SUMMARY
Purpose
To report the mature data of a prospective Phase II trial designed to evaluate the efficacy of an epidermal growth factor receptor inhibitor cetuximab (CTX) added to the concurrent therapy of weekly paclitaxel/carboplatin (PC) and daily radiation therapy (RT).
Methods and Materials
From 2005 to 2009, a total of 43 patients were enrolled in the study. The median follow-up was 31 months (range, 9–59 months). All patients had Stage III/IV disease at presentation, and 67% had oropharyngeal primaries. The weekly IV dose schedules were CTX 250 mg/m2 (400 mg/m2 IV loading dose 1 week before RT), paclitaxel 40 mg/m2, and carboplatin AUC 2. RT was given at 1.8 Gy per day to 70.2 Gy. Intensity-modulated RT was used in 70% of cases.
Results
All patients completed the planned RT dose, 74% without any treatment breaks. The planned CTX and PC cycles were completed in 70% (91% with at least seven of planned nine cycles) and 56% (93% with at least seven of planned eight cycles) of patients, respectively. Toxicity included Grade 3 mucositis (79%), rash (9%), leucopenia (19%), neutropenia (19%), and RT dermatitis (16%). The complete response (CR) rate at the completion of therapy was 84%. The estimated 3-year local regional control rate was 72%. Six patients with an initial CR subsequently experienced a local recurrence, 10 patients experienced distant progression. The median overall survival and disease-free survivals have not been reached. The 3-year actuarial overall survival and disease-free survival were 59% and 58%, respectively.
Conclusions
The addition of CTX to weekly PC and daily RT was well tolerated and resulted in encouraging local control and survival rates.
INTRODUCTION
The management of patients presenting with locally advanced squamous cell carcinomas of the head and neck (SCCHN) has evolved significantly over the past two decades. Organ preservation trials have documented the efficacy of chemotherapy and radiation therapy (RT) instead of primary surgery in resectable disease. The concurrent application of chemotherapy and RT is aimed at improving local regional control in an effort to positively affect long-term survival. Meta-analyses of multiple of Phase III randomized trials have documented a 4% to 5 % absolute survival advantage associated with the use of chemotherapy in addition to locoregional RT (1, 2).
A majority of these trials have used platinum-based regimens (3). Although cisplatin given every 3 weeks during RT has been used in most trials, the advantages seen with this agent have come at a cost of increased toxicity (4). Given the radiation sensitizing properties, favorable toxicity profile and activity in SCCHN, paclitaxel and carboplatin (PC) have formed the backbone of combination regimens designed to decrease toxicity while still maintaining survival advantages. Our institution has previously reported the results of a Phase II trial that documented the efficacy of weekly PC delivered concurrently with daily RT for patients diagnosed with locally advanced SCCHN. This regimen achieved a 3-year locoregional control and overall survival (OS) rates of 63% and 48%, respectively, and 94% of patients completed prescribed therapy (5).
Although concurrent chemoradiation regimens have improved outcomes, locoregional control remains the dominant pattern of disease progression. It is well understood that 90% of SCCHN cell lines express high levels of the epidermal growth factor receptor (EGFR), and that the inhibition of this receptor is associated with radiosensitization (6, 7). Cetuximab (CTX) is an IgG1 monoclonal antibody that exclusively targets EGFR and inhibits tumor cell proliferation. The addition of this agent to RT has been shown in a Phase III trial to significantly improve the local control and OS for SCCHN patients when compared to RT alone (8).
Here we report the mature results of a prospective, Phase II study evaluating the efficacy and toxicity of the addition of CTX to concurrent weekly PC and daily RT in patients with locally advanced SCCHN.
METHODS AND MATERIALS
Eligibility criteria and pretreatment staging
The study and consent were approved by the Institutional Review Board of the University of Maryland School of Medicine as Greenebaum Cancer Center Protocol 0442. From July 2005 to March 2008, a total of 43 patients with previously untreated, locally advanced SCCHN (Stage III–IV, M0; American Joint Committee on Cancer [AJCC] 2002) were enrolled into the study. Each patient was evaluated by a multidisciplinary physician team including a surgeon, medical oncologist, and radiation oncologist before providing signed study consent. Patients were deemed eligible if they presented with unresectable disease or if planned surgery would have a significant adverse impact on long-term speech and/or swallowing function. All patients had primary tumors involving the oropharynx, larynx, hypopharynx, or nasopharynx.
Eligibility criteria included age >18 years, no prior chemotherapy or head-and-neck RT, Karnofsky Performance Status ≥70, and normal hematopoietic, hepatic, and renal functions. All patients were required to undergo a physical examination, panedoscopy, and radiographic studies that included computed tomography (CT) scans. In addition, a majority of patients underwent positron emission tomography (PET)/CT for staging and 3 months after therapy.
Systemic therapy
One week before concurrent chemoradiation, CTX loading dose of 400 mg/m2 IV was infused over 2 h. This was followed by weekly infusions at 250 mg/m2. Premedication consisted of diphenhydramine 50 mg IV. CTX was discontinued if any Grade 3 or 4 hypersensitivity reaction was observed, and it was also held if protocol-specified toxicity occurred that was attributable to PC chemotherapy.
The concurrent chemotherapy regimen consisted of paclitaxel 40 mg/m2 and carboplatin AUC 2 delivered with the weekly CTX. These therapies were administered before RT on either a Monday or a Tuesday during the treatment schedule. Premedication consisted of dexamethasone (20 mg PO 12 and 6 h before therapy), diphenhydramine (50 mg IV), ranitidine (50 mg IV), granisetron (1 mg IV), and appropriate hydration. During the course of therapy, full-dose weekly chemotherapy was administered respecting hematologic parameters (WBC > 3,500/ml and platelet count >-100,000/ml). A 50% dose reduction was required for both agents if the WBC before therapy was between 2,500/ml and 3,499/ml or the platelet count was 75,000 to 99,000/ml. Chemotherapy was held if either the WBC was less than 2,500/ml or the platelet count was less than 75,000/ml.
Radiation therapy
RT was delivered at 1.8 Gy per day, 5 days per week, to a total dose of 70.2 Gy to all gross disease. Uninvolved nodal chains received 50 Gy, whereas chains harboring grossly involved nodes received 60 Gy. RT treatment plan generation used either three-dimensional conformal RT (3D-CRT) or intensity-modulated radiation therapy (IMRT) planning techniques. IMRT was delivered via a sequential cone-down technique, and no dose painting was allowed. A 1-cm safety margin was used around the primary tumor and enlarged lymph nodes, and a further 0.3- to 0.5-cm margin was used for uncertainties associated with organ motion and patient setup error.
Toxicity assessment
Adverse events were coded according to the Common Terminology Criteria of Adverse Events version 3. Infusion reactions were scored according to the allergic reaction/hypersensitivity grading scale.
Response assessment
Weekly treatment evaluations included history and physical examinations, documentation of performance status, CBC, and toxicity scoring. After the completion of therapy, all patients were routinely evaluated by all 3 specialists. All patients underwent PET/CT and physical examination, and a repeat endoscopy with mandatory biopsy of the primary site if any suspicious lesions were noted. Any persistent nodes documented on either radiographic or physical examination at the 3-month time point necessitated a subsequent neck dissection.
A complete response (CR) was defined a complete disappearance of all disease 3 months after completion of therapy. A partial response required a greater than 50% reduction in all tumor masses, whereas the appearance of new tumor growth or a 25% increase in known disease was defined as progressive disease.
Statistical analysis
The primary endpoint was locoregional control rate assessed 3 months after completion of protocol therapy. Locoregional control was defined as patients having achieved a complete response of their primary tumor and regional lymph nodes and subsequent control. Secondary endpoints included disease-free survival (DFS) and OS. DFS was defined from the time of achieving locoregional control (3 months after therapy) until either the first sign of documented disease recurrence or death from any cause. The endpoints of actuarial locoregional control, DFS, and OS were estimated using the Kaplan–Meier statistical method. Locoregional control and OS were calculated from diagnosis.
Because our previous experience achieved a locoregional control of 63%, it was assumed that an 80% locoregional control rate with the protocol regimen would be a meaningful improvement. To determine whether this increase would be of statistical significance, and assuming a dropout rate of 10%, it was determined that a patient population of approximately 60 patients was required. Simon’s two-stage optimal design was used for patient accrual. At least 13 of the first 19 qualified patients had to have achieved local control before more were enrolled. However, a total of 43 patients were enrolled before the protocol accrual was stopped. An interim analysis using the sequential conditional probability ratio test (power, 0.90; level of significance, 0.10; probability of discordance, 0.10) determined that there was enough evidence of a locoregional control rate difference before the full accrual of 60 patients.
RESULTS
Study population
The median follow-up and age were 31 months (range, 6–59 months) and 58 years (range, 42–75 years). Of the patients, 86% were male and 77% were Caucasian/white (Table 1). A majority of patients had primary tumors located in the oropharynx (67%). All patients enrolled had Stage III/IV, M0 disease (AJCC 2002). Two-thirds of patients presented with T3 or T4 tumors (44%/23%), and 92% had N2/N3 nodal involvement. Patients presenting with technically resectable disease were offered this therapy in an attempt to preserve speech and/or swallowing function.
Table 1.
Patient characteristics
| n | % | |
|---|---|---|
| Median age, y (range) | 58 (42–75) | |
| Sex | ||
| Male | 37 | 86 |
| Female | 6 | 14 |
| Race | ||
| African American | 10 | 23 |
| Caucasian/white | 33 | 77 |
| T stage | ||
| T1 | 8 | 19 |
| T2 | 6 | 14 |
| T3 | 19 | 44 |
| T4 | 10 | 23 |
| N stage | ||
| N1 | 4 | 9 |
| N2 | 31 | 72 |
| N3 | 8 | 19 |
| KPS | ||
| 100 | 24 | 56 |
| 90 | 9 | 21 |
| 80 | 9 | 21 |
| 70 | 1 | 2 |
| Primary site | ||
| Oropharynx | 29 | 67 |
| Larynx | 6 | 14 |
| Hypopharynx | 4 | 9 |
| Nasopharynx | 4 | 9 |
| PEG tube | ||
| Yes | 37 | |
| No | 6 | |
Abbreviations: KPS = Karnofsky performance status
PEG = per-cutaneous endoscopic gastrostomy
Protocol compliance and toxicity
All patients received the planned RT dose. Thirty patients received IMRT, whereas 13 received RT based on 3D-CRT techniques. A total of 32 patients (74%) completed therapy without any unscheduled treatment breaks. Among the 11 patients who had an unscheduled treatment delay, the average length of treatment prolongation was 4 days.
A total of 24 patients (56%) received all planned weekly doses of carboplatin and paclitaxel. An additional 16 patients (37%) received seven of eight weekly doses, whereas 2 patients had two doses and 1 patient had three doses held because of hematologic toxicities. One patient experienced a Grade 4 infusion reaction to the initial dose of paclitaxel, and therefore this agent was discontinued for the remainder of his treatment. A total of 31 patients (72%) received all weekly infusions delivered at full dose, whereas 10 (23%) had one infusion at reduced doses as prescribed by protocol because of hematologic toxicities.
Of the patients enrolled in the study, 70% received all planned doses of CTX. Six patients (14%) received eight cycles, and an additional 3 patients received seven cycles.
Three patients (7%) experienced an anaphylactic reaction to the loading dose of CTX (2 patients Grade 4, 1 patient Grade 3), and this agent was subsequently withheld for the entire course of therapy. One patient experienced a Grade 3 rash that necessitated withholding multiple weekly doses.
All 43 patients enrolled in the study were assessable for toxicity (Table 2). The most common Grade 2 or 3 non-hematologic toxicities encountered at least once during the course of therapy included mucositis, dysphagia, RT dermatitis, cetuximab-associated rash, and hypomagnesemia. A percutaneous endoscopic gastrostomy (PEG) tube was placed in 37 patients (86%) before the initiation or during therapy.
Table 2.
Acute toxicities
| Toxicity | Grade 2 (%) | Grade 3 (%) | Grade 4 (%) |
|---|---|---|---|
| Mucositis | 21 | 79 | |
| Dysphagia | 58 | 21 | |
| Fever | 12 | 5 | |
| Leukopenia | 37 | 19 | 2 |
| Cetuximab rash | 74 | 9 | |
| Radiation dermititis | 58 | 16 | |
| Cetuximab hypersensitivity | 4 | ||
| Dehydration | 65 | 5 | |
| Neutropenia | 12 | 19 | 2 |
| Hypomagnesemia | 16 | 7 | |
| Anemia | 12 | 2 | |
| Thrombocytopenia | 2 | ||
| Xerostomia | 77 | 2 |
There was no correlation between the severity of the CTX rash (p = 0.24) or RT dermatitis (p = 0.12) and the type of RT planning technique used.
Response and survival
A total of 36 patients (84%) achieved a complete response after all therapy. Sixteen patients who achieved a CR at their primary site underwent neck dissections for potential residual disease found on either clinical examination and/or at 3-month post-therapy imaging. Five of these patients had residual disease found in their neck dissection specimens. Six patients with initial CR subsequently experienced a local recurrence and 10 patients systemic metastasis without local failure.
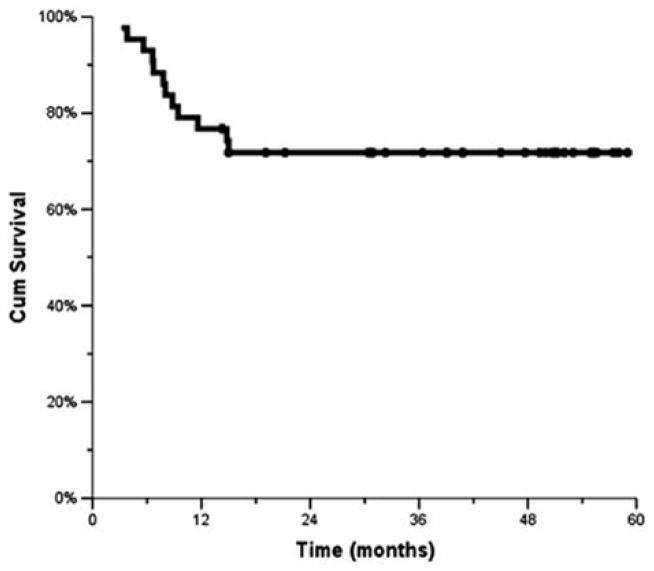
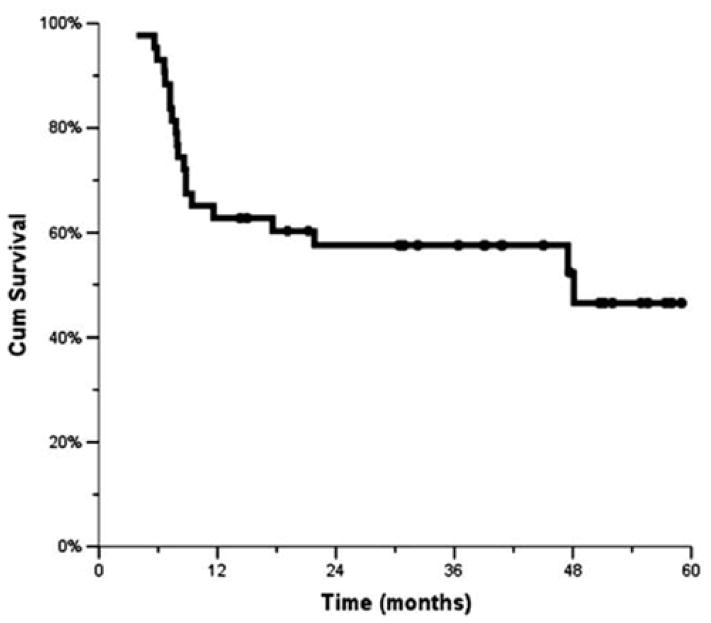
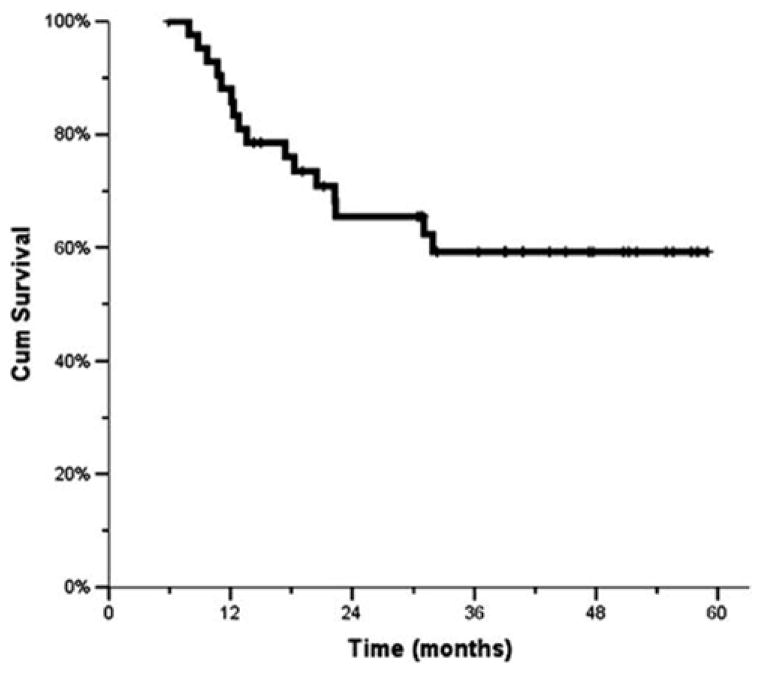
The median time to locoregional failure has not been reached. The 1-, 2-, and 3-year locoregional control rates were 77%, 72%, and 72%, respectively (Fig. 1). The median DFS for the study population was 48 months, with the 1-, 2-, and 3-year rates being 63%, 58%, and 58%, respectively (Fig. 2). The median OS has not been reached. The 1-, 2-, and 3-year OSs were 88%, 65% and 59%, respectively (Fig. 3).
Fig. 1.
Local control (Kaplan–Meier)
Fig. 2.
Disease-free survival (Kaplan–Meier).
Fig. 3.
Overall survival (Kaplan–Meier)
Human papilloma virus–associated tumors
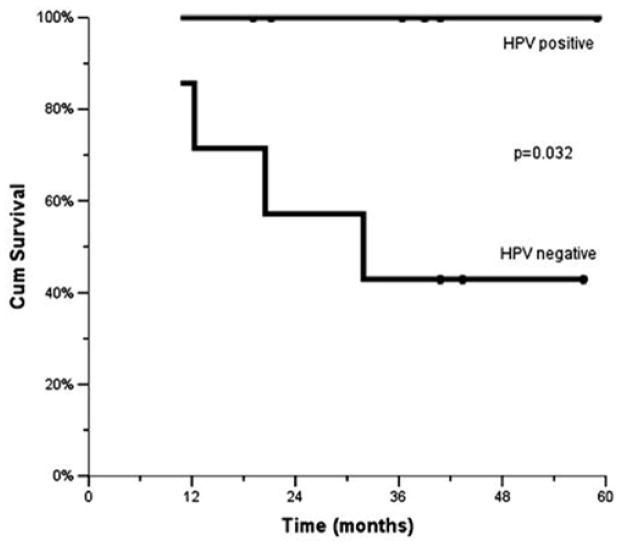
Routine human papilloma virus (HPV) testing was not performed, because the prognostic implications associated with HPV related oropharyngeal tumors were not elucidated at the time of study design. We retrospectively evaluated the HPV status of oropharyngeal tumor samples. Of the 14 oropharyngeal patients with available tumor specimens, 7 patients had HPV-positive tumors determined by immuno-histochemical staining. The 3-year OS of HPV-positive vs. HPV-negative patients were 100% and 43% (p = 0.032), respectively (Fig. 4).
Fig. 4.
Overall survival based on humanpapilloma virus (HPV)
DISCUSSION
Locally advanced SCCHN have historically presented physicians with a significant therapeutic challenge. Although many of these trials have documented advantages in terms of organ preservation, local control, and survival compared with results achieved with RT alone, there remains significant room for improvement, as locoregional failure continues to be a dominant pattern of relapse (9). This reality has led investigators to consider alternative treatment strategies in an attempt to improve upon the results obtained with standard concurrent chemotherapy and RT schemes.
The Radiation Therapy Oncology Group (RTOG) 9003 trial demonstrated the local control advantage associated with hyperfractionation and concomitant boost schedules (i.e., altered fractionation) compared with other RT alone schedules (10). RTOG 0129 randomized patients receiving concurrent cisplatin chemotherapy to either once a day or concomitant boost RT schedules. The results of this Phase III trial failed to document any survival advantage associated with altered fractionation when delivered in conjunction with concurrent chemotherapy (11).
Investigators have identified the epidermal growth factor receptor (EGFR) to be abnormally activated in many epithelial malignancies including head and neck cancers. Ang et al. studied a cohort of tissue samples from patients treated with RT alone and discovered that EGFR overexpression was a strong, independent predictor of tumor control (12). Laboratory investigations have also elucidated the cytotoxic effects of EGFR blockade. CTX (IgG1 monoclonal antibody against the ligand binding domain of EGFR) has been shown to effectively inhibit downstream phosphorylation and activation of receptor-associated kinases, which results in inhibition of cell growth, induction of apoptosis, and decreases in matrix metalloproteinases and vascular endothelial growth factor production (6, 7).
Based these findings, Bonner et al. performed a Phase III trial that tested the efficacy of the combination of CTX and RT vs. RT alone in patients with locally advanced SCCHN. This landmark study provided Level 1 evidence of the benefit associated with combining EGFR inhibition and RT as it documented a significant survival (3-year OS 57% vs. 45%) and local control advantage (56% vs. 48%) (13). Of particular note was the fact that these advances came without a significant increase either acute hematologic or mucosal toxicities.
The encouraging results from this study have provided a sound rationale for incorporating targeted therapies into the established concurrent chemoradiation regimens. The initial attempts of this approach highlighted the need to proceed cautiously. Pfister et al. published an institutional Phase II trial of concurrent CTX, cisplatin, and concomitant boost RT (14). This study was closed early because of an unacceptably high rate of adverse events, with 4 patients experiencing Grade 4 cardiac toxicities. It is worth noting that, despite these toxicities, the 3-year OS of 76% and 3-year locoregional control rate of 71% were viewed as encouraging, given the fact that 86% of these patients presented with Stage IV disease. Ultimately, the authors concluded that these results supported the continued investigation of strategies designed to incorporate CTX into the combined-modality paradigm.
More recently, investigators from the Brown University reported the preliminary toxicity results from a Phase II trial that evaluated the addition of CTX to a weekly carboplatin, paclitaxel, and daily RT (15). This trial included a 1-month induction phase that consisted of CTX alone. Three patients experienced Grade 3 or 4 toxicities (2 had Grade 4 hypersensitivity reactions and 1 patient developed a Grade 3 rash), and all 3 patients withdrew from therapy before initiating definitive chemoradiation. Similar to our study, IMRT was allowed but not mandated in this study. The investigators concluded that the rates of acute toxicity were similar to their previous treatment regimen with the exception of an increase in dermatologic toxicities and hypersensitivity reactions. They were unable to determine whether the increase in skin toxicity was a result of IMRT use or CTX. The results of our study suggest that CTX was not associated with an increase in skin toxicity when compared with the results of our previously published experience with PC and RT, which found a 30% incidence of Grade 3 skin toxicity (5). The addition of CTX on the current trial was associated with a 16% Grade 3 skin toxicity, and there was no difference seen between patients treated with three-dimensional conformal RT and IMRT.
Over the past decade it has become increasingly apparent that the HPV plays a crucial role in the pathogenesis of a portion of SCCHN. Some reports have identified that up to 50% of newly diagnosed tumors of the oropharynx are HPV related (16). Multiple retrospective series have now documented improved OS for HPV-related SCCHN. The Eastern Cooperative Oncology Group reported the results of a Phase II clinical trial (E2339) that used induction chemotherapy (cis-platin and caclitaxel) followed by definitive chemoradiation for patients with resectable oropharyngeal cancer. The response rates to both the induction chemotherapy and concurrent chemoradiation were higher in HPV+ tumors (17). The multivariate analysis revealed that HPV+ patients had a significantly lower risk of progression and death.
More recently, Ang et al. have reported that HPV status can also predict for long-term survival in patients undergoing definitive chemoradiation in a retrospective analysis of the Phase III RTOG 0129 trial. Investigators examined the HPV status of 317 patients (73%) and found that 60% of the patients with oropharyngeal primary tumors were HPV+. There were dramatic 2-year progression-free survival (72% vs. 51%, p < 0.0001) and OS (88% vs. 67%, p < 0.0001) differences in favor of HPV+ patients (11). Although our data are limited by the number of tumor specimens that were available for analysis, the findings were consistent with these previously reported series. Taken together, these data suggest that the improved survival associated with these HPV-related malignancies is a direct reflection of their increased responsiveness to chemotherapy and RT.
Our study represents one of the first published sets of mature results of a prospective trial designed to investigate the addition of CTX to a weekly concurrent chemotherapy and RT treatment scheme. The 3-year survival and locoregional control results achieved in this study do suggest an improvement when compared with the results achieved in our previous institutional trials that used weekly PC and once-daily RT (5). We previously reported a 3-year local control rate of 63% and 3-year OS of 48% for patients treated with this regimen. By comparison, the current protocol demonstrates a CR rate of 85%, a 3-year local control of 72%, and a 3-year OS of 59%. It is important to note that this regimen was not associated with any unexpected cardiovascular events. Undoubtedly this may reflect the benefit of appropriate patient selection based on previous published experience from Bonner and Pfister (8, 13, 14).
CONCLUSION
In conclusion, the addition of CTX to concurrent chemo-radiation provides encouraging results and acceptable toxicity profile. However, this strategy must be validated in a prospective Phase III trial.
Footnotes
Conflict of interest: none.
References
- 1.Pignon JP, Bourhis J, Domenge C, et al. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: Three meta-analyses of updated individual data. MACH-NC collaborative group. meta-analysis of chemotherapy on head and neck cancer. Lancet. 2000;355:949–955. [PubMed] [Google Scholar]
- 2.Pignon JP, le Maitre A, Maillard E, et al. Meta-Analysis of Chemotherapy in Head and Neck Cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 3.Calais G, Alfonsi M, Bardet E, et al. Randomized trial of radiation therapy vs. concomitant chemotherapy and radiation therapy for advanced-stage oropharynx carcinoma. J Natl Cancer Inst. 1999;91:2081–2086. doi: 10.1093/jnci/91.24.2081. [DOI] [PubMed] [Google Scholar]
- 4.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 5.Suntharalingam M, Haas ML, Conley BA, et al. The use of carboplatin and paclitaxel with daily radiotherapy in patients with locally advanced squamous cell carcinomas of the head and neck. Int J Radiat Oncol Biol Phys. 2000;47:49–56. doi: 10.1016/s0360-3016(00)00408-9. [DOI] [PubMed] [Google Scholar]
- 6.Milas L, Mason K, Hunter N, et al. In vivo enhancement of tumor radio response by C225 antiepidermal growth factor receptor antibody. Clin Cancer Res. 2000;6:701–708. [PubMed] [Google Scholar]
- 7.Baselga J. The EGFR as a target for anticancer therapy focus on cetuximab. Eur J Cancer. 2001;37(Suppl 4):S16–S22. doi: 10.1016/s0959-8049(01)00233-7. [DOI] [PubMed] [Google Scholar]
- 8.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-Year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11:21–28. doi: 10.1016/S1470-2045(09)70311-0. [DOI] [PubMed] [Google Scholar]
- 9.Denis F, Garaud P, Bardet E, et al. Final results of the 9401 French Head and Neck Oncology and Radiotherapy Group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced-stage oropharynx carcinoma. J Clin Oncol. 2004;22:69–76. doi: 10.1200/JCO.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 10.Fu KK, Pajak TF, Trotti A, et al. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: First report of RTOG 9003. Int J Radiat Oncol Biol Phys. 2000;48:7–16. doi: 10.1016/s0360-3016(00)00663-5. [DOI] [PubMed] [Google Scholar]
- 11.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milas L, Fan Z, Andratschke NH, et al. Epidermal growth factor receptor and tumor response to radiation: In vivo preclinical studies. Int J Radiat Oncol Biol Phys. 2004;58:966–971. doi: 10.1016/j.ijrobp.2003.08.035. [DOI] [PubMed] [Google Scholar]
- 13.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetux-imab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 14.Pfister DG, Su YB, Kraus DH, et al. Concurrent cetuximab, cis-platin, and concomitant boost radiotherapy for locoregionally advanced, squamous cell head and neck cancer: A pilot phase II study of a new combined-modality paradigm. J Clin Oncol. 2006;24:1072–1078. doi: 10.1200/JCO.2004.00.1792. [DOI] [PubMed] [Google Scholar]
- 15.Birnbaum A, Dipetrillo T, Rathore R, et al. Cetuximab, pacli-taxel, carboplatin, and radiation for head and neck cancer: A toxicity analysis. Am J Clin Oncol. 2010;33:144–147. doi: 10.1097/COC.0b013e3181979093. [DOI] [PubMed] [Google Scholar]
- 16.D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 17.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]