For height, we used a stadiometer graduated in centimeters to take two consecutive measurements recorded to the nearest 0.1 cm and calculated the mean.
Depressive Symptoms
We assessed depressive symptoms using the Patient Health Questionnaire (PHQ)-9, which is the depression module of the three-page PHQ. The PHQ-9 is a self-report questionnaire that was developed according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth edition (DSM-IV) system. It consists of 9 items describing depressive symptoms that participants rate on a Likert-type scale from 0 (not at all) to 3 (nearly every day). The overall score on the PHQ-9 ranges from 0 to 27. Scores of 0–4 indicate no symptoms, 5–9 mild symptoms, 10–14 moderate symptoms, 15–19 moderately severe symptoms, and 20–27 severe symptoms. The PHQ-9 is quick to complete and easy to administer even to individuals with lower education levels. The PHQ-9 is a valid and reliable screening tool (Kroenke, Spitzer, & Williams, 2001; Martin, Reif, Psych, & Braehler, 2006; Phelan et al., 2010) and has been used previously in HF patients (Holzapfel et al., 2008; Muller-Tasch et al., 2007). We categorized patients with PHQ-9 scores ≥ 5 as having depressive symptoms and compared them with those who did not have these symptoms (PHQ-9 scores ≤ 4).
Inflammatory Markers
We measured IL-6, TNFR2, and CRP as markers of inflammation in the present study. Inflammation is a complex response to internal or external stressors and can occur in many disorders such as infection, injury, neoplasm, and disease states. IL-6 and TNF-α are pro-inflammatory lymphokines that are produced by T cells, macrophages, and natural killer cells. These cytokines play a role in the immune response by stimulating immune cells to exert their effects locally or remotely. TNF-α exerts its effects through binding to its receptors TNFR1 and TNFR2, which, when cleaved from the cell membrane into circulation, can be detected in a soluble form (sTNFR1 and sTNFR2; Pasic et al., 2003). Because the levels of these receptors have less variability over time and can be measured more reliably in plasma than TNF-α, we examined sTNFR2 as a marker of TNF-α activity in the present study (Dibbs, Thornby, White, & Mann, 1999; Ferrari et al., 1995). CRP is an acute-phase protein that is primarily produced by the liver in response to inflammation and to IL-6 (Whooley et al., 2007).
To measure these inflammatory markers, we collected blood samples using a vacutainer tube containing ethylenediaminetetraacetic acid (EDTA) after participants had rested in a sitting position for at least 15 min. Plasma was obtained following centrifugation at 1,500 × g, aliquoted, and stored at −80 °C until we were ready for analysis. We measured plasma levels of IL-6 and sTNFR2 using commercially available enzyme-linked immunosorbent assay (ELISA) kits, according to the manufacturer’s protocols (Quantikine HS IL-6, Quantikine sTNFR2, R & D Systems, Minneapolis, MN). The high-sensitivity IL-6 assay was performed on undiluted plasma with a lower detection limit of 0.2 pg/ml. For sTNFR2, assays were performed on plasma diluted 1:30. Taking the dilution into account, the lower detection limit for the assay was 234 pg/ml. Plasma CRP levels were determined using a high-sensitivity ELISA (hsCRP, ALPCO Diagnostics, Salem, NH), which examines steady-state levels of CRP in the low reference range. Plasma samples were assayed for CRP as described by Aziz, Fahey, Detels, and Butch (2003), utilizing a 1:200 dilution, which yields a lower detection limit of 0.2 mg/L. We performed all inflammatory-marker assays in duplicate and report the mean value of the two measurements as the final result.
Statistical Analysis
Data were analyzed using Statistical Package for the Social Sciences (SPSS) for Windows (version 19.0; SPSS, Inc., Chicago, IL). Descriptive statistics (mean, standard deviation, and median, proportion) were used to report the characteristics of the study sample and analyze mean depression scores. Student’s t-test and χ2 tests were used to compare baseline characteristics between the groups for continuous and categorical variables, respectively. Significance was accepted as a two-sided p value of .05.
Normality of distribution was satisfied for continuous variables except sTNFR2, IL-6, and CRP; therefore, these variables were log10 transformed, after which distributional assumptions were achieved using the Shapiro-Wilk test.
Pearson correlation coefficients were calculated for analyses of correlations between the inflammatory markers and depressive symptoms and sociodemographic (age, gender,) and clinical characteristics (EF, NYHA class, HF etiology, statin), and BMI. Linear multivariate regression equations were calculated to evaluate the potential impact of depressive symptoms, age, gender, EF, BMI, statin, and HF etiology on inflammation. sTNFR2, IL-6, and CRP were each entered as the dependent variable in one of three analyses with depressive symptoms as the independent variable. Depression scores were used in the correlation and regression analyses to represent depressive symptoms. Crude (unadjusted) regression coefficients were estimated as well as adjusted regression coefficients based on multivariate modeling of multiple factors. Residual analysis was conducted to identify sources of model misspecification, outliers, and possibly influential observations. Sensitivity analysis was performed to discern the impact of influential cases on the results. In predicting the depressive symptoms, step-type regression analysis was used to obtain the optimal model. Power analysis showed that the sample size of this study (N = 55) was sufficient to detect a large effect size of 0.25 at p = .05 at a power level of 95% with seven predictors included in each regression model.
Results
Sample Socioeconomic and Clinical Characteristics
The 55 participants were mostly White (n = 33, 60%), married (n = 37, 68%) men (n = 41, 74.5%), and had a mean age of 71.6 ± 11.3 (range, 43–88) years. The mean EF was 29.87 ± 7.08% with ischemic cardiomyopathy being the most prevalent cause of HF (n = 40, 72.7%). The majority of participants had NYHA Class II (n = 27, 49.1%) and Class III (n = 26, 47.3%) HF.
Of the 22 participants (40%) who met the criteria for experiencing any depressive symptoms (based on PHQ-9 score ≥5), 17 (30.9%) had mild depression, 2 (3.6%) had moderate depression, and 3 (5.5%) had moderately severe depression. Only two participants (3.6%) with depressive symptoms were taking antidepressants and none had PHQ-9 scores indicating severe symptoms of depression. The sociodemographic and clinical characteristics of participants with and without depressive symptoms were comparable (Table 1), although we did observe a trend toward a significant difference in gender, marital status, and education. We present descriptive statistics for the inflammatory markers for participants as a whole in Table 2. We found no differences between the two groups for any of the three inflammatory markers. When we compared variables between genders, we found similar depression scores and levels of inflammatory markers. However, males were significantly more likely to have worse NYHA classification and EF and higher BMI than females (data not shown).
Table 1.
Sociodemographic and Clinical Characteristics of Heart Failure (HF) Patients With and Without Depressive Symptoms.
| Characteristic | All patients (N = 55) | Depressed (n = 22) | Nondepressed (n = 33) | p value |
|---|---|---|---|---|
| Depression score,a mean ± SD | 4.2 ± 3.8 | 7.8 ± 3.5 | 1.8 ± 1.2 | |
| Age, mean ± SD | 71.6 ± 11.3 | 72.3 ± 13.0 | 71.2 ± 10.2 | .73 |
| Sex, % Men | 74.5 | 63.6 | 81.8 | .09 |
| Married,% | 61.8 | 45.5 | 72.7 | .07 |
| Race, % White | 60.0 | 54.5 | 63.6 | .26 |
| Education, % < high school | 45.5 | 54.5 | 39.4 | .07 |
| Medications, % | ||||
| ACE inhibitors | 56.6 | 52.4 | 59.4 | .45 |
| ARB | 28.3 | 33.3 | 25.0 | .83 |
| Beta blockers | 94.3 | 90.5 | 96.9 | .29 |
| Diuretics | 80.0 | 85.7 | 74.2 | .40 |
| Aldactone | 36.5 | 38.1 | 35.5 | .69 |
| Statin | 71.0 | 71.4 | 71.0 | .61 |
| HF etiology, % ischemic | 72.7 | 80.0 | 77.4 | .83 |
| Dyslipidemia, % | 83.0 | 86.4 | 80.6 | .86 |
| Hypertension, % | 72.2 | 68.2 | 75.0 | .47 |
| Diabetes, % | 35.8 | 45.5 | 29.0 | .15 |
| BMI, mean ± SD | 28.0 ± 6.1 | 27.7 ± 5.7 | 28.2 ± 6.4 | .79 |
| NYHA class | 2.5 ±.5 | 2.73 ±.63 | 2.42 ±.50 | .05 |
| EF, mean ± SD | 29.9 ± 7.2 | 30.8 ± 8.3 | 29.2 ± 6.1 | .46 |
Note. ACE inhibitor = angiotensin-converting enzyme; ARB = angiotensin II receptor blockers; BMI = body mass index; EF = left ventricular ejection fraction; NYHA class = New York Heart Association classification of heart failure.
Depression scores are from the Patient Health Questionnaire (PHQ)-9. Scores of 0–4 indicate no symptoms, 5–9 mild symptoms, 10–14 moderate symptoms, 15–19 moderately severe symptoms, and 20–27 severe symptoms.
Table 2.
Levels of Inflammatory Markers in Heart Failure (HF) Patients (N = 55).
| Inflammatory marker | Min–Max | 25th percentile |
50th percentile |
75th percentile |
|---|---|---|---|---|
| sTNFR2 pg/ml | 924–7,329 | 2,002.1 | 2,525.5 | 3,720.2 |
| IL-6 pg/ml | 0.6–20.0 | 1.5 | 2.4 | 4.4 |
| CRP mg/dl | 0.1–25.5 | 0.9 | 1.6 | 4.5 |
Note. CRP = C-reactive protein; IL-6 = interleukin 6; sTNFR2 = soluble tumor necrosis factor receptor 2.
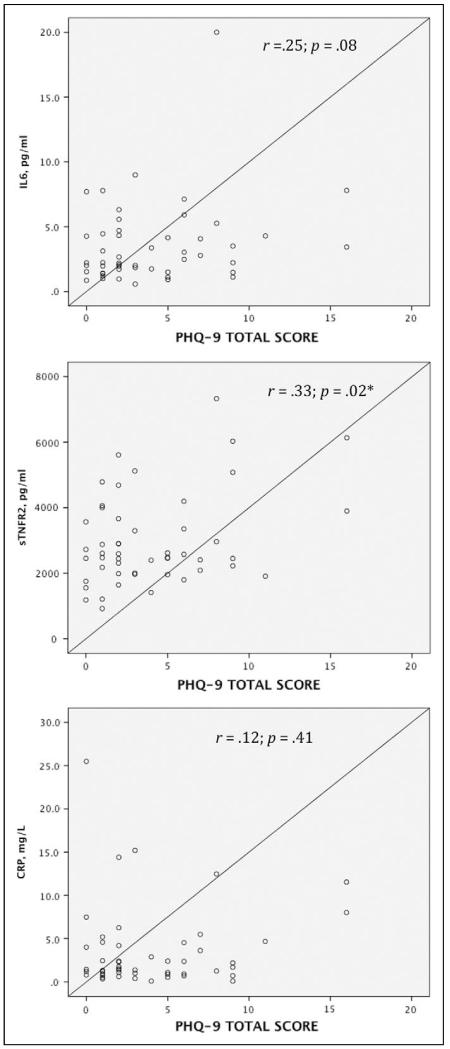
The Pearson correlation coefficient for the three inflammatory markers showed that only the level of sTNFR2 (r = .33; p = .019) was correlated with depressive symptoms. No association was found between levels of CRP and IL-6 and depressive symptoms. Results from these bivariate analyses showed significant correlations between IL-6 and CRP levels (r = .59; p = .00). sTNFR2 level was not correlated with IL-6 (r = .25; p = .07) or CRP (r = .13; p = .36) levels.
Analyses of the three linear regression models including each of the inflammatory markers yielded different results. In the first model, higher depression scores (β = .35; p = .01) along with age (β = .43; p = .004) predicted sTNFR2 levels independent significantly of gender, EF, BMI, HF etiology, and statin use. These two variables explained 38% of the variance in the sTNFR2 levels. When we controlled for the same variables in the second regression model, depressive symptoms (β = .30; p = .04) main and EF (β = .40; p = .01) emerged as the predictors of IL-6 levels and accounted for 22% of the variance in those levels. In the third model, neither depression scores nor the other covariates predicted CRP levels. Scatter plots show that outliers did not greatly influence the regression model (Figure 1).
Figure 1.
Scatter plots for the inflammatory markers interleukin-6 (IL-6), soluble tumor necrosis factor receptor 2 (sTNFR2), and C-reactive protein (CRP) versus total scores on the Patient Health Questionnaire-9 (PHQ-9) measuring depressive symptoms. *Significant relationship at p < 0.05.
Discussion
Our findings indicate that HF patients with depressive symptoms, as assessed by PHQ-9 scores, had significantly higher levels of circulating sTNFR2 and IL-6, but not CRP, compared to their counterparts who did not have these symptoms. This association was independent of age, gender, HF severity, BMI, HF etiology, and statin use. Although we cannot determine directionality, our observations do have clinical implications and supplement existing literature that suggests a key role for inflammation in both depression and the pathophysiology and progression of HF.
The notion that disturbed production of cytokines is an important diagnostic and prognostic marker in HF is well established (Bozkurt et al., 2010). In nondepressed HF patients, increased circulating levels of TNF-α, IL-6, sTNFR1, and sTNFR2 are associated with new HF and can adversely impact myocardial function and sympatheto-neurohormonal activation (Deswal et al., 2001; Nozaki, Yamaguchi, Shirakabe, Nakamura, & Tomoike, 1997; Torre-Amione et al., 1996). sTNF-R1, sTNFR2, and IL-6 levels are also increased in direct proportion to NYHA functional class (Deswal et al., 2001; Ferrari et al., 1995) and independently predict mortality (Deswal et al., 2001; Marcucci et al., 2006). However, compared to TNF-α, sTNFR1and other HF prognosticators, sTNFR2 was a more powerful predictor of patients’ survival (Ferrari et al., 1995). Similarly, when TNF-α, IL-6 and their receptors were entered in a mortality prediction model, sTNFR2 was the only predictor of mortality, along with NYHA class and LVEF (Deswal et al., 2001). These authors concluded that, compared to TNF-α and sTNFR1, sTNFR2 appears to be the best indicator of its ligand activity and the strongest prognosticator of survival. In a more recent study, authors suggested that plasma sTNFR2 may serve as a surrogate marker for increased severity of HF and poor outcomes (Bozkurt et al., 2010).
Investigators have similarly observed increased inflammation in depressed individuals, and the evidence to support these observations in patients with HF continues to evolve. For example, some authors have reported independent relationships between different inflammatory markers such as soluble intercellular adhesion molecule (sICAM; Wirtz et al., 2010), sTNFR1 (Moorman et al., 2007), TNF-α/IL-10 (Parissis et al., 2004), and TNF-α and depression severity (Ferketich et al., 2005) in patients with HF. These and other reports showing the increased risk of morbidity and mortality associated with depression in HF patients have led to the conclusion that depression may enhance the already heightened inflammatory state that characterizes HF and is likely to adversely impact outcomes through these inflammatory processes (Pasic et al., 2003). Two studies have validated this proposition. In these studies, researchers prospectively investigated the prognostic implications of inflammation in a group of HF patients and found that disrupted production of inflammatory proteins was associated with increased risk of higher depression scores (Redwine et al., 2007; Wirtz et al., 2010), rehospitalization, and cardiovascular mortality (Redwine et al., 2007). However, these studies were limited by their small sample sizes and, thus, larger studies are needed to firmly establish these relationships.
In the present study, we also observed a relationship between IL-6, but not CRP, levels and depressive symptoms. The relationships between both of these inflammatory markers and depressive symptoms remain inconclusive in the literature in patients both with and without HF. Among otherwise healthy adult subjects, depression was associated with higher levels of CRP in one study (Ford & Erlinger, 2004). Contrary to these findings, Douglas, Taylor, and O’Malley (2004) found no relationship between CRP and depressive symptoms in subjects with no HF. Similarly, other researchers reported no association between depression and either CRP or IL-6 levels in patients with coronary heart disease (Rumsfeld et al., 2005). A recent meta-analysis confirmed these conflicting findings for IL-6 in clinical (e.g., coronary artery diseases, cancer, and endstage renal disease) and community samples (Howren, Lamkin, & Suls, 2009). In the few studies involving patients with HF, however, results show inconsistent trends in the relationships of CRP and IL-6 levels with depression. The authors of the majority of these studies noted the absence of relationships between depression/depressive symptoms and IL-6 (Ferketich et al., 2005; Parissis et al., 2009; Redwine et al., 2007; Wirtz et al., 2010) and CRP (Redwine et al., 2007; Wirtz et al., 2010). In one recent study, however, researchers found an association between depressive symptoms and levels of IL-6 and CRP (Johansson et al., 2011). As noted, our results concerning CRP and IL-6 and depressive symptoms are supported by some, but not all, previous findings. While this variation may be explained by the small sample sizes and use of different depression assessment tools, the consistency of CRP results among most of these and the present study may imply that this particular inflammatory biomarker might not be the most useful to investigate in HF patients with depression. The variation in the findings regarding IL-6, by contrast, highlights the need for further studies with larger samples and comparable screening approaches. Reiterating a proposition that Redwine et al. (2007) first made, future studies should implement an alternative approach that takes into account the inflammatory system and its regulation as an integrated system rather than investigating inflammatory markers in isolation.
The prominent role that cytokines appear to play in depression has led investigators to examine the effects of antidepressants on the cytokine system (Maes, 2001; Pasic et al., 2003). Clinical studies have shown the benefits of antidepressant therapy on reducing interferon (INF)-γ release and increasing the production of the anti-inflammatory cytokine IL-10 (Kubera et al., 2001, 2004; Maes et al., 1999). Results of in vitro studies supported these findings: exposure of tricyclic antidepressant (TCA)-incubated monocytes to lipopolysaccharides (LPS) resulted in significant reduction in IL-1β, TNF-α, and to a less degree, IL-6. Investigators noted similar observations for IL-2 and INF-γ release from T-lymphocytes (Xia, DePierre, & Nassberger, 1996). In HF, selective serotonin reuptake inhibitors (SSRIs) are believed safer than TCAs, but the underlying mechanisms of these drugs are not fully known. While studies are lacking on the effects of antidepressants in HF patients, one recent study that examined the effect of different antidepressants on inflammation showed for the first time that TCAs and serotonin norepinephrine reuptake inhibitors (SNRI) were associated with lower levels of TNF-α and CRP in these patients compared to SSRIs (Tousoulis et al., 2009). These findings highlight the need for additional research to support recommendations for pharmacologic (and nonpharmacologic) therapies to treat depression in HF.
Limitations
The relatively small sample size of the present study may have diminished the statistical power to detect possible associations between depression and the other inflammatory markers and limited simultaneous adjustment for other confounders. Despite this limitation, however, we were still able to detect a significant relationship between sTNFR2 and depressive symptoms. Our findings are preliminary and need further exploration through larger-scale studies. Our sample consisted primarily of Caucasian men with stable HF resulting from ischemic cardiomyopathy. Moreover, participants were recruited from community outpatient settings serving a limited geographical area in Los Angeles. As a result, our sample can be described as a homogeneous one that may not represent the overall HF population, including those in the inpatient setting. In addition, the cross-sectional design of this study precludes determination of the directionality of the cause–effect relationship between depressive symptoms and inflammation.
Conclusion
Our findings in the present study add to a growing body of evidence supporting the proposition that heightened inflammation, particularly as indicated by increased levels of TNF-α, is common in HF patients with depression and may explain the adverse effects depression has on HF. While existing studies have mainly examined TNF-α and IL-6 in depressed HF patients, prospective and longitudinal larger scale studies are needed to better understand the role that the receptors of these cytokines play in these adverse effects and to explore therapeutics to modulate their effects. Clinically, this study suggests that health care providers and nurses should be more active in screening for and treating depressive symptoms in HF patients, regardless of the severity of these symptoms, to identify those who may be at risk of increased inflammation and poor outcomes.
Acknowledgment
The authors would like to acknowledge Kelli Nicholas and the patients, doctors, and staff of the Cardiovascular Consultants Medical Group; the staff and management at the Northridge Hospital laboratory; and Jaime Moran and Norr Santz at the Inflammatory Biology Core Laboratory of the UCLA Claude D. Pepper Older Americans Independence Center for their support in this study.
Funding
This work was funded by the National Institutes of Health, National Institute of Nursing Research (T32 NR007077) and supported in part by the Inflammatory Biology Core Laboratory of the UCLA Claude D. Pepper Older Americans Independence Center funded by the National Institute of Aging (5P30 AG028748).
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Alonso-Martinez JL, Llorente-Diez B, Echegaray-Agara M, Olaz-Preciado F, Urbieta-Echezarreta M, Gonzalez-Arencibia C. C-reactive protein as a predictor of improvement and readmission in heart failure. European Journal of Heart Failure. 2002;4:331–336. doi: 10.1016/s1388-9842(02)00021-1. [DOI] [PubMed] [Google Scholar]
- Aziz N, Fahey JL, Detels R, Butch AW. Analytical performance of a highly sensitive C-reactive protein-based immunoassay and the effects of laboratory variables on levels of protein in blood. Clinical and Diagnostic Laboratory Immunology. 2003;10:652–657. doi: 10.1128/CDLI.10.4.652-657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozkurt B, Mann DL, Deswal A. Biomarkers of inflammation in heart failure. Heart Failure Review. 2010;15:331–341. doi: 10.1007/s10741-009-9140-3. [DOI] [PubMed] [Google Scholar]
- Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and cytokine receptors in advanced heart failure: An analysis of the cytokine database from the Vesnarinone trial (VEST) Circulation. 2001;103:2055–2059. doi: 10.1161/01.cir.103.16.2055. [DOI] [PubMed] [Google Scholar]
- Dibbs Z, Thornby J, White BG, Mann DL. Natural variability of circulating levels of cytokines and cytokine receptors in patients with heart failure: Implications for clinical trials. Journal of the American College of Cardiology. 1999;33:1935–1942. doi: 10.1016/s0735-1097(99)00130-8. [DOI] [PubMed] [Google Scholar]
- Douglas KM, Taylor AJ, O’Malley PG. Relationship between depression and C-reactive protein in a screening population. Psychosomatic Medicine. 2004;66(5):679–683. doi: 10.1097/01.psy.0000138132.66332.85. [DOI] [PubMed] [Google Scholar]
- Ferketich AK, Ferguson JP, Binkley PF. Depressive symptoms and inflammation among heart failure patients. American Heart Journal. 2005;150:132–136. doi: 10.1016/j.ahj.2004.08.029. [DOI] [PubMed] [Google Scholar]
- Ferrari R, Bachetti T, Confortini R, Opasich C, Febo O, Corti A, Visioli O. Tumor necrosis factor soluble receptors in patients with various degrees of congestive heart failure. Circulation. 1995;92:1479–1486. doi: 10.1161/01.cir.92.6.1479. [DOI] [PubMed] [Google Scholar]
- Ford DE, Erlinger TP. Depression and C-reactive protein in US adults: Data from the third national health and nutrition examination survey. Archives of Internal Medicine. 2004;164:1010–1014. doi: 10.1001/archinte.164.9.1010. [DOI] [PubMed] [Google Scholar]
- Holzapfel N, Muller-Tasch T, Wild B, Junger J, Zugck C, Remppis, Lowe B. Depression profile in patients with and without chronic heart failure. Journal of Affective Disorders. 2008;105:53–62. doi: 10.1016/j.jad.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosomatic Medicine. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Jiang W, Alexander J, Christopher E, Kuchibhatla M, Gaulden LH, Cuffe MS, O’Connor CM. Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Archives of Internal Medicine. 2001;161:1849–1856. doi: 10.1001/archinte.161.15.1849. [DOI] [PubMed] [Google Scholar]
- Johansson P, Lesman-Leegte I, Svensson E, Voors A, van Veldhuisen DJ, Jaarsma T. Depressive symptoms and inflammation in patients hospitalised for heart failure. American Heart Journal. 2011;161:1053–1059. doi: 10.1016/j.ahj.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. Journal of General Internal Medicine. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubera M, Kenis G, Bosmans E, Kajta M, Basta-Kaim A, Scharpe S, Maes M. Stimulatory effect of antidepressants on the production of IL-6. International Immunopharmacology. 2004;4:185–192. doi: 10.1016/j.intimp.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Kubera M, Kenis G, Budziszewska B, Bosmans E, Scharpe S, Basta-Kaim A, Maes M. Lack of modulatory effect of imipramins on glugocorticoid-induced suppression of interferon-gamma and interleukin-10 production in vitro. Polish Journal of Pharmacology. 2001;53:289–294. [PubMed] [Google Scholar]
- Maes M. The immunoregulatory effects of antidepressants. Human Psychopharmacology. 2001;16:95–103. doi: 10.1002/hup.191. [DOI] [PubMed] [Google Scholar]
- Maes M, Song C, Lin AH, Bonaccorso S, Kenis G, De Johng R, Scharpe S. Negative immunoregulatory effects of antidepressants: Inhibition of interferon-gamma and stimulation of interleukin-10 secretion. Neuropsychopharmacology. 1999;20:370–379. doi: 10.1016/S0893-133X(98)00088-8. [DOI] [PubMed] [Google Scholar]
- Marcucci R, Gori AM, Giannotti F, Baldi M, Verdiani V, Del Pace S, Abbate R. Markers of hypercoagulability and inflammation predict mortality in patients with heart failure. Journal of Thrombosis and Haemostasis. 2006;4:1017–1022. doi: 10.1111/j.1538-7836.2006.01916.x. [DOI] [PubMed] [Google Scholar]
- Martin A, Rief W, Klaiberg A, Braehler E. Validity of the brief patient health questionnaire mood scale (PHQ-9) in the general population. General Hospital Psychiatry. 2006;28:71–77. doi: 10.1016/j.genhosppsych.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Moorman AJ, Mozaffarian D, Wilkinson CW, Lawler RL, McDonald GB, Crane BA, Levy WC. In patients with heart failure elevated soluble TNF-receptor 1 is associated with higher risk of depression. Journal of Cardiac Failure. 2007;13:738–743. doi: 10.1016/j.cardfail.2007.06.301. [DOI] [PubMed] [Google Scholar]
- Muller-Tasch T, Peters-Klimm F, Schellberg D, Holzapfel N, Barth A, Junger J, Herzog W. Depression is a major determinant of quality of life in patients with chronic systolic heart failure in general practice. Journal of Cardiac Failure. 2007;13:818–824. doi: 10.1016/j.cardfail.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Nozaki N, Yamaguchi S, Shirakabe M, Nakamura H, Tomoike H. Soluble tumor necrosis factor receptors are elevated in relation to severity of congestive heart failure. Japanese Circulation Journal. 1997;61:657–664. doi: 10.1253/jcj.61.657. [DOI] [PubMed] [Google Scholar]
- Parissis JT, Adamopoulos S, Rigas A, Kostakis G, Karatzas D, Venetsanou K, Kremastinos DT. Comparison of circulating proinflammatory cytokines and soluble apoptosis mediators in patients with chronic heart failure with versus without symptoms of depression. American Journal of Cardiology. 2004;94:1326–1328. doi: 10.1016/j.amjcard.2004.07.127. [DOI] [PubMed] [Google Scholar]
- Parissis JT, Farmakis D, Nikolaou M, Birmpa D, Bistola V, Paraskevaidis I, Kremastinos DT. Plasma B-type natriuretic peptide and anti-inflammatory cytokine interleukin-10 levels predict adverse clinical outcome in chronic heart failure patients with depressive symptoms: A 1-year follow-up study. European Journal of Heart Failure. 2009;11:967–972. doi: 10.1093/eurjhf/hfp125. [DOI] [PubMed] [Google Scholar]
- Pasic J, Levy WC, Sullivan MD. Cytokines in depression and heart failure. Psychosomatic Medicine. 2003;65:181–193. doi: 10.1097/01.psy.0000058372.50240.38. [DOI] [PubMed] [Google Scholar]
- Phelan E, Williams B, Meeker K, Bonn K, Frederick J, Logerfo J, Snowden M. A study of the diagnostic accuracy of the PHQ-9 in primary care. BMC Family Practice. 2010;11:63–71. doi: 10.1186/1471-2296-11-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redwine LS, Mills PJ, Hong S, Rutledge T, Reis V, Maisel A, Irwin MR. Cardiac-related hospitalization and/or death associated with immune dysregulation and symptoms of depression in heart failure patients. Psychosomatic Medicine. 2007;69:23–29. doi: 10.1097/PSY.0b013e31802e2f35. [DOI] [PubMed] [Google Scholar]
- Roger VL, Go A,S, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Wylie-Rosett J, on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2011 update: A report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumsfeld JS, Jones PG, Whooley MA, Sullivan MD, Pitt B, Weintraub WS, Spertus JA. Depression predicts mortality and hospitalization in patients with myocardial infarction complicated by heart failure. American Heart Journal. 2005;150:961–967. doi: 10.1016/j.ahj.2005.02.036. [DOI] [PubMed] [Google Scholar]
- Sato Y, Takatsu Y, Kataoka K, Yamada T, Taniguchi R, Sasayama S, Matsumori A. Serial circulating concentrations of C-reactive protein, interleukin (IL)-4, and IL-6 in patients with acute left heart decompensation. Clinical Cardiology. 1999;22:811–813. doi: 10.1002/clc.4960221211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood A, Blumenthal JA, Trivedi R, Johnson KS, O’Connor CM, Adams KF, Jr., Hinderliter AL. Relationship of depression to death or hospitalization in patients with heart failure. Archives of Internal Medicine. 2007;167:367–373. doi: 10.1001/archinte.167.4.367. [DOI] [PubMed] [Google Scholar]
- Sullivan M, Simon G, Spertus J, Russo J. Depression-related costs in heart failure care. Archives of Internal Medicine. 2002;162:1860–1866. doi: 10.1001/archinte.162.16.1860. [DOI] [PubMed] [Google Scholar]
- Torre-Amione G, Kapadia S, Benedict C, Oral H, Young JB, Mann DL. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: A report from the studies of left ventricular dysfunction (SOLVD) Journal of the American College of Cardiology. 1996;27:1201–1206. doi: 10.1016/0735-1097(95)00589-7. [DOI] [PubMed] [Google Scholar]
- Tousoulis D, Droias A, Antoniades C, Vasiliadou C, Marinou K, Latsios G, Stefanadis C. Antidepressive treatment as a modulator of inflammatory process in patients with heart failure: Effects on proinflammatory cytokines and acute phase protein levels. International Journal of Cardiology. 2009;134:238–243. doi: 10.1016/j.ijcard.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Vaccarino V, Kasl SV, Abramson J, Krumholz HM. Depressive symptoms and risk of functional decline and death in patients with heart failure. Journal of the American College of Cardiology. 2001;38:199–205. doi: 10.1016/s0735-1097(01)01334-1. [DOI] [PubMed] [Google Scholar]
- Vasan RS, Sullivan LM, Roubenoff R, Dinarello CA, Harris T, Benjamin EJ, Wilson PW. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: The Framingham Heart Study. Circulation. 2003;107:1486–1491. doi: 10.1161/01.cir.0000057810.48709.f6. [DOI] [PubMed] [Google Scholar]
- Villacorta H, Masetto AC, Mesquita ET. C-reactive protein: An inflammatory marker with prognostic value in patients with decompensated heart failure. Arquivos Brasilerios de Cardiology. 2007;88:585–589. doi: 10.1590/s0066-782x2007000500014. [DOI] [PubMed] [Google Scholar]
- Whooley MA, Caska CM, Hendrickson BE, Rourke MA, Ho J, Ali S. Depression and inflammation in patients with coronary heart disease: Findings from the heart and soul study. Biological Psychiatry. 2007;62:314–320. doi: 10.1016/j.biopsych.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz PH, Redwine LS, Linke S, Hong S, Rutledge T, Greenberg BH, Mills PJ. Circulating levels of soluble intercellular adhesion molecule-1 (sICAM-1) independently predict depressive symptom severity after 12 months in heart failure patients. Brain Behavior and Immunity. 2010;24:366–369. doi: 10.1016/j.bbi.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Xia Z, DePierre JW, Nassberger L. Tricyclic antide-pressants inhibit IL-6, IL-1 beta and TNF-alpha release in human blood monocytes and IL-2 and interferon-gamma in T cells. Immunopharmacology. 1996;34:27–37. doi: 10.1016/0162-3109(96)00111-7. [DOI] [PubMed] [Google Scholar]