Abstract
Background
The Drug Enforcement Agency estimates that 80% of cocaine seized in the United States contains the veterinary pharmaceutical levamisole (LVM). One problem with LVM is that it is producing life-threatening neutropenia in an alarming number of cocaine abusers. The neuropharmacological profile of LVM is also suggestive of an agent with modest reinforcing and stimulant effects that could enhance cocaine’s addictive effects.
Methods
We tested the hypothesis that LVM (ip) enhances the rewarding and locomotor stimulant effects of cocaine (ip) using rat conditioned place preference (CPP) and locomotor assays. Effects of LVM by itself were also tested.
Results
LVM (0–10 mg/kg) produced CPP at 1 mg/kg (P < 0.05) and locomotor activation at 5 mg/kg (P < 0.05). For CPP combination experiments, a statistically inactive dose of LVM (0.1 mg/kg) was administered with a low dose of cocaine (2.5 mg/kg). Neither agent produced CPP compared to saline (P > 0.05); however, the combination of LVM and cocaine produced enhanced CPP compared to saline or either drug by itself (P < 0.01). For locomotor experiments, the same inactive dose of LVM (0.1 mg/kg, ip) was administered with low (10 mg/kg) and high doses (30 mg/kg) of cocaine. LVM (0.1 mg/kg) enhanced locomotor activation produced by 10 mg/kg of cocaine (P < 0.05) but not by 30 mg/kg (P > 0.05).
Conclusions
LVM can enhance rewarding and locomotor-activating effects of low doses of cocaine in rats while possessing modest activity of its own.
Keywords: levamisole, cocaine, addiction, conditioned place preference, locomotor, psychostimulant
1. INTRODUCTION
Cocaine accounts for 25% of emergency department visits for drug misuse; furthermore, 1.6 million individuals aged 12 or older are estimated to be cocaine users, and relapse occurs in greater than 50% of cocaine addicts (SAMHSA, 2013; Gerlach et al., 2014). Health risks for cocaine abusers are being exacerbated by levamisole (LVM), an antiparasitic drug and veterinary pharmaceutical (Auffenberg et al., 2013). LVM has been identified as a prevalent cocaine adulterant; in fact, the Drug Enforcement Agency (DEA) estimates that up to 80% of cocaine seized in the United States contains LVM (Wolford et al., 2012). One problem with LVM is that it produces severe neutropenia, and it is this effect that is responsible for the large number of cocaine abusers presenting with life-threatening immunological deficits. Hazards of LVM-tainted cocaine are being reported by the news media, scientific publications, and government agencies (Zhu et al., 2009; Chang et al., 2010; Ullrich et al., 2011). In addition to the estimate that LVM is present in 8 out of 10 cocaine samples, DEA data also indicate an average concentration of 10% LVM is detected in cocaine. Buchanan et al (2010) also demonstrated the presence of LVM (as high as 10%) in a patient’s crack cocaine pipe, confirming LVM as an adulterant.
Speculation about the presence of LVM in cocaine centers around two overlapping hypotheses. One explanation is that drug manufacturers add LVM to increase the amount of ‘final product’ to increase profits. LVM has characteristics that are ideal for an adulterant, namely that it is cheap, has similar physicochemical properties to cocaine, and is easily accessible as a veterinary pharmaceutical in regions in which the laced cocaine originates. A second explanation is that LVM can modify addictive effects of cocaine. The neuropharmacological profile of LVM, while still incomplete, is suggestive of an agent that could enhance the rewarding, reinforcing, and stimulant effects of cocaine while possessing modest activity of its own. Prior work indicates that LVM activates nicotinic receptors, especially the α3β4 subtype, and inhibits monoamine oxidase (MAO) activity, and both nicotinic agonists and MAO inhibitors enhance in vivo actions of COC by increasing DA transmission (Hernando et al., 2012; Levandoski et al., 2003; Agarwal et al., 1990). Patients taking LVM as an adjunctive cancer therapy have reported mood-elevating effects associated with elevation of monoamine transmission (Goldin et al., 1982).
Here, we tested the hypotheses that LVM enhances the rewarding and locomotor-activating effects of cocaine in rats and displays modest rewarding and locomotor-stimulant effects of its own. We are aware of only one other experimental study that has investigated this drug-drug interaction (Tallarida et al., 2014). That study was our own recent work and was conducted in planarians, a type of flatworm with mammalian-relevant neurotransmitter systems that demonstrates quantifiable pharmacological responses (e.g., abstinence-induced withdrawal, environmental place conditioning, stereotypical activity, sensitization, etc.) to different classes of abused drugs (Raffa and Rawls, 2008; Buttarelli et al., 2008; Pagan et al., 2008, 2009, 2013; Eriksson and Panula, 1994). In that study, we demonstrated that LVM interacted synergistically with cocaine and specifically enhanced place conditioning and stereotypical effects of cocaine (Tallarida et al., 2014). Here, we have taken the important step of providing the first experimental evidence that LVM enhances rewarding and locomotor-stimulant effects of low doses of cocaine in mammals.
2. EXPERIMENTAL PROCEDURES
2.1. Subjects and drugs
Male Sprague-Dawley rats (225–250 g, Harlan Laboratories, Frederic, MD, USA) were pair-housed, maintained on a 12-hr light/dark cycle, and provided access to food and water ad libitum. Procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at Temple University. (−)-Cocaine hydrochloride (cocaine) was generously provided by the National Institute on Drug Abuse (Bethesda, MD, USA). Levamisole hydrochloride (LVM) was purchased from Sigma-Aldrich (St. Louis, MO, USA). All drugs were dissolved in physiological saline and injected intraperitoneally (ip). The dose range (0.1–10 mg/kg) was based on prior work showing that it can increase endogenous levels of opiate alkaloids and attenuate the opioid withdrawal syndrome in rats (Spector et al., 1998). The dose range of cocaine (2.5–30 mg/kg) was based on prior work showing that 10 mg/kg of cocaine produces more robust and consistent CPP than lower and higher doses in rats (Zakharova et al., 2009).
2.2. Behavioral experiments
2.2.1. Conditioned place preference (CPP) experiments
CPP chambers (27.5” W × 8.75” D × 16” H) were obtained from San Diego Instruments (San Diego, CA, USA). The chambers consisted of two compartments separated by a removable door, and CPP experiments were conducted as previously described (Schroeder et al., 2014). One compartment was black with a rough floor and the other compartment was white with black stripes consisting of a smooth floor. The movement of each rat was captured by 4 × 16 photo beam arrays, stored in the place preference system, and later were exported to Microsoft excel for analysis. A 6-day biased design consisting of three different phases w: 1) a pre-test in the absence of drug on day 1; 2) 4 days of drug conditioning; and 3) a post-test on day 6 in the absence of drug. On day 1, rats were acclimated for 30 min to the test room and then placed individually into the chamber (in the absence of the removal door) and allowed free access to both sides of the chamber for 30 min.
The time spent in each compartment of the chamber was measured, and the compartment in which the rat spent the least amount of time (i.e., the non-preferred compartment) was designated as the drug-paired side. During the conditioning phase (days 2–5), rats were once again acclimated to the test room for 30 min in the morning and then injected with cocaine, LVM, or a combination of cocaine and LVM and confined to their non-preferred side. In the afternoon, 4 h later, rats were injected with saline and confined to their preferred side. Control animals were conditioned with saline on each side of the chambers. On day 6 following the 30-min acclimation interval, rats were placed back into the chambers and allowed free access to both compartments for 30 min. A preference score was calculated for each rat as the difference in time spent on the drug paired side on post-test and pre-test days (i.e., preference score = time spent on drug-paired side post-test – time spent on drug-paired side pre-test).
2.2.2. Locomotor Experiments
LVM was tested by itself and in combination with cocaine. In the initial set of experiments, LVM (1, 5, 10 mg/kg) or saline was injected once and locomotor activity was measured. For combination experiments, a statistically inactive dose of LVM (0.1 mg/kg, determined from the initial set of experiments) was administered with two different doses of cocaine (10, 30 mg/kg).
For assessment of locomotor activity, rats were placed individually into activity chambers and allowed to acclimate for 60 min. Basal activity was measured for 60 min prior to drug injection, followed by measuring of activity for 60 min. The Digiscan DMicro system (Accuscan, Inc., Columbus, OH) was used to measure locomotor activity as described (Schroeder et al., 2014; Tallarida et al., 2013; Lisek et al., 2012; Rasmussen et al., 2011). Chambers consisted of transparent plastic boxes (45 cm × 20 cm × 20 cm) set inside metal frames equipped with 16 infrared light emitters and detectors. The beam height was 4.5 cm, and the space between beams was 2.5 cm. The number of photocell beam breaks was recorded by a computer interface.
2.3. Data analysis
For CPP experiments investigating multiple doses of LVM, comparisons of group means (± S.E.M.) were evaluated by one-way ANOVA, and in cases of significance, followed by Dunnett’s post-hoc test. For CPP experiments investigating a combination of LVM and cocaine, comparisons of group means (± S.E.M.) were evaluated by two-way ANOVA (LVM dose×cocaine dose), and in cases of significance, followed by a Bonferroni post-hoc test. For locomotor experiments, time-course data were analyzed by two-way ANOVA (treatment×time) followed by a Bonferroni test. Cumulative data were analyzed by either one-way ANOVA (followed by Dunnett’s test) or a Student’s t-test in cases of two groups. Values of P < 0.05 were considered statistically significant.
3. RESULTS
3.1. LVM produces CPP and enhances CPP produced by a submaximal dose of cocaine
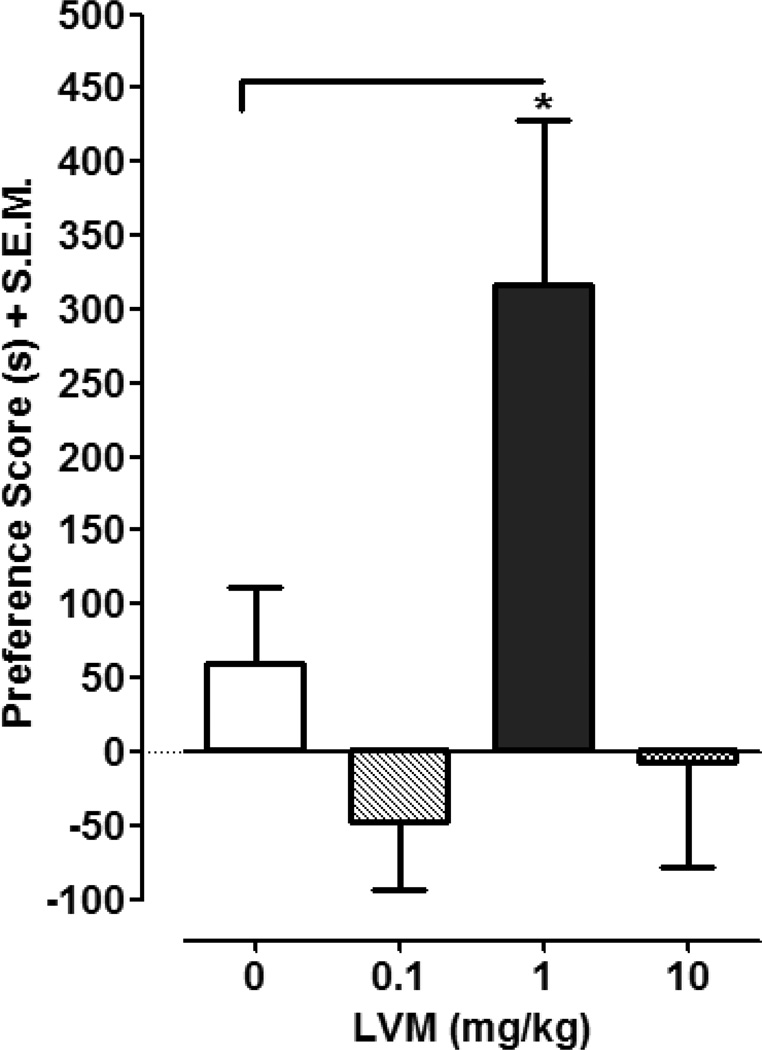
One-way ANOVA conducted on the preference data in Fig.1 revealed a significant main effect [F (3, 26) = 4.935, P < 0.01]. Post-hoc analysis indicated that LVM (1 mg/kg) produced significant CPP compared to saline (P < 0.05). The preference shift produced by LVM (1 mg/kg) was 317 ± 112 s compared to 59 ± 53 s for saline-treated controls. Lower (0.1 mg/kg) and higher (10 mg/kg) doses of LVM did not produce CPP compared to saline (P > 0.05).
Fig. 1. LVM produces CPP.
CPP was assessed in rats conditioned with saline or LVM (0.1, 1, 10 mg/kg). Data were presented as mean preference score + S.E.M. (difference in time spent in drug-paired environment between post-test and pre-test). N=7–8 rats per group. *p < 0.05 compared to saline control group.
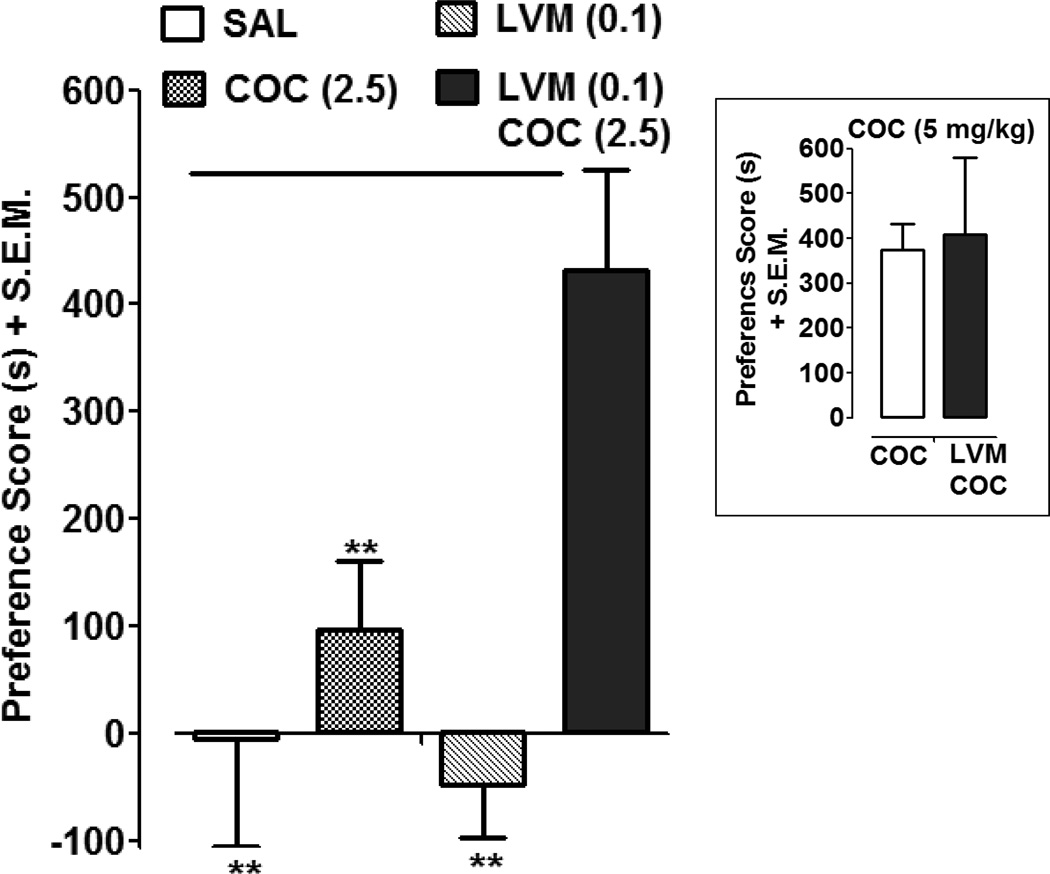
Fig. 2 presents the effects of a combination of a low dose of cocaine (2.5 mg/kg) and a dose of LVM (0.1 mg/kg) that was statistically ineffective in producing CPP (Fig. 1). Two-way ANOVA of the place preference data revealed significant drug interaction and treatment effects (Drug Interaction: F (1,26) = 5.62, P < 0.05; LVM dose: F (1,26) = 3.42, P > 0.05; cocaine dose: F (1,26) = 13.40, P < 0.01). Bonferroni post-hoc analysis indicated that rats conditioned with a combination of cocaine (2.5 mg/kg) and LVM (0.1 mg/kg) displayed a significant preference shift as compared to saline-conditioned rats (P < 0.01, LVM/COC versus SAL); cocaine alone (2.5 mg/kg) (P < 0.01, LVM/COC versus SAL); and LVM alone (0.1 mg/kg) (P < 0.01, LVM/COC versus LVM). Neither cocaine (2.5 mg/kg) nor LVM (0.1 mg/kg) produced significant CPP compared to saline controls (P > 0.05). LVM (0.1 mg/kg) did not significantly enhance CPP produced by a higher dose of cocaine (P > 0.05, Student’s t-test).
Fig. 2. A combination of LVM and cocaine produces enhanced CPP.
CPP was assessed in rats conditioned with saline, cocaine (COC) (2.5 mg/kg), LVM (0.1 mg/kg), or a combination of COC (2.5 mg/kg) and LVM (0.1 mg/kg). Data were presented as mean preference score + S.E.M. (difference in time spent in drug-paired environment between post-test and pre-test). N=7–8 rats per group. **p < 0.01 compared to LVM (0.1 mg/kg)/ COC (2.5 mg/kg). Inset) Effect of LVM (0.1 mg/kg) on CPP produced by a higher dose of COC (5 mg/kg). N=7 rats per group.
3.2. LVM produces locomotor activation and enhances locomotor stimulant effects of a low dose of cocaine
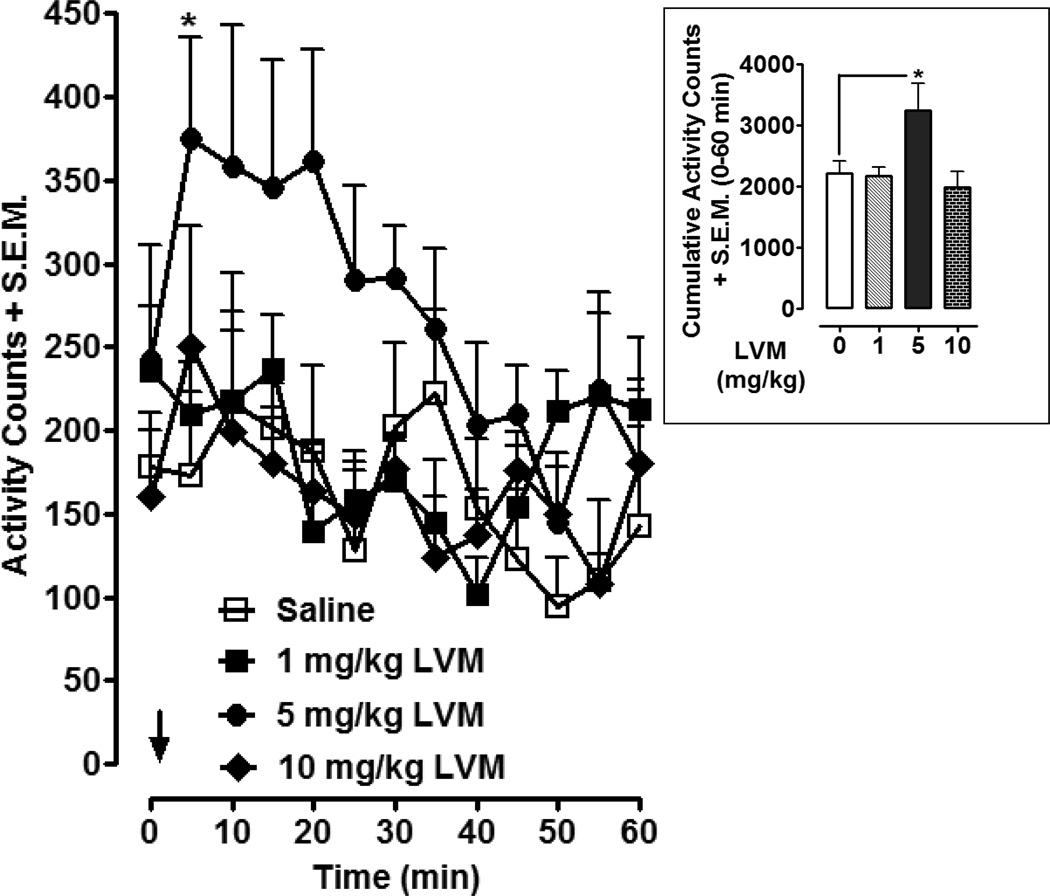
Effects of different doses of LVM on locomotor activity are presented in Fig 3. Two-way ANOVA conducted on time-course data revealed significant effects of treatment [F (3, 260) = 14.51, P < 0.0001] and time [F (12, 260) = 2.33, P < 0.01]. Post-hoc analysis revealed that the median dose of LVM (5 mg/kg) produced significant locomotor activation compared to saline treatment 5 min following injection (P < 0.05). For cumulative data (Fig. 3 inset), calculated as the total number of activity counts from the time of injection until 60 min later, one-way ANOVA indicated a significant main effect [F (3, 20) = 3.886, P < 0.05]. Post-hoc analysis indicated that 5 mg/kg of LVM produced significant locomotor activation compared to saline-treated controls (P < 0.05). Locomotor activation produced by lower (1 mg/kg) and higher (10 mg/kg) was not significantly different than that produced by saline (P > 0.05).
Fig. 3. LVM produces locomotor activation.
Locomotor activity was assessed in rats injected with saline or LVM (1, 5, 10 mg/kg). Time-course data were presented as mean activity counts + S.E.M. following LVM or saline injection (arrow). N= 6 rats per group. *p < 0.05 compared to saline control group. Inset) Cumulative data were presented as total activity counts + S.E.M. (0 to 60 min following LVM or saline injection). *p < 0.05 compared to saline control group.
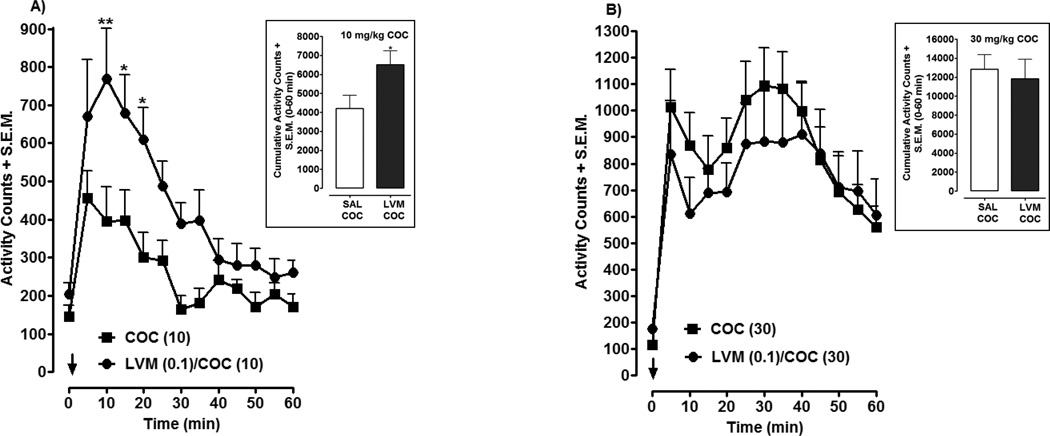
For combined administration (Fig. 4), a dose of LVM (0.1 mg/kg) that was statistically ineffective in producing CPP or locomotor activation by itself (Figs. 1, 3) was injected in combination with two doses of cocaine (10, 30 mg/kg). Two-way ANOVA conducted on the time-course data for the 10 mg/kg cocaine experiment revealed significant effects of treatment [F (1, 182) = 41.34, P < 0.0001] and time [F (12, 182) = 9.11, P < 0.0001] (Fig. 4A). Post-hoc analysis revealed that the combination of LVM (0.1 mg/kg) and cocaine (10 mg/kg) produced significantly greater locomotor activity than 10 mg/kg of cocaine alone at the following time points following drug injection: (10 min, P < 0.01; 15 min, P < 0.05; 20 min, P < 0.05). Evaluation of cumulative data (Fig. 4 inset), calculated as the total number of activity counts from the time of injection until 60 min later, confirmed that LVM enhanced cocaine’s locomotor stimulant effect, as a Student’s t-test revealed that the combination of LVM and cocaine produced greater locomotor activation than cocaine by itself (P < 0.05). However, in experiments in which a higher dose of cocaine (30 mg/kg) was tested in combination with LVM (0.1 mg/kg), the locomotor activation produced by cocaine was not enhanced by the presence of LVM (P > 0.05) (Fig. 4B).
Fig. 4. LVM enhances locomotor activation produced by cocaine (COC).
Locomotor activity was assessed in rats injected with COC by itself (10 mg/kg, 4A or 30 mg/kg, 4B) and in combination with low-dose LVM (0.1 mg/kg). Time-course data were presented as mean activity counts + S.E.M. following drug injection (arrow). N= 8 rats per group. *p < 0.05 or **p < 0.01 compared to COC alone group. Insets) Cumulative data were presented as total activity counts + S.E.M. (0 to 60 min following drug injection). *p < 0.05 compared to COC group.
4. DISCUSSION
LVM can produce severe immunological deficits in cocaine abusers but its effects on cocaine addiction have not been investigated in mammals (Wolford et al., 2012). Evidence that LVM activates nicotinic and monamingeric systems (Hernando et al., 2012; Levandoski et al., 2003; Agarwal et al., 1990; Goldin et al., 1982) led us to hypothesize that LVM can enhance rewarding and stimulant effects of cocaine and produce modest reinforcing and locomotor-stimulant effects of its own. That is indeed what we found, as the present study provides the first experimental information in mammals that LVM enhances some of cocaine’s in vivo effects. We demonstrated that LVM alone can produce place conditioning effects and locomotor activation. For combined administration, we found that some dose combinations of LVM and cocaine produce an enhancement of CPP and locomotor activation, which suggests that LVM can enhance cocaine’s rewarding and stimulant effects. Thus, for cocaine abusers, LVM may increase the addictive effects of cocaine and produce life-threatening neutropenia (Wolford et al., 2012; Zhu et al., 2009; Chang et al., 2010; Ullrich et al., 2011).
The ability of cocaine to produce rewarding effects in place conditioning assays and stimulant effects in locomotor and motility assays is established across different species, including rats, mice and invertebrates (Nader et al., 2014; Tallarida et al., 2014; Pagan et al., 2008, 2009, 2013; Rawls et al., 2010, 2011; Aguilar et al., 2009; Huber et al., 2011). In contrast, for LVM, its in vivo profile remains incomplete despite being synthesized by Janssen Pharmaceuticals almost five decades ago (Raeymakers et al., 1967). This is due in part to the therapeutic history of LVM, which was originally marketed as an antiparasitic drug for humans but withdrawn from the US market in 1999 due to the risk of agranulocytosis (Keiser and Utzinger, 2008). More recently, LVM has been studied in combination with chemotherapy agents and is now used in veterinary medicine to treat parasitic infections in livestock, especially in South American regions where cocaine is manufactured, which is presumably related to its prevailing association with cocaine (Auffenberg et al., 2013).
Our behavioral results indicate that certain doses of LVM produce modest CPP and locomotor activation in rats. Previously reported biological actions of LVM are indicative of an agent that can produce direct or indirect augmentation of monoamine activity, and mood-elevating effects have been reported in cancer patients taking LVM as an adjunctive therapy (Goldin et al., 1982). At the cellular level, LVM activates nicotinic acetylcholine receptors (nAChRs), with agonist effects at α3β4 nAChRs shown in C. elegans and partial agonist activity demonstrated at human α3β4 and α3β2 nAChRs (Hernando et al., 2012; Levandoski et al., 2003). Agonists of nAChRs produce positive reinforcing and locomotor-stimulant effects by increasing dopaminergic transmission in the mesolimbic pathway, thereby leading to enhanced levels of extracellular dopamine in the nucleus accumbens (Hendrickson et al., 2013; Baker et al., 2013; Benwell and Balfour, 1992). High densities of α3β4 nAChRs are located in the medial habenula (MH; Grady et al., 2009; Clarke et al., 1985), and the level of activity of these MH α3β4 nAChRs influences mesolimbic dopamine output in the nucleus accumbens and contributes to nicotine’s positive reinforcing and dopamine-activating effects (McCallum et al., 2012; Glick et al., 2006, 2011). This evidence suggests that the behavioral effects of LVM observed here may be due to a nicotine-type profile in which LVM enhances mesolimbic DA output through activation of α3β4 nAChRs in the MH or α4β2 and α6β2 nAChRs in the VTA (Chen et al., 2012; Zhao-Shea et al., 2011; Gotti et al., 2010; Rollema et al., 2007; Picciotto et al., 1998; Pidoplichko et al., 1997). For the case of combined exposure with cocaine, those actions of LVM may create a hyperactive mesolimbic DA system that interacts synergistically or additively with cocaine’s dopamine reuptake block, leading to enhancement of dopamine transmission in the nucleus accumbens and augmentation of reinforcement and locomotor activation. Future experiments, including the testing of LVM and LVM/cocaine combinations in the presence of an α3β4 nAChR antagonist such as 18-Methoxycoronaridine (18-MC), are planned to probe a mechanistic role for nAChR systems in the behavioral effects demonstrated here (Glick et al., 2011). Other mechanisms are also possible, including inhibition of monoamine oxidase that could elevate dopamine, norepinephrine and serotonin levels, leading to locomotor activation and reinforcement and enhancement of cocaine’s action (Agarwal et al., 1990; Vanhoutte et al., 1977). Prior evidence also indicates that LVM increases endogenous opioid levels and inhibits the opioid withdrawal syndrome in rats (Spector et al., 1998), raising the possibility of a ‘speedball’ type of interaction in which LVM, by increasing opioid tone, inhibits GABA interneurons in the VTA leading to disinhibition of the mesolimbic DA system (Lamas et al., 1998).
It should be pointed out that LVM by itself produced CPP at only one of the doses tested (i.e., 1 mg/kg) and that doses at both the lower and higher ends of the spectrum were ineffective. A similar inverted U-type response was also detected for locomotor experiments, as only a single median dose (i.e., 5 mg/kg) produced locomotor activation. An interesting analysis of those data is that CPP was detected at doses of LVM that were lower than those required to produce acute locomotor activation (i.e.,1 versus 5 mg/kg). In fact, this is not an unusual dose-effect profile, as with more established drugs, including cocaine, CPP is often detected at lower doses than are needed for locomotor activation (Zakharova et al., 2009). For CPP our most effective doses for cocaine are 5–10 mg/kg, and these doses produce only limited hyperactivity following acute exposure (Parikh et al., 2014; Xu et al., 2013; Gregg et al., 2013; Zakharova et al., 2009; Hummel and Unterwald, 2003). Higher doses (e.g., 30 mg/kg) produce diminished responses due to their aversive effects (Bardo et al., 1995; Tzschentke, 2007; Zakharova et al., 2009).
For our combination experiments, enhanced in vivo responses were detected for only some dose-combinations of LVM and cocaine. For both CPP and locomotor experiments, greater effects were detected for combinations in which a dose of LVM that was ineffective by itself was administered with low, submaximal doses of cocaine (≤ 5–10 mg/kg). The inability of LVM to enhance CPP or locomotor activation produced by high doses of cocaine may have been due to a ‘ceiling effect’, a phenomenon in which a drug produces a maximum effect so that increasing the drug dosage or, as in the present case, adding another type of drug does not increase overall efficacy (Lufty and Cowan, 2004; Tallarida, 2011, 2012). The present findings are for the most part consistent with what we demonstrated in our planarian study (Tallarida et al., 2014), where we found that LVM enhances place conditioning produced by submaximal concentrations of cocaine but not by higher, more efficacious, concentrations of cocaine. The main difference between the planarian and rat studies was that LVM produced significant place conditioning in rats but not in planarians, a discrepancy that may be related to species differences or weak-to-modest efficacy for LVM that is highly dependent on experimental variables. While the connection between the present findings and actual cocaine abuse is unclear, the DEA has estimated that an average concentration of 10% LVM is detected in cocaine samples (Buchanan et al., 2010). This suggests that combinations involving low ratios of LVM may be most relevant to the actual clinical situation. Future work will use self-administration assays to assess how different doses of LVM influence the reinforcing strength of cocaine during actual cocaine consumption and relapse to cocaine seeking during cocaine abstinence.
It does warrant mention that isobolographic analysis was not used in the present study to analyze the drug-drug interaction between LVM and cocaine (Tallarida, 2011, 2012). When an ineffective dose of drug A is combined with drug B and significantly enhances the effect of drug B, then we have the clearest example of synergism. It is not necessary to formally show an isobologram in this case. If so, it would be a horizontal line rather than the familiar double-intercept line (Tallarida, 2012). In that case the combination point is well below the line because the enhanced response means that a lesser quantity of the active drug is needed to get the specified effect. The isobole is done when both drugs exert an effect.
In conclusion, the identification of a drug-drug interaction between cocaine and LVM is an important first step in exploring mechanism and impact. Understanding the pharmacology of a prevalent, dangerous combination is important because poly-drug consumption (e.g., heroin + cocaine, cocaine + alcohol, etc.) often increases dangers posed by each individual drug and makes it more challenging to identify efficacious medications to manage dependence and relapse. An example of this challenge is the impact that alcohol dependence has on the efficacy of modafinil in treating cocaine dependence (Anderson et al., 2009; Vocci and Elkashef, 2005). The present study, by identifying an in vivo interaction between cocaine and LVM, provides a foundation to examine mechanism of action and determine if promising therapeutics for cocaine dependence and relapse maintain their efficacy against combinations of cocaine and LVM.
Research Highlights.
Interactions between levamisole and cocaine were investigated in rats.
Levamisole produced conditioned place preference in rats.
Levamisole produced locomotor activation in rats.
Levamisole enhanced cocaine-induced place conditioning and locomotor activation in rats.
Levamisole may enhance addictive effects of cocaine.
Acknowledgements
None.
Role of Funding Source
This study was supported by Dr. Rawls’ internal PI funding at Temple University School of Medicine and by the Center for Substance Abuse P30 grant DA01342.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosures
Contributors
Authors Scott M. Rawls and Chris Tallarida designed the studies. Author Chris Tallarida conducted conditioned place preference and locomotor activity experiments. Authors Ronald Tallarida and Scott M. Rawls conducted the statistical analyses for the experiments. Author Scott Rawls managed the literature searches and summaries of previous related work. Author Scott M. Rawls wrote drafts of the manuscript, which were subsequently circulated to all authors for their comments, critiques and suggestions. All authors contributed to and have approved the final manuscript.
Conflict of Interest
All authors declare that they have no conflicts of interest.
REFERENCES
- Agarwal A, Shukla OP, Ghatak S, Tekwani BL. Biogenic amines, metabolites and monoamine oxidase in the filarial worm Setaria cervi. Int. J. Parasitol. 1990;20:873–881. doi: 10.1016/0020-7519(90)90025-i. [DOI] [PubMed] [Google Scholar]
- Aguilar MA, Rodríguez-Arias M, Miñarro J. Neurobiological mechanisms of the reinstatement of drug-conditioned place preference. Brain Res. Rev. 2009;59:253–277. doi: 10.1016/j.brainresrev.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Anderson AL, Reid MS, Li SH, Holmes T, Shemanski L, Slee A, Smith EV, Kahn R, Chiang N, Vocci F, Ciraulo D, Dackis C, Roache JD, Salloum IM, Somoza E, Urschel HC, Elkashef AM. Modafinil for the treatment of cocaine dependence. Drug Alcohol Depend. 2009;104:133–139. doi: 10.1016/j.drugalcdep.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffenberg C, Rosenthal LJ, Dresner N. Levamisole: a common cocaine adulterant with life-threatening side effects. Psychosomatics. 2013;54:590–593. doi: 10.1016/j.psym.2013.02.012. [DOI] [PubMed] [Google Scholar]
- Baker LK, Mao D, Chi H, Govind AP, Vallejo YF, Iacoviello M, Herrera S, Cortright JJ, Green WN, McGehee DS, Vezina P. Intermittent nicotine exposure upregulates nAChRs in VTA dopamine neurons and sensitises locomotor responding to the drug. Eur. J. Neurosci. 2013;37:1004–1011. doi: 10.1111/ejn.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Rowlett JK, Harris MJ. Conditioned place preference using opiate and stimulant drugs: a meta-analysis. Neurosci. Biobehav. Rev. 1995;19:39–51. doi: 10.1016/0149-7634(94)00021-r. [DOI] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ. The effects of acute and repeated nicotine treatment on nucleus accumbens dopamine and locomotor activity. Br. J. Pharmacol. 1992;105:849–856. doi: 10.1111/j.1476-5381.1992.tb09067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan JA, Oyer RJ, Patel NR, Jacquet GA, Bornikova L, Thienelt C, Shriver DA, Shockley LW, Wilson ML, Hurlbut KM, Lavonas EJ. A confirmed case of agranulocytosis after use of cocaine contaminated with levamisole. J. Med. Toxicol. 2010;6:160–164. doi: 10.1007/s13181-010-0060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttarelli FR, Pellicano C, Pontieri FE. Neuropharmacology and behavior in planarians: translations to mammals. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 2008;147:399–408. doi: 10.1016/j.cbpc.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Chang A, Osterloh J, Thomas J. Levamisole: a dangerous new cocaine adulterant. Clin. Pharmacol. Ther. 2010;88:408–411. doi: 10.1038/clpt.2010.156. [DOI] [PubMed] [Google Scholar]
- Chen Y, Broad LM, Phillips KG, Zwart R. Partial agonists for α4β2 nicotinic receptors stimulate dopaminergic neuron firing with relatively enhanced maximal effects. Br. J. Pharmacol. 2012;165:1006–1016. doi: 10.1111/j.1476-5381.2011.01628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PB, Schwartz RD, Paul SM, Pert CB, Pert A. Nicotinic binding in rat brain: autoradiographic comparison of [3H]acetylcholine, [3H]nicotine, and [125I]-alpha-bungarotoxin. J. Neurosci. 1985;5:1307–1315. doi: 10.1523/JNEUROSCI.05-05-01307.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson KS, Panula P. gamma-Aminobutyric acid in the nervous system of a planarian. J. Comp. Neurol. 1994;345:528–536. doi: 10.1002/cne.903450405. [DOI] [PubMed] [Google Scholar]
- Gerlach KK, Dasgupta N, Schnoll S, Henningfield J. Epidemiology of stimulant misuse and abuse: implications for future epidemiologic and neuropharmacologic research. Neuropharmacology. 2014;87C:91–96. doi: 10.1016/j.neuropharm.2014.04.020. [DOI] [PubMed] [Google Scholar]
- Glick SD, Ramirez RL, Livi JM, Maisonneuve IM. 18-Methoxycoronaridine acts in the medial habenula and/or interpeduncular nucleus to decrease morphine self-administration in rats. Eur. J. Pharmacol. 2006;537:94–98. doi: 10.1016/j.ejphar.2006.03.045. [DOI] [PubMed] [Google Scholar]
- Glick SD, Sell EM, McCallum SE, Maisonneuve IM. Brain regions mediating α3β4 nicotinic antagonist effects of 18-MC on nicotine self-administration. Eur. J. Pharmacol. 2011;669:71–75. doi: 10.1016/j.ejphar.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin A, Chirigos MA, Macdonald JS, Fefer A, Mihich E. Biologic-response modifiers and adjuvant chemotherapy: consideration of selected preclinical investigations in relation to clinical potential. Recent Results Cancer Res. 1982;80:351–356. doi: 10.1007/978-3-642-81685-7_57. [DOI] [PubMed] [Google Scholar]
- Gotti C, Guiducci S, Tedesco V, Corbioli S, Zanetti L, Moretti M, Zanardi A, Rimondini R, Mugnain,i M, Clementi F, Chiamulera C, Zoli M. Nicotinic acetylcholine receptors in the mesolimbic pathway: primary role of ventral tegmental area alpha6beta2* receptors in mediating systemic nicotine effects on dopamine release, locomotion, and reinforcement. J. Neurosci. 2010;30:5311–5325. doi: 10.1523/JNEUROSCI.5095-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady SR, Moretti M, Zoli M, Marks MJ, Zanardi A, Pucci L, Clementi F, Gotti C. Rodent habenulo-interpeduncular pathway expresses a large variety of uncommon nAChR subtypes, but only the alpha3beta4* and alpha3beta3beta4* subtypes mediate acetylcholine release. J. Neurosci. 2009;29:2272–2282. doi: 10.1523/JNEUROSCI.5121-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg RA, Tallarida CS, Reitz AB, Rawls SM. Mephedrone interactions with cocaine: prior exposure to the 'bath salt' constituent enhances cocaine-induced locomotor activation in rats. Behav. Pharmacol. 2013;8:684–688. doi: 10.1097/FBP.0000000000000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson LM, Guildford MJ, Tapper AR. Neuronal nicotinic acetylcholine receptors: common molecular substrates of nicotine and alcohol dependence. Front. Psychiatry. 2013;4:29. doi: 10.3389/fpsyt.2013.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernando G, Bergé I, Rayes D, Bouzat C. Contribution of subunits to Caenorhabditis elegans levamisole-sensitive nicotinic receptor function. Mol. Pharmacol. 2012;82:550–560. doi: 10.1124/mol.112.079962. [DOI] [PubMed] [Google Scholar]
- Huber R, Panksepp JB, Nathanie T, Alcaro A, Panksepp J. Drug-sensitive reward in crayfish: an invertebrate model system for the study of SEEKING, reward, addiction, and withdrawal. Neurosci. Biobehav. Rev. 2011;35:1847–1853. doi: 10.1016/j.neubiorev.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel M, Unterwald EM. Intra-accumbens pertussis toxin sensitizes rats to the locomotor activating effects of a single cocaine challenge. Brain Res. 2003;965:100–107. doi: 10.1016/s0006-8993(02)04142-2. [DOI] [PubMed] [Google Scholar]
- Keiser J, Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis. JAMA. 2008;299:1937–1948. doi: 10.1001/jama.299.16.1937. [DOI] [PubMed] [Google Scholar]
- Lamas X, Negus SS, Gatch MB, Mello NK. Effects of heroin/cocaine combinations in rats trained to discriminate heroin or cocaine from saline. Pharmacol. Biochem. Behav. 1998;60:357–364. doi: 10.1016/s0091-3057(98)00021-5. [DOI] [PubMed] [Google Scholar]
- Levandoski MM, Piket B, Chang J. The anthelmintic levamisole is an allosteric modulator of human neuronal nicotinic acetylcholine receptors. Eur. J. Pharmacol. 2003;471:9–20. doi: 10.1016/s0014-2999(03)01796-5. [DOI] [PubMed] [Google Scholar]
- Lisek R, Xu W, Yuvasheva E, Chiu YT, Reitz AB, Liu-Chen LY, Rawls SM. Mephedrone ('bath salt') elicits conditioned place preference and dopamine-sensitive motor activation. Drug Alcohol Depend. 2012;126:257–262. doi: 10.1016/j.drugalcdep.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutfy K, Cowan A. Buprenorphine: a unique drug with complex pharmacology. Curr. Neuropharmacol. 2004;2:395–402. doi: 10.2174/1570159043359477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum SE, Cowe MA, Lewis SW, Glick SD. α3β4 nicotinic acetylcholine receptors in the medial habenula modulate the mesolimbic dopaminergic response to acute nicotine in vivo. Neuropharmacology. 2012;63:434–440. doi: 10.1016/j.neuropharm.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader MA, Balster RL, Henningfield JE. William L. Woolverton: a case history in unraveling the behavioral pharmacology of stimulants. Neuropharmacology. 2014 doi: 10.1016/j.neuropharm.2014.05.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagán OR, Deats S, Baker D, Montgomery E, Wilk G, Tenaglia M, Semon J. Planarians require an intact brain to behaviorally react to cocaine, but not to react to nicotine. Neuroscience. 2013;246:265–270. doi: 10.1016/j.neuroscience.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagán OR, Rowlands AL, Azam M, Urban KR, Bidja AH, Roy DM, Feeney RB, Afshari LK. Reversal of cocaine-induced planarian behavior by parthenolide and related sesquiterpene lactones. Pharmacol. Biochem. Behav. 2008;89:160–170. doi: 10.1016/j.pbb.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Pagán OR, Rowlands AL, Fattore AL, Coudron T, Urban KR, Bidja AH, Eterović VA. A cembranoid from tobacco prevents the expression of nicotine-induced withdrawal behavior in planarian worms. Eur. J. Pharmacol. 2009;615:118–124. doi: 10.1016/j.ejphar.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Naughton SX, Shi X, Kelley LK, Yegla B, Tallarida CS, Rawls SM, Unterwald EM. Cocaine-induced neuroadaptations in the dorsal striatum: glutamate dynamics and behavioral sensitization. Neurochem. Int. 2014;75C:54–65. doi: 10.1016/j.neuint.2014.05.016. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Léna C, Marubio LM, Pich EM, Fuxe K, Changeux JP. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Pidoplichko VI, DeBiasi M, Williams JT, Dani JA. Nicotine activates and desensitizes midbrain dopamine neurons. Nature. 1997;390:401–404. doi: 10.1038/37120. [DOI] [PubMed] [Google Scholar]
- Raeymakers HM, Roevens LFC, Janssen PAJ. Tet. Lett. 1967;16:1467–1470. doi: 10.1016/s0040-4039(00)90983-3. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Rawls SM. A Model For Drug Action And Abuse. Austin, TX: Landes Bioscience; 2008. [Google Scholar]
- Rasmussen B, Unterwald EM, Rawls SM. Glutamate transporter subtype 1 (GLT-1) activator ceftriaxone attenuates amphetamine-induced hyperactivity and behavioral sensitization in rats. Drug Alcohol Depend. 2011;118:484–488. doi: 10.1016/j.drugalcdep.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls SM, Patil T, Tallarida CS, Baron S, Kim M, Song K, Ward S, Raffa RB. Nicotine behavioral pharmacology: clues from planarians. Drug Alcohol Depend. 2011;118:274–279. doi: 10.1016/j.drugalcdep.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls SM, Karaca F, Madhani I, Bhojani V, Martinez RL, Abou-Gharbia M, Raffa RB. β-lactamase inhibitors display anti-seizure properties in an invertebrate assay. Neuroscience. 2010;169:1800–1804. doi: 10.1016/j.neuroscience.2010.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, Lu Y, Mansbach RS, Mather RJ, Rovetti CC, Sands SB, Schaeffer E, Schulz DW, Tingley FD, Williams KE. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52:985–994. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Schroeder JA, Tolman NG, McKenna FF, Watkins KL, Passeri SM, Hsu AH, Shinn BR, Rawls SM. Clavulanic acid reduces rewarding, hyperthermic and locomotor-sensitizing effects of morphine in rats: a new indication for an old drug? Drug Alcohol Depend. 2014;142:41–45. doi: 10.1016/j.drugalcdep.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector S, Munjal I, Schmidt DE. Effects of the immunostimulant, levamisole, on opiate withdrawal and levels of endogenous opiate alkaloids and monoamine neurotransmitters in rat brain. Neuropsychopharmacology. 1998;19:417–427. doi: 10.1016/S0893-133X(98)00034-7. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings NSDUH Series H-46. Rockville, MD: Substance Abuse and Mental Health Services Administration, U. S. Dept. of Health and Human Services; 2013. HHS Publication No. (SMA) 13–4795. [Google Scholar]
- Tallarida CS, Egan E, Alejo GD, Raffa R, Tallarida RJ, Rawls SM. Levamisole and cocaine synergism: a prevalent adulterant enhances cocaine's action in vivo. Neuropharmacology. 2014;79:590–595. doi: 10.1016/j.neuropharm.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallarida CS, Corley G, Kovalevich J, Yen W, Langford D, Rawls SM. Ceftriaxone attenuates locomotor activity induced by acute and repeated cocaine exposure in mice. Neurosci. Lett. 2013;556:155–159. doi: 10.1016/j.neulet.2013.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallarida RJ. Revisiting the isobole and related quantitative methods for assessing drug synergism. J. Pharmacol. Exp. Ther. 2012;342:2–8. doi: 10.1124/jpet.112.193474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallarida RJ. Quantitative methods for assessing drug synergism. Genes Cancer. 2011;2:1003–1008. doi: 10.1177/1947601912440575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict. Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Ullrich K, Koval R, Koval E, Bapoje S, Hirsh JM. Five consecutive cases of a cutaneous vasculopathy in users of levamisole-adulterated cocaine. J. Clin. Rheumatol. 2011;17:193–196. doi: 10.1097/RHU.0b013e31820e6822. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM, Van Nueten JM, Verbeuren TJ, Laduron PM. Differential effects of the isomers of tetramisole on adrenergic neurotransmission in cutaneous veins of dog. J. Pharmacol. Exp. Ther. 1977;200:127–140. [PubMed] [Google Scholar]
- Vocci FJ, Elkashef A. Pharmacotherapy and other treatments for cocaine abuse and dependence. Curr. Opin. Psychiatry. 2005;18:265–270. doi: 10.1097/01.yco.0000165596.98552.02. [DOI] [PubMed] [Google Scholar]
- Wolford A, McDonald TS, Eng H, Hansel S, Chen Y, Bauman J, Sharma R, Kalgutkar AS. Immune-mediated agranulocytosis caused by the cocaine adulterant levamisole: a case for reactive metabolite(s) involvement. Drug Metab. Dispos. 2012;40:1067–1075. doi: 10.1124/dmd.112.045021. [DOI] [PubMed] [Google Scholar]
- Xu W, Wang Y, Ma Z, Chiu YT, Huang P, Rasakham K, Unterwald E, Lee DY, Liu-Chen LY. L-isocorypalmine reduces behavioral sensitization and rewarding effects of cocaine in mice by acting on dopamine receptors. Drug Alcohol Depend. 2013;133:693–703. doi: 10.1016/j.drugalcdep.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharova E, Miller J, Unterwald E, Wade D, Izenwasser S. Social and physical environment alter cocaine conditioned place preference and dopaminergic markers in adolescent male rats. Neuroscience. 2009;163:890–897. doi: 10.1016/j.neuroscience.2009.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao-Shea R, Liu L, Soll LG, Improgo MR, Meyers EE, McIntosh JM, Grady SR, Marks MJ, Gardner PD, Tapper AR. Nicotine-mediated activation of dopaminergic neurons in distinct regions of the ventral tegmental area. Neuropsychopharmacology. 2011;36:1021–1032. doi: 10.1038/npp.2010.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu NY, Legatt DF, Turner AR. Agranulocytosis after consumption of cocaine adulterated with levamisole. Ann. Intern. Med. 2009;150:287–289. doi: 10.7326/0003-4819-150-4-200902170-00102. [DOI] [PubMed] [Google Scholar]