Abstract
Background
Urinary schistosomiasis is caused by an intravascular infection with parasitic Schistosoma haematobium worms. The adult worms typically migrate to the venous plexus of the human bladder and excrete eggs which the infected person passes in their urine. Chronic infection can cause substantial morbidity and long‐term complications as the eggs become trapped in human tissues causing inflammation and fibrosis. We summarised evidence of drugs active against the infection. This is new edition of a review first published in 1997.
Objectives
To evaluate the efficacy and safety of drugs for treating urinary schistosomiasis.
Search methods
We searched the Cochrane Infectious Diseases Group Specialized Register, MEDLINE, CENTRAL, EMBASE and LILACS and reference lists of articles up to 23 May 2014.
Selection criteria
Randomized controlled trials (RCTs) of antischistosomal drugs and drug combinations compared to placebo, no intervention, or each other.
Data collection and analysis
Two researchers independently screened the records, extracted the data and assessed risk of bias. The primary efficacy outcomes were parasitological failure (defined as the continued presence of S. haematobium eggs in the urine at time points greater than one month after treatment), and percent reduction of egg counts from baseline. We presented dichotomous data as risk ratios (RR), and continuous data as mean difference (MD), alongside their 95% confidence intervals (CIs). Where appropriate we combined trials in meta analyses or tables. We assessed the quality of evidence using the GRADE approach.
Main results
We included 30 RCTs enrolling 8165 participants in this review. Twenty‐four trials were conducted in children in sub‐Saharan Africa, and 21 trials were over 20 years old. Many studies were assessed as being at unclear risk of bias due to inadequate descriptions of study methods.
Praziquantel
On average, a single 40 mg/kg dose of praziquantel reduced the proportion of people still excreting eggs in their urine by around 60% compared to placebo at one to two months after treatment (treatment failure: RR 0.42, 95% CI 0.29 to 0.59, 864 participants, seven trials, high quality evidence). The proportion of people cured with praziquantel varied substantially between trials, from 22.5% to 83.3%, but was higher than 60% in five of the seven trials. At one to two months following praziquantel treatment at 40 mg/kg, the mean number of schistosome eggs in the urine was reduced by over 95% in five out of six trials (678 participants, six trials, high quality evidence).
Splitting praziquantel 40 mg/kg into two doses over 12 hours probably has no benefits over a single dose, and in a single trial of 220 participants the split dose caused more vomiting (RR 0.5, 95% CI 0.29 to 0.86) and dizziness (RR 0.39, 95% CI 0.16 to 0.94).
Metrifonate
A single dose of metrifonate 10 mg/kg reduced egg excretion (210 participants, one trial, at eight months), but was only marginally better than placebo at achieving cure at one month (RR 0.83, 95% CI 0.74 to 0.94, 142 participants, one trial). In a single trial comparing one, two and three doses, the absolute number of participants cured improved from 47% after one dose to 81% after three doses (93 participants, one trial, low quality evidence).
Two small trials compared 40 mg/kg single dose praziquantel with two or three doses of 10 mg/kg metrifonate and found no clear evidence of differences in cure (metrifonate 2 x 10 mg/kg at one month: RR 1.03, 95% CI 0.8 to 1.34, 72 participants, one trial; metrifonate 3 x 10 mg/kg at three months: RR 0.33, 95% CI 0.07 to 1.57, 100 participants, one trial. In one trial both drugs performed badly and in one trial both performed well.
Other drugs
Three trials have evaluated the antimalarial artesunate; with inconsistent results. Substantial antischistosomal effects were only seen in one of the three trials, which was at unclear risk of bias due to poor reporting of the trial methods. Similarly, another anti‐malarial mefloquine has been evaluated in two small trials with inconsistent effects.
Adverse events were described as mild for all evaluated drugs, but adverse event monitoring and reporting was generally of low quality.
Authors' conclusions
Praziquantel 40 mg/kg is the most studied drug for treating urinary schistosomiasis, and has the strongest evidence base.
Potential strategies to improve future treatments for schistosomiasis include the combination of praziquantel with metrifonate, or with antimalarial drugs with antischistosomal properties such as artesunate and mefloquine. Evaluation of these combinations requires rigorous, adequately powered trials using standardized outcome measures.
15 April 2019
Update pending
Studies awaiting assessment
The CIDG is currently examining a new search conducted in April 2019 for potentially relevant studies. These studies have not yet been incorporated into this Cochrane Review.
Plain language summary
Drugs for treating urinary schistosomiasis
What is urinary schistosomiasis and how is it treated?
Urinary schistosomiasis is a disease caused by infection of people with the parasitic worm Schistosoma haematobium. These worms live in blood vessels around the infected person's bladder and the worm releases eggs which are released in the person's urine. If the urine is passed into ponds or lakes, the eggs can hatch and infect people that are washing or swimming there. Infection can cause blood in the urine and if left untreated can eventually lead to anaemia, malnutrition, kidney failure, or bladder cancer. Urinary schistosomiasis is diagnosed by looking for worm eggs in the urine.
The disease occurs mainly in school‐aged children and young adults in sub‐Saharan Africa. The drug currently recommended for treatment is praziquantel, which can be given as a single dose, but other drugs such as metrifonate, artesunate, and mefloquine have also been evaluated.
After examining the research published up to 23th May 2014, we included 30 randomized controlled trials, enrolling 8165 children and adults.
What does the research say?
On average, the standard dose of praziquantel cures around 60% of people at one to two months after treatment (high quality evidence), and reduces the number of schistosome eggs in the urine by over 95% (high quality evidence).
Metrifonate, an older drug no longer in use, had little effect when given as a single dose but an improved effect when given as multiple doses two weeks apart. Two trials compared three doses of metrifonate with the single dose of praziquantel and found similar effects.
Two more recent trials evaluated a combination of artesunate and praziquantel compared to praziquantel alone. In one trial artesunate improved cure and in one it made no difference.
Authors conclusions
Future treatments for schistosomiasis could include combining praziquantel with metrifonate, or with artesunate, but these need to be evaluated in high quality trials.
Summary of findings
Summary of findings for the main comparison. Praziquantel 40 mg/kg versus placebo for treating urinary schistosomiasis.
| Praziquantel 40 mg/kg versus placebo for treating urinary schistosomiasis | |||||
| Patient or population: People with urinary schistosomiasis Settings: Endemic areas in sub‐Saharan Africa Intervention: Praziquantel 40 mg/kg (single dose) versus placebo | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Placebo | Praziquantel 40 mg/kg | ||||
|
Parasitological failure At 1 to 2 months |
91 per 100 | 38 per 100 (26 to 54) | RR 0.42 (0.29 to 0.59) | 864 (7 trials) | ⊕⊕⊕⊕ high1,2,3,4 |
| Percentage egg reduction At 1 to 2 months | Mean change in egg excretion in the control groups ranged from a 53.2% reduction to a 138% increase. | Mean egg excretion in the intervention groups was reduced by > 98% in all trials | Not pooled | 678 (6 trials) |
⊕⊕⊕⊕ high1,2,3,5 |
|
Microhaematuria At 8 weeks |
53 per 100 | 28 per 100 (17 to 45) | RR 0.53 (0.33 to 0.84) | 119 (1 trial) | ⊕⊕⊝⊝ low6,7,8 |
|
Haemoglobin At 6 to 8 months |
The mean haemoglobin ranged across control groups from 11.3 to 11.9 G/dL | The mean haemoglobin in the intervention groups was 0.08 G/dL lower (0.24 lower to 0.09 higher) | ― | 727 (2 trials) | ⊕⊕⊕⊝ moderate3, 9,10 11 |
| Adverse events | ― | ― | ― | 1591 (9 trials) | ⊕⊕⊝⊝ low12 |
| The basis for the assumed risk is the mean risk in the control groups across trials. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 No serious risk of bias: Several trials were at unclear or low risk of selection bias. However, a sensitivity analysis excluding these trials still found a strong effect. 2 No serious inconsistency: Six of the seven trials found large consistent effects. The seventh trial found no difference, this may be explained by the different diagnostic criteria used in this trial. 3 No serious indirectness: These seven trials are all conducted in children in endemic areas of sub‐Saharan Africa. 4 No serious imprecision: The result is statistically significant and the 95% CI is narrow around a clinically important effect. 5 No serious imprecision: The trials are small and most did not report tests of statistical significance, however the differences are large. 6 No serious risk of bias: This trial was well conducted. 7 Downgraded by 1 for serious indirectness: Only a single trial reports this outcome. Further trials from different settings would be needed to be confident in this effect. 8 Downgraded by 1 for serious imprecision: This trial is underpowered. 9 Downgraded by 1 for serious risk of bias: both trials had inadequate sequence generation and allocation concealment. 10 No serious inconsistency: Low statistical heterogeneity. 11 No serious imprecision: only two trials reported this outcome. CIs are narrow. The effect is not statistically significant and does not appear to be clinically important, when compared to the baseline data. 12 Downgraded by 2 for serious risk of bias: Three trials do not comment on adverse events. Six trials made comments that praziquantel was generally well tolerated and no statistically significant differences were noted. However, adverse events were poorly reported in all six trials such that meta‐analysis, and assessment of other quality criteria was not possible.
Summary of findings 2. Praziquantel 40 mg/kg single dose versus 30 mg/kg single dose.
| Praziquantel 40 mg/kg compared to praziquantel 30 mg/kg for treating urinary schistosomiasis | |||||
| Patient or population: people with urinary schistosomiasis Settings: endemic areas in Sub‐Saharan Africa Intervention: praziquantel 40 mg/kg (single dose) Comparison: praziquantel 30 mg/kg (single dose) | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Praziquantel 30 mg/kg single dose | Praziquantel 40 mg/kg single dose | ||||
| Parasitological failure At 1 month | 32 per 100 | 24 per 100 (19 to 32) | RR 0.76 (0.59 to 0.99) | 401 (4 trials) | ⊕⊕⊝⊝ low1,2,3,4 |
| Mean percent egg reduction At 1 month | The mean reduction in control groups ranged from an 85% reduction to a 99% reduction. | The mean reduction in the intervention groups was > 95% in all trials | Not pooled | 362 (4 trials) |
⊕⊕⊝⊝ low1,3,5,6 |
| Parasitological failure At 6 months | 29 per 100 |
28 per 100 (22 to 36) |
RR 0.97 (0.76 to 1.23) |
669 (6 trials) |
⊕⊕⊕⊝ moderate 1,3,7,8 |
| Mean percent egg reduction At 6 months | The mean reduction in control groups ranged from an 97% reduction to a 99% reduction. | The mean reduction in the intervention groups ranged from a 46% reduction15 to a 99% reduction | Not pooled | 362 (4 trials) |
⊕⊕⊝⊝ low1,3,9,10 |
| Haematuria | 26 per 100 | 23 per 1000 (12 to 44) | RR 0.89 (0.47 to 1.67) | 117 (1 trial) | ⊕⊝⊝⊝ very low11,12,13 |
| Proteinuria | 15 per 100 | 13 per 100 (5 to 31) | RR 0.85 (0.34 to 2.12) | 117 (1 trial) | ⊕⊝⊝⊝ very low11,12,13 |
| Adverse events | ― | ― | Not estimable | 992 (8 trials) | ⊕⊕⊝⊝ low14 |
| *The basis for the assumed risk (for example, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Downgraded by 1 for serious risk of bias: None of the trials described a method of allocations concealment or blinding outcome assessors. 2 No serious inconsistency: No statistical heterogeneity in the relative effect of the two praziquantel doses. However, treatment failure with praziquantel 40 mg/kg ranged from 0% to than more than 50%. 3 No serious indirectness: All trials were conducted in sub‐Saharan Africa, in patients aged from seven to 20 years. 4 Downgraded by 1 for serious imprecision: None of the individual studies found statistical significant differences, and overall, the meta‐analysis remains underpowered to confidently detect an effect. 5 No serious inconsistency: Three of the four trials report the difference was not statistically significant. The fourth trial did not report significance but effects were similar. 6 Downgraded by 1 for serious imprecision: We were unable to pool the data, and as such cannot exclude a small difference in effect between the two doses in a pooled analysis. 7 No serious inconsistency. Low statistical heterogeneity. 8 No serious imprecision. The effect is of no clinically important difference between the two doses, and the 95% CIs are narrow. 9 Downgraded by 1 for serious inconsistency: In one trial praziquantel 40 mg/kg had a very low percent egg reduction of 46%. The reasons for this are unclear. 10 Unable to assess precision as the data were not pooled. 11 Downgraded by 1 for serious risk of bias: This trial did not adequately describe allocation concealment. Participants and clinicians were not blinded. 12 Downgraded by 1 for serious indirectness: Only one trial from one setting. 13 Downgraded by 1 for serious imprecision. This trial is underpowered to detect an effect. The 95% CI is wide and includes clinically important benefits and no effect. 14 Downgraded by 2 for serious risk of bias. Six out of ten trials comparing praziquantel 40 mg/kg to lower doses did not comment on adverse events, and of the remaining only two used prospective active surveillance to monitor adverse events. Only two trials out of ten described blinding for clinicians or participants.
Summary of findings 3. Praziquantel 40 mg/kg multiple doses versus single dose.
| Praziquantel 40 mg/kg multiple doses compared to single dose for treating urinary schistosomiasis | ||||||
| Patient or population: patients with treating urinary schistosomiasis Settings: endemic settings Intervention: Praziquantel 40 mg/kg multiple doses (every three months for two years) Comparison: Praziquantel 40 mg/kg single dose | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Praziquantel 40 mg/kg single dose | Praziquantel 40 mg/kg multiple doses | |||||
|
Parasitological failure At 2 years |
90 per 100 | 244 per 100 (132 to 450) | RR 2.71 (1.47 to 5.00) | 62 (1 trial) | ⊕⊝⊝⊝ very low1,2,3,4 | |
|
Mean percent egg reduction At 2 years |
This study reports a81% reduction after a single dose of praziquantel | This study reports a96% reduction after multiple doses of praziquantel | ― | 62 (1 trial) |
⊕⊝⊝⊝ very low1,2,3,4 | |
|
Parasitological failure At 3 years |
63 per 100 | 56 per 100 (37 to 89) | RR 0.92 (0.59 to 1.42) | 43 (1 trial) | ⊕⊝⊝⊝ very low1,2,3,4 | |
|
Haematuria At 3 years |
48 per 100 | 34 per 100 (20 to 56) | RR 0.7 (0.42 to 1.17) | 43 (1 trial) | ⊕⊝⊝⊝ very low1,2,3,4 | |
| Adverse events | ― | This study reports a96% reduction after multiple doses of praziquantel | ― | 43 (1 trial) |
⊕⊝⊝⊝ very low5 | |
| *The basis for the assumed risk (for example, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded by 2 for serious risk of bias. The one trial reporting the outcome did not report adequately on sequence generation and blinding. Allocation was not concealed, and loss to follow up was very high. 2 No serious inconsistency: only one trial. 3 No serious indirectness: only one trial. 4 Downgraded by 1 for serious imprecision: This single trial is small and underpowered to reliably detect an effect. 5 This trial did not report on adverse events.
Summary of findings 4. Metrifonate 3 x 7.5 mg/kg given two weeks apart versus placebo.
| Metrifonate compared to placebo for treating urinary schistosomiasis | |||||
| Patient or population: patients with treating urinary schistosomiasis Settings: endemic settings Intervention: metrifonate 3 x 7.5 mg/kg given two weeks apart Comparison: placebo | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Placebo | Metrifonate 3 x 7.5 mg/kg given two weeks apart | ||||
|
Parasitological failure At 2 to 2.5 months |
40 per 100 |
16 per 100 (12 to 22) |
RR 0.41 (0.3 to 0.56) |
93 (1 trial) |
⊕⊕⊝⊝ low1,2,3,4 |
|
Mean percent egg reduction At 2 to 2.5 months |
Egg excretion increased by 131% in the placebo group in this study | Egg excretion was reduced by 100% in this trial | ‐ | 93 (1 trial) |
⊕⊕⊝⊝ low1,2,3,4 |
|
Parasitological failure At 6 months |
96 per 100 |
29 per 100 (23 to 36) |
RR 0.3 (0.24 to 0.37) |
400 (1 trial) | ⊕⊕⊕⊝ moderate2,3,5,6 |
|
Mean percent egg reduction At 6 months |
13% increase | 94% reduction | ― | 400 (1 trial) |
⊕⊕⊕⊝ moderate2,3,5,7 |
| Adverse events | ― | ― | ― | 493 (2 trials) |
8 |
| *The basis for the assumed risk (for example, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Downgraded by 1 for serious risk of bias; the single trial reporting this outcome did not adequately describe sequence generation, allocation concealment and blinding of participants, clinicians or outcome assessors. 2 No serious inconsistency. Only one trial.
3 No serious indirectness. This single trial was conducted in children in rural sub‐Saharan Africa. 4 Downgraded by 1 for serious imprecision. The trial was underpowered.
5 Downgraded by 1 for serious risk of bias. The trial did not report on sequence generation and allocation concealment. The study described blinding of participants, clinicians and outcome assessors.
6 No serious imprecision. CIs are narrow and both CI limits have clinically important effects. The trial is adequately powered for this outcome.
7 No serious imprecision. The difference in effect between metrifonate and placebo group is large.
8 None of the trials reported on adverse events.
Summary of findings 5. Artesunate versus placebo.
| Artesunate compared to placebo for treating urinary schistosomiasis | |||||
| Patient or population: patients with treating urinary schistosomiasis Settings: endemic settings Intervention: artesunate 4 mg/kg for three days Comparison: placebo | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Placebo | Artesunate | ||||
|
Parasitological failure At 8 weeks |
87 per 100 | 46 per 100 (14 to 148) | RR 0.53 (0.16 to 1.71) | 251 (2 trials) | ⊕⊕⊝⊝ very low1,2,3,4 |
|
Mean percent egg reduction At 8 weeks |
Mean change in egg excretion ranged from range from 47.1% reduction to 111.5% increase. | Reduction in egg excretion ranged from 52.1% to a 69.3% | ― | 276 (2 trials) |
⊕⊝⊝⊝ low1,3,5,6 |
|
Microhaematuria At 8 weeks |
53 per 100 | 65 per 100 (45 to 94) | RR 1.22 (0.85 to 1.76) | 119 (1 trial) | ⊕⊕⊝⊝ low7,8,9,10 |
| Adverse events | ― | ― | ― | 276 (2 trials) |
⊕⊕⊝⊝ low11,12 |
| *The basis for the assumed risk (for example, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Downgraded by 1 for serious risk of bias. One trial described sequence generation, allocation concealment and blinding adequately, whereas the second study did not.
2 Downgraded by 1 for serious inconsistency. One of the trials (at high risk of bias) reported a large effect, while the other trial (at low risk of bias) detected no effect.
3 No serious indirectness. The trials were conducted in Gabon and Nigeria in patients of a similar age range.
4 Downgraded by 1 for serious imprecision. The CI is very wide and reaches from no benefit to a significant benefit after treatment.
5 No for serious inconsistency. Percent egg reductions the studies reported were similar.
6 Downgraded by 1 for serious imprecision. The meta analysis is underpowered.
7 No serious risk of bias. The one trial reporting the outcome reported adequately on sequence generation, allocation concealment and blinding.
8 No serious inconsistency: only one trial.
9 No serious indirectness: This trial was conducted in school children in Gabon.
10 Downgraded by 2 for very serious imprecision: only one trial reporting 74 events in 119 participants evaluated this outcome.
11Downgraded by 1 for serious risk of bias: only one trial was blinded. Both trials reported on adverse events, but the methods are unclear.
12 Downgraded by 1 for imprecision. One study reported on clinically diagnosed outcomes per treatment group, but was underpowered to confidently detect a difference.
Summary of findings 6. Praziquantel and artesunate versus praziquantel.
| Praziquantel plus artesunate compared to praziquantel alone for treating urinary schistosomiasis | |||||
| Patient or population: patients with urinary schistosomiasis Settings: Countries endemic for urinary schistosomiasis Intervention: Praziquantel plus artesunate Comparison: Praziquantel alone | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Praziquantel 40 mg/kg single dose alone | Praziquantel 40 mg/kg single dose plus artesunate 4 mg/kg/d for 3 days | ||||
| Parasitological failure at 8 weeks | 27 per 100 |
17 per 100 (10 to 27) |
RR 0.62 (0.38 to 0.99) | 265 (2 trials) | ⊕⊕⊝⊝ low1,2,3,4 |
| Percent egg reduction | Egg reduction in the Praziquantel groups ranged from 52.1% reduction to a 97.11% reduction. | Egg reduction in the Praziquantel and ARS groups ranged from 93.5% to 98.8% | ― | 265 (2 trials) |
⊕⊝⊝⊝ very low1,2,5,6 |
| Microhaematuria | 28 per 100 |
19 per 100 (11 to 33) |
RR 0.69 (0.4 to 1.18) | 177 (1 trial) | ⊕⊕⊝⊝ low7,8 |
| Adverse events | ― | ― | ― | 156 (1 trial) |
⊕⊝⊝⊝ very low9,10 |
| *The basis for the assumed risk (for example, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Downgraded by 1 for serious risk of bias: only one out of two studies did report adequate random sequence generation, allocation concealment and blinding or participants and clinicians, while the other study did not provide enough information to allow a judgement. 2 No serious inconsistency. Both studies favour the combination of Praziquantel and ARS over Praziquantel alone. 3 No serious indirectness. The trials were conducted in rural communities in Gabon and Nigeria, in children (6 to 15 years) and young adults (4 to 20 years) 4 Downgraded by 1 for serious imprecision: Only two studies were included in this comparison. The effect size, described by the 95% CI ranged from a very small, clinically non‐important effect to a clinically important effect. 5 Downgraded by 1 for serious inconsistency: egg reduction varied widely between the two trials. 6 Downgraded by 1 for serious imprecision: Only two studies reported this outcome. 7 No serious risk of bias. The one study that reporting this outcome described adequate random sequence generation, allocation concealment and blinding. 8 Downgraded by 2 for serious imprecision: only one small study reported this outcome, the outcome is not statistically significant with wide 95% CI. 9 Downgraded by 2 for serious risk of bias. This study did not provide enough information to allow a judgement regarding sequence generation, allocation concealment and blinding. 10Downgraded by 1 for serious imprecision. Only one study reported on adverse events. The study was underpowered, and no difference in adverse events was detected between treatment groups.
Background
Urinary schistosomiasis, also called bilharzia or snail fever, is an intravascular infection caused by parasitic Schistosoma haematobium worms. It is endemic in sub‐Saharan Africa, the Arabian peninsula and the Middle East. According to the World Health Organization (WHO), at least 243 million people required treatment for schistosomiasis in 2011 (WHO 2013), and more than 700 million people live in endemic areas (WHO 2014).
The WHO currently recommends regular chemoprophylaxis with praziquantel for populations at risk to prevent the long term consequences of infection. These programmes usually target school children (Table 7), but may be extended to the whole community in high risk settings (King 2011).
1. Population based treatment according to prevalence among schoolchildren (WHO).
|
Category |
Prevalence among school‐aged children | Action to be taken | Comment |
| High‐risk community | 50% by parasitological methods (intestinal or urinary schistosomiasis; or 30% by questionnaire for visible haematuria (urinary schistosomiasis) |
Treat all school‐age children (enrolled and not enrolled) once a year | Also treat adults considered to be at risk (from special groups to entire communities living in endemic areas) |
| Moderate‐risk community | > 10 to < 50% by parasitological methods (intestinal and urinary schistosomiasis); or 30% by questionnaire for visible haematuria (urinary schistosomiasis) |
Treat all school‐age children (enrolled or not enrolled) once every two years | Also treat adults considered to be at risk (special groups only) |
| Low–risk community | < 10% by parasitological methods (intestinal and urinary schistosomiasis) | Treat all school‐age children (enrolled and not enrolled) twice during their primary schooling age (for example, once on of suspected cases entry and once on exit) |
Praziquantel should be available in dispensaries and clinics for treatment of suspected cases. |
Description of the condition
Human infection with S. haematobium is acquired through contact with water bodies containing cercariae, the larval form of the parasite. The cercariae are able to penetrate human skin and migrate via blood vessels to the liver, where they mature into male and female forms for reproduction. Typically, they then migrate further to the venous plexus of the urinary bladder, and begin to produce eggs which the infected person excretes in their urine (Gryseels 2006). If these eggs reach water, they hatch into miracidia, infect specific freshwater snails which act as intermediate hosts, before emerging as cercariae that can infect humans (Gray 2011; Ross 2002).
Any illness associated with acute infection is typically mild, but chronic schistosomiasis can cause considerable morbidity with chronic pain, anaemia, fatigue, under nutrition and reduced exercise tolerance (King 2005). A review of 124 observational studies and 11 randomized controlled trials (RCTs) in 2005 estimated that up to 15% of people infected with any form of schistosomiasis suffer disabling long‐term complications (King 2005). The main pathological process occurs when schistosome eggs become trapped in the tissue around the bladder and ureters causing chronic inflammation, which may obstruct the ureters, damage the kidneys, and lead to bladder cancer. Occasionally, eggs can become trapped in other tissues such as the brain and spinal cord (WHO 1985).
Two‐thirds of all infected persons are schoolchildren (aged five to 14 years), and the intensity of infection with S. haematobium is highest in children aged ten to 14 years (WHO 1985).
The standard test for urinary schistosomiasis is urine filtration and microscopic examination of the urine sample (WHO 1991). The urine sample is passed through a filter paper and the eggs retained on the filter are counted either with or without staining. Sedimentation and centrifugation is less commonly used for urine concentration (Cook 2003). High urine egg counts are related to high infection intensity.
Parasitologists define cure when eggs can no longer be detected in one or more urine samples using standard methods. Besides parasitological cure, researchers also record the relative reduction in egg output after treatment compared to pre‐treatment levels. This outcome, expressed as % egg reduction, is an indirect estimate of a reduction of the worm burden (Cook 2003).
Blood and protein excretion in the urine is usually elevated in urinary schistosomiasis and decreases when the infection resolves. The most commonly used test is a dipstick test. Ultrasound can demonstrate organ involvement of the urinary tract as well as its resolution.
Description of the intervention
Praziquantel is the current treatment for urinary schistosomiasis recommended by the WHO (WHO 2006). Historically, metrifonate was also used but this fell out of favour due to the need for multiple doses (Feldmeier 1999; WHO 1998). More recently, there has been interest in the antischistosomal properties of artemisinin derivates and mefloquine, more commonly used for treating malaria (Utzinger 2004).
Praziquantel is an pyrazinoisoquinoline derivative with activity against adult worms of all schistosome species (S. mansoni, S. intercalatum and S. japonicum), but not against maturing worms. Praziquantel has a rapid onset of action. It is well‐tolerated, can be given as a single dose (Utzinger 2004) and paediatric formulations are available (Stothard 2013).
Metrifonate, an organophosphorous cholinesterase inhibitor, is active against S. haematobium but not against other schistosome species (Utzinger 2004).
Artemisinin, extensively used as potent antimalarial, has highest activity against immature schistosomes. Artemsinins are safe and well‐tolerated (Utzinger 2004).
How the intervention might work
After treatment with praziquantel, the worms appear to die quickly but egg excretion continues for several weeks. There are several possible reasons for this:
Firstly, some worms might not have been mature at the time of praziquantel treatment and therefore not killed by praziquantel (Cioli 2003). Maturation of the worms after infection takes four to six weeks, and after two months eggs can be detected in the urine.
Secondly, the patient might have been re‐infected (Cioli 2003).
Thirdly, dead eggs still wander out of the tissue into the urine several weeks after clearing adult worms (Taylor 1988 ZWE). Therefore, a follow‐up four to six weeks after treatment is useful (Renganathan 1998). There is also considerable variation in daily urinary egg output (Cook 2003).
Although there is concern that S. haematobium might develop resistance against praziquantel (Fenwick 2006), there is no clinically relevant evidence for resistance up to now (Doenhoff 2008).
In endemic settings, reinfection with S. haematobium is likely, and cure (often defined as complete cessation of egg excretion) is not a sustainable long term goal. However, reduction of infection intensity results in clinical improvement, low morbidity and prevention of long term complications. Therefore, WHO promotes morbidity control rather than cure as an objective for schistosomiasis control programmes (WHO 2002).
Why it is important to do this review
At present, praziquantel as the only drug in use that is exposed to resistance development. It is therefore important to monitor its performance and to assess the effects of other drugs against urinary schistosomiasis.
Dosing regimens for subgroups such as highly infected patient groups, incremental benefits of drug combinations, double dosing and optimal interval between doses have to be determined to inform control programmes for urinary schistosomiasis.
Paediatric schistosomiasis has gained attention as a public health problem, and evaluation of existing treatment studies is indicated.
Objectives
To evaluate the efficacy and safety of drugs for treating urinary schistosomiasis.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials.
Types of participants
Patients diagnosed with urinary schistosomiasis by:
detection of macro or microhaematuria;
identification of schistosome eggs by urine microscopy;
detection of parasite antigens in blood or urine.
Types of interventions
Intervention
Drugs used to treat urinary schistosomiasis. Drugs considered as obsolete (such as ambilhar, oltipraz and niridazole) were not included. Metrifonate was included.
Control
Placebo, no intervention, an alternative regimen of the same drug, or an alternative drug used to treat urinary schistosomiasis.
Types of outcome measures
Primary outcomes
Parasitological failure at one month post‐treatment (as defined by the trial authors);
Percent egg reduction at one month.
Secondary outcomes
Parasitological failure at time‐points > one month;
Percent egg reduction from baseline at > one month;
Clinical outcomes: resolutions of signs and symptoms (for example, haematuria and proteinuria);
Anaemia (decrease of the number of red blood cells or the quantity of haemoglobin in the blood);
Growth outcomes (gain in body weight, body length).
Adverse events
Serious adverse events;
Other adverse events
Search methods for identification of studies
We attempted to identify all relevant trials regardless of language and publication status (published, unpublished, in press, under review and in progress).
Electronic searches
We searched the following databases using the search terms outlined in Appendix 1: The Cochrane Infectious Diseases Group Specialized Register (23 May 2014); Cochrane Central Register of Controlled Trials (CENTRAL), published in The Cochrane Library (2014, Issue 4); MEDLINE (1966 to 23 May 2014); EMBASE (1974 to 23 May 2014); and LILACS (1982 to 23 May 2014). We also searched the metaRegister of Controlled Trials (mRCT) using 'Schistosoma haematobium' as the search term (23 May 2014).
Searching other resources
We checked the reference lists of all studies identified by the above methods for additional studies relevant to this review.
Data collection and analysis
Selection of studies
Vittoria Lutje, the Cochrane Infectious Diseases Group (CIDG) Information Retrieval Specialist, searched the literature and retrieved trial titles and abstracts.
VK and FZ independently screened the results of the search and retrieved full trial reports of all potentially relevant trials. Then, VK and FZ independently assessed each trial for inclusion using an eligibility form based on the inclusion criteria. We resolved any discrepancies by discussion with PG.
Data extraction and management
VK and FZ independently extracted data using pre‐tested standardized forms. We resolved any differences through discussion with PG. For each trial we extracted details of the trial methods, participants, interventions and outcomes.
VK and FZ extracted the number of participants randomized and number of participants followed up in each treatment arm. For dichotomous outcomes, we extracted the number of participants experiencing the event in each group. For continuous outcomes summarized as geometric means, we extracted means and their standard error, if reported. If the data were presented as arithmetic means, we extracted arithmetic means and their standard deviations (SD), if reported, for each treatment group. Where continuous data were summarized as medians and ranges, these were extracted and entered into tables.
VK and FZ double‐entered the data and cross‐checked to minimise errors. VK tried to contact trial authors for clarification or insufficient of missing data when necessary and summarised data reported in multiple publications as one single data set.
Assessment of risk of bias in included studies
VK and FZ independently assessed the risk of bias of each trial using an assessment form based on the Cochrane Collaboration's 'Risk of bias' tool (Higgins 2008). DS verified the assessment results.
We assessed the risk of bias for six domains: sequence generation; allocation concealment; blinding (of participants, personnel, and outcome assessors); incomplete outcome data; selective outcome reporting; and other sources of bias. We categorized these judgments as low, high or unclear risk of bias.
For sequence generation, allocation concealment and blinding, we quoted the method as described in the trial in the Characteristics of included studies tables. For blinding, we stated the blinding method and who was blinded separately for different outcomes. For incomplete outcome data, we assigned a judgement for different outcomes (for example, loss to follow‐up at different time points).
We resolved disagreements by discussion or consultation. Where risk of bias was unclear, we attempted to contact the trial authors for clarification.
Measures of treatment effect
We presented dichotomous outcomes as risk ratios (RR), and continuous outcomes as mean differences or geometric mean ratios. All results are shown with a 95% confidence interval (CI).
Unit of analysis issues
For trials including more than two comparison groups, we split and analysed as individual pair‐wise comparisons. When conducting meta‐analysis we ensured that participants and cases in the placebo group were not counted more than once, by dividing the placebo cases and participants evenly between the intervention groups.
Dealing with missing data
The primary analysis is a complete case analysis where the number of evaluable participants at each time point is used as the denominator.
Assessment of heterogeneity
We assessed heterogeneity by inspecting forest plots for overlapping CIs and outlying data. We applied the Chi2 test with a P value < 0.10 to indicate statistically significant heterogeneity, and the I2 statistic with a value of greater than 50% to indicate moderate heterogeneity.
Assessment of reporting biases
We planned to evaluate the possibility of publication bias by constructing funnel plots, but there were too few trials within each comparison to make this meaningful.
Data synthesis
We analysed the data in pair‐wise comparisons using Review Manager (RevMan). We stratified the primary analysis by drug dose and the time point after treatment. Data were combined in meta‐analyses using a fixed‐effect model. If we detected moderate heterogeneity but still considered combination of the trials to be appropriate we used a random‐effects model. We presented data which could not be presented in forest plots in tables (medians, means without measure of variance, ranges).
We assessed quality of evidence using the GRADE approach, and displayed the results in 'Summary of Findings' tables. The GRADE approach defines quality as a measure of 'our confidence in the effect estimates' and defines four levels of quality; high, moderate, low and very low. The evidence from RCTs is rated as 'high quality' but can be downgraded where there are major concerns about: 1) the risk of bias of the trials; 2) inconsistency between the trial results; 3) a mismatch between the question being asked and the trial setting, population, intervention or control; 4) the trial being underpowered; or 5) evidence of publication bias.
Subgroup analysis and investigation of heterogeneity
We planned to conduct the following subgroup analyses to explore the potential causes of heterogeneity. However, there were too few trials within each comparison to make this meaningful: patient age (children versus adults), intensity of infection, endemicity.
Sensitivity analysis
Data were insufficient to assess the robustness of results by sensitivity analyses to evaluate risk of bias components and the effects of missing data.
Results
Description of studies
Results of the search
Following database searches, we identified 116 individual citations, and a further 40 potential studies after we checked trial abstracts. Following abstract screening, we assessed 71 full text articles for inclusion. Figure 1 shows the flow diagram of these trials.
1.
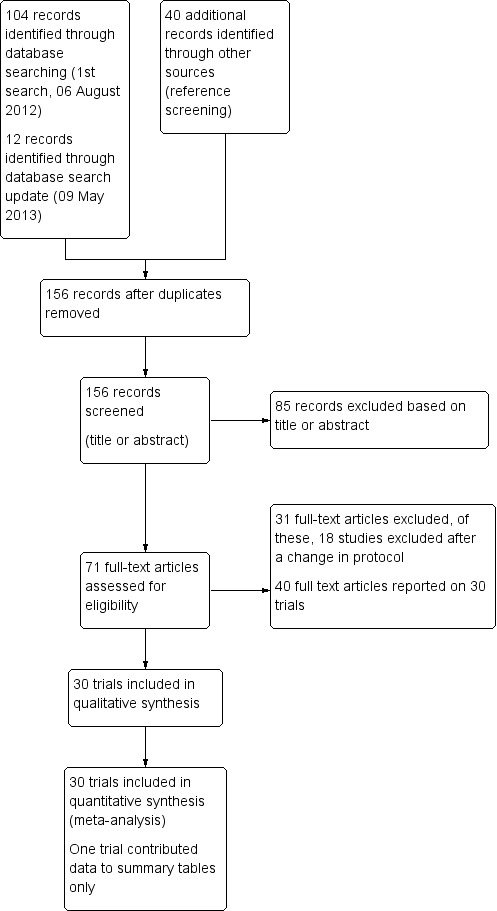
Study flow diagram
Included studies
We included 30 RCTs, enrolling 8965 participants, and reported in 39 publications. Twenty trials were over 20 years old, and only eight were published since the year 2000.
Settings
All but one trial were conducted in sub Saharan Africa; 13 trials from East Africa: Somalia (one) Sudan (three), Tanzania (two), Kenya (six), Malawi (one); 13 trials from West Africa: Cameroon (two), Gabon (three), Niger (two), Mali (one), Nigeria (two), Cote d' Ivoire (one), Ghana (one), Gambia (one); and three trials from southern Africa: Zimbabwe (two), and Zambia (one). Most trials were based in rural settings, but two were conducted in peri‐urban or semi‐rural settings, three were from urban settings, and in one trial the setting was not described. The remaining trial was conducted in an urban setting in Saudi Arabia.
Twenty trials were based in schools and one in a college, seven in villages, farms or settlements, one in antenatal clinics and two in referral hospitals.
Participants
Twenty‐four trials enrolled school‐age children and young adults, although the exact age‐range varied; age six to 20 years (16 trials), age five to 18 years (three trials), age two to 23 years (five trials). Two trials enrolled adults only, and four trials didn't clearly state the age range.
All trials diagnosed S. haematobium infection by detection of eggs or miracidia on urine microscopy. Sixteen trials reported egg counts as geometric mean egg counts, four trials as arithmetic mean egg counts, three trials reported both. One study reported geometric mean miracidial counts. Six trials used ranges or medians.
Interventions
Eight trials compared praziquantel with placebo, and 14 trials published between 1981 and 2009 compared different doses or regimens of praziquantel.
Five trials compared metrifonate with placebo, and seven trials published between 1983 and 1990 directly compared the efficacy of praziquantel and metrifonate.
More recently, three trials published between 2001 and 2009 evaluated artesunate as single agent or in combination with praziquantel, and two trials published in 2009 and 2011 evaluated mefloquine.
Excluded studies
We excluded 65 studies for the reasons given in the 'Characteristics of excluded studies' table.
Risk of bias in included studies
Many trials lacked adequate descriptions of methods to allow judgements on risk of bias, and so have been classified as unclear (see Figure 2).
2.
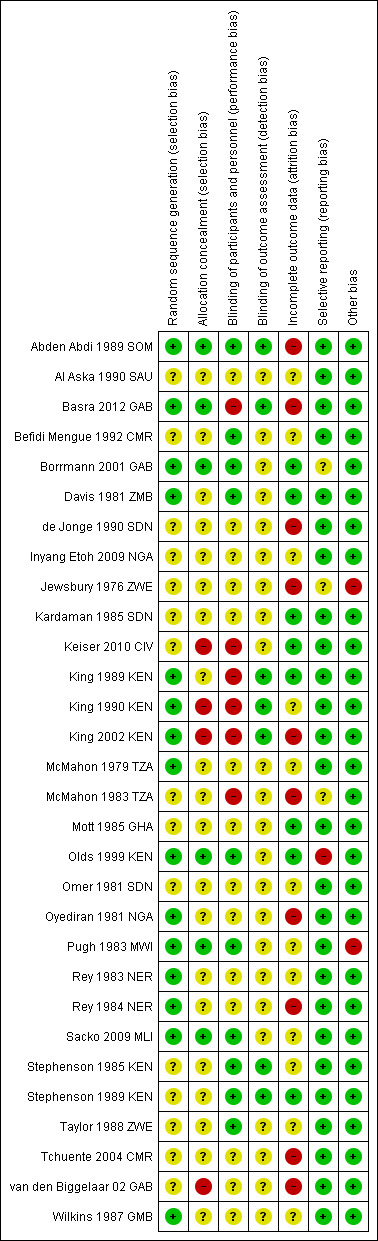
Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.
Allocation
Fourteen trials adequately described a random method of sequence generation, but only six described a method of allocation concealment and could be considered at low risk of selection bias (Abden Abdi 1989 SOM; Basra 2012 GAB; Borrmann 2001 GAB; Olds 1999 KEN; Pugh 1983 MWI; Sacko 2009 MLI).
Blinding
Ten trials reported adequate attempts to blind participants and trial staff to treatment allocation, six trials were unblinded and blinding was unclear in the remaining trials. Seven trials reported adequate blinding of outcome assessors.
Incomplete outcome data
Many trials had high levels of attrition, particularly at later time points. When trials presented cure or failure rates as percentages, we were unable to assess attrition. We considered the risk of attrition bias to be unclear in 13 trials and high in nine trials.
Selective reporting
We found evidence of reporting bias in one trial, as trial authors did not present pre‐specified outcomes. In three trials, selective reporting was at unclear risk of bias.
Other potential sources of bias
Trial authors reported baseline imbalances in two trials, which we identified as sources of other bias.
The trials were mostly funded by funds, trusts or international agencies (see Characteristics of included studies tables). Eight trials did not declare funding, four received drug donations and only two trials declared funding by pharmaceutical companies (both Dafra Pharma).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
Section A: Praziquantel
Praziquantel 40 mg/kg single dose versus placebo (comparison 1)
On average, a single 40 mg/kg dose of praziquantel reduces the proportion of people still excreting eggs at one to two months after treatment by around 60% compared to placebo, and reduces the mean number of eggs excreted by over 95%.
Eight trials compared a single 40 mg/kg dose of praziquantel with placebo or no treatment in schoolchildren in sub‐Saharan Africa. We have listed the definitions of parasitological failure in Table 8.
2. Definion of cure, reporting and calculation of egg counts.
| Study ID | Definition cure | Reporting of egg counts/10 mL urine | Methods to calculate egg counts | Comment |
| Abden Abdi 1989 SOM | Patients without schistosome eggs in their urine after treatment | Mean (SD), % ER | Not reported | No hatching test employed, cured might be underestimated because of dead eggs |
| Al Aska 1990 SAU | Clinical improvement Disappearance of ova from the urine on three successive examinations |
Mean, range | Not reported | ― |
| Basra 2012 GAB | Three consecutive urine samples without presence of eggs | Median, interquartile range | Not reported | ― |
| Befidi Mengue 1992 CMR | Cure not reported | GMEC | Not reported | Hb and weight as outcomes |
| Borrmann 2001 GAB | Two negative egg counts on two consecutive days | GMEC | Arithmetric mean of two egg counts per participant before and after treatment including 0 egg counts (cured patients). Geometric means of these arithmetic means. | We received the data file from the study author Day to day variation in egg counts explains 10% cure rate with placebo. |
| Davis 1981 ZMB | Defined as three negative urine defined as the absence of hatched miracidia, although recently dead or black eggs might be present. | Geometric mean miracidial count | At follow‐up: If the first urine specimen contained hatched miracidia, then random 10 mL samples were taken from further bladder collections, the miracidial count was recorded, and the geometric mean of the counts was compared directly with the geometric mean of the pretreatment counts. | Quantitaive hatching test. if the first sedimented urine specimen was negative, then two further urine specimens taken on consecutive days were sedimented and examined. |
| de Jonge 1990 SDN | No definition of cure given, presumably absence of urinary egg excretion | Minimum and maximum value, median, 90%value | Not reported | Excretion of eggs following treatment |
| Inyang Etoh 2009 NGA | No definition of cure given, cure rates and egg reduction rates as end points | Mean ± SD | "Treatment‐related changes in egg counts were investigated using paired Student’s t test." | ― |
| Jewsbury 1976 ZWE | No definition of cure given | "median urine egg count" | Not reported | ― |
| Kardaman 1985 SDN | No definition of cure given, "negative" | GMEC | Not reported | "It would appear that the cure rate determined in any trial is dependent on the pretreatment egg count and on the ...urine examination techniques used." |
| Keiser 2010 CIV | Absence of urinary egg excretion Cure rate (CR, defined as the percentage of children excreting no S. haematobium eggs 26 days after treatment among children with confirmed parasites at baseline) |
GMEC |
S. haematobium egg counts before and after treatment were averaged for every child (arithmetic mean) and the GM egg count for each treatment group was calculated. Because egg counts are over dispersed, they were logarithmically transformed log [count+1], and the GM was expressed as the antilogarithm of the mean. Egg reduction rate (ERR) defined as reduction of geometric mean (GM) egg count among S. haematobium positive children after treatment, compared with the respective GM pretreatment. The ERR was calculated as (1 ‐ [GM egg count after treatment/GM egg counts at enrolment] x 100 |
(ERR; defined as reduction of geometric mean egg count among S. haematobium–positive children after treatment, compared with the respective geometric mean pretreatment) |
| King 1989 KEN | No definition of cure given | AMEC GMEC |
Not reported | Infection was identified and quantified by Nucleopore filtration |
| King 1990 KEN | No definition of cure given | AMEC GMEC |
Not reported | Infection was identified and quantified by Nucleopore filtration |
| King 2002 KEN | Cure defined as egg‐negative | GMEC | Not reported | ― |
| McMahon 1979 TZA | Probable cure rate: excretion of no or only non viable eggs in the urine | GMEC, 95%confidence limit of the mean | Not reported | ― |
| McMahon 1983 TZA | People were considered cured when no eggs or non‐viable eggs were excreted in the urine | Screening: GMEC of miracidia/10 mL urine reduction in egg excretion |
"In non cured cases the reduction of egg excretion was calculated." | ― |
| Mott 1985 GHA | Absence of S. haematobium eggs in two random 5 mL samples of urine from the same specimen | GMEC 5 mL urine samples reduction in GMEC |
Not reported | ― |
| Olds 1999 KEN | No definition given | GMEC | "Egg counts are geometric means in subjects who remained infected. Reduction in egg no. after treatment in infected children was significant in all infections at 45 days." | ― |
| Omer 1981 SDN | 100% reduction of egg excretion (absence of egg excretion in the urine) or 98% egg reduction and neg miracidial hatching test |
GMEC | Not reported | Only children with GMEC > 60/10 mL (in three egg counts) included |
| Oyediran 1981 NGA | No definition of cure given | GMEC mean ± SD | Not reported | Only children with GMEC > 60/10 mL (in three egg counts) included |
| Pugh 1983 MWI | No definition of cure given | AMEC % egg count reduction |
Percentage reduction in egg output was determined by comparing the arithmetic and geometric means of pooled egg counts before and after treatment. The geometric mean was obtained by recording the logarithm of egg counts and using the n +1 transformation for a series of counts after treatment that included zeros. | We did not use a hatching test to determine the viability of excreted ova since percentage reduction in egg output rather than parasitological cure was our main criterion of efficacy. |
| Rey 1983 NER | No definition of cure given | AMEC "nombre moyenne" average number |
Not reported | If possible, a hatching test was that at the last control (6 months) |
| Rey 1984 NER | No definition of cure given, "negativation" | AMEC moyenne des nombres d'oeufs/10 mL urine Number average |
Not reported | ― |
| Sacko 2009 MLI | The cure rate was calculated as the proportion of infected individuals who became parasitologically negative (0 egg/10 mL urine based on three urine samples) at three months post treatment | GMEC | Individual egg counts were calculated as the mean number of eggs per 10 mL of urine in the three urine samples. To compare the effect of the treatment on the intensity of the infection at 3, 6 and 18 months geometric mean egg/10 mL for all urine samples examined for S. haematobium eggs were calculated as log10(x+1) to allow egg count of 0 to be included in the analysis. | ― |
| Stephenson 1985 KEN | no definition of cure given | AMEC | Not reported | ― |
| Stephenson 1989 KEN | ― | AMEC GMEC |
Not reported | ― |
| Taylor 1988 ZWE | Cure defined as negative egg counts "infections as were cured by a negative GMEC at 1,3 and 6 months" |
GMEC | Not reported | "in cases were only one egg was found in three (urine) examinations the egg count was always taken as positive." |
| Tchuente 2004 CMR | The parasitologic cure rates were calculated as the proportion of children excreting eggs at the first survey before treatment and who were not excreting eggs in their urine after treatment. | GMEC | Geometric mean (GM) values of all
individuals were used to assess average egg counts of each group. The GM was calculated as the antilogarithm of the
mean of all log transformed egg counts + 1. The intensity reduction rate was calculated as [1 − (GM egg counts per 10 mL of urine after treatment/GM egg counts per 10 mL before treatment)] × 100 |
The parasitological cure rates were calculated as the proportion of children excreting eggs at the first survey before treatment and who were not excreting eggs in their urine after treatment. |
| van den Biggelaar 02 GAB | Negative for both eggs and circulating antigen failure: pos. for eggs or circulating antigen |
GMEC interquartile range | Not reported | ― |
| Wilkins 1987 GMB | No definition of cure given | GMEC | When appropriate a log10 transformation was used in statistical analysis to make their skewed distribution approximate to normal. This was reversed for the presentation of results to give a geometric mean which included zero values. | ― |
Parasitological failure
Praziquantel 40 mg/kg as a single dose reduced parasitological treatment failure by around 60% at one to two months compared to placebo (RR 0.42, 95% CI 0.29 to 0.59; 864 participants, seven trials, Analysis 1.1). The absolute level of treatment failure with praziquantel ranged from 16.6% (McMahon 1979 TZA) to 77.5% (de Jonge 1990 SDN). Treatment failure with placebo was greater than 80% in all seven trials and over 90% in four trials.
1.1. Analysis.
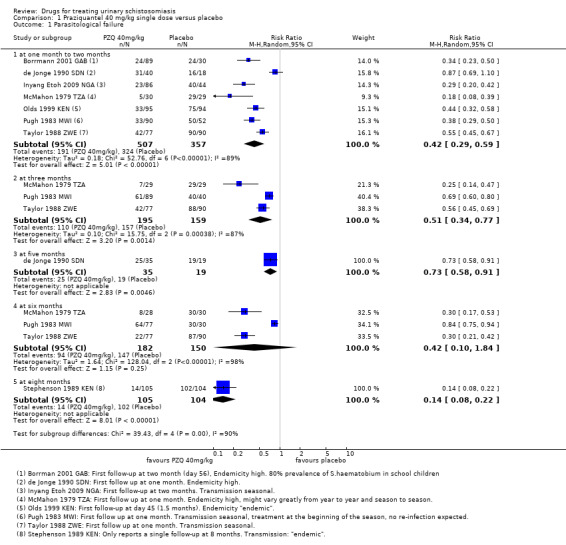
Comparison 1 Praziquantel 40 mg/kg single dose versus placebo, Outcome 1 Parasitological failure.
Four trials reported follow‐up beyond two months (Analysis 1.1). Failure rate increased over time in two trials, as might be expected in areas of schistosomiasis transmission as people become re‐infected (McMahon 1979 TZA; Pugh 1983 MWI). However, treatment outcomes improved in Taylor 1988 ZWE over time, with moderate reductions in treatment failure at one month and three months and a 70% reduction at six months. The trial authors stated that this improvement might have been due to excretion of remaining eggs from the urinary tract over time.
The fourth trial, de Jonge 1990 SDN, found no difference in treatment failure between praziquantel and placebo at any time point. The trial authors used a more sensitive diagnostic method (three urine samples, filtration of the whole volume up to 350 mL when the 10 mL urine sample contained fewer than 10 eggs) and a strict definition of cure (no excretion of eggs, no viability testing of eggs). This may explain the high failure rates observed despite high percent egg reductions comparable to other trials.
Stephenson 1989 KEN reported treatment failure at eight months, its only time point. A single dose of praziquantel reduced treatment failure by 86% compared to placebo (RR 0.14, 95% CI 0.08 to 0.22; 209 participants, one trial, Analysis 1.1).
Six trials reported parasitological failure stratified by intensity of infection; the categorisation of strata varied between trials (642 participants, see Appendix 2). At the first follow‐up at four to six weeks, three out of four trials had a tendency to higher failure in participants with higher infection intensity. The pattern attenuated at later time points.
Percent egg reduction
Seven trials reported mean urine egg counts per 10 mL urine at baseline, and at one to two months after a single dose of praziquantel 40 mg/kg or placebo (867 participants, seven trials, see Table 9), although we were only able to reliably interpret this data for six trials (678 participants).
3. Praziquantel 40 mg/kg single dose versus placebo: % egg reduction at one and two months.
| Study ID | Subgroup | Timepoint | Measure | Praziquantel 40 mg/kg single dose | Placebo | P value difference between groups | ||||
|
Egg count/10 mL (Range/95% CI) N |
% egg reduction |
Egg count/10 mL (Range/95% CI) N |
% egg reduction | |||||||
| Baseline | Follow‐up | Baseline | Follow‐up | |||||||
| de Jonge 1990 SDN | ― | 1 month | Median | 66 N = 48 |
1 N = 40 |
98.5 | 124 N = 21 |
58 N = 18 |
53.2 | P = 0.29 not significant |
| McMahon 1979 TZA | ― | 1 month | Miracidial count (95% CI) |
288.4 (33.2 to 2508.9) N = 32 |
1.1 (0 to 8.3) N = 30 | 99.6 | 324.9 (22.1 to 4783.3) N = 37 |
187.5 (6.3 to 5601.3) N = 29 |
42.3 | Not reported |
| Pugh 1983 MWI | ― | 1 month | GMEC AMEC |
385.5 780.9 N = 97 |
1.8/ 1.8 |
99.5 99.7 |
136.8 188.8 N = 52 |
119.9 437.2 |
12.35 (GMEC) ‐ 131.5 (AMEC) (increase) |
Not reported |
| Taylor 1988 ZWE | light infections < 50/10 mL |
1 month | GMEC N = (both light and heavy) |
15.1 N = 77 (both groups) |
0.4 | 99.7 | 15.7 N = 90 (both groups) |
37.5 | ‐138 (increase) |
Not reported |
| heavy infections < 100/10 mL |
1 month | GMEC N = (both light and heavy) |
204.7 N = 77 (both groups) |
4.0 | 98.1 | 191.9 N = 90 (both groups) |
147.0 | 23.39 | Not reported | |
| Olds 1999 KEN | ― | 45 days | GMEC | Not reported N = 95 |
1.4 | ― | N = 94 | 29.8 | ― | Not reported |
| Borrmann 2001 GAB | ― | 8 weeks | GMEC (range) |
38.51 (1 to 3313) N = 90 |
1.11 N = 89 |
97.11 | 21.57 (1 to 778) N = 30 |
11.41 N = 30 |
47.1 | Significant |
| Inyang Etoh 2009 NGA2 | without placebo | 8 weeks | ― | 42.0 ± 1.7 N = 52 |
9.8 ± 0.5 N = 42 |
76.7 | 34.1 ± 0.8 N = 52 |
72.0 ± 2.3 N = 44 |
‐ 111.5 (increase) |
P < 0.0012 |
1P for therapeutic efficacy (not defined) Praziquantel versus placebo
2 Treatment group: Praziquantel 40 mg/kg without placebo. Inyang Etoh 2009 NGA also reports a second treatment group (Praziquantel 40 mg/kg with placebo), data not shown.
The mean egg count was reduced by more than 95% at one to two months following praziquantel in five trials, and by 75% in one trial. In the placebo groups the change in mean egg count ranged from a 53% decrease to a 115% increase.
Percent egg reduction in the praziquantel group remained high (> 95%) in all three trials reporting at three months, and in all four trials at six months. Percent egg reduction was variable in the placebo group, ranging from 26% increase to 54% reduction at three months and from 5% to 64% reduction at six months (see Table 10). One additional trial, Stephenson 1989 KEN, reported percent egg reduction at eight months as its only time point (209 participants, see Table 10). Percent egg reduction after praziquantel was 99% compared to 5% with placebo.
4. Praziquantel 40 mg/kg single dose versus placebo: % egg reduction at later time points.
| Study ID | Subgroup | Time point | Measure | Praziquantel 40 mg/kg single dose | Placebo | P value for difference between groups | ||||
| Egg count /10 mL urine | % egg reduction | Egg count/10 mL urine | % egg reduction | |||||||
| Baseline | Follow‐up | Baseline | Follow‐up | |||||||
| McMahon 1979 TZA | ― | 3 months | miracidial count (95% CI) |
288.4 (33.2 to 2508.9) N = 32 |
1.1 (0 to 16.3) | 99.6 | 324.9 (22.1 to 4783.3) N = 37 |
149.4 (6.3 to 3556.6) |
54 | Not reported |
| Pugh 1983 MWI | ― | 3 months | GMEC AMEC |
385.5 780.9 N = 97 |
1.9 1.9 |
99.5 (GMEC) 99.75(AMEC) |
136.8 188.8 N = 52 |
85.9 270.3 |
37.2 (GMEC) 43.16 (AMEC) |
Not reported |
| Taylor 1988 ZWE | light infections < 50/10 mL |
3 months | GMEC | 15.1 N = 77 (for both groups) |
0.4 | 97.35 | 15.7 N = 90 |
19.8 | ‐26.11 (increase) |
Not reported |
| heavy infections < 100/10 mL |
GMEC | 204.7 N = 77 (for both groups) |
2.0 | 99.02 | 191.9 N = 90 |
94.7 | 50.65 | Not reported | ||
| de Jonge 1990 SDN | ― | 5 months | median | 66 N = 48 |
0 | 100 | 124 N = 21 |
95 | 23.38 | P = 0.27 not significant |
| McMahon 1979 TZA | ― | 6 months | miracidial count (95% CI) |
288.4 (33.2 to 2508.9) N = 32 |
1.1 (0‐20.3) |
99.6 | 324.9 (22.1 to 4783.3) N = 37 |
188.6 (13.9 to 2563.5) | 41.95 | Not reported |
| Pugh 1983 MWI | ― | 6 months | GMEC AMEC |
385.5 780.9 N = 97 |
2.4 20.1 |
99.3 (GMEC) 97.4 (AMEC) |
136.8 188.8 N = 52 |
69.7 261.8 |
49.0 GMEC ‐38.7 (increase) AMEC |
Not reported |
| Befidi Mengue 1992 CMR | ― | 6 months | GMEC | 41/10 mL N = 238 |
2/10 mL | 95.1 | 39/10 mL N = 198 |
14/10 mL | 64.1 | |
| Taylor 1988 ZWE | light infections < 50/10 mL |
6 months | GMEC | 15.1 N = 77 (for both groups) |
0.2 | 98.67 | 15.7 N = 90 |
11.7 | 25.5 | Not reported |
| heavy infections < 100/10 mL |
204.7 N = 77 (for both groups) |
0.6 | 99.7 | 191.9 N = 90 |
75.5 | 60 | Not reported | |||
| Stephenson 1989 KEN | ― | 8 months | GMEC AMEC |
57/ 112 N = 105 |
0.2/ 1 |
99.64 (GMEC) 99.1 (AMEC) |
38/ 85 N = 104 |
36/ 102 |
5.26 (GMEC) ‐20 (increase) (AMEC) |
Not reported1 |
1Praziquantel 40 mg/kg single dose: significant egg reduction in praziquantel group (before, after treatment) P < 0.0002. no significant reduction in the placebo group (before, after treatment).
Five trials reported percent egg reduction stratified by intensity of infection (764 participants, Appendix 2). At four to six weeks, all trials reported percent egg reductions over 90% across the strata. Percent egg reduction as a relative measure was at least as high in heavy infections as in mild infections, but post‐treatment egg counts as an absolute measure tended to be higher in people with high intensity infections. This pattern persisted at later time points.
Clinical resolution
At eight weeks the proportion of patients with persistent haematuria (defined as > 5 erythrocytes/mL) was lower in those given praziquantel than placebo in one small trial which reported this (RR 0.53, 95% CI 0.33 to 0.84; 119 participants, one trial, Analysis 1.2). There were substantial reductions in the mean number of erythrocytes in the urine in three trials at one to two months, but we could not combine these data in a meta‐analysis (357 participants, three trials, see Appendix 3).
1.2. Analysis.
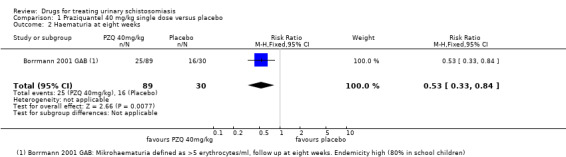
Comparison 1 Praziquantel 40 mg/kg single dose versus placebo, Outcome 2 Haematuria at eight weeks.
Proteinuria was reduced by 65% to 84% at one to two months after praziquantel compared to increases in the placebo groups (238 participants, two trials, see Appendix 3).
Two trials reported mean haemoglobin at baseline and at six to eight months after treatment with no difference between groups (mean difference ‐0.08, 95% CI ‐0.24 to 0.09; 727 participants, two trials, Analysis 1.3).
1.3. Analysis.
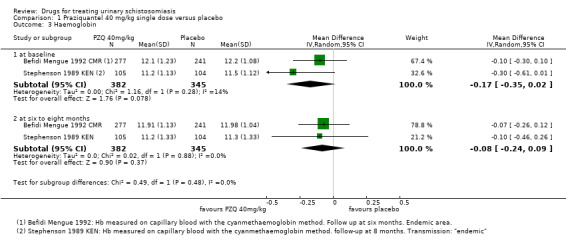
Comparison 1 Praziquantel 40 mg/kg single dose versus placebo, Outcome 3 Haemoglobin.
Three trials measured a variety of growth parameters (Befidi Mengue 1992 CMR; Olds 1999 KEN; Stephenson 1989 KEN). Two trials reported little or no effect on the outcomes measured (Befidi Mengue 1992 CMR; Olds 1999 KEN). The third trial (Stephenson 1989 KEN) reports 14 measures, some of which are reported as statistically significant, but all appear to be of no or only borderline clinical importance (see Appendix 4). Most notably, there is a reported increase in children's physical fitness as measured by the Harvard Step test. The difference in mean improvement between groups was 6.8% at five weeks (mean end scores 81.2% praziquantel versus 75.5% placebo). Scores between 68% and 82% are considered average. Children that took praziquantel also gained 1.2 kg more weight than those in the control group, however baseline differences between groups were of a similar magnitude to this effect.
Adverse events
Of nine trials, six (with 1286 participants) commented on adverse events. Only four described the methods used for data collection, but rarely reported them in detail (see Appendix 5). Adverse events were usually monitored in the first days after medication. Only two trials actually reported numbers of adverse events, and only abdominal pain was reported by both trials. The absolute number of adverse events was low and none were more common with praziquantel than placebo (see Analysis 1.4). The other trials summarized narratively with comments such as "both treatments were well tolerated" (see Appendix 5).
1.4. Analysis.
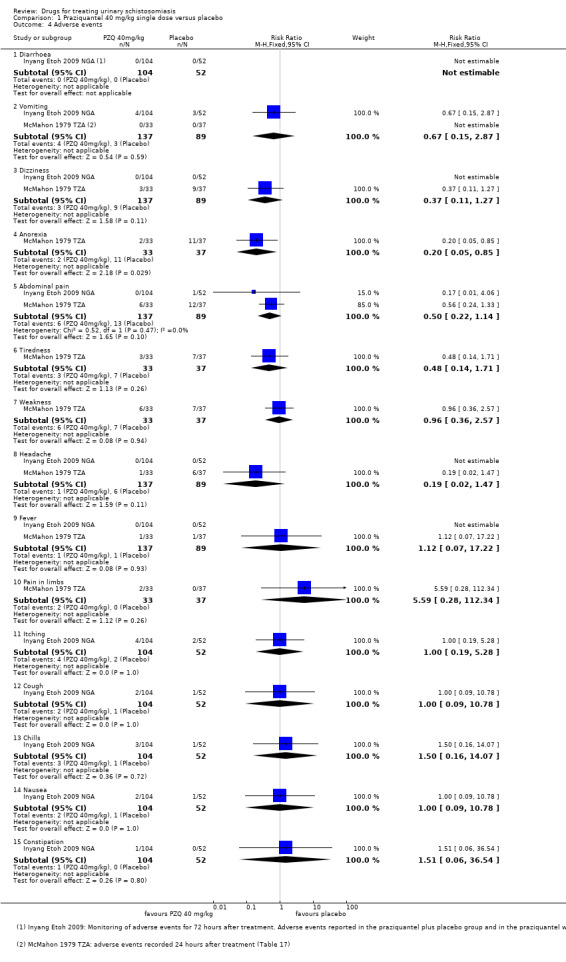
Comparison 1 Praziquantel 40 mg/kg single dose versus placebo, Outcome 4 Adverse events.
Praziquantel 40 mg/kg versus lower doses (comparison 2)
Praziquantel doses of 20 to 40 mg/kg result in similar reductions in mean egg excretion, but 40 mg/kg is marginally superior at achieving cure.
Ten trials compared praziquantel 40 mg/kg with lower doses: 30 mg/kg (seven trials), 20 mg/kg (three trials), and 10 mg/kg (three trials). All trials were conducted in sub‐Saharan Africa in schoolchildren, apart from one trial, which recruited college students and army recruits.
Treatment with praziquantel 40 mg/kg had fewer treatment failures than lower doses when measured at four to six weeks after treatment (versus 30 mg/kg; RR 0.76, 95% CI 0.59 to 0.99; 401 participants, four trials, Analysis 2.1, versus 20 mg/kg; RR 0.74, 95% CI 0.59 to 0.93; 338 participants, two trials, Analysis 2.1). However, there was no difference between 40 mg/kg and 30 mg/kg at two to three months (517 participants, five trials, Analysis 2.2), or six months after treatment (699 participants, six trials, Analysis 2.3).
2.1. Analysis.
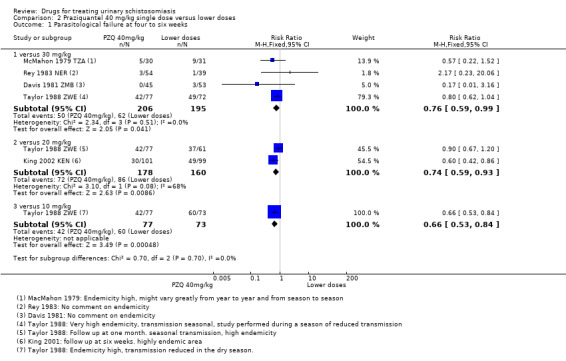
Comparison 2 Praziquantel 40 mg/kg single dose versus lower doses, Outcome 1 Parasitological failure at four to six weeks.
2.2. Analysis.
Comparison 2 Praziquantel 40 mg/kg single dose versus lower doses, Outcome 2 Parasitological failure at two to three months.
2.3. Analysis.
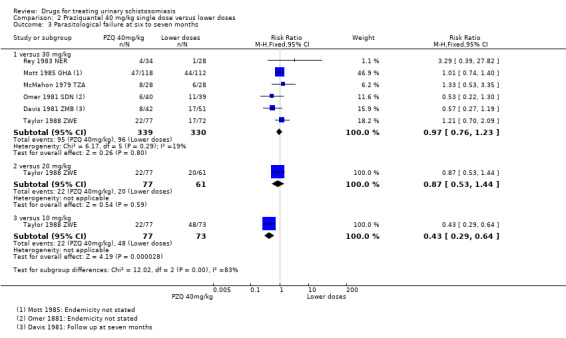
Comparison 2 Praziquantel 40 mg/kg single dose versus lower doses, Outcome 3 Parasitological failure at six to seven months.
In the five trials comparing praziquantel 40 mg/kg and 30 mg/kg, the mean number of eggs excreted was reduced by greater than 90% with both doses and without significant differences between groups (495 participants, five trials, see Table 11).
5. Praziquantel 40 mg/kg single dose versus 30 mg/kg single dose: % egg reduction.
| Study ID | Subgroup | Time point | Measure | Praziquantel 40 mg/kg (SD) | Praziquantel 30 mg/kg (SD) | P value difference between groups | ||||
| Egg count/10 mL urine | % reduction | Egg count/10 mL urine | % reduction | |||||||
| Baseline | Follow‐up | Baseline | Follow‐up | |||||||
| McMahon 1979 TZA | ― | 1 month | GMEC (95 Confidence limits of mean) N |
288.4 (33.2 to 2508.9) N = 33 |
1.1 (0‐8.3) N = 30 |
99.61 | 308.5 (31.2 to 3034.7) N = 32 |
1.2 (0 to 15.4) N = 31 |
99.6 | Not significant P value not reported |
| Rey 1983 NER1 | ― | 1 month | AMEC N |
7.5 ± 1.7 N = 57 |
0.24 N = 54 |
96.8 | 7.5 ± 1.7 N = 46 |
0.74 N = 39 |
90.13 | Not significant |
| Taylor 1988 ZWE2 | heavy infection < 100/10 mL |
1 month | GMEC N |
204.7 N = 77 for both groups |
4.0 | 98.04 | 185.4 N = 72 for both groups |
3.1 | 98.32 | Not reported |
| light infection > 50/10 mL |
1 month | GMEC | 15.1 | 0.4 | 97.35 | 15.9 | 0.6 | 96.23 | ||
| Oyediran 1981 NGA3 | ― | 1 month | GMEC mean ± SE, N = |
Stratum 1 87.4 ± 23.46 N = 15 Stratum 2 339.4 ± 32.61 N = 5 Stratum 3 518.00 ± 0.71 N = 2 N = 22 |
N = 21 | 97.69 ± 0.98 | Stratum 1: 111.67 ± 47.14 N = 15 Stratum 2: 306.83 ± 54.29 (N = 6) Stratum 3: 1507.00 ± 1400.07 N = 2 N = 23 |
N = 19 | 85.65 ± 13.08 | Not significant Not reported |
| King 1989 KEN | 2‐3 months | AMEC (± SD) GMEC N = |
377 255 N = 64 |
31 (± 21) 2 N = 54 |
91.7 (AMEC) 99.2 (GMEC) |
327 204 N = 69 |
22 ± 17 2 N = 60 |
93.27 (AMEC) 99 (GMEC) |
Not significant Not reported |
|
| McMahon 1979 TZA | 3 months | GMEC (95 Confidence limits of mean) N |
288.4 (33.2 to 2508.9) N = 33 |
1.1 (0‐16.3) N = 29 |
99.61 | 308.5 (31.2 to 3034.7) N = 31 |
0.9 (0 to 13.4) N = 31 |
97.08 | Not significant Not reported |
|
| Rey 1983 NER | 3 months | AMEC N = |
7.5 ± 1.7 N = 57 |
0.42 N = 52 |
94.4 | 7.5 ± 1.7 N = 46 |
1.21 N = 42 |
83.86 | Not reported | |
| Taylor 1988 ZWE3 | heavy infections < 100/10 mL | 3 months | GMEC N = |
204.7 N = 77 for both groups |
2.0 | 99.02 | 185.4 N = 72 for both groups |
1.1 | 99.4 | Not reported |
| light infections > 50/10 mL | 3 months | GMEC | 15.1 | 0.4 | 97.35 | 15.9 | 0.4 | 97.48 | ||
| Oyediran 1981 NGA3 | ― | 3 months | GMEC mean ± SE, N = |
Stratum 1 87.4 ± 23.46 N = 15 Stratum 2 339.4 ± 32.61 N = 5 Stratum 3 518.00 ± 0.71 N = 2 N = 22 |
97.55 ± 0.85 (N = 18) | Stratum 1 111.67 ± 47.14 N = 15 Stratum 2 306.83 ± 54.29 N = 6 Stratum 3 1507.00 ± 1400.07 N = 2 N = 23 |
99.01 ± 0.47 (N = 19) | Not significant Not reported |
||
| McMahon 1979 TZA | ― | 6 months | GMEC (95 Confidence limits of mean) |
288.4 (33.2 to 2508.9) N = 33 |
1.1 (0 to 20.3) N = 28 |
99.6 | 308.5 (31.2 to 3034.7) N = 32 |
1.4 (0 to 39.5) N = 28 |
99.46 | Not significant Not reported |
| Rey 1983 NER | ― | 6 months | AMEC | 7.5 ± 1.7 N = 57 |
4 N = 34 |
46.6 | 7.5 ± 1.7 N = 462 |
0.18 N = 28 |
97.6 | Not reported |
| Taylor 1988 ZWE3 | heavy infections < 100/10 mL | 6 months | GMEC N = |
204.7 (N = 77) | 0.6 | 99.7 | 185.4 (N = 72) | 0.7 | 99.62 | Not significant Not reported |
| light infections > 50/10 mL | 6 months | GMEC N = | 15.1 (N = 77) | 0.2 | 98.67 | 15.9 (N = 72) | 0.1 | 99.37 | ||
| Oyediran 1981 NGA4 | ― | 6 months | GMEC mean ± SE, (N =) |
Stratum 1 87.4 ± 23.46 (N = 15) Stratum 2 339.4 ± 32.61 (N = 5) Stratum 3 518.00 ± 0.71 (N = 2) (N = 22) |
(N = 15) | 93.09 ± 0.12 | Stratum 1 111.67 ± 47.14 (N = 15) Stratum 2 306.83 ± 54.29 (N = 6) Stratum 3 1507.00 ± 1400.07 (N = 2) (N = 23) |
(N = 17) | 98.72 ± 0.28 | Not significant Not reported |
| ― | 9 months | (N = 6) | 92.4 ± 5.92 | (N = 8) | 96.49 ± 1.59 | |||||
| ― | 12 months | (N = 3) | 99.3 ± 0.26 | (N = 4) | 99.28 ± 0.46 | |||||
1Baseline data not reported separately per group.
2A reduction as low as 46% after praziquantel 40 mg/kg was not observed by any other study that reported this outcome. At six months, five other studies reported % egg reduction above 90% (see Table 10 and Table 11)
3Heavy and light infections together; N = 77 for Praziquantel 40 mg/kg and N = 72 for Praziquantel 30 mg/kg.
4 GMEC/10 mL urine, stratum 1: 60 to 250, stratum 2: 251 to 500, stratum 3 > 500.
In trials comparing 40 mg/kg and 20 mg/kg, again the mean number of eggs excreted was reduced by more than 95% for both doses and differences in percent egg reduction appeared small (636 participants, four trials, see Appendix 2). Treatment with praziquantel 40 mg/kg appeared to result in greater percent egg reductions than 10 mg/kg (357 participants, three trials, see Appendix 2).
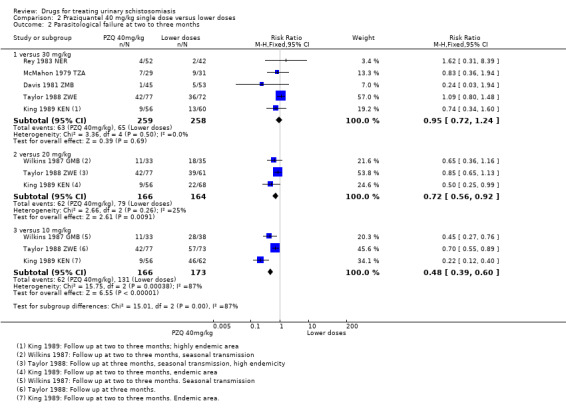
One small trial from Kenya (King 1989 KEN) reported similar numbers of participants with persistent haematuria or proteinuria at three months with praziquantel 40 mg/kg, 30 mg/kg and 20 mg/kg, but 40 mg/kg was superior to 10 mg/kg (haematuria at three months: RR 0.35, 95% CI 0.21 to 0.58, 119 participants, one trial, Analysis 2.4; proteinuria at three months: RR 0.25, 95% CI 0.12 to 0.51; 119 participants, one trial, Analysis 2.5). A larger trial by the same authors comparing 40 mg/kg and 20 mg/kg (King 2002 KEN) detected fewer participants with haematuria at six weeks following praziquantel 40 mg/kg (RR 0.63, 95% CI 0.47 to 0.86; 245 participants, one trial, Analysis 2.6), and fewer participants with proteinuria (RR 0.66, 95% CI 0.46 to 0.96; 245 participants, one trial, Analysis 2.7). These differences were still observed at nine months (haematuria: RR 0.59, 95% CI 0.44 to 0.78; 215 participants, one trial, Analysis 2.8; proteinuria RR 0.67, 95% CI 0.5 to 0.9; 214 participants, one trial, Analysis 2.9). King 2002 KEN also reported ultrasound findings (bladder thickening, bladder irregularity and hydronephrosis) before and after treatment with praziquantel 40 mg/kg and 20 mg/kg respectively, but the results were inconclusive (264 participants, see Appendix 6).
2.4. Analysis.
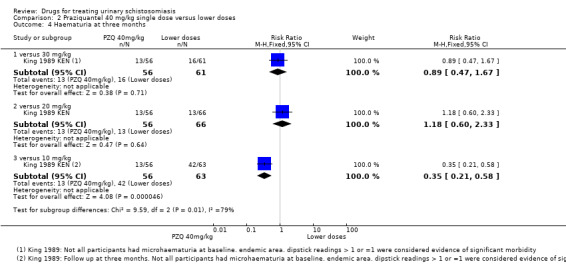
Comparison 2 Praziquantel 40 mg/kg single dose versus lower doses, Outcome 4 Haematuria at three months.
2.5. Analysis.
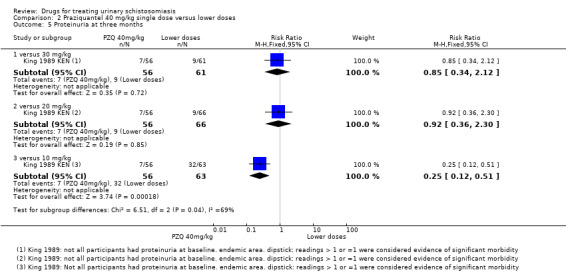
Comparison 2 Praziquantel 40 mg/kg single dose versus lower doses, Outcome 5 Proteinuria at three months.
2.6. Analysis.
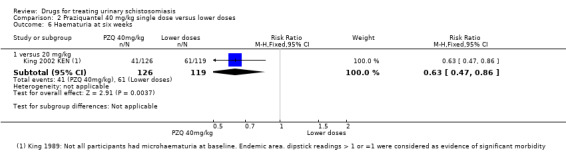
Comparison 2 Praziquantel 40 mg/kg single dose versus lower doses, Outcome 6 Haematuria at six weeks.
2.7. Analysis.
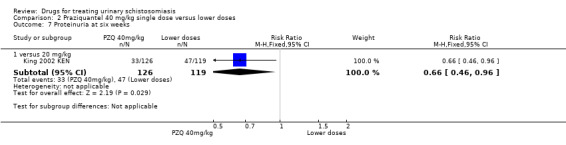
Comparison 2 Praziquantel 40 mg/kg single dose versus lower doses, Outcome 7 Proteinuria at six weeks.
2.8. Analysis.
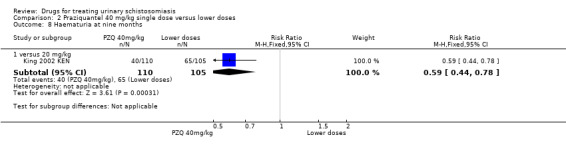
Comparison 2 Praziquantel 40 mg/kg single dose versus lower doses, Outcome 8 Haematuria at nine months.
2.9. Analysis.
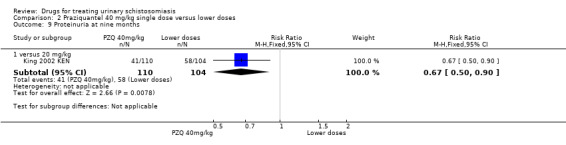
Comparison 2 Praziquantel 40 mg/kg single dose versus lower doses, Outcome 9 Proteinuria at nine months.
Six of these trials did not comment on adverse events. Four trials described the methods of data collection, but often in insufficient detail; two out of four trials used active, prospective surveillance for adverse events (Appendix 5). Two trials stated for all treatment arms collectively that adverse events after praziquantel treatment were mild and transient. Two trials reported numbers of adverse events with no differences between groups (163 participants, Analysis 3.2).
3.2. Analysis.
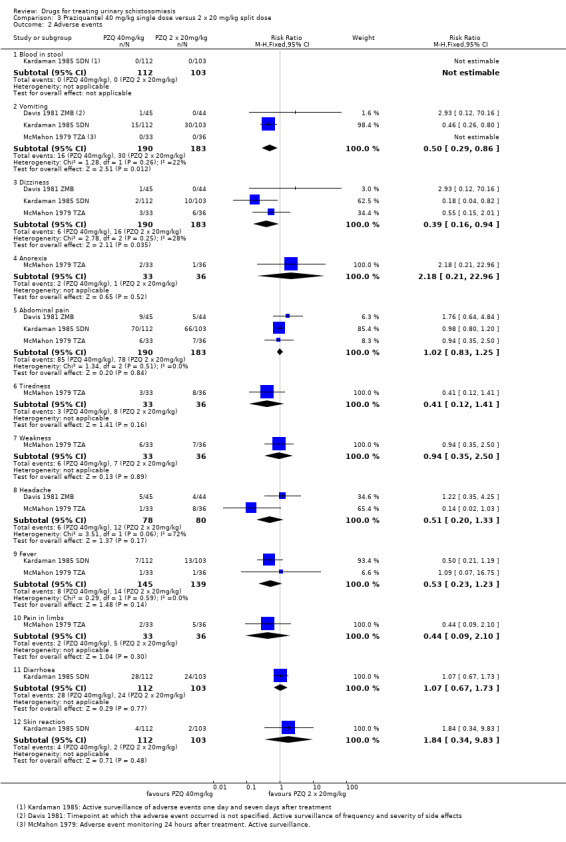
Comparison 3 Praziquantel 40 mg/kg single dose versus 2 x 20 mg/kg split dose, Outcome 2 Adverse events.
Praziquantel 40 mg/kg single dose versus split dose (comparison 3)
Splitting the dose of praziquantel 40 mg/kg into two 20 mg/kg doses over 24 hours has not been shown to improve tolerability and may actually cause more vomiting and dizziness.
Three trials compared the single 40 mg/kg dose with a split dose regimen giving two doses of 20 mg/kg over 24 hours. There was no statistically significant difference in treatment failure at one month (RR 0.75, 95% CI 0.51 to 1.11; 374 participants, three trials), three months (RR 0.74, 95% CI 0.45 to 1.2; 361 participants, three trials), or six months (RR 0.83, 95% CI 0.51 to 1.35; 234 participants, three trials, Analysis 3.1). Similarly percent egg reduction was over 90% for both groups (332 participants, three trials, see Appendix 2).
3.1. Analysis.
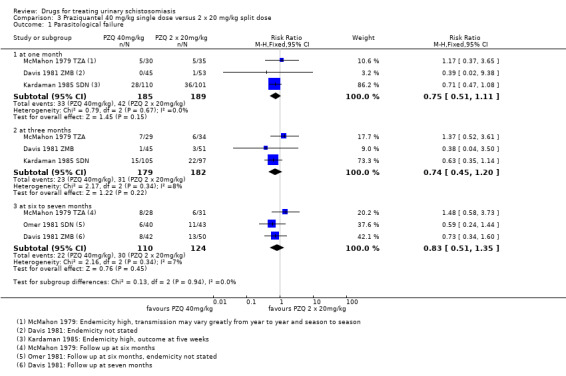
Comparison 3 Praziquantel 40 mg/kg single dose versus 2 x 20 mg/kg split dose, Outcome 1 Parasitological failure.
These trials enrolled 191 participants for a single dose of praziquantel 40 mg/kg and 195 participants for a split dose of 2 x 20 mg/kg. All trials used active surveillance for adverse events (see Appendix 5). Adverse events were generally reported to be mild and transient. However one trial reports significantly more vomiting and dizziness with the split dose compared to the single dose (vomiting: RR 0.5, 95% CI 0.29 to 0.86; dizziness: RR 0.39, 95% CI 0.16 to 0.94; 373 participants, three trials, Analysis 3.2).
Praziquantel 40 mg/kg single dose versus multiple doses (comparison 4 and 5)
There are too few trials to determine the optimal frequency and timing of repeated praziquantel dosing.
Two trials compared the standard single dose of praziquantel (40 mg/kg) with two or three doses given at two or three week intervals, and found no statistically significant differences in parasitological failure (Analysis 4.1, Analysis 4.2), percentage egg reduction (Appendix 2), or clinical resolution (Appendix 3; Analysis 4.3).
4.1. Analysis.
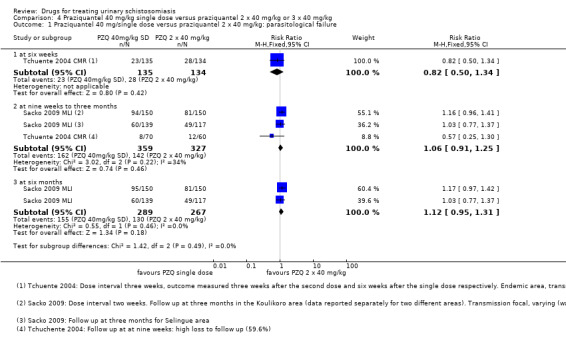
Comparison 4 Praziquantel 40 mg/kg single dose versus praziquantel 2 x 40 mg/kg or 3 x 40 mg/kg, Outcome 1 Praziquantel 40 mg/single dose versus praziquantel 2 x 40 mg/kg: parasitological failure.
4.2. Analysis.
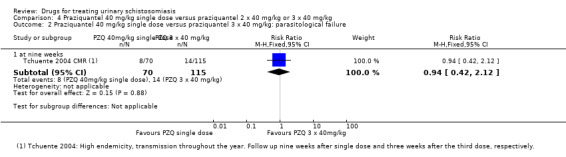
Comparison 4 Praziquantel 40 mg/kg single dose versus praziquantel 2 x 40 mg/kg or 3 x 40 mg/kg, Outcome 2 Praziquantel 40 mg/kg single dose versus praziquantel 3 x 40 mg/kg: parasitological failure.
4.3. Analysis.
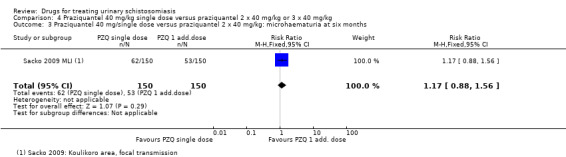
Comparison 4 Praziquantel 40 mg/kg single dose versus praziquantel 2 x 40 mg/kg or 3 x 40 mg/kg, Outcome 3 Praziquantel 40 mg/single dose versus praziquantel 2 x 40 mg/kg: microhaematuria at six months.
One additional very small trial from a high transmission setting in Gabon (van den Biggelaar 02 GAB), compared praziquantel 40 mg/kg every three months for two years to a single dose of praziquantel 40 mg/kg given at the beginning of the trial. At two years, patients who received only one dose of praziquantel had almost three times the risk of treatment failure compared to multiple doses (RR 2.71, 95% CI 1.47 to 5.00; 62 participants, one trial, Analysis 5.1). Percent egg reduction was 96% after multiple doses and 80% after a single dose of praziquantel at two years (90 participants, see Table 12). These effects were no longer apparent one year after the last praziquantel dose.
5.1. Analysis.
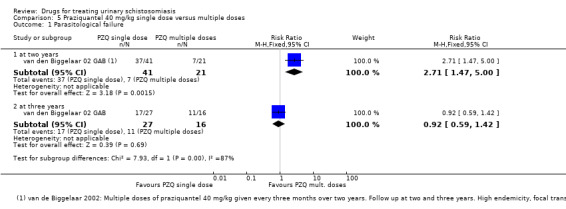
Comparison 5 Praziquantel 40 mg/kg single dose versus multiple doses, Outcome 1 Parasitological failure.
6. Praziquantel 40 mg/kg multiple doses versus single dose: % egg reduction.
| Study ID | Time point | Measure | Praziquantel 40 mg/kg single dose | % egg reduction | Praziquantel 40 mg/kg multiple doses | % egg reduction | Comments | ||
| Egg count/10 mL | Egg count/10 mL | ||||||||
| Baseline | Follow‐up | Baseline | Follow‐up | ||||||
| van den Biggelaar 02 GAB1 | 2 years | GMEC (IQR) |
47 N = 45 |
9 (2‐45) | 80.85 | 47 N = 45 |
2 (1‐3) | 95.74 | Significant P = 0.002 |
1Baseline egg counts not reported separately per treatment group; no difference at baseline stated. Praziquantel 40 mg/kg given every 3 months over 2 years. Location: Gabon, endemic area.
These trials did not report on adverse events.
Section B: Metrifonate
Metrifonate single dose versus placebo (comparison 6)
A single dose of metrifonate 10 mg/kg probably reduces egg excretion but is only marginally better than placebo at achieving cure.
Two trials compared a single dose of metrifonate to placebo, although one trial only reported outcomes at a single time point eight months after treatment (Stephenson 1989 KEN).
In the first trial (Pugh 1983 MWI), 80% of those treated with metrifonate continued to excrete eggs one month after treatment which was only marginally better than placebo (RR 0.83, 95% CI 0.74 to 0.94; 142 participants, one trial, Analysis 6.1), and no difference was seen at six months (RR 0.94, 95% CI 0.87 to 1.02; 102 participants, one trial, Analysis 6.1).
6.1. Analysis.
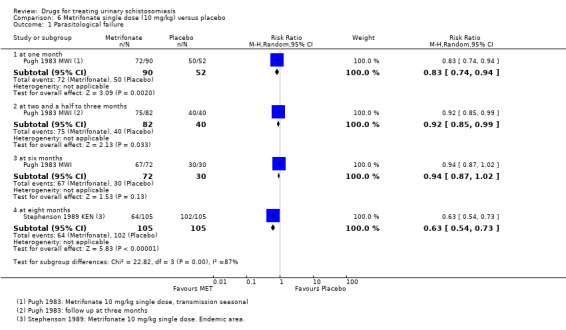
Comparison 6 Metrifonate single dose (10 mg/kg) versus placebo, Outcome 1 Parasitological failure.
In the second trial (Stephenson 1989 KEN), 61% of those treated with metrifonate continued to excrete eggs eight months after treatment compared with almost 100% who received placebo (RR 0.63, 95% CI 0.54 to 0.73, 210 participants, one trial, Analysis 6.1). Egg excretion was also reduced by more than 90% eight months after treatment compared to just 5% with placebo (210 participants, see Appendix 2).
The second trial also reported mean haemoglobin at baseline and eight months (with no difference between groups, Analysis 6.2), and various measures of nutrition and growth (see Appendix 4). However, this trial had three arms and the nutritional measures are reported for the metrifonate and praziquantel groups combined. Consequently, we were unable to evaluate the effect of metrifonate. Trial authors did not report adverse events.
6.2. Analysis.
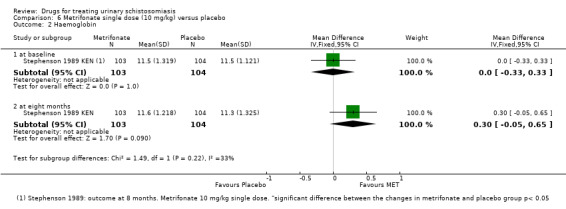
Comparison 6 Metrifonate single dose (10 mg/kg) versus placebo, Outcome 2 Haemoglobin.
Metrifonate multiple doses versus placebo (comparison 7)
Subsequently trials evaluated multiple doses of metrifonate given two weeks apart, which improved the proportion of patients being cured.
Two trials evaluated three doses of metrifonate 7.5 mg/kg given two weeks apart (Jewsbury 1976 ZWE; Stephenson 1985 KEN), and reported much reduced treatment failures compared to placebo at 11 weeks (RR 0.41, 95% CI 0.30 to 0.56; 93 participants, one trial, Analysis 7.1) and six months respectively (RR 0.30, 95% CI 0.24 to 0.37; 400 participants, one trial, Analysis 7.1).
7.1. Analysis.
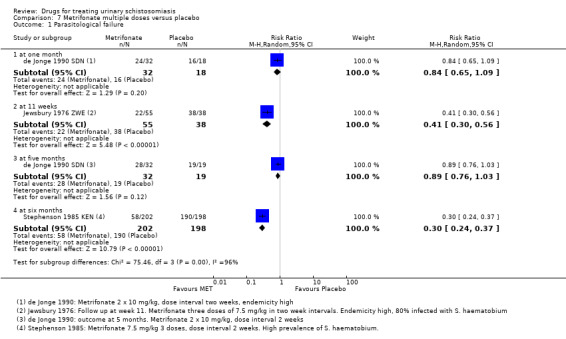
Comparison 7 Metrifonate multiple doses versus placebo, Outcome 1 Parasitological failure.
A third small trial (de Jonge 1990 SDN) comparing two 10 mg/kg doses given two weeks apart with placebo found very low levels of cure and no difference compared to placebo at one month or five months (51 participants, one trial, Analysis 7.1). However, this is the same trial that found very high levels of treatment failure with praziquantel, which may be a result of the highly sensitive method used for detecting low level egg excretion and the strict definition of cure.
All three trials found substantial reductions in the number of eggs being excreted at their various time points (> 90% reductions in all three trials, see Table 13).
7. Metrifonate 20 mg/kg given as divided dose versus placebo: % egg reduction.
| Study ID | Time point | Measure | Metrifonate 21.5 mg, 20 mg/kg given as divided dose | Placebo or no treatment | P value difference between groups | ||||
| Egg count/10 mL urine | % egg reduction | Egg count/10 mL urine | % egg reduction | ||||||
| Baseline | Follow‐up | Baseline | Follow‐up | ||||||
| de Jonge 1990 SDN1 | 1 month | median N = (reports min, max, 90th percentile and median of egg counts/10 mL) |
95 N = 38 |
1 N = 32 |
98.94 | 124 N = 21 |
58 N = 18 |
53.22 | Not significant P = 0.29 |
| Jewsbury 1976 ZWE2 | 11 weeks | median N = |
101 N = 32 |
0 | 100 | 26 N = 38 |
60 | ‐130.77 (increase) |
Not reported |
| 11 weeks | median N = |
40 N = 23 |
0 | 100 | |||||
| de Jonge 1990 SDN1 | 5 months | median N = (reports min, max, 90th percentile and median of egg counts/10 mL) |
124 N = 38 |
1 N = 32 |
99.19 | 124 N = 21 |
95 N = 19 |
23.38 | Not significant P = 0.27 |
| Stephenson 1985 KEN3 | 6 months | AMEC N = |
109 N = 202 |
7 | 94 | 110 N = 198 |
124 | ‐12.7 (increase) |
Not reported |
1Metrifonate 2 x 10 mg/kg, dose interval two weeks. Placebo: multivitamins.
2Reports two groups with metrifonate 7.5 mg x 3, dose interval two weeks. Control group: nil.
3 Metrifonate 3 x 7.5 mg/kg, dose interval one to two weeks.
Stephenson 1985 KEN also reported mean haemoglobin, with slightly higher values at six months after metrifonate compared to placebo (mean difference 0.3 G/dL, 95% CI 0.14 to 0.46; 400 participants, one trial, Analysis 7.2). The authors noted that hookworm endemicity was high, and metrifonate also has an effect on hookworm which could account for this finding.
7.2. Analysis.
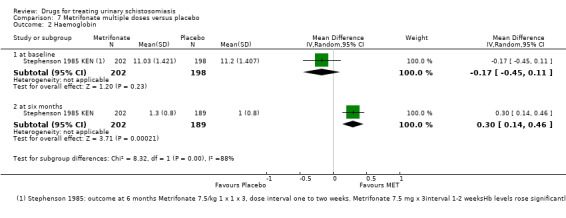
Comparison 7 Metrifonate multiple doses versus placebo, Outcome 2 Haemoglobin.
None of the trials reported on adverse events.
Direct comparisons of different metrifonate regimens (comparisons 8 and 9)
In one trial, multiple doses of 10 mg/kg were superior to a single dose.
One three‐arm trial directly compared a single dose of 10 mg/kg with two or three doses given two weeks apart. Parasitological failure at one month was 53% with a single dose, 40% with two doses, and 19% with three doses. The difference was statistically significant for three doses versus one dose (RR 0.36, 95% CI 0.17 to 0.77; 93 participants, one trial, Analysis 8.1), but not two doses versus one dose (RR 0.75, 95% CI 0.5 to 1.13; 112 participants, one trial, Analysis 8.1). Results were similar at four months (Analysis 8.2).
8.1. Analysis.
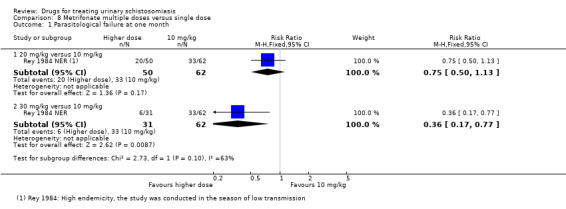
Comparison 8 Metrifonate multiple doses versus single dose, Outcome 1 Parasitological failure at one month.
8.2. Analysis.
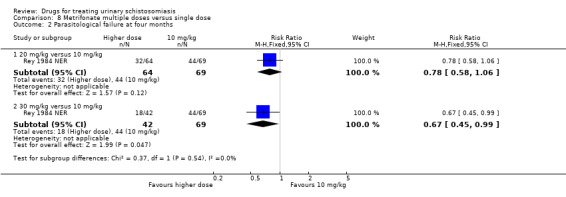
Comparison 8 Metrifonate multiple doses versus single dose, Outcome 2 Parasitological failure at four months.
The percent egg reduction was also improved from 37% after a single dose to 88% after three doses, although this was not maintained at the four months' follow‐up (see Appendix 2). This trial did not report on adverse events.
One additional trial (Abden Abdi 1989 SOM) compared three doses of 7.5 mg/kg given two weeks apart with three doses of 5 mg/kg given in one day. The trial detected no difference for parasitological failure at one month, three months or six months (201 participants, one trial, Analysis 9.1). Egg reduction at one month was above 90% after both metrifonate doses and was sustained (> 90%) at two, three and six months (201 participants, see Appendix 2). This trial recorded adverse events by active surveillance (Appendix 5). It did not detect a significant difference for any of the symptoms between treatment groups (201 participants, one trial, Analysis 9.2) The adverse events were mild and transient.Headache and abdominal pain were most common.
9.1. Analysis.
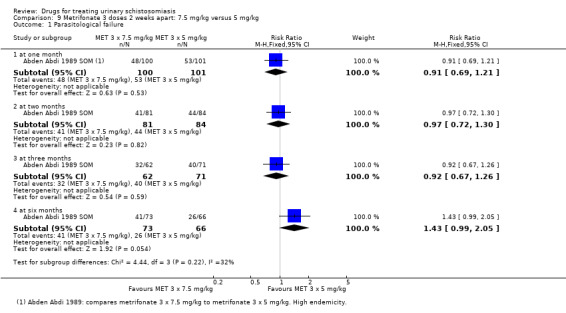
Comparison 9 Metrifonate 3 doses 2 weeks apart: 7.5 mg/kg versus 5 mg/kg, Outcome 1 Parasitological failure.
9.2. Analysis.
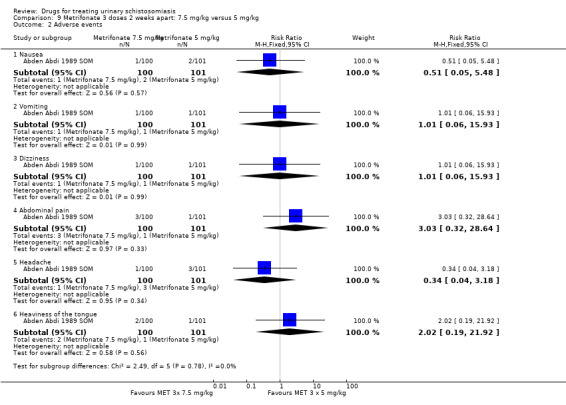
Comparison 9 Metrifonate 3 doses 2 weeks apart: 7.5 mg/kg versus 5 mg/kg, Outcome 2 Adverse events.
Section C: Praziquantel versus metrifonate
Praziquantel 40 mg/kg single dose versus metrifonate 10 mg/kg single dose (comparison 10)
Single dose praziquantel 40 mg/kg was more effective than single dose metrifonate 10 mg/kg in curing patients and reducing egg excretion.
Three trials compared the standard dose of praziquantel 40 mg/kg with a single dose of metrifonate 10 mg/kg, although one trial only reported outcomes at eight months after treatment (Stephenson 1989 KEN).
In the first trial (Pugh 1983 MWI), parasitological failure at one month was halved with praziquantel 40 mg/kg compared to metrifonate 10 mg/kg (RR 0.46, 95% CI 0.34 to 0.61; 183 participants, one trial, Analysis 10.1). Treatment failure increased in both groups over the following five months which the authors suspect was due to egg excretion by maturing worms, as transmission and re‐infection were low in the trial setting (Analysis 10.1). The second trial (Wilkins 1987 GMB), also found praziquantel to be superior to metrifonate at two to three months as its only time point (RR 0.45, 95% CI 0.27 to 0.75; 72 participants, one trial, Analysis 10.1).
10.1. Analysis.
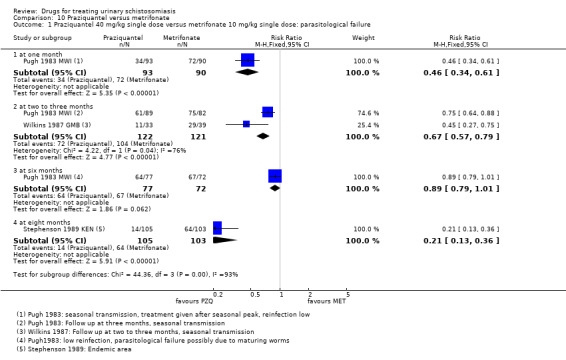
Comparison 10 Praziquantel versus metrifonate, Outcome 1 Praziquantel 40 mg/kg single dose versus metrifonate 10 mg/kg single dose: parasitological failure.
The third trial (Stephenson 1989 KEN), found substantial reductions in both treatment failure (RR 0.21, 95% CI 0.13 to 0.36; 208 participants, one trial, Analysis 10.1) and egg excretion (see Appendix 2), with praziquantel compared to metrifonate. Haemoglobin levels measured in this trial were higher in the praziquantel treatment arm both at baseline and at follow‐up (208 participants, one trial, Analysis 10.2). The trial did not detect a difference in growth parameters between groups but does not report them separately (see Appendix 4).
10.2. Analysis.
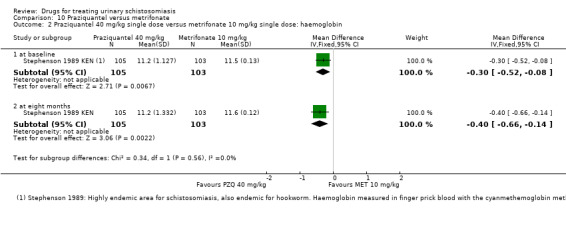
Comparison 10 Praziquantel versus metrifonate, Outcome 2 Praziquantel 40 mg/kg single dose versus metrifonate 10 mg/kg single dose: haemoglobin.
None of the trials reported on adverse events.
Praziquantel 40 mg/kg single dose versus multiple doses of metrifonate 10 mg/kg
Two small trials found no difference in parasitological treatment failure or egg excretion between single dose praziquantel 40 mg/kg and two or three doses of metrifonate 10 mg/kg.
Two small trials compared praziquantel 40 mg/kg single dose to two and three doses of metrifonate 10 mg/kg given two weeks apart. The trials detected no difference in parasitological treatment failure at different time points and with different metrifonate regimens. However, in one trial both drugs performed poorly (de Jonge 1990 SDN), and in one trial both performed well (Al Aska 1990 SAU) (see Analysis 10.3). The trial where both drugs performed poorly for parasitological failure has been discussed above and this is likely to be due to the very sensitive method for detecting eggs. In this trial, both drugs reduced mean egg excretion by over 98% at one month and five months (see Appendix 2), and a decrease in haematuria by over 90% at one month. Reduction in proteinuria was almost 80% in both groups (see Appendix 3).
10.3. Analysis.
Comparison 10 Praziquantel versus metrifonate, Outcome 3 Praziquantel 40 mg/kg single dose versus metrifonate 20 and 30 mg/kg given as split doses: parasitological failure.
Only Al Aska 1990 SAU reported adverse events; dizziness was more common after praziquantel (RR 2.9, 95% CI 1.59 to 5.3; 100 participants, one trial, Analysis 10.4). Dizziness (20% in the praziquantel group and 10% in the metrifonate group) and abdominal pain (12% both in the praziquantel and metrifonate group) were the most common side effects (Appendix 5).
10.4. Analysis.
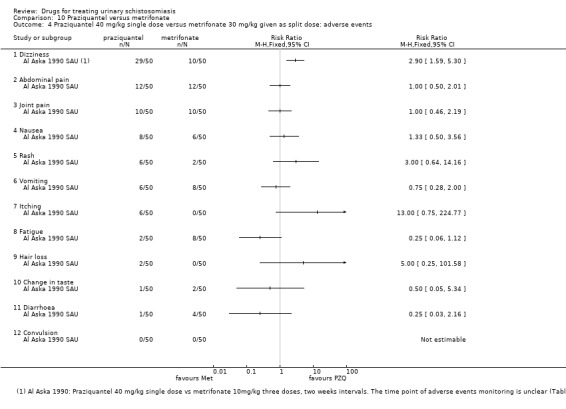
Comparison 10 Praziquantel versus metrifonate, Outcome 4 Praziquantel 40 mg/kg single dose versus metrifonate 30 mg/kg given as split dose: adverse events.
Additional comparisons of praziquantel and metrifonate
One small trial compared a single dose of praziquantel 30 mg/kg to three doses of metrifonate 10 mg/kg given two weeks apart and found no difference in parasitological failure at two months, but a statistically significant difference in favour of praziquantel at four months (RR 0.24, 95% CI 0.07 to 0.8; 52 participants, one trial, Analysis 10.5). Egg reduction at four months was above 98% in both treatment groups (Appendix 2). In this trial, abdominal pain was more common in the metrifonate group (RR 0.33, 95% CI 0.12 to 0.92; 60 participants, one trial, Analysis 10.6), while no difference was detected for the eight other clinically diagnosed symptoms reported.
10.5. Analysis.
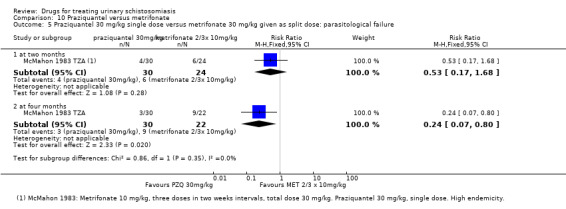
Comparison 10 Praziquantel versus metrifonate, Outcome 5 Praziquantel 30 mg/kg single dose versus metrifonate 30 mg/kg given as split dose: parasitological failure.
10.6. Analysis.
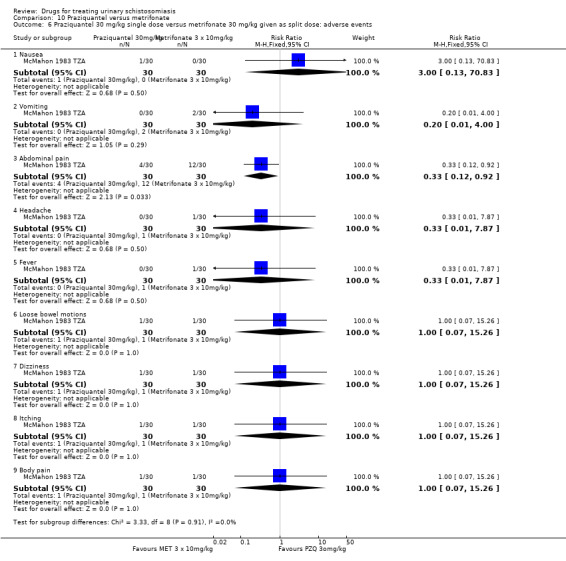
Comparison 10 Praziquantel versus metrifonate, Outcome 6 Praziquantel 30 mg/kg single dose versus metrifonate 30 mg/kg given as split dose: adverse events.
One large population‐based trial from Kenya compared praziquantel 40 mg/kg given once a year to metrifonate 10 mg/kg given three times a year. After one year, this trial detected no difference in treatment failure, haematuria or proteinuria (1400 participants, one trial, Analysis 10.7), but mean egg excretion was reduced by over 80% in both groups at one year (Appendix 2). There continued to be no difference in parasitological failure at two years, but praziquantel was superior in the third year (RR 0.62, 95% CI 0.42 to 0.93; 827 participants one trial, Analysis 10.8). Ultrasound findings, recorded in a sub‐sample of children, were inconclusive (373 participants, Appendix 6).
10.7. Analysis.
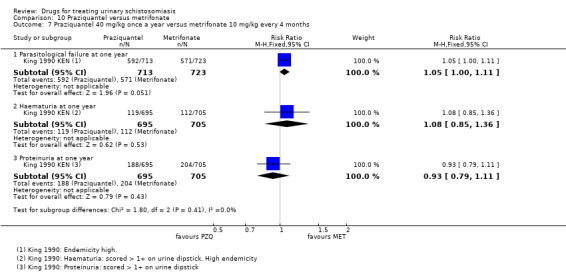
Comparison 10 Praziquantel versus metrifonate, Outcome 7 Praziquantel 40 mg/kg once a year versus metrifonate 10 mg/kg every 4 months.
10.8. Analysis.
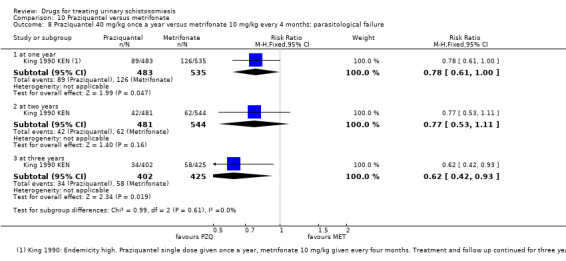
Comparison 10 Praziquantel versus metrifonate, Outcome 8 Praziquantel 40 mg/kg once a year versus metrifonate 10 mg/kg every 4 months: parasitological failure.
One further small trial compared a single dose of praziquantel 40 mg/kg with a combination of praziquantel 10 mg/kg and metrifonate 10 mg/kg. At two to three months there was no difference in treatment failure (72 participants, one trial, Analysis 10.9). Percent egg reduction was 99.4% after praziquantel alone and 92.9% after the combination treatment (see Appendix 2).
10.9. Analysis.
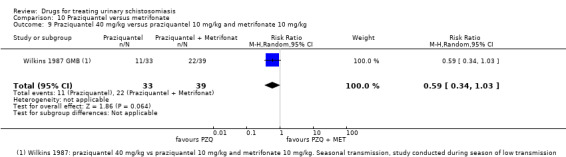
Comparison 10 Praziquantel versus metrifonate, Outcome 9 Praziquantel 40 mg/kg versus praziquantel 10 mg/kg and metrifonate 10 mg/kg.
Section D: Artesunate
Artesunate versus placebo (comparison 11)
The two placebo controlled trials of artesunate had inconsistent results, and the single trial at low risk of bias found only a modest effect on egg excretion compared to placebo.
Two trials compared artesunate 4 mg/kg once daily for three days with placebo. The two trials had inconsistent results on parasitological failure, with one trial finding no difference between artesunate and placebo, and one finding lower treatment failures with artesunate at eight weeks (251 participants, two trials, Analysis 11.1). The trial finding an effect was at unclear risk of both selection and detection bias due to an inadequate description of trial methods (Inyang Etoh 2009 NGA).
11.1. Analysis.
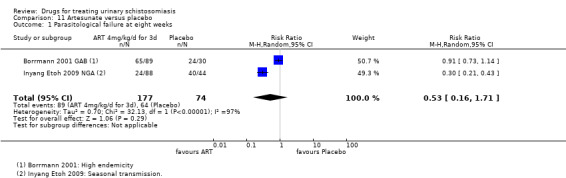
Comparison 11 Artesunate versus placebo, Outcome 1 Parasitological failure at eight weeks.
Both trials found that artesunate reduced egg excretion compared to placebo (Table 14), but the percent reduction was low compared to that seen in placebo controlled trials of praziquantel (percent egg reductions of between 52% and 69%).
8. Artesunate versus placebo: % egg reduction.
| Study ID | Time point | Measure | Artesunate 4 mg/kg/d for 3 days | Placebo | P value difference between groups | ||||
| Egg count/10 mL urine |
% egg reduction |
Egg count/10 mL |
% egg reduction |
||||||
| Baseline | Follow‐up | Baseline | Follow‐up | ||||||
| Borrmann 2001 GAB | 8 weeks | GMEC (range) 95% CI N = |
35.22 (1‐4360) N = 90 |
10.8 N = 89 |
69.34 | 21.56 (1‐778) N = 30 |
11.41 N = 30 |
47.1 | Not significant |
| Inyang Etoh 2009 NGA1 | 8 weeks | Mean ova count ± SD N = |
39.8 ± 1.1 N = 52 |
19.1 ± 1.0 N = 44 |
52.1 | 34.1 ± 0.8 N = 52 |
72.0 ± 2.3 N = 44 |
111.5 (increase) |
P for "therapeutic efficacy" < 0.001 |
1Treatment group: Praziquantel 40 mg/kg without placebo. Inyang Etoh 2009 NGA also reports a second treatment group (Praziquantel 40 mg/kg with placebo), data not shown.
The trial at unclear risk of bias also reported improved reductions in haematuria and proteinuria compared to placebo, while the trial at low risk of bias (Borrmann 2001 GAB) found no effect on proteinuria (see Appendix 3). No differences in adverse events were reported (see Appendix 5, Analysis 11.3).
11.3. Analysis.
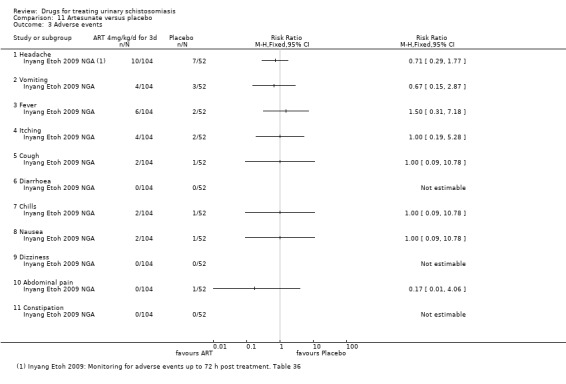
Comparison 11 Artesunate versus placebo, Outcome 3 Adverse events.
Praziquantel versus artesunate (comparison 12)
The results of the three trials are inconsistent, with the single trial at low risk of bias finding only a modest reduction in egg excretion with artesunate.
Three trials (Borrmann 2001 GAB; Inyang Etoh 2009 NGA; Keiser 2010 CIV) compared artesunate 4 mg/kg/d for three days with praziquantel 40 mg/kg single dose.
The three trials had mixed results. In two trials artesunate performed poorly, with parasitological treatment failures of over 70% at one month and two months respectively (Borrmann 2001 GAB; Keiser 2010 CIV). In these trials praziquantel was clearly superior (Analysis 12.1). In the third trial (Inyang Etoh 2009 NGA), at unclear risk of bias due to inadequate description of trial methods, artesunate performed similarly to praziquantel with 28% treatment failures at two months (Analysis 12.1).
12.1. Analysis.
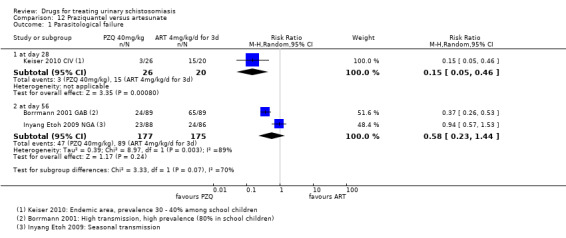
Comparison 12 Praziquantel versus artesunate, Outcome 1 Parasitological failure.
The percent egg reduction with artesunate varied across the three trials from 52% to 85% (see Appendix 2). In the single trial where both praziquantel and artesunate performed well at reducing treatment failures, both drugs had fairly modest effects on egg excretion (Inyang Etoh 2009 NGA).
Only the trial at unclear risk of bias (Inyang Etoh 2009 NGA) reported substantial effects of artesunate on haematuria and proteinuria (see Appendix 3). In the trial at low risk of bias (Borrmann 2001 GAB) praziquantel was clearly superior at reducing microhematuria (RR 0.43, 95% CI 0.3 to 0.62; 178 participants, one trial, Analysis 12.2).
12.2. Analysis.
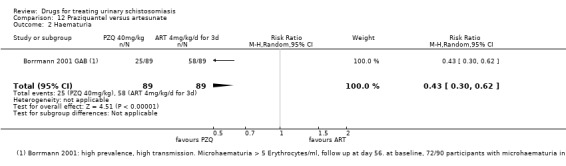
Comparison 12 Praziquantel versus artesunate, Outcome 2 Haematuria.
All trials reported on adverse events with no significant differences noted between groups (see Appendix 5, Analysis 12.3).
12.3. Analysis.
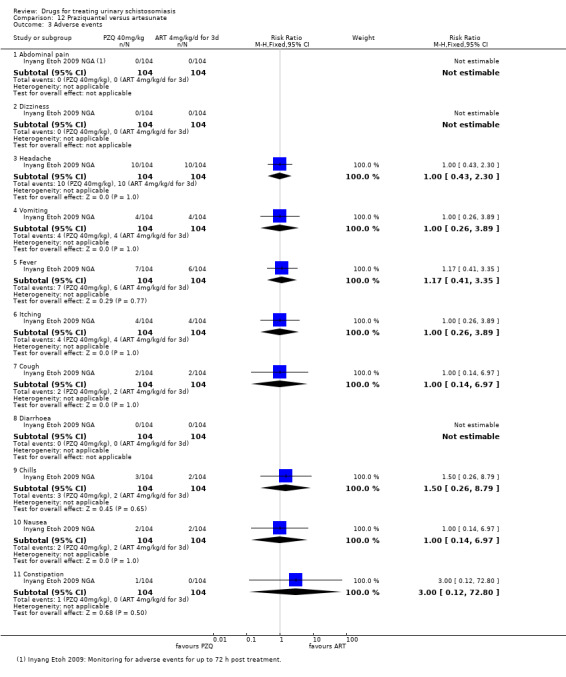
Comparison 12 Praziquantel versus artesunate, Outcome 3 Adverse events.
Praziquantel versus praziquantel plus artesunate (comparison 13)
The results of the two trials were inconsistent but the trial at low risk of bias found no benefit with adding artesunate to praziquantel.
Two of the trials comparing artesunate with praziquantel also had a treatment arm where patients received both drugs (Borrmann 2001 GAB; Inyang Etoh 2009 NGA). Again, in the trial at low risk of bias (Borrmann 2001 GAB) adding artesunate to praziquantel did not substantially reduce treatment failures or percent egg reduction at eight weeks compared to praziquantel alone, whereas in the trial at unclear risk of bias (Inyang Etoh 2009 NGA), adding artesunate improved outcomes (Analysis 13.1; Table 15; Appendix 2). No differences in adverse events were reported (see Appendix 5).
13.1. Analysis.
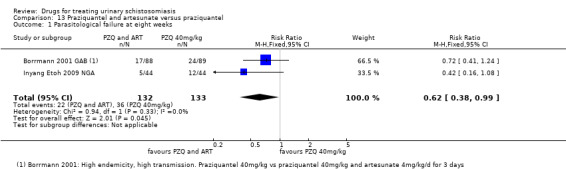
Comparison 13 Praziquantel and artesunate versus praziquantel, Outcome 1 Parasitological failure at eight weeks.
9. Praziquantel and Artesunate versus Praziquantel: % egg reduction.
| Study ID | Time point | Measure | Praziquantel 40 mg/kg single dose and artesunate 4 mg/kg/d for 3 days | Praziquantel 40 mg/kg single dose | P value difference between groups | ||||
| Egg count/10 mL | % egg reduction | Egg count/10 mL | % egg reduction | ||||||
| Baseline | Follow‐up | Baseline | Follow‐up | ||||||
| Borrmann 2001 GAB | 8 weeks | GMEC (range), (95% CI) N = |
31.5 (1 to 3225) N = 90 |
0.36 N = 88 |
98.8 | 38.51 (1 to 3313) N = 90 |
1.11 (0.7 to 1.7) N = 89 |
97.11 | Not significant |
| Inyang Etoh 2009 NGA1 | 8 weeks | mean ± SD N = |
62.2 ± 2.1 N = 52 |
4.0 (± 15.2) N = 44 | 93.6 | 39.8 (± 1.1) N = 52 |
19.1 (± 1.0) N = 44 |
52.1 | Not reported |
1Treatment group: Praziquantel 40 mg/kg without placebo. Inyang Etoh 2009 NGA also reports a second treatment group (Praziquantel 40 mg/kg with placebo), data not shown.
Section E: Others
Mefloquine versus sulfadoxine‐pyrimethamine (comparison 14)
In a single trial comparing the use of mefloquine and sulfadoxine‐pyrimethamine as intermittent preventive treatment for malaria in pregnancy, a re‐analysis of the small number of mothers infected with S. haematobium found more women were cured at one month after mefloquine compared to sulfadoxine‐pyrimethamine (RR 0.57, 95% CI 0.4 to 0.83; 44 participants, one trial, Analysis 14.1), and an egg reduction of 80% four weeks after treatment and 98% ten weeks after treatment (see Appendix 2).
14.1. Analysis.
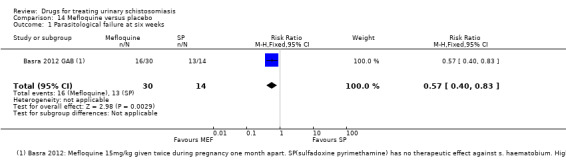
Comparison 14 Mefloquine versus placebo, Outcome 1 Parasitological failure at six weeks.
Praziquantel versus mefloquine alone or mefloquine in combination with artesunate (comparison 15 and 16)
A single small trial (Keiser 2010 CIV) reported lower treatment failures with praziquantel 40 mg/kg alone than with mefloquine 25 mg/kg (RR 0.15, 95% CI 0.05 to 0.43; 45 participants, one trial, Analysis 15.1) or with mefloquine in combination with artesunate 4 mg/kg/d for three days (RR 0.23, 95% CI 0.07 to 0.74; 44 participants, one trial, Analysis 16.1). At four weeks, this trial reports a percent egg reduction of 74% at four weeks with mefloquine alone (19 participants), 96% with mefloquine and artesunate combined, and 97% with praziquantel (Appendix 2).
15.1. Analysis.
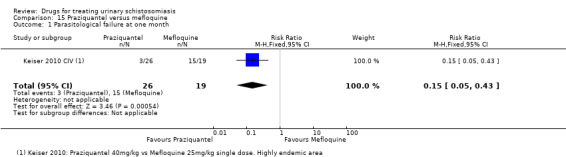
Comparison 15 Praziquantel versus mefloquine, Outcome 1 Parasitological failure at one month.
16.1. Analysis.
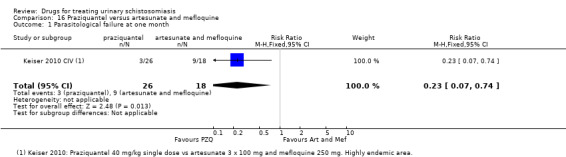
Comparison 16 Praziquantel versus artesunate and mefloquine, Outcome 1 Parasitological failure at one month.
Keiser 2010 CIV recorded adverse events by active, prospective surveillance. Adverse events were mild to moderate and common in all groups. There were no statistically significant differences in any individual adverse event (Appendix 5).
Praziquantel versus praziquantel and albendazole (comparison 17)
One trial (Olds 1999 KEN) compared a single dose of praziquantel 40 mg/kg with a combination of single dose praziquantel 40 mg/kg plus albendazole 400 mg at day 45 (RR 0.9, 95% CI 0.62 to 1.3; 193 participants, one trial, Analysis 17.1). The authors concluded that albendazole does not influence the effect of praziquantel.
17.1. Analysis.
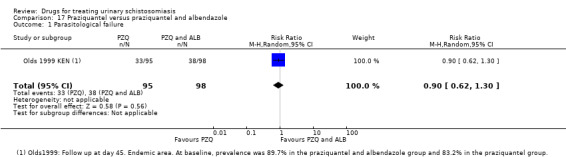
Comparison 17 Praziquantel versus praziquantel and albendazole, Outcome 1 Parasitological failure.
Adverse events were monitored by active, prospective surveillance and described as mild and transient. Diarrhoea, headache and abdominal pain were observed most frequently, but adverse events were reported for participants treated for S. haematobium and S. mansoni together (Appendix 5).
Discussion
For a summary of the main results of the review and GRADE assessment of the quality of evidence see: Table 1; Summary of findings table 2; Table 2; Table 3; Table 4; Table 5; and Table 6.
Summary of main results
On average, a single 40 mg/kg dose of praziquantel reduced the proportion of people still excreting S. haematobium eggs in their urine by around 60% compared to placebo at one to two months after treatment (high quality evidence), and reduced the mean number of schistosome eggs in the urine by over 95% in five out of six trials (high quality evidence). Splitting praziquantel 40 mg/kg into two doses over 12 hours probably has no benefits over a single dose.
Two small trials compared a single 40 mg/kg dose of praziquantel with two or three doses of 10 mg/kg metrifonate and found no differences in cure. In one trial both drugs performed badly and in one trial both performed well.
Three trials evaluated the antimalarial artesunate, and two trials evaluated mefloquine, with inconsistent results.
Overall completeness and applicability of evidence
The WHO currently recommend that schistosomiasis is treated with a single dose of praziquantel of at least 40 mg/kg (WHO 2006). In this review we found no trials evaluating doses higher than 40 mg in urinary schistosomiasis, but doses of 40 mg/kg or even 30 mg/kg are effective at reducing egg excretion and achieving cure.
Of all the drugs that have been evaluated for treating urinary schistosomiasis, praziquantel has by far the strongest evidence base. It has been evaluated across a wide range of endemic countries, and most trials were conducted in children who bear the highest burden of disease. However, few trials included children younger than five years of age, and Stothard 2013 suggested that higher doses of praziquantel might be required for this group. We would have liked to explore this possibility through an analysis stratified by age, but the data did not allow this and no firm conclusions can be made. In addition, most trials concentrated on parasitological efficacy, and few reported clinical outcomes such as improvement in haematuria or anaemia. Data on resolution of long‐term morbidity after treatment, as nutritional outcomes and sonographic findings are very rare, and follow‐up is limited to less than one year.
The absolute proportion of people cured by praziquantel varied between trials while percent egg reduction was relatively homogenous. This may be explained by low sensitivity and negative predictive value of the diagnostic test, compounded with the fact that egg yield varies during the day and with physical activity. This means that patients with few eggs in their urine may be variably declared as positive or negative in different settings. The proportional reduction in the mean egg counts from before to after treatment is less prone to this error. It also appears that some trials based post‐treatment egg reduction on the whole trial population (including cured patients with zero egg counts), while other trials based the post‐treatment calculations on those patients still excreting eggs. We were unable to combine egg reduction values in meta‐analysis, and assess statistical significance, due to the poor reporting of standard deviations and methods for calculating the mean (Table 8).
None of the included trials suggested drug resistance as a possible cause of high parasitological failure, or of recurrent schistosomiasis over prolonged follow‐up. In high transmission areas two mechanisms could explain rising parasitological failure over time: maturation of immature worms (which escape the action of praziquantel) to egg producing adults, and reinfection.
Previously the WHO also recommended metrifonate at 7.5 mg/kg for three doses (given two weeks apart), but this drug is now largely unavailable (Danso‐Appiah 2008). We found some evidence that repeated doses of metrifonate had reasonable antischistosomal effects but we found no trials directly comparing this dose with the standard dose of praziquantel. Combining praziquantel with metrifonate is one possible strategy for improving parasitological cure as they attack S. haematobium by different mechanisms (Utzinger 2004). However, we only found one small trial evaluating a combination approach and this used a low dose of praziquantel rather than the standard 40 mg/kg (Wilkins 1987 GMB).
Antimalarials (such as artesunate and mefloquine) given alone or in combination with praziquantel are another potential future treatment option, but the current evidence base is limited to a few trials with inconsistent results. As many locations in sub‐Saharan Africa are co‐endemic for schistosomiasis and malaria, there are also concerns about development of Plasmodium parasite resistance to artemisinins, especially as they would be used in a single dose and without a companion antimalarial drug (Utzinger 2004). Any change in policy would need to fully consider this potential public health harm.
Quality of the evidence
We used the GRADE approach to assess the quality for the evidence.
We consider the evidence for substantial benefits with praziquantel compared to placebo to be of high quality, meaning we have confidence in this result. Many of the included trials are old, but reassuringly the findings of the most recent trial conducted in 2005/2006 are consistent with the older studies.
However, we consider most of the evidence for other comparisons in this review to be of low or even very low quality. Most of the trials evaluating metrifonate are old and precede guidelines on transparent reporting of clinical trials. As such, many trials lacked adequate descriptions of methods to allow judgements on risk of bias, and so risk of bias has been classified as unclear. Trials were also generally small and underpowered to reliably detect or exclude effects.
Of the three trials reporting on the antischistosomal effects of artesunate, only one was at low risk of bias and this trial found little effect with artesunate compared to placebo (Borrmann 2001 GAB). Although the metanalysis suggests artesunate may improve cure when added to praziquantel, this evidence was of low quality due to inconsistency between trials, and the single trial showing a large effect being at unclear risk of bias for all domains.
Potential biases in the review process
Our information specialist followed a detailed, reproducible search strategy, and we searched reference lists of included trials. However, some trials might not be available online, and therefore an electronic search will not identify them.
In many cases, clarification of information with authors was not possible as no contact e‐mail addresses were available as the trials were very old.
Agreements and disagreements with other studies or reviews
Two recent systematic reviews evaluated the use of artemisinins in treating urinary schistosomiasis (Liu 2011; Pérez del Villar 2012), and both concluded that the combination of artesunate plus praziquantel is superior to praziquantel alone, While we find some evidence to support this we conclude that this evidence is only of low quality and encourage further high quality and adequately powered trials before any change in treatment policy. Of note, the trial at lowest risk of bias (Borrmann 2001 GAB), found no significant difference in cure between artesunate alone and placebo, or between praziquantel plus artesunate and praziquantel alone.
One further systematic review evaluated single or repeated doses of praziquantel, and found no evidence of benefit with repeated dosing compared to a single dose in people with S. haematobium infection (King 2011). We would agree that repeating doses two or three weeks apart does not seem to provide benefit over a single dose based on two trials with 686 participants. However, repeating doses at three monthly intervals over two years did seem to provide some additional benefits in a single small trial and further trials could evaluate this.
Authors' conclusions
Implications for practice.
Praziquantel is the most studied drug for treating urinary schistosomiasis and has the strongest evidence base. Although there is some evidence that 30 mg/kg may be sufficient, operationally this would prove difficult as 40 mg/kg is used to treat people with intestinal schistosomiasis, and the two diseases often overlap.
Implications for research.
Potential strategies to improve future treatments for schistosomiasis include the combination of praziquantel with metrifonate, or with antimalarials with antischistosomal properties such as artesunate and mefloquine. Evaluation of these combinations requires rigorous. adequately powered trials using standardized outcome measures. It is both important and urgent that these parameters be agreed upon and applied. Trial protocols with standardised diagnostic methods, time points of follow‐up and efficacy outcomes would enable us to combine trials in meta‐analysis and to reduce heterogeneity between trials.
What's new
| Date | Event | Description |
|---|---|---|
| 7 July 2014 | New search has been performed | The review has been updated and revised with a new author team. |
| 7 July 2014 | New citation required but conclusions have not changed | A new author team was put in place for this review update. |
Acknowledgements
We would like to thank the authors of the previous version of this review Anthony Danso‐Appiah, Jürg Utzinger, and Jianping Liu, who stood down for this update.
We thank the trial authors for their research, which enabled us to conduct a systematic review and particularly trial authors that responded to our requests for further information. We acknowledge Paul Garner for his expertise, experience and encouragement; Anne Marie Stephani, whose ready technical assistance with Review Manager (RevMan) was most helpful; and to Vittoria Lutje for her approachability and support. The editorial base of the Cochrane Infectious Diseases Group is funded by the UK Department for International Development for the benefit of developing countries.
Appendices
Appendix 1. Search strategy
| Search set | CIDG SR* | CENTRAL | MEDLINE** | EMBASE** | LILACS** |
| 1 | Schistosoma haematobium | SCHISTOSOMIASIS HAEMATOBIA | SCHISTOSOMA HAEMATOBIA | SCHISTOSOMA‐HAEMATOBIA | Schistosoma haematobium |
| 2 | praziquantel | urinary schistosomiasis | urinary schistosomiasis | urinary schistosomiasis | urinary schistosomiasis |
| 3 | metrifonate | 1 OR 2 | 1 OR 2 | 1 OR 2 | 1 or 2 |
| 4 | albendazole | praziquantel | praziquantel | praziquantel | praziquantel |
| 5 | artesunate | metrifonate | metrifonate | metrifonate | metrifonate |
| 6 | artemether | albendazole | albendazole | albendazole | albendazole |
| 7 | 2‐6/OR | artesunate | artesunate | artesunate | artesunate |
| 8 | 1 AND 7 | artemether | artemether | artemether | artemether |
| 9 | ‐‐ | 4‐8/OR | 4‐8/OR | 4‐8/OR | 4‐8/OR |
| 10 | ‐‐ | 3 AND 9 | 3 AND 9 | 3 AND 9 | 3 AND 9 |
| 11 | ‐‐ | ‐‐ | Limit 10 to human | Limit 10 to human | ‐‐ |
*Cochrane Infectious Diseases Group Specialized Register.
**Search terms used in combination with the search strategy for retrieving trials developed by The Cochrane Collaboration (Lefebvre 2008); upper case: MeSH or EMTREE heading; lower case: free text term.
Appendix 2. Appendix: Additional tables for egg reduction data
Praziquantel 40 mg/kg single dose versus placebo: parasitological failure stratified by severity of infection
| Trial ID | Time point | Stratification | GMEC or miracidial count/10 mL urine | Praziquantel 40 mg/kg single dose | Placebo | P value difference between groups |
| Parasitological failure | Parasitological failure | |||||
| Taylor 1988 ZWE | 1 month | light | < 100 N = 77 for all strata |
37.7% | 100% | Not reported |
| heavy | > 100 | 91.7% | 100% | |||
| McMahon 1979 TZA | 1 month | light | 60 to 250 N = 101 for all strata |
13.6% | ― | ― |
| moderate | 251 to 500 | 12.1% | ― | |||
| heavy | ≥ 500 | 34.5% | ― | |||
| King 2002 KEN | 6 weeks | light | 0 to 99 N = 48 |
6/48 12% |
― | ― |
| moderate | 100 to 399 N = 27 |
13/27 50% |
― | |||
| heavy | ≥ 400 N = 26 |
15/26 59% |
― | |||
| McMahon 1983 TZA | 2 months | light | 250 to 500 miracidia N = 10 |
1/10 | ― | ― |
| moderate | 501 to 1000 miracidia N = 10 |
2/10 | ― | |||
| heavy | > 1000 miracidia N = 10 |
1/10 | ― | |||
| McMahon 1979 TZA | 3 months | light | 60 to 250 N = 101 for all strata |
15.9% | ― | ― |
| moderate | 251 to 500 | 8.1% | ― | |||
| heavy | ≥ 500 | 46.4% | ― | |||
| King 1989 KEN | 3 months | light | 1 to 99 N = 9 |
0% | ― | ― |
| moderate | 100 to 399 N = 29 |
10% | ― | |||
| heavy | ≥ 400 N = 18 |
33% | ― | |||
| Taylor 1988 ZWE | 3 months | light | < 100 N = 77 for all strata |
43.1% N = 90 for all strata |
98.3% | Not reported |
| heavy | > 100 | 79.2% | 95.5% | |||
| McMahon 1983 TZA | 4 months | light | 250 to 500 miracidia N = 10 |
1/10 | ― | ― |
| moderate | 501 to 1000 miracidia N = 10 |
1/10 | ― | |||
| heavy | > 1000 miracidia N = 10 |
1/10 | ― | |||
| McMahon 1979 TZA | 6 months | light | 60 to 250 N = 101 for all strata |
18.6% | ― | ― |
| moderate | 251 to 500 | 26.3% | ― | |||
| heavy | ≥ 500 | 28% | ― | |||
| Taylor 1988 ZWE | 6 months | light | < 100 N = 77 for all strata |
25% | 96.1% | Not reported |
| heavy | > 100 | 36.8% | 100% | |||
| Omer 1981 SDN | 6 months | light | 60 to 249 N = 11 |
1/11 | ― | ― |
| moderate | 250 to 499 N = 11 |
3/11 | ― | |||
| heavy | > 500 N = 14 |
2/14 | ― |
Praziquantel 40 mg/kg single dose versus placebo: % egg reduction stratified by severity of infection
| Trial ID | Time point | Stratum | By GMEC/10 mL/urine or by "egg count" | Praziquantel 40 mg/kg single dose | Placebo | P value difference between groups | ||||
| GMEC/10 mL urine | % egg reduction | GMEC/10 mL urine | % egg reduction | |||||||
| Baseline | Follow‐up | Baseline | Follow‐up | |||||||
| Pugh 1983 MWI | 1 month | light | 20 to 124 | 51.7 N = 21 |
2.1 | 95.93 | 52.7 N = 20 |
35.6 | 32.45 | Not reported |
| moderate | 125 to 499 | 234.7 N = 30 |
1.5 | 99.36 | 248.0 N = 32 |
256.2 | ‐ 3.2 % (increase) | |||
| heavy | 500 to 1999 | 907.6 N = 38 |
1.7 | 99.86 | ― | ― | ― | |||
| very heavy | > 2000 | 3433.3 N = 8 |
2.8 | 99.9 | ― | ― | ― | |||
| Taylor 1988 ZWE1 | 1 month | light | < 50 | 15.1 N = n.r. |
0.4 | 97.35 | 15.7 | 37.5 | 138.85 (increase) | ― |
| heavy | > 100 | 204.7 N = n.r. |
4.0 | 98 | 191.9 | 147.0 | 23.4 | ― | ||
| King 2002 KEN | 6 weeks | light | 0 to 99 | N = 48 | 2.07 | 93 | ― | ― | ― | ― |
| moderate | 100 to 399 | N = 27 | 2.67 | 99 | ― | ― | ― | ― | ||
| heavy | ≥ 400 | N = 26 | 3.49 | 99.6 | ― | ― | ― | ― | ||
| Pugh 1983 MWI2 | 3 months | light | 20 to 124 | 51.7 N = 21 |
1.9 | 96.32 | 52.7 N = 20 |
36.8 | 30.17 | Not reported |
| moderate | 125 to 499 | 234.7 N = 30 |
1.9 | 99.19 | 248.0 N = 32 |
145.7 | 41.25 | |||
| heavy | 500 to 1999 | 907.6 N = 38 |
1.8 | 99.8 | ― | ― | ― | |||
| very heavy | > 2000 | 3433.3 N = 8 |
2.2 | 99.93 | ― | ― | ― | |||
| Tchuente 2004 CMR | 3 months | light | < 50 | 8.22 N = 183 |
0.86 | 89.53% | ― | ― | ― | ― |
| heavy | > 50 | 115.59 N = 63 |
4.11 | 96.4% | ― | ― | ― | ― | ||
| Taylor 1988 ZWE | 3 months | light | < 50 | 15.1 N = n.r. |
0.4 | 97.35 | 15.7 | 19.8 | 26.1 increase | ― |
| heavy | > 100 | 204.7 N = n.r. |
2.0 | 99 | 191.9 | 94.7 | 50.65 | ― | ||
| Pugh 1983 MWI | 6 months | light | 20 to 124 | 51.7 N = 21 |
2.3 | 95.5 | ― | ― | ― | ― |
| moderate | 125 to 499 | 234.7 N = 30 |
2.0 | 99.14 | ― | ― | ― | ― | ||
| heavy | 500 to 1999 | 907.6 N = 38 |
2.6 | 99.7 | ― | ― | ― | ― | ||
| very heavy | > 2000 | 3433.3 N = 8 |
2.8 | 99.9 | ― | ― | ― | ― | ||
| Taylor 1988 ZWE | 6 months | light | < 50 | 15.1 N = n.r. |
0.2 | 98.67 | 15.7 | 11.7 | 25.5 | ― |
| heavy | > 100 | 204.7 N = n.r. |
0.6 | 99.7 | 191.9 | 75.5 | 60.6 | ― | ||
1Stratum I: light infections < 50 eggs/10 mL, praziquantel (N = 77), placebo (N = 90). "Pretreatment light infections exhibited better cure rates for S. haematobium than pretreatment heavy infections". Praziquantel (N = 77), placebo (N = 90).
2Baseline imbalance in terms of intensity of infection "In accordance with local ethical guidelines the placebo group consisted only of children with light (20 to 124 ova/10 mL or moderate (125 to 4999 ova/10 mL) infections before treatment."
Praziquantel 40 mg/kg single dose versus 20 mg/kg single dose: % egg reduction
| Trial ID | Subgroup | Time point | Measure | Praziquantel 40 mg/kg single dose | Praziquantel 20 mg/kg single dose | P value difference between groups | ||||
| Egg count/10 mL urine |
% egg reduction |
Egg count/10 mL urine | % egg reduction | |||||||
| Baseline | Follow‐up | Baseline | Follow‐up | |||||||
| Taylor 1988 ZWE | light infections < 50/10 mL |
1 month | GMEC | 15.1 N = 77 (in both groups) |
0.4 | 97.35 | 15.5 N = 61 |
0.5 | 96.77 | Not reported |
| heavy infections > 100/10 mL |
1 month | GMEC | 204.7 N = 77 (in both groups) |
4.0 | 98.04 | 177.3 N = 61 |
3.4 | 98.08 | ||
| King 2002 KEN | ― | 6 weeks | GMEC (± CI) N = |
Not reported | 2.54 (1.84 to 3.5) N = 145 |
98 | Not reported | 4.42 (3.1 to 6.3) N = 146 |
95 | Not reported |
| Wilkins 1987 GMB | ― | 2 to 3 months | GMEC median AMEC N = |
54/63/298 N = 33 |
0.3/0/1 | 99.4 100 99.6 |
53/87/313/ N = 35 |
0.8/0.3/7 | 98.4 99.7 97.6 |
0.31 |
| King 1989 KEN | ― | 3 months | GMEC AMEC ± SD GMEC N = |
255 377/ N = 64 |
2 31(± 21) N = 56 |
99.2 (GMEC) 91.7 (AMEC) |
210 327/ N = 75 |
2 13 ± 9/ N = 68 |
99.04 (GMEC) 96 (AMEC) |
Not significant |
| Taylor 1988 ZWE | light infections < 50/10 mL |
3 months | GMEC | 15.1 N = 77 (in both groups) |
0.4 | 97.35 | 15.5 N = 61 |
0.6 | 96.12 | Not reported |
| heavy infections > 100/10 mL |
3 months | GMEC | 204.7 N = 77 (in both groups) |
2.0 | 99.02 | 177.3 N = 61 |
3.6 | 97.97 | ||
| Taylor 1988 ZWE | light infections < 50/10 mL |
6 months | GMEC | 15.1 N = 77 (in both groups) |
0.2 | 98.67 | 15.5 N = 61 |
0.2 | 98.7 | Not significant |
| heavy infections > 100/10 mL |
6 months | GMEC | 204.7 N = 77 (in both groups) |
0.6 | 99.7 | 177.3 N = 61 |
2.7 | 98.5 | ||
Praziquantel 40 mg/kg single dose versus 10 mg/kg single dose: % egg reduction
| Trial ID | Subgroup | Time point | Measure | Praziquantel 40 mg/kg single dose | Praziquantel 10 mg/kg single dose | P value difference between groups | ||||
| Egg count/10 mL urine | % egg reduction | Egg count/10 mL urine | % egg reduction | |||||||
| Baseline | Follow‐up | Baseline | Follow‐up | |||||||
| Taylor 1988 ZWE | light infections < 50/10 mL |
1 month | GMEC | 15.1 N = 77 |
0.4 | 97.35 | 14.8 N = 73 |
2.3 | 84.46 | Not reported |
| heavy infections > 100/10 mL |
1 month | GMEC | 204.7 N = 77 |
4.0 | 98.04 | 197.5 N = 73 |
34.7 | 82.43 | Not reported | |
| King 1989 KEN | 2 to 3 months | GMEC AMEC ± SE N = |
245 378 N = 64 |
2/ 31 ± 21 N = 56 |
99.183 (GMEC) 91.79 (AMEC) |
255 377 N = 72 |
20/ 102 ± 22 N = 62 |
92.156 (GMEC) 72.94 (AMEC) |
0.001 | |
| Wilkins 1987 GMB | 2 to 3 months | GMEC median AMEC N = |
54 63 298 N = 33 |
0.3/ 0/ 1/ |
99.4 (GMEC) 100 (median) 99.6 (AMEC) |
61/82/297N = 38 | 7.9/ 5.9/ 33/ |
87.0 (GMEC) 92.8 (median) 88.8 (AMEC) |
Not reported | |
| Taylor 1988 ZWE | light infections < 50/10 mL |
3 months | GMEC | 15.1 | 0.4 | 97.35 | 14.8 | 2.2 | 85.14 | Not reported |
| heavy infections > 100/10 mL |
3 months | GMEC | 204.7 | 2.0 | 99.02 | 197.5 | 27.6 | 86.02 | Not reported | |
| Taylor 1988 ZWE | light infections < 50/10 mL |
6 months | GMEC | 15.1 | 0.2 | 98.67 | 14.8 | 1.2 | 91.89 | Not reported |
| heavy infections > 100/10 mL |
6 months | GMEC | 204.7 | 0.6 | 97.07 | 197.5 | 14.4 | 92.7 | Not reported | |
Praziquantel 40 mg/kg single dose versus split dose: % egg reduction
| Trial ID | Time point | Measure | Praziquantel 40 mg/kg single dose | Praziquantel 40 mg/kg split dose (2 x 20 mg/kg in one day) | P value difference between groups | ||||
| Egg count/10 mL urine | % egg reduction | Egg count/10 mL urine | % egg reduction | ||||||
| Baseline | Follow‐up | Baseline | Follow‐up | ||||||
| Kardaman 1985 SDN | 5 weeks | GMEC N = |
Not reported N = 114 |
28 | ― | Not reported N = 106 |
26 | ― | No significant difference, P value not reported |
| McMahon 1979 TZA | 1 month | GMEC+ 95 range |
288.4 (33.2 to 2508.9) N = 33 |
1.1 (0 to 8.3) | 99.61 | 352.8 (37.0 to 3361.8) N =36 |
0.8 (0 to 62) | 99.7 | P value not reported |
| Oyediran 1981 NGA1 | 1 month | GMEC mean + SD (N = ) |
Stratum 1 87.4 ± 23.46 (N = 15) Stratum 2 339.4 ± 32.61 (N = 5) Stratum 3 518.00 ± 0.71 (N = 2) N = 22 |
― | 97.69 ± 0.98 (N = 21) |
Stratum 1 84.93 ± 34.71 (N = 15) Stratum 2 296.00 ± 26.19 (N = 5) Stratum 3 526.00 (N = 1) N = 21 |
98.69 ± 0.39 (N = 19) |
No significant difference, P value not reported |
|
| Kardaman 1985 SDN | 3 months | GMEC | ‐ N = 114 |
15 | ― | ‐ N =106 |
9 | ― | No significant difference, P value not reported |
| McMahon 1979 TZA | 3 months | GMEC+ 95% CIs | 288.4 (33.2 to 2508.9) N = 33 |
1.1 (0‐16.3) | 99.61 | 352.8 (37.0 to 3361.8) N = 36 |
0.5 (0 to 3.9) | 99.85 | P value not reported |
| Oyediran 1981 NGA1 | 3 months | GMEC mean + SD (N = ) |
Stratum 1 87.4 ± 23.46 (N = 15) Stratum 2 339.4 ± 32.61 (N = 5) Stratum 3 518.00 ± 0.71 (N = 2) N = 22 |
― | 97.55 ± 0.85 (N = 18) | Stratum 1 84.93 ± 34.71 (N = 15) Stratum 2 296.00 ± 26.19 (N = 5) Stratum 3 526.00 (N = 1) N = 21 |
― | 99.48 ± 0.3 (N = 16) |
N o significant difference, P value not reported |
| McMahon 1979 TZA | 6 months | GMEC+ 95 % CIs | 288.4 (33.2 to 2508.9) N =33 |
1.1 (0 to 20.3) | 99.61 | 352.8 (37.0 to 3361.8) N =36 |
0.6 (0 to 11.3) | 99.82 | P value not reported |
| Oyediran 1981 NGA1 | 6 months | GMEC mean + SD (N = ) |
Stratum 1 87.4 ± 23.46 (N = 15) Stratum 2 339.4 ± 32.61 (N = 5) Stratum 3 518.00 ± 0.71 (N = 2) N = 22 |
― | 93.09 ± 0.12 (N = 15) | Stratum 1 84.93 ± 34.71 (N = 15) Stratum 2 296.00 ± 26.19 (N = 5) Stratum 3 526.00 (N = 1) N = 21 |
― | 99.52 ± 0.21 (N = 13) |
No significant difference in egg counts between treatment groups, P value not reported. |
| 9 months | ― | 92.4 ± 5.92 (N = 6) | ― | 98.12 ± 1.13 (N = 6) |
|||||
| 12 months | ― | 99.3 ± 0.26 (N = 3) | ― | 98.68 ± 0.51 (N = 5) |
|||||
1GMEC/10 mL urine, Stratum 1: 60 to 250 GMEC/10 mL urine; Stratum 2: 251 to 500 GMEC/10 mL; Stratum 3 > 500 GMEC/10 mL.
Praziquantel 40 mg/kg single dose versus praziquantel 2 x 40 mg/kg or praziquantel 3 x 40 mg/kg given three weeks apart: % egg reduction
| Trial ID | Time point | Measure | Praziquantel 40 mg/kg (single dose) | Praziquantel 2 x 40 mg/kg | P value for differences between groups | ||||
| Egg count /10 mL urine | Egg count/10 mL urine | ||||||||
| Baseline | Follow‐up | % egg reduction | Baseline | Follow‐up | % egg reduction | ||||
| Tchuente 2004 CMR | 6 weeks | GMEC | All classes 15.83 Light infection 8.22 Heavy infection 115.59 N = 135 |
0.29/ 0.2/ 0.64 |
98.18/ 97.63/ 99.44 |
All classes 19.00/ Light infection 9.5/ Heavy infection 129.05 N = 246 |
0.25/ 0.19/ 0.43/ |
98.69/ 97.99/ 99.67 |
Follow‐up at 6 weeks, 6 weeks after a single dose (cohort 3) Follow‐up at 6 weeks, 3 weeks after the second dose (cohort 1) |
| 6 weeks | GMEC | All classes 15.83 Light infection 8.22 Heavy infection 115.59 N = 135 |
0.29/ 0.2/ 0.64/ |
98.18/ 97.63/ 99.44/ |
All classes 16.96 Light infection 7.3 Heavy infection 173.02 N = 134 |
0.27/ 0.17/ 0.64/ |
98.39/ 97.7/ 99.63 |
F ollow‐up at 6 weeks, 6 weeks after a single dose (cohort 3) P > 0.066 Follow‐up at 6 weeks, 3 weeks after the second dose (cohort 2) |
|
| 9 weeks | GMEC | All classes 15.83 Light infection 8.22 Heavy infection 115.59 N = 135 |
0.17/ 0.15/ 0.23 N = 70 |
99.06/ 98.29/ 99.80 |
All classes 16.96 Light infection 7.3 Heavy infection 173.02 N = 134 |
0.43/ 0.2/ 1.23 N = 60 |
97.88/ 97.59/ 99.29 |
Follow‐up at 9 weeks, 9 weeks after a single dose (cohort 3) Follow‐up at 9 weeks, 6 weeks after the second dose (cohort 2) |
|
| Praziquantel 40 mg/kg (single dose) | Praziquantel 3 x 40 mg/kg | Comments | |||||||
| 9 weeks | GMEC | All classes 15.83 Light infection 8.22 Heavy infection 115.59 N = 135 |
0.17/ 0.15/ 0.23 N = 70 |
99.06/ 98.29/ 99.80 |
All classes 0.19 Light infection 0.06 Heavy infection 0.51 N = 246 |
― | 99.18/ 99.61/ 99.36 |
Follow‐up at 9 weeks, 9 weeks after a single dose (cohort 3) Follow‐up at 9 weeks: 3 weeks after the last (third) dose (cohort 1) |
|
Praziquantel 40 mg/kg single dose x 2, interval three weeks: one arm received praziquantel single at baseline, one arm received a second dose at three weeks, one arm received the second dose at three weeks and a third dose at six weeks. Follow‐up for all groups at six weeks and nine weeks.
Strata: light infection < 50/10 mL, heavy infection > 50/10 mL.
Metrifonate 10 mg/kg single dose versus placebo: % egg reduction
| Trial ID | Time point | Measure | Metrifonate 10 mg/kg single dose | Placebo | ||||
| Egg count/10 mL urine | % egg reduction | Egg count/10 mL urine | % egg reduction | |||||
| Baseline | Follow‐up | Baseline | Follow‐up | |||||
| Stephenson 1989 KEN | 8 months | GMEC AMEC N = |
47/ 94 N = 105 |
4/ 23 N = 103 |
91.48/ 76 |
38/ 85/ N = 105 |
36/ 102 N = 104 |
5.26/ ‐20 (increase) |
Metrifonate 2 x 10 and 3 x 10 mg/kg given two weeks apart versus 10 mg/kg single dose: % egg reduction
| Trial ID | Time point | Measure | Metrifonate 2 x 10 mg/kg | Metrifonate 10 mg/kg single dose | ||||
| Egg count/10 mL urine | % egg reduction | Egg count/10 mL urine | % egg reduction | |||||
| Baseline | Follow‐up | Baseline | Follow‐up | |||||
| Rey 1984 NER | 1 month | AMEC N = |
93.2 N = 99 |
16.9 N = 49 |
81.9 N = 50 |
30.4 N = 125 |
19.1 N = 62 |
37.2 |
| 4 months | AMEC N = |
93.2 N = 99 |
58.4 N = 35 |
37.34 | 30.4 N = 125 |
22.8 N = 69 |
25 | |
| Trial ID | Time point | Measure | Metrifonate 3 x 10 mg/kg | Metrifonate 10 mg/kg single dose | ||||
| Egg count/10 mL urine | % egg reduction | Egg count/10 mL urine | % egg reduction | |||||
| Baseline | Follow‐up | Baseline | Follow‐up | |||||
| Rey 1984 NER | 1 month | AMEC N = |
17.8 N = 62 |
2.0 N = 31 |
88.7 | 30.4 N = 125 |
19.1 N = 62 |
37.2 |
| 4 months | AMEC N = |
17.8 N = 62 |
10.8 N = 42 |
39.32 | 30.4 N = 125 |
22.8 N = 69 |
25 | |
Metrifonate 3 doses two weeks apart: 7.5 mg/kg versus 5 mg/kg: % egg reduction
| Trial ID | Time point | Measure | Metrifonate 7.5 mg/kg x 3 | Metrifonate 5 mg/kg x 3 | P value difference between groups | ||||
| Egg count/10 mL urine | % egg reduction | Egg count/10 mL urine | % egg reduction | ||||||
| Baseline | Follow‐up | Baseline | Follow‐up | ||||||
| Abden Abdi 1989 SOM | 1 month | Egg count/10 mL urine mean (SD) % egg reduction mean (SD) |
1010 (1550) | ― | 97 (5) | 997 (1700) | ― | 96 (6) | P > 0.7 (difference in egg counts at 1 and 6 months) |
| 2 months | 1010 (1550) | ― | 97 (6) | 997 (1700) | ― | 96 (7) | |||
| 3 months | 1010 (1550) | ― | 95 (8) | 997 (1700) | ― | 94 (8) | |||
| 6 months | 1010 (1550) | ― | 93 (11) | 997 (1700) | ― | 92 (11) | |||
1N = 101 for both groups together
Praziquantel 40 mg/kg single dose versus metrifonate 10 mg/kg single dose: % egg reduction
| Trial ID | Time point | Measure | Praziquantel 40 mg/kg single dose | Metrifonate 10 mg/kg single dose | P value differences between groups | ||||
| Egg count/10 mL urine | % egg reduction | Egg count/10 mL urine | % egg reduction | ||||||
| Baseline | Follow‐up | Baseline | Follow‐up | ||||||
| Stephenson 1989 KEN | 8 months | AMEC GMEC |
112/ 57 N = 105 |
1/ 0.2 |
99 | 94/ 47 N = 103 |
23/ 4 |
76 | Significant P < 0.0001 |
Praziquantel 40 mg/kg single dose versus metrifonate 2 x 10 mg/kg given two weeks apart: % egg reduction
| Trial ID | Time point | Measure | Praziquantel 40 mg/kg single dose | Metrifonate 2 x 10 mg/kg | P value differences between groups | ||||
| Egg count/10 mL urine | % egg reduction | Egg count/10 mL urine | % egg reduction | ||||||
| Baseline | Follow‐up | Baseline | Follow‐up | ||||||
| de Jonge 1990 SDN | 1 month | median N = |
66 N = 48 |
1 N = 40 |
98.48 | 95 N = 38 |
1 N = 32 |
98.94 | Not reported |
| 5 months | median N = |
66 N = 48 |
0 N = 35 |
100 | 95 N = 38 |
1 N = 32 |
98.94 | ||
Praziquantel 30 mg/kg single dose versus metrifonate 3 x 10 mg/kg given two weeks apart: % egg reduction
| Trial ID | Time point | Measure | Praziquantel 30 mg/kg single dose | Metrifonate 3 x 10 mg | P value difference between groups | ||
| N | % egg reduction | N | % egg reduction | ||||
| McMahon 1983 TZA | 4 months | GMEC of miracidia/10 mL urine | 30 | 99 | 30 | 98 | Not reported |
Praziquantel 40 mg/kg 1x/year versus metrifonate 10 mg/kg 3x/year: % egg reduction
| Trial ID | Time point | Measure | Praziquantel 40 mg/kg 1x/year | Metrifonate 10 mg/kg 3x/year | P value difference between groups | ||||
| Egg count/10 mL urine | % egg reduction | Egg count/10 mL urine | % egg reduction | ||||||
| Baseline | Follow‐up | Baseline | Follow‐up | ||||||
| King 1990 KEN1 | 12 months | AMEC (± SD) GMEC |
Light 33 19 |
6 ± 2 | 81.81 | Light 33 19 |
4 ± 3 ― |
87.87 | Not significant |
| Moderate 193 86 |
3 ± 2 | 98.44 | Moderate 193 86 |
5 ± 2 ― |
97.4 | ||||
| Heavy 597 581 |
8 ± 4 | 98.65 | Heavy 597 581 |
9 ± 3 ― |
89.49 | ||||
1Baseline data reported for both treatment groups together.
Praziquantel 40 mg/kg single dose versus praziquantel 10 mg/kg and metrifonate 10 mg/kg: % egg reduction
| Trial ID | Time point | Praziquantel 40 mg/kg single dose | Praziquantel 10 mg/kg and Metrifonate 10 mg/kg | P value difference between groups | ||||
| GMEC/10 mL | % egg reduction | GMEC/10 mL | % egg reduction | |||||
| Baseline | Follow‐up | Baseline | Follow‐up | |||||
| Wilkins 1987 GMB1 | 2 to 3 months | 54 N = 33 |
70.3 | 99.4 | 67 N = 39 |
4.8 | 92.9 | Not reported |
1AMEC and median also reported.
Praziquantel versus artesunate: % egg reduction
| Trial ID | Time point | Measure | Praziquantel 40 mg/kg single dose | Artesunate 4 mg/kg/d for 3 days | P value difference between groups | ||||
| Egg count/10 mL urine | % egg reduction | Egg count/10 mL urine | % egg reduction | ||||||
| Baseline | Follow‐up | Baseline | Follow‐up | ||||||
| Keiser 2010 CIV | 26 days | GMEC (range) N = |
32.0 (1 to 457) N = 26 |
1.1 (1 to 5) | 97 | 40.2 (2 to 562) N = 20 |
6.2 (1 to 267) | 85 | Significant P < 0.001 |
| Borrmann 2001 GAB | 8 weeks | GMEC (range) | 38.51 (1 to 3313) N = 90 |
1.11 N = 89 |
97.11 | 35.22 (1 to 4360) N = 90 |
10.8 N = 89 |
69.34 | Significant, P value not reported |
| Inyang Etoh 2009 NGA1 | 8 weeks | mean ± SD N = |
42.0 ± 1.7 N = 52 |
9.8 ± 0.5 N = 42 |
76.7 | 39.8 ± 1.1 N = 52 |
19.1 ± 1.0 N = 44 |
52.1 | Not reported |
1Treatment group: Praziquantel 40 mg/kg without placebo. Inyang Etoh 2009 NGA also reports a second treatment group (Praziquantel 40 mg/kg with placebo), data not shown.
Mefloquine versus placebo: % egg reduction
| Trial ID | Time point | Measure | Praziquantel 40 mg/kg single dose | Mefloquine 25 mg/kg single dose | P value difference between groups | ||||
| Egg count/10 mL urine | % egg reduction | Egg count/10 mL urine | % egg reduction | ||||||
| Baseline | Follow‐up | Baseline | Follow‐up | ||||||
| Keiser 2010 CIV | 28 days | GMEC range N = |
32.0 (1 to 457) N = 26 |
1.1 (1 to 5) N = 26 |
97 | 30.1 (1 to 2039) N = 19 |
1.7 (1 to 73) N = 19 |
74 | Significant P < 0.001 |
Praziquantel versus mefloquine: % egg reduction
| Trial ID | Time point | Measure | Praziquantel 40 mg/kg single dose | Mefloquine 25 mg/kg single dose | P value difference between groups | ||||
| Egg count/10 mL urine | % egg reduction | Egg count/10 mL urine | % egg reduction | ||||||
| Baseline | Follow‐up | Baseline | Follow‐up | ||||||
| Keiser 2010 CIV | 28 days | GMEC range N = |
32.0 (1 to 457) N = 26 |
1.1 (1 to 5) N = 26 |
97 | 30.1 (1 to 2039) N = 19 |
1.7 (1 to 73) N = 19 |
74 | Significant P < 0.001 |
Praziquantel versus mefloquine and artesunate: % egg reduction
| Trial ID | Time point | Measure | Praziquantel 40 mg/kg single dose | Artesunate 100 mg plus Mefloquine 250 mg fixed dose combination (FDC) x 3 in one day | P value difference between groups | ||||
| Egg count/10 mL urine | percent egg reduction | Egg count/10 mL urine | Percent egg reduction | ||||||
| Baseline | Follow‐up | Baseline | Follow‐up | ||||||
| Keiser 2010 CIV | 28 days | GMEC range N = |
32.0 (1 to 457) N = 26 |
1.1 (1 to 5) N = 26 |
97 | 42.0 (1 to 688) N = 18 |
1.7 (1 to 73) N = 18 |
96% | Not significant P = 0.13 |
Appendix 3. Appendix: Additional tables for haematuria and proteinuria
Praziquantel 40 mg/kg single dose versus placebo: haematuria and proteinuria
| Outcome | Trial ID | Time point | Measure | Praziquantel 40 mg/kg single dose | Placebo | P value difference between groups | ||||
| Baseline | Follow‐up | Mean % change | Baseline | Follow‐up | Mean % change | |||||
| Haematuria | de Jonge 1990 SDN | 1 month | median erythrocytes/mykrol (95% CI) | 159 (34 to 627) N = 56 | 1(0 to 2) N = 56 |
‐99.37 | 290 (54 to 1224) N = 26 |
323 (51 to 864) | +11.37 | Not reported |
| Borrmann 2001 GAB1 | 8 weeks | erythrocytes/mL (95% CI) | NR N = 89 |
‐ 1101 (‐137 to ‐84) | ― | NR N = 30 |
‐391 (‐86 to ‐8) | ― | Significant P < 0.001 |
|
| Inyang Etoh 2009 NGA | 8 weeks | units unclear mean (± SD) | 47.6(± 2.0) N = 52 | 7.6(± 0.9) N = 42 |
‐84.033 | 38.0 (±1.6) N = 52 |
59.6 (± 2.2) N = 44 | +56.84 | P < 0.001 | |
| Proteinuria | de Jonge 1990 SDN | 1 month | median, g/L (95% CI) |
0.42 (0.22 to 0.62) N = 56 | 0.09 (0.05 to 0.12) | ‐78 | 0.24 (0.09 to 0.59) N = 26 |
0.32 (0.14 to 0.35) | +33.3 | Not reported |
| Inyang Etoh 2009 NGA2 | 8 weeks | mean (± SD) units unclear |
160.2 (± 5.2) N = 52 | 24.8 ± 1.9 N = 42 |
‐84 | 185.2 ± 5.0 N = 52 |
213.9 ± 5.3 N = 44 | +15.49 | P < 0.001 | |
Footnotes
1Mean change from baseline in erythrocytes/mL urine (95% CI).
2Data shown here are for the treatment group praziquantel 40 mg/kg without placebo. Inyang Etoh 2009 NGA also reports a treatment group for praziquantel with placebo, (data not shown).
Praziquantel 40 mg/kg single dose versus Praziquantel 2 x 40 mg/kg: haematuria
| Outcome | Trial ID | Time point | Measure | Praziquantel 40 mg/kg single dose | Praziquantel 2 x 40 mg (one additional dose) | P value difference between groups | ||||
| Baseline | Follow‐up | % change | Baseline | Follow‐up | % change | |||||
| Prevalence of haematuria1 |
Sacko 2009 MLI Koulikoro |
12 weeks | N = 310 | 81.3 % (N = ?) |
15.5 % | ‐80.9 | 75.5 % (N = ?) |
12.9 % | ‐82.9 | 0.51 |
|
Sacko 2009 MLI Selingue |
12 weeks | N = 293 | ― | 41.4 % | ‐44.4 | 75.5% (N = ?) |
35.6 % | ‐52.8 | 0.03 | |
|
Sacko 2009 MLI Koulikoro |
6 months | N = 300 | 67.7% (N = 150) |
7.6% | ‐88.8 | 71.1% (N = 150) |
2.2 % | ‐96.9 | 0.32 | |
|
Sacko 2009 MLI Selingue |
6 months | N = 275 | N = ? | 19.7% | ‐70.9 | 71.1% (N = ?) |
16.4 % | ‐76.9 | 0.47 | |
1Microhaematuria as diagnosed by dipstick (Haemastix)
Praziquantel 40 mg/kg single dose versus Metrifonate 20 mg/kg given as split dose: haematuria and proteinuria
| Outcome | Trial ID | Time point | Measure | Praziquantel 40 mg/kg single dose | Metrifonate 10 mg/kg two doses | P value difference between groups | ||||
| Baseline | Follow‐up | % change | Baseline | Follow‐up | % change | |||||
| Haematuria | de Jonge 1990 SDN | 1 month | median (95% CI) Erythrocytes/µl |
159
(34 to 627) N = 56 |
1 (0 to 2) | ‐99.37 | 503
(72 to 930) N = 42 |
31 (1 to 112) | ‐93.93 | Not reported |
| Proteinuria | de Jonge 1990 SDN | 1 month | median (95% CI) Proteinuria g/L |
0.42
(0.22 to 0.62) N = 56 |
0.09 (0.05 to 0.12) | ‐78.57 | 0.44
(0.2 to 0.73) N = 42 |
0.09 (0.07 to 0.15) | ‐79.54 | Not reported |
ARS versus placebo: haematuria and proteinuria
| Outcome | Trial ID | Time point | Measure | Artesunate 4mg/kg/day for 3 days | Placebo | P value difference between groups | ||||
| Baseline | Follow‐up | % change | Baseline | Follow‐up | % change | |||||
| Haematuria | Borrmann 2001 GAB | 8 weeks | mean change from baseline Ery/mL (95% CI) |
― | ― | ‐34 (‐59 to ‐9) |
― | ― | ‐39 (‐86 to +8) |
Not reported |
| Inyang Etoh 2009 NGA1 | 8 weeks | mean haematuria + SD | 61.8 ± 2.2 N = 52 |
25.7 ± 1.6 N = 44 |
‐58.41 | 38.0 ± 1.6 N = 52 |
59.6 ± 2.2 N = 44 |
‐36.24 | Not reported | |
| Proteinuria | Inyang Etoh 2009 NGA1 | 8 weeks | mean ± SD unit unclear (mg/dL) |
191.1 ± 5.2 N = 52 |
102.1 ± 4.4 N = 44 |
‐46.57 | 185.2 ± 5.0 N = 52 |
213.9 ± 5.3 N = 44 |
‐13.41 | Not reported |
Footnotes
1Treatment group: Praziquantel 40 mg/kg without placebo. Inyang Etoh 2009 NGA also reports a second treatment group (Praziquantel 40 mg/kg with placebo), data not shown.
Praziquantel versus ARS: haematuria and proteinuria
| Outcome | Trial ID | Subgroup | Time point | Measure | Praziquantel 40 mg/kg single dose | Artesunate 4 mg/kg/day for 3 days | P value difference between groups | ||||
| Baseline | Follow‐up | % change | Baseline | Follow‐up | % change | ||||||
| Haematuria | Borrmann 2001 GAB | ― | 8 weeks | Mean change from baseline Ery/mL (95% CI) |
N = 90 | N = 89 | ‐ 110 (‐137‐84) |
N = 90 | N = 89 | ‐ 34 (‐59 to ‐9) |
Significant P < 0.001 |
| Inyang Etoh 2009 NGA | with placebo | 8 weeks | Mean haematuria ± SD | 55.9 ± 2 N = 52 |
13.6 ± 1.2 N = 44 |
‐75.6 | 50.9 ± 1.9 N = 52 |
11.1 ± 0.9 N = 44 |
‐78.19 | Not reported | |
| without placebo | 47.6 ± 2 N = 52 |
7.6 ± 0.9 N = 42 |
‐84 | 61.8 ± 2.2 N = 52 |
25.7 ± 1.6 N = 44 |
‐58.41 | |||||
| Proteinuria | Inyang Etoh 2009 NGA | with placebo | 8 weeks | Mean proteinuria ± SD | 190.9 ± 5.2 N = 52 |
65.7 ± 3.3 N = 44 |
‐65 | 177.3 ± 5.1 N = 52 |
85.5 ± 3.9 N = 44 |
‐51.7 | Not reported |
| without placebo | 160.2 ± 5.2 N = 52 |
24.8 ± 1.9 N = 42 |
‐84.5 | 191.1 ± 5.2 N = 52 |
102.1 ± 4.4 N = 44 |
‐46.5 | Not reported | ||||
Praziquantel versus Praziquantel and ARS: haematuria and proteinuria
| Outcome | Trial ID | Time point | Measure | Praziquantel 40 mg/kg single dose |
Artesunate 4 mg/kg/d for 3 days Praziquantel 40 mg/kg single dose |
P value difference between groups | ||||
| Baseline | Follow‐up | % change | Baseline | Follow‐up | % change | |||||
| Haematuria | Borrmann 2001 GAB | 8 weeks | Mean change from baseline Ery/mL (95% CI) |
― | ― | ‐110 (‐137 to ‐84) |
― | ― | ‐102 (‐128 to ‐77) | Not reported |
| Inyang Etoh 2009 NGA1 | 8 weeks | Mean haematuria ± SD | 55.9 (± 2) N = 44 |
13.6 (± 1.2) | ‐75.65 | 73.0 (± 2.3) N = 44 |
8.8 (± 8.7) | ‐87.94 | Not reported | |
| Proteinuria | Inyang Etoh 2009 NGA | 8 weeks | Mean proteinuria ± SD | 190.9 ± 5.2 N = 52 |
65.7 ± 3.3 N = 44 |
‐65.58 | 267.5 ± 5.4 N = 52 |
4.0 ± 15.2 N = 44 |
‐ 98.5 | Not reported |
1Treatment group: Praziquantel 40 mg/kg with placebo. Inyang Etoh 2009 NGA also reports a second treatment group (Praziquantel 40 mg/kg without placebo), data not shown.
Appendix 4. Appendix: Additional tables for growth outcomes
Praziquantel 40 mg/kg single dose versus placebo: growth outcomes
| Trial ID | Time point | Measure | Praziquantel 40 mg/kg single dose | Placebo | P value difference between praziquantel 40 mg/kg and placebo after treatment | |||||
| Baseline | Follow‐up | growth greater than placebo | P value difference before and after treatment | Baseline | Follow‐up | Difference before and after treatment | ||||
| Stephenson 1989 KEN1 | 5 weeks | Harvard step test score mean ± SEM |
76 ± +‐1.41 | 81.2 ± 1 | 6.8% points | P = 0.0002 | 77.1 ± +‐1.51 | 75.5 ± 1.95 | Not significant | P < 0.05 significant |
| resting heart rate beats/min mean ± SEM |
81.1+‐ ± 1.66 | 77.9 ± 1.1 | 4.3 beats/min (decrease) |
P = 0.004 | 85.8+‐ ± 1.7 | 86.9 ± 1.96 | Not significant | P = 0.003 significant |
||
| appetite mean ± SEM |
709+‐ ± 58 | 841± 65.2 | 139 mL | P = 0.014 | 811+‐ ± 93.4 | 803 ± 78 | Not significant | Nnot significant | ||
| Befidi Mengue 1992 CMR2 | 6 months | height median cm (SD) |
134.1 (12.3) | 135.8 (12.5) | 1.6 | 132.8 (12.0) | 134.5 (12.3) | 1.7 | Not significant | |
| weight median kg (SD) |
29.2 (7.4) | 31.3 (8.2) | 2.1 | 28.3 (7.2) | 30.2 (7.9) | 1.9 | Not significant | |||
| middle upper arm circumference (MUAC) median cm (SD) |
18.4 (2.0) | 19.0 (2.2) | 0.6 | 18.3 (2.1) | 18.7 (2.2) | 0.4 | Significant | |||
| triceps skinfold median unit not specified (SD) |
6.7 (1.5) | 7.1 (1.9) | 0.4 | 6.6 (1.9) | 6.9 (2.1) | 0.3 | Not significant | |||
| mean muscle mass median unit not specified (SD) |
16.3 (1.8) | 16.8 (2.0) | 0.47 | 16.2 (1.9) | 16.6 (1.9) | 0.36 | Not significant | |||
| ht for age (%) | 93.6 | 92.7 | ‐0.72 | 94.1 | 93.3 | ‐0.75 | Not significant | |||
| wt for age (%) | 81.3 | 82.2 | 0.92 | 82.5 | 83.1 | 0.65 | Not significant | |||
| wt for ht (%) | 97.49 | 100.78 | 3.28 | 96.87 | 99.44 | 2.57 | Not significant | |||
| Stephenson 1989 KEN3 | 8 months | height (cm) mean ± SEM |
134.8 ± 1.12 | 138.2 ± 1.10 | 0.1 cm | 0.0002 | 135.9 ± 1.23 | 139.3 ± 1.22 | 0.0002 | Not significant |
| weight (kg) mean ± SEM |
27.3 ± 0.72 | 30.4 ± 0.76 | 1.2kg | 0.0002 | 28.4 ± 0.78 | 30.3 ± 0.83 | 0.0002 | P = 0.0001 significant |
||
| triceps skinfold thickness mean ± SEM |
7.4 ± 0.21 | 8.8 ± 0.25 | 1.4 mm | 0.0002 | 8.2 ± 0.27 | 8.1 ± 0.27 | Not significant | P = 0.0001 significant |
||
| subscapular skinfold thickness mean ± SEM |
5.6 ± 0.16 | 6.9 ± 0.2 | 1.4 mm | 0.0002 | 6.0 ± 0.2 | 5.9 ± 0.2 | Not significant | P = 0.0001 significant |
||
| mid upper arm circumference (MUAC) mean ± SEM |
17.5 ± 0.22 | 18.5 ± 0.24 | 0.7 cm | 0.0002 | 17.8 ± 0.21 | 18.0 ± 0.22 | 0.0002 | P = 0.0001 significant |
||
| ht for age (%) mean ± SEM |
93.0 ± 0.44 | 92.8 ± 0.43 | 0.1% points | 0.02 | 92.9 ± 0.44 | 92.6 ± 0.44 | 0.001 | Not significant | ||
| wt for age (%) mean ± SEM |
72.7 ± 0.98 | 74.9 ± 1.00 | 3.3% points | 0.0002 | 73.8 ± 1.04 | 72.5 ± 1.03 | 0.0002 | Not significant | ||
| wt for ht (%) mean ± SEM |
89.5 ± 0.77 | 93.0 ± 0.84 | 3.7% points | 0.0002 | 90.6 ± 0.72 | 90.4 ± 0.74 | Not significant | P = 0.0001 significant |
||
| MUAC/age (%) mean ± SEM |
81.6 ± 0.7 | 83.1 ± 0.73 | 0.7% points | 0.0002 | 82.2 ± 0.7 | 80.5 ± 0.69 | 0.0002 | P = 0.0001 significant |
||
| triceps skinfold/age mean ± SEM |
68.5 ± 1.67 | 80.0 ± 1.82 | 13.2% points | 0.0002 | 75.6 ± 1.89 | 73.9 ± 1.88 | 0.014 | P = 0.0001 significant |
||
| subscapular skinfold/age mean ± SEM |
86.8 ± 1.79 | 102.7 ± 2.24 | 21.4% points | 0.0002 | 91.3 ± 1.96 | 85.7 ± 1.78 | 0.0002 | P = 0.0001 significant |
||
| Olds 1999 KEN | Reports that anthropometric data were measured at baseline and day 45, no significant difference was found before and after treatment with albendazole and praziquantel. Data not shown in the publication. | |||||||||
1Stephenson 1989 KEN reports growth greater than placebo. Sixteenparticipants per group are followed up at 5 weeks. Appetite measured by mL of porridge intake. No significant difference between metrifonate and praziquantel groups for the outcomes resting heart rate, Harvard step test and appetite.
2Befidi Mengue 1992 CMR has 238 participants in the praziquantel group and 198 in the placebogroup. "MUAC was the only anthropometric measure with a significant difference between Praziquantel and Placebo group.". Nno significant differences (before and after interventions) between the groups for height, weight, TSS; TSS:triceps skinfold thickness, MUAC: middle upper arm circumference.
3Stephenson 1989 KEN At follow up at eight months, there are 105 participants in the praziquantel group and 104 in the placebo group.
Praziquantel 40 mg/kg or metrifonate 10 mg/kg single dose versus placebo: growth outcomes
| Trial ID | Time point | Measure |
Praziquantel 40 mg/kg or Metrifonate 10 mg/kg single dose |
Placebo | P value for difference between treatment and placebo group | |||||
| Baseline | Follow up | growth greater than placebo | P value for difference before and after treatment | baseline | follow up | P value p for difference before and after treatment | ||||
| Stephenson 1989 KEN1 | 5 weeks | height (cm) mean ± SEM |
133.8 ± 2.02 | 134.4 ± 2.05 | 0.2cm | 0.0002 | 135 ± 3.14 | 134.4 ± 3.14 | 0.0002 | P=0.056 not significant |
| weight (kg) mean ± SEM |
25.9 ± 1.18 | 27 ± 1.22 | 0.2kg | 0.0002 | 26.8 ± 1.99 | 27.7 ± 1.98 | 0.0002 | P=0.11 not significant |
||
| triceps skinfold/age (%) mean ± SEM |
70.8 ± 3 | 71 ± 3.11 | 2.9% points | Not significant | 75 ± 6.59 | 72.3 ± 6.47 | 0.052 | P=0.048 significant |
||
| wt for ht (%) mean ± SEM |
87 ± 1.38 | ‐89.7 ± 1.32 | 0.9% points | 0.0002 | 87.5 ± 1.47 | 89.3 ± 1.17 | 0.004 | P=0.084 not significant |
||
1Stephenson 1989 KEN reports growth greater than placebo for the treatment group (praziquantel 40 mg/kg group (N = 16) and metrifonate 10 mg/kg group (N = 16) together). THe treatment group has 32 participants, the placebo group 16.
Metrifonate 10 mg/kg single dose versus placebo: growth outcomes
| Trial ID | Time point | Measure | Metrifonate 10 mg/kg | Placebo | P value difference treatment and placebo | |||||
| Baseline | Follow‐up | growth greater than placebo | P value difference between baseline and follow‐up | Baseline | Follow‐up | P value difference between baseline and follow‐up | ||||
| Stephenson 1989 KEN1 | 5 weeks | Harvard step test score mean ± SEM |
76.2 ± 1.6 | 83.8 ± 1.22 | 9.2%points | 0.0002 | 77.1 ± 1.51 | 75.5 ± 1.95 | Not significant | Significant |
| resting heart rate beats/min mean ± SEM |
82 ± 2.44 | 80.2 ± 1.65 | 2.9beats/min | 0.004 | 85.8 ± 1.7 | 86.9 ± 1.96 | Not significant | Not significant | ||
| appetite mean ± SEM |
797 ± 78.2 | 917 ± 68.4 | 128mL | 0.051 | 811 ± 93.4 | 803 ± 78 | P = 0.014 | P = 0.051 Not significant. |
||
| Stephenson 1989 KEN | 8 months | height (cm) mean ± SEM |
139.0 ± 1.27 | 142.6 ± 1.25 | 0.2cm | 0.0002 | 135.9 ± 1.23 | 139.3 ± 1.22 | 0.0002 | Not significant |
| weight (kg) mean ± SEM |
30.1 ± 0.83 | 33.4 ± 0.87 | 1.4kg | 0.0002 | 28.4 ± 0.78 | 30.3 ± 0.83 | 0.0002 | P < 0.05 significant |
||
| triceps skinfold thickness mean ± SEM |
8.2 ± 0.28 | 9.7 ± 0.32 | 1.5mm | 0.0002 | 8.2 ± 0.27 | 8.1 ± 0.27 | Not significant | P < 0.05 significant |
||
| subscapular skinfold thickness mean ± SEM |
6.1 ± 0.2 | 7.4 ± 0.25 | 1.4mm | 0.0002 | 6.0 ± 0.2 | 5.9 ± 0.2 | Not significant | P < 0.05 significant |
||
| middle upper arm circumference (MUAC) mean ± SEM |
18.3 ± 0.22 | 19.2 ± 0.24 | 0.7cm | 0.0002 | 17.8 ± 0.21 | 18.0 ± 0.22 | 0.0002 | P < 0.05 significant |
||
| ht for age (%) mean ± SEM |
93.2 ± 0.43 | 93.3 ± 0.43 | 0.1% points | Not significant | 92.9 ± 0.44 | 92.6 ± 0.44 | 0.001 | Significant | ||
| wt for age (%) mean ± SEM |
73.8 ± 1.04 | 76.2 ± 1.08 | 3.7% points | 0.0002 | 73.8 ± 1.04 | 72.5 ± 1.03 | 0.0002 | Significant | ||
| wt for ht mean ± SEM |
89.9 ± 0.78 | 93.3 ± 0.78 | 3.6% points | 0.0002 | 90.6 ± 0.72 | 90.4 ± 0.74 | Not significant. | Significant | ||
| MUAC/age mean ± SEM |
82.5 ± 0.7 | 83.9 ± 0.76 | 3%points | 0.0002 | 82.2 ± 0.7 | 80.5 ± 0.69 | 0.0002 | Significant | ||
| triceps skinfold/age mean ± SEM |
74.4 ± 1.83 | 85.7 ± 1.93 | 13.1% points | 0.0002 | 75.6 ± 1.89 | 73.9 ± 1.88 | 0.014 | Significant | ||
| subscapular skinfold/age mean ± SEM |
6.1 ± 0.2 | 7.4 ± 0.25 | 1.4 mm | 0.0002 | 91.3 ± 1.96 | 85.7 ± 1.78 | 0.0002 | Significant | ||
1Stephenson 1989 KEN 16 participants both in the Metrifonate 10 mg group is and placebo group
2Stephenson 1989 KEN 103 particpants in the Metrifonate 10 mg group, 104 participants in the placebogroup
Praziquantel 40 mg/kg single dose versus metrifonate 10 mg/kg single dose: growth outcomes
| Trial ID | Time point | Measure | Praziquantel 40 mg/kg single dose | Metrifonate 10 mg/kg single dose | P value (difference between praziquantel and metrifonate) | ||||||
| Baseline | Follow‐up | Growth greater than placebo | P values | Baseline | Follow‐up | Growth greater than placebo | P value | ||||
| Stephenson1 1989 KEN | 5 weeks | HST score mean ± SEM |
76 ± 1.41 | 81.2 ± 1 | 6.8% points | 0.0002 | 76.2 ± 1.6 | 83.8 ± 1.22 | 9.2% points | 0.0002 | Significant |
| resting heart rate beats/min mean ± SEM |
81.1 ± 1.66 | 77.9 ± 1.1 | ‐ 4.3 beats/min (decrease) |
0.004 | 82 ± 2.44 | 80.2 ± 1.65 | 2.9 beats/min | 0.004 | Significant | ||
| appetite mean ± SEM |
709 ± 58 | 841 ± 65.2 | 139 mL | 0.014 | 797 ± 78.2 | 917 ± 68.4 | 128 mLl | 0.051 | Not significant | ||
| Stephenson1 1989 KEN | 8 months | height (cm) mean ± SEM |
134.8 ± 1.12 | 138.2 ± 1.10 | 0.1 cm | 0.0002 | 139.0 ± 1.27 | 142.6 ± 1.25 | 0.2 cm | 0.0002 | 0.0002 |
| weight (kg) mean ± SEM |
27.3 ± 0.72 | 30.4 ±0.76 | 1.2 kg | 0.0002 | 30.1 ± 0.83 | 33.4 ± 0.87 | 1.4 kg | 0.0002 | 0.0002 | ||
| triceps skinfold thickness mean ±SEM |
7.4 ± 0.21 | 8.8 ± 0.25 | 1.4 mm | 0.0002 | 8.2 ± 0.28 | 9.7 ± 0.32 | 1.5 mm | 0.0002 | 0.0002 | ||
| subscapular skinfold thickness mean ± SEM |
5.6 ± 0.16 | 6.9 ± 0.2 | 1.4 mm | 0.0002 | 6.1 ± 0.2 | 7.4 ± 0.25 | 1.4 mm | 0.0002 | 0.0002 | ||
| Middle upper arm circumference (MUAC) mean ± SEM |
17.5 ± 0.22 | 18.5 ± 0.24 | 0.7 cm | 0.0002 | 18.3 ± 0.22 | 19.2 ± 0.24 | 0.7 cm | 0.0002 | 0.0002 | ||
| ht for age (%) mean ± SEM |
93.0 ± 0.44 | 92.8 ± 0.43 | 0.1% points | 0.02 | 93.2 ± 0.43 | 93.3 ± 0.43 | 0.1% points | Not significant | Not significant | ||
| wt for age (%) mean ± SEM |
72.7 ± 0.98 | 74.9 ± 1.00 | 3.3% points | 0.0002 | 73.8 ± 1.04 | 76.2 ± 1.08 | 3.7% points | 0.0002 | 0.0002 | ||
| wt for ht (%) mean ± SEM |
89.5 ± 0.77 | 93.0 ± 0.84 | 3.7% points | 0.0002 | 89.9 ± 0.78 | 93.3 ± 0.78 | 3.6% points | 0.0002 | 0.0002 | ||
| MUAC/age (%) mean ± SEM |
81.6 ± 0.7 | 83.1 ± 0.73 | 0.7% points | 0.0002 | 82.5 ± 0.7 | 83.9 ± 0.76 | 3% points | 0.0002 | 0.0002 | ||
| triceps skinfold/age mean ± SEM |
68.5 ± 1.67 | 80.0 ± 1.82 | 13.2% points | 0.0002 | 74.4 ± 1.83 | 85.7 ± 1.93 | 13.1% points | 0.0002 | 0.0002 | ||
| subscapular skinfold/age mean ± SEM |
86.8 ± 1.79 | 102.7 ± 2.24 | 21.4% points | 0.0002 | 6.1 ± 0.2 | 7.4 ± 0.25 | 19.7% points | 0.0002 | 0.0002 | ||
Stephenson 1989 KEN at 5 weeks, there were 16 participants in the praziquantel group and 16 in the metrifonate group. At 8 months, there were 105 participants in the praziquantel group and 103 in the metrifonate group.
Appendix 5. Appendix: Additional tables for adverse events
Praziquantel 40 mg/kg single dose versus placebo: adverse events
| Trial ID | N (praziquantel treatment arm) | Adverse event monitoring | Blinding | Summary of adverse events findings |
| Pugh 1983 MWI | N = 97 (Praziquantel 40 mg/kg) | No comment | Blinded for participants and clinicians | "Treatment was well tolerated". |
| Borrmann 2001 GAB | N = 300 90 (Praziquantel 40 mg/kg) 90 (ARS 4 mg/kg for 3 days) 90 (Praziquantel 40 mg/kg and ARS 4 mg/kg for 3 days) 30 (placebo) |
Adverse events recorded on day 1, 3 and 7 (changes in the participants condition, compared with baseline) |
Blinded for participants and clinicians | No difference between treatment groups regarding the number of adverse events, or distribution of particular adverse events. All treatment regimens well tolerated. Six moderate and 127 mild adverse events reported. Most common adverse events:abdominal pain (overall 14%) and headache (12%). |
| Inyang Etoh 2009 NGA | N = 312 104 (Praziquantel; 52 with placebo, 52 without placebo) 104 (ARS 4 mg/kg for 3 days; 52 with placebo, 52 without placebo 52 (Praziquantel 40 mg/kg and ARS 4 mg/kg for 3 days) 52 (placebo) |
Careful monitoring for adverse events by trial physician, any potential adverse events were noted and monitored for up to 72 hours post treatment. Final assessment on day 56 for 2 consecutive days. | Unclear | No difference between treatment groups. No severe adverse events reported within one hour of medication, no child required immediate medical care. Good tolerance of all treatment regimens. 97 incidences of adverse events reported. 33 cases of headache. |
| McMahon 1979 TZA | N = 101 32 (Praziquantel 40 mg/kg) 33 (Praziquantel 30 mg/kg) 36 (Praziquantel 2 x 20 mg/kg) |
All children examined clinically before and four and 24 hours after treatment. Symptoms recorded after both general and specific queries (anorexia, nausea, vomiting, abdominal pain, diarrhoea, giddiness, tiredness, weakness, body pain, headache and fever) |
Unclear | No difference between treatment groups and placebo group, no side effects related to the drug or to infection intensity common side effects equally frequent before and after treatment |
| Olds 1999 KEN | N = 193 98 (Praziquantel and albendazole) 95 (Praziquantel) |
Surveillance for 48 hours for specific side effects (drug‐related side effect compared with parasite egg counts, symptoms over the past two weeks, physical examination and treatment group). Participants asked for overall rating of side effects and limitations of activity recording of request for medication for symptoms and hospitalisation. |
Blinded for clinicians, participants not blinded for outcome assessors |
Data not reported for S. haematobium separately. Vomiting, abdominal pain, headache, interference with normal activity, diarrhoea, bloody diarrhoea, request for additional medication for symptoms, and total side effects were all higher in children with documented schistosomiasis. |
| Taylor 1988 ZWE | N = 283 77 (Praziquantel 40 mg/kg) 72 (Praziquantel 30 mg/kg) 61 (Praziquantel 20 mg/kg) 73 (Praziquantel 10 mg/kg) |
No comment | Blinded for clinicians, participants not blinded for outcome assessors |
"Side effects were not monitored but it appeared that those receiving smaller doses received less abdominal discomfort." |
Footnotes
Befidi Mengue 1992 CMRand Stephenson 1989 KEN did not comment on adverse events.
Praziquantel 40 mg/kg single dose versus 30 mg/kg single dose: adverse events
| Trial ID | N | Adverse event monitoring | Blinding | Summary of adverse event finding |
| Rey 1983 NER | N = 103 57 (Praziquantel 40 mg/kg) 46 (Praziquantel 30 mg/kg) |
Not described | Unclear | 6% had mild adverse events, no difference between groups. |
| Davis 1981 ZMB | N = 98 N = 45 (Praziquantel 40 mg/kg), N = 53 (Praziquantel 30 mg/kg) |
Active surveillance of frequency and severity of side effects after treatment by direct questioning. Prospective: paired examinations of pre and post treatment measurements and haematological and clinical chemical variables before and after treatment |
Single blind no blinding out outcome assessors |
Very good tolerance and patient acceptability for praziquantel; low incidence and severity of side effects. |
| McMahon 1979 TZA | N = 65 N = 32 (Praziquantel 40 mg/kg) 33 (Praziquantel 30 mg/kg) |
All children examined clinically before and four and 24 hours after treatment. Symptoms recorded after both general and specific queries. Active surveillance, likely to be prospective. |
Unclear | No difference between groups. Common side effects equally frequent before and after treatment and in treated and placebo groups. No side effects related to the drug or to infection intensity. |
| Omer 1981 SDN | N = 100 N = 50 (Praziquantel 40 mg/kg), N = 50 (Praziquantel 30 mg/kg) |
Side effects recorded before and after treatment. Method of monitoring not described. Monitoring of vital signs (pulse rate, respiratory rate, blood pressure) at 24 and 48 hours. |
Unclear for participants, clinicians and outcome assessors |
Difference between groups not reported. Mild diarrhoea on day one following treatment in 31% of patients. All other symptoms mild and transient. |
| Oyediran 1981 NGA | N = 22 Praziquantel 40 mg/kg N = 23 (Praziquantel 30 mg/kg) N = 21 (Praziquantel 2 x 20 mg/kg) N = 24 (placebo) |
Clinical examination pre‐treatment and 18 to 24 hours post treatment to detect any unwanted side effects of praziquantel (pulse rate, systolic and diastolic blood pressure). Haematological and biochemical blood tests before (standard blood count, Hb electrophoresis, Bilirubin, SGPT, SGOT) and 18 to 24 hours after treatment (packed cell volume, total and differential white blood count, Bilirubin, SGOT, SGPT). Therapeutic doses of chloroquine (for Malaria) and levo‐tetramisole (ascariasis) given to all subjects. |
Unclear | Difference in clinically diagnosed side effects not reported. No difference between groups in post treatment haematological and biochemical findings (within normal limits). Very good tolerance of praziquantel, very few side effects (two cases of moderate abdominal pain). |
| Taylor 1988 ZWE1 | 283 77 (Praziquantel 40 mg/kg), 72 (Praziquantel 30 mg/kg) |
No comment | Blinded for clinicians, participants. Not blinded for outcome assessors. |
"Side effects were not monitored but it appeared that those receiving smaller doses received less abdominal discomfort." |
Footnotes
King 2002 KEN, Mott 1985 GHA, King 2002 KEN and Wilkins 1987 GMB did not report adverse events.
1Taylor 1988 ZWE also reported a treatment arm of 61 participants who received Praziquantel 20 mg/kg and a treatment arm of 73 participants who received Praziquantel 10 mg/kg.
Praziquantel single dose versus split doses: adverse events
| Trial ID | N (praziquantel treatment arm) | Adverse event monitoring | Blinding | Summary of adverse events findings |
| Kardaman 1985 SDN | N = 220 114 (Praziquantel 40 mg/kg) 106 (Praziquantel 2 x 20 mg/kg) |
Monitoring Pre‐treatment clinical examination and interview of all patients by a clinician at the school on the day prior to treatment. recording of pre‐treatment symptoms per group. Post‐treatment interview of patients the morning after treatment by clinicians. recording or post‐treatment drug‐induced side‐effects per group. Second check seven days after treatment. Prospective, active surveillance. |
Unclear | 80% of children with some mild, transitory drug‐induced side‐effects. Most common complaint abdominal pain (63%) (further complaints diarrhoea, nausea, vomiting). Vomiting and dizziness significantly less common with a single dose than a split dose (resolved within 24 hours). |
| McMahon 1979 TZA | N = 68 32 (Praziquantel 40 mg/kg) 36 (Praziquantel 2 x 20 mg/kg) |
All children examined clinically before, and four and 24 hours after treatment. Symptoms recorded after both general and specific queries (anorexia, nausea, vomiting, abdominal pain, diarrhoea, giddiness, tiredness, weakness, body pain, headache and fever). Prospective, active surveillance, likely to be prospective. |
Unclear | No side effects related to the drug or to infection intensity. Common side effects equally frequent before and after treatment and in treated and placebo groups. |
| Davis 1981 ZMB | N = 98 45 (Praziquantel 40 mg/kg), 53 (Praziquantel 2 x 20 mg/kg) |
Active surveillance of frequency and severity of side effects after treatment direct questioning. Prospective monitoring: paired examinations of pre and post treatment measurements and haematological and clinical chemical variables before and after treatment. |
Single blind No blinding of outcome assessors |
Very good tolerance and patient acceptability for praziquantel. Low incidence and severity of side effects. |
Metrifonate 3 doses two weeks apart: 7.5 mg/kg versus 5 mg/kg: adverse events
| Trial ID | N (metrifonate treatment arm) | Adverse event monitoring | Blinding | Summary of adverse events findings |
| Abden Abdi 1989 SOM | N = 201 100 (3 x 7.5 mg/kg) 101 (3 x 5 mg/kg) |
Patients left as soon as they had received the drug; good monitoring during the first day (3 x 5 mg/kg given in one day) Questioning about side effects when patient returned for the next treatment with a check list; spontaneous reports were also noted (active and passive surveillance). |
Double blinded | Side effects in 7% of patients in the 3 x 7.5 mg/kg treatment group and 9% patients in the 3 x 5 mg/kg treatment group; mostly mild and transient, headache and abdominal pain were most frequently noted (Analysis 9.2). |
Praziquantel 40 mg/kg single dose versus 3 x metrifonate 10 mg/kg: adverse events
| Trial ID | N (metrifonate treatment arm) | Adverse event monitoring | Blinding | Summary of adverse events findings |
| Al Aska 1990 SAU | N = 100 50 (metrifonate 3 x 10 mg/kg) 50 (praziquantel 1 x 40 mg/kg) |
Recording of drug side effects at the second visit (time point unclear) | Unclear | Dizziness was more common in the praziquantel group (20%) than in the metrifonate group (10%). No difference between groups for abdominal pain (12% in both groups ). |
de Jonge 1990 SDN did not comment on adverse events.
Praziquantel 30 mg/kg single dose versus 3 x metrifonate 10 mg/kg: adverse events
| Trial ID | N (metrifonate treatment arm) | Adverse event monitoring | Blinding | Summary of adverse events findings |
| McMahon 1983 TZA | N = 60 30 (metrifonate 3 x 10 mg/kg) 30 (praziquantel 1 x 30 mg/kg) |
— | No | No major side effects. "Abdominal pain was more common and more severe after metrifonate." |
Artesunate versus placebo, praziquantel and artesunate versus praziquantel: adverse events
| Trial ID | N (treatment arms) | Adverse event monitoring | Blinding of participants and staff | Summary of adverse events findings |
| Borrmann 2001 GAB | N = 300 90 (praziquantel 40 mg/kg) 90 (artesunate 4 mg/kg for 3 days) 90 (praziquantel 40 mg/kg and artesunate 4 mg/kg for 3 days) 30 (placebo) |
Adverse events recorded on day 1, 3 and 7 (changes in the participants condition, compared with baseline). Prospective surveillance. Unclear if active or passive monitoring. |
Blinded for participants and clinicians | All treatment regimens well tolerated. Six moderate and 127 mild adverse events reported. No difference between treatment groups regarding the number of adverse events, or distribution of particular adverse events, most common adverse events: abdominal pain (overall 14%) and headache (12%). |
| Inyang Etoh 2009 NGA | N = 312 104 (praziquantel; 52 with placebo, 52 without placebo) 104 (artesunate 4 mg/kg for 3 days; 52 with placebo, 52 without placebo) 52 (praziquantel 40 mg/kg and artesunate 4 mg/kg for 3 days) 52 (placebo) |
Careful monitoring for adverse events by trial physician, any potential adverse events were noted and monitored for up to 72 hours post treatment, excellent compliance for reporting. Final assessment on day 56 for 2 consecutive days. Unclear if active or passive monitoring. |
Unclear | No difference between treatment groups. No severe adverse events reported within one hour of medication, no child required immediate medical care. 97 incidences of adverse events reported 33 cases of headache Good tolerance of all treatment regimens |
Praziquantel versus artesunate: adverse events
| Trial ID | N (treatment arms) | Adverse event monitoring | Blinding of participants and staff | Summary of adverse events findings |
| Keiser 2010 CIV | N = 83 19 (mefloquine 25 mg/kg) 18 (artesunate 3 x 100 mg and mefloquine 250 mg FDC) 20 (artesunate 4 mg/kg for 3 days) 26 (praziquantel 40 mg/kg) No placebo arm |
Observation for AE for 3 hours after the first dose AE monitoring until 96 hours after the first dosing. Interview with standardized questionnaire at 24, 48, 72 and 96 hours. Clinical examination in case AE occurred. Classification of AE as mild, moderate, severe or life threatening. |
Not blinded | No difference between the four treatment groups (headache, coughing, vomiting, vertigo or chills). Abdominal pain more frequent in the mefloquine group (98%, P < 0.001), mefloquine‐artesunate (83%, P = 0.008, artesunate (60%, P = 0.37) than in the praziquantel group (46%). More participants had at least one AE in any of the assessments in the mefloquine group (100%), in the mefloquine‐artesunate group (94%) and in the artesunate group (80%) than in the praziquantel group (61%), No serious or life‐threatening adverse events, no hospital admissions due to AE no neuropsychological AE, no trial discontinuation due to AE. |
| Borrmann 2001 GAB | N = 300 90 (praziquantel 40 mg/kg) 90 (artesunate 4 mg/kg for 3 days) 90 (praziquantel 40 mg/kg and artesunate 4 mg/kg for 3 days) 30 (placebo) |
Adverse events recorded on day 1, 3 and 7 (changes in the participants condition, compared with baseline). Prospective surveillance. |
Blinded for participants and clinicians | All treatment regimens well tolerated. Six moderate and 127 mild adverse events were reported. No difference between treatment groups regarding the number of adverse events, or distribution of particular adverse events, most common adverse events: abdominal pain (overall 14%) and headache (12%). |
| Inyang Etoh 2009 NGA | N = 312 104 (praziquantel; 52 with placebo, 52 without placebo) 104 (artesunate 4 mg/kg for 3 days; 52 with placebo, 52 without placebo. 52 (praziquantel 40 mg/kg and artesunate 4 mg/kg for 3 days) 52 (placebo) |
Careful monitoring for adverse events by trial physician, any potential adverse events were noted and monitored for up to 72 hours post treatment. Final assessment on day 56 for two consecutive days. Unclear if active or passive surveillance |
Unclear | No difference between treatment groups No severe adverse events reported within one hour of medication, no child required immediate medical care. 97 incidences of adverse events reported 33 cases of headache Good tolerance of all treatment regimens |
Praziquantel versus mefloquine: adverse events
| Trial ID | N (treatment arms) | Adverse event monitoring | Blinding of participants and staff | Summary of adverse events findings |
| Keiser 2010 CIV | N = 83 19 (mefloquine 25 mg/kg) 18 (artesunate 3 x 100 mg and mefloquine 250 mg FDC) 20 (artesunate 4 mg/kg for 3 days) 26 (praziquantel 40 mg/kg) No placebo arm |
Observation for AE for 3 hours after the first dose AE monitoring until 96 hours after the first dosing interview with standardized questionnaire at 24, 48, 72 and 96 hours. Clinical examination in case AE occurred. Classification of AEs as mild, moderate, severe or life threatening. |
Not blinded | No difference between the four treatment groups (headache, coughing, vomiting, vertigo or chills), abdominal pain more frequent in the mefloquine group (98%, P < 0.001), mefloquine‐artesunate (83%, P = 0.008, artesunate (60%, P = 0.37) than in the praziquantel group (46%). More participants had at least one AE in any of the assessments in the mefloquine group (100%), in the mefloquine‐artesunate group (94%) and in the artesunate group (80%) than in the praziquantel group (61%). No serious or life‐threatening adverse events, no hospital admissions due to AE, no neuropsychological AE, no trial discontinuation due to AE. |
Praziquantel versus Praziquantel and ALB: adverse events
| Trial ID | Participants | Adverse events monitoring | Blinding | Summary of adverse events findings |
| Olds 1999 KEN | N = 193 98 (Praziquantel and albendazole) 95 (Praziquantel) |
AE monitoring for 48 hours for pre‐defined AE | Double blind | 65% of side effects for S. haematobium reported within four to six hours. Symptoms usually resolved within two hours with or without relief medication. Data not reported for S. haematobium separately. |
Appendix 6. Appendix: Ultrasound findings
Praziquantel 40 mg/kg single dose versus 20 mg/kg single dose: ultrasound findings
| Trial ID | Time point | Measure | Praziquantel 40 mg/kg (SD) | Praziquantel 20 mg/kg (SD) | ||
| Baseline | Follow‐up | Baseline | Follow‐up | |||
| King 2002 KEN1 | 9 months | Number of participants with haematuria/total N = 264 for both groups |
22/32 (69%) | 16/32 (50%) | 20/32 (62%) | 17/32 (53%) |
| Mild hydronephrosis (%) | 25% | 15% | 21% | 18% | ||
| Moderate hydronephrosis (%) | 5% | 4% | 11% | 0% | ||
| Severe hydronephrosis (%) | 5% | 2% | 3% | 2% | ||
| Mild bladder abnormalities (%) | 11% | 4% | 17% | 6% | ||
| Severe bladder abnormalities (%) | 8% | 0% | 6% | 0% | ||
1Haematuria measured by urine dipstick, "no statistical difference", "study might be underpowered, for ultrasound findings" all severe bladder abnormalities were eliminated.
Praziquantel 40 mg/kg 1x/year versus metrifonate 10 mg/kg 3x/year: ultrasound findings
| Trial ID | Time point | Measure |
Praziquantel 40 mg/kg 1x/year |
Metrifonate 10 mg/kg 3x/year |
Comments |
| King 1990 KEN | 12 months | Bladder wall thickness | 35% improvement 8% deterioration |
29% improvement 9% deterioration |
Subsample N = 373 Praziquantel N = 141 Metrifonate N = 126 |
| Bladder deformity (granulomata) | 21% improvement 6% deterioration |
15% improvement 2% deterioration |
|||
| Hydronephrosis | 12% improvement 13% deterioration |
9% improvement 7% deterioration |
Data and analyses
Comparison 1. Praziquantel 40 mg/kg single dose versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological failure | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 at one month to two months | 7 | 864 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.29, 0.59] |
| 1.2 at three months | 3 | 354 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.34, 0.77] |
| 1.3 at five months | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.58, 0.91] |
| 1.4 at six months | 3 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.10, 1.84] |
| 1.5 at eight months | 1 | 209 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.08, 0.22] |
| 2 Haematuria at eight weeks | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.33, 0.84] |
| 3 Haemoglobin | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 at baseline | 2 | 727 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.35, 0.02] |
| 3.2 at six to eight months | 2 | 727 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.24, 0.09] |
| 4 Adverse events | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Diarrhoea | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Vomiting | 2 | 226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.15, 2.87] |
| 4.3 Dizziness | 2 | 226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.11, 1.27] |
| 4.4 Anorexia | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.05, 0.85] |
| 4.5 Abdominal pain | 2 | 226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.22, 1.14] |
| 4.6 Tiredness | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.14, 1.71] |
| 4.7 Weakness | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.36, 2.57] |
| 4.8 Headache | 2 | 226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.02, 1.47] |
| 4.9 Fever | 2 | 226 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.07, 17.22] |
| 4.10 Pain in limbs | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.59 [0.28, 112.34] |
| 4.11 Itching | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.19, 5.28] |
| 4.12 Cough | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.09, 10.78] |
| 4.13 Chills | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.16, 14.07] |
| 4.14 Nausea | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.09, 10.78] |
| 4.15 Constipation | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.06, 36.54] |
Comparison 2. Praziquantel 40 mg/kg single dose versus lower doses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological failure at four to six weeks | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 versus 30 mg/kg | 4 | 401 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.59, 0.99] |
| 1.2 versus 20 mg/kg | 2 | 338 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.59, 0.93] |
| 1.3 versus 10 mg/kg | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.53, 0.84] |
| 2 Parasitological failure at two to three months | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 versus 30 mg/kg | 5 | 517 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.72, 1.24] |
| 2.2 versus 20 mg/kg | 3 | 330 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.56, 0.92] |
| 2.3 versus 10 mg/kg | 3 | 339 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.39, 0.60] |
| 3 Parasitological failure at six to seven months | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 versus 30 mg/kg | 6 | 669 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.76, 1.23] |
| 3.2 versus 20 mg/kg | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.53, 1.44] |
| 3.3 versus 10 mg/kg | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.29, 0.64] |
| 4 Haematuria at three months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 versus 30 mg/kg | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.47, 1.67] |
| 4.2 versus 20 mg/kg | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.60, 2.33] |
| 4.3 versus 10 mg/kg | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.21, 0.58] |
| 5 Proteinuria at three months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 versus 30 mg/kg | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.34, 2.12] |
| 5.2 versus 20 mg/kg | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.36, 2.30] |
| 5.3 versus 10 mg/kg | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.12, 0.51] |
| 6 Haematuria at six weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 versus 20 mg/kg | 1 | 245 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.47, 0.86] |
| 7 Proteinuria at six weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 versus 20 mg/kg | 1 | 245 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.46, 0.96] |
| 8 Haematuria at nine months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 versus 20 mg/kg | 1 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.44, 0.78] |
| 9 Proteinuria at nine months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 versus 20 mg/kg | 1 | 214 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.50, 0.90] |
| 10 Adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Vomiting | 2 | 163 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.05, 13.51] |
| 10.2 Dizziness | 2 | 163 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.11, 4.62] |
| 10.3 Anorexia | 1 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 4.85 [0.24, 97.31] |
| 10.4 Abdominal pain | 2 | 163 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.23, 5.56] |
| 10.5 Tiredness | 1 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.10, 1.09] |
| 10.6 Weakness | 1 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.39, 3.44] |
| 10.7 Headache | 2 | 163 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.08, 2.85] |
| 10.8 Fever | 1 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 2.91 [0.12, 68.95] |
| 10.9 Pain in limbs | 1 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.08, 1.86] |
2.10. Analysis.
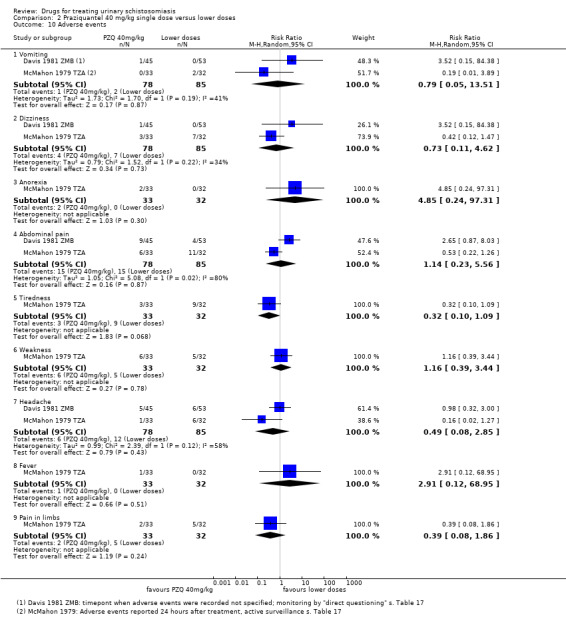
Comparison 2 Praziquantel 40 mg/kg single dose versus lower doses, Outcome 10 Adverse events.
Comparison 3. Praziquantel 40 mg/kg single dose versus 2 x 20 mg/kg split dose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological failure | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 at one month | 3 | 374 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.51, 1.11] |
| 1.2 at three months | 3 | 361 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.45, 1.20] |
| 1.3 at six to seven months | 3 | 234 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.51, 1.35] |
| 2 Adverse events | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Blood in stool | 1 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Vomiting | 3 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.29, 0.86] |
| 2.3 Dizziness | 3 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.16, 0.94] |
| 2.4 Anorexia | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.18 [0.21, 22.96] |
| 2.5 Abdominal pain | 3 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.83, 1.25] |
| 2.6 Tiredness | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.12, 1.41] |
| 2.7 Weakness | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.35, 2.50] |
| 2.8 Headache | 2 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.20, 1.33] |
| 2.9 Fever | 2 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.23, 1.23] |
| 2.10 Pain in limbs | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.09, 2.10] |
| 2.11 Diarrhoea | 1 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.67, 1.73] |
| 2.12 Skin reaction | 1 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.84 [0.34, 9.83] |
Comparison 4. Praziquantel 40 mg/kg single dose versus praziquantel 2 x 40 mg/kg or 3 x 40 mg/kg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Praziquantel 40 mg/single dose versus praziquantel 2 x 40 mg/kg: parasitological failure | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 at six weeks | 1 | 269 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.50, 1.34] |
| 1.2 at nine weeks to three months | 2 | 686 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.91, 1.25] |
| 1.3 at six months | 1 | 556 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.95, 1.31] |
| 2 Praziquantel 40 mg/kg single dose versus praziquantel 3 x 40 mg/kg: parasitological failure | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 at nine weeks | 1 | 185 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.42, 2.12] |
| 3 Praziquantel 40 mg/single dose versus praziquantel 2 x 40 mg/kg: microhaematuria at six months | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.88, 1.56] |
Comparison 5. Praziquantel 40 mg/kg single dose versus multiple doses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological failure | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 at two years | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [1.47, 5.00] |
| 1.2 at three years | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.59, 1.42] |
| 2 Haematuria | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.42, 1.17] |
5.2. Analysis.
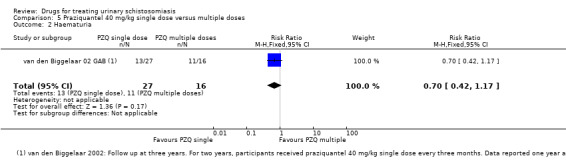
Comparison 5 Praziquantel 40 mg/kg single dose versus multiple doses, Outcome 2 Haematuria.
Comparison 6. Metrifonate single dose (10 mg/kg) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological failure | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 at one month | 1 | 142 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.74, 0.94] |
| 1.2 at two and a half to three months | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.85, 0.99] |
| 1.3 at six months | 1 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.87, 1.02] |
| 1.4 at eight months | 1 | 210 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.54, 0.73] |
| 2 Haemoglobin | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 at baseline | 1 | 207 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.33, 0.33] |
| 2.2 at eight months | 1 | 207 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.05, 0.65] |
Comparison 7. Metrifonate multiple doses versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological failure | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 at one month | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.65, 1.09] |
| 1.2 at 11 weeks | 1 | 93 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.30, 0.56] |
| 1.3 at five months | 1 | 51 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.76, 1.03] |
| 1.4 at six months | 1 | 400 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.24, 0.37] |
| 2 Haemoglobin | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 at baseline | 1 | 400 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.45, 0.11] |
| 2.2 at six months | 1 | 391 | Mean Difference (IV, Random, 95% CI) | 0.30 [0.14, 0.46] |
Comparison 8. Metrifonate multiple doses versus single dose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological failure at one month | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 20 mg/kg versus 10 mg/kg | 1 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.50, 1.13] |
| 1.2 30 mg/kg versus 10 mg/kg | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.17, 0.77] |
| 2 Parasitological failure at four months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 20 mg/kg versus 10 mg/kg | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.58, 1.06] |
| 2.2 30 mg/kg versus 10 mg/kg | 1 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.45, 0.99] |
Comparison 9. Metrifonate 3 doses 2 weeks apart: 7.5 mg/kg versus 5 mg/kg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological failure | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 at one month | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.69, 1.21] |
| 1.2 at two months | 1 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.72, 1.30] |
| 1.3 at three months | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.67, 1.26] |
| 1.4 at six months | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.99, 2.05] |
| 2 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Nausea | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.05, 5.48] |
| 2.2 Vomiting | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.06, 15.93] |
| 2.3 Dizziness | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.06, 15.93] |
| 2.4 Abdominal pain | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.32, 28.64] |
| 2.5 Headache | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.04, 3.18] |
| 2.6 Heaviness of the tongue | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.19, 21.92] |
Comparison 10. Praziquantel versus metrifonate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Praziquantel 40 mg/kg single dose versus metrifonate 10 mg/kg single dose: parasitological failure | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 at one month | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.34, 0.61] |
| 1.2 at two to three months | 2 | 243 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.57, 0.79] |
| 1.3 at six months | 1 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.79, 1.01] |
| 1.4 at eight months | 1 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.13, 0.36] |
| 2 Praziquantel 40 mg/kg single dose versus metrifonate 10 mg/kg single dose: haemoglobin | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 at baseline | 1 | 208 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.52, ‐0.08] |
| 2.2 at eight months | 1 | 208 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.66, ‐0.14] |
| 3 Praziquantel 40 mg/kg single dose versus metrifonate 20 and 30 mg/kg given as split doses: parasitological failure | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 2 x 10 mg/kg Metrifonate at one month | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.80, 1.34] |
| 3.2 2 x 10 mg/kg Metrifonate at five months | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.64, 1.05] |
| 3.3 3 x 10 mg/kg Metrifonate at three months | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.07, 1.57] |
| 3.4 3 x 10 mg/kg Metrifonate at six months | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.2 [0.02, 1.65] |
| 4 Praziquantel 40 mg/kg single dose versus metrifonate 30 mg/kg given as split dose: adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Dizziness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Abdominal pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Joint pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Nausea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Rash | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 Vomiting | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.7 Itching | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.8 Fatigue | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.9 Hair loss | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.10 Change in taste | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.11 Diarrhoea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.12 Convulsion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Praziquantel 30 mg/kg single dose versus metrifonate 30 mg/kg given as split dose: parasitological failure | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 at two months | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.17, 1.68] |
| 5.2 at four months | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.07, 0.80] |
| 6 Praziquantel 30 mg/kg single dose versus metrifonate 30 mg/kg given as split dose: adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Nausea | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 70.83] |
| 6.2 Vomiting | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.00] |
| 6.3 Abdominal pain | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.12, 0.92] |
| 6.4 Headache | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.87] |
| 6.5 Fever | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.87] |
| 6.6 Loose bowel motions | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 15.26] |
| 6.7 Dizziness | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 15.26] |
| 6.8 Itching | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 15.26] |
| 6.9 Body pain | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 15.26] |
| 7 Praziquantel 40 mg/kg once a year versus metrifonate 10 mg/kg every 4 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Parasitological failure at one year | 1 | 1436 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [1.00, 1.11] |
| 7.2 Haematuria at one year | 1 | 1400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.85, 1.36] |
| 7.3 Proteinuria at one year | 1 | 1400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.79, 1.11] |
| 8 Praziquantel 40 mg/kg once a year versus metrifonate 10 mg/kg every 4 months: parasitological failure | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 at one year | 1 | 1018 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.61, 1.00] |
| 8.2 at two years | 1 | 1025 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.53, 1.11] |
| 8.3 at three years | 1 | 827 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.42, 0.93] |
| 9 Praziquantel 40 mg/kg versus praziquantel 10 mg/kg and metrifonate 10 mg/kg | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.34, 1.03] |
Comparison 11. Artesunate versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological failure at eight weeks | 2 | 251 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.16, 1.71] |
| 2 Haematuria | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.85, 1.76] |
| 3 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Vomiting | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Itching | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Cough | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Diarrhoea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.7 Chills | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.8 Nausea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.9 Dizziness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.10 Abdominal pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.11 Constipation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
11.2. Analysis.
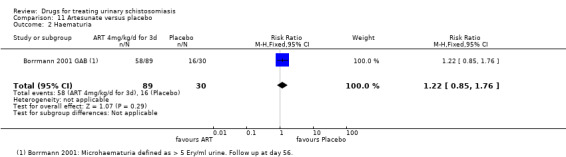
Comparison 11 Artesunate versus placebo, Outcome 2 Haematuria.
Comparison 12. Praziquantel versus artesunate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological failure | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 at day 28 | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.05, 0.46] |
| 1.2 at day 56 | 2 | 352 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.23, 1.44] |
| 2 Haematuria | 1 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.30, 0.62] |
| 3 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Abdominal pain | 1 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Dizziness | 1 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Headache | 1 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.43, 2.30] |
| 3.4 Vomiting | 1 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.26, 3.89] |
| 3.5 Fever | 1 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.41, 3.35] |
| 3.6 Itching | 1 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.26, 3.89] |
| 3.7 Cough | 1 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.14, 6.97] |
| 3.8 Diarrhoea | 1 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.9 Chills | 1 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.26, 8.79] |
| 3.10 Nausea | 1 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.14, 6.97] |
| 3.11 Constipation | 1 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 72.80] |
Comparison 13. Praziquantel and artesunate versus praziquantel.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological failure at eight weeks | 2 | 265 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.38, 0.99] |
| 2 Haematuria at eight weeks | 1 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.40, 1.18] |
| 3 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Abdominal pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Dizziness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Vomiting | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Itching | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.7 Cough | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.8 Diarrhoea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.9 Chills | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.10 Nausea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.11 Constipation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
13.2. Analysis.
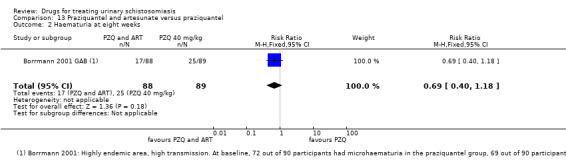
Comparison 13 Praziquantel and artesunate versus praziquantel, Outcome 2 Haematuria at eight weeks.
13.3. Analysis.
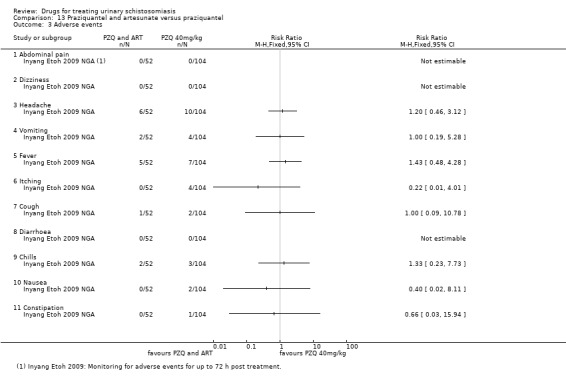
Comparison 13 Praziquantel and artesunate versus praziquantel, Outcome 3 Adverse events.
Comparison 14. Mefloquine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological failure at six weeks | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.40, 0.83] |
Comparison 15. Praziquantel versus mefloquine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological failure at one month | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.05, 0.43] |
Comparison 16. Praziquantel versus artesunate and mefloquine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological failure at one month | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.07, 0.74] |
Comparison 17. Praziquantel versus praziquantel and albendazole.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological failure | 1 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.62, 1.30] |
Comparison 18. Praziquantel versus praziquantel and artesunate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological failure at eight weeks | 2 | 265 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [1.01, 2.60] |
| 2 Haematuria at eight weeks | 1 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.85, 2.50] |
| 3 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Abdominal pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Dizziness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Vomiting | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Itching | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.7 Cough | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.8 Diarrhoea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.9 Chills | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.10 Nausea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.11 Constipation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
18.1. Analysis.
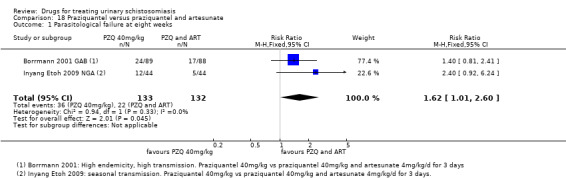
Comparison 18 Praziquantel versus praziquantel and artesunate, Outcome 1 Parasitological failure at eight weeks.
18.2. Analysis.
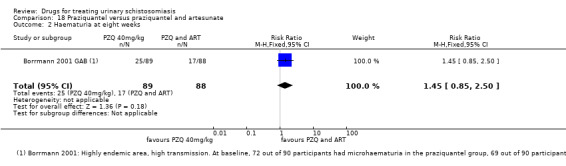
Comparison 18 Praziquantel versus praziquantel and artesunate, Outcome 2 Haematuria at eight weeks.
18.3. Analysis.
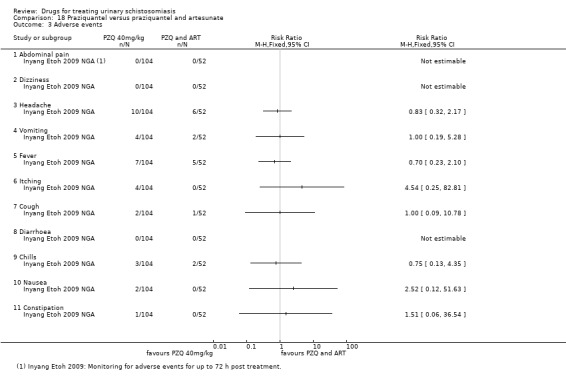
Comparison 18 Praziquantel versus praziquantel and artesunate, Outcome 3 Adverse events.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abden Abdi 1989 SOM.
| Methods | RCT Diagnostics: egg excretion in a single, mid‐day urine sample, mixing an aliquot of 10 mL urine, filtration (nucleopore) Follow‐up at 1, 2, 3 and 6 months |
|
| Participants | Children aged 11 to 12 years on average Number randomized 300 Number analysed for primary outcome at one month 201, at six months 139 Inclusion criteria: excreting 20 or more S. haematobium eggs per 10 mL urine Exclusion criteria: concomitant disease |
|
| Interventions | 1. Metrifonate 3 x 7.5 mg/kg dose interval two weeks 2. Metrifonate 3 x 5 mg/kg within one day 3. Placebo |
|
| Outcomes | Cure rate Percentage egg reduction Adverse events |
|
| Notes | Location: Somalia, southern part Setting: rural, five villages Endemicity: high Dates: not stated Source of funding: SAREC (Swedish agency for research cooperation with developing countries) Authors' conclusion: Both metrifonate regimens have similar efficacy |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized, randomly assigned, table of random numbers. |
| Allocation concealment (selection bias) | Low risk | All doses were kept in coded envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind, placebo controlled "and the distributor of the drug and the participants were all blind to the type of treatment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of the lab technician. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High loss to follow‐up, 33% at one month, 53% at six months, balanced between treatment arms |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No evidence of other bias. |
Al Aska 1990 SAU.
| Methods | RCT Diagnostics: ova excretion in 10 mL midday urine after sedimentation Follow‐up: three and six months |
|
| Participants | Adult patients referred to hospital, age not stated. Saudi and Jemeni Number randomized: not reported Number analysed: 100 Inclusion criteria: S. haematobium infection Exclusion criteria: none stated Co‐infection with S. mansoni |
|
| Interventions | 1. Praziquantel 40 mg/kg single dose 2. Metrifonate 10 mg/kg three doses in intervals of two weeks |
|
| Outcomes | Cure rates Failure rates |
|
| Notes | Location: Saudi Arabia Setting: King Abdul Aziz University hospital, Riyadh. Patient referral Endemicity: not reported Dates: not stated Funding: not stated Authors' conclusion: Metrifonate and praziquantel in the stated dosage are effective against S. haematobium, side effectives are minor and transient |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "allocated randomly". |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned, no placebo mentioned. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up not reported. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | Few baseline characteristics reported. |
Basra 2012 GAB.
| Methods | RCT Diagnostics: Ova excretion, microscopy in 10 mL urine after filtration, AMEC Follow‐up: six weeks |
|
| Participants | Pregnant women attending ANC clinics, aged 19 to 25 years Number randomized 65 Number analysed 44 Inclusion criteria: S. haematobium infection, pregnancy Exclusion criteria: intake of antihelminthic and antimalarial drug within the previous two months, HIV pos |
|
| Interventions | 1. Praziquantel 40 mg/kg single dose 2. Metrifonate 10 mg/kg two doses, dose interval two weeks |
|
| Outcomes | Cure rates Failure rates Egg counts at baseline, four and six weeks |
|
| Notes | Location: Gabon Setting: two ANC health care centres Endemicity: highly endemic for S. haematobium and malaria Dates: Sept 2009 to Dec 2011 Funding: European and Developing Countries Clinical Trial Partnership (EDCCTP), Malaria in Prengnancy consortium, Karl Landsteiner Gesellschaft Authors' conclusion: Mefloquine IPTp is effective against S. haematobium in pregnant women. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomizations list was computer‐generated and provided by the independent MIPPAD trial management team. |
| Allocation concealment (selection bias) | Low risk | Trial assignment was concealed via sealed opaque envelopes which were opened only after enrolment of a patient by a trial investigator. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High loss to follow‐up, unbalanced (in the intervention group 18/48 = 37.5%, in the control group 3/48 = 6.25%) reasons partly stated. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting. |
| Other bias | Low risk | No risk of other bias. |
Befidi Mengue 1992 CMR.
| Methods | RCT Diagnostics: urine sample preserved with 5 mg sodium azide, sedimentation for one hour, examination of sediment, egg count Follow‐up: six months (as only time point) |
|
| Participants | Male primary school students, aged six to 15 years Number randomized 653, 436 in groups of interest for this review Exclusion: heavy S. haematobium infections (> 499 eggs/10 mL) Inclusion: positive for S. haematobium |
|
| Interventions | 1. Praziquantel 40 mg/kg single dose 2. Placebo |
|
| Outcomes | Geometric mean egg counts Weight Height Height for age Weigth for age Weight for height MUAC Triceps skinfold thickness Mean muscle mass Hb (reported in a separate publication Befidi Mengue 1993, see reference Befidi Mengue 1992 CMR) with slightly higher numbers of participants: 771 randomized, 518 in treatment groups of interest of this review). |
|
| Notes | Location: Cameron, Eastern Province, Bertuoa Setting: urban (capital city of Eastern province), primary school Endemicity: polyparasitism is common Dates: not reported Funding: USAID Cameroon health constraints to rural production project 1608 ‐ 1408 Authors' conclusion: only demonstrable effect of a single praziquantel treatment on MUAC |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned, method not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The placebo tablets were physically identical to the praziquantel tablets. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up unclear, as numbers followed up not reported. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | Low risk | No evidence for other bias. |
Borrmann 2001 GAB.
| Methods | RCT Diagnostics: two urine samples. filtration of 10 mL of urine through polycarbonate filters (Millipore), staining with Trypan blue Follow‐up at day 56 (as only time point) |
|
| Participants | School children aged six to 15 years Participants randomized: 300 Inclusion: S. haematobium positive, asymptomatic S. haematobium infection Exclusion: symptomatic schistosomiasis, recent schistosomiasis treatment, serious underlying disease, pregnancy or lactation, anaemia (Hb < 7 G/dL) |
|
| Interventions | 1. Praziquantel 40 mg/kg single dose 2. Artesunate 4 mg/kg once daily for three days 3. Artesunate 4 mg/kg once daily for three days and praziquantel 40 mg/kg single dose 4. Placebo |
|
| Outcomes | Cure rates Failure rates Egg reduction rates Microhaematuria (Adverse events day seven) |
|
| Notes | Location: Gabon, province Moyen Ogone Setting: rural villages Endemicity: high (prevalence 80% in school children) Dates: Oct. 2000 to Feb 2001 Funding: tablet donation Sanofi (Artesunate), Medochemie (Praziquantel) Authors' conclusions: Efficacy of artesunate for S. haematobium treatment as single medication or in combination is low. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomization code was generated by computer. |
| Allocation concealment (selection bias) | Low risk | The trial drugs were prepared in plastic bags, which were labelled sequentially with treatment numbers according to the randomization code. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind. Praziquantel placebo and artesunate placebo were identical in appearance to the respective active substance tablets. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low loss to follow‐up (7.6%). |
| Selective reporting (reporting bias) | Unclear risk | Haemoglobin measurements, proteinuria and leucocyturia at day 56 not reported. |
| Other bias | Low risk | No evidence for other bias. |
Davis 1981 ZMB.
| Methods | RCT Diagnostics: three successive daily schistosome egg counts made on a random 10 mL urine sub sample of the total bladder content by a filtration staining technique; quantitative hatching technique (enumeration of miracidia, recently dead eggs and black eggs) Follow‐up: three consecutive daily urine samples, quantitative hatching test Follow‐up: at 1, 3, 7, 12 and 24 months |
|
| Participants | School children aged seven to 17 years Number followed up after one month 151, number randomized not reported Inclusion: S. haematobium positive Exclusion: pregnant or lactating women, no serious acute coexistent diseases or complications, no other treatment during the past six months, older than six years |
|
| Interventions | 1. Praziquantel 30 mg/kg single dose 2. Praziquantel 40 mg/kg single dose 3. Praziquantel 20 mg/kg 2 x daily |
|
| Outcomes | Cure rate Failure rate |
|
| Notes | Location: Zambia, Ndola Setting: eight rural schools Dates: not reported Endemicity: high Funding: Parasitic Disease Programme for Research and Training in Tropical diseases Authors' conclusion: treatment groups clinically and statistically comparable |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned, random number table. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Single blind technique. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low loss to follow‐up (3.7% to 6%) at 1, 3 and 7 months. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting (some investigations at baseline not reported). |
| Other bias | Low risk | No evidence of other bias. |
de Jonge 1990 SDN.
| Methods | RCT Diagnostics: urine collection after 250 mL soda drink at midday. Trypan blue staining technique (if the egg concentration was less than 10 eggs per 10 mL urine, the whole volume (up to 350 mL) was filtered). Follow‐up one and five months |
|
| Participants | Male primary school children aged six to 11 years Patients randomized 160, participants randomized into treatment groups of interest for this review: 107 Inclusion: co‐infection with S. haematobium and S. mansoni Exclusion: not reported |
|
| Interventions | 1. Praziquantel 40 mg/kg single dose 2. Metrifonate 2 x 10 mg/kg, dose interval 14 weeks 3. Oxaminique 60 mg/kg single dose 4. Multivitamin single dose |
|
| Outcomes | Failure Egg count |
|
| Notes | Location: Sudan Gezira Setting: rural, village primary schools Funding: Science and Technology for Development, EC, WHO, UNDP, World bank, Special Programme for Training & Research. Gesellschaft für technische Zusammenarbeit Dates: not reported Endemicity: high for both S. mansoni and S. haematobium Authors' conclusion: discussion of correlation of parasitological outcomes and CAA titres |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly divided". |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Multivitamin as placebo, but blinding not mentioned. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up high, at one months up to 23%, at five months up to 28%. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | Low risk | No evidence for other bias. |
Inyang Etoh 2009 NGA.
| Methods | RCT Diagnostics: collection of two urine samples at midday (12.00 to 14.00) after exercise on two consecutive days, agitation of urine sample, preservation of eggs, staining (1% aqueous solution, carbol fuchsin), filtration, egg counts Follow‐up at eight weeks (as only time point) |
|
| Participants | School children aged four to 20 years (nursery school, primary and junior secondary schools, students) Number randomized 260 children into five groups Inclusion: healthy, able to swallow the medication Exclusion: serious underlying disease, recent treatment for schistosomiasis, > 20 yrs, < 4 yrs old |
|
| Interventions | 1. Praziquantel 40 mg/kg single dose and placebo 2. Praziquantel 40 mg/kg single dose only 3. Artesunate 4 mg/kg 1 x daily for three days and placebo 4. Artesunate 4 mg/kg 1 x daily for three days only 5. Praziquantel 40 mg/kg single dose and artesunate 4 mg/kg 1 x daily for three days 6. Placebo and placebo |
|
| Outcomes | Cure Egg counts and egg reduction rate Haematuria Proteinuria |
|
| Notes | Location: Nigeria, Adim community, Cross RIver State Setting: school students Dates: August 2005 to June 2006 Endemicity: seasonal transmission Funding: partly funded by the management of the University of Calabar Authors' conclusion: both praziquantel and artesunate in the stated doses are safe, well‐tolerated and effective in the trial area. Combined treatment is more effective and single treatment with any of the drugs. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised". |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Placebo not identical in appearance. Blinding not mentioned. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up of 15.4% and 19.2% at day 56. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No evidence of other bias. |
Jewsbury 1976 ZWE.
| Methods | RCT Diagnostics: three urine samples on three consecutive days, determination of egg counts and cure rates Follow‐up at week 11 and week 36 |
|
| Participants | Children, aged three to 15 years (and older) Number of children randomized: 179 Number of children analysed 114 (complete case analysis) Inclusion: S. haematobium positive Exclusion: not reported |
|
| Interventions | 1. Metrifonate 7.5 mg x 3, dose interval two weeks 2. Control: no intervention |
|
| Outcomes | Cure rate Failure rate Median urine egg counts |
|
| Notes | Location: Zimbabwe near Salibury Setting: rural, four farms Dates: not reported Endemicity: high (pre‐infection rate with S. haematobium 80%) Funding: Drug donation by Bayer Authors' conclusion: Metrifonate is safe and effective for the treatment of S. haematobium |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised". |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Participant numbers not reported at week 11, high loss to follow‐up of 46% at week 36. |
| Selective reporting (reporting bias) | Unclear risk | Data of week 11 not reported. |
| Other bias | High risk | Baseline imbalance; for the infected, untreated control group, an infection rate of 89.4% is given at baseline. |
Kardaman 1985 SDN.
| Methods | RCT Diagnostics: centrifugation, sediment taken for egg counts Follow‐up at five weeks and three months |
|
| Participants | School children aged seven to 11 years Number of children included: 237 Inclusion: co‐infection S. haematobium and S. mansoni Exclusion: receiving medication for any other infection, treatment for schistosomiasis during the preceding 6 months. |
|
| Interventions | 1. Praziquantel 40 mg/kg single dose 2. Praziquantel 2 x 20 mg/kg in one day, dose interval four to six hours |
|
| Outcomes | Cure Failure Adverse events |
|
| Notes | Location: Sudan, Galaga Village Setting: rural, primary schools Dates: not reported Endemicity: high (mixed infections common) Funding: Parasitic disease programme, WHO Authors' conclusion: Results of two regimens not significantly different. Treatment for this setting has to be repeated every six months. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up at five weeks up to 4.7%, at three months up to 8.4%. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | Low risk | No evidence for other bias. |
Keiser 2010 CIV.
| Methods | RCT Diagnostics: collection of two urine specimen at midday (10.00 to 14.00), samples were rigorously shaken, filtration of 10 mL through a 13 mL filter with 25 µm diameter Follow‐up at 26 days |
|
| Participants | School children aged eight to 12 years Participants randomized 83 Inclusion: confirmed S. haematobium infection Exclusion: not reported |
|
| Interventions | 1. Praziquantel 40 mg/kg single dose 2. Mefloquine 25 mg/kg single dose 3. Artesunate 4 mg/kg 1 x daily for three days 4. Artesunate 3 x 100 mg and mefloquine 250 mg |
|
| Outcomes | Cure rates Failure rate Egg count Egg reduction rate Adverse effects |
|
| Notes | Location: Cote d' Ivoire, district Agboville Setting: rural, school children Dates: November to December 2009 Funding: support Dafra Pharma, Mepha for drug donations Endemicity: highly endemic, 40% among school children Authors' conclusion: High cure rates with praziquantel, promising results for mefloquine ‐ artesunate (in the standard dose for malaria) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "using a computer generated randomisation code". Seven children were added to one treatment group in a non‐randomized manner. |
| Allocation concealment (selection bias) | High risk | Not implemented (email correspondence with author). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up during the trial (day 26). |
| Selective reporting (reporting bias) | Low risk | Urinary findings day 26 not reported (not available, email correspondence with author). |
| Other bias | Low risk | No evidence of other sources of bias. |
King 1989 KEN.
| Methods | RCT Diagnostics: collection of midday urine sample (10.00 to 13.00), urine filtration technique with nucleopore filters, egg count Follow‐up at two to three months |
|
| Participants | Primary school students aged five to 17 years and adult participants over 20 years Number of patients randomized 280 (34 adults, 246 children) Inclusion: egg count > 50 eggs/10 mL urine Exclusion: not reported |
|
| Interventions | 1. Praziquantel 10 mg/kg single dose 2. Praziquantel 20 mg/kg single dose 3. Praziquantel 30 mg/kg single dose 4. Praziquantel 40 mg/kg single dose |
|
| Outcomes | Cure Egg counts Severity of infection Proteinuria Haematuria |
|
| Notes | Location: Kenya, Kwale district Setting: rural, primary schools Dates: not reported Endemicity: high Funding: Edna McConnell Clark Foundation Authors' conclusion: low dose (20 mg/kg) is as effective as standard dose (40 mg/kg) of praziquantel (reductions in parasite burden and morbidity) for population based control programmes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation, pre‐randomized cards. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinicians not blinded to the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors and laboratory staff blinded to the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up at two to three months 9% to 14%, balanced between groups. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No evidence for other sources of bias. |
King 1990 KEN.
| Methods | RCT Diagnostics: sample collection of midday urine (10.00 to 13.00), nucleopore filtration, egg counts Follow‐up at one, two and three years |
|
| Participants | Primary school children aged four to 21 years Number randomized 1813 Inclusion: S. haematobium positive Exclusion: not reported |
|
| Interventions | 1. Praziquantel 40 mg/kg single dose once a year 2. Metrifonate 10 mg/kg single dose three times a year, dose interval four months |
|
| Outcomes | Haematuria Proteinuria Ultrasound (hydronephrosis, bladder thickening, bladder deformity) |
|
| Notes | Location: Kenya, Coast Province, Kwale Province, Msambweni Area Setting: rural, primary schools, nine villages Dates: 1984 Endemicity: high (prevalence in school children 60% to 85%) Funding: Edna McConnell Clark Foundation, WHO, Rockefeller Foundation Authors' conclusion: Both regimens had significant effects on the prevalence of hematuria, proteinuria, and bladder abnormalities. no significant differences between the two drugs. No effect on hydronephrosis at twelve months. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation with pre‐randomized cards. |
| Allocation concealment (selection bias) | High risk | "Treatment allocation was not concealed to the investigators" (email correspondence with author). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants (different taste and appearance of commercially purchased drugs) email response). no blinding of clinicians |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Evaluators were effectively blinded to the treatment status of the children they were testing (email correspondence with author). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No evidence of other bias. |
King 2002 KEN.
| Methods | RCT Diagnostics: Collection of two mid‐day (10:00 to 14:00) on different days, filtration, Nucleopore) Intensity of infection assigned according to the highest one day egg count in the repeated daily testing. Follow‐up at six weeks and nine months |
|
| Participants | School children and adults, aged four to 23 years Number of participants randomized 291 Inclusion: S. haematobium positive Exclusion: not reported |
|
| Interventions | 1. Praziquantel 40 mg/kg single dose 2. Praziquantel 20 mg/kg single dose |
|
| Outcomes | Cure Egg count Ultrasound findings (Hydronephrosis, bladder thickening and bladder irregularity) |
|
| Notes | Location: Kenya, Coastal Province, Kwale District Setting: rural, village schools Dates: 1992 to 1993 Endemicity: high Funding: WHO, TDR, Rockefeller Foundation Joint Funding Venture and National Institutes of Health Authors' conclusion: Praziquantel 20 mg and praziquantel 40 mg are equally effective in reducing structural urinary tract morbidity over nine months. A praziquantel dose of 20 mg/kg may be sufficient for practical control of renal and bladder morbidity due to S. haematobium in certain settings: not reported (trial might be underpowered for ultrasound findings). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Infected students were then individually randomised to therapy...by computer random number generation." |
| Allocation concealment (selection bias) | High risk | Allocation was not concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of personnel: "Dosing assignment lists were transmitted to clinical staff responsible for treatment". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessors (clinicians, parasitologists). "Assignments were masked form staff parasitologists and physicians responsible for follow‐up until the end of the study." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up 31% at six weeks. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting. |
| Other bias | Low risk | Important baseline characteristics (egg counts) not reported at baseline. |
McMahon 1979 TZA.
| Methods | RCT Diagnostics: Collection of three midday (10.00 to 13.00) urine samples on three consecutive days, sedimentation in a conical flask for 30 mins, taking of a 10 mL sample of the bottom of the flask, centrifugation and processing of the deposit 5 mL boiled, cooled water added to deposit, miracidia hatching test, fixing and staining of miracidia (alcohol and eosin), microscopy and count. Follow‐up at one, three and six months. |
|
| Participants | School children aged seven to 15 years No. of children randomized: 138 Inclusion: S. haematobium positive Exclusion: not reported |
|
| Interventions | 1. Praziquantel 30 mg/kg single dose 2. Praziquantel 40 mg/kg single dose 3. Praziquantel 2 x 20 mg in one day, dose interval four hours 4. Placebo |
|
| Outcomes | Cure Egg counts Adverse effects |
|
| Notes | Location: Tanzania, Tanga region Setting: school, rural area Endemicity: high, transmission may vary greatly form year to year and season to season. Dates: not reported Funding: MRC/WHO/Tanzania Helminthiasis Research Unit, Tanga Authors' conclusion: Praziquantel in the given doses is not toxic. Praziquantel 40 mg did not affect the therapeutic response in children with large egg loads. As cure rates are influenced by pre‐treatment egg loads, trials of higher doses in patients with high egg loads needed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly sub‐divided into four groups according to previously arranged blocks. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up 10% to 15% at 1, 3 and 6 months. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | Baseline characteristics not reported. |
McMahon 1983 TZA.
| Methods | RCT Diagnostics: collection of two midday (10.00 to 14.00) samples on two consecutive days for initial diagnosis, of three samples for follow‐up), quantitative hatching technique, sedimentation of 10 mL urine Follow‐up at two and four months |
|
| Participants | School children and adults Number of participants randomized: 90 Inclusion: 250 miracidia/10 mL urine Exclusion: not reported |
|
| Interventions | 1. Praziquantel 30 mg/kg single dose 2. Metrifonate 10 mg/kg 1 x daily, dose interval 14 days 3. Niridazole 25 mg/kg 1 x daily for six days, dose interval one day |
|
| Outcomes | Cure rates Egg reduction rates Adverse effects |
|
| Notes | Location: Tanzania, Tanga region Setting: not stated Endemicity: high Dates: not reported Funding: MRC/WHO/Tazania Helminthiasis Research unit, Tanga, Biltricide (Praziqantel) was supplied by Bayer. Authors conclusion: Praziquantel was more effective than metrifonate and niridazole. Side effects were minor. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned; use of different regimens, no use of placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up partly high, not balanced (at four months 0% in the praziquantel group, 26% in the metrifonate and 30% in the niridazole group. |
| Selective reporting (reporting bias) | Unclear risk | No evidence of selective reporting. |
| Other bias | Low risk | Few baseline characteristics reported. |
Mott 1985 GHA.
| Methods | RCT Diagnostics: collection or one urine sample, two random samples out of this urine sample were processed. quantitative urine filtration technique Follow‐up at three and six months |
|
| Participants | Residents "entire population of five settlements", aged six years or older Number of people randomized 266 Inclusion: S. haematobium infected Exclusion: pregnancy, alcoholism, severe debilitating disease |
|
| Interventions | 1. Praziquantel 30 mg/kg single dose 2. Praziquantel 40 mg/kg single dose |
|
| Outcomes | Cure rate Egg count, egg reduction rate (Urinary results not reported by treatment group) |
|
| Notes | Location: Ghana, Lake Volta Setting: rural, five settlements Dates: not reported Endemicity: not reported Funding: Parasitic Diseases Programme WHO/UNDP/Wold bank/ WHO Special Programme for Research and Training in Tropical diseases Authors' conclusions: Similar efficacy of Praziquantel 30 mg and 40 mg in this trial. Praziquantel reduces clinical signs (macrohaematuria) and morbidity in urinary schistosomiasis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up at six months 11.6%. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | Baseline characteristics not reported per group. |
Olds 1999 KEN.
| Methods | RCT Diagnostics: Eggs from 2 x 10 mL samples were filtered on membranes (Nucleopore) Follow at 45 days, 90 days, six months and one year |
|
| Participants | School children aged four to 18 years Number of participants pos for S. haematobium: 380 Inclusion: S. haematobium positive Exclusion: pregnancy or marriage, failure to submit two stool specimens prior to initial therapy, known allergy to praziquantel or albendazole, treatment within the past six months |
|
| Interventions | 1. Praziquantel 40 mg/kg single dose and albendazole 400 mg single dose 2. Praziquantel 40 mg/kg single dose and placebo 3. Albendazole 400 mg single dose and placebo 4. Placebo and placebo |
|
| Outcomes | Cure Egg count Ultrasound Weight, height, skinfold thickness, MUAC Hb Adverse effects |
|
| Notes | Location: Kenya, Kwale District, Coast province for S. haematobium (multi centre trial for different Schistosoma species, conducted in different countries) Setting: rural Endemicity: endemic ascariasis, hookworm, trichuris, S. haematobium Dates: not reported Funding: WHO/TDR Tropical disease research Authors' conclusion: Combined mass treatment of children with albendazole and praziquantel produced not more side effects than treatment with praziquantel alone. Combined mass treatment should have an important impact on schistosoma and hookworm prevalence and intensity and improves Hb levels. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized in one of four treatment groups, block design with block size of 80. |
| Allocation concealment (selection bias) | Low risk | Randomization lists were prepared by WHO/TDR using a randomized block design. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind, placebo controlled; physically identical placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up 10% at six months, loss to follow‐up 17% at one year (for all groups). |
| Selective reporting (reporting bias) | High risk | Hb values, proteinuria, hematuria, ultrasound findings not reported. |
| Other bias | Low risk | No evidence for other bias. |
Omer 1981 SDN.
| Methods | RCT Diagnosis: sedimentation concentration technique, miracidial hatching Follow‐up at seven days, one month, three to four months, six months |
|
| Participants | Patients presenting to the Hospital of Tropical diseases, Karthoum, aged eight to 16 years Number of patients randomized: 152 Inclusion: mixed S. haematobium and S. mansoni infections Exclusion: under eight years of age, advanced stage of disease, severe anaemia, poor general health |
|
| Interventions | 1. Praziquantel 30 mg/kg single dose 2. Praziquantel 40 mg/kg single dose 3. Praziquantel 2 x 20 mg/kg within one day |
|
| Outcomes | Cure rates Egg counts Adverse events Laboratory parameters at day 0 or 1 and at day 1 or 2, not of interest for this review |
|
| Notes | Location: Sudan, Karthoum Setting: Hospital of Tropical Diseases, Karthoum Endemicity: not reported Dates: 1978 to 1979 Funding: not reported Authors' conclusion: Praziquantel is easily applicable, safe and effective in the treatment of mixed (S. haematobium and S. mansoni) infections |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up at six months 17% to 22%, balanced. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No evidence of other bias. |
Oyediran 1981 NGA.
| Methods | RCT Diagnostics: collection of a midday urine sample (12.00 to 2.00), taking a 10 mL sub sample, filtration of the urine, staining with Ninhydrin, counting of the eggs retained on the filter paper Follow‐up at one, three and six months |
|
| Participants | Primary school children aged nine to 16 years Participants randomized: 90 Inclusion criteria: mean egg count 80 eggs/10 mL, viable eggs, aged over six years Exclusion criteria: under six years, concurrent acute or serious illness, antischistosomal treatment within the past six months |
|
| Interventions | Praziquantel 30 mg/kg single dose Praziquantel 40 mg/kg single dose Praziquantel 2 x 20 mg/kg, dose interval three to four hours Placebo |
|
| Outcomes | Egg counts | |
| Notes | Nigeria, Oyo State Setting: Primary Schools Dates: not reported Funding: not reported Authors' conclusion: No significant difference in efficacy between the three dosage regimens, trials on the effects of lower doses required. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Placebo single dose The treatment group received a split dose of praziquantel, blinding not mentioned. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High loss to follow‐up, not balanced (at one month 4 to 17%, at three months 17 to 23%, at six month 26 to38%, at twelve months 76% to 87%). |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No evidence of other bias. |
Pugh 1983 MWI.
| Methods | RCT Diagnostics: collection of two midday urine samples on two consecutive days filtration, staining and egg count Follow‐up at one, three and six months. Further follow‐up reported at nine, 12, 15 and 24 months in a separate publication (Pugh 1983 MWI) |
|
| Participants | School children aged five to 18 years Number of participants randomized: 499 Inclusion: mean egg count (S. haematobium) > 19/10 mL Exclusion: malaise, febrile illness, treatment with schistosomacidal drugs in the past six months |
|
| Interventions | 1. Praziquantel 40 mg/kg single dose 2. Niridazole 25 mg/kg single dose and metrifonate 10 mg/kg single dose 3. Metrifonate 10 mg/kg single dose 4. Niridazole 25 mg/kg single dose 5. Placebo |
|
| Outcomes | Cure Geometric mean egg counts Egg reduction rates |
|
| Notes | Location: Malawi, Pirimiti Area, Phalombe plain Setting: rural Endemicity: seasonal Funding: Overseas Development Administration, U.K. MoH Malawi. Praziquatel supplied by Bayer Authors' conclusion: Praziquantel is superior to the other drugs studied in this trial, it is the most efficient and convenient drug available. Maintained low egg output at 24 months was presumably influenced by low levels of transmission during the second year of the trial, which was very dry. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of a randomized x‐y list. |
| Allocation concealment (selection bias) | Low risk | "An independent worker had sole and confidential access to a randomised x‐y list." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as double blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up low at one months: 0% to 4.1%, at three months 8% to 11% in treatment groups, up to 23% in the placebo group; at six months 20% in the treatment group. Loss to follow‐up high at 24 months, about 40% to 70 %. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | High risk | Baseline imbalance in terms of intensity of infection. "In accordance to with local ethical guidelines the placebo group consisted only of children with light (20‐124 ova/10mL or moderate (125 to 4999 ova/10 mL) infections before treatment. Important baseline characteristics not reported (age, weight). |
Rey 1983 NER.
| Methods | RCT Diagnostics: collection of two urine samples, filtration (Swinex 13 Filter Millipore, 13 mm diameter), fixation and staining (Lugol), egg counts Length of follow‐up: one, three and six months |
|
| Participants | Participants: recruits aged 18 to 20 years and college students aged 15 to 19 years Number of participants randomized: 207 (co‐infection with S. mansoni likely, but not investigated) Inclusion: S. haematobium positive Exclusion: not reported |
|
| Interventions | 1. Praziquantel 30 mg/kg daily dose 2. Praziquantel 40 mg/kg daily dose 3. Oltipraz 17.5 mg/kg 2 x daily in one day |
|
| Outcomes | Failure Egg reduction rates |
|
| Notes | Location: Niger Setting: not reported Endemicity: not reported Dates: not reported Funding: not reported Authors' conclusion: No significant difference found between praziquantel 30 mg/kg and praziquantel 40 mg/kg. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized, tirage au sort. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned, no use of placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up acceptable at one month (9% to 15%) and three months 9% to 11%, high at six months (39% to 47%). |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | Baseline characteristics not reported. |
Rey 1984 NER.
| Methods | RCT Diagnostics: urine filtration, normal filtration paper, egg counts (no further details given) Follow‐up for children (aged five to 15 years) at 1, 5 and 6 months, for adults (> 15 years) at six months only |
|
| Participants | Children older than five years and adults Participants treated and controlled: 268 randomized, 143 participants at one month, randomized Inclusion: not reported Exclusion: not reported |
|
| Interventions | 1. Metrifonate 10 mg/kg single dose 2. Metrifonate 10 mg/kg two doses with a dose interval of two weeks 3. Metrifonate 10 mg/kg three doses with a dose interval of two weeks |
|
| Outcomes | Cure rate Egg reduction |
|
| Notes | Location: Niger, near Niamey Setting: not reported Endemicity: high, the trial was conducted in the season of low transmission Dates: not reported Funding: not reported Authors' conclusions: Recommendation against the combined metrifonate niridazole treatment. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "au hasard ", random number table. |
| Allocation concealment (selection bias) | Unclear risk | No comment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No comment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No comment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up high: at one month 50%, at four months 39%. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No evidence of other bias, funding not stated. |
Sacko 2009 MLI.
| Methods | RCT Diagnostics: Collection of three urine samples between 10 am and 2 PM on three consecutive days. 10 mL of urine passed through a nucleopore filter, Swinnex filter support. Egg counts. Follow‐up at 3, 6 and 18 months |
|
| Participants | School children aged seven to 14 years Number of participants randomized: 603 Inclusion: not reported Exclusion: not reported |
|
| Interventions | Praziquantel 40 mg/kg single dose Praziquantel 40 mg/kg two doses, interval two weeks |
|
| Outcomes | Cure rate Egg reduction Haematuria |
|
| Notes | Location: Mali, Niger River Basin Setting: rural, primary schools Endemicity: not reported Dates: not reported Funding: not reported Authors' conclusion: Significantly reduced prevalence of microhematuria with praziquantel x 2, this could indicate reduction of morbidity |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized (SPSS generated random number tables). |
| Allocation concealment (selection bias) | Low risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind, placebo‐controlled. Placebo tablets were of the same form and colour as praziquantel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up unclear, as number randomized were not reported, only the numbers at first follow‐up at three months. |
| Selective reporting (reporting bias) | Low risk | Follow‐up data at six and 18 months reported in graphs, not in numbers. |
| Other bias | Low risk | No evidence for other bias. |
Stephenson 1985 KEN.
| Methods | RCT Diagnostics: nucleopore filter method of Peters and others collection of a midday urine sample (complete bladder content, 11.00 to 12.00) after 200 mL of fruit drink, nucleopore filter method of Peters and others, staining with 0.5 trypan blue, egg counts in 10 mL of urine adjusted for the total volume of each urine specimen Follow‐up for six months |
|
| Participants | Primary school children aged six to 16 years Number of participants randomized: 400 Inclusion: light to moderate S. haematobium infections at exam 1 Exclusion: not reported |
|
| Interventions | 1. Metrifonate 7.5 mg/kg three doses, dose interval one to two weeks 2. Placebo: gelatin capsules |
|
| Outcomes | Parasitological failure and cure Egg counts Egg reduction rate Haemoglobin Anthropometric measures weight, height, weight for height, middle upper arm circumference, triceps and subscapular skinfold thickness Liver size Spleen size |
|
| Notes | Location: Kenya, Kwale District, Coast Province Setting: rural, four primary schools Endemicity: highly endemic Dates: not reported Funding: not reported Authors' conclusion: S. haematobium infections can precipitate or aggravate anaemia in vulnerable children (poor iron intake, high endemicity of other parasites). S. haematobium treatment improves Hb levels. S. haematobium treatment may improve child growth (in populations were hookworm infections and Protein Energy Malnutrition is common). S. haematobium treatment may be associated with regression of splenomegaly and hepatomegaly in children treated for S. haematobium infection. Population‐based treatment is recommended. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Allocated at random. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Use of placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Examinations 1 and 2 were carried out in a blind fashion with the same team of workers. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up unclear, as results were reported as proportions. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting. |
| Other bias | Low risk | No evidence of other sources of bias. |
Stephenson 1989 KEN.
| Methods | RCT Diagnostics: collection of a midday urine sample (complete bladder content, 11.00 to 12.00) after 200 mL of fruit drink, nucleotome filter method of Peters and others, staining with 0.5 trypan blue, egg counts in 10 mL of urine adjusted for the total volume of each urine specimen Follow‐up at eight months (as only time point) Latham 1990, a sub‐study nested within Stephenson 1989 KEN, followed up patients at five weeks (as only time point) |
|
| Participants | Primary school children, 98% Muslim of the Wadigo tribe, aged eight to 13 years Number of participants randomized: not reported Number of participants analysed: 312 Inclusion: light to moderate infections Exclusion: anaemia (Hb < 8 G/dL, severe infections) Latham 1990 included 48 boys aged seven to 15 years with no sign of puberty, high egg counts, Hb > 8 G/dL, cooperation for physical fitness test |
|
| Interventions | 1. Praziquantel 40 mg/kg single dose 2. Metrifonate 10 mg/kg single dose 3. Placebo As a nested study, Latham had the same study arms. |
|
| Outcomes | Parasitological failure Egg counts (geometric and arithmetic) Anthropometric measurements: weight, height, MUAC, triceps skinfold thickness, subscapular skinfold thickness, Haemoglobin Liver size Spleen size Latham 1990 (reference see Stephenson 1989 KEN) reports parasitological failure, egg reduction rate and anthropometric measures: weight, height, skinfold thickness, MUAC at five weeks at five weeks, and additionally reports on Physical fitness: Harvard Step test, Appetite (quantity of porridge consumed) Questionnaire of clinical symptoms |
|
| Notes | Location: Kenya, Kwale district, Coast Province Setting: rural, primary schools Endemicity: endemic for S. haematobium, hookworm and malaria Dates: March 1986 to April 1986 Funding: Edna McConnell Clark Foundation, grant 284‐0120 Authors' conclusion: Both metrifonate and praziquantel are effective in reducing egg excretion and are both recommended for population based treatment. Praziquantel is more effective. S. haematobium treatment with a single dose of either metrifonate or praziquantel may improve child growth in areas were hookworms and malnutrition are common and appears to have a beneficial effect on hepatomegaly and splenomegaly. Treatment of moderate to heavy S. haematobium infections with metrifonate or praziquantel in undernourished schoolboys can improve physical fitness, growth rates and appetite within approximately one month. Recommendation for widespread population based chemotherapy in highly endemic areas as Kwale district. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Allocated at random. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Use of placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Examinations carried out in a blind fashion. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up 10%, 3 participants not accounted for. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No evidence for other source of bias. |
Taylor 1988 ZWE.
| Methods | RCT Diagnostics: urine sample collection; three midday urine samples (10.00 to 14.00), filtration (13 mm nytrl filter), staining with Lugol Follow‐up at 1, 3 and 6 months |
|
| Participants | School children aged ten to 15 years, mixed infection with S. haematobium and S. mansoni Number of participants randomized: 373 Inclusion: mixed S. haematobium and S. mansoni infection Exclusion: not reported |
|
| Interventions | 1. Praziquantel 10 mg/kg single dose 2. Praziquantel 20 mg/kg single dose 3. Praziquantel 30 mg/kg single dose 4. Praziquantel 40 mg/kg single dose 4. Control: Nil |
|
| Outcomes | Parasitological cure Egg count |
|
| Notes | Location: Zimbabwe Setting rural, primary school Endemicity: seasonal transmission Date: not reported Funding: Rockefeller Foundation (financial support) Authors' conclusion: Doses of 20 to 40 mg praziquantel may be equally effective in S. haematobium infection |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Single blind manner "only the principal investigator knew which children had been assigned to which treatment group." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up unclear, as only means and percentages of cure are reported. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No evidence for other source of bias. |
Tchuente 2004 CMR.
| Methods | RCT Diagnostics: collection of two urine samples on two consecutive days in 50 mL plastic screw cap vials, processing in field laboratory, agitation of urine (from dispersal of eggs) filtration of 10 mL (Nucleopore filter), egg counts Length of follow‐up 3, 6 and 9 weeks |
|
| Participants | School children, age not reported Number of participants randomized: 592 Inclusion: S. haematobium positive Exclusion: not reported |
|
| Interventions | 1. Praziquantel 40 mg/kg single dose 2. Praziquantel 40 mg/kg two single doses, dose interval three weeks 3. Praziquantel 40 mg/kg three single doses, dose interval three weeks |
|
| Outcomes | Cure rates Egg counts, egg reduction rates Proteinuria |
|
| Notes | Location: Cameroon, Loum Setting: urban, schools Date: April to June 2002 Endemicity: endemic all year, prevalence amongst school children 41.8%, trial carried out during high transmission period Funding: European Commission INCO‐DC (ICA‐4‐CT‐2001‐10079) Authors' conclusion: No significant differences between the three dosing regimens, persistent high cure rates with a single dose of Praziquantel. Findings suggest efficacy of praziquantel against immature schistosoma stages. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Assigned to random groups. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No use of placebo mentioned. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High loss to follow‐up of 13% at six weeks, very high loss to follow‐up of 58.6% at nine weeks (change in schools schedules). |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No evidence of other bias. |
van den Biggelaar 02 GAB.
| Methods | RCT Diagnostics: collection of urine samples on three different days, filtration of 10 mL urine, nucleopore pore size 13 µm), staining with ninhydrin, eggs count Follow‐up at two and three years, length of follow‐up three years |
|
| Participants | School children aged five to 14 years Participants randomized: 135 Inclusion: positive for S. haematobium eggs Exclusion: not reported |
|
| Interventions | Praziquantel 40 mg/kg single dose Praziquantel 40 mg/kg in repeated doses, dose interval three months, over two years |
|
| Outcomes | Cure rates, failure rates Egg counts Microhaematuria |
|
| Notes | Location: Gaboon, near Lambarene Setting: rural, village schools Endemicity: high Funding: not reported Dates: not reported Authors' conclusion: relate to immunologic outcomes also measured by this trial, but not of interest for this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Allocated randomly. |
| Allocation concealment (selection bias) | High risk | "The allocation of children to the treatment group was open." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Use of placebo (given every three months) not mentioned. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High loss to follow‐up (not balanced, reasons not given): at 24 months 8%, 23%, 44% in different treatment groups; at 36 months 40%, 64%, 77%. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No evidence of other bias. |
Wilkins 1987 GMB.
| Methods | RCT Diagnostics: Follow‐up at two to three months |
|
| Participants | Residents aged two to 19 years, median age 9.5 years Participants randomized: not reported |
|
| Interventions | 1. Praziquantel 10 mg/kg 2. Praziquantel 20 mg/kg 3. Praziquantel 40 mg/kg 4. Metrifonate 10 mg/kg 5. Praziquantel 10 mg/kg and metrifonate 10 mg/kg |
|
| Outcomes | Egg counts Side effects |
|
| Notes | Location: Gambia Upper River Division, Nyanamari Setting: rural Endemicity: seasonal, trial conducted during season of low transmission Dates: not reported Funding: not reported Authors' conclusion: Mass treatment of intensely infected groups should be based on the standard dose of praziquantel, with metrifonate as second choice. Note: only one of the two trials reported in this publication, the Nyanamari trial, fulfilled the inclusion criteria. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects...were stratified into four age groups and within each age stratum were ordered by intensity of egg counts. They were then placed sequentially into groups of five. Computer generated random sets of the numbers one to five were used to allocated on subject in each group of five to each of the five regimens used." |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Placebo and blinding not mentioned. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up unclear, as cure rates are reported as percentages. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | Baseline characteristics not reported. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aryeetey 1999 | Study of health education and community participation. |
| Ayoya 2007 | No comparison group (treatment groups receive praziquantel with or without iron supplements and multivitamins). |
| Bausch 1995 | Not a RCT. |
| Beasley 1999 | This study compares a combination of praziquantel and albendazole with placebo. This outcome is not of interest for this review. |
| Bejon 2008 | Study of gastrointestinal helminths, not urinary schistosomiasis. |
| Bhargava 2003 | This study does not report baseline criteria for control group, as the control group was not screened at baseline. |
| Boulanger 2007 | No comparison group (both groups receive artesunate). |
| Burchard 1984 | This study compares praziquantel 2 x 30 mg/kg to oltipraz, which is obsolete. Details of this trial can be seen in earlier versions of this review. |
| Clarke 1969 | Not a RCT. |
| Clarke 1973 | Not a RCT, allotted to groups, "for practical reasons, the infected children in the two senior grades were set aside for treatment with i.m. hycanthone". |
| Creasey 1986 | This study compares different doses of praziquantel (8 mg/kg, 15 mg/kg and 20 mg/kg) combined with oxaminique in patients with S. haematobium and S. mansoni co‐infections. A comparison of the praziquantel dosages used is not of interest for this review. |
| Danso‐Appiah 2009 | Systematic review. |
| Davis 1966 | This study evaluates different doses of ambilhar which is now obsolete. Details of this trial can be seen in earlier versions of this review. |
| Davis 1979 | Outcomes are not reported per treatment group, only for the total number of participants randomized. |
| De Clercq 2002 | Not a RCT, "systematically allocated". |
| Druilhe 1981 | Not a RCT. |
| el Hawey 1990 | No comparison group. |
| el Tayeb 1988 | This study compares praziquantel 2 x 20 mg/kg to oltipraz 2 x 15 mg/kg, which is now obsolete. Details of this trial can be seen in earlier versions of this review. |
| el‐Zayadi 1985 | No outcome of interest reported. |
| Erikstrup 2008 | This is a study of HIV and S. haematobium or S. mansoni co‐infection, no outcomes of interest for this review are reported. |
| Fontanilles 1964 | Conference speech. |
| Forsyth 1964 | Not a RCT. "At three of the schools, every sixth injected child received "curative" treatment..." |
| Garba 2001 | Study of health education. |
| Garba 2004 | This study evaluates mass treatment with praziquantel without comparison group. |
| Hammad 1997 | This cross‐sectional study evaluates the diagnosis of urinary schistosomiasis by reagent strip and parasitological methods. |
| Jewsbury 1977 | No comparison group (sequence of treatment, then prophylaxis within one group). |
| Jinabhai 2001 | This study compares a combination of praziquantel and albendazole with placebo. This outcome is not of interest for this review. |
| Jordan 1966 | Quasi‐RCT. "children were allocated to Groups 1‐4 corresponding to different regimens of treatment, in rotation down the list (pre‐treatment results in descending order), thus ensuring four groups matched for egg output." |
| Kardaman 1983 | No comparison group. |
| Kern 1984 | Study of intestinal manifestations of schistosomiasis, very low number for S. haematobium positive patients, outcome data not reported separately. |
| King 1989 | Review article. |
| King 1992 | Data reported in other publications. |
| Kurz 1986 | This study evaluates metrifonate in hookworm infections. |
| Latham 1983 | No comparison group. |
| Lucas 1969 | This study reports ultrasound findings in patients with urinary schistosomiasis after treatment with Niridazole to a untreated control. Niridazole is now obsolete. |
| Mwanakasale 2009 | Study of iron supplementation in S. haematobium treatment with no outcomes of interest for this review. |
| N'Goran 2003 | Study of S. haematobium prevention. |
| Nagaty 1962 | This trial studies the therapy of drug side effects in urinary schistosomiasis treatment. |
| Odongo‐Aginya 1996 | Not a RCT, study of S. mansoni. |
| Olsen 2007 | Review article. |
| Pitchford 1978 | No comparison group. |
| Podgore 1994 | Study of S. haematobium prevention. |
| Rabarijaona 2001 | Epidemiological survey. |
| Rey 1984 | This study compares oltipraz 30 mg/kg to a combination of metrifonate 10 mg/kg and niridazole 25 mg/kg. Niridazole and oltipraz are now obsolete. |
| Rugemalila 1984 | Study of S. mansoni. |
| Schutte 1983 | No comparison group. |
| Sellin 1986 | This study compares metrifonate 10 mg/kg to oltipraz 30 mg/kg, which is now obsolete. |
| Sissoko 2009 MLI | This study compared praziquantel to a combination of artesunate with sulfamethoxypyrazine pyrimethamine; it is therefore not possible to attribute observed effects to artesunate alone. |
| Snyman 1997 | Study of calcitriol as experimental antischistosomal treatment. |
| Snyman 1998 | Study of levimasole as experimental antischistosomal treatment. |
| Squires 2000 | Review article. |
| Stephenson 1985 | No comparison group (compares children of moderate and severe infection intensity with uninfected children, using the same treatment regimen for infected children). |
| Taylor 2001 | This study compares a combination of praziquantel and albendazole with placebo. This outcome is not of interest for this review, whereas a comparison the combination of praziquantel and albendazole versus praziquantel would be of interest. |
| Teesdale 1980 | Not a RCT. |
| Thigpen 2011 | Not a RCT. |
| Urbani 1997 | Epidemiological survey. |
| Utzinger 2001 | Review article. |
| van Lieshout 1994 | Study of S. mansoni. |
| Wilkins 1987 Simoto trial | Not a RCT, alternate allocation. |
| Wolfe 1967 | Not a RCT. |
| Xiao 2002 | Review article. |
| Zwingenberger 1990 | Case study. |
Differences between protocol and review
While inclusion criteria of the first protocol included all RCTs which studied antischistosomal drugs, we decided to change the protocol. We excluded trials which evaluated obsolete drugs as ambilhar, oltipraz and niridazole. We also excluded studies which compared a combination of praziquantel and albendazole to placebo only, as this comparison is not of interest for this review. We included trials evaluating metronidazole.
We did not contact researchers or organizations looking for unpublished studies, as stated in the protocol. We did not report parasitological outcomes at three months as primary outcomes.
The older version of this review concluded that both metrifonate and praziquantel were effective in treating urinary schistosomiasis, even if metrifonate had operational disadvantages. As implications for further research, evaluation of different metrifonate doses and regimens and of evaluation of artemisinin drugs and of combination therapy is recommended.
While we agree with these conclusions, the data on egg reduction allow some further recommendations. We have newly included three trials evaluating artemisinin drugs, and one recent trial using mefloquine, and present this new evidence here.
Additional analysis carried out in this edition of the review, which was not in the previous edition (Danso‐Appiah 2008), is the presentation of egg reduction rates in summary tables.
Contributions of authors
VK developed the protocol with input from PG and DS. VK and FZ assessed eligibility and extracted the data. We resolved any disagreements through discussion with DS and PG. VK entered the data and drafted the manuscript with input from DS, PG and PO. DS, PG and PO assisted in interpretation of the results and revisions of the text.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK.
External sources
This review was supported by the Department for I nternational Development , UK.
Declarations of interest
We have no known conflicts of interest.
Unchanged
References
References to studies included in this review
Abden Abdi 1989 SOM {published data only}
- Aden‐Abdi Y, Gustafsson LL, Elmi SA. A simplified dosage schedule of metrifonate in the treatment of Schistosoma haematobium infection in Somalia. European Journal of Clinical Pharmacology 1987;32(5):437‐41. [DOI] [PubMed] [Google Scholar]
Al Aska 1990 SAU {published data only}
- Al‐Aska AK, Al‐Mofleh IA, Al‐Rashed R, Hafez MA, Al‐Nozha M, Abu‐Aisha H, et al. Praziquantel, oxamniquine, and metrifonate in the treatment of schistosomiasis in Riyadh. Annals of Saudi Medicine 1990;10(3):296‐8. [Google Scholar]
Basra 2012 GAB {published data only}
- Basra A, Mombo‐Ngoma G, Melser MC, Diop DA, Würbel H, Mackanga JR, et al. Efficacy of mefloquine intermittent preventive treatment in pregnancy against Schistosoma haematobium infection in Gabon: a nested randomized controlled assessor‐blinded clinical trial. Clinical Infectious Diseases 2012;56(6):e68‐75. [DOI] [PubMed] [Google Scholar]
Befidi Mengue 1992 CMR {published data only}
- Befidi‐Mengue R N, Ratard R C, D'Alessandro A, Rice J, Befidi‐Mengue R, Kouemeni L E, et al. The impact of Schistosoma haematobium infection and of praziquantel treatment on the growth of primary school children in Bertoua, Cameroon. Journal of Tropical Medicine and Hygiene 1992;95(6):404‐9. [PubMed] [Google Scholar]
- Befidi‐Mengue RN, Ratard RC, Beltran G, D'Alessandro A, Rice J, Befidi‐Mengue R, et al. Impact of Schistosoma haematobium infection and of praziquantel treatment on anaemia of primary school children in Bertoua, Cameroon. Journal of Tropical Medicine and Hygiene 1993;96(4):225‐30. [PubMed] [Google Scholar]
Borrmann 2001 GAB {published data only}
- Borrmann S, Szlezak N, Faucher JF, Matsiegui PB, Neubauer R, Binder RK, et al. Artesunate and praziquantel for the treatment of Schistosoma haematobium infections: a double‐blind, randomized, placebo‐controlled study. Journal of Infectious Diseases 2001;184(10):1363‐6. [DOI] [PubMed] [Google Scholar]
Davis 1981 ZMB {published data only}
- Davis A, Biles JE, Ulrich AM, Dixon H. Tolerance and efficacy of praziquantel in phase IIA and IIB therapeutic trials in Zambian patients. Arzneimittel‐Forschung 1981;31(3a):568‐74. [PubMed] [Google Scholar]
de Jonge 1990 SDN {published data only}
- Doehring E, Ehrich JH, Vester U, Feldmeier H, Poggensee U, Brodehl J. Proteinuria, hematuria, and leukocyturia in children with mixed urinary and intestinal schistosomiasis. Kidney International 1985;28(3):520‐5. [DOI] [PubMed] [Google Scholar]
- Doehring E, Poggensee U, Feldmeier H. The effect of metrifonate in mixed Schistosoma haematobium and Schistosoma mansoni infections in humans. American Journal of Tropical Medicine and Hygiene 1986;35(2):323‐9. [DOI] [PubMed] [Google Scholar]
- Jonge N, Schommer G, Feldmeier H, Krijger FW, Dafalla AA, Bienzle U, et al. Mixed Schistosoma haematobium and S. mansoni infection: effect of different treatments on the serum level of circulating anodic antigen (CAA). Acta Tropica 1990;48(1):25‐35. [DOI] [PubMed] [Google Scholar]
Inyang Etoh 2009 NGA {published data only}
- Inyang‐Etoh PC, Ejezie GC, Useh MF, Inyang‐Etoh EC. Efficacy of a combination of praziquantel and artesunate in the treatment of urinary schistosomiasis in Nigeria. Transactions of the Royal Society of Tropical Medicine and Hygiene 2009;103(1):38‐44. [DOI] [PubMed] [Google Scholar]
Jewsbury 1976 ZWE {published data only}
- Jewsbury JM, Cooke MJ. Prophylaxis of schistosomiasis ‐ field trial of metrifonate for the prevention of human infection. Annals of Tropical Medicine and Parasitology 1976;70(3):361‐3. [DOI] [PubMed] [Google Scholar]
Kardaman 1985 SDN {published data only}
- Kardaman MW, Fenwick A, Igail AB, Tayeb M, Daffalla AA, Dixon HG. Treatment with praziquantel of schoolchildren with concurrent Schistosoma mansoni and S. haematobium infections in Gezira, Sudan. Journal of Tropical Medicine and Hygiene 1985;88(2):105‐9. [PubMed] [Google Scholar]
Keiser 2010 CIV {published data only}
- Keiser J, N'Guessan NA, Adoubryn KD, Silué KD, Vounatsou P, Hatz C, et al. Efficacy and safety of mefloquine, artesunate, mefloquine‐artesunate, and praziquantel against Schistosoma haematobium: randomized, exploratory open‐label trial. Clinical Infectious Diseases 2010;50(9):1205‐13. [DOI] [PubMed] [Google Scholar]
King 1989 KEN {published data only}
- King CH, Wiper DW 3rd, Stigter KV, Peters PA, Koech D, Ouma JH, et al. Dose‐finding study for praziquantel therapy of Schistosoma haematobium in Coast Province, Kenya. American Journal of Tropical Medicine and Hygiene 1989;40(5):507‐13. [DOI] [PubMed] [Google Scholar]
King 1990 KEN {published data only}
- King CH, Lombardi G, Lombardi C, Greenblatt R, Hodder S, Kinyanjui H, et al. Chemotherapy based control of schistosomiasis haematobia. I. Metrifonate versus praziquantel in control of intensity and prevalence of infection. American Journal of Tropical Medicine and Hygiene 1988;39(3):295‐305. [DOI] [PubMed] [Google Scholar]
- King CH, Lombardi G, Lombardi C, Greenblatt R, Hodder S, Kinyanjui H, et al. Chemotherapy based control of schistosomiasis haematobia. II. Metrifonate vs. praziquantel in control of infection associated morbidity. American Journal of Tropical Medicine and Hygiene 1990;42(6):587‐95. [DOI] [PubMed] [Google Scholar]
- King CH, Muchiri E, Ouma JH, Koech D. Chemotherapy based control of schistosomiasis haematobia. IV. Impact of repeated annual chemotherapy on prevalence and intensity of Schistosoma haematobium infection in an endemic area of Kenya. American Journal of Tropical Medicine and Hygiene 1991;45(4):498‐508. [DOI] [PubMed] [Google Scholar]
King 2002 KEN {published data only}
- King CH, Muchiri EM, Mungai P, Ouma JH, Kadzo H, Magak P, et al. Randomized comparison of low‐dose versus standard‐dose praziquantel therapy in treatment of urinary tract morbidity due to Schistosoma haematobium infection. American Journal of Tropical Medicine and Hygiene 2002;66(6):725‐30. [DOI] [PubMed] [Google Scholar]
McMahon 1979 TZA {published data only}
- McMahon JE, Kolstrup N. Praziquantel: a new schistosomicide against Schistosoma haematobium. British Medical Journal 1979;2(6202):1396‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
McMahon 1983 TZA {published data only}
- McMahon JE. A comparative trial of praziquantel, metrifonate and niridazole against Schistosoma haematobium. Annals of Tropical Medicine and Parasitology 1983;77(2):139‐42. [DOI] [PubMed] [Google Scholar]
Mott 1985 GHA {published data only}
- Mott KE, Dixon H, Osei‐Tutu E, England EC, Davis A. Effect of praziquantel on hematuria and proteinuria in urinary schistosomiasis. American Journal of Tropical Medicine and Hygiene 1985;34(6):1119‐26. [DOI] [PubMed] [Google Scholar]
Olds 1999 KEN {published data only}
- Olds GR, King C, Hewlett J, Olveda R, Wu G, Ouma J, et al. Double‐blind placebo‐controlled study of concurrent administration of albendazole and praziquantel in schoolchildren with schistosomiasis and geohelminths. Journal of Infectious Diseases 1999;179(4):996‐1003. [DOI] [PubMed] [Google Scholar]
Omer 1981 SDN {published data only}
- Omer AH. Praziquantel in the treatment of mixed S. haematobium and S. mansoni infections. Arzeneimittel‐Forschung 1981;31(3a):605‐8. [PubMed] [Google Scholar]
Oyediran 1981 NGA {published data only}
- Oyediran AB, Kofie BA, Bammeke AO, Bamgboye EA. Clinical experience with praziquantel in the treatment of Nigerian patients infected with S. haematobium. Arzneimittel‐Forschung 1981;31(3a):581‐4. [PubMed] [Google Scholar]
Pugh 1983 MWI {published data only}
- Pugh RN, Teesdale CH. Long‐term efficacy of single‐dose oral treatment in schistosomiasis haematobium. Transactions of the Royal Society of Tropical Medicine and Hygiene 1984;78(1):55‐9. [DOI] [PubMed] [Google Scholar]
- Pugh RN, Teesdale CH. Single dose oral treatment in urinary schistosomiasis: a double blind trial. British Medical Journal 1983;286(6363):429‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rey 1983 NER {published data only}
- Rey JL, Sellin B, Gazere O, Ott D, Reges M, Garrouty P. Comparison study of praziquantel efficiency (30 mg/kg and 40 mg/kg) in a single dose and oltipraz (35 mg/kg) in two doses on Schistosoma haematobium in Niger [Comparaison au Niger de l' efficacite sur Schistosoma haematobium du Praziquantel (30 mg/kg et 40 mg/kg) en une prise et de l' Oltipraz (35 mg/kg) en deux prises]. Medicine et Maladies Infectieuses 1983;13(6):328‐31. [Google Scholar]
Rey 1984 NER {published data only}
- Rey JL, Nouhou H, Sellin B. Comparison of three metrifonate dosages in mass chemotherapy of Schistosoma haematobium [Comparaison de trois posologies de Metrifonate en chimiotherapie de masse contre Schistosoma haematobium]. Médicine Tropicale 1984;44(1):57‐60. [PubMed] [Google Scholar]
Sacko 2009 MLI {published data only}
- Sacko M, Magnussen P, Traoré M, Landouré A, Doucouré A, Reimert CM, et al. The effect of single dose versus two doses of praziquantel on Schistosoma haematobium infection and pathology among school‐aged children in Mali. Parasitology 2009;136(13):1851‐7. [DOI] [PubMed] [Google Scholar]
Stephenson 1985 KEN {published data only}
- Stephenson LS, Latham MC, Kinoti SN, Oduori ML. Regression of splenomegaly and hepatomegaly in children treated for Schistosoma haematobium infection. American Journal of Tropical Medicine and Hygiene 1985;34(1):119‐23. [DOI] [PubMed] [Google Scholar]
- Stephenson LS, Latham MC, Kurz KM, Kinoti SN, Oduori ML, Crompton DW. Relationships of Schistosoma haematobium, hookworm and malarial infections and metrifonate treatment to growth of Kenyan school children. American Journal of Tropical Medicine and Hygiene 1985;34(6):1109‐18. [DOI] [PubMed] [Google Scholar]
- Stephenson LS, Latham MC, Kurz KM, Kinoti SN, Oduori ML, Crompton DW. Relationships of Schistosoma haematobium, hookworm and malarial infections and metrifonate treatment to hemoglobin level in Kenyan school children. American Journal of Tropical Medicine and Hygiene 1985;34(3):519‐28. [DOI] [PubMed] [Google Scholar]
Stephenson 1989 KEN {published data only}
- Latham MC, Stephenson LS, Kurz KM, Kinoti SN. Metrifonate or praziquantel treatment improves physical fitness and appetite of Kenyan schoolboys with Schistosoma haematobium and hookworm infections. American Journal of Tropical Medicine and Hygiene 1990;43(2):170‐9. [DOI] [PubMed] [Google Scholar]
- Stephenson LS, Kinoti SN, Latham MC, Kurz KM, Kyobe J. Single dose metrifonate or praziquantel treatment in Kenyan children. I. Effects on Schistosoma haematobium, hookworm, hemoglobin levels, splenomegaly, and hepatomegaly. American Journal of Tropical Medicine and Hygiene 1989;41(4):436‐44. [DOI] [PubMed] [Google Scholar]
- Stephenson LS, Latham MC, Kurz KM, Kinoti SN. Single dose metrifonate or praziquantel treatment in Kenyan children. II. Effects on growth in relation to Schistosoma haematobium and hookworm egg counts. American Journal of Tropical Medicine and Hygiene 1989;41(4):445‐53. [DOI] [PubMed] [Google Scholar]
Taylor 1988 ZWE {published data only}
- Taylor P, Murare HM, Manomano K. Efficacy of low doses of praziquantel for Schistosoma mansoni and S. haematobium. Journal of Tropical Medicine and Hygiene 1988;91(1):13‐7. [PubMed] [Google Scholar]
Tchuente 2004 CMR {published data only}
- Tchuenté LA, Shaw DJ, Polla L, Cioli D, Vercruysse J. Efficacy of praziquantel against Schistosoma haematobium infection in children. American Journal of Tropical Medicine and Hygiene 2004;71(6):778‐82. [PubMed] [Google Scholar]
van den Biggelaar 02 GAB {published data only}
- Biggelaar AH, Borrmann S, Kremsner P, Yazdanbakhsh M. Immune responses induced by repeated treatment do not result in protective immunity to Schistosoma haematobium: interleukin (IL)‐5 and IL‐10 responses. Journal of Infectious Diseases 2002;186(10):1474‐82. [DOI] [PubMed] [Google Scholar]
Wilkins 1987 GMB {published data only}
- Wilkins HA, Moore PJ. Comparative trials of regimens for the treatment of urinary schistosomiasis in The Gambia. Journal of Tropical Medicine and Hygiene 1987;90(2):83‐92. [PubMed] [Google Scholar]
References to studies excluded from this review
Aryeetey 1999 {published data only}
- Aryeetey ME, Aholu C, Wagatsuma Y, Bentil G, Nkrumah FK, Kojima S. Health education and community participation in the control of urinary schistosomiasis in Ghana. East African Medical Journal 1999;76(6):324‐9. [PubMed] [Google Scholar]
Ayoya 2007 {published data only}
- Ayoya MA. Effect of iron and/or multiple micronutrient supplementation added to praziquantel treatment of anemic Malian school children infected with schistosoma haematobium (Mali). Dissertation Abstracts International: Section B: The Sciences and Engineering 2007, issue 3‐b.
Bausch 1995 {published data only}
- Bausch D, Cline BL. The impact of control measures on urinary schistosomiasis in primary school children in northern Cameroon: a unique opportunity for controlled observations. American Journal of Tropical Medicine and Hygiene 1995;53(6):577‐80. [DOI] [PubMed] [Google Scholar]
Beasley 1999 {published data only}
- Beasley NM, Tomkins AM, Hall A, Kihamia CM, Lorri W, Nduma B, et al. The impact of population level deworming on the haemoglobin levels of schoolchildren in Tanga, Tanzania. Tropical Medicine and International Health 1999;4(11):744‐50. [DOI] [PubMed] [Google Scholar]
Bejon 2008 {published data only}
- Bejon P, Mwangi TW, Lowe B, Peshu N, Hill AV, Marsh K. Helminth infection and eosinophilia and the risk of Plasmodium falciparum malaria in 1‐ to 6‐year‐old children in a malaria endemic area. PLoS Neglected Tropical Diseases 2008;2(1):e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhargava 2003 {published data only}
- Bhargava A, Jukes M, Lambo J, Kihamia CM, Lorri W, Nokes C, et al. Anthelmintic treatment improves the hemoglobin and serum ferritin concentrations of Tanzanian schoolchildren. Food and Nutrition Bulletin 2003;24(4):332‐42. [DOI] [PubMed] [Google Scholar]
Boulanger 2007 {published data only}
- Boulanger D, Dieng Y, Cisse B, Remoue F, Capuano F, Dieme JL, et al. Antischistosomal efficacy of artesunate combination therapies administered as curative treatments for malaria attacks. Transactions of the Royal Society of Tropical Medicine and Hygiene 2007;101(2):113‐6. [DOI] [PubMed] [Google Scholar]
Burchard 1984 {published data only}
- Burchard GD, Kern P, Baltes R, Dietrich M. Comparative trial of oltipraz versus praziquantel in the treatment of urinary schistosomiasis in the Gabon. Tropenmedizin und Parasitologie 1984;35(2):91‐4. [PubMed] [Google Scholar]
Clarke 1969 {published data only}
- Clarke Vde V, Blair DM, Weber MC. Field trial of hycanthone (Etrenol Winthrop) in the treatment of urinary and intestinal bilharziasis. Central African Journal of Medicine 1969;15(1):1‐6. [PubMed] [Google Scholar]
Clarke 1973 {published data only}
- Clarke Vde V, Weber MC, Blair DM. Suppressive therapy in the control of bilharziasis: a comparative trial in African school children. Central African Journal of Medicine 1973;Sep:32‐8. [PubMed] [Google Scholar]
Creasey 1986 {published data only}
- Creasey AM, Taylor P, Thomas JE. Dosage trial of a combination of oxamniquine and praziquantel in the treatment of schistosomiasis in Zimbabwean schoolchildren. Central African Journal of Medicine 1986;32(7):165‐7. [PubMed] [Google Scholar]
Danso‐Appiah 2009 {published data only}
- Danso‐Appiah A, Garner P, Olliaro PL, Utzinger J. Treatment of urinary schistosomiasis: methodological issues and research needs identified through a Cochrane systematic review. Parasitology 2009;136(13):1837‐49. [DOI] [PubMed] [Google Scholar]
Davis 1966 {published data only}
- Davis A. Field trials of ambilhar in the treatment of urinary bilharziasis in schoolchildren. Bulletin of the World Health Organization 1966;35(6):827‐35. [PMC free article] [PubMed] [Google Scholar]
Davis 1979 {published data only}
- Davis A, Wegner DH. Multicentre trials of praziquantel in human schistosomiasis: design and techniques. Bulletin of the World Health Organisation 1979;57(5):767‐71. [PMC free article] [PubMed] [Google Scholar]
De Clercq 2002 {published data only}
- Clercq D, Vercruysse J, Kongs A, Verlé P, Dompnier JP, Faye PC. Efficacy of artesunate and praziquantel in Schistosoma haematobium infected schoolchildren. Acta Tropica 2002;82(1):61‐6. [DOI] [PubMed] [Google Scholar]
Druilhe 1981 {published data only}
- Druilhe P, Bourdillon F, Froment A, Kyelem J M. [Control of urinary schistosomiasis by 3 annual courses of metrifonate]. Annales de la Société belge de médecine tropicale 1981;61(1):99‐109. [PubMed] [Google Scholar]
el Hawey 1990 {published data only}
- el‐Hawey AM, Massoud AM, el‐Rakieby A, Rozeik MS, Nassar MO. Side effects of praziquantel in bilharzial children on a field level. Journal of the Egyptian Society of Parasitology 1990;20(2):599‐605. [PubMed] [Google Scholar]
el Tayeb 1988 {published data only}
- Tayeb M, Daffalla AA, Kardaman MW, See R, Fenwick A. Praziquantel and oltipraz: the treatment of schoolchildren infected with Schistosoma mansoni and/or Schistosoma haematobium in Gezira, Sudan. Annals of Tropical Medicine and Parasitology 1988;82(1):53‐7. [DOI] [PubMed] [Google Scholar]
el‐Zayadi 1985 {published data only}
- el‐Zayadi A, Fakhfakh A, Fawzy Montasser M, Soltan YA. Effect of the oral antibilharzial niridazole (Ambilhar) on patients with concomitant schistosomiasis and HBs antigenaemia. Hepatogastroenterology 1985;32(4):168‐70. [PubMed] [Google Scholar]
Erikstrup 2008 {published data only}
- Erikstrup C, Kallestrup P, Zinyama‐Gutsire RBL, Gomo E, Dam GJ, Deelder AM, et al. Schistosomiasis and infection with human immunodeficiency virus 1 in rural Zimbabwe: systemic inflammation during co‐infection and after treatment for schistosomiasis. American Journal of Tropical Medicine and Hygiene 2008;79(3):331‐7. [PubMed] [Google Scholar]
Fontanilles 1964 {published data only}
- Fontanilles F. New aspects of the chemotherapy of bilharziasis with Ambilhar (Niridazole). Egyptian Journal of Bilharziasis 1974;1(1):29‐34. [PubMed] [Google Scholar]
Forsyth 1964 {published data only}
- Forsyth DM, Bradley DJ. Long‐term results of treating urinary schistosomiasis endemic in primary‐school children. Lancet 1964;2(7352):171‐3. [DOI] [PubMed] [Google Scholar]
Garba 2001 {published data only}
- Garba A, Aboubacar A, Barkire A, Vera C, Sellin B, Chippaux JP. [Impact of health education programs on the control of urinary bilharziasis in Niger]. Santé (Montrouge, France) 2001;11(1):35‐42. [PubMed] [Google Scholar]
Garba 2004 {published data only}
- Garba A, Campagne G, Tassie JM, Barkire A, Vera C, Sellin B, et al. [Long‐term impact of a mass treatment by praziquantel on morbidity due to Schistosoma haematobium in two hyperendemic villages of Niger]. Bulletin de la Société de pathologie exotique 2004;97(1):7‐11. [PubMed] [Google Scholar]
Hammad 1997 {published data only}
- Hammad TA, Gabr NS, Talaat MM, Orieby A, Shawky E, Strickland GT. Hematuria and proteinuria as predictors of Schistosoma haematobium infection. American Journal of Tropical Medicine and Hygiene 1997;57(3):363‐7. [DOI] [PubMed] [Google Scholar]
Jewsbury 1977 {published data only}
- Jewsbury JM, Cooke MJ, Weber MC. Field trial of metrifonate in the treatment and prevention of schistosomiasis infection in man. Annals of Tropical Medicine and Parasitology 1977;71(1):67‐83. [DOI] [PubMed] [Google Scholar]
Jinabhai 2001 {published data only}
- Jinabhai CC, Taylor M, Coutsoudis A, Coovadia HM, Tomkins AM, Sullivan KR. Epidemiology of helminth infections: implications for parasite control programmes, a South African perspective. Public Health Nutrition 2001;4(6):1211‐9. [DOI] [PubMed] [Google Scholar]
Jordan 1966 {published data only}
- Jordan P. Schistosomiasis in Tanzania: long term results of TWSb and lucanthone hydrochloride combined in suppressive therapy in Schistosoma haematobium infection. Transactions of the Royal Society of Tropical Medicine and Hygiene 1966;60(1):83‐8. [Google Scholar]
Kardaman 1983 {published data only}
- Kardaman MW, Amin MA, Fenwick A, Cheesmond AK, Dixon HG. A field trial using praziquantel (BiltricideR) to treat Schistosoma mansoni and Schistosoma haematobium infection in Gezira, Sudan. Annals in Tropical Medicine and Parasitology 1983;77(3):297‐304. [DOI] [PubMed] [Google Scholar]
Kern 1984 {published data only}
- Kern P, Burchard GD, Dietrich M. Comparative study of oltipraz versus praziquantel for treatment of schistosomiasis with intestinal manifestation in the Gabon (Schistosoma intercalatum and S. haematobium). Tropenmedizin und Parasitologie 1984;35(2):95‐9. [PubMed] [Google Scholar]
King 1989 {published data only}
- King CH, Mahmoud AA. Drugs five years later: praziquantel. Annals of Internal Medicine 1989;110(4):290‐6. [DOI] [PubMed] [Google Scholar]
King 1992 {published data only}
- King CH, Muchiri EM, Ouma JH. Age‐targeted chemotherapy for control of urinary schistosomiasis in endemic populations. Memórias do Instituto Oswaldo Cruz 1992;87(Suppl 4):203‐10. [DOI] [PubMed] [Google Scholar]
Kurz 1986 {published data only}
- Kurz KM, Stephenson LS, Latham MC, Kinoti SN. The effectiveness of metrifonate in reducing hookworm infection in Kenyan school children. American Journal of Tropical Medicine and Hygiene 1986;35(3):571‐4. [DOI] [PubMed] [Google Scholar]
Latham 1983 {published data only}
- Latham MC, Stephenson LS, Hall A, Wolgemuth JC, Elliot TC, Crompton DW. Parasitic infections, anaemia and nutritional status: a study of their interrelationships and the effect of prophylaxis and treatment on workers in Kwale District, Kenya. Transactions of the Royal Society of Tropical Medicine and Hygiene 1983;77(1):41‐8. [DOI] [PubMed] [Google Scholar]
Lucas 1969 {published data only}
- Lucas AO, Akpom CA, Cockshott WP, Bohrer SP. Reversibility of the urological lesions of schistosomiasis in children after specific therapy. Annals of the New York Academy of Sciences 1969;160(2):629‐44. [DOI] [PubMed] [Google Scholar]
Mwanakasale 2009 {published data only}
- Mwanakasale V, Siziya S, Mwansa J, Koukounari A, Fenwick A. Impact of iron supplementation on schistosomiasis control in Zambian school children in a highly endemic area. Malawi Medical Journal 2009;21(1):12‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
N'Goran 2003 {published data only}
- N'Goran EK, Utzinger J, Gnaka HN, Yapi A, N'Guessan NA, Kigbafori SD, et al. Randomized, double‐blind, placebo‐controlled trial of oral artemether for the prevention of patent Schistosoma haematobium infections. American Journal of Tropical Medicine and Hygiene 2003;68(1):24‐32. [PubMed] [Google Scholar]
Nagaty 1962 {published data only}
- Nagaty HF, Khalil HM. The use of tricyclamol chloride as a preventive measure for the side reactions of lucanthone hydrochloride in treatment of urinary schistosomiasis cases. Central African Journal of Medicine 1962;8:136‐8. [PubMed] [Google Scholar]
Odongo‐Aginya 1996 {published data only}
- Odongo‐Aginya EI, Doehring M, Lakwo TL, Etyono S, Luyinda LB, Roth J, et al. Integrated control trial of schistosomiasis at Nakiwogo fishing village near Entebbe, Uganda. East African Medical Journal 1996;73(8):495‐8. [PubMed] [Google Scholar]
Olsen 2007 {published data only}
- Olsen A. Efficacy and safety of drug combinations in the treatment of schistosomiasis, soil‐transmitted helminthiasis, lymphatic filariasis and onchocerciasis. Transactions of the Royal Society of Tropical Medicine and Hygiene 2007;101(8):747‐58. [DOI] [PubMed] [Google Scholar]
Pitchford 1978 {published data only}
- Pitchford RJ, Lewis M. Oxamniquine in the treatment of various schistosome infections in South Africa. South African Medical Journal 1978;53(17):677‐80. [PubMed] [Google Scholar]
Podgore 1994 {published data only}
- Podgore JK, Abu‐Elyazeed RR, Mansour NS, Youssef FG, Hibbs RG, Gere JA. Evaluation of a twice‐a‐week application of 1% niclosamide lotion in preventing Schistosoma haematobium reinfection. American Journal of Tropical Medicine and Hygiene 1994;51(6):875‐9. [DOI] [PubMed] [Google Scholar]
Rabarijaona 2001 {published data only}
- Rabarijaona LP, Andriamaroson BJ, Ravaoalimalala VE, Ravoniarimbinina P, Migliani R. [Identification of communities endemic for urinary bilharziosis by the "Lot Quality Assurance Sampling" method in Madagascar]. Archives de l'Institut Pasteur de Madagascar 2001;67(1‐2):41‐5. [PubMed] [Google Scholar]
Rey 1984 {published data only}
- Rey JL, Sellin E, Sellin B, Simonkovich E, Mouchet F. Comparative efficacy of oltipraz (1 dose, 30 mg/kg) and the combination of niridazole (25 mg/kg) and metrifonate (10 mg/kg) against S. haematobium [Efficacite comparee de l' oltipraz (1 dose 30 mg/kg) et de l' association niridazole (25 mg/kg) ‐ metrifonate (10 mg/kg) contre S. haematobium]. Medicine Tropicale 1984;44(2):155‐8. [PubMed] [Google Scholar]
Rugemalila 1984 {published data only}
- Rugemalila JB, Asila J, Chimbe A. Randomized comparative trials of single doses of the newer antischistosomal drugs at Mwanza, Tanzania. I. Praziquantel and oxamniquine for the treatment of schistosomiasis mansoni. Journal of Tropical Medicine and Hygiene 1984;87(6):231‐5. [PubMed] [Google Scholar]
Schutte 1983 {published data only}
- Schutte CH, Osman Y, Deventer JM, Mosese G. Effectiveness of praziquantel against the South African strains of Schistosoma haematobium and S. mansoni. South African Medical Journal 1983;64(1):7‐10. [PubMed] [Google Scholar]
Sellin 1986 {published data only}
- Sellin B, Rey JL, Simonkovic E, Sellin E, Mouchet F. Chemotherapy trial in the battle against S. haematobium in an irrigated sahelian zone in Niger [Essai de lutte par chimiotherapie contre schistosoma haematobium en zone irriguee sahelienne au niger]. Medecine Tropicale 1986;46(1):21‐30. [PubMed] [Google Scholar]
Sissoko 2009 MLI {published data only}
- Sissoko MS, Dabo A, Traoré H, Diallo M, Traoré B, Konaté D, et al. Efficacy of artesunate + sulfamethoxypyrazine/pyrimethamine versus praziquantel in the treatment of Schistosoma haematobium in children. PLoS One 2009;4(10):e6732. [DOI] [PMC free article] [PubMed] [Google Scholar]
Snyman 1997 {published data only}
- Snyman JR, Sommers K, Steinmann MA, Lizamore DJ. Effects of calcitriol on eosinophil activity and antibody responses in patients with schistosomiasis. European Journal of Clinical Pharmacology 1997;52(4):277‐80. [DOI] [PubMed] [Google Scholar]
Snyman 1998 {published data only}
- Snyman JR, Sommers de K. Effect of levamisole on the immune response of patients with schistosomiasis after treatment with praziquantel. Clinical Drug Investigation 1998;15(6):483‐9. [DOI] [PubMed] [Google Scholar]
Squires 2000 {published data only}
- Squires N. Interventions for treating schistosomiasis haematobium. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD000053] [DOI] [PubMed] [Google Scholar]
Stephenson 1985 {published data only}
- Stephenson LS, Latham MC, Kurz MK, Miller D, Kinoti SN, Oduori ML. Urinary iron loss and physical fitness of Kenyan children with urinary schistosomiasis. Americal Journal of Tropical Medicine and Hygiene 1985;34(2):322‐30. [DOI] [PubMed] [Google Scholar]
Taylor 2001 {published data only}
- Taylor M, Jinabhai CC, Couper I, Kleinschmidt I, Jogessar VB. The effect of different anthelmintic treatment regimens combined with iron supplementation on the nutritional status of schoolchildren in KwaZulu‐Natal, South Africa: a randomized controlled trial. Transactions of the Royal Society of Tropical Medicine and Hygiene 2001;95(2):211‐6. [DOI] [PubMed] [Google Scholar]
Teesdale 1980 {published data only}
- Teesdale CH, Chitsulo L, Mkandawire AC. Concurrent dosage with niridazole and metrifonate for the treatment of Bilharziasis in school children in Malawi. Central African Journal of Medicine 1980;26(9):202‐5. [PubMed] [Google Scholar]
Thigpen 2011 {published data only}
- Thigpen MC, Filler SJ, Kazembe PN, Parise ME, Macheso A, Campbell CH, et al. Associations between peripheral Plasmodium falciparum malaria parasitemia, human immunodeficiency virus, and concurrent helminthic infection among pregnant women in Malawi. American Journal of Tropical Medicine and Hygiene 2011;84(3):379‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Urbani 1997 {published data only}
- Urbani C, Touré A, Hamed AO, Albonico M, Kane I, Cheikna D, et al. Intestinal parasitic infections and schistosomiasis in the valley of the Senegal river in the Islamic Republic of Mauritania [Parasitoses intestinales et schistosomiases dans la vallee du fleuve Senegal en Republique Islamique de Mauritanie]. Médicine Tropicale 1997;57(2):157‐60. [PubMed] [Google Scholar]
Utzinger 2001 {published data only}
- Utzinger J, Xiao S, N'Goran EK, Bergquist R, Tanner M. The potential of artemether for the control of schistosomiasis. International Journal for Parasitology 2001;31(14):1549‐62. [DOI] [PubMed] [Google Scholar]
van Lieshout 1994 {published data only}
- Lieshout L, Jonge N, el‐Masry N, Mansour MM, Bassily S, Krijger FW, et al. Monitoring the efficacy of different doses of praziquantel by quantification of circulating antigens in serum and urine of schistosomiasis patients. Parasitology 1994;108(Pt 5):519‐26. [DOI] [PubMed] [Google Scholar]
Wilkins 1987 Simoto trial {published data only}
- Wilkins HA, Moore PJ. Comparative trials of regimens for the treatment of urinary schistosomiasis in The Gambia. Journal of Tropical Medicine and Hygiene 1987;90(2):83‐92. [PubMed] [Google Scholar]
Wolfe 1967 {published data only}
- Wolfe HL. Treatment of urinary schistosomiasis with niridazole (Ambilhar) in 576 African schoolchildren. Lancet 1967;1(7486):350‐4. [DOI] [PubMed] [Google Scholar]
Xiao 2002 {published data only}
- Xiao S, Tanner M, N'Goran EK, Utzinger J, Chollet J, Bergquist R, et al. Recent investigations of artemether, a novel agent for the prevention of schistosomiasis japonica, mansoni and haematobia. Acta Tropica 2002;82(2):175‐81. [DOI] [PubMed] [Google Scholar]
Zwingenberger 1990 {published data only}
- Zwingenberger K, Feldmeier H, Bienzle U, Steiner A. Mixed Schistosoma haematobium/Schistosoma intercalatum infection. Annals of Tropical Medicine and Parasitology 1990;84(1):85‐7. [DOI] [PubMed] [Google Scholar]
Additional references
Cioli 2003
- Cioli D, Pica‐Mattoccia L. Praziquantel. Parasitology Research 2003;90(Suppl 1):S3‐9. [DOI] [PubMed] [Google Scholar]
Cook 2003
- Manson P, Cook GC, Zumla A. Manson's Tropical diseases. 21st Edition. Philadelphia: Saunders, 2003. [Google Scholar]
Doenhoff 2008
- Doenhoff MJ, Cioli D, Utzinger J. Praziquantel: mechanisms of action, resistance and new derivates for schistosomiasis. Current Opinion in Infectious Diseases 2008;21(6):659‐67. [DOI] [PubMed] [Google Scholar]
Feldmeier 1999
- Feldmeier H, Chitsulo L. Therapeutic and operational profiles of metrifonate and praziquantel in Schistosoma haematobium infection. Arzneimittelforschung 1999;49(7):557‐65. [DOI] [PubMed] [Google Scholar]
Fenwick 2006
- Fenwick A, Webster JP. Schistosomiasis: challenges for control, treatment and drug resistance. Current Opinion in Infectious Diseases 2006;19(6):577‐82. [DOI] [PubMed] [Google Scholar]
Gray 2011
- Gray DJ, Ross AG, Li YS, McManus DP. Diagnosis and management of schistosomiasis. British Medical Journal 2011;342:d2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gryseels 2006
- Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet 2006;368(9541):1106‐18. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JP, Altman DG. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons Ltd, 2008:187‐242. [Google Scholar]
King 2005
- King C, Dickman K, Tisch D. Reassessment of the cost of chronic helminthic infection: a meta‐analysis of disability‐related outcomes in endemic schistosomiasis. Lancet 2005;365(9470):1561‐9. [DOI] [PubMed] [Google Scholar]
King 2011
- King CH, Olbyrch S, Soon M, Singer ME, Carter J, Colley DG. Utility of repeated praziquantel dosing in the treatment of schistosomiasis in high‐risk communities in Africa: a systematic review. PLoS Neglected Tropical Diseases 2011;5(9):e1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2008
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, Ltd., 2008:95‐147. [Google Scholar]
Liu 2011
- Liu R, Dong HF, Guo Y, Zhao QP, Jiang MS. Efficacy of praziquantel and artemisinin derivatives for the treatment and prevention of human schistosomiasis: a systematic review and meta‐analysis. Parasites and Vectors 2011;4:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pérez del Villar 2012
- Pérez del Villar L, Burguillo FJ, López‐Abán J, Muro A. Systematic review and meta‐analysis of artemisinin based therapies for the treatment and prevention of schistosomiasis. PLoS One 2012;7(9):e45867. [DOI] [PMC free article] [PubMed] [Google Scholar]
Renganathan 1998
- Renganathan E, Cioli D. An international initiative on praziquantel use. Parasitology Today 1998;14(10):390‐1. [DOI] [PubMed] [Google Scholar]
Review Manager (RevMan) [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Ross 2002
- Ross AG, Bartley PB, Sleigh AC, Olds GR, Li Y, Williams GM, et al. Schistosomiasis. New England Journal of Medicine 2002;346(16):1212‐20. [DOI] [PubMed] [Google Scholar]
Stothard 2013
- Stothard RJ, Sousa‐Figueiredo JC, Betson M, Bustinduy A, Reinhard‐Rupp J. Schistosomaisis in African infants and preschool children: let them now be treated!. Trends in Parasitology 2013;29(4):197‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Utzinger 2004
- Utzinger J, Keiser J. Schistosomiasis and soil‐transmitted helminthiasis: common drugs for treatment and control. Expert Opinion on Pharmacotherapy 2004;5(2):263‐85. [DOI] [PubMed] [Google Scholar]
WHO 1985
- World Health Organization. The control of schistosomiasis. Report of a WHO Expert Committee 1985; Vol. WHO Technical Report Series 728:1‐114. [PubMed]
WHO 1991
- World Health Organization. Basic laboratory methods in medical parasitology 1) Parasitology ‐ laboratory manuals. http://whqlibdoc.who.int/publications/9241544104_(part1).pdf 1991:1‐111.
WHO 1998
- World Health Organization. The use of essential drugs. http://apps.who.int/iris/bitstream/10665/42135/1/WHO_TRS_882.pdf 1998; Vol. Eighth report of the WHO Expert Committee:1‐77. [PubMed]
WHO 2002
- World Health Organization. Prevention and control of schistosomiasis and soil‐transmitted helminthiasis. Report of a WHO Expert Committee. http://whqlibdoc.who.int/trs/WHO_TRS_912.pdf 2002:1‐57. [PubMed]
WHO 2006
- World Health Organization. Preventive chemotherapy in human helminthiasis. Coordinated use of antihelminthic drugs in control interventions: a manual for health professionals and programme managers. http://whqlibdoc.who.int/publications/2006/9241547103_eng.pdf 2006:1‐62.
WHO 2013
- World Health Organization. Schistosomiasis Fact sheet no. 115. http://www.who.int/mediacentre/factsheets/fs115/en/index.html (accessed 25 September 2013).
WHO 2014
- World Health Organization. Schistosomiasis. A major public health problem. http://www.who.int/schistosomiasis/en/index.html (accessed 12 February 2014).
References to other published versions of this review
Danso‐Appiah 2008
- Danso‐Appiah A, Utzinger J, Liu J, Olliaro P. Drugs for treating urinary schistosomiasis. Cochrane Database of Systematic Reviews 2008, Issue 3 . [DOI: 10.1002/14651858.CD000053] [DOI] [PubMed] [Google Scholar]
Squires N
- Squires N. Interventions for treating Schistosomiasis haemtobium. Cochrane Database of Systematic Reviews 1997, Issue 3. [DOI: 10.1002/14651858.] [DOI] [PubMed] [Google Scholar]


