Abstract
We used event-related potentials (ERPs) to study age effects of perceptual (basic-level) vs. perceptual-semantic (superordinate-level) categorization on cognitive control using the go/nogo paradigm. Twenty-two younger (11 M; 21±2.2 years) and 22 older adults (9 M; 63±5.8 years) completed two visual go/nogo tasks. In the single car task (SiC) (basic), go/nogo responses were made based on single exemplars of a car (go) and a dog (nogo). In the object animal task (ObA) (superordinate), responses were based on multiple exemplars of objects (go) and animals (nogo). Each task consisted of 200 trials: 160 (80%) ‘go’ trials that required a response through button pressing and 40 (20%) ‘nogo’ trials that required inhibition/withholding of a response. ERP data revealed significantly reduced nogo-N2 and nogo-P3 amplitudes in older compared to younger adults, whereas go-N2 and go-P3 amplitudes were comparable in both groups during both categorization tasks. Although the effects of categorization levels on behavioral data and P3 measures were similar in both groups with longer response times, lower accuracy scores, longer P3 latencies, and lower P3 amplitudes in ObA compared to SiC, N2 latency revealed age group differences moderated by the task. Older adults had longer N2 latency for ObA compared to SiC, in contrast, younger adults showed no N2 latency difference between SiC and ObA. Overall, these findings suggest that age differentially affects neural processing related to cognitive control during semantic categorization. Furthermore, in older adults, unlike in younger adults, levels of categorization modulate neural processing related to cognitive control even at the early stages (N2).
Keywords: Semantic, Categorization, Cognitive control, Aging, Event-related potentials
Introduction
Categorization (e.g., categorizing items as animate vs. inanimate) is a basic cognitive skill that allows meaningful organization of objects in our surrounding environment (Cicirelli, 1976; Kinces, Chadaide, Varga, Antal, & Paulus, 2006; Long, Liu, Qiu, Shen, Li & Li, 2010; Mack & Palmeri, 2011). Broadly speaking, objects can be categorized at three levels: basic, superordinate, and subordinate (Mervis & Rosch, 1981). For example, a ‘dog’ at the basic level, can be categorized at the superordinate level as an ‘animal’ and at the subordinate level as a ‘golden retriever’. Basic and superordinate categorizations are frequently used in day-to-day functioning, whereas subordinate categorization is more specific and relates to in-depth/expert knowledge (e.g., knowledge used by dog experts; car experts; bird experts).
It is well recognized that categorization of object information enhances memory retention and recall in both younger and older adults (Cicirelli, 1976; Nieuwenhuis, Holroyd, Mol, & Coles, 2004; Schmitt, Murphy, & Sanders, 1981). Less well understood is how object categorization influences other cognitive processes such as those related to response inhibition, response conflict, and response monitoring that fall into the broad concept of cognitive control (Botvinick, Braver, Barch, Carter, & Cohen 2001; Inzlicht, Bartholow, & Hirsch, 2015; Miyake et al., 2000). The ability to successfully withhold/block a pre-potent/dominant tendency to respond is one of the core functions of cognitive control (Miyake et al., 2000), which often involves conflict monitoring and outcome monitoring to an extent. For instance, when walking down a street, we stop if we see an approaching car but continue to walk if we simply see other people walking around. Similarly, when shopping at a grocery store in the produce section, we stop when we see an item on our grocery list (e.g., apples). As we navigate our ever-changing environment, we monitor conflicts between current actions and intentions when competing sources of information are present and withhold our ongoing behavioral responses as needed, and many of these decisions are made based on how objects we encounter are categorized. While there is some evidence in children (7–8 and 10–11 years old) and in young adults (18–31 years old) that basic and superordinate object categorizations differentially affect processing related to response inhibition/conflict (Maguire, White, & Brier, 2011; Maguire et al., 2009; Tachibana, Aragane, & Sugita, 1996), little is known about how levels of categorization interact with cognitive control in normal older adults (55 years and older). Given the general consensus that aging impacts cognitive control to variable degrees (e.g., Braver et al., 2001; Hasher, Stoltzfus, Zacks, & Rypma, 1991; Hasher & Zacks, 1988; Kane, Hasher, Stoltzfusz, Zack, & Connelly, 1994; Kramer, Humphrey, Larish, Logan, & Strayer, 1994; McDowd, 1997; Paxton, Barch, Racine, & Braver, 2008), examining the effects of object categorization on cognitive control in older adults will provide useful information about how these operations interact and change with age.
Object categorizations at basic and superordinate levels have varying perceptual and semantic processing requirements. Research has shown that basic categorization depends heavily on perceptual information (Biederman, & Gerhardstein, 1993; Jolicoeur, Gluck, & Kosslyn, 1984; Large, Kiss, & McMullen, 2004; Scott, Tanaka, Sheinberg, & Curran, 2006), whereas superordinate categorization depends on both perceptual and semantic information related to object knowledge (Jolicoeur et al., 1984; Large et al., 2004; Murphy & Brownell, 1985; Murphy & Lassaline, 1997; Rosch, Mervis, Gray, Johnson, & Boyes-Braem, 1976). More specifically, basic-level visual object categorization depends on global, coarse perceptual discrimination (Biederman & Gerhardstein, 1993), given that members within the same basic-level category share many perceptual features (e.g., different breeds of dogs), whereas members from different basic-level categories (e.g., dogs vs. cars; dogs vs. birds) share fewer perceptual features. In comparison to basic categorization, superordinate categorization extends beyond perceptual similarities since members of the same superordinate category share relatively few perceptual features, and is conceptually more complex. For instance, the category ‘animal’ includes perceptually different entities such as a snake and a dog. Thus, categorization at this level depends heavily on semantic information beyond basic perceptual processing (e.g., Large et al., 2004; Maguire et al., 2009) and involves additional neural resources as have been shown in fMRI studies (e.g., Chiang et al., 2013; Raposo, Mendes, & Marques, 2012).
Following Rosch et al.’s (1976) seminal study, basic-level categorization during tasks that involve overt response selection has been shown by many to be the fastest and most accurate for typical objects (Grill-Spector & Kanwisher, 1993; Johnson & Mervis, 1997; Murphy & Brownell, 1985; Murphy & Lassaline, 1997; Tanaka & Taylor, 1991) with some exceptions. These exceptions include ultra-rapid stimulus exposure (stimulus duration less than 30 ms) and paradigms that incorporate time pressure (i.e., requiring responses to be made within certain reaction time deadlines), which demonstrate faster superordinate categorization than perceptual/ basic-level categorization (Fabre-Thorpe, Delorme, Marlot, & Thorpe, 2001; Macé, Joubert, Nespoulous, and Fabre-Thorpe, 2009; Rogers and Patterson, 2007; Thorpe, Fize, & Marlot, 1996). However, we know little about how varying levels of semantic categorization (basic vs. superordinate) affect the ability to withhold/regulate responses, especially in older adults.
In the aging literature, cognitive control processes related to response inhibition and response conflict have been examined using a variety of paradigms (Kok, 1999; Kramer et al., 1994) such as stroop, negative priming, and go/nogo. This paper focuses on the study of cognitive control using the go/nogo paradigm. The standard go/nogo task requires individuals to respond to a certain type of stimuli (go) and inhibit/refrain from responding to another type of stimuli (nogo), pre defined by a specific set of rules and criteria. The accuracy data, i.e. commission errors during nogo trials in particular, are indicative of the efficiency of cognitive control, but the neural underpinnings of cognitive control may not be directly associated with the behavioral measure (Vallesi, Stuss, McIntosh, & Picton, 2009). Objective techniques such as electroencephalography (EEG) have therefore been applied to explore neural mechanisms underlying cognitive control (e.g., Kok, 1999). Go/nogo tasks in particular elicit predictable changes in known event-related potential (ERP) components that are considered to be markers of response inhibition and/or response conflict and response monitoring (Huster, Enriquez-Geppert, Lavallee, Falkenstein, & Herrmann, 2013), and are thus useful for studying age-related changes in cognitive control during semantic categorization.
The ERP literature on cognitive control in normal older adults using the go/nogo task is vast, but the majority has examined perceptual discrimination using stimuli such as numbers, letters, shapes, and natural images (Beste, Willemssen, Saft, & Falkenstein, 2010; Falkenstein, Hoormann, & Hohnsbein, 1999; Nieuwenhuis et al., 2004; Pfefferbaum & Ford, 1988; Thorpe et al., 1996; Vallesi, 2011; Vallesi et al. 2009; see Friedman, Hamberger, & Ritter, 1993; Tachibana et al., 1996 for some exceptions). These go/nogo studies bear close resemblance to basic-level semantic categorization that involves visual processing and discrimination (e.g., discriminating cars from dogs; cars from birds). In these go/nogo studies, two major ERP components have been consistently found. The first component is a fronto-central negative deflection developing between 200 and 400 ms post-stimulus onset (N2) and the second is a subsequent centro-parietal positive deflection (P3) between 300 and 600 ms (Eimer, 1993; Falkenstein et al., 1999; Pfefferbaum, Ford, Weller, & Kopel, 1985; Kok, 1986; Simson, Vaughan, & Ritter, 1977). The negative deflection (N2) in the frontal electrodes is considered to be a marker of response inhibition by some (Falkenstein, 2006; also see Folstein & Van Petten, 2008 for a review), while others have suggested its role in conflict monitoring (Donkers & van Boxtel, 2004; Nieuwehuis, Yeung, van den Wildenberg, & Ridderinkhof, 2003; Botvinick, Cohen, & Carter, 2004). The positive deflection (P3) is considered to be an index of evaluation of stimuli and attentional allocation by some (Donchin, Karis, Bashore, Coles, & Gratton, 1986; Polich & Herbst, 2000), while others have suggested its role in response monitoring, be it a decision to respond during go trials or to withhold responses during nogo trials (e.g., Falkenstein et al., 1999). While the exact functional significance of each of these two ERP components in relation to cognitive control continues to be debated, there is a general consensus that both are markers of cognitive control to some degree (Smith, Johnstone & Barry, 2007, 2008).
Overall, consistent patterns of delayed latency and reduced amplitude in nogo-N2 have been observed in older adults compared to younger adults (e.g., Falkenstein, Hoormann, & Hohnsbein, 2002; Beste et al., 2010), supporting weakening of neural processing related to cognitive control with age. For nogo-P3 latency, the typical finding in studies involving older adults is prolonged latency (Pfefferbaum & Ford, 1985; Pfefferbaum & Ford, 1988; Pfefferbaum, Ford, Wenegrat, Roth, & Kopell, 1984; Falkenstein et al., 2002). The findings of nogo-P3 amplitude have been less consistent, varying from reduced nogo-P3 amplitude (Pfefferbaum et al., 1984) to either no age-related differences (Falkenstein et al., 2002) or increased nogo-P3 amplitude in older compared to younger adults (Vallesi, 2011). While these findings provide some indications of age-related alterations in cognitive control that can be expected during basic categorization, it is unclear whether the need to use perceptual and semantic processes for superordinate categorization will aid or impede cognitive control processes in older adults, especially given the predilection towards meaning-based processing of information with age (e.g., Radvansky & Dijkstra, 2007).
Two previous ERP studies by Maguire et al. (2009, 2011), one including young adults and the other including children, have examined the effects of varying levels of visual object categorization on cognitive control in typically developing normal populations. In these studies, participants completed go/nogo tasks that compared perceptual (basic) vs. perceptual-semantic (superordinate) categorization. In the young adults study, they found no significant amplitude differences in nogo-N2 between basic and superordinate conditions, whereas nogo-P3 had smaller amplitude for superordinate compared to basic categorization (Maguire et al., 2009). In the study of children, they compared the performance of 7–8 year olds to that of 10–11 year olds. They found that similar to young adults, 10–11 year olds had smaller nogo-P3 amplitude for superordinate categorization compared to basic categorization, whereas the 7–8 year olds showed no differences. To gain insights into the effects of categorization on cognitive control in later adulthood, we examined known neural markers related to cognitive control (the N2 and the P3 ERPs) in cognitively normal younger and older adults corresponding to two visual go/nogo tasks that have been previously used in the Maguire et al. studies. These two visual go/nogo tasks allowed us to contrast perceptual (basic) versus perceptual-semantic (superordinate) categorization. The overall goal of the present study is not to parse out the exact functional significance of N2 or P3 in relation to the go/nogo task, but to uncover whether and how they are influenced differentially by age during categorization.
The primary questions addressed were: (i) does age affect cognitive control measured using N2 and P3 ERP components during go/nogo tasks that involve perceptual (basic) versus perceptual-semantic (superordinate) categorization? and (ii) do young and old adults demonstrate similar categorization task effects? We expected delayed latency and reduced amplitude in older compared to younger adults in both N2 and P3 components, with more prominent changes during superordinate compared to basic categorization. We predicted that N2 and P3 nogo amplitudes will be larger in both groups compared to go amplitudes regardless of the task since nogo trials will require the ability to successfully withhold/block a pre-potent response tendency.
METHODS
Participants
Twenty-two young adults (11 males; mean age = 21.3 years, SD = 2.2) and 22 older adults (9 males; mean age = 63 years, SD = 5.8) participated in the study. All participants were right-handed and native English speakers, and none had a history of neurological or psychiatric disorders, traumatic brain injury, learning disabilities, communication disorders, or uncorrected visual or hearing impairments. Young adults were recruited from the University of Texas at Dallas (UTD) and the University of Illinois at Urbana-Champaign (UIUC) undergraduate student bodies. Older adults were recruited from an ongoing study of normal aging at UTD that rigorously screened participants using a neuropsychological battery to exclude those with cognitive impairment. All participants provided written informed consent as per the Institutional Review Boards at UTD, UIUC, and the University of Texas Southwestern Medical Center.
Experimental Paradigm and Procedures
Participants completed two go/nogo tasks (Maguire et al., 2009, 2011) that required varying levels of perceptual and semantic categorization during a single visit with short breaks between the tasks.
In the first task, the single car task (SiC), participants made go/nogo decisions based on a single image (line-drawing) of a car (go) and a dog (nogo). We used basic-level labels (‘car’ and ‘dog’) in the SiC task to encourage correct discrimination using basic classification (car vs. dog) as opposed to superordinate classification (vehicle vs. animal). The images were repeated so the perceptual properties of the items remained identical over the course of the task so as to facilitate coarse perceptual discrimination between items. Participants were given the following instructions: “You are going to see some dogs and cars. When you see a dog, do not push the button. Press the button for anything that is not a dog. Be as quick and as accurate as possible.” The pictures of the car and the dog were presented 160 and 40 times, respectively.
In the second task, the object animal task (ObA), participants made go/nogo decisions based on multiple exemplars (line-drawings) of objects (go) and animals (nogo) involving superordinate categorization. This superordinate-level categorization task included 160 different exemplars of objects (go) (40 food items, 40 cars, 20 clothing items, 20 kitchen items, 20 human body parts, and 20 tools) and 40 different exemplars of animals of varying visual typicality (nogo). Participants were given the following instructions: “You are going to see some objects and animals. When you see an animal, do not push the button. Press the button for anything that is not an animal. Be as quick and as accurate as possible.” Each of the 200 items was displayed only once during the course of the task. Overall, each task consisted of 200 trials: 160 (80%) ‘go’ trials that required a response through button pressing and 40 (20%) ‘nogo’ trials that required inhibition/withholding of a response. The infrequent nogos compared to frequent gos were implemented to accentuate pre-potent response tendency.
The sequence of the stimuli in each task was pseudo-randomized and the task order was counterbalanced for each participant to mitigate order or practice effect. A button box was placed under the right index finger to register go responses and record reaction time (RT). Each stimulus was presented for 300 ms followed by a 1700 ms blank fixation period. The development of these tasks can be found in detail in Maguire et al. (2009).
EEG Data Acquisition and Processing
While the participants performed the two go/nogo tasks, continuous EEG was recorded from a 64-electrode elastic cap (Neuroscan Quickcap) using a Neuroscan SynAmps amplifier and Scan 4.5 software (sampling rate: 1 kHz, DC-200 Hz) with impedances typically below 10 kΩ. The reference electrode was located at the midline between Cz and CPz. Vertical electroocculogram (VEOG) was recorded at sites above and below the left eye. Data were processed off-line using Neuroscan Edit software. Poorly functioning electrodes were identified by visual inspection and excluded from analysis (typically fewer than 5% of all electrodes; Picton et al., 2000). The continuous EEG data were high-pass filtered at 0.15Hz and corrected for eye blinks using spatial filtering in Neuroscan. The data were epoched between 200 ms before the onset of the stimuli until 1500 ms after the presentation of the stimuli. An algorithm computing the average based on spherical splines fitted to the data (as described in Ferree, Brier, Hart, & Kraut, 2009) was applied to interpolate EEG data to the sites of the bad electrodes. Additionally, we applied a digital low-pass filter with a cutoff value of 30 Hz (linear finite impulse response function) to minimize high frequency noise, such as muscle activity. Epochs with peak signal amplitudes of more than 75 µV were rejected. The rejection rates were generally 20–30% in go and 10–15% in nogo (more than two-thirds of all trials were retained, as suggested by Picton et al., 2000). The EEG data were re-referenced to the average potential over the entire scalp. Baseline correction was done by subtracting the mean amplitude of the pre-stimulus interval (−200 ms to 0 ms) from each time point. Individual ERP data were averaged for the two tasks separately (both go and nogo response types). Only correct trials were included in the ERP averages and responses with reaction times (RT) shorter than 200 ms or longer than 1000 ms were excluded from further analysis.
ERP Analysis
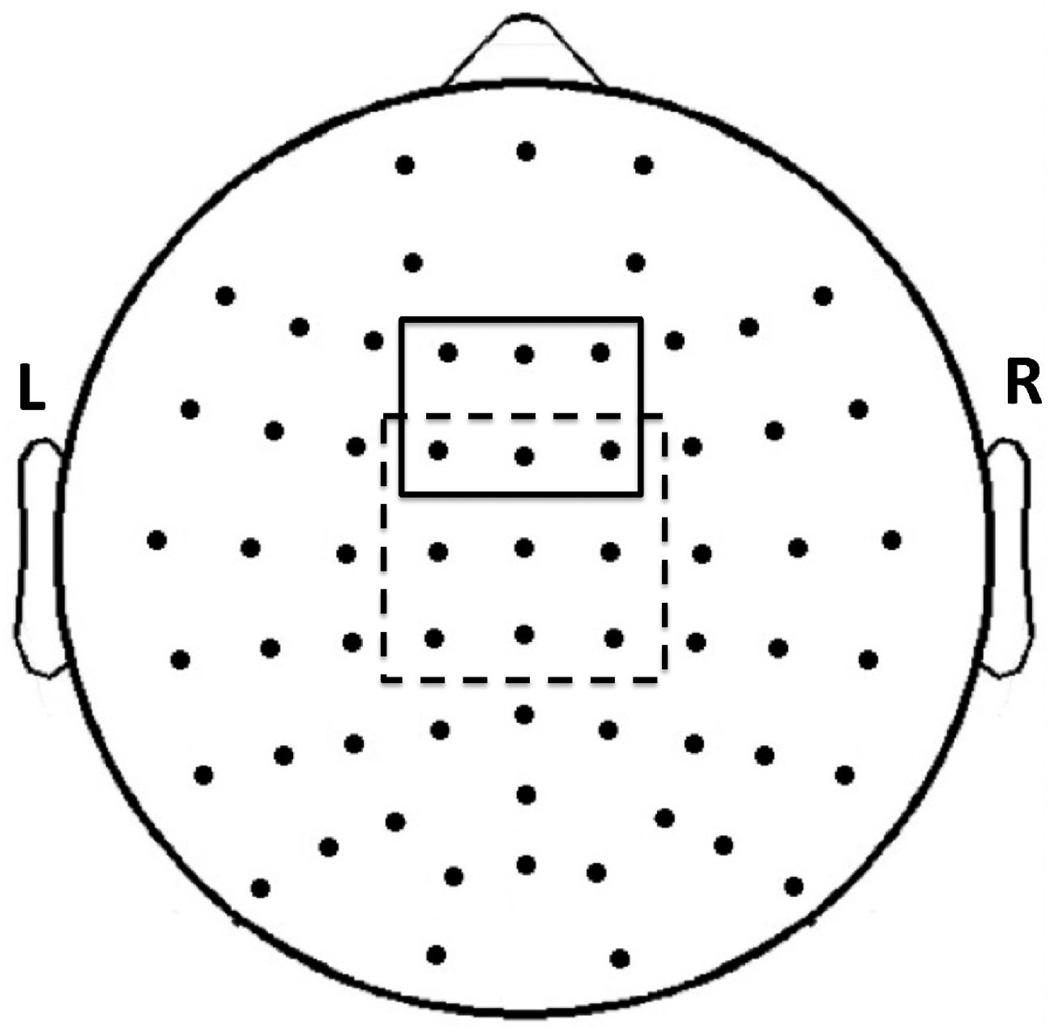
Visual inspection of the ERP waveforms revealed typical ERPs, including N2 and P3 components, in both younger and older adult groups. We focused on N2 and P3 components around the midline. We first measured peak latency between 150 and 300 ms for N2 at 6 midline electrodes (F1, Fz, F2, FC1, FCz, FC2) and between 250 and 600 ms for P3 at 9 midline electrodes (FCZ, FC1, FC2, CZ, C1, C2, CPz, CP1, CP2) for go and nogo trials (see Fig. 1). We calculated peak latency-adjusted (time window: mean latency ± 1 SD) mean amplitude for each group to better estimate amplitude differences independent of latency variability across young and old subjects. Electrode sites and time windows were selected based on (1) previous N2/P3 studies, in particular the Maguire et al. studies from which the two tasks were adopted, and (2) the consistency with which N2 and P3 were observed across participants at each electrode location. We averaged mean amplitude and peak latency across the six electrodes in the frontal and fronto-central scalp regions for examining N2 and across the nine electrodes in the fronto-central, central, and centro-parietal scalp regions for examining P3.
Figure 1. Electrodes used for ERP analysis.
Electrodes used for N2 analysis (enclosed by solid lines) and P3 analysis (enclosed by dotted lines). Six electrodes were included for N2 analysis over the frontal (F1, Fz, F2) and fronto-central (FC1, FCz, FC2) scalp sites. Nine electrodes were included for P3 analysis over the fronto-central (FC1, FCz, FC2), central (C1, Cz, C2), and centro-parietal (CP1, CPz, CP2) scalp sites.
Statistical Analysis
A standard general linear model (GLM) was applied to each behavioral and ERP outcome measure to assess the effects of response type (go/nogo), task (SiC/ObA), and age group (young/old), as well as all second- and third-order interactions among these experimental factors on the means. Young adult data obtained from the UTD and UIUC undergraduate students were compared statistically. No behavioral or ERP differences (p > .05) were observed between the UTD and UIUC groups; hence data from these sites were merged into one young adult data set for all analyses.
Behavioral outcome measures included RT and error rate. [Note: go and nogo errors are also referred to as omission errors (i.e., a subject incorrectly inhibits during go trials/misses) and commission errors (i.e., a subject fails to inhibit during nogo trials/false alarms), respectively.] Because RT was measured only in the go trials, the GLM for RT did not include response type (go/nogo). ERP outcome measures included peak latency and latency adjusted mean amplitude for N2 and P3.
The GLMs were implemented in SAS (Cary, NC), using the mixed model procedure with the Kenward-Roger degree-of-freedom method and default residual maximum likelihood estimation of variance components. For the ERP measures, combinations of each level of response type (go and nogo) and task (SiC and ObA) were applied to each subject. Consequently, the GLMs included subject as a random term to account for within- and between-subject sources of error variability. Additionally, due to the unequal number of go/nogo trials (160 versus 40 trials) and the subject-specific attrition rates for trials themselves, the variance of trial-averaged responses was unequal. Therefore, we employed weights in the GLMs for the ERP measures to take into account the unequal variances of subjects’ measured responses to each level of experimental factor. Weights were determined by the number of trials used for the calculation of each ERP measure (trial types separately including SiC-go, SiC-nogo, ObA-go, and ObA-nogo).
Primary interest was in the higher-order interactions from the GLMs of the ERP measures, because we hypothesized differential response-type means that depended on age and/or task. P-values reported in the Results section derive from t-statistics of contrasts of experimental factor means, including interaction contrasts.
RESULTS
Behavioral Data
The average RT for each age group (young/old) and task (SiC/ObA), as well as error rates for each response type (go/nogo), age group (young/old) and task (SiC/ObA), are listed in Table 1. Older adults had significantly slower mean RT compared to younger adults (407.7 ms vs. 330 ms, respectively, p < .001); the mean RT for ObA was significantly slower compared to SiC (410 ms vs. 327.8 ms, respectively, p < .001). The interaction was not significant (p > .1) (Table 2).
Table 1.
Results of task performance.
| Measures by task Mean (Standard Deviation) |
Younger | Older |
|---|---|---|
| SiC | ||
| Go RT (ms) | 293 (53) | 362 (64) |
| Go omission errors (%) | 2 (2.1) | 3.8 (4.3) |
| Nogo commission errors (%) | 11.8 (6.1) | 9.9 (7.2) |
| ObA | ||
| Go RT (ms) | 366 (73) | 454 (65) |
| Go omission errors (%) | 2.7 (2.5) | 7 (5.7) |
| Nogo commission errors (%) | 13.5 (9.4) | 10.8 (9.3) |
SiC: single cars task; ObA: object animal task.
Table 2.
Statistical results of task performance.
| Effects | Go-RT | Error Rates |
|---|---|---|
| Response type | N.A. | F(1,126) = 77.31, p < .0001** |
| Response type × group | N.A. | F(1,126) = 9.47, p = .003** |
| Task | F(1,42) = 118.5, p < .0001** | F(1,126) = 3.46, p = .065^ |
| Task × group | F(1,42) = 1.61, p = .21 | F(1,126) = 0.22, p = .64 |
| Response type × task | N.A. | F(1,126) = 0.13, p = .72 |
| Response type × task × group | N.A. | F(1,126) = 0.96, p = .33 |
| Group | F(1,42) = 19.25, p < .0001** | F(1,42) = 0.09, p = .76 |
p < .05
p < .01
trend.
N.A.: not applicable.
The error rate for ObA showed a trend to be higher than that for SiC (8.5% vs. 6.9%, respectively, p = .065), demonstrating that longer response times and higher error rates occur in the more difficult and more semantically involved task. Interestingly, error rates (i.e., omission and commission errors) in response types (i.e., go and nogo) depended on the age group. For example, older adults had a higher omission error rate compared to younger adults (5.4% vs. 2.4%, respectively, p < .003), but the older adults did not show a significant difference in commission error rate relative to younger adults (10.4% vs. 12.7%, respectively, p = .28). The 3% increase for omission errors and the non-significant difference for commission errors in the older group relative to the younger adults are explained as a significant interaction (group/response-type interaction, p = .003). All the test results, including both significant and non-significant ones, are reported in Table 2.
ERP Data
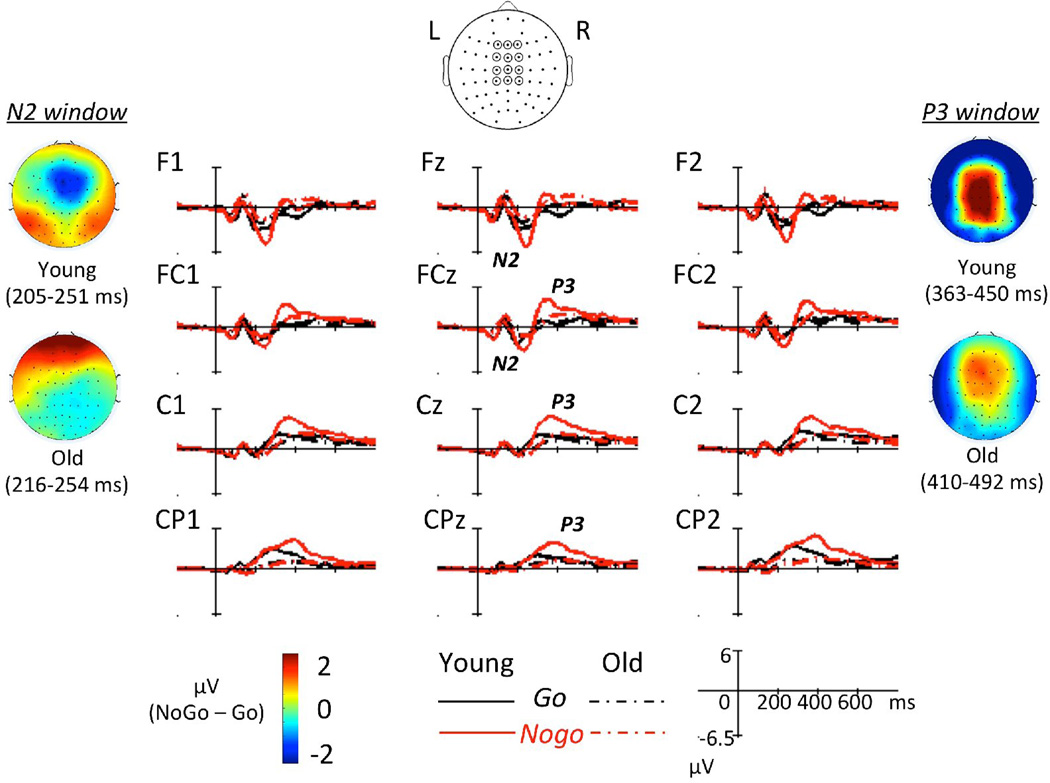
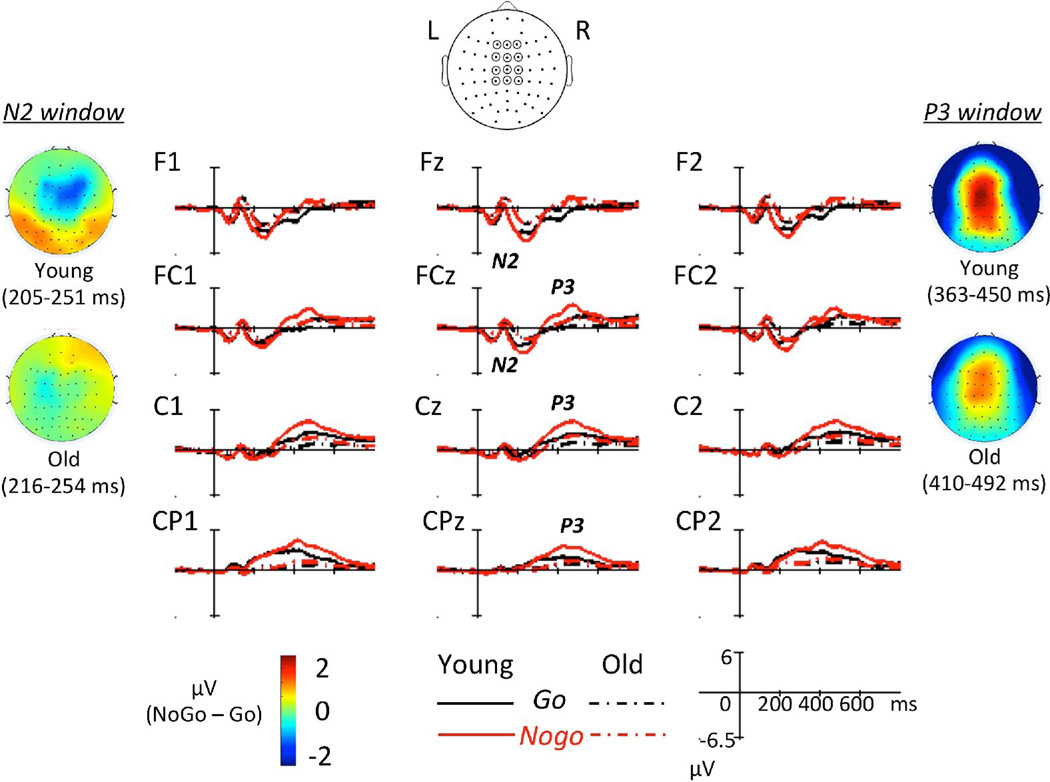
Grand average ERPs for each group and response type and N2/P3 topographies are illustrated in Figure 2 for the SiC task and in Figure 3 for the ObA task. The group latency-adjusted time windows for N2 mean amplitude were 205–251 ms in young and 216–254 ms in older adults. The group latency-adjusted time windows for P3 mean amplitude were 363–450 ms in young and 410–492 ms in older adults. N2 and P3 topographies illustrated in Figure 2 and Figure 3 correspond to these time windows. Additionally, averages (± SDs) for N2 latency, N2 amplitude, P3 latency, and P3 amplitude for each group, task, and response type are listed in Table 3.
Figure 2.
Group average ERPs with N2/P3 topography (Nogo – Go) in the SC task.
Figure 3.
Group average ERPs with N2/P3 topography (Nogo – Go) in the OA task.
Table 3.
Results of ERP measures.
| Measure by task Mean (Standard Deviation) |
Younger | Older |
|---|---|---|
| N2 latency (ms) | ||
| SiC | ||
| Go | 213 (26) | 224 (19) |
| Nogo | 239 (18) | 232 (20) |
| ObA | ||
| Go | 222 (27) | 239 (16) |
| Nogo | 236 (21) | 246 (21) |
| N2 amplitude (µV) | ||
| SiC | ||
| Go | −2.23 (2.3) | −1.6 (2.12) |
| Nogo | −3.61 (2.24) | −1.41 (1.72) |
| ObA | ||
| Go | −2.58 (2) | −1.8 (1.94) |
| Nogo | −3.53 (2.2) | −1.83 (2.18) |
| P3 latency (ms) | ||
| SiC | ||
| Go | 386 (58) | 415 (42) |
| Nogo | 368 (33) | 441 (42) |
| ObA | ||
| Go | 433 (49) | 463 (41) |
| Nogo | 440 (34) | 485 (40) |
| P3 amplitude (µV) | ||
| SiC | ||
| Go | 1.44 (1.44) | 1.09 (0.9) |
| Nogo | 3.63 (2.82) | 1.79 (1.03) |
| ObA | ||
| Go | 1.51 (1.98) | 0.7 (0.77) |
| Nogo | 3 (2.02) | 1.48 (1.27) |
SiC: single cars task; ObA: object animal task.
N2 Latency
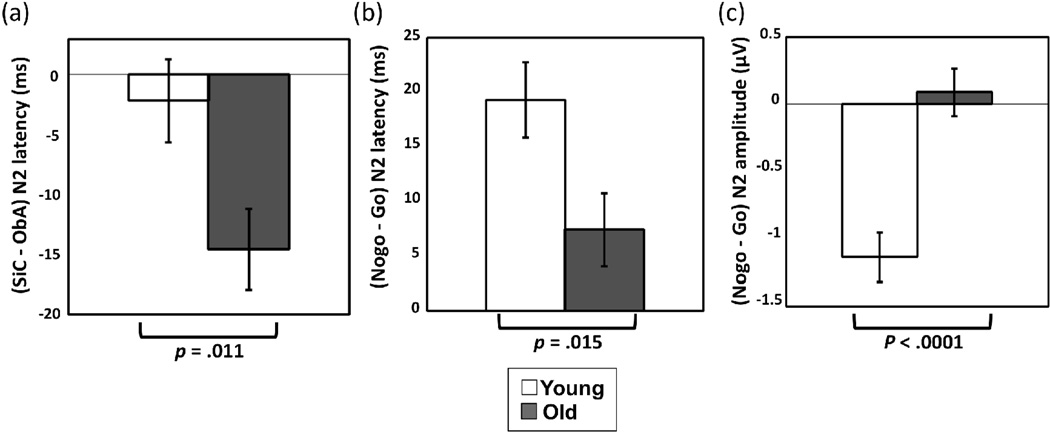
Mean N2 latency was significantly longer in nogo compared to go (238 ms vs. 225 ms, respectively, p < .0001) and longer in ObA compared to SiC (235 ms vs. 227 ms, respectively, p = .0007); however, these main effects were qualified by interactions. Longer N2 latency in ObA compared to SiC was found only in the older group (243 ms vs. 228 ms, respectively, p < .0001), but not in the younger group (229 ms vs. 226 ms, respectively, p = .53), contributing to a significant two-way group/task interaction (p = .011, Table 4, Figure 4a). Additionally, the older adults had longer N2 latency compared to younger adults in the ObA task (p = .024) but not in the SiC task (p = .76). Furthermore, a significant two-way group/response type interaction (p = .015, Table 4) was also observed. The difference between go-N2 and nogo-N2 latency in younger adults (217 vs. 237 ms, p < .0001) was larger in magnitude compared to older adults (232 vs. 239, p = .029) (Figure 4b). No other effects were significant (Table 4).
Table 4.
Statistical results of the ERP measures.
| Effects | N2-latency | N2-amplitude |
|---|---|---|
| Response type | F(1,126) = 30.64, p < .0001** | F(1,126) = 16.96, p < .0001** |
| Response type × group | F(1,126) = 6.07, p = .015* | F(1,126) = 22.97, p < .0001** |
| Task | F(1,126) = 12.22, p = .0007** | F(1,126) = 2.76, p = .099 |
| Task × group | F(1,126) = 6.68, p = .011* | F(1,126) = 0.36, p = .55 |
| Response type × task | F(1,126) = 1.19, p = .28 | F(1,126) = 0.1, p = .75 |
| Response type × task x×group | F(1,126) = 1.49, p = .22 | F(1,126) = 1.4, p = .24 |
| Group | F(1,47.1) = 2.02, p = .16 | F(1,43.3) = 4.65, p = .037* |
| Effects | P3-latency | P3-amplitude |
| Response type | F(1,125) = 1.84, p = .18 | F(1,126) = 75.13, p < .0001** |
| Response type × group | F(1,125) = 5.82, p = .017* | F(1,126) = 13.72, p = .0003** |
| Task | F(1,125) = 70.89, p < .0001** | F(1,126) = 4.35, p = .039* |
| Task × group | F(1,125) = 1.44, p = .23 | F(1,126) = 0.1, p = .76 |
| Response type × task | F(1,125) = 1.01, p = .32 | F(1,126) = 0.87, p = .35 |
| Response type × task × group | F(1,125) = 1.55, p = .22 | F(1,126) = 1.47, p = .23 |
| Group | F(1,50.4) = 15.18, p = .0003** | F(1,46) = 7.99, p = .007** |
p < .05
p < .01.
Figure 4.
Illustration of the interaction effects in N2 latency and amplitude
The bar graphs represent N2 difference [SiC–ObA] (a), N2 latency difference [nogo–go] (b), and N2amplitude difference [nogo–go] (c) across groups. The p-value at the bottom refers to the group × task interaction in (a) and the group × response type interaction in (b) and(c). Error bars represent standard errors.
N2 Amplitude
The magnitude of N2 amplitude was larger in younger compared to older adults (−2.99 µV vs. −1.67 µV, respectively, p = .037) and larger in nogo compared to go trials (−2.59 µV vs. −2.06 µV, respectively, p < .0001). Nevertheless, larger nogo-N2 compared to go-N2 amplitude (in absolute value) was found in the younger (−3.57 µV vs. −2.4 µV, respectively, p < .0001) but not in the older adults (−1.62 µV vs. −1.71 µV, respectively, p = .63), contributing to a significant two-way group/response type interaction (p < .0001, Table 4, Figure 4c). Additionally, nogo-N2 amplitude was larger in younger compared to older adults (p = .004), but no group difference in go-N2 amplitude was observed (p = .25). See Table 4 for all test results, including both significant and non-significant results.
P3 Latency
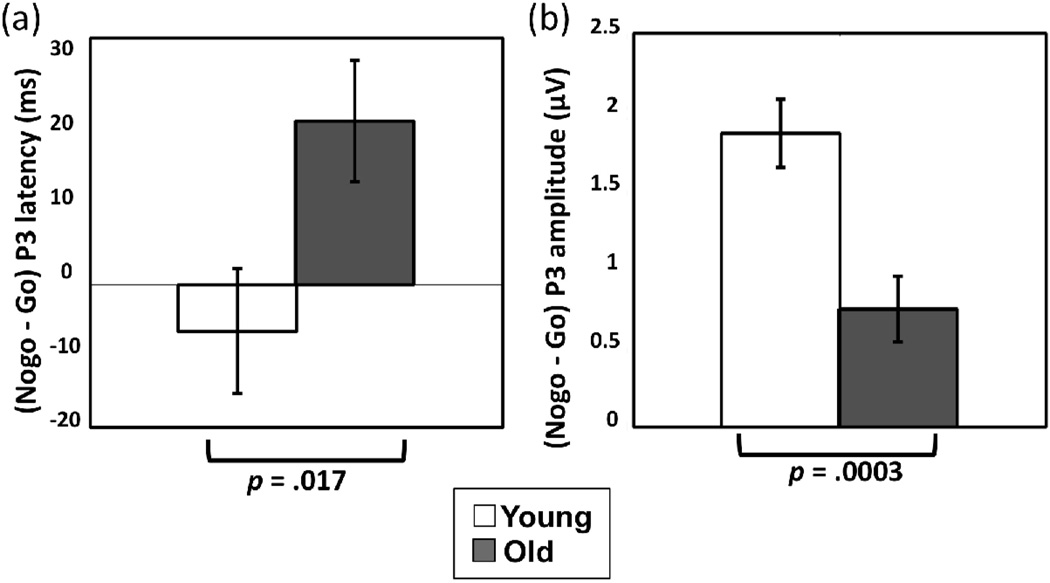
P3 latency was longer in ObA than in SiC (455 ms vs. 403 ms, respectively, p < .0001), and overall P3 latency was longer for older adults than younger adults (451 ms vs. 407 ms, respectively, p = .0003). A significant two-way group/response type interaction was observed (p = .017, Table 4), showing that whereas older adults had longer nogo-P3 than go-P3 latency (463 ms vs. 439 ms, p = .008), younger adults showed no significant difference between nogo and go (404 ms and 410 ms, respectively, p = .46) (Figure 5a). Additionally, the magnitude of group difference in P3 latency (old > young) was larger for nogo trials (a difference of 59 ms, p < .0001) compared to go trials (a difference of 29 ms, p = .011). No other effects were significant (Table 4).
Figure 5.
Illustration of the interaction effects in P3 latency and amplitude
The bar graphs represent P3 latency [nogo–go] (a) and P3 amplitude difference [nogo–go] (b) across groups. The p-value at the bottom refers to the group × response type interaction. Error bars represent standard errors.
P3 Amplitude
P3 amplitude was larger in SiC than ObA (1.99 µV vs. 1.67 µV, respectively, p = .039). While P3 amplitude was smaller in magnitude, overall, for older adults compared to younger adults (1.27 µV vs. 2.39 µV, respectively, p = .007) and larger in nogo than go (2.47 µV vs. 1.19 µV, respectively, p < .0001), these main effects were qualified by an interaction. The magnitude change between nogo and go responses for P3 amplitude was larger for younger adults compared to older adults (a difference of 1.87 µV vs. .75 µV, p < .0001 and p = .0005, respectively), contributing to a significant two-way group/response type interaction (p = .0003; Table 4, Figure 5b). Additionally, larger nogo-P3 amplitude was observed in younger compared to older adults (3.31 µV vs. 1.63 µV, respectively, p = .0004) with no significant group differences in go-P3 amplitude (1.47 µV vs. 0.9 µV, respectively, p = .15). No other effects were significant (Table 4).
DISCUSSION
We examined the effects of age on behavioral measures and known neural markers of cognitive control (the N2 and the P3 ERPs) across two go/nogo tasks that involved basic-level categorization (SiC) and superordinate-level categorization (ObA). Two major findings emerged: (i) significantly reduced N2 and P3 nogo amplitudes were observed in older compared to younger adults during both categorization tasks, despite comparable N2 and P3 go amplitudes in both groups, and (ii) N2 latency was modulated by task in older but not in younger adults, suggesting that categorization levels differentially impact young and old.
No age-related behavioral signs of impairment in cognitive control measured by the accuracy data on nogo trials were observed in older as compared to younger adults. That is, both groups had comparable commission error rates (false alarms) during nogo trials suggesting that both groups withheld their behavioral responses equally well. However, older adults had higher rates of omission errors (misses) during go trials despite longer reaction times compared to younger adults. Previous go/nogo studies that have found no differences in commission errors between young and old have not found age group differences in omission errors either; however, these studies were done using equal numbers of go and nogo trials (e.g., Falkenstein et al., 2002; Vallesi et al., 2009). Our rationale for using unequal trial distribution with 80% go trials and 20% nogo trials was to enhance the pre-potent response tendencies and to increase the demand for cognitive control to better estimate age-related differences. Most likely, our trial distribution manipulation did not yield age group differences in commission errors during nogo trials because the older adults in our study employed a conservative response strategy since our instructions emphasized both speed and accuracy. Older adults not only responded more slowly (prolonged RT), but also withheld button pushes more frequently (increased omission error rates) compared to younger adults in an attempt to avoid errors (Hsieh & Fang, 2012; Friedman, Kazmerski, & Fabiani, 1997). It is likely that the older adults continued to use this conservative response strategy (withholding responses) during nogo trials as well, thereby minimizing the rate of commission errors (false alarms) and masking behavioral differences between groups.
Unlike the accuracy data for nogo trials, the ERP data showed effects of age on N2 amplitude, which was observed as a group by response type interaction. In younger adults, nogo- N2 amplitude was larger than go-N2 amplitude, suggesting increased cognitive effort/control in withholding the pre-potent tendency to respond with a button push, a typical finding in go/nogo studies (Benikos, Johnstone, & Roodenrys, 2013; Beste et al., 2010; Falkenstein et al., 2002; Gajewski & Falkenstein, 2013; Maguire et al., 2009). In contrast, older adults appeared to process both go and nogo trials with comparable effort, resulting in a lack of differences between go-N2 and nogo-N2 amplitudes, which is contrary to our expectations. However, despite the fact that older adults had significantly lower nogo-N2 amplitudes compared to younger adults, both groups had comparable go-N2 amplitudes. These findings might be interpreted as weakening of neural processing related to response inhibition with age (Falkenstein, 2006; also see Folstein & Van Petten, 2008) when the demand for inhibitory response is increased, as would be the case with frequent gos (80%) compared to less frequent nogos (20%). Alternatively, the lack of difference between go-N2 and nogo-N2 in older adults might reflect weakened conflict monitoring in general (Dronkers & Van Boxtel, 2004; Nieuwenhuis et al., 2003). Studies have found that N2 (particularly in frontocentral electrodes) is larger for infrequent compared to frequent trials during the go/nogo tasks (Eimer, 1993; Jodo & Kayama, 1992; Kok, 1986), even when the infrequent trials do not require inhibition (Donkers & van Boxtel, 2004; Nieuwehuis et al., 2003), suggesting its relationship to conflict monitoring. While our N2 amplitude findings support changes in neural processing related to cognitive control with age in general, whether these results are specifically due to weakened cognitive effort during inhibition trials i.e., a response inhibition deficit, or due to weakened conflict monitoring of infrequent trials in general, which happens to be nogo trials in the current study, needs to be verified in future studies using an equal number of go and nogo trials.
Similar to N2 amplitude, an interaction was observed between age and response type for both P3 amplitude and latency. Older adults had reduced nogo-P3 amplitude compared to younger adults, but both groups had comparable go-P3 amplitudes. Furthermore, in older adults, smaller differences between go and nogo P3 amplitudes were observed compared to younger adults. Since go-P3 amplitudes were comparable between groups, reduced nogo-P3 amplitudes observed in older adults compared to younger adults might reflect impaired evaluation or attentional allocation during nogo trials (Donchin et al., 1986; Polich & Herbst, 2000) or impaired monitoring of the outcome during nogo trials (e.g., Falkenstein et al., 1999) or both. Given that we used latency adjusted time windows to estimate mean amplitude, the amplitude differences observed between conditions and groups cannot be explained by latency variability across groups. The P3 latency findings also suggest more prominent age-related changes in processing during nogo trials. Older adults had longer nogo-P3 latency compared to go-P3 latency, whereas younger adults had comparable go and nogo P3 latencies. Furthermore, the nogo-P3 latency difference between groups was larger in magnitude compared to the go-P3 latency group difference. Overall, reduced nogo-P3 amplitude and prolonged nogo-P3 latency in older adults are consistent with the observations of previous studies (Pfefferbaum et al., 1985; Pfefferbaum & Ford, 1988; Pfefferbaum et al., 1984; Falkenstein et al., 2002) and support slower and weakened neural processing related to cognitive control with age (Fabiani, Low, Wee, Sable, & Gratton, 2006; Gazzaley, Cooney, Rissman, & D’Esposito, 2005; Hasher & Zacks, 1988).
In addition to age and response type interactions, differential age effects of task were observed in N2 latency. Older adults had longer N2 latency for ObA compared to SiC, whereas the N2 latencies across tasks were comparable in young adults. Furthermore, the N2 latency was longer in the older compared to younger adults for ObA but not in SiC. Basic categorization is considered to be an entry point following sensory and perceptual processing, when the perceived stimulus first makes contact with object representations stored in memory, well before the object is categorized at the superordinate level (Jolicoeur et al., 1984). Older adults seem to be able to complete the initial sensory and perceptual processing related to the SiC task involving basic categorization similar to younger adults, but semantic processing demands placed by the ObA task involving superordinate categorization appears to slow processing in general, resulting in prolonged N2 latency during both the go and nogo trials. Whether prolonged N2 latency reflects a deficit in coping with the demands of semantic processing in the ObA task, or is related to strategy preference towards meaning-based processing of information with age (e.g., Radvansky & Dijkstra, 2007), cannot be disentangled. Also, the extent to which these findings are specific to semantic categorization levels as opposed to a function of task complexity in general is debatable since the ObA task is inherently more complex compared to the SiC task (Jolicoeur et al., 1984; Large et al., 2004; Murphy & Brownell, 1985; Murphy & Lassaline, 1997; Rosch et al., 1976).
Apart from the unique N2 latency task effects observed in the older group, both groups exhibited similar task effects in accuracy, reaction time, P3 amplitude, and P3 latency. Behavioral response in the basic categorization task (SiC) was faster and more accurate compared to the superordinate categorization task (ObA) in both age groups, supporting numerous previously conducted behavioral studies (Grill-Spector & Kanwisher, 1993; Johnson & Mervis, 1997; Murphy & Brownell, 1985; Murphy & Lassaline, 1997; Tanaka & Taylor, 1991) with the exception of those that have examined ultra-rapid object categorization (e.g., Thorpe et al., 1996; Large et al., 2004; Rogers & Patterson, 2007). In our study, each stimulus was presented for 300 ms, allowing individuals enough time to process perceptual differences first, as opposed to ultra-rapid stimulus exposure studies that have observed entry-level shift (from basic-level to superordinate-level advantage) (Thorpe et al., 1996; Fabre-Thorpe et al., 2001; Macé et al., 2009; Rogers & Patterson, 2007). In terms of ERP, shorter P3 latency and larger P3 amplitude were observed in SiC compared to ObA in both groups irrespective of the response type. While our categorization effects on P3 are similar to that observed in the Maguire et al., (2009) study and a study conducted by Schapkin, Falkenstein, Marks, & Griefahn (2006), our N2 latency findings in older as compared to younger adults provide unique insights into the differential age effects of categorization levels on neural processing during tasks that require varying levels of cognitive control.
Conclusion
Our findings support the view that neural processing related to cognitive control is affected by response type and task with age during visual object categorization. Although older adults were able to withhold their behavioral responses with the same accuracy as younger adults across both tasks, age-related alterations in neurophysiological markers related to cognitive control were observed in both early (nogo-N2 amplitude) and later (nogo-P3 latency and amplitude) stages of processing in older adults. Differential effects of categorization levels were observed in older adults in early stages of processing (N2 latency), whereas both groups exhibited similar task effects in the later stages of processing (P3). Given that in real life situations the environment is not as well controlled (e.g., more distractors and noise and greater demand on conceptual processing) as in the laboratory setting in which these data were acquired, we speculate that older adults will exhibit more obvious behavioral manifestations of weakened cognitive control in situations that demand more advanced semantic processing. Not only do our findings advance knowledge of underlying changes in neural mechanisms associated with normal aging in terms of cognitive control and semantic categorization, but they also have future applications in studying processing related to response conflict and response inhibition in pathological aging that harbingers certain neurodegenerative disorders.
Highlights.
We studied age effects on cognitive control during semantic categorization
Examined ERPs related to two visual go/nogo tasks that varied in categorization levels
Nogo-N2 and nogo-P3 amplitudes were reduced in older compared to younger adults
N2 latency showed differential age effects of categorization
Acknowledgments
The authors thank Dr. Elizabeth K. Bartz, Rajen Patel, Monique Salinas, Erin Venza, Audette Rackley, Claire Gardner, Amy Strohman, Ewa Nawacki, Natalie Gannon, and Lukasz Pazdan for their invaluable assistance in data collection.
Funding Source: This work was funded by grants from the National Institute of Health (RC1-AG035954), the RGK Foundation, and the Berman Research Initiative at the University of Texas at Dallas and CHAD pilot grant at the University of Illinois at Urbana-Champaign. The funding sources had no involvement in study design, data collection, analysis and interpretation of data or in the writing of the report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benikos N, Johnstone SJ, Roodenrys SJ. Varying task difficulty in the Go/Nogo task: the effects of inhibitory control, arousal, and perceived effort on ERP components. Int J Psychophysiol. 2013;87(3):262–272. doi: 10.1016/j.ijpsycho.2012.08.005. http://dx.doi.org/10.1016/j.ijpsycho.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Beste C, Willemssen R, Saft C, Falkenstein M. Response inhibition subprocesses and dopaminergic pathways: basal ganglia disease effects. Neuropsychologia. 2010;48(2):366–373. doi: 10.1016/j.neuropsychologia.2009.09.023. [DOI] [PubMed] [Google Scholar]
- Biederman I, Gerhardstein PC. Recognizing depth-rotated objects: evidence and conditions for three-dimensional viewpoint invariance. J Exp Psychol Hum Percept Perform. 1993;19(6):1162–1182. doi: 10.1037//0096-1523.19.6.1162. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108(3):624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8(12):539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Keys BA, Carter CS, Cohen JD, Kaye JA, Reed BR. Context processing in older adults: evidence for a theory relating cognitive control to neurobiology in healthy aging. J Exp Psychol Gen. 2001;130(4):746–763. [PubMed] [Google Scholar]
- Chiang H-S, Motes MA, Mudar RA, Rao NK, Mansinghani S, Brier MR, Hart J. Semantic processing and response inhibition. Neuroreport. 2013;24(16):889–893. doi: 10.1097/WNR.0000000000000014. [DOI] [PubMed] [Google Scholar]
- Cicirelli VG. Categorization behavior in aging subjects. J Gerontol. 1976;31(6):676–680. doi: 10.1093/geronj/31.6.676. [DOI] [PubMed] [Google Scholar]
- Donchin E, Karis D, Bashore T, Coles M, Gratton G. Cognitive psychophysiology and human information processing. In: Coles MGH, Donchin E, Porges S, editors. Psychophysiology: Systems, Processes, and Applications. New York: Guilford Press; 1986. [Google Scholar]
- Donkers FCL, van Boxtel GJM. The N2 in go/no-go tasks reflects conflict monitoring not response inhibition. Brain Cogn. 2004;56(2):165–176. doi: 10.1016/j.bandc.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Eimer M. Effects of attention and stimulus probability on ERPs in a Go/Nogo task. Biol Psychol. 1993;35(2):123–138. doi: 10.1016/0301-0511(93)90009-w. [DOI] [PubMed] [Google Scholar]
- Fabiani M, Low KA, Wee E, Sable JJ, Gratton G. Reduced suppression or labile memory? Mechanisms of inefficient filtering of irrelevant information in older adults. J Cogn Neurosci. 2006;18(4):637–650. doi: 10.1162/jocn.2006.18.4.637. [DOI] [PubMed] [Google Scholar]
- Fabre-Thorpe M, Delorme A, Marlot C, Thorpe S. A limit to the speed of processing in ultra-rapid visual categorization of novel natural scenes. J CognNeurosci. 2001;13(2):171–180. doi: 10.1162/089892901564234. [DOI] [PubMed] [Google Scholar]
- Falkenstein M. Inhibition, conflict and the Nogo-N2. Clin Neurophysiol. 2006;117(8):1638–1640. doi: 10.1016/j.clinph.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Hohnsbein J. ERP components in Go/Nogo tasks and their relation to inhibition. Acta Psychol (Amst) 1999;101(2–3):267–291. doi: 10.1016/s0001-6918(99)00008-6. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Hohnsbein J. Inhibition-related ERP components: variation with modality, age, and time-on-task. Journal of Psychophysiology. 2002;16(3):167–175. [Google Scholar]
- Ferree TC, Brier MR, Hart J, Jr, Kraut MA. Space-time-frequency analysis of EEG data using within-subject statistical tests followed by sequential PCA. Neuroimage. 2009;45(1):109–121. doi: 10.1016/j.neuroimage.2008.09.020. http://dx.doi.org/10.1016/j.neuroimage.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Folstein JR, Van Petten C. Influence of cognitive control and mismatch on theN2 component of the ERP: a review. Psychophysiology. 2008;45(1):152–170. doi: 10.1111/j.1469-8986.2007.00602.x. http://dx.doi.org/10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D, Hamberger M, Ritter W. Event-related potentials as indicators of repetition priming in young and older adults: amplitude, duration, and scalp distribution. Psychol Aging. 1993;8(1):120–125. doi: 10.1037//0882-7974.8.1.120. http://dx.doi.org/10.1037/0882-7974.8.1.120. [DOI] [PubMed] [Google Scholar]
- Friedman D, Kazmerski V, Fabiani M. An overview of age-related changes in the scalp distribution of P3b. Electroencephalogr Clin Neurophysiol. 1997;104(6):498–513. doi: 10.1016/s0168-5597(97)00036-1. [DOI] [PubMed] [Google Scholar]
- Gajewski PD, Falkenstein M. Effects of task complexity on ERP components in Go/Nogo tasks. Int J Psychophysiol. 2013;87(3):273–278. doi: 10.1016/j.ijpsycho.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, Rissman J, D'Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nat Neurosci. 2005;8(10):1298–1300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kanwisher N. Visual recognition: as soon as you know it is there, you know what it is. Psychol Sci. 1993;16(2):152–160. doi: 10.1111/j.0956-7976.2005.00796.x. http://dx.doi.org/10.1111/j.0956-7976.2005.00796.x. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Welsh RC, Boduroglu A, Park DC. Cultural differences in neural function associated with object processing. Cogn Affect Behav Neurosci. 2006;6(2):102–109. doi: 10.3758/cabn.6.2.102. http://dx.doi.org/10.3758/CABN.6.2.102. [DOI] [PubMed] [Google Scholar]
- Hasher L, Stoltzfus ER, Zacks RT, Rypma B. Age and inhibition. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1991;17(1):163–169. doi: 10.1037//0278-7393.17.1.163. [DOI] [PubMed] [Google Scholar]
- Hasher L, Zacks RT. Working memory, comprehension, and aging: a review and a new view. In: Bower GH, editor. The psychology of learning and motivation. Vol. 22. New York: Academic Press; 1988. pp. 193–225. [Google Scholar]
- Hsieh S, Fang W. Elderly adults through compensatory responses can be just as capable as young adults in inhibiting the flanker influence. Biol Psychol. 2012;90(2):113–126. doi: 10.1016/j.biopsycho.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Huster RJ, Enriquez-Geppert S, Lavallee CF, Falkenstein M, Herrmann CS. Electroencephalography of response inhibition tasks: functional networks and cognitive contributions. Int J Psychophysiol. 2013;87(3):217–233. doi: 10.1016/j.ijpsycho.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Inzlicht M, Bartholow BD, Hirsh JB. Emotional foundations of cognitive control. Trends in Cognitive Sciences. 2015;19(3):126–132. doi: 10.1016/j.tics.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodo E, Kayama Y. Relation of a negative ERP component to response inhibition in a Go/No-go task. Electroencephalogr Clin Neurophysiol. 1992;82(6):477–482. doi: 10.1016/0013-4694(92)90054-l. [DOI] [PubMed] [Google Scholar]
- Johnson KE, Mervis CB. Effects of varying levels of expertise on the basic level of categorization. J Exp Psychol Gen. 1997;126(3):248–277. doi: 10.1037//0096-3445.126.3.248. [DOI] [PubMed] [Google Scholar]
- Jolicoeur P, Gluck MA, Kosslyn SM. Pictures and names: making the connection. Cogn Psychol. 1984;16(2):243–275. doi: 10.1016/0010-0285(84)90009-4. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Hasher L, Stoltzfus ER, Zacks RT, Connelly SL. Inhibitory attentional mechanisms and aging. Psychol Aging. 1994;9(1):103–112. doi: 10.1037//0882-7974.9.1.103. [DOI] [PubMed] [Google Scholar]
- Kincses TZ, Chadaide Z, Varga ET, Antal A, Paulus W. Task-related temporal and topographical changes of cortical activity during ultra-rapid visual categorization. Brain Res. 2006;1112(1):191–200. doi: 10.1016/j.brainres.2006.07.044. [DOI] [PubMed] [Google Scholar]
- Kok A. Effects of degradation of visual stimulation on components of the event-related potential (ERP) in go/nogo reaction tasks. Biol Psychol. 1986;23(1):21–38. doi: 10.1016/0301-0511(86)90087-6. [DOI] [PubMed] [Google Scholar]
- Kok A. Varieties of inhibition: manifestations in cognition, event-related potentials and aging. Acta Psychol (Amst) 1999;101(2-3):129–158. doi: 10.1016/s0001-6918(99)00003-7. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Humphrey DG, Larish JF, Logan GD, Strayer DL. Aging and inhibition: beyond a unitary view of inhibitory processing in attention. Psychol Aging. 1994;9(4):491–512. [PubMed] [Google Scholar]
- Large M-E, Kiss I, McMullen PA. Electrophysiological correlates of object categorization: back to basics. Brain Res Cogn Brain Res. 2004;20(3):415–426. doi: 10.1016/j.cogbrainres.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Long C, Liu Q, Qiu J, Shen X, Li S, Li H. Neural signs of flexible categorization: evidence from the flexibility of inclusion of humans in animal/non-animal categorization. Brain Res. 2010;1337:64–73. doi: 10.1016/j.brainres.2010.04.023. [DOI] [PubMed] [Google Scholar]
- Mace MJM, Joubert OR, Nespoulous J-L, Fabre-Thorpe M. The time-course of visual categorizations: you spot the animal faster than the bird. PLoS One. 2009;4(6) doi: 10.1371/journal.pone.0005927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack ML, Palmeri TJ. The timing of visual object categorization. Front Psychol. 2011;2:165–165. doi: 10.3389/fpsyg.2011.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire MJ, Brier MR, Moore PS, Ferree TC, Ray D, Mostofsky S, Kraut MA. The influence of perceptual and semantic categorization on inhibitory processing as measured by the N2-P3 response. Brain Cogn. 2009;71(3):196–203. doi: 10.1016/j.bandc.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire MJ, White J, Brier MR. How semantic categorization influences inhibitory processing in middle-childhood: an event related potentials study. Brain Cogn. 2011;76(1):77–86. doi: 10.1016/j.bandc.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowd JM. Inhibition in attention and aging. J Gerontol B Psychol Sci Soc Sci. 1997;52(6):265–273. doi: 10.1093/geronb/52b.6.p265. [DOI] [PubMed] [Google Scholar]
- Mervis CB, Rosch E. Categorization of natural objects. Annual Review of Psychology. 1981;32(1):89–115. [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cogn Psychol. 2000;41(1):49–100. doi: 10.1006/cogp.1999.0734. doi: http://dx.doi.org/10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Murphy GL, Brownell HH. Category differentiation in object recognition: typicality constraints on the basic category advantage. J Exp Psychol Learn Mem Cogn. 1985;11(1):70–84. doi: 10.1037//0278-7393.11.1.70. [DOI] [PubMed] [Google Scholar]
- Murphy GL, Lassaline ME. Hierarchical structure in concepts and the basic level of categorization. In: Shanks KLDR, editor. Knowledge, concepts and categories. Cambridge, MA, US: The MIT Press; 1997. pp. 93–131. [Google Scholar]
- Nieuwenhuis S, Holroyd CB, Mol N, Coles MG. Reinforcement-related brain potentials from medial frontal cortex: origins and functional significance. Neurosci Biobehav Rev. 2004;28(4):441–448. doi: 10.1016/j.neubiorev.2004.05.003. http://dx.doi.org/10.1016/j.neubiorev.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, van den Wildenberg W, Ridderinkhof KR. Electrophysiological correlates of anterior cingulate function in a go/no-go task: effects of response conflict and trial type frequency. Cogn Affect Behav Neurosci. 2003;3(1):17–26. doi: 10.3758/cabn.3.1.17. [DOI] [PubMed] [Google Scholar]
- Paxton JL, Barch DM, Racine CA, Braver TS. Cognitive control, goal maintenance, and prefrontal function in healthy aging. Cereb Cortex. 2008;18(5):1010–1028. doi: 10.1093/cercor/bhm135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM. ERPs to stimuli requiring response production and inhibition: effects of age, probability and visual noise. Electroencephalogr Clin Neurophysiol. 1988;71(1):55–63. doi: 10.1016/0168-5597(88)90019-6. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, Weller BJ, Kopell BS. ERPs to response production and inhibition. Electroencephalogr Clin Neurophysiol. 1985;60(5):423–434. doi: 10.1016/0013-4694(85)91017-x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, Wenegrat BG, Roth WT, Kopell BS. Clinical application of the P3 component of event-related potentials. I. Normal aging. Electroencephalogr Clin Neurophysiol. 1984;59(2):85–103. doi: 10.1016/0168-5597(84)90026-1. [DOI] [PubMed] [Google Scholar]
- Picton TW, Bentin S, Berg P, Donchin E, Hillyard SA, Johnson R, Jr, et al. Guide-lines for using human event-related potentials to study cognition: recording standards and publication criteria. Psychophysiology. 2000;37(2):127–152. [PubMed] [Google Scholar]
- Polich J, Herbst KL. P300 as a clinical assay: rationale, evaluation, and findings. Int J Psychophysiol. 2000;38(1):3–19. doi: 10.1016/s0167-8760(00)00127-6. [DOI] [PubMed] [Google Scholar]
- Radvansky GA, Dijkstra K. Aging and situation model processing. Psychon Bull Rev. 2007;14(6):1027–1042. doi: 10.3758/bf03193088. [DOI] [PubMed] [Google Scholar]
- Raposo A, Mendes M, Marques JF. The hierarchical organization of semantic memory: executive function in the processing of superordinate concepts. Neuroimage. 2012;59(2):1870–1878. doi: 10.1016/j.neuroimage.2011.08.072. [DOI] [PubMed] [Google Scholar]
- Rogers TT, Patterson K. Object categorization: reversals and explanations of the basic-level advantage. J Exp Psychol Gen. 2007;136(3):451–469. doi: 10.1037/0096-3445.136.3.451. [DOI] [PubMed] [Google Scholar]
- Rosch E, Mervis CB, Gray WD, Johnson DM, Boyes-Braem P. Basic objects in natural categories. Cogn Psychol. 1976;8(3):382–439. doi: http://dx.doi.org/10.1016/0010-0285(76)90013-X. [Google Scholar]
- Schapkin SA, Falkenstein M, Marks A, Griefahn B. Executive brain functions after exposure to nocturnal traffic noise: effects of task difficulty and sleep quality. Eur J Appl Physiol. 2006;96(6):693–702. doi: 10.1007/s00421-005-0049-9. [DOI] [PubMed] [Google Scholar]
- Schmitt FA, Murphy MD, Sanders RE. Training older adult free recall rehearsal strategies. J Gerontol. 1981;36(3):329–337. doi: 10.1093/geronj/36.3.329. [DOI] [PubMed] [Google Scholar]
- Scott LS, Tanaka JW, Sheinberg DL, Curran T. A reevaluation of the electrophysiological correlates of expert object processing. J Cogn Neurosci. 2006;18(9):1453–1465. doi: 10.1162/jocn.2006.18.9.1453. [DOI] [PubMed] [Google Scholar]
- Simson R, Vaughan HG, Ritter W. The scalp topography of potentials in auditory and visual discrimination tasks. Electroencephalography & Clinical Neurophysiology. 1977;42(4):528–535. doi: 10.1016/0013-4694(77)90216-4. [DOI] [PubMed] [Google Scholar]
- Smith JL, Johnstone SJ, Barry RJ. Movement-related potentials in the Go/NoGo task: the P3 reflects both cognitive and motor inhibition. Clini Neurophysiol. 2008;119(3):704–714. doi: 10.1016/j.clinph.2007.11.042. [DOI] [PubMed] [Google Scholar]
- Smith JL, Johnstone SJ, Barry RJ. Response priming in the Go/NoGo task: the N2reflects neither inhibition nor conflict. Clini Neurophysiol. 2007;118(2):343–355. doi: 10.1016/j.clinph.2006.09.027. [PubMed:17140848] [DOI] [PubMed] [Google Scholar]
- Tachibana H, Aragane K, Sugita M. Age-related changes in event-related potentials in visual discrimination tasks. Electroencephalogr Clin Neurophysiol. 1996;100(4):299–309. doi: 10.1016/0168-5597(96)95108-4. [DOI] [PubMed] [Google Scholar]
- Tanaka JW, Taylor M. Object categories and expertise: Is the basic level in the eye of the beholder? Cogn Psychol. 1991;23(3):457–482. [Google Scholar]
- Thorpe S, Fize D, Marlot C. Speed of processing in the human visual system. Nature. 1996;381(6582):520–522. doi: 10.1038/381520a0. http://dx.doi.org/10.1038/381520a0. [DOI] [PubMed] [Google Scholar]
- Vallesi A. Targets and non-targets in the aging brain: A go/nogo event-related potential study. Neurosci Lett. 2011;487(3):313–317. doi: 10.1016/j.neulet.2010.10.046. [DOI] [PubMed] [Google Scholar]
- Vallesi A, Stuss DT, McIntosh AR, Picton TW. Age-related differences in processing irrelevant information: evidence from event-related potentials. Neuropsychologia. 2009;47(2):577–586. doi: 10.1016/j.neuropsychologia.2008.10.018. [DOI] [PubMed] [Google Scholar]