Abstract
Background
Anaemia is a global public health problem. Children under five years of age living in developing countries (mostly Africa and South‐East Asia) are highly affected. Although the causes for anaemia are multifactorial, malaria has been linked to anaemia in children living in malaria‐endemic areas. Administering intermittent preventive antimalarial treatment (IPT) to children might reduce anaemia, since it could protect children from new Plasmodium parasite infection (the parasites that cause malaria) and allow their haemoglobin levels to recover.
Objectives
To assess the effect of IPT for children with anaemia living in malaria‐endemic areas.
Search methods
We searched the Cochrane Infectious Diseases Group Specialized Register, Cochrane Central of Controlled Trials (CENTRAL), published in The Cochrane Library; MEDLINE; EMBASE; and LILACS. We also searched the World Health Organization (WHO) International Clinical Trial Registry Platform and metaRegister of Controlled Trials (mRCT) for ongoing trials up to 4 December 2014.
Selection criteria
Randomized controlled trials (RCTs) evaluating the effect of IPT on children with anaemia.
Data collection and analysis
Two review authors independently extracted data and assessed risk of bias. We analysed data by conducting meta‐analyses, stratifying data according to whether participants received iron supplements or not. We used GRADE to assess the quality of evidence.
Main results
Six trials with 3847 participants met our inclusion criteria. Trials were conducted in areas of low malaria endemicity (three trials), and moderate to high endemicity (three trials). Four trials were in areas of seasonal malaria transmission. Iron was given to all children in two trials, and evaluated in a factorial design in a further two trials.
IPT for children with anaemia probably has little or no effect on the proportion anaemic at 12 weeks follow‐up (four trials, 2237 participants, (moderate quality evidence).
IPT in anaemic children probably increases the mean change in haemoglobin levels from baseline to follow‐up at 12 weeks on average by 0.32 g/dL (MD 0.32, 95% CI 0.19 to 0.45; four trials, 1672 participants, moderate quality evidence); and may improve haemoglobin levels at 12 weeks (MD 0.35, 95% CI 0.06 to 0.64; four trials, 1672 participants, low quality evidence). For both of these outcomes, subgroup analysis did not demonstrate a difference between children receiving iron and those that did not.
IPT for children with anaemia probably has little or no effect on mortality or hospital admissions at six months (three trials, 3160 participants moderate quality evidence). Subgroup analysis did not show a difference between those children receiving iron supplements and those that did not.
Authors' conclusions
Trials did show a small effect on average haemoglobin levels but this did not appear to translate into an effect on mortality and hospital admissions. Three of the six trials were conducted in low endemicity areas where transmission is low and thus any protective effect is likely to be modest.
16 April 2019
Update pending
Studies awaiting assessment
The CIDG is currently examining a new search conducted up to 16 Nov, 2017 for potentially relevant studies. These studies have not yet been incorporated into this Cochrane Review.
Plain language summary
Antimalarial drugs as a treatment of anaemia in children living in malaria‐endemic areas.
Children living in malaria areas may develop severe anaemia, often caused by malaria infection, and this can cause death if not treated properly. Intermittent preventive treatment (IPT) is a course of malaria treatment given regularly to these children in order to prevent infection and malaria illness. It has been suggested that IPT could be used to treat children with anaemia in these areas. We aimed to find all the studies looking at treating anaemic children with IPT in order to see what the overall effect is. We examined the evidence available up to 4 December 2014.
We included six trials in this review, with a total number of 3847 participants. In all the trials, one group received IPT and the control group received placebo. Three trials were done in low malaria endemicity areas and the other three in high endemicity areas. In some trials, iron supplements were also given to children, which is also a treatment for anaemia, and we took this into consideration when analysing the data.
Our results did not find that the number of children who died or were admitted to hospital was lower in the group receiving IPT, irrespective of whether they received iron (moderate quality evidence); and there was no difference in the number of children with anaemia at the end of follow‐up (moderate quality evidence). Average haemoglobin levels were higher in the IPT group compared to the placebo group, but the effect was modest (low quality evidence).
Although our results show that there are small benefits in haemoglobin levels when treating anaemic children with IPT, we did not detect an effect on death or hospital admissions. However, three of the six included trials were conducted in low endemicity areas where malaria transmission is low and thus any protective effect is likely to be modest.
Summary of findings
Summary of findings for the main comparison. Summary of findings table 1.
| Intermittent preventive treatment compared to placebo for children with anaemia | |||||
| Patient or population: Children with anaemia Settings: Malaria‐endemic areas Intervention: IPT (± iron and folic acid) Comparison: Placebo (± iron and folic acid) | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Placebo | IPT | ||||
|
Death or hospital admission Follow up at 6 months |
34 per 1000 | 31 per 1000 (24 to 38) | RR 0.9 (0.71 to 1.13) | 3160 (3 trials) | ⊕⊕⊕⊝ moderate1,2,3,4 |
|
Children with anaemia
(Hb < 11 g/dL) Follow up at 12 weeks |
579 per 1000 | 561 per 1000 (510 to 620) | RR 0.97 (0.88 to 1.07) | 2237 (4 trials) | ⊕⊕⊕⊝ moderate2,5,6,7 |
|
Mean change in Hb from baseline Follow up: 12 weeks |
The mean change ranged across control groups from 0.32 to 5.4 g/dL | The mean change in the intervention groups was 0.32 g/dL higher (0.19 to 0.45 higher) | ‐ | 1672 (4 trials) | ⊕⊕⊕⊝ moderate2,8,9,10 |
|
Mean Hb Follow up at 12 weeks |
The mean Hb concentration ranged across control groups from 9.91 to 10.7 g/dL | The mean Hb concentration in the intervention groups was 0.35 g/dL higher (0.06 to 0.64 higher) | ‐ | 1672 (4 trials) | ⊕⊕⊝⊝ low8,9,10,11 |
| *The basis for the assumed risk is the median control group risk across trials. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; IPT: intermittent preventive treatment; AL: artemether lumefantrine. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 No serious risk of bias: The largest trial was at low risk of bias. The two smaller trials were at high risk of attrition bias, but exclusion of these trials does not change the result. 2 No serious inconsistency: Statistical heterogeneity was low. 3 No serious indirectness: The three trials were conducted in the Gambia, Kenya and Malawi, one trial gave IPT (AL monthly) to children discharged from hospital following severe malarial anaemia, and two trials gave IPT (SP monthly) to anaemic children attending hospital, outpatient clinics or recruited in the community. There was no significant result for subgroup differences between areas with high versus areas with low endemicity. 4 Downgraded by 1 for serious imprecision: The 95% CI around the absolute risk difference is very narrow and excludes clinically important effects. However, much larger trials would be necessary to fully exclude small benefits with IPT. 5 Downgraded by 1 for serious risk of bias: high risk of attrition bias for Bojang 2010 GMB and Desai 2003 KEN. 6 No serious indirectness: All the trials gave IPT (SP) monthly to anaemic children attending hospital, outpatient clinics or recruited in the community. There was no significant result for subgroup differences between areas with high versus areas with low endemicity. 7 No serious imprecision: No effect was seen and the meta‐analysis is adequately powered to detect an effect.
8 No serious imprecision. A small effect was seen although this disappears when we removed trials at high risk of bias from the analysis. 9 Downgraded by 1 for serious risk of bias: High risk for attrition bias for Bojang 2010 GMB and Desai 2003 KEN. 10 No serious indirectness: Three trials gave IPT (SP) monthly and one trial gave CQ weekly to anaemic children attending hospital, outpatient clinics or recruited in the community. There was no significant result for subgroup differences between areas with high versus areas with low endemicity. 11 Downgraded for serious inconsistency: Heterogeneity (I² statistic = 76%; Chi² statistic = 16.93; P = 0.002) is present. One trial is an outlier (Desai 2003 KEN).
Background
Description of the condition
Anaemia is a public health problem that affects people worldwide. Between 1993 and 2005, an estimated 1.62 billion people worldwide had anaemia, which corresponded to 24.8% of the world's population (WHO 2008). The reported global prevalence was 47.4% in children aged under five. Children in Africa and South‐East Asia carried the highest reported burden of anaemia: 67.6% and 65.5% respectively (WHO 2008). Causes of anaemia are multifactorial and include poor nutritional status, micronutrient deficiencies (especially iron deficiency, but also vitamin A, vitamin B and folic acid), intestinal helminth infection, HIV infection and haemoglobinopathies (Calis 2008). However, malaria is probably the most important cause of anaemia in malaria‐endemic countries (Antony 2008; Balarajan 2011; Crawley 2004). Anaemia is also more common in children from low‐income and illiterate families, compared to children coming from wealthier households (Balarajan 2011).
Malaria causes anaemia mainly by destruction of red blood cells (haemolysis) (Looareesuwan 1987) but also by causing an increase in the splenic pool of red blood cells and decreased production of red blood cells (Crawley 2004; Phillips 1992). Acute loss of red blood cells may lead to severe anaemia. Chronic anaemia can slow growth and result in learning difficulties and behavioural changes in affected children (Grantham‐McGregor 2001; Lozoff 1991).
The symptoms of anaemia vary according to the severity, the age of the affected person, and whether the anaemia is acute or chronic. People with anaemia report fatigue, shortness of breath and palpitations. Clinical signs include paleness of the mucosal linings, such as the tongue, conjunctiva, palm and nail bed (Kalter 1997). Although palm pallor is commonly used for classification of disease in children (Meremikwu 2009), diagnosis of anaemia is based on laboratory tests. The World Health Organization (WHO) has defined anaemia in pre‐school aged children as a haemoglobin (Hb) concentration of less than 11 g/dL (WHO 2008) and severe anaemia, often a complication of severe malaria, as a Hb concentration of less than 5 g/dL (WHO 2000). In a study assessing the short and long term outcome of severe anaemia in Malawian children, children hospitalized and treated for severe anaemia had a significantly higher mortality rate (in‐hospital and post‐discharge) than children who were seen in hospital for other conditions and those from the community (Phiri 2008). Furthermore, researchers estimate that severe anaemia probably accounts for more than half of all childhood deaths from malaria in Africa (Crawley 2004). Children who are affected may need to be admitted to hospital and may need blood transfusions (Obonyo 2007).
A Cochrane Review has shown that long lasting insecticide‐treated net (LLIN) use was highly effective in reducing childhood mortality and morbidity from malaria and had a positive effect on anaemia in children (Lengeler 2009). These vector control strategies are a core component of the malaria control programmes globally and especially in Africa (WHO 2012a). Other measures to prevent anaemia include prompt and effective treatment of malaria infections, intestinal helminths and human immunodeficiency virus (HIV), increased use of measures to prevent mother‐to‐child transmission of HIV and provision of micronutrient supplementation (Balarajan 2011; Crawley 2004).
Description of the intervention
Intermittent preventive treatment (IPT) is the administration of a full course of antimalarial treatment to a population at risk of malaria during a specific time period, regardless of whether or not they are known to be infected (Greenwood 2006). IPT policies were first implemented in pregnant women (IPTp) living in areas with a high rate of seasonal malaria transmission. This treatment consisted of a single dose of sulphadoxine/pyrimethamine (SP) given two or three times during the pregnancy, and was introduced as an alternative to chemoprophylaxis with chloroquine (CQ), due to the increasing CQ resistance and unpopularity of the drug (Greenwood 2010). The WHO also recommends that IPT in infants (IPTi) up to the age of 12 months, should be administered together with the second and third diphtheria‐pertussis‐tetanus (DPT) and measles vaccination of infants in areas that have a moderate to high transmission rate of malaria (WHO 2010; WHO 2012a).
IPT was first made available for children (IPTc) after it had been shown that most children in highly seasonal malaria areas suffer from malaria and its related complications during the rainy season (Dicko 2011). Two recent systematic reviews have demonstrated that IPTc reduces episodes of clinical malaria in areas with a high rate of seasonal malaria transmission (Meremikwu 2012; Wilson 2011). Currently, the WHO recommends seasonal malarial chemoprevention (SMC) or IPTc, in seasonal malarial areas during the transmission season (WHO 2012b). This consists of a complete treatment course of SP and amodiaquine (AQ), given to children aged between three to 59 months, at monthly intervals, during the high risk period of malaria transmission. Children may receive up to four doses of this antimalarial treatment during the malaria transmission season with the aim of maintaining therapeutic drug levels during the period of high transmission. This strategy excludes areas with SP resistance outbreak (WHO 2013).
How the intervention might work
Children with severe anaemia, for whom routine management like blood transfusions and hematinics is insufficient to improve the Hb level, might benefit from IPT, since it has been shown to augment the effect of hematinics on Hb recovery when administered together in anaemic children (Akech 2008; Phiri 2011; Verhoef 2002). In addition, IPT enables hematological recovery by preventing and treating new malaria infections (White 2004). Combining the effect of IPT, LLIN, and other programs like deworming and iron supplementation might add significant benefit in reducing the burden of anaemia in pre‐school aged children. Iron supplementation is often recommended for children with anaemia, although there are concerns about an association between iron supplementation and increased malaria morbidity and mortality (WHO 2006). However, a recent Cochrane Review concluded that there is high quality evidence that iron supplementation, even when given together with antimalarial treatment, does not increase the risk of clinical malaria morbidity or mortality (Okebe 2011).
A Cochrane Review reported that IPT, when given to treat malaria, also increased Hb levels of children (Meremikwu 2012). They also concluded that there is moderate quality evidence that children given IPT were less likely to have moderately severe anaemia at follow‐up (Hb < 8 g/dL) compared to placebo (Risk ratio (RR) 0.71, 95% confidence interval (CI) 0.52 to 0.98).
Why it is important to do this review
The prevalence of anaemia in pre‐school aged children remains high, especially in children living in Africa and South‐East Asia. The Cochrane Review of IPT in areas with seasonal transmission of malaria showed promising effects on preventing and treating anaemia in children (Meremikwu 2012). Although the review included all pre‐school aged children living in malaria‐endemic regions, it did not examine the effects of IPT on children diagnosed with anaemia.
Since the two systematic reviews on IPT for malaria (Meremikwu 2012; Wilson 2011) have conflicting results on the effect of IPT on anaemia, a formal assessment of existing studies in a systematic review can provide physicians, policy makers and researchers with reliable evidence on the use of IPT in anaemic children living in malaria‐endemic areas with a high seasonal transmission rate.
Objectives
To assess the effect of intermittent preventive antimalarial treatment for children with anaemia living in malaria‐endemic areas.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) and cluster‐RCTs.
Types of participants
Children with anaemia (Hb < 11 g/dL; WHO 2008) living in malaria‐endemic areas.
Types of interventions
Intervention
IPT for malaria
Control
No IPT for malaria
Co‐interventions, such as hematinics or LLINs, should be identical in both intervention and control groups.
Types of outcome measures
Primary outcomes
All‐cause mortality and hospital admission
Secondary outcomes
Anaemia at follow‐up (Hb < 11g/dL)
Mean change in Hb (g/dL) from baseline to follow‐up
Mean Hb at follow‐up (g/dL)
Search methods for identification of studies
We attempted to identify all relevant studies regardless of the language and publication status (published, unpublished, in press and ongoing).
Electronic searches
We searched the following databases up to 4 December 2014 using the search terms and strategy described in Appendix 1: Cochrane Infectious Diseases Group Specialized Register, Cochrane Central of Controlled Trials (CENTRAL), published in The Cochrane Library (2014, Issue 11); MEDLINE; EMBASE; and LILACS. We also searched the WHO International Clinical Trial Registry Platform and metaRegister of Controlled Trials (mRCT) for ongoing trials using "anaemia", "children", "intermittent preventive treatment" and "malaria" as search terms.
Searching other resources
Reference lists
We checked the reference lists of all included studies for relevant trials.
Data collection and analysis
Selection of studies
Two review authors (MA and AR) independently screened the results of the literature search for potentially eligible trials. We retrieved the full text articles of relevant studies and independently assessed eligibility using an eligibility form. We contacted trial authors in cases of missing or unclear information. We resolved discrepancies through discussion or alternatively through consulting the third review author, AMK. We ensured that multiple publications of the same trial were only included once. We listed excluded studies, together with the reasons for exclusion, in table format.
Data extraction and management
Two review authors (MA and AR) extracted data independently using pre‐piloted, electronic data extraction forms. We resolved any disagreements through discussion. We contacted the trial authors in case of missing data.
For each included trial, we extracted data on the trial design, participants, intervention, control intervention, outcomes (included outcomes, measurement of outcomes) and results. For RCTs, we extracted the number of participants randomized to each treatment arm and the number of participants monitored for each outcome of interest. For dichotomous data, we extracted the number of events in each of the treatment arms. For continuous data, we extracted the arithmetic mean, standard deviations (SDs) and the number of participants in each group. We reported the measure of effect for each outcome, RRs or mean differences, with 95% CIs.
Assessment of risk of bias in included studies
Two review authors (MA and AR) independently assessed risk of bias for each included trial by using the Cochrane Collaboration's 'Risk of bias' assessment tool (Higgins 2011). All discrepancies were resolved through discussion or consultation with AMK.
We classified risk of bias judgements as either low, high or unclear risk of bias. We assessed the following components for risk of bias in each included trial as follows:
Sequence generation
We regarded a trial as having: low risk of bias if the sequence generation was truly random (for example, computer‐generated table of random numbers, tossing a coin); high risk of bias if sequence generation contained a non‐random component (for example, alternate randomization, randomization by birth date); or unclear risk of bias if the trial authors did not clearly describe the randomization process.
Allocation concealment
We regarded trials as having: low risk of selection bias if allocation was truly concealed (for example, central allocation of participants, use of sequentially numbered, opaque, sealed envelopes); high risk of bias if the allocation process was not concealed (for example, open randomization, unsealed or non‐opaque envelopes); or unclear risk of bias if the trial authors did not describe the process of allocation concealment in sufficient detail.
Blinding of participants and personnel
We determined whether blinding was present, who was blinded and the methods used to blind trial participants and personnel. We regarded a trial as having: low risk of bias if blinding was present, or if the absence of blinding was unlikely to affect the outcomes; high risk of bias if blinding was absent and likely to affect the results; or at unclear risk of bias if blinding was not clearly described.
Blinding of outcome assessors
We described whether blinding of outcome assessors was present and how they were blinded. We regarded a trial as having: low risk of detection bias if they were blind to knowledge about which intervention the participants received; high risk of bias if blinding was absent; and unclear risk if blinding was not clearly described.
Incomplete outcome data
We regarded trials as having: low risk of attrition bias if there was no missing data or if missing data was balanced across groups and attrition rates were less that 20%; high risk of bias if there was missing data (attrition rate higher than 20%) or if missing data was more prevalent in one of the groups; or unclear risk of bias if trial authors did not clearly state whether outcome data was missing.
Selective outcome reporting
We regarded a trial as having low risk of reporting bias if it was evident that all pre‐specified outcomes were reported on; high risk of bias if it was evident that not all pre‐specified outcomes were reported on; or unclear risk of bias if it was unclear whether all outcomes have been reported on.
Other bias
We described any important feature of included trials that could have affected the result.
Measures of treatment effect
We compared dichotomous data using RRs. For continuous data summarized by arithmetic means and SDs, we presented mean difference values. We presented all results with their associated 95% CIs.
Unit of analysis issues
We included two trials with a two‐by‐two factorial trial design, that is containing four treatment groups (IPT plus iron; iron only; IPT only; placebo). We included all four groups in the meta‐analyses, by making use of subgroups (IPT plus iron versus IPT without iron).
Dealing with missing data
We applied available case analysis to continuous outcomes and only included data on the known results. The denominator was the total number of participants who had data recorded for the specific outcome.
For dichotomous outcomes, we performed analyses on an intention‐to‐treat basis. We included all participants randomized to each group in the analyses and analysed participants in the group to which they were randomized.
We calculated missing SDs from 95% CIs, if available. Where 95% CIs were not reported with the mean Hb level at follow‐up, we borrowed the SDs reported for the mean Hb levels at baseline (Higgins 2011). Where mean values were not reported for the outcome (mean change in Hb from baseline to follow‐up) we calculated means by subtracting the mean value at baseline from the mean value at follow‐up. We imputed the corresponding SD by calculating a correlation coefficient from a reported mean and SD (mean change in Hb from baseline to follow‐up) in Rohner 2010, after consultation with a statistician. This study examined the effect of IPT on malaria and anaemia in children, but included both anaemic and non‐anaemic children and thus we excluded it from this review.
Assessment of heterogeneity
We inspected forest plots for overlapping CIs and assessed statistical heterogeneity in each meta‐analysis using the I² and Chi² statistics. We regarded heterogeneity as moderate if I² statistic values were between 30% and 60%; substantial if they were between 50% and 90%; and considerable if they were between 75% and 100%. We regarded a P value of 0.10 or less indicative of statistically significant heterogeneity.
Assessment of reporting biases
We did not formally assess reporting biases, since we only included six trials in the review.
Data synthesis
We used RevMan 2014 for data analysis. If considerable heterogeneity was present, we combined data using random‐effects meta‐analysis and reported an average treatment effect, since this was considered to be clinically meaningful. We presented results using forest plots.
Evidence quality
We assessed the quality of evidence using the GRADE approach (Guyatt 2011). We rated each outcome as either high (we are very confident that the true effect lies close to that of the estimate of the effect); moderate (we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect); low (our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect); or very low quality of evidence (we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect) (Balshem 2011).
RCTs are regarded as high quality evidence but can be downgraded within the following five categories: study limitations, imprecision, inconsistency, indirectness and publication bias. Studies can also be upgraded if there is a large effect, a dose‐response effect, or if all plausible residual confounding would reduce a demonstrated effect or would suggest a spurious effect if no effect was observed (Balshem 2011). We summarized our findings in a 'Summary of findings' table.
Subgroup analysis and investigation of heterogeneity
We performed the following subgroup analyses:
Additional interventions to treat anaemia (such as hematinics or folic acid)
Hospital recruitment versus community recruitment
We performed subgroup analyses for the following outcomes:
All‐cause mortality and hospital admissions at six months follow‐up
Anaemia at 12 weeks follow‐up
Mean change in Hb from baseline to follow‐up (12 weeks)
Mean Hb at follow‐up (12 weeks)
We assessed differences between subgroups using the Chi2 test with a P value of 0.05 or less indicating statistically significant differences between subgroups.
We documented the drugs used for IPT in the footnotes for each forest plot. There was no evidence of heterogeneity by drug type, which might be anticipated with emerging sulfadoxine‐pyrimethamine (SP) resistance, so we did not subgroup by drug type.
Sensitivity analysis
Due to the limited amount of included trials, we did not perform sensitivity analysis.
Results
Description of studies
See Table 2; Characteristics of included studies; Characteristics of excluded studies.
1. Summary of trial characteristics.
| Trial | Bojang 2010 GMB | Cox 2013 GMB | Desai 2003 KEN | Phiri 2012 MWI | Verhoef 2002 KEN | Tomashek 2001 TNZ |
| Sample size (n randomized) | 1200 | 96 | 554 | 1431 | 328 | 238 |
| Country | The Gambia (Banjul) | The Gambia | Kenya (Western Kenya) | Malawi (southern Malawi) | Kenya (Eastern province) | Tanzania (Kigoma region) |
| Endemicity | low* | low* | high* | high* | low* | moderate/high (50% parasitaemia)** |
| Age | 3 months to 9 years | 12 to 72 months | 2 to 36 months | 4 to 59 months | 2 to 36 months | 6 to 59 months |
| Anaemia | Hb < 7 g/dL | Hb 69 to 110 g/L | Hb 7.0 to 10.9 g/dL | All children treated for severe malarial anaemia with transfusion and completed the course of intravenous quinine with subsequent Hb > 5g/dL | Hb 60 to 110 g/L | Hb 5.0 to 8.0 g/dL |
| Malaria | Not criteria for inclusion. Children with malaria were treated | Uncomplicated malaria | No malaria (aparasitaemic) or parasite counts < 20,000 parasites/mm3 | No clinical malaria | Not described as part of eligibility | |
| Recruitment | Hospital or OPD admission | Active and passive case finding of children in community | Community: resident children screened | Hospital admissions | Community: randomly selected | Health care worker diagnosed children with clinical anaemia and referred them to the trial |
| Trial intervention |
|
|
|
|
|
|
| Iron and other supplementation during trial period (all participants) | Yes: oral iron for 28 days | No | No ‐ part of trial intervention | Not reported | No – part of trial intervention | Yes: Iron and folic acid for 12 weeks |
| Baseline treatment for all participants | Some were treated with quinine and SP or CQ and SP. Not all children | Either CQ and SP or AL | Single dose of SP | 3 day course of AL in hospital (6 doses) | None | Single dose of SP; mebendazole for participants > 12 months |
*endemicity derived from the Malaria Atlas Project (Gething 2011).
**as reported in the trial (Tomashek 2001 TNZ).
Abbreviations:
Hb: Haemoglobin
OPD: Out patient department
IPT: Intermittent preventive treatment
SP:Sulfadoxine‐pyrimethamine
CQ: Chloroquine
AL: Arthemeter‐lumefantrine
VAC: Vitamin A and C
Results of the search
Our search yielded 88 records. We excluded 74 studies after screening abstracts and a further eight studies after assessing eligibility of full texts. We included six RCTs (3847 participants). See Figure 1.
1.
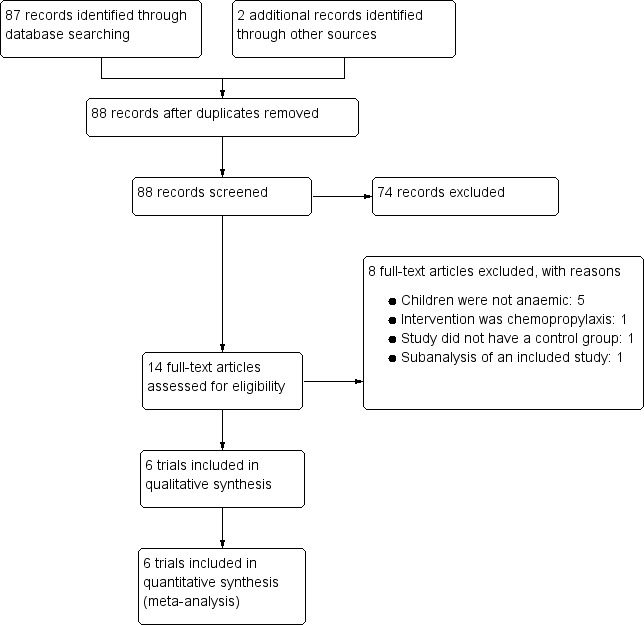
Study flow diagram.
Included studies
Trial designs
All included trials were individually RCTs. Two trials with a factorial design (Desai 2003 KEN; Verhoef 2002 KEN) had four trial arms. One trial had three arms (Tomashek 2001 TNZ) and the remaining three trials had two trial arms. Most of the included RCTs had an intervention period of approximately 12 weeks, at which time outcomes were assessed, and some trials had an extended follow‐up period of up to one year post enrolment.
Location
All included trials were conducted in malaria‐endemic areas. Three trials were conducted in low endemicity areas: two in The Gambia (Bojang 2010 GMB; Cox 2013 GMB) and one in eastern Kenya (Verhoef 2002 KEN). Three trials were conducted in high endemicity areas: one in western Kenya (Desai 2003 KEN), one in southern Malawi (Phiri 2012 MWI) and one in western Tanzania (Tomashek 2001 TNZ).
Participants
The age of the anaemic children ranged between two months and nine years. In five trials, children had mild to moderate anaemia (Hb 5 to 11 g/dL) at enrolment. The remaining trial enrolled children with Hb levels less than 7 g/dL (Bojang 2010 GMB). In one trial, children had severe malaria (Phiri 2012 MWI), two trials included children with uncomplicated malaria (Cox 2013 GMB; Desai 2003 KEN), for two trials malaria was not part of the inclusion criteria (Bojang 2010 GMB; Tomashek 2001 TNZ) and one trial excluded children with clinical malaria (Verhoef 2002 KEN). Three trials (Bojang 2010 GMB; Phiri 2012 MWI; Tomashek 2001 TNZ) recruited children that attended outpatient clinics or were admitted to hospital, while three trials recruited children from the community (Cox 2013 GMB; Desai 2003 KEN; Verhoef 2002 KEN).
Interventions
Baseline treatments
In four trials, children in intervention and placebo groups received baseline treatment for malaria or anaemia. In two of these trials, all children were given a single dose of SP (Desai 2003 KEN; Tomashek 2001 TNZ). In one trial (Cox 2013 GMB) children received either CQ and SP or artemether‐lumefantrine (AL) before randomization to intervention and placebo groups. Phiri 2012 MWI treated all children with intravenous quinine and blood transfusion while in hospital and AL at discharge. In addition, Tomashek 2001 TNZ administered a single dose of mebendazole to all children over the age of 12 months.
In Bojang 2010 GMB only children with malaria were treated with quinine and SP or CQ and SP. Verhoef 2002 KEN was the only trial where no baseline treatment was given to children.
Trial interventions
All included RCTs compared IPT to placebo. The types of IPT used were the following:
SP (500 mg sulfadoxine/25 mg pyrimethamine per tablet, given at an approximate dose of 25 mg sulfadoxine/1.25 mg pyrimethamine per kg) given monthly for an average of 12 weeks or until the end of the malaria transmission period (Bojang 2010 GMB; Desai 2003 KEN; Tomashek 2001 TNZ; Verhoef 2002 KEN). A drug sensitivity study of SP in a multicentre trial in Eastern Kenya and Kigoma Tanzania (Gorissen 2000) between 1998 and 2000 showed efficacy of more than 85%. However, these findings differed from other studies done in East Africa, which reported on resistance of Plasmodium falciparum to SP between 1995 and 1997. This finding did not have a strong association with the clinical evidence (Jelinek 1997; Terlouw 2003). In contrast to East Africa, reports on declining SP efficacy and its resistance on P. falciparum were already reported by several studies in The Gambia by 1998 (Dunyo 2006; von Seidlein 2000).
AL tablets (20 mg artemether, 120 mg lumefantrine per tablet, children weighing 15 kg or more received two tablets, less than 15 kg received one tablet) given as a three day course (six doses) monthly for 12 weeks (Phiri 2012 MWI). Artemisinin combination therapies (ACTs) were adopted in most African countries after 2005. In Malawi, an earlier study (2004 to 2006) had already shown efficacy of above 85% (Bell 2009). This finding was supported by another study in the same country which found less genetic amplification of P. falciparum to Coartem (Haildar 2009).
CQ syrup (5 mg/kg) given as a weekly dose (three day course) for 12 weeks (Cox 2013 GMB). CQ sensitivity studies conducted in the early 2000s in The Gambia and Mali revealed low efficacy of CQ (Tekete 2009) with evidence of increased resistance of CQ to P. falciparum (Ord 2007).
Four trials gave iron as part of the intervention. In two trials, all children received iron, regardless of whether they were in the intervention or placebo group (Bojang 2010 GMB; Tomashek 2001 TNZ). Bojang 2010 GMB administered iron for 28 days and Tomashek 2001 TNZ gave iron and folic acid for 12 weeks. In the two‐by‐two factorial trials (Desai 2003 KEN; Verhoef 2002 KEN), children were randomized to receive either iron or placebo.
In addition, children in one of the three intervention groups in Tomashek 2001 TNZ received vitamin A and C (VAC) three times a week.
Co‐interventions
Two trials did not report the use of LLINs (Tomashek 2001 TNZ; Verhoef 2002 KEN). In Cox 2013 GMB, LLIN distribution was part of the standard malaria prevention programme and in Desai 2003 KEN all households were issued with LLINs, but both studies did not assess the use of LLINs. Bojang 2010 GMB assessed LLIN use at the end of the transmission period (20.7% in IPT group; 15.3% in placebo) and the reported bed net use in Phiri 2012 MWI was similar in both groups, overall use was 51% (35% treated net, 16% untreated net).
Outcomes
Trials reported on a variety of outcomes (Table 3).
2. Summary of outcomes reported in trials.
| Outcomes | Trial | Bojang 2010 GMB | Cox 2013 GMB | Desai 2003 KEN | Phiri 2012 MWI | Verhoef 2002 KEN | Tomashek 2001 TNZ |
| Haematological outcomes | Mean Hb at end of follow‐up | Mean Hb level at end of transmission period | ‐ | Hb concentration (measured in g/dL) | ‐ | Hb concentration at the end of follow‐up (12 weeks)* | Mean Hb |
| Mean change of Hb from baseline to follow‐up | ‐ | Mean change of Hb from baseline to follow‐up | ‐ | ‐ | ‐ | ‐ | |
| Children with anaemia at follow‐up | Proportion of children with moderate or severe anaemia at the end of the transmission period* | ‐ | Hematological recovery (Hb ≥ 11g/dL before or at week 12) Severe anaemia (Hb < 7 g/dL before or at week 12) |
‐ | Anaemia (Hb < 11.0 g/dL) | Prevalence of anaemia (Hb < 11.0 g/dL) | |
| Other | ‐ | Change in erythropoietic response Hb change from baseline to follow‐up in two placebo arms to investigate the effect of antimalarial therapy |
MCV (measured in fL) sTfR concentration (measured in µg/mL) |
‐ | Iron deficiency (serum ferritin concentration < 12 µg/L) | Mean TfR level Prevalence of iron deficiency (TfR < 8.5 µg/mL) |
|
| Malaria outcomes | Clinical malaria | Clinical episodes of malaria during the surveillance period | ‐ | Clinical malaria (axillary temperature 37.5 with co‐existing malaria parasitaemia) | ‐ | Proportion of children with at least one malaria attack (defined as presence of fever, that is temperature ≥ 37.5°C, and a positive dipstick result)* | ‐ |
| Malaria parasitaemia | Prevalence of parasitaemia and splenomegaly | Prevalence of submicroscopic malaria parasitaemia | Prevalence of malaria parasitaemia Parasite density (parasites/mm3) |
‐ | ‐ | ‐ | |
| Hospital admissions or clinic visits related to malaria | ‐ | ‐ | ‐ | Hospital re‐admission because of all‐cause severe anaemia or severe malaria Clinic visits because of microscopically confirmed non‐severe malaria |
‐ | ‐ | |
| Other | ‐ | ‐ | ‐ | ‐ | Time to first occurrence of malaria attack | ‐ | |
| All‐cause mortality and hospital admission | ‐ | ‐ | ‐ | Composite outcome of all‐cause mortality and hospital readmission because of all‐cause severe anaemia or severe malaria between 1 and 6 months* All‐cause hospital admission All‐cause mortality |
‐ | ‐ | |
| Visits to healthcare facilities | Outpatient attendance | ‐ | Clinic visits (incidence, number of episodes) | All‐cause sick child clinic visits | ‐ | ‐ | |
| Other | Nutritional status at the end oft he transmission period Compliance with treatment regimen |
Change in urinary neopterin | ‐ | ‐ | Adverse drug reactions | ‐ | |
*indicates primary outcomes
Abbreviations:
Hb: haemoglobin
TfR: transferrin receptor
Haematological outcomes
Four trials reported on the mean Hb concentration at 12 weeks follow‐up or at the end of the transmission period. Only one trial (Cox 2013 GMB) reported on the mean change in Hb concentration from baseline to follow‐up.
Four trials reported on the number of children with anaemia at 12 weeks or at the end of the transmission period. Anaemia was defined as Hb < 11 g/dL in three trials (Desai 2003 KEN; Tomashek 2001 TNZ; Verhoef 2002 KEN). Bojang 2010 GMB reported the number of children with an Hb < 7 g/dL and Desai 2003 KEN reported the outcome separately for children with an Hb < 11 g/dL and those with Hb < 7 g/dL.
Other reported outcomes included mean corpuscular volume (MCV) at 12 week follow‐up (Desai 2003 KEN), serum transferrin receptor (TfR) concentration at 12 weeks follow‐up (Desai 2003 KEN; Tomashek 2001 TNZ), iron deficiency measured as serum ferritin < 12 µg/L in Verhoef 2002 KEN and as TfR < 8.5 µg/mL in Tomashek 2001 TNZ and change in erythropoietic response (Cox 2013 GMB).
All‐cause mortality and hospital admissions
All‐cause mortality plus hospital readmissions due to severe anaemia (Hb < 5 g/dL or clinical indication for blood transfusion) or severe malaria (re‐admittance due to confirmed malaria treated with parenteral quinine) at three and six months was the primary outcome in Phiri 2012 MWI. All‐cause mortality and hospital readmission because of all‐cause severe anaemia or severe malaria were also reported on separately.
Malaria outcomes
Three trials reported clinical malaria. One trial (Bojang 2010 GMB) did not define clinical malaria. Desai 2003 KEN defined clinical malaria as an axillary temperature of 37.5°C or higher with co‐existing malaria parasitaemia. Verhoef 2002 KEN reported the proportion of children with at least one malaria attack, defined as a temperature of 37.5°C or higher and a positive dipstick result.
Two trials reported malaria parasitaemia (Bojang 2010 GMB; Desai 2003 KEN). Desai 2003 KEN also reported parasite densities. Cox 2013 GMB reported on the prevalence of submicroscopic parasitaemia (detection of parasites' DNA).
Phiri 2012 MWI reported on clinic visits due to microscopically confirmed non‐severe malaria and Verhoef 2002 KEN on the time to first occurrence of malaria attack.
Other outcomes
Three trials reported visits to healthcare facilities (outpatients, clinics, pharmacies) (Bojang 2010 GMB; Desai 2003 KEN; Phiri 2012 MWI). Other outcomes reported across trials included nutritional status at the end of the transmission period, compliance with treatment regime (Bojang 2010 GMB), adverse drug reactions (Verhoef 2002 KEN) and change in urinary neopterin (Cox 2013 GMB).
Excluded studies
We excluded eight trials (see Characteristics of excluded studies). Five of these did not include only anaemic children, one did not have a control group, another trial was a sub‐analysis of an included trial and the intervention of one study was chemoprophylaxis and not IPT.
Risk of bias in included studies
Overall, risk of bias in included trials was low (see Figure 2 and Figure 3).
2.
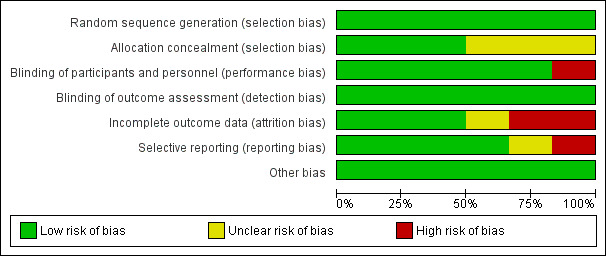
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials.
3.
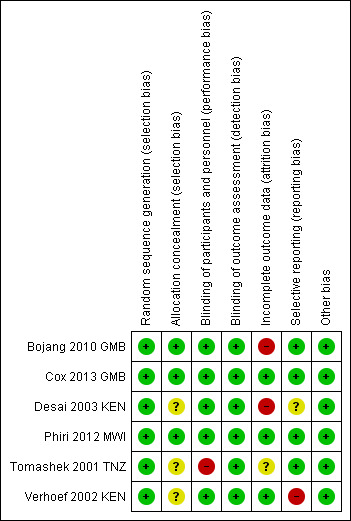
Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.
Allocation
All trials used adequate methods to generate a random sequence.
Three RCTs (Bojang 2010 GMB; Cox 2013 GMB; Phiri 2012 MWI) had adequate allocation concealment. The remaining three trials had insufficient information to make a judgement about risk of bias and were thus judged as having unclear risk of bias.
Blinding
Five RCTs were at low risk of performance bias and adequately blinded participants and personnel. We judged one trial (Tomashek 2001 TNZ) to have high risk of bias, since one of the groups did not receive IPT placebo and the personnel had access to lists of group assignment.
All included trials were at low risk of detection bias and adequately blinded outcome assessors.
Incomplete outcome data
Two trials had high risk of attrition bias. Bojang 2010 GMB had a high rate of loss to follow‐up at the end of the transmission period, 23% (136/600) in the intervention group and 21.5% (127/600) in the placebo group. Although the overall loss to follow‐up rate in Desai 2003 KEN was not high, there was a significant difference in loss to follow‐up at 12 weeks between the placebo group and the other intervention groups: 4% (6/135) in the IPT + iron group; 8.6% (12/139) in the iron group; 6.6% (9/136) in the IPT group, and 20.5% (28/136) in the double placebo group. Follow‐up data at 24 weeks follow‐up was missing.
For Tomashek 2001 TNZ there was also a difference in children lost to follow‐up. In the placebo group, the loss to follow‐up was 6% (5/82), in the IPT group it was 10% (8/81) and in the IPT + VAC group it was 13% (10/75). Reasons for missing children were not stratified according to groups and we judged the risk of bias to be unclear.
The other three trials had low risk of attrition bias.
Selective reporting
Verhoef 2002 KEN did not report on the primary outcome (mean Hb concentration at follow‐up) and was therefore at high risk of reporting bias. One trial (Desai 2003 KEN) did not pre‐specify their outcomes in the methods section and we therefore judged risk of bias to be unclear. The remaining three RCTs had low risk of reporting bias.
Other potential sources of bias
We did not identify any other sources of bias in the six included trials.
Effects of interventions
See: Table 1
All‐cause mortality and hospital admissions at six months
We included three trials (Bojang 2010 GMB; Desai 2003 KEN; Phiri 2012 MWI) with a total of 3160 children in the fixed‐effects meta‐analysis (Analysis 1.1). Results for all‐cause mortality and hospital admissions were included for two trials (Bojang 2010 GMB; Phiri 2012 MWI). Bojang 2010 GMB assessed outcomes at the end of the malaria transmission period and at the end of the following dry season. We included results from the dry season follow‐up for this outcome. For Desai 2003 KEN, we only included mortality data since the hospital admissions were not reported. We extracted mortality data from the study flow‐chart.
1.1. Analysis.
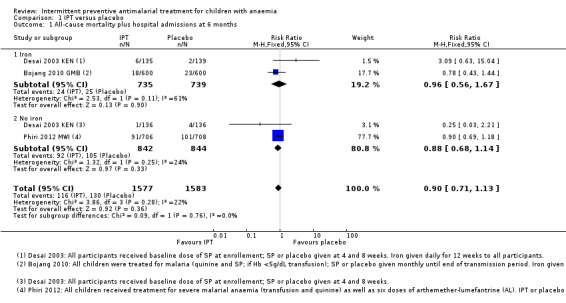
Comparison 1 IPT versus placebo, Outcome 1 All‐cause mortality plus hospital admissions at 6 months.
IPT did not reduce the risk of death or hospital admission compared to placebo (three trials, 3160 participants; Analysis 1.1; I² = 22%). Subgroup analysis did not show a significant difference between children receiving iron and children receiving no iron (Chi² = 0.09, P = 0.76, I² = 0%). There was heterogeneity in the subgroup that received iron (Chi² = 2.53, P = 0.11, I² = 61%), but this subgroup included only two trials and random‐effects meta‐analysis did not change the overall effect (RR 0.89, 95% CI 0.61 to 1.31).
There was no significant difference between subgroups for areas with high endemicity versus areas with low endemicity (Chi² = 0.23, P = 0.63, I² = 0%; Analysis 2.1).
2.1. Analysis.
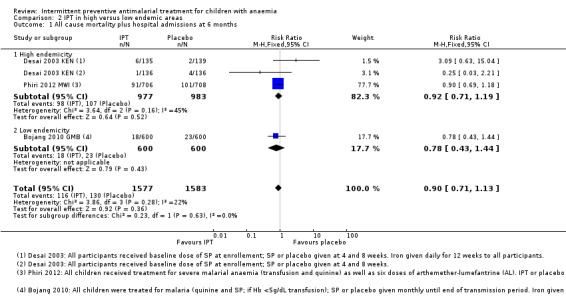
Comparison 2 IPT in high versus low endemic areas, Outcome 1 All cause mortality plus hospital admissions at 6 months.
Subgroup analysis further stratifying subgroups according to recruitment (hospital admission versus selected from community) did not show a difference between groups and we thus combined hospital admissions and community recruitments in the main analyses.
Children with anaemia at 12 weeks
We included four RCTs (Bojang 2010 GMB; Desai 2003 KEN; Tomashek 2001 TNZ; Verhoef 2002 KEN) with a total of 2237 children in the fixed‐effects meta‐analysis (Analysis 1.2). Bojang 2010 GMB assessed outcomes at the end of the malaria transmission period, therefore length of follow‐up depended on time of enrolment.
1.2. Analysis.
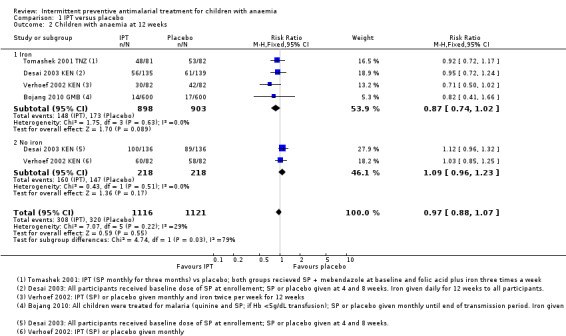
Comparison 1 IPT versus placebo, Outcome 2 Children with anaemia at 12 weeks.
Overall, IPT did not have an effect on the risk of anaemia compared to placebo (four trials, 2237 participants, Analysis 1.2; I² = 29%).
Overall the heterogeneity was not remarkable in the meta‐analysis, but there was some suggestion that the subgroup with iron and the subgroup without iron were slightly different (test for subgroup difference: (Chi² = 4.74, P = 0.03, I² = 78.9%). However, the point estimate and CIs between those receiving iron and those not receiving iron was similar, and the subgroup analysis was underpowered to be confident of any conclusion (Analysis 1.2).
There was no significant difference between subgroups for areas with high endemicity versus areas with low endemicity (Chi² = 1.43, P = 0.23, Analysis 2.2; I² = 30.2%).
2.2. Analysis.
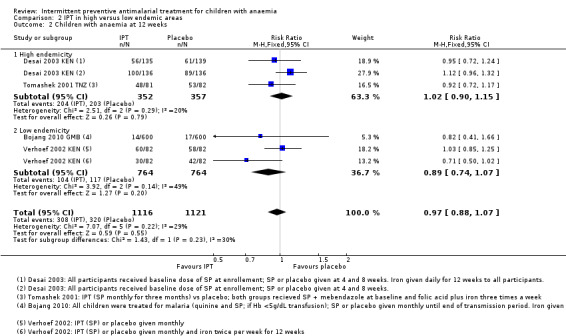
Comparison 2 IPT in high versus low endemic areas, Outcome 2 Children with anaemia at 12 weeks.
Subgroup analysis further stratifying subgroups according to recruitment (hospital admission versus selected from community) did not show a difference between groups and we thus combined hospital admissions and community recruitments in the main analyses.
We did not include one intervention arm of Tomashek 2001 TNZ, where children received SP plus VAC, in the meta‐analysis. The risk for anaemia was not significantly different in these children compared to those that only received SP and vitamin placebo (one trial, 138 participants, Analysis 3.1).
3.1. Analysis.
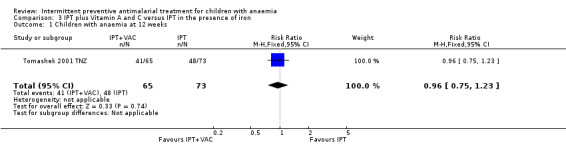
Comparison 3 IPT plus Vitamin A and C versus IPT in the presence of iron, Outcome 1 Children with anaemia at 12 weeks.
Mean change in Hb (baseline to 12 weeks)
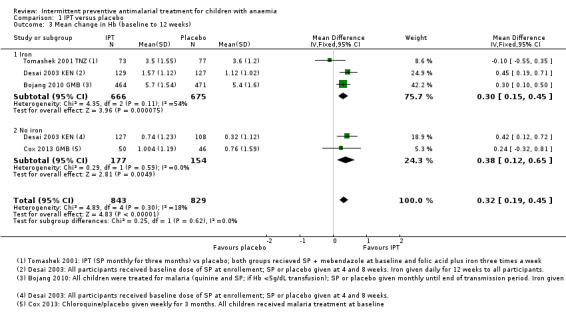
We included four trials (Bojang 2010 GMB; Cox 2013 GMB; Desai 2003 KEN; Tomashek 2001 TNZ) with a total of 1672 children in the fixed effects‐meta‐analysis (Analysis 1.3). Only one trial (Cox 2013 GMB) reported on this outcome and we calculated the values for the other four trials. Bojang 2010 GMB assessed outcomes at the end of the malaria transmission period, therefore length of follow‐up depended on time of enrolment.
1.3. Analysis.
Comparison 1 IPT versus placebo, Outcome 3 Mean change in Hb (baseline to 12 weeks).
Overall, the mean change in Hb concentration from baseline to follow‐up was 0.32 g/dL higher in the IPT group compared to the placebo group (Mean difference (MD) 0.32, 95% CI 0.19 to 0.45; four trials, 1672 participants; Analysis 1.3; I² = 18%)
Subgroup analysis did not show a difference between children receiving iron and children receiving no iron (Chi² = 0.25, P = 0.62, I² = 0%).
There was also no significant difference between subgroups for areas with high endemicity versus areas with low endemicity (Chi² = 0.17, P = 0.68, I² = 0%; Analysis 2.3).
2.3. Analysis.
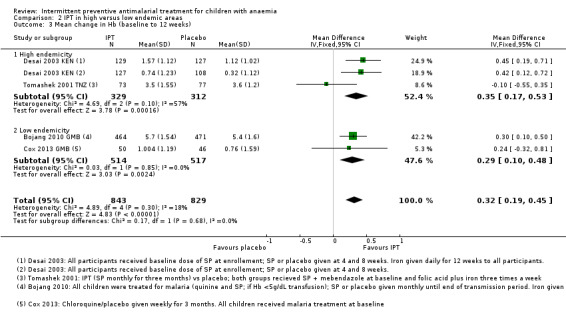
Comparison 2 IPT in high versus low endemic areas, Outcome 3 Mean change in Hb (baseline to 12 weeks).
Subgroup analysis further stratifying subgroups according to recruitment (hospital admission versus selected from community) did not show a difference between groups and we thus combined hospital admissions and community recruitments in the main analyses.
We did not include one intervention arm of Tomashek 2001 TNZ, where children received SP plus VAC, in the meta‐analysis. The mean change in Hb from baseline to follow‐up in these children was not different from the mean change in Hb in children that only received SP (MD 0.00, 95%CI ‐0.48 to 0.48; one trial, 138 participants, Analysis 3.2).
3.2. Analysis.
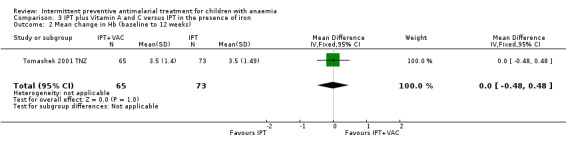
Comparison 3 IPT plus Vitamin A and C versus IPT in the presence of iron, Outcome 2 Mean change in Hb (baseline to 12 weeks).
Mean Hb at 12 weeks
We included four trials (Bojang 2010 GMB; Cox 2013 GMB; Desai 2003 KEN; Tomashek 2001 TNZ) with a total of 1672 children in the random‐effects meta‐analysis (Analysis 1.4). Bojang 2010 GMB assessed outcomes at the end of the malaria transmission period, therefore length of follow‐up depended on time of enrolment. Cox 2013 GMB did not report on this outcome, but we calculated the value using the reported baseline and the change from baseline values.
1.4. Analysis.
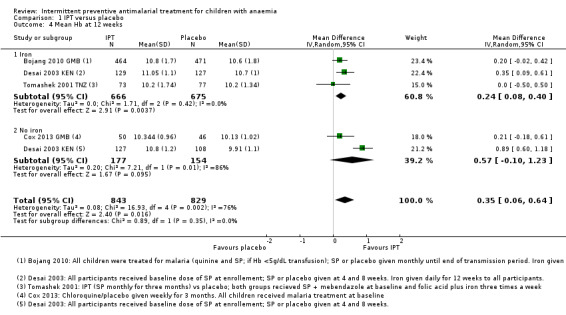
Comparison 1 IPT versus placebo, Outcome 4 Mean Hb at 12 weeks.
Overall, the mean Hb at 12 weeks follow‐up was on average 0.35 g/dL higher in the IPT group compared to the placebo group (MD 0.35, 95% CI 0.06 to 0.64; four trials, 1672 participants, Analysis 1.4; T² = 0.08; I² = 76%). One trial (Desai 2003 KEN) caused heterogeneity. If we remove this trial from the analysis, heterogeneity is reduced to 0%.
Subgroup analysis did not show a difference between children receiving iron and children receiving no iron (Chi² = 0.89, P = 0.35, I² = 0%).
There was also no significant difference between subgroups for areas with high endemicity versus areas with low endemicity (Chi² = 0.85, P = 0.36, I² = 0%; Analysis 2.4).
2.4. Analysis.
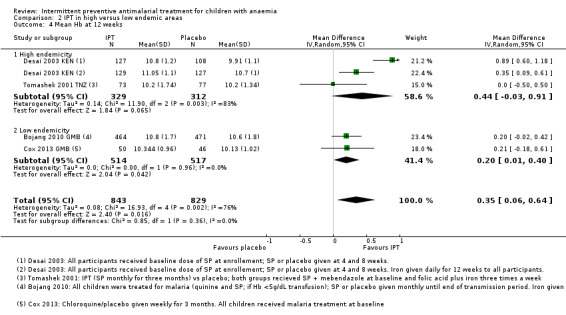
Comparison 2 IPT in high versus low endemic areas, Outcome 4 Mean Hb at 12 weeks.
Subgroup analysis further stratifying subgroups according to recruitment (hospital admission versus selected from community) did not show a difference between groups and we thus combined hospital admissions and community recruitments in the main analyses.
We did not include one intervention arm of Tomashek 2001 TNZ, where children received SP plus VAC, in the meta‐analysis. The mean Hb in these children was not different from the mean Hb in children that only received SP (one trial, 138 participants, Analysis 3.3).
3.3. Analysis.
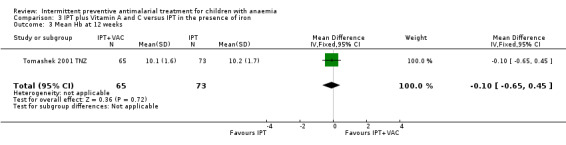
Comparison 3 IPT plus Vitamin A and C versus IPT in the presence of iron, Outcome 3 Mean Hb at 12 weeks.
Discussion
Summary of main results
The included trials did not demonstrate a difference in mortality or hospital admissions at six months when administering IPT, compared to not administering IPT to anaemic children living in malaria‐endemic areas (moderate quality evidence); and also did not demonstrate a difference in the prevalence of anaemia at 12 weeks amongst children that received IPT compared to those that did not receive IPT (moderate quality evidence). IPT for anaemic children living in malaria‐endemic areas probably increases the mean change in Hb levels from baseline to follow‐up at 12 weeks (moderate quality evidence); and may improve Hb levels at 12 weeks (low quality evidence).
Overall completeness and applicability of evidence
All six included trials were conducted in malaria‐endemic areas, four trials in seasonal and two in perennial malaria transmission areas. Three of the six trials were conducted in areas of low endemicity. Anaemic children were recruited either in hospital, where they were treated for severe anaemia, or in the community through a screening procedure. Hb levels considered eligible for inclusion in a study differed across trials. While some trials only recruited children with severe anaemia (Hb 5.0 to 8.0 g/dL; or Hb < 7 g/dL), others only included children with mild anaemia (Hb 7.0 to 10.9 g/dL). In all but two trials, children received treatment for either anaemia or malaria before randomization, which might explain why the subsequent effect of IPT was not very big. We found a small, statistically significant effect on mean Hb levels at follow‐up, and on mean change in Hb from baseline to follow‐up. However, an increase in Hb levels of 0.32 g/dL at twelve weeks is too small to add a clinically significant effect on children with moderate or severe anaemia.
In the past decade, a decrease in malaria endemicity has been recorded in most of sub‐Saharan Africa. There has been a significant decline in malaria prevalence which coincides with a decline in the transmission potentials measured through the recent P. falciparum infectious inoculations rates downturn by the Anopheles mosquito (Killeen 2007). These in turn have resulted in a further downturn in new malaria infections that may have contributed to the recent shift in anaemia morbidity decline in settings with malaria transmission (Kabanywanyi 2012). Malaria mortality and cumulative probability of deaths have thus also continued to decline steadily (Rumisha 2014). A combination of many malaria interventions have resulted in these reductions, including improved access to effective antimalarial combination therapy, vector control using LLINs and indoor residual spraying as well as intermittent presumptive treatments in infants and pregnant women (Alba 2014).
The results of this Cochrane Review thus need to be interpreted in light of these changes in malaria endemicity. Of the included trials, only three were published in the last five years (Bojang 2010 GMB; Cox 2013 GMB; Phiri 2012 MWI), with two of these trials having been conducted in The Gambia, where malaria endemicity is regarded as being low. The remaining trials were all published over ten years ago; one of these in an area of high malaria endemicity (Desai 2003 KEN) and one where, at the time of the trial, malaria endemicity was moderate to high (Tomashek 2001 TNZ). There could be larger effects of IPT in areas where malaria endemicity is higher.
In addition, the mild effect of IPT in improving Hb levels may be associated with the fact that there are other causes for severe anaemia apart from malaria. Calis 2008 found additional associations between severe anaemia and bacteraemia, hookworm, HIV infection, and Vitamin A and B12 deficiency. They also found an inverse association between iron deficiency and severe anaemia.
Quality of the evidence
We assessed the quality of the body of evidence by using the GRADE approach (Guyatt 2011). We made judgements on the quality of evidence for each outcome by looking at trial limitations, inconsistency, imprecision, indirectness and the likelihood of publication bias (Balshem 2011). Our results show that there is moderate quality evidence that IPT did not have an effect on death or hospital admissions at six months; and anaemia at 12 weeks; and that the mean change in Hb from baseline to 12 weeks was slightly higher amongst children receiving IPT. This means that, when looking at these three outcomes, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. We found low quality evidence that the mean Hb level at follow‐up was slightly higher amongst children receiving IPT, meaning that further research is very likely to have an important impact on our confidence in the estimate of the effect and is likely to change the estimate. We have presented the reasons for downgrading the quality of evidence in the footnotes of Table 1.
Potential biases in the review process
We attempted to minimise bias in the review process by conducting a comprehensive search of published and unpublished literature, without language restrictions. Two review authors independently screened abstracts, extracted data and assessed risk of bias. We resolved any discrepancies by involving a third party. We were unable to create funnel plots to assess reporting biases, since less than 10 RCTs met the inclusion criteria.
Agreements and disagreements with other studies or reviews
We are not aware of another review on the treatment of anaemic children with IPT, but two recent reviews on IPT for malaria (Meremikwu 2012; Wilson 2011) included anaemia as one of the outcomes. Although the included children in these reviews did not necessarily have anaemia at enrolment, our findings on the prevalence of anaemia resonate with those of Wilson 2011 (RR 0.84, 95% CI 0.59 to 1.21), but are contradictory to those of Meremikwu 2012. Meremikwu 2012 found a reduced risk of moderate anaemia (RR 0.71, 95% CI 0.52 to 0.98; 8805 participants, five trials) as well as a reduced risk of severe anaemia (RR 0.24, 95% CI 0.06 to 0.94; 5964 participants, two trials) in children with malaria receiving IPT compared to those not receiving IPT. The markedly reduced risk of anaemia in Meremikwu 2012 could be due to subgroup analysis (severe and moderate anaemia groups) which was not done in Wilson 2011. Neither of the reviews found a significant difference in Hb levels at the end of follow‐up in children receiving IPT compared to children not receiving IPT (Meremikwu 2012; Wilson 2011). We found a small, statistically significant effect of IPT on Hb levels.
Authors' conclusions
Implications for practice.
The trials did not demonstrate a difference in all‐cause mortality and hospital admissions at six months; and prevalence of anaemia at 12 weeks amongst children that received IPT compared to those that did not receive IPT. IPT probably increases the Hb levels of anaemic children. Despite the small benefits of IPT on Hb levels of anaemic children, it does not warrant routine administration of IPT to anaemic children. However, one needs to take into consideration that the majority of trials were conducted in low endemicity areas, where any effect is likely to be modest.
Implications for research.
Only one trial was adequately powered to detect a difference in the risk of all‐cause mortality and hospital admissions due to severe anaemia or severe malaria. Future trials should be adequately powered to detect differences in patient‐orientated outcomes (for example, all‐cause mortality) and consideration should be given to malaria endemicity.
Acknowledgements
We thank Paul Garner, Dave Sinclair, Marty Richardson, Taryn Young and Vittoria Lutje for their comments and support. The editorial base of the Cochrane Infectious Diseases Group is funded by UKaid from the UK Government for the benefit of developing countries.
AR is supported in part by the Effective Health Care Research Consortium, which is funded by UKaid from the UK Government.
Appendices
Appendix 1. Search strategy
| Search set | CIDG SR1 | CENTRAL | MEDLINE2 | EMBASE2 | LILACS2 |
| 1 | Anemia OR anaemia | Anemia (MeSH, ti, ab ) | Anemia (MeSH, ti, ab ) OR anaemia (ti, ab) | Anemia (Emtree, ti, ab ) OR anaemia (ti, ab) | Anemia OR anaemia |
| 2 | malaria | Malaria (MeSH, ti,ab) | Malaria (MeSH, ti,ab) | Malaria (Emtree, ti,ab) | malaria |
| 3 | Child* OR infant* | 1 OR 2 | 1 OR 2 | 1 OR 2 | Child$ OR infant$ |
| 4 | (Intermittent Preventive treatment) OR IPT* | Child* OR infant*(ti,ab) | Child* OR infant*(ti,ab) | Child* OR infant*(ti,ab) | (Intermittent Preventive treatment) OR IPT$ |
| 5 | 1 or 2 | 3 and 4 | 3 and 4 | 3 and 4 | 1 or 2 |
| 6 | 3 and 4 and 5 | (Intermittent preventive treatment) ti, ab | (Intermittent preventive treatment) ti, ab | (Intermittent preventive treatment) ti, ab | 3 and 4 and 5 |
| 7 | ‐ | IPT*(ti,ab) | IPT*(ti,ab) | IPT*(ti,ab) | |
| 8 | ‐ | 6 or 7 | 6 or 7 | 6 or 7 | |
| 9 | ‐ | 5 and 8 | 5 and 8 | 5 and 8 | |
| 10 | ‐ | ‐ | ‐ | ‐ |
1Cochrane Infectious Diseases Group Specialized Register.
2Search terms used in combination with the search strategy for retrieving trials developed by The Cochrane Collaboration (Lefebvre 2011).
Data and analyses
Comparison 1. IPT versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality plus hospital admissions at 6 months | 3 | 3160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.71, 1.13] |
| 1.1 Iron | 2 | 1474 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.56, 1.67] |
| 1.2 No iron | 2 | 1686 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.68, 1.14] |
| 2 Children with anaemia at 12 weeks | 4 | 2237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.88, 1.07] |
| 2.1 Iron | 4 | 1801 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.74, 1.02] |
| 2.2 No iron | 2 | 436 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.96, 1.23] |
| 3 Mean change in Hb (baseline to 12 weeks) | 4 | 1672 | Mean Difference (IV, Fixed, 95% CI) | 0.32 [0.19, 0.45] |
| 3.1 Iron | 3 | 1341 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [0.15, 0.45] |
| 3.2 No iron | 2 | 331 | Mean Difference (IV, Fixed, 95% CI) | 0.38 [0.12, 0.65] |
| 4 Mean Hb at 12 weeks | 4 | 1672 | Mean Difference (IV, Random, 95% CI) | 0.35 [0.06, 0.64] |
| 4.1 Iron | 3 | 1341 | Mean Difference (IV, Random, 95% CI) | 0.24 [0.08, 0.40] |
| 4.2 No iron | 2 | 331 | Mean Difference (IV, Random, 95% CI) | 0.57 [‐0.10, 1.23] |
Comparison 2. IPT in high versus low endemic areas.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All cause mortality plus hospital admissions at 6 months | 3 | 3160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.71, 1.13] |
| 1.1 High endemicity | 2 | 1960 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.71, 1.19] |
| 1.2 Low endemicity | 1 | 1200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.43, 1.44] |
| 2 Children with anaemia at 12 weeks | 4 | 2237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.88, 1.07] |
| 2.1 High endemicity | 2 | 709 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.90, 1.15] |
| 2.2 Low endemicity | 2 | 1528 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.74, 1.07] |
| 3 Mean change in Hb (baseline to 12 weeks) | 4 | 1672 | Mean Difference (IV, Fixed, 95% CI) | 0.32 [0.19, 0.45] |
| 3.1 High endemicity | 2 | 641 | Mean Difference (IV, Fixed, 95% CI) | 0.35 [0.17, 0.53] |
| 3.2 Low endemicity | 2 | 1031 | Mean Difference (IV, Fixed, 95% CI) | 0.29 [0.10, 0.48] |
| 4 Mean Hb at 12 weeks | 4 | 1672 | Mean Difference (IV, Random, 95% CI) | 0.35 [0.06, 0.64] |
| 4.1 High endemicity | 2 | 641 | Mean Difference (IV, Random, 95% CI) | 0.44 [‐0.03, 0.91] |
| 4.2 Low endemicity | 2 | 1031 | Mean Difference (IV, Random, 95% CI) | 0.20 [0.01, 0.40] |
Comparison 3. IPT plus Vitamin A and C versus IPT in the presence of iron.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Children with anaemia at 12 weeks | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.75, 1.23] |
| 2 Mean change in Hb (baseline to 12 weeks) | 1 | 138 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.48, 0.48] |
| 3 Mean Hb at 12 weeks | 1 | 138 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.65, 0.45] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bojang 2010 GMB.
| Methods |
Trial design: Individually randomized, controlled double‐blind trial Multicentre trial: Yes Trial duration: 2 years |
|
| Participants |
Recruitment: Children presenting to the out‐patient clinic or ward at the Royal Teaching Hospital, Banjul; Medical Research Council Hospital, Fajara; and major health centres at Birkama, Essau and Faji Kunda during 2003 and 2004 transmission period (July to December), Sibanor was added during the 2004 period. Inclusion criteria:
Other co‐morbidities: Not reported Sample size: 1200 enrolled |
|
| Interventions |
Total number of intervention groups: 2 Presumptive treatment for all participants:
Interventions:
Dose and timing of intervention:
Duration of intervention period: until the end of the transmission season (July to December) Place and person delivering intervention:
Co‐interventions:
Additional treatments:
Co‐interventions equal in each arm? (if not, describe): Yes |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
Measurement time points:
How were outcomes assessed?
Interviews with mothers at end of dry season |
|
| Notes |
Country: The Gambia Setting: Urban and peri‐urban Transmission area: Seasonal transmission Source of funding: Gates Malaria partnership Conflict of interest stated: Authors state that they have no competing interests |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "children were individually randomised into either the SP or the placebo group in a 1:1 ratio at the time of admission, using permuted blocks of 12 generated by computer using the STATA program. Blockswere not split across centres". |
| Allocation concealment (selection bias) | Low risk | "Tablets (enough for 6 doses) were packed into envelopes bearing the randomisation number by MRC staff not involved in the trial in any other way. The next envelope in sequence was assigned to the child at the time of their admission to hospital". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "None of the investigators, health care centre staff or laboratory staff participating in the trial had access to the code during the trial". Placebo and active tablet were identical in shape and colour. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "None of the investigators, health care centre staff or laboratory staff participating in the trial had access to the code during the trial". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up was similar in both groups (SP: 23%; placebo: 21.5%), but was over 20% in each group. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported and checked with protocol. |
| Other bias | Low risk | Discrepancy between text and flow‐chart regarding number of children seen at follow‐up. |
Cox 2013 GMB.
| Methods |
Trial design: Population‐based RCT (proof of concept study) Multicentre trial: No Trial duration: 2007 to 2008 |
|
| Participants |
Recruitment: Eligible children identified through active and passive malaria surveillance in participating communities (West Kiang district, lower river region, The Gambia) Inclusion criteria:
Exclusion criteria:
Other co‐morbidities: Not reported Sample size: Enrolled into trial: 132; randomized to receive IPT/placebo: 96 |
|
| Interventions |
Baseline treatment for all children:
Total number of intervention groups: 2
Dose and timing of intervention:
Duration of intervention period: 90 days (12 weeks) Place and person delivering intervention:
Co‐interventions:
Additional treatments:
Children with positive tests were treated with either CQ/SP (2007) or ACT (2007 and 2008) Co‐interventions equal in each arm? (if not, describe): Yes |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
Measurement time points:
How were outcomes assessed?
|
|
| Notes |
Country: The Gambia Setting: Rural Transmission area: Seasonal transmission Source of funding: UK Medical Research Council Conflict of interest stated: Authors stated no competing interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The block randomisation to the post‐malaria treatment of weekly CQ or placebo in both 2007 and 2008 was double blinded and was carried out in blocks of eight. The randomisation codes were generated by a staff member independent of the study team and held by the external trial monitor". |
| Allocation concealment (selection bias) | Low risk | "Treatment codes were labelled A to H and placed in sequentially numbered, opaque, sealed envelopes held by the study nurses. Allocation to the treatment was by matching the code in the envelope to a bottle of the intervention labelled with the same code and then labelled with the subject ID". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both participants and personnel were blinded, intervention and placebo syrups were in similar amber‐coloured bottles with matching caps and labels. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinded trial, treatment codes held by external trial monitor. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for, loss to follow‐up: 2% in IPT group; 7% in placebo group. All lost due to second malaria episode. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported on. |
| Other bias | Low risk | No other sources of bias identified. |
Desai 2003 KEN.
| Methods |
Trial design: RCT with 2 X 2 factorial design Multicentre trial: no Trial duration: Children were screened between April to November 1999 |
|
| Participants |
Recruitment: All resident children in 15 villages in Asembo, Bondo district, Kenya were screened Inclusion criteria:
Other co‐morbidities: Not reported Sample size: 554 participants randomized; 546 enrolled; 491 followed up at 12 weeks; 468 followed up at 24 weeks. |
|
| Interventions |
Total number of intervention groups: 4 Presumptive treatment for all participants: single dose of SP (500 mg sulphadoxine and 25 mg pyrimethamine per tablet). Children ≤ 10kg received half a tablet, children > 10kg received one tablet
Dose, and timing of intervention: IPT with SP (or placebo) at 4 and 8 weeks; iron (or placebo) given daily for 12 weeks (3 to 6 mg/kg/day, orally). IPT given as crushed tablets mixed with water. Duration of intervention period: 12 weeks Place and person delivering intervention:
Co‐interventions:
Additional treatments: Children with symptomatic malaria (temp ≥ 37.5°C with any malaria parasitaemia or parasitaemia > 5000 parasites/mm3) received oral quinine (10 mg/kg, 3 times/day for 7 days). Children who developed severe malaria, severe anaemia (Hb < 5.0g/dL) or other severe disease requiring hospitalization were referred for further treatment. Co‐interventions equal in each arm? (if not, describe): Yes |
|
| Outcomes |
Outcomes not specified according to primary and secondary outcomes:
Measurement time points: Every 4 weeks How were outcomes assessed?
|
|
| Notes |
Country: Western Kenya Setting: Rural Transmission area: Perennial transmission Source of funding: US agency for International Development, Netherlands Foundation for the advancement of Tropical research Conflict of interest stated: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Balanced block randomisation (8 children/block) and a random number listing generated independently before the study". |
| Allocation concealment (selection bias) | Unclear risk | Children were assigned to 1 of the 4 groups sequentially according to the random number listing by one author. Drugs and placebos were identical. Code to true drug and placebo assignment was revealed only after completion of analysis. Still unclear whether allocation was concealed sufficiently – did they use numbered envelopes or bottles? |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo and trial drugs were identical; participants (or their mothers) and staff administering the drugs were thus not aware of the study group. The code was only broken after data analysis. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo and trial drugs were identical; staff assessing outcomes were thus not aware of the trial group. The code was only broken after data analysis. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up at 12 weeks: 4% (IPT + iron), 8.6% (iron), 6.6% (IPT) and 20.5% (double placebo). Reported reason: mostly migration (23/28 for double placebo group), loss to follow‐up at 24 weeks – data missing. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes not listed in Methods section (protocol?). |
| Other bias | Low risk | No. |
Phiri 2012 MWI.
| Methods |
Trial design: Randomized double‐blind, placebo‐controlled trial Multicentre trial: Yes Trial duration: June 2006 to August 2009 |
|
| Participants |
Recruitment: Children were recruited from four hospital in southern Malawi: Queen Elizabeth Central hospital (Blantyre), Chikwawa District hospital, Thyolo District hospital, Zomba Central hospital. Inclusion criteria:
Exclusion criteria:
Other co‐morbidities: 8% of children were infected with HIV Sample size: 1431 randomized, 1414 allocated to groups; analysed 4310 |
|
| Interventions |
Presumptive treatment for all participants: Six doses of AL as part of the standard 3 day course in hospital. Children < 15 kg received one tablet; children > 15 kg received 2 tablets, once every 12 hours for 3 days. Total number of intervention groups: 2
Dose and timing of intervention:
Duration of intervention period: 3 months Place and person delivering intervention:
Co‐interventions:
Additional treatments:
Co‐interventions equal in each arm? (if not, describe): Yes |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
Measurement time points:
How were outcomes assessed?
|
|
| Notes |
Country: Malawi Setting: Urban/peri‐urban/rural Transmission area: Perennial Source of funding: Netherlands African Partnership for Capacity Development and Clinical Interventions against Poverty‐related Diseases; UBS Optimus foundation; Gates Malaria Partnership. Conflict of interest stated: Authors declared no conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list of random numbers, "stratified by hospital and weight group (< 15 kg and 15 kg or more) in randomly varying block sizes of two, four, or six". |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered envelopes containing AL or placebo. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Authors did not mention that placebo and AL were identical tablets, but described the trial as "double‐blind". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Masking was maintained and the code only broken once all data sets were closed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for. Loss to follow‐up rates similar across groups (7% at 6 months). |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported on. |
| Other bias | Low risk | No. |
Tomashek 2001 TNZ.
| Methods |
Trial design: Randomized double‐blind trial Multicentre trial: No Trial duration: March 1998 to May 1998 |
|
| Participants |
Recruitment: Children living in refugee camp diagnosed with clinical anaemia Inclusion criteria:
Exclusion criteria
Other co‐morbidities: Hookworm Sample size: 238 randomized, 215 analysed |
|
| Interventions |
Presumptive treatment for all participants:
Total number of intervention groups: 3
Dose, and timing of intervention:
Duration of intervention period: 3 months Place and person delivering intervention:
Co‐interventions:
Additional treatments:
Co‐interventions equal in each arm? (if not, describe): No |
|
| Outcomes |
Outcomes not specified as primary or secondary outcomes
Measurement time points:
How were outcomes assessed?
|
|
| Notes |
Country: Tanzania Setting: Rural Transmission area: Not reported Source of funding: Woodruff Foundation, Atlanta, Georgia. Conflict of interest stated: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization list. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Personnel distributing medications had access to the list of group assignment; group 1 did not receive SP placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The study coordinator and the study nurse, who were responsible for distributing medications, were the only team members with access to the register containing participants' names and group assignment. All other research team members were blinded to participants’ treatment group assignment". Laboratory workers were not aware of group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All participants accounted for. Loss to follow‐up rates: Group 1: 6%, Group 2: 10%; Group 3: 13% reasons for loss to follow‐up not stratified according to groups. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported on. |
| Other bias | Low risk | No. |
Verhoef 2002 KEN.
| Methods |
Trial design: Double‐blind, placebo controlled trial with a 2x2 factorial design Multicentre trial: No Trial duration: 1998 to 2000 |
|
| Participants |
Recruitment: Children were recruited (randomly selected) from communities neighbouring the research clinic in the Mtito Andei Division, Eastern Province, Kenya at the start of the rainy season Inclusion criteria:
Other co‐morbidities: Not reported Sample size: 328 randomized, 307 analysed |
|
| Interventions |
Total number of intervention groups: 4
Dose, and timing of intervention:
Duration of intervention period: 12 weeks Place and person delivering intervention:
Co‐interventions:
Additional treatments:
Co‐interventions equal in each arm? (if not, describe): Yes |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
Measurement time points:
How were outcomes assessed?
Surveillance and monitoring of illness episodes and adverse effects at twice weekly meetings with community healthworker (questionnaires) |
|
| Notes |
Country: Kenya Setting: Unclear – rural? Transmission area: Seasonal transmission Source of funding: Netherlands foundation for the Advancement of Tropical Research Conflict of interest stated: Declared no conflicts. Author contacted for follow‐up Hb concentration values |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The allocation schedule was generated by one of us (HV) for each block, by means of tables with randomised permutations, and only after acceptance of all children making up a block". |
| Allocation concealment (selection bias) | Unclear risk | "The order of the children listed in each block was concealed from the person generating the allocation schedule" – unclear, was this not the same person? |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded, placebos and active compounds were indistinguishable in taste and appearance. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | None of the field investigators was aware of the code until after crude analysis and a plan for further analysis had been prepared. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for. Loss to follow‐up rates similar across groups. |
| Selective reporting (reporting bias) | High risk | Do not report on mean Hb concentrations (primary outcome), only on the difference in Hb concentration between groups. |
| Other bias | Low risk | No. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bojang 1997 GMB | Intervention is chemoprophylaxis, not IPT. |
| Browne 2005 | Anaemia not part of inclusion criteria ‐ study assessed effects on preventing anaemia. |
| Grobusch 2007 | Anaemia not part of inclusion criteria. |
| Kweku 2008 | Anaemia not part of inclusion criteria. |
| Massaga 2003 | Anaemia not part of the inclusion criteria. |
| Nakibuuka 2009 | No control group ‐ comparing two malaria treatment regimes. |
| Rohner 2010 | Anaemia not part of the inclusion criteria. |
| Terlouw 2004 | Subanalysis of Desai 2003 KEN. |
Differences between protocol and review
1. We planned to include only children under the age of five (as stated in protocol), but when screening the studies, we noticed that some of them included children up to the age of nine years and we thus decided to include all children.
2. In the protocol, we prespecified the following outcomes:
Primary outcomes:
Mean Hb at follow‐up (g/dL)
Mean change in Hb from baseline at follow‐up
Secondary outcomes:
Complete recovery from severe anaemia (Hb > 5 g/dL)
Complete recovery from moderate anaemia (Hb > 8 g/dL)
Blood transfusions
All‐cause mortality
Admission due to severe anaemia
During our fellowship (MK and AR) at the editorial base of the Cochrane Infectious Diseases Group (CIDG) in Liverpool, we discussed these outcomes with some of the CIDG editors. We also consulted Higgins 2011 and agreed that mortality and hospital admission would be more meaningful outcomes to clinicians and decision‐makers, than mean Hb levels. We thus changed the primary and secondary outcomes to the following:
Primary outcomes:
All‐cause mortality and hospital admission
Secondary outcomes:
Anaemia at follow‐up (Hb < 11 g/dL)
Mean change in Hb (g/dL) from baseline to follow‐up
Mean Hb at follow‐up (g/dL)
3. We planned to perform the following subgroup analyses:
IPTi versus IPTc
Additional interventions to treat anaemia (such as, hematinics or folic acid)
The use of LLINs or not
Due to the lack of heterogeneity and the limited amount of included trials, we only performed the following subgroup analyses:
Additional interventions to treat anaemia (such as, hematinics or folic acid)
Hospital recruitment versus community recruitment
4. Due to the limited number of trials included in the analyses, we did not do sensitivity analyses.
Contributions of authors
MA, AR and AMK developed the protocol. MA and AR screened search outputs, selected trials for inclusion, extracted data, assessed risk of bias, analysed data and prepared the draft manuscript. AMK critically engaged with the manuscript and provided comments. All review authors have seen and approved the final manuscript.
Sources of support
Internal sources
Centre for Evidence‐based Health Care, Stellenbosch University, South Africa.
Ifakara Health Institute, Tanzania.
Liverpool School of Tropical Medicine, UK.
External sources
Effective Health Care Research Consortium, UK.
Department for International Development (DfID), UK.
Declarations of interest
The review authors have no known conflicts of interest.
Unchanged
References
References to studies included in this review
Bojang 2010 GMB {published data only}
- Bojang KA, Milligan PJM, Conway DJ, Sisay‐Joof F, Jallow M, Nwakanma DC, et al. Prevention of the recurrence of anaemia in Gambian children following discharge from hospital. PLoS ONE 2010;5(6):e11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cox 2013 GMB {published data only}
- Cox SE, Nweneka CV, Doherty CP, Fulford AJ, Moore SE, Prentice AM. Randomised controlled trial of weekly chloroquine to re‐establish normal erython iron flux and haemoglobin recovery in postmalarial anaemia. BMJ Open 2013;3(7):e002666. [DOI] [PMC free article] [PubMed] [Google Scholar]
Desai 2003 KEN {published data only}
- Desai MR, Mei VJ, Kariuki SK, Wannemuehler KA, Phillips‐Howard PA, Nahlen BL, et al. Randomized, controlled trial of daily iron supplementation and intermittent sulfadoxine‐pyrimethamine for the treatment of mild childhood anaemia in Western Kenya.. Journal of Infectious Diseases 2003;187(4):658‐66. [DOI] [PubMed] [Google Scholar]
Phiri 2012 MWI {published data only}
- Phiri K, Esan M, Hensbroek MB, Khairallah C, Faragher B, ter Kuile FO. Intermittent preventive therapy for malaria with monthly artemether‐lumefantrine for the post‐discharge management of severe anaemia in children aged 4‐59 months in southern Malawi: a multicentre, randomised, placebo‐controlled trial.. Lancet Infectious Diseases 2012;12(3):191‐200. [DOI] [PubMed] [Google Scholar]
Tomashek 2001 TNZ {published data only}
- Tomashek KM, Woodruff BA, Gotway CA, Bloland P, Mbaruku G. Randomised intervention study comparing several regimens for the treatment of moderate anemia among refugee children in Kigoma Region, Tanzania. American Journal of Tropical Medicine and Hygiene 2001;64(3‐4):164‐71. [DOI] [PubMed] [Google Scholar]
Verhoef 2002 KEN {published data only}
- Verhoef H, West CE, Nzyuko SM, Vogel S, Valk R, Wanga MA, et al. Intermittent administration of iron and sulfadoxine‐pyrimethamine to control anaemia in Kenyan children: a randomised controlled trial. Lancet 2002;360(9337):908‐14. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bojang 1997 GMB {published data only}
- Bojang KA, Palmer A, Boele van Hensbroek M, Banya WAS, Greenwood BM. Management of severe malarial anaemia in Gambian children. Transactions of the Royal Society of Tropical Medicine and Hygiene 1997;91(5):557‐61. [DOI] [PubMed] [Google Scholar]
Browne 2005 {published data only}
- Browne E, Bam V, Agyei‐Baffour P, Boateng S, Sawyerr P, Mensah C, et al. Intermittent malaria treatment and iron supplementation for control of malaria and anaemia in infants in forest belt of Ghana: a randomised trial. 4th MIM Pan‐African Malaria Conference; 13‐19 Nov 2005; Yaoundé, Cameroon. 2005.
Grobusch 2007 {published data only}
- Grobusch MP, Lell B, Schwarz NG, Gabor J, Dörnemann J, Pötschke M, et al. Intermittent preventive treatment against malaria in infants in Gabon—a randomized, double‐blind, placebo‐controlled trial. Journal of Infectious Diseases 2007;196(11):1595–602. [DOI] [PubMed] [Google Scholar]
Kweku 2008 {published data only}
- Kweku M, Liu D, Adjuik M, Binka F, Seidu M, Greenwood B, et al. Seasonal intermittent preventive treatment for the prevention of anaemia and malaria in Ghanaian children: a randomized, placebo controlled trial. PLoS ONE 2008;3(12):e4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Massaga 2003 {published data only}
- Massaga JJ, Kitua AY, Lemnge MM, Akida JA, Malle LN, Rønn AM, et al. Effect of intermittent treatment with amodiaquine on anaemia and malarial fevers in infants in Tanzania: a randomised placebo controlled trial. Lancet 2003;361(9372):1853–60. [DOI] [PubMed] [Google Scholar]
Nakibuuka 2009 {published data only}
- Nakibuuka V, Ndeezi G, Nakiboneka D, Ndugwa CM, Tumwine JK. Presumptive treatment with sulphadoxine‐pyrimethamine versus weekly chloroquine for malaria prophylaxis in children with sickle cell anaemia in Uganda: a randomized controlled trial. Malaria Journal 2009;8:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rohner 2010 {published data only}
- Rohner F, Zimmermann MB, Amon RJ, Vounatsou P, Tschannen AB, N'Goran EK, et al. In a randomized controlled trial of iron fortification, anthelmintic treatment, and intermittent preventive treatment of malaria for anemia control in Ivorian children, only anthelmintic treatment shows modest benefit. Journal of Nutrition 2010;140(3):635–41. [DOI] [PubMed] [Google Scholar]
Terlouw 2004 {published data only}
- Terlouw DJ, Desai MR, Wannemuehler KA, Kariuki SK, Pfeiffer CM, Kager PA, et al. Relation between the response to iron supplementation and sickle cell hemoglobin phenotype in preschool children in western Kenya. American Journal of Clinical Nutrition 2004;79(3):466‐72. [DOI] [PubMed] [Google Scholar]
Additional references
Akech 2008
- Akech SO, Hassall O, Pamba A, Idro R, Williams TN, Newton CR, et al. Survival and haematological recovery of children with severe malaria transfused in accordance to WHO guidelines in Kilifi, Kenya. Malaria Journal 2008;7:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alba 2014
- Alba S, Nathan R, Schulze A, Mshinda H, Lengeler C. Child mortality patterns in rural Tanzania: an observational study on the impact of malaria control interventions. International Journal of Epidemiology 2014;43(1):204‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Antony 2008
- Antony AC. Severe anemia in Malawian children. New England Journal of Medicine 2008;358(21):2291; author reply 2291. [PubMed] [Google Scholar]
Balarajan 2011
- Balarajan Y, Ramakrishnan U, Ozaltin E, Shankar AH, Subramanian SV. Anaemia in low‐income and middle‐income countries. Lancet 2011;378(9809):2123‐35. [DOI] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [DOI] [PubMed] [Google Scholar]
Bell 2009
- Bell DJ, Wootton D, Mukaka M, Montgomery J, Kayange N, Chimpeni P, et al. Measurement of adherence, drug concentrations and the effectiveness of artemether‐lumefantrine, chlorproguanil‐dapsone or sulphadoxine‐pyrimethamine in the treatment of uncomplicated malaria in Malawi. Malaria Journal 2009;8:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Calis 2008
- Calis JC, Phiri KS, Faragher EB, Brabin BJ, Bates I, Cuevas LE, et al. Severe anemia in Malawian children. New England Journal of Medicine 2008;358(9):888‐99. [DOI] [PubMed] [Google Scholar]
Crawley 2004
- Crawley J. Reducing the burden of anemia in infants and young children in malaria‐endemic countries of Africa: from evidence to action. American Journal of Tropical Medicine and Hygiene 2004;71(2 Suppl):25‐34. [PubMed] [Google Scholar]
Dicko 2011
- Dicko A, Diallo AI, Tembine I, Dicko Y, Dara N, Sidibe Y, et al. Intermittent preventive treatment of malaria provides substantial protection against malaria in children already protected by an insecticide‐treated bednet in Mali: a randomised, double‐blind, placebo‐controlled trial. PLoS Medicine 2011;8(2):e1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dunyo 2006
- Dunyo S, Ord R, Hallett R, Jawara M, Walraven G, Mesa E, et al. Randomised trial of chloroquine/sulphadoxine‐pyrimethamine in Gambian children with malaria: impact against multidrug‐resistant P. falciparum. PLoS Clinical Trials 2006;1(3):e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gething 2011
- Gething PW, Patil AP, Smith DL, Guerra CA, Elyazar IRF, Johnston GL, et al. A new world malaria map: Plasmodium falciparum endemicity in 2010. Malaria Journal 2011;10:378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gorissen 2000
- Gorissen E, Ashruf G, Lamboo M, Bennebroek J, Gikunda S, Mbaruku G, et al. In vivo efficacy study of amodiaquine and sulfadoxine/pyrimethamine in Kibwezi, Kenya and Kigoma, Tanzania. Tropical Medicine & International Health 2000;5(6):459‐63. [DOI] [PubMed] [Google Scholar]
Grantham‐McGregor 2001
- Grantham‐McGregor S, Ani C. A review of studies on the effect of iron deficiency on cognitive development in children. Journal of Nutrition 2001;131(2S‐2):649S‐68S. [DOI] [PubMed] [Google Scholar]
Greenwood 2006
- Greenwood B. Review: Intermittent preventive treatment ‐ a new approach to the prevention of malaria in children in areas with seasonal malaria transmission. Tropical Medicine & International Health 2006;11(7):983‐91. [DOI] [PubMed] [Google Scholar]
Greenwood 2010
- Greenwood B. Anti‐malarial drugs and the prevention of malaria in the population of malaria endemic areas. Malaria Journal 2010;9(Suppl 3):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI] [PubMed] [Google Scholar]
Haildar 2009
- Haildar K, Mohandas N. Malaria,erthrocytic infection and anaemia. Hematology Am. Soc Hematol Educ Program 2009;10(1):87‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. The Cochrane Collaboration. Available from www.cochrane‐handbook.org.
Jelinek 1997
- Jelinek T, Rønn AM, Curtis J, Duraisingh MT, Lemnge MM, Mhina J, et al. High prevalence of mutations in the dihydrofolate reductase gene of Plasmodium falciparum in isolates from Tanzania without evidence of an association to clinical sulfadoxine/pyrimethamine resistance. Tropical Medicine & International Health 1997;2(11):1075‐9. [DOI] [PubMed] [Google Scholar]
Kabanywanyi 2012
- Kabanywanyi AM. Pharmaco‐Epidemiology of Artemisinin‐Based Combination Therapy in the Context of Impact Evaluation of Artemether‐Lumefantrine on Malaria Morbidity and Mortality During Programmatic Implementation in Rural Tanzania [PhD thesis]. Basel, Switzerland: University of Basel, 2012. [Google Scholar]
Kalter 1997
- Kalter HD, Burnham G, Kolstad PR, Hossain M, Schillinger JA, Khan NZ, et al. Evaluation of clinical signs to diagnose anaemia in Uganda and Bangladesh, in areas with and without malaria. Bulletin of the World Health Organization 1997;75(Suppl 1):103‐11. [PMC free article] [PubMed] [Google Scholar]
Killeen 2007
- Killeen GF, Tami A, Kihonda J, Okumu FO, Kotas ME, Grundmann H, et al. Cost‐sharing strategies combining targeted public subsidies with private‐sector delivery achieve high bed net coverage and reduced malaria transmission in Kilombero Valley, southern Tanzania. BMC Infectious Diseases 2007;7:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Search for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lengeler 2009
- Lengeler C. Insecticide‐treated bed nets and curtains for preventing malaria. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD000363.pub2] [DOI] [PubMed] [Google Scholar]
Looareesuwan 1987
- Looareesuwan S, Merry AH, Phillips RE, Pleehachinda R, Wattanagoon Y, Ho M, et al. Reduced erythrocyte survival following clearance of malarial parasitaemia in Thai patients. British Journal of Haematology 1987;67(4):473‐8. [DOI] [PubMed] [Google Scholar]
Lozoff 1991
- Lozoff B, Jimenez E, Wolf AW. Long‐term developmental outcome of infants with iron deficiency. New England Journal of Medicine 1991;325(10):687‐94. [DOI] [PubMed] [Google Scholar]
Meremikwu 2009
- Meremikwu M, Ehiri JE. Chapter 27: Integrated management of childhood illness. Maternal and child health: global challenges, programs and policies. New York: Springer, 2009:497‐514. [Google Scholar]
Meremikwu 2012
- Meremikwu M, Donegan S, Sinclair D, Esu E, Oringanje C. Intermittent preventive treatment for malaria in children living in areas with seasonal transmission. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD003756.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Obonyo 2007
- Obonyo CO, Vulule J, Akhwale WS, Grobbee DE. In‐hospital morbidity and mortality due to severe malarial anemia in western Kenya. American Journal of Tropical Medicine and Hygiene 2007;77(6 Suppl):23‐8. [PubMed] [Google Scholar]
Okebe 2011
- Okebe JU, Yahav D, Shbita R, Paul M. Oral iron supplements for children in malaria‐endemic areas. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD006589.pub3] [DOI] [PubMed] [Google Scholar]
Ord 2007
- Ord R, Alexander N, Dunyo S, Hallett R, Jawara M, Targett G, et al. Seasonal carriage of pfcrt and pfmdr1 alleles in Gambian Plasmodium falciparum imply reduced fitness of chloroquine‐resistant parasites. Journal of Infectious Diseases 2007;196(11):1613‐9. [DOI] [PubMed] [Google Scholar]
Phillips 1992
- Phillips RE, Pasvol G. Anaemia of Plasmodium falciparum malaria. Baillière's Clinical Haematology 1992;5(2):315‐30. [DOI] [PubMed] [Google Scholar]
Phiri 2008
- Phiri KS, Calis JCJ, Faragher B, Nkhoma E, Ng'oma K, Mangochi B, et al. Long term outcome of severe anaemia in Malawian children. PLoS One 2008;3(8):e2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Phiri 2011
- Phiri K, Esan M, Hensbroek MB, Khairallah C, Faragher B, ter Kuile FO. Intermittent preventive therapy for malaria with monthly artemether‐lumefantrine for the post‐discharge management of severe anaemia in children aged 4‐59 months in southern Malawi : a multicentre, randomised, placebo‐controlled trial. Lancet Infectious Diseases 2011;12(3):191‐200. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rumisha 2014
- Rumisha SF, Smith TA, Masanja H, Abdulla S, Vounatsou P. Relationship between child survival and malaria transmission: an analysis of the malaria transmission intensity and mortality burden across Africa (MTIMBA) project data in Rufiji demographic surveillance system, Tanzania. Malaria Journal 2014;13:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tekete 2009
- Tekete M, Djimde AA, Beavogui AH, Maiga H, Sagara I, Fofana B, et al. Efficacy of chloroquine, amodiaquine and sulphadoxine‐pyrimethamine for the treatment of uncomplicated falciparum malaria: revisiting molecular markers in an area of emerging AQ and SP resistance in Mali. Malaria Journal 2009;8:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Terlouw 2003
- Terlouw DJ, Nahlen BL, Courval JM, Kariuki SK, Rosenberg OS, Oloo AJ, et al. Sulfadoxine‐pyrimethamine in treatment of malaria in Western Kenya: increasing resistance and underdosing. Antimicrobial Agents and Chemotherapy 2003;47(9):2929‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Verhoef 2002
- Verhoef H, West CE, Nzyuko SM, Vogel S, Valk R, Wanga MA, et al. Intermittent administration of iron and sulfadoxine‐pyrimethamine to control anaemia in Kenyan children: a randomised controlled trial. Lancet 2002;360(9337):908‐14. [DOI] [PubMed] [Google Scholar]
von Seidlein 2000
- Seidlein L, Milligan P, Pinder M, Bojang K, Anyalebechi C, Gosling R, et al. Efficacy of artesunate plus pyrimethamine‐sulphadoxine for uncomplicated malaria in Gambian children: a double‐blind, randomised, controlled trial. Lancet 2000;355(9201):352‐7. [DOI] [PubMed] [Google Scholar]
White 2004
- White NJ. Antimalarial drug resistance. Journal of Clinical Investigation 2004;113(8):1084‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2000
- WHO, Communicable Diseases Cluster. Severe falciparum malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 24/07/2012;94(Suppl 1):S1‐90. [PubMed] [Google Scholar]
WHO 2006
- WHO, UNICEF. Iron supplementation of young children in regions where malaria transmission is intense and infectious disease highly prevalent. http://www.who.int/nutrition/publications/micronutrients/WHOStatement_%20iron%20suppl.pdf (23/07/2012):1‐2.
WHO 2008
- Benoist B, McLean E, Egli I, Cogswell M (editors). Worldwide prevalence of anaemia 1993‐2005. http://whqlibdoc.who.int/publications/2008/9789241596657_eng.pdf (05/02/2013):1‐40.
WHO 2010
- WHO. WHO policy recommendation on intermittent preventive treatment during infancy with sulphadoxine‐pyrimethamine (SP‐IPTi) for Plasmodium falciparum malaria control in Africa. March 2010. http://www.who.int/malaria/news/WHO_policy_recommendation_IPTi_032010.pdf (06/11/2012):1‐3.
WHO 2012a
- WHO. World Malaria Report 2012. http://www.who.int/malaria/publications/world_malaria_report_2012/report/en/index.html (30/07/2013):1‐195.
WHO 2012b
- WHO Global Malaria Programme. WHO policy recommendation: seasonal malaria chemoprevention (SMC) for Plasmodium falciparum malaria control in highly seasonal transmission areas of the Sahel sub‐region in Africa. http://www.who.int/malaria/publications/atoz/smc_policy_recommendation_en_032012.pdf (01/02/2013):1‐4.
WHO 2013
- WHO. Seasonal malaria chemoprevention with sulfadoxine–pyrimethamine plus amodiaquine in children: A field guide. August 2013. http://www.who.int/malaria/publications/atoz/9789241504737/en/ (13/09/2013):1‐56.
Wilson 2011
- Wilson AL, IPTc Taskforce. A systematic review and meta‐analysis of the efficacy and safety of intermittent preventive treatment of malaria in children (IPTc). PLoS One 2011;6(2):e16976. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Athuman 2013
- Athuman M, Kabanywanyi AM, Rohwer AC. Intermittent preventive antimalarial treatment for children with anaemia. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD010767] [DOI] [PMC free article] [PubMed] [Google Scholar]


