Summary
The two major lineages of classical dendritic cells (cDCs) express and require either IRF8 or IRF4 transcription factors for their development and function. IRF8-dependent cDCs promote anti-viral and T-helper 1 (Th1) cell responses, whereas IRF4-expressing cDCs have been implicated in controlling both Th2 and Th17 cell responses. Here, we have provided evidence that Kruppel-like factor 4 (Klf4) is required in IRF4-expressing cDCs to promote Th2 but not Th17 cell responses in vivo. Conditional Klf4 deletion within cDCs impaired Th2 cell responses during Schistosoma mansoni infection, Schistosoma egg antigen (SEA) immunization, and house dust mite challenge (HDM), without affecting cytotoxic T lymphocyte (CTL), Th1 and Th17 cell responses to herpes simplex virus, Toxoplasma gondii and Citrobacter rodentium infections. Further, Klf4 deletion reduced IRF4 expression in pre-cDCs and resulted in selective loss of IRF4-expressing cDCs subsets in several tissues. These results indicate that Klf4 guides a transcriptional program promoting IRF4-expressing cDCs heterogeneity.
Introduction
Conventional dendritic cells (cDCs) are professional antigen presenting cells that play a key role in shaping appropriate immune responses (Banchereau and Steinman, 1998; Merad et al., 2013; Satpathy et al., 2012b; Mildner and Jung, 2014). Several transcription factors have been implicated in cDCs development, but basis for specification and commitment of cDC subsets is still incompletely understood (Belz and Nutt, 2012; Murphy, 2013). One major subset of cDCs identified by the expression of CD8α in spleen, and CD24 or CD103 in the periphery, requires the transcription factors IRF8 (Hambleton et al., 2011; Tailor et al., 2008), BATF3 (Edelson et al., 2010; Hildner et al., 2008; Ginhoux et al., 2009), NFIL3 (Kashiwada et al., 2011) and ID2 (Hacker et al., 2003; Spits et al., 2000). Selective loss of CD8α+ and CD103+ cDCs in Batf3−/− mice demonstrated the specialization of this cDC subset in promoting anti-viral immunity, tumor rejection and protection against Toxoplasma gondii infection (Mashayekhi et al., 2011; Hildner et al., 2008; Tussiwand et al., 2012; Pinto et al., 2011; Torti et al., 2011). The second major branch of cDCs is characterized by the expression of IRF4 and CD11b and is developmentally impacted by the transcription factors RelB, Traf6, Notch2, Irf2 and Irf4 (Mildner and Jung, 2014).
The function of CD11b+ cDCs in controlling different classes of immune responses has been recently examined (Lewis et al., 2011; Satpathy et al., 2013; Persson et al., 2013; Schlitzer et al., 2013; Williams et al., 2013; Gao et al., 2013; Kumamoto et al., 2013; Zhou et al., 2014). Conditional Notch2 deletion in cDCs impaired development of CD11b+ cDCs expressing CD4 and the endothelial cell-selective adhesion molecule (ESAM) (Lewis et al., 2011; Satpathy et al., 2013). These mice are susceptible to infection with Citrobacter rodentium, resulting from a requirement for interleukin-23 (IL-23) production by Notch2-dependent cDCs early during infection (Satpathy et al., 2013). Similarly, conditional deletion of Irf4 in cDCs causes a reduction in the numbers of CD11b+ cDCs, and reduced IL-23 production leading to impaired Th17 cell development in both lung and intestine (Persson et al., 2013; Schlitzer et al., 2013). Consistently, mice lacking IRF4 expression in cDCs are therefore susceptible to pulmonary infection with Aspergillus fumigatus (Schlitzer et al., 2013). Subsequent studies showed that Irf4-dependent CD11b+ cDCs are involved in Th2 cell responses in skin and lung (Gao et al., 2013; Kumamoto et al., 2013; Williams et al., 2013; Zhou et al., 2014). However, other reports have demonstrated that IRF4 acts in both migration of CD11b+ cDCs (Bajana et al., 2012) and expression of major histocompatibility complex (MHC)-II and co-stimulatory molecules by cDCs (Vander et al., 2014). As a result, it is unclear whether there is a specific cDC subset dedicated to Th2 cell priming that is selectively dependent on IRF4, or whether IRF4 acts in all CD11b+ cDCs, affecting their migration and maturation in both Th17 and Th2 cell responses.
We identified Kruppel like factor 4 (Klf4) as a potential candidate for regulating the development of the IRF4-expressing CD11b+ cDCs. Klf4 can act as a repressor or activator of transcription and regulates development in several epithelial tissues, including skin, lung, and intestine (Segre et al., 1999; Dang et al., 2000; Katz et al., 2002; Dang et al., 2000; Ghaleb et al., 2005; Feinberg et al., 2007; Alder et al., 2008; Zheng et al., 2009; McConnell and Yang, 2010). In hematopoietic cells, Klf4 is expressed on myeloid cells, is required for monocyte development (Feinberg et al., 2007; Alder et al., 2008; Kurotaki et al., 2013) as well as for in vitro M2 macrophage polarization (Feinberg et al., 2007; Kurotaki et al., 2013; Terry and Miller, 2014). Klf4 conditional deficient mice have reduced CD11b+ cDCs in spleen, however the nature of the defect was not further analyzed with respect to cDC subsets or function (Park et al., 2012). Here we showed that Klf4 is required within IRF4-expressing cDC subsets for normal priming of Th2 cell responses. Our results indicated that the IRF4-expressing cDC lineage is functionally heterogeneous, with Klf4 promoting a DC transcriptional program controlling Th2 cell responses.
Results
Conditional deletion of Klf4 alters development of IRF4-expressing pre-cDCs
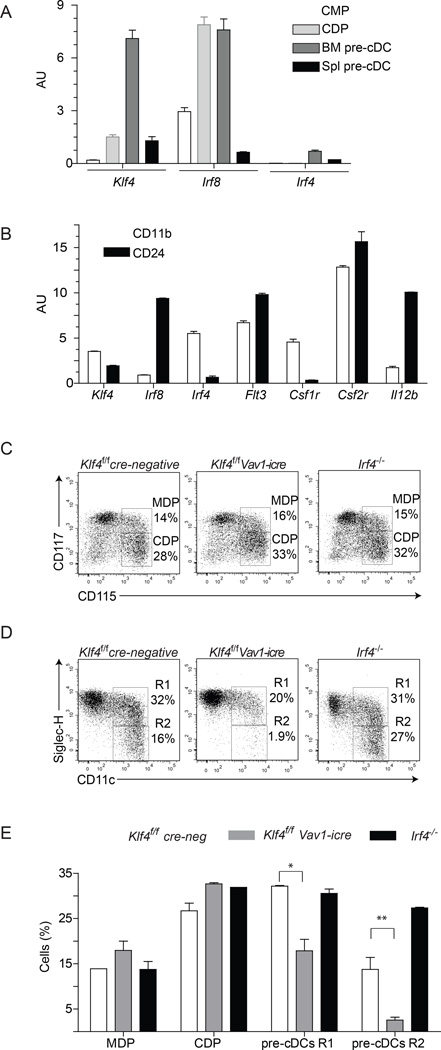
Klf4 expression was transiently up-regulated at the bone marrow (BM) pre-cDC stage, while Irf8 was induced in common DC progenitors (CDPs) (Liu et al., 2009) (Figure 1A). Klf4 expression within mature splenic cDC subsets was reduced compared to Irf8 and Irf4 (Figure 1B). We crossed the Klf4fl/fl allele (Katz et al., 2002) onto Vav1-icre, Itgax-cre and Lyz2-cre deleter strains (Caton et al., 2007; de Boer et al., 2003; Clausen et al., 1999). Vav1-icre induced general hematopoietic Klf4 deletion as expected, whereas Itgax-cre deleted Klf4 only within cDCs (Figure S1A). Deletion of Klf4 by Vav1-icre resulted in loss of Ly6Chi monocyte development (Figure S1B–C), as previously reported (Feinberg et al., 2007). Neither Lyz2-cre- nor Itgax-cre-mediated deletion of Klf4 impaired Ly6Chi monocyte development, confirming an early developmental requirement for Klf4 in monocyte differentiation and validating the use of Itgax-cre mice for a cDC restricted deletion of Klf4 (Figure S1B–C). Klf4 deletion by Vav1-icre reduced the expression of IRF4 on pre-cDC (Figure S1E) and impaired development of SiglecH− pre-cDCs (Figure 1C–E), which also had reduced IRF4 expression (Figure S1E). Macrophage and DC precursors (MDPs) and CDPs were unaltered in Klf4fl/fl Vav1-icre mice (Figure 1C–E, S1I). CD11c is induced at the pre-cDC stage (Naik, 2010; Liu et al., 2007) and comparison of Klf4fl/fl Itgax-cre and cre-negative progenitors showed few changes in gene expression in CDPs, but increased differences in pre-cDCs (Figure S1F–I). Deletion of Klf4 by Vav1-icre or Itgax-cre reduced IRF4 expression in progenitors, but still allowed the divergence of DC progenitors into two major subsets of IRF8+ and IRF4+ cDCs in BM cultures (Figure S1D).
Figure 1. KLF-4 deletion impairs development of bone-marrow pre-cDC progenitors.
(A) Relative expression of Klf4, Irf8, and Irf4 determined by microarray analysis is shown for the indicated stages of DC progenitors. (B) Relative expression determined by microarray analysis of the indicated genes is shown for splenic CD24+ Sirp-α− CD11b− (CD24) and CD24− Sirp-α+ CD11b+ (CD11b) DCs. (C,D) DC progenitors in BM were analyzed from wild type mice (WT), Klf4fl/fl (cre-neg) or Klf4fl/fl mice crossed onto Vav1-icre (Harker et al., 2002), or Irf4fl/fl mice crossed into B6.C-Tg(CMV-cre)1Cgn/J (The Jackson Laboratory) backgrounds. CDP, MDP and pre-cDC were gated as described (Liu et al., 2009) in the Methods and analyzed for the indicated markers. (E) The percent of the progenitors from the indicated genotype analyzed in (C,D) are shown. The experiment was repeated 4 times and 2 mice per genotype were analyzed Error bars, ± s.d., n = 5, Student’s t-test. *P < 0.05; ***P < 0.001; NS, P > 0.05. See also Supplemental Figure S1
Klf4 regulates development of specific subsets of IRF4-expressing cDCs in peripheral tissues
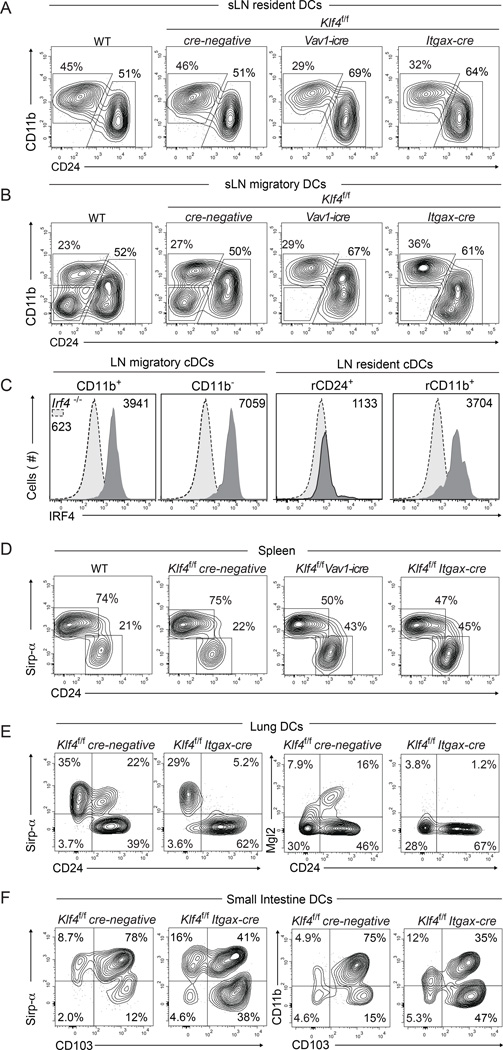
We next examined various lymphoid and peripheral tissue-resident DC subsets under conditional deletion of Klf4 (Figure 2 and S2). Consistent with a previous report (Park et al., 2012), there was approximately 50% reduction in total CD11c+ MHC-II+ cells in spleen (Figure 2D, S2G), mostly compromising Sirp-α+ CD24− cDCs (Figure 2D and Figure S2G). Approximately a 50% reduction of CD11b+ cDCs occurred across all lymphoid and peripheral tissues examined (Figure 2, and Figure S2). In skin draining lymph nodes (sLN), one subset within the migratory cDCs was completely absent (Figure 2A,B, and S2B). In skin, Langerhans cells (LC) and at least four other cDCs subsets can be distinguished on the basis of CD207, CD103 and CD11b (Henri et al., 2010). The CD103+CD207+ cDC subset represents the Batf3-depedendent cDC lineage, but three other subsets (CD103−CD207+, CD207−CD11b+ and CD103−CD11b−CD207− cDCs) are independent of Batf3 (Edelson et al., 2010; Ginhoux et al., 2009) and have distinct transcriptional identities (Miller et al., 2012; Robbins et al., 2008; Bar-On and Jung, 2010). Among these migratory cDC subsets, deletion of Klf4 completely impaired development of CD11b− CD24− cDCs, previously called "Double Negative" (DN) cDCs (Malissen et al., 2014; Henri et al., 2010) and characterized as CD207− CD11b− Sirp-α+ CX3CR1+ (Figure 2B, S2A and B and data not shown). In the dermis, deletion of Klf4 reduced CD11b+ cDCs and increased CD24+ and CD103+ cDCs (Figure S2D).
Figure 2. A discrete subset of migratory cDCs in skin-draining lymph nodes requires Klf4.
(A,B) Two-color histograms for CD11b and CD24 expression are gated on live cells for the resident (A) and migratory (B) gates shown in (S2A). (C) Single color histograms for intracellular IRF4 expression of the indicated subsets gated as in Figure 2A,B for resident and migratory DCs isolated from Klf4f/f cre-negative mice. Dotted line shows Irf4−/− control cDCs. Numbers represent geometrical mean values of IRF4 expression on the gated subsets. (D) Splenocytes from mice of the indicated genotypes were pre-gated as cDCs (MHC-IIhi CD11chi) and analyzed for CD24 and Sirp-α expression. Numbers indicate the percent of cells in the gate. (E and F) Two-color histograms for Sirp-α, CD24, CD11b, CD103 and Mgl2 as indicated in the plots, are shown for live cells from lung (E) and liver (F) of mice of the indicated genotype. Cells were pre-gated as CD45+ MHC-IIhi CD11c+ CD64−. Numbers indicate percent cells in the gates. All stainings were repeated in at least 3 independent experiments using at least 2 mice per genotype. See also Supplemental Figure S2
In the lung, Klf4-deficiency selectively eliminated a Sirp-α+ CD24+ subset (Figure E and Figure S2J,K)), which comprised 20% of cDCs (Figure S2J,K), and reduced Sirp-α+ CD24− cDCs. Further, Mgl-2 expression identified the Klf4-dependent Sirp-α+ CD24+ cDCs in lung (Figure 2E), but not in sLN (Figure S2C). In mediastinal lymph nodes, Klf4-deficiency severely reduced Sirp-α+ cDCs, which expressed PD-L2 but not Mgl2 (Figure S2l,M and data not shown). In the liver, Klf4-deficiency reduced the frequency of Sirp-α+ CD24− cDCs, which also expressed Mgl-2 (Figure S2H). In mesenteric lymph nodes, lamina propria and thymus, Sirp-α+ cDCs were reduced and CD24+ CD103+ respectively increased in the absence of Klf4 (Figure S2E,F Figure 2F and Figure S2I). These results highlight the requirement for Klf4 on the IRF4-expressing, but not IRF8-dependent cDCs and suggest heterogeneous expression of PD-L2 and Mgl2 across the different peripheral tissues (Figure 2,E,S2 and data not shown). Expression levels of IRF4 and IRF8 were analyzed on Klf4f/f cre-negative mice (Figure 2C and data not shown). Among the different IRF4 expressing cDCs, the Klf4-dependent DN subset showed the highest expression level for IRF4 (Figure 2C).
A cell-intrinsic requirement for Klf4 in development of CD11b− cDCs in sLNs
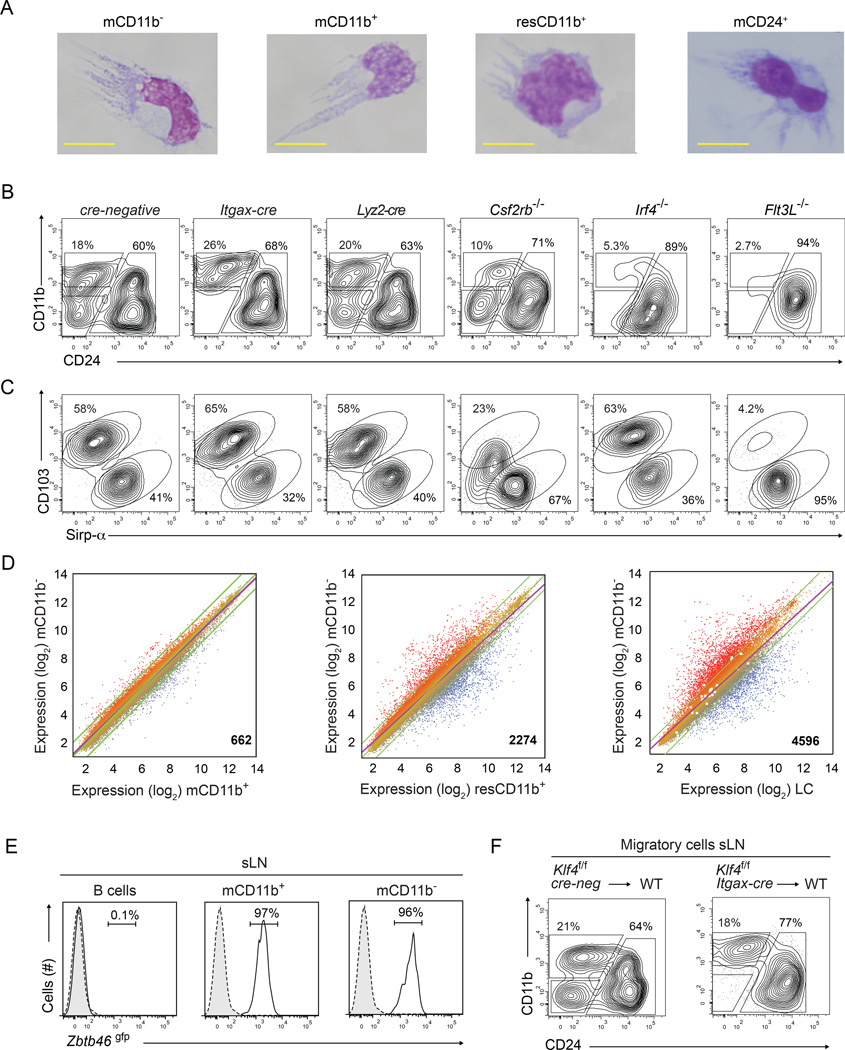
Previous studies concluded that dermal CD11b−MHC-IIhi cells were conventional DCs based on hematopoietic origin and dependence on Flt3L (Henri et al., 2010; Mollah et al., 2014), although these properties also identify progenitors of other hematopoietic lineages (McKenna et al., 2000; Mackarehtschian et al., 1995). Thus, we independently assessed the identity of the sLN Klf4-dependent cells (Figure 3). Klf4-dependent CD11b− cells had morphology similar to other migratory and resident cDC subsets (Figure 3A, Figure S3A), were not affected by Lyz2-cre-mediated Klf4 deletion, and were present in Csf2rb−/− mice (Figure 3B,C). In contrast, IRF8-dependent CD103+ cDCs develop in Csf2rb−/− mice but have reduced surface CD103 expression (Figure 3C) (Edelson et al., 2011). Moreover, the Klf4-dependent CD11b− cells in sLNs were absent in Flt3l−/− and Irf4−/− mice (Figure 3B,C), confirming their identity as IRF4-dependent cDCs. Among migratory cDCs, IRF4 expression was highest on the Klf4-dependent DCs (Figure S1W). Klf4-deficiency did not impair the development of CD24+ cDCs, which were absent in Flt3l−/− mice (Figure 3B,C), nor the development of LCs, which were severely reduced in Il34−/− mice (Wang et al., 2012; Greter et al., 2012) (Figure S3F). Klf4-dependent CD11b− cells were most similar in gene expression to migratory CD11b+ cDCs, and more distantly related to resident CD11b+ cDCs and LCs (Figure 3D, Figure S3G). In Zbtb46gfp mice, the Klf4-dependent CD11b− cells expressed GFP at levels similar to migratory CD11b+ cDCs and resident cDCs in sLN (Figure 3E, and Figure S3H–J). These results suggest that Klf4-dependent CD11b− cells are bona fide dendritic cells.
Figure 3. Klf4-dependent CD11b− cDCs require IRF4 and FLT3L, express Zbtb46gfp and most closely resemble CD11b+ cDCs.
(A) Wright's stain of sort-purified migratory CD11b− cDCs (mCD11b−), CD11b+ (mCD11b+), or CD24+ (mCD24+) and resident CD11b+(resCD11b+) cDCs. Images were collected from 2 independent experiments, cells from 2 mice were pooled and at least 10 cells were evaluated per subset. (B,C) Representative two-color histograms for CD11b, CD24 (B) and CD103, Sirp-α (C) for cells pre-gated as migratory CD24+ cDCs as in Figure 2B are shown for sLN cells harvested from mice of the indicated genotypes. Analysis was performed in at least 2 independent experiments and at least 3 mice per genotype were analyzed. (D) Microarray analysis of gene expression presented as M-plots for sort-purified mCD11b+ cDCs, mCD11b− cDCs, CD24+ migratory cDCs, and resident CD11b+ cDCs harvested from sLN. Colors indicate higher (red) or lower (blue) expression. Shown at the bottom right is the number of genes differing between paired samples by more than 2-fold. (E) Shown are single-color histograms for Zbtb46gfp expression for B cells, mCD11b+ or mCD11b− cDCs from sLN of Zbtb46+/gfp mice (Satpathy et al., 2012a). Numbers are the percent of live cells within the indicated gate. (F) Chimeras were generated by reconstitution of lethally irradiated C57BL/6 mice (WT) using BM from Klf4fl/fl cre-negative or Klf4fl/fl Itgax-cre mice as indicated. Shown are two-color histograms as in (B) for cells pre-gated as migratory cDCs as in Figure S2A. Experiment was performed twice and at least 4 mice per group were analyzed. See also Supplemental Figure S3
To test for a non-cell intrinsic action of Klf4, we analyzed single- and mixed-BM chimeras reconstituted with Klf4fl/flItgax-cre and Zbtb46gfp BM (Figure 3F, and Figure S3B–D). A hematopoietic requirement of Klf4 was confirmed by the selective loss of migratory CD11b− cDCs in sLN (Figure 3F) and the reduction of splenic CD11b+ cDCs (Figure S3B) in chimeras reconstituted with Itgax-cre Klf4fl/fl BM. We also excluded an indirect action of Klf4 deficiency on other hematopoietic lineages in regulating development of migratory CD11b− cDCs, since this subset was positive for GFP expression in chimeras reconstituted with a 10:1 mixture of Itgax-cre Klf4fl/fl BM and Zbtb46gfp BM (Satpathy et al., 2012a) (Figure S3C, D). As a control, we observed that similar ratios of GFP-positive and -negative cells that developed within the CD24+ and CD11b+ migratory DC subsets (Figure S3D). Collectively, these results argue for a cell-intrinsic requirement for Klf4 acting on migratory CD11b− cDCs.
Klf4-dependent migratory cDCs transport antigen from skin to sLNs
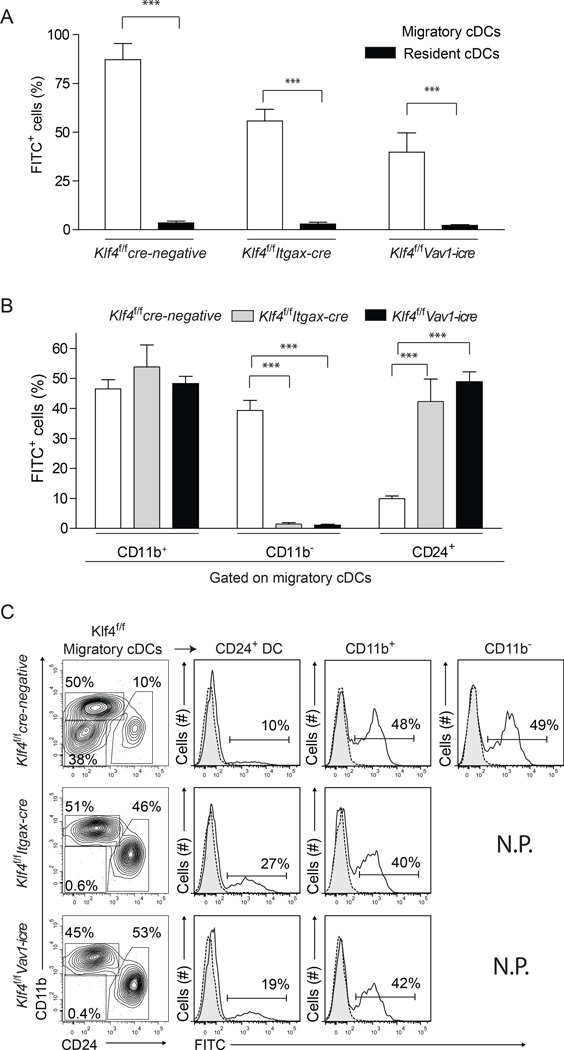
cDCs residing in the migratory gate transport the majority of fluorescein isothiocyanate (FITC) from skin to sLNs and the Klf4-dependent CD11b− migratory cDC subset accounted for a substantial fraction of total antigen transport (Figure 4A–C). Itgax-cre Klf4fl/fl and Vav1-icre Klf4fl/fl mice showed reduced percentage and absolute numbers of FITC+ cells present in sLNs following FITC painting (Figure 3A,B and S4A). In control mice, direct examination of FITC+ cells showed roughly similar contributions to antigen transport by CD11b+ and Klf4-dependent CD11b− cDCs, which together accounted for about 90% of FITC+ cells (Figure 4B,C, Figure S4B). In the absence of migratory CD11b− cDCs, the relative contribution to antigen transport by CD24+ cDCs was increased from 10% to roughly 40% (Figure 4B,C and Figure S4B). In contrast to Irf4−/− mice (Bajana et al., 2012; Gao et al., 2013), deletion of Klf4 did not affect antigen transport, as Klf4-independent migratory CD11b+ cDCs subset transported substantial antigen from skin to sLNs, in both Itgax-cre Klf4fl/fl and Vav1-icre Klf4fl/fl mice (Figure 4B,C and S4B).
Figure 4. Klf4-dependent CD11b− cDCs account for substantial antigen transport from skin to lymph nodes.
(A) Shown is the contribution to total FITC+ cells by migratory (open bars) and resident (closed bars) cDCs in skin draining lymph nodes (sLN) 16 h after FITC painting, as a percentage of total FITC+ sLN cells. Error bars, ± s.d., n = 6, Student’s t-test. *P < 0.05; ***P < 0.001; NS, P > 0.05. (B) Shown is contribution to total FITC+ cells by each migratory DC subset as a percentage of total FITC+ sLN cells in mice of the indicated genotypes 16 h after FITC painting. (C) FITC transport by individual migratory cDC subsets is show as single color histogram from mice of the indicated genotypes and gates (left panels). N.P. Not Present. Numbers indicate the percent of cells in the indicated gates. The experiment was performed 3 times and 2 mice per group were used. See also Supplemental Figure S4
Deletion of Klf4 in cDCs selectively impairs Th2 cell responses to pathogens
IRF4-expressing DCs produce of IL-23 that drives ILC3 and Th17 cell activation and protect against Citrobacter rodentium and Aspergillus fumigatus infection (Schlitzer et al., 2013; Satpathy et al., 2013; Persson et al., 2013). We tested the role of Klf4-dependent cDCs in IL-23 production and Th17 cell response by infecting Itgax-cre Klf4fl/fl mice with C. rodentium. We confirmed that susceptible to C. rodentium infection was increased in mice where Notch2 was deleted by Itgax-cre (cNotch2−/−). These mice lost 10–15% of their body weight and succumbed to infection around day 12 (Figure 5A, B). In contrast, Itgax-cre Klf4fl/fl mice did not lose weight and survived infection similar to wild type Klf4fl/fl cre-negative control mice, suggesting normal in vivo activation of ILC3 and Th17 cell responses. While cNotch2−/− mice have severely reduced splenic ESAM+ cDCs, Klf4 deficiency did not compromise development of this subset (Figure S5A–C).
Figure 5. Conditional deletion of Klf4 in DCs selectively impairs Th2 cell responses to S. mansoni.
(A, B) Survival (A) and weight loss (B) of mice following oral inoculation of C. rodentium (2 × 109 colony-forming units). Mice used were Klf4fl/fl (n=20) or Notch2fl/fl (n=5) (Satpathy et al., 2013) crossed to Itgax-cre (n=10) (Caton et al., 2007) (Notch2c−/−) or Klf4fl/fl cre-negative (cre-neg) (n=10) as indicated Experiment was repeated 2 times. (C) Survival of mice following skin inoculation with 100 Schistosoma mansoni cercariae. Mice used were Klf4fl/fl crossed to Itgax-cre (n=7) or a cre-negative background, (n=11), or were Il4−/− (Kopf et al., 1993) (n=7) experiment was repeated 3 times. (D) Shown are histograms for intracellular GATA3 and IL-4 staining for CD4+ T cells isolated from mice of the indicated gentoypes purified by MACS beads and passed twice in vitro with anti-CD3 and anti-CD28 under Th2-inducing (solid lines) or Th1-inducing conditions (dotted and shaded) as described (Schraml et al., 2009). Mice used were Klf4fl/fl crossed to Itgax-cre, Vav1-icre, cre-negative, or Batf−/−Batf3−/− double deficient mice (Tussiwand et al., 2012). Numbers are the percent of cells in the indicated gates. (E) MACs purified T cells from mice of the indicated genotypes as in (D) were polarized under Th1-, Th17- or Th2-inducing conditions as described (Schraml et al., 2009). Shown are the percent of T cells positive for intracellular expression of the indicated cytokine or factor for each genotype as a percent of the maximum expression obtained for cre-negative control T cell samples. Two mice per genotype were used per experiment and the experiment was repeated 4 times. Error bars, ± s.d., n = 8, Student’s t-test. *P < 0.05; ***P < 0.001; NS, P > 0.05. See also Supplemental Figure S5
Protection against the helminth pathogen Schistosoma mansoni requires a Th2 cell response (Brunet et al., 1997; Fallon et al., 2000; Herbert et al., 2004). We infected Klf4fl/fl Itgax-cre and Il4−/− mice with S. mansoni (Figure 5C). Il4−/− mice succumbed to infection after 50 days with an overall mortality of approximately 75% at 60 days after infection (Figure 5C), as expected (Brunet et al., 1997; Fallon et al., 2000; Pearce et al., 1996). Klf4fl/fl Itgax-cre mice succumbed to infection with a similar rate and kinetic as Il4−/− mice, while wild type control mice survive beyond 100 days after challenge (Figure 5C).
Since Itgax-cre can induce deletion in cells other than DCs, including T cells (Figure S1A) (Abram et al., 2014), we tested Th2 and Th17 cell polarization after Klf4 deletion. Deletion of Klf4 by either Vav1-icre or Itgax-cre did not decrease IL-17, IL-4, and IL-10 production inTh17 or Th2 cells compared to cre-negative controls (Figure 5D, E), but Batf−/− Batf3−/− T cells were strongly impaired in all of these cytokines, as expected (Tussiwand et al., 2012). Further, deletion of Klf4 by either Vav1-icre or Itgax-cre also did not reduce intracellular expression of either GATA3 (Figure 5D, E). Thus, deletion of Klf4 in T cells by Vav-icre leaves Th2 cell polarization intact, suggesting that susceptibility to S. mansoni infection occurred through a mechanism other than a T-cell intrinsic action.
We also immunized mice with S. mansoni egg antigen (SEA) (Sabin et al., 1996; Everts et al., 2012; Kane et al., 2008; Pearce et al., 1991). IL-4 and IL-5 were both produced by T cells harvested from sLNs 7 days after SEA immunization of wild type cre-negative control mice, but this was substantially and selectively reduced in Klf4fl/fl Itgax-cre mice (Figure S5D–G). Moreover, switching of germinal center B cells from surface immunoglobulin IgM to IgG1 after SEA-immunization was reduced in Klf4fl/fl Itgax-cre mice relative to cre-negative control mice (Figure S7H, I).
To test if Klf4 deletion within cDCs impaired the function of the IRF8- and Batf3-dependent cDCs, we examined infections by T.gondii and herpes simplex virus (HSV) (Mashayekhi et al., 2011; Tussiwand et al., 2012). Control mice cre-negative Klf4fl/fl and Itgax-cre Klf4fl/fl mice were similarly resistant to T. gondii infection, while Batf3−/− mice were as expected highly susceptible (Figure S5J,K). Second, Itgax-cre Klf4fl/fl mice, but not Batf3−/− mice, generated a robust Th1 cell response specific for the Gb2 peptide of herpes simplex virus (HSV) seven days after infection (Figure S5L,M). Also, cross-presentation of cell-associated antigens was preserved in Itgax-cre Klf4fl/fl mice (Figure S5N) suggesting that IRF8-expressing DC were phenotypically and functionally fully preserved in Itgax-cre Klf4fl/fl mice.
We also excluded that klf4 deletion resulted in a general priming defect in the remaining IRF4-dependent cDCs by performing several DC-T cell cultures, where we did not observe any difference using either BM derived or isolated DCs from control mice cre-negative, Itgax-cre or Vav1-icre Klf4fl/fl mice (Figure S5O). Further, Klf4-dependent DCs did not show superior in vitro priming ability, but only increased expression of the co-stimulatory molecules CD80 and CD86 after SEA (data not shown).
Collectively, since deletion of Klf4 in Itgax-cre Klf4fl/fl mice did not compromise the development of other myeloid subsets other than cDCs and these mice had normal Th17, Th1 and CTL cell response to several infections and antigens, Klf4 appears to be selectively required on DCs for inducing Th2 immunity.
Deletion of Klf4 in cDCs prevents house dust mite-induced allergic inflammation
Development of asthma is associated with a Th2 cell type of immunity characterized by the accumulation of eosinophils (Robinson, 2000; Robinson et al., 1992). In mice, intra-nasal challenge with extracts of house dust mite (HDM) causes acute allergic peribronchial Th2 cell inflammation mediated by CD11b+ cDCS (Hammad et al., 2009; Plantinga et al., 2013; Williams et al., 2013). Since pulmonary macrophages express CD11c, we used Klf4fl/fl Lyz2-cre mice as a control for potential requirements for Klf4 in pulmonary macrophages. Klf4fl/fl Itgax-cre mice showed a dramatic reduction in pulmonary accumulation of eosinophils compared to both cre-negative Klf4fl/fl control mice and Klf4fl/fl Lyz2-cre mice, which had robust eosinophilia (Figure 6A, B). Control cre-negative and Klf4fl/fl Lyz2-cre mice, but not Klf4fl/fl Itgax-cre mice, had typical asthmatic features with peribronchial inflammation with substantial leukocyte infiltrations (Figure 6C).
Figure 6. Conditional deletion of Klf4 in DCs prevents development of HDM-induced allergic inflammation.
(A) HDM sensitized mice of the indicated genotypes were challenged intra-nasally with PBS or HDM extract. After 3 days bronchial alveolar lavage (BAL) was analyzed for eosinophils by FACS. Shown are eosinophils as a percentage (left panel) or total numbers recruited into BAL (right panel) and pre-gated as CD45+ live cells. Mice used were Klf4fl/fl crossed to Itgax-cre (n=8) or Lyz2-cre (n=8) or a cre-negative background (n=8) 3 mice per genotype used per experiment. Experiment was repeated 3 times. Error bars, ± s.d., n = 8, Student’s t-test. *P < 0.05; ***P < 0.001; NS, P > 0.05. (B) Mice of the indicated genotypes were challenged as in (A) and BAL analyzed by flow cytometry after 3 days. Shown are two-color histograms pre-gated on CD45+ BAL cells. Numbers indicate percentage of cells in the indicate gates for eosinophils (SiglecF+ CD11c−) or pulmonary macrophages (SiglecF+ CD11c+) respectively. (C) Shown are hematoxylin and eosin stained sections of lungs from mice described in (A) as indicated. Scale bar indicates 200µm.
Discussion
Klf4 regulates DCs development beginning in the pre-cDC, where it impacts the IRF4-expressing branch of cDCs. Deletion of Klf4 by Vav1-icre altered the expression of relatively few genes, but among these, IRF4 was severely down-regulated as measured by microarray expression and intracellular staining. Reduction of IRF8 and induction of IRF4 occurs at the SiglecH− pre-cDCs stage, a stage that was severely reduced in Vav1-icre Klf4fl/fl mice. However, IRF4 expression was not altered in mature cDCs or in Flt3L generated BM-derived cDCs implying a developmental rather than an absolute requirement for Klf4 in the induction of IRF4 expression.
In mature cDCs, Klf4 deletion selectively compromised the development of particular subsets of IRF4-epxressing cDCs. The subset most clearly dependent on Klf4 was the previously described migratory subset of CD11b−CD24− (DN) cDCs in sLN. This subset had highest expression levels of IRF4 among migratory cDCs. However, it is still unclear whether impaired Klf4-dependent cDCs development is caused by reduced IRF4-expression at a progenitor stage or due to some other action of Klf4.
The DN migratory cDCs identified here as being Klf4-dependent have been described recently to acquire fluorescently labeled antigen from non-viable Nippostrongylus larvae and suggested to be involved in Th2 cell responses based on in vitro analysis (Ochiai et al., 2014; Connor et al., 2014). Our results would support those conclusions by drawing a correlation between the loss of the DN cDC subset and reduced in vivo Th2 cell responses in Klf4 deficient mice. We also observed a higher co-stimulatory potential of the Klf4-dependent DN migratory cDCs relative to other cDC subsets after SEA stimulation. Nonetheless, despite the genetic requirement for Klf4 for DN cDC development, it is still possible that Klf4 controls Th2 responses by actions in other cDC subsets that are not seemingly affected by the deletion. Excluding such possibilities awaits the development of deleter strains with higher DC-subset specificity.
IRF4-expressing cDCs were recently shown to control Th2 cell responses induced in skin and lungs (Schlitzer et al., 2013; Gao et al., 2013; Kumamoto et al., 2013; Zhou et al., 2014; Williams et al., 2013), although no mechanism for this action was determined. Irf4−/− cDCs fail to migrate to sLN (Bajana et al., 2012) and show reduced expression of co-stimulatory molecules (Vander et al., 2014) which could explaining reduced T-cell priming at least in skin. Klf4 deletion selectively impaired Th2 cell responses without preventing migration of the remaining cDCs.
IRF4-expressing cDCs also control Th17 cell responses in lungs and intestine (Schlitzer et al., 2013; Lewis et al., 2011; Satpathy et al., 2013). We find that in the lung, Klf4 deletion selectively eliminated an IRF4-dependent cDC subset characterized by the expression of Sirp-α+ CD24+ Mgl-2+. Conceivably, Klf4-dependent Sirp-α+ CD24+ lung cDCs might mediate Th2 cell responses to house dust mite, while Klf4-independent Sirp-α+ CD24− cDCs mediate Th17 cell immunity to fungal infections. Alternately, Klf4 may act on both IRF4-expressing cDC subsets but selectively influence Th2 but not Th17 cell priming. Similarly, in the small intestine, Th17 cell immunity against C. Rodentium infection has been attributed to the action of a Notch2-dependent subset of IRF4-expressing cDCs (Lewis et al., 2011; Satpathy et al., 2013). Klf4 deletion did not increase susceptibility to C. Rodentium infection and did not impair development of the splenic Notch2-dependent ESAM-expressing cDC subset. Thus, in the intestine as well, distinct transcriptional programs regulated by either Notch2 or Klf4 appear to control different types of immunity mediated by the IRF4-expressing cDCs.
Our results show a genetic requirement for Klf4 in cDCs for Th2 cell immunity however, do not reveal the cellular mechanism mediating Th2 cell responses. The in vivo trigger for Th2 cell development has been elusive, with many mechanisms proposed over time. Conceivably, a particular cDC subset could directly induce Th2 development, for example by production of a cytokine or expressing a specific surface receptor. Alternately, the cDC subset could recruit an accessory cell that favors and creates the necessary microenvironment for Th2 cell priming. Indeed, recent evidence suggests the involvement of innate lymphoid cells type 2 (ILC2) in the lung (Halim et al., 2014; Licona-Limon et al., 2013; Halim et al., 2012) and of eosinophils in the intestine (Chu et al., 2014). It will be important to determine if Klf4-dependent cDCs utilize these cells, since such a mechanism would not be re-capitulated by in vitro studies using isolated co-cultures of cDCs and T cells.
Experimental Procedures
Mice
All animals were bred and maintained in a specific pathogen-free animal facility according to institutional guidelines and with protocols approved by the Animal Studies Committee at Washington University in St. Louis. Mice of the following genotypes were purchased from Jackson Laboratories: wild type mice C57BL6 (C57BL/6J), Itgax-cre (B6.Cg-Tg(Itgax-cre)1-1Reiz/J) (Caton et al., 2007), Vav1-icre (B6.Cg-Tg(Vav1-cre)A2Kio/J) (Harker et al., 2002), Lyz2-cre (B6.129P2-Lyz2tm1(cre)Ifo/J) (Clausen et al., 1999), Cx3cr1gfp (B6.129P-Cx3cr1tm1Litt/J) (Jung et al., 2000). Irf4−/− were generated in our facility by backcrossing Irf4fl/fl mice (B6.129S1-Irf4tm1Rdf/J) to the cytomegalovirus (CMV) promoter expressing CMV-cre mice (B6.C-Tg(CMV-cre)1Cgn/J), both purchased from Jackson Laboratories. Csf2rb−/− (B6.129S1-Csf2rbtm1Cgb/J) and Flt3L−/− (C57BL/6-flt3Ltm1Imx) were obtained from Taconic. Irf8−/− mice were obtained from the European Mutant Mouse Archive and maintained on the C57BL/6 background. Notch2fl/flItgax-cre were generated and maintained as described (Satpathy et al., 2013). Klf4fl/fl mice were obtained from MMRC (MMRRC line 29877) and backcrossed to Itgax-cre, Vav1-icre, Lyz2-cre. Zbtb46gfp were generated as previously described (Satpathy et al., 2012a). Batf3−/− were generated as previously described (Hildner et al., 2008). IL4−/−(B6.129P2-Il4tm1Cgn/J) (Kopf et al., 1993) mice were obtained from EJ Pearce (Pearce et al., 1996). For BM chimera experiments, the CD45.1+ B6.SJL (B6.SJL-PtprcaPepcb/BoyJ) mice were purchased from Jackson Laboratories. Unless otherwise indicated, experiments used sex and age matched littermates between 6 to12 weeks of age.
Infection models
All animal model studies described were done in accordance with institutional guidelines and with protocols approved by the Animal Studies Committee at Washington University in St. Louis.
For Citrobacter rodentium infection Mice were orally inoculated with 2 × 109 colony-forming units of C. rodentium, strain DBS100 (American Type Culture Collection) as described (Lee et al., 2012). Survival and weight loss were monitored over 30 days. Mice used for C. rodentium experiments weighed less than 22 g.
For Toxoplasma gondii infection, the type II Prugniaud strain of T. gondii expressing a transgene encoding firefly luciferase and GFP (PRU-FLuc-GFP) provided by J. (Saeij et al., 2005) was used. The parasites were grown in human foreskin fibroblasts cultures as described (Mashayekhi et al., 2011) For infection, freshly egressed parasites were filtered, counted, and 100 tachyzoites per mouse were injected intraperitoneally. Survival of mice was monitored over 30 days; parasite burden was measured every 2 days as described below.
Snails infected with S. mansoni (strain NMRI, NR-21962) were provided by the Schistosome Research Reagent Resource Center for distribution by BEI Resources, NIAID NIH. Mice were each infected by percutaneous exposure to 100 cercariae. S, mansoni Egg Antigen (SEA) was made from parasite eggs isolated from the livers of infected mice, as previously described (MacDonald et al., 2001). Mice were each immunized with 50 µg of SEA s.c. into a rear footpad. For Herpes simplex virus 1 (HSV-1) infection mice were infected with the KOS strain subcutaneously (s.c.) with 1.5 × 105 plaque-forming units (p.f.u.) per mouse in the footpad.
In vitro T-cell re-stimulation after SEA and HSV infection
One week after HSV infection, spleens and popliteal lymph nodes (LN) were collected and re-stimulated with the HSV peptide HSV-gB2 (498–505) (Anaspec). Briefly, 2 × 106 splenocytes or LN cells were re-stimulated for 5 h in the presence of brefeldin A at 1 µg ml−1 and analyzed by FACS for intracellular IFN-γ and TNF-α production as described later.
Similarly, one week after SEA injection, 3 × 105 cells harvested from popliteal lymph nodes were collected and restimulated under different conditions: SEA (20µg/ml), plate-bound anti-CD3, PMA and Ionomycin (50ng/ml and 1µg/ml) or media alone. For detection of IL-4, 2.5µg /ml of anti-IL-4R antibody (M1) was added to the culture. Supernatants were collected after 72h and analyzed using the BD CBA mouse Th1/Th2 Kit™ (BD Biosciences), and data were analyzed with FCAP Array software (Soft Flow, Inc.).
House dust mite (HDM) induced asthma
HDM (Dermatophagoidespteronyssinus extracts, Greer Laboratories) was dissolved in PBS. To induce allergic airway inflammation, mice were sensitized i.n. 10 µg HDM subsequently challenged with 50 µg HDM i.n. on days 7, and 3 days after challenge, lungs, BAL, and mediastinal LNs were collected.
Statistical analysis
Differences between groups in survival were analyzed by the log-rank test. Analysis of all other data was done with an unpaired, two-tailed Student’s t-test with a 95% confidence interval (Prism; GraphPad Software, Inc.). P values less than 0.05 were considered significant. *0.01 < P < 0.05; **0.001 < P < 0.01; ***P < 0.001; ****P < 0.0001.
Supplementary Material
Acknowledgements
This work was supported by the Howard Hughes Medical Institute (K.M. Murphy). NIH grant AI32573 to EJP. BE was supported by a VENI grant from the Netherlands Organization for Scientific Research. We thank Susan Gilfillan and Marco Colonna for Il34−/− mice (Wang et al., 2012). We thank Victor Cortez for help in processing LP tissues.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicting financial interests.
References
- Abram CL, Roberge GL, Hu Y, Lowell CA. Comparative analysis of the efficiency and specificity of myeloid-Cre deleting strains using ROSA-EYFP reporter mice. J. Immunol. Methods. 2014;408:89–100. doi: 10.1016/j.jim.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages 1. Nature. 2000;404:193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- Alder JK, Georgantas RW, III, Hildreth RL, Kaplan IM, Morisot S, Yu X, McDevitt M, Civin CI. Kruppel-like factor 4 is essential for inflammatory monocyte differentiation in vivo. J. Immunol. 2008;180:5645–5652. doi: 10.4049/jimmunol.180.8.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajana S, Roach K, Turner S, Paul J, Kovats S. IRF4 promotes cutaneous dendritic cell migration to lymph nodes during homeostasis and inflammation. J. Immunol. 2012;189:3368–3377. doi: 10.4049/jimmunol.1102613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Bar-On L, Jung S. Defining in vivo dendritic cell functions using CD11c-DTR transgenic mice. Methods Mol Biol. 2010;595:429–442. doi: 10.1007/978-1-60761-421-0_28. [DOI] [PubMed] [Google Scholar]
- Belz GT, Nutt SL. Transcriptional programming of the dendritic cell network. Nat. Rev. Immunol. 2012;12:101–113. doi: 10.1038/nri3149. [DOI] [PubMed] [Google Scholar]
- Brunet LR, Finkelman FD, Cheever AW, Kopf MA, Pearce EJ. IL-4 protects against TNF-alpha-mediated cachexia and death during acute schistosomiasis. J. Immunol. 1997;159:777–785. [PubMed] [Google Scholar]
- Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8− dendritic cells in the spleen. J Exp. Med. 2007;204:1653–1664. doi: 10.1084/jem.20062648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu DK, Jimenez-Saiz R, Verschoor CP, Walker TD, Goncharova S, Llop-Guevara A, Shen P, Gordon ME, Barra NG, Bassett JD, Kong J, Fattouh R, McCoy KD, Bowdish DM, Erjefalt JS, Pabst O, Humbles AA, Kolbeck R, Waserman S, Jordana M. Indigenous enteric eosinophils control DCs to initiate a primary Th2 immune response in vivo. J. Exp. Med. 2014;211:1657–1672. doi: 10.1084/jem.20131800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- Connor LM, Tang SC, Camberis M, Le Gros G, Ronchese F. Helminth-Conditioned Dendritic Cells Prime CD4+ T Cells to IL-4 Production In Vivo. J. Immunol. 2014 doi: 10.4049/jimmunol.1400374. [DOI] [PubMed] [Google Scholar]
- Dang DT, Pevsner J, Yang VW. The biology of the mammalian Kruppel-like family of transcription factors. Int. J. Biochem. Cell Biol. 2000;32:1103–1121. doi: 10.1016/s1357-2725(00)00059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer J, Williams A, Skavdis G, Harker N, Coles M, Tolaini M, Norton T, Williams K, Roderick K, Potocnik AJ, Kioussis D. Transgenic mice with hematopoietic and lymphoid specific expression of Cre. Eur. J. Immunol. 2003;33:314–325. doi: 10.1002/immu.200310005. [DOI] [PubMed] [Google Scholar]
- Edelson BT, Bradstreet TR, KC W, Hildner K, Herzog JW, Sim J, Russell JH, Murphy TL, Unanue ER, Murphy KM. Batf3-dependent CD11b(low/−) peripheral dendritic cells are GM-CSF-independent and are not required for Th cell priming after subcutaneous immunization. PLoS One. 2011;6:e25660. doi: 10.1371/journal.pone.0025660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelson BT, KC W, Juang R, Kohyama M, Benoit LA, Klekotka PA, Moon C, Albring JC, Ise W, Michael DG, Bhattacharya D, Stappenbeck TS, Holtzman MJ, Sung SS, Murphy TL, Hildner K, Murphy KM. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. J. Exp. Med. 2010;207:823–836. doi: 10.1084/jem.20091627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everts B, Hussaarts L, Driessen NN, Meevissen MH, Schramm G, van der Ham AJ, van der HB, Scholzen T, Burgdorf S, Mohrs M, Pearce EJ, Hokke CH, Haas H, Smits HH, Yazdanbakhsh M. Schistosome-derived omega-1 drives Th2 polarization by suppressing protein synthesis following internalization by the mannose receptor. J. Exp. Med. 2012;209:1753–1767. S1. doi: 10.1084/jem.20111381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon PG, Richardson EJ, McKenzie GJ, McKenzie AN. Schistosome infection of transgenic mice defines distinct and contrasting pathogenic roles for IL-4 and IL-13: IL-13 is a profibrotic agent. J. Immunol. 2000;164:2585–2591. doi: 10.4049/jimmunol.164.5.2585. [DOI] [PubMed] [Google Scholar]
- Feinberg MW, Wara AK, Cao Z, Lebedeva MA, Rosenbauer F, Iwasaki H, Hirai H, Katz JP, Haspel RL, Gray S, Akashi K, Segre J, Kaestner KH, Tenen DG, Jain MK. The Kruppel-like factor KLF4 is a critical regulator of monocyte differentiation. EMBO J. 2007;26:4138–4148. doi: 10.1038/sj.emboj.7601824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Nish SA, Jiang R, Hou L, Licona-Limon P, Weinstein JS, Zhao H, Medzhitov R. Control of T helper 2 responses by transcription factor IRF4-dependent dendritic cells. Immunity. 2013;39:722–732. doi: 10.1016/j.immuni.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaleb AM, Nandan MO, Chanchevalap S, Dalton WB, Hisamuddin IM, Yang VW. Kruppel-like factors 4 and 5: the yin and yang regulators of cellular proliferation. Cell Res. 2005;15:92–96. doi: 10.1038/sj.cr.7290271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Liu K, Helft J, Bogunovic M, Greter M, Hashimoto D, Price J, Yin N, Bromberg J, Lira SA, Stanley ER, Nussenzweig M, Merad M. The origin and development of nonlymphoid tissue CD103+ DCs. J Exp. Med. 2009;206:3115–3130. doi: 10.1084/jem.20091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greter M, Lelios I, Pelczar P, Hoeffel G, Price J, Leboeuf M, Kundig TM, Frei K, Ginhoux F, Merad M, Becher B. Stroma-derived interleukin-34 controls the development and maintenance of langerhans cells and the maintenance of microglia. Immunity. 2012;37:1050–1060. doi: 10.1016/j.immuni.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker C, Kirsch RD, Ju XS, Hieronymus T, Gust TC, Kuhl C, Jorgas T, Kurz SM, Rose-John S, Yokota Y, Zenke M. Transcriptional profiling identifies Id2 function in dendritic cell development. Nat Immunol. 2003;4:380–386. doi: 10.1038/ni903. [DOI] [PubMed] [Google Scholar]
- Halim TY, MacLaren A, Romanish MT, Gold MJ, McNagny KM, Takei F. Retinoic-acid-receptor-related orphan nuclear receptor alpha is required for natural helper cell development and allergic inflammation. Immunity. 2012;37:463–474. doi: 10.1016/j.immuni.2012.06.012. [DOI] [PubMed] [Google Scholar]
- Halim TY, Steer CA, Matha L, Gold MJ, Martinez-Gonzalez I, McNagny KM, McKenzie AN, Takei F. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014;40:425–435. doi: 10.1016/j.immuni.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambleton S, Salem S, Bustamante J, Bigley V, Boisson-Dupuis S, Azevedo J, Fortin A, Haniffa M, Ceron-Gutierrez L, Bacon CM, Menon G, Trouillet C, McDonald D, Carey P, Ginhoux F, Alsina L, Zumwalt TJ, Kong XF, Kumararatne D, Butler K, Hubeau M, Feinberg J, Al Muhsen S, Cant A, Abel L, Chaussabel D, Doffinger R, Talesnik E, Grumach A, Duarte A, Abarca K, Moraes-Vasconcelos D, Burk D, Berghuis A, Geissmann F, Collin M, Casanova JL, Gros P. IRF8 mutations and human dendritic-cell immunodeficiency. N. Engl. J. Med. 2011;365:127–138. doi: 10.1056/NEJMoa1100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat. Med. 2009;15:410–416. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker N, Naito T, Cortes M, Hostert A, Hirschberg S, Tolaini M, Roderick K, Georgopoulos K, Kioussis D. The CD8alpha gene locus is regulated by the Ikaros family of proteins. Mol. Cell. 2002;10:1403–1415. doi: 10.1016/s1097-2765(02)00711-6. [DOI] [PubMed] [Google Scholar]
- Henri S, Poulin LF, Tamoutounour S, Ardouin L, Guilliams M, de Bovis B, Devilard E, Viret C, Azukizawa H, Kissenpfennig A, Malissen B. CD207+ CD103+ dermal dendritic cells cross-present keratinocyte-derived antigens irrespective of the presence of Langerhans cells. J Exp. Med. 2010;207:189–206. doi: 10.1084/jem.20091964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert DR, Holscher C, Mohrs M, Arendse B, Schwegmann A, Radwanska M, Leeto M, Kirsch R, Hall P, Mossmann H, Claussen B, Forster I, Brombacher F. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity. 2004;20:623–635. doi: 10.1016/s1074-7613(04)00107-4. [DOI] [PubMed] [Google Scholar]
- Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, Schreiber RD, Murphy TL, Murphy KM. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane CM, Jung E, Pearce EJ. Schistosoma mansoni egg antigen-mediated modulation of Toll-like receptor (TLR)-induced activation occurs independently of TLR2, TLR4, and MyD88. Infect. Immun. 2008;76:5754–5759. doi: 10.1128/IAI.00497-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwada M, Pham NL, Pewe LL, Harty JT, Rothman PB. NFIL3/E4BP4 is a key transcription factor for CD8{alpha}+ dendritic cell development. Blood. 2011;117:6193–6197. doi: 10.1182/blood-2010-07-295873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JP, Perreault N, Goldstein BG, Lee CS, Labosky PA, Yang VW, Kaestner KH. The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development. 2002;129:2619–2628. doi: 10.1242/dev.129.11.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf M, Le Gros G, Bachmann M, Lamers MC, Bluethmann H, Kohler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993;362:245–248. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- Kumamoto Y, Linehan M, Weinstein JS, Laidlaw BJ, Craft JE, Iwasaki A. CD301b(+) dermal dendritic cells drive T helper 2 cell-mediated immunity. Immunity. 2013;39:733–743. doi: 10.1016/j.immuni.2013.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurotaki D, Osato N, Nishiyama A, Yamamoto M, Ban T, Sato H, Nakabayashi J, Umehara M, Miyake N, Matsumoto N, Nakazawa M, Ozato K, Tamura T. Essential role of the IRF8-KLF4 transcription factor cascade in murine monocyte differentiation. Blood. 2013;121:1839–1849. doi: 10.1182/blood-2012-06-437863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Cella M, McDonald KG, Garlanda C, Kennedy GD, Nukaya M, Mantovani A, Kopan R, Bradfield CA, Newberry RD, Colonna M. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat. Immunol. 2012;13:144–151. doi: 10.1038/ni.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KL, Caton ML, Bogunovic M, Greter M, Grajkowska LT, Ng D, Klinakis A, Charo IF, Jung S, Gommerman JL, Ivanov II, Liu K, Merad M, Reizis B. Notch2 receptor signaling controls functional differentiation of dendritic cells in the spleen and intestine. Immunity. 2011;35:780–791. doi: 10.1016/j.immuni.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licona-Limon P, Kim LK, Palm NW, Flavell RA. TH2, allergy and group 2 innate lymphoid cells. Nat. Immunol. 2013;14:536–542. doi: 10.1038/ni.2617. [DOI] [PubMed] [Google Scholar]
- Liu K, Victora GD, Schwickert TA, Guermonprez P, Meredith MM, Yao K, Chu FF, Randolph GJ, Rudensky AY, Nussenzweig M. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324:392–397. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Waskow C, Liu X, Yao K, Hoh J, Nussenzweig M. Origin of dendritic cells in peripheral lymphoid organs of mice. Nat Immunol. 2007;8:578–583. doi: 10.1038/ni1462. [DOI] [PubMed] [Google Scholar]
- MacDonald AS, Straw AD, Bauman B, Pearce EJ. CD8− dendritic cell activation status plays an integral role in influencing Th2 response development. J. Immunol. 2001;167:1982–1988. doi: 10.4049/jimmunol.167.4.1982. [DOI] [PubMed] [Google Scholar]
- Mackarehtschian K, Hardin JD, Moore KA, Boast S, Goff SP, Lemischka IR. Targeted disruption of the flk2/flt3 gene leads to deficiencies in primitive hematopoietic progenitors. Immunity. 1995;3:147–161. doi: 10.1016/1074-7613(95)90167-1. [DOI] [PubMed] [Google Scholar]
- Malissen B, Tamoutounour S, Henri S. The origins and functions of dendritic cells and macrophages in the skin. Nat. Rev. Immunol. 2014;14:417–428. doi: 10.1038/nri3683. [DOI] [PubMed] [Google Scholar]
- Mashayekhi M, Sandau MM, Dunay IR, Frickel EM, Khan A, Goldszmid RS, Sher A, Ploegh HL, Murphy TL, Sibley LD, Murphy KM. CD8a+ Dendritic Cells Are the Critical Source of Interleukin-12 that Controls Acute Infection by Toxoplasma gondii Tachyzoites. Immunity. 2011;35:249–259. doi: 10.1016/j.immuni.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell BB, Yang VW. Mammalian Kruppel-like factors in health and diseases. Physiol Rev. 2010;90:1337–1381. doi: 10.1152/physrev.00058.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna HJ, Stocking KL, Miller RE, Brasel K, De Smedt T, Maraskovsky E, Maliszewski CR, Lynch DH, Smith J, Pulendran B, Roux ER, Teepe M, Lyman SD, Peschon JJ. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood. 2000;95:3489–3497. [PubMed] [Google Scholar]
- Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu. Rev. Immunol. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mildner A, Jung S. Development and function of dendritic cell subsets. Immunity. 2014;40:642–656. doi: 10.1016/j.immuni.2014.04.016. [DOI] [PubMed] [Google Scholar]
- Miller JC, Brown BD, Shay T, Gautier EL, Jojic V, Cohain A, Pandey G, Leboeuf M, Elpek KG, Helft J, Hashimoto D, Chow A, Price J, Greter M, Bogunovic M, Bellemare-Pelletier A, Frenette PS, Randolph GJ, Turley SJ, Merad M, Gautier EL, Jakubzick C, Randolph GJ, Best AJ, Knell J, Goldrath A, Miller J, Brown B, Merad M, Jojic V, Koller D, Cohen N, Brennan P, Brenner M, Shay T, Regev A, Fletcher A, Elpek K, Bellemare-Pelletier A, Malhotra D, Turley S, Jianu R, Laidlaw D, Collins J, Narayan K, Sylvia K, Kang J, Gazit R, Rossi DJ, Kim F, Rao TN, Wagers A, Shinton SA, Hardy RR, Monach P, Bezman NA, Sun JC, Kim CC, Lanier LL, Heng T, Kreslavsky T, Painter M, Ericson J, Davis S, Mathis D, Benoist C. Deciphering the transcriptional network of the dendritic cell lineage. Nat. Immunol. 2012;13:888–899. doi: 10.1038/ni.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollah SA, Dobrin JS, Feder RE, Tse SW, Matos IG, Cheong C, Steinman RM, Anandasabapathy N. Flt3L dependence helps define an uncharacterized subset of murine cutaneous dendritic cells. J. Invest Dermatol. 2014;134:1265–1275. doi: 10.1038/jid.2013.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KM. Transcriptional control of dendritic cell development. Adv. Immunol. 2013;120:239–267. doi: 10.1016/B978-0-12-417028-5.00009-0. [DOI] [PubMed] [Google Scholar]
- Naik SH. Generation of large numbers of pro-DCs and pre-DCs in vitro 1. Methods Mol Biol. 2010;595:177–186. doi: 10.1007/978-1-60761-421-0_11. [DOI] [PubMed] [Google Scholar]
- Ochiai S, Roediger B, Abtin A, Shklovskaya E, Fazekas de St GB, Yamane H, Weninger W, Le Gros G, Ronchese F. CD326loCD103loCD11blo Dermal Dendritic Cells Are Activated by Thymic Stromal Lymphopoietin during Contact Sensitization in Mice. J. Immunol. 2014;193:2504–2511. doi: 10.4049/jimmunol.1400536. [DOI] [PubMed] [Google Scholar]
- Onai N, Obata-Onai A, Schmid MA, Ohteki T, Jarrossay D, Manz MG. Identification of clonogenic common Flt3(+) M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat. 2007;8:1207–1216. doi: 10.1038/ni1518. [DOI] [PubMed] [Google Scholar]
- Park CS, Lee PH, Yamada T, Burns A, Shen Y, Puppi M, Lacorazza HD. Kruppel-like factor 4 (KLF4) promotes the survival of natural killer cells and maintains the number of conventional dendritic cells in the spleen. J. Leukoc. Biol. 2012;91:739–750. doi: 10.1189/jlb.0811413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EJ, Caspar P, Grzych JM, Lewis FA, Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J. Exp. Med. 1991;173:159–166. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EJ, Cheever A, Leonard S, Covalesky M, Fernandez-Botran R, Kohler G, Kopf M. Schistosoma mansoni in IL-4-deficient mice. Int. Immunol. 1996;8:435–444. doi: 10.1093/intimm/8.4.435. [DOI] [PubMed] [Google Scholar]
- Persson EK, Uronen-Hansson H, Semmrich M, Rivollier A, Hagerbrand K, Marsal J, Gudjonsson S, Hakansson U, Reizis B, Kotarsky K, Agace WW. IRF4 Transcription-Factor-Dependent CD103(+)CD11b(+) Dendritic Cells Drive Mucosal T Helper 17 Cell Differentiation. Immunity. 2013;38:958–969. doi: 10.1016/j.immuni.2013.03.009. [DOI] [PubMed] [Google Scholar]
- Pinto AK, Daffis S, Brien JD, Gainey MD, Yokoyama WM, Sheehan KC, Murphy KM, Schreiber RD, Diamond MS. A temporal role of type I interferon signaling in CD8+ T cell maturation during acute West Nile virus infection. PLoS Pathog. 2011;7:e1002407. doi: 10.1371/journal.ppat.1002407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantinga M, Guilliams M, Vanheerswynghels M, Deswarte K, Branco-Madeira F, Toussaint W, Vanhoutte L, Neyt K, Killeen N, Malissen B, Hammad H, Lambrecht BN. Conventional and Monocyte-Derived CD11b(+) Dendritic Cells Initiate and Maintain T Helper 2 Cell-Mediated Immunity to House Dust Mite Allergen. Immunity. 2013 doi: 10.1016/j.immuni.2012.10.016. [DOI] [PubMed] [Google Scholar]
- Robbins SH, Walzer T, Dembele D, Thibault C, Defays A, Bessou G, Xu H, Vivier E, Sellars M, Pierre P, Sharp FR, Chan S, Kastner P, Dalod M. Novel insights into the relationships between dendritic cell subsets in human and mouse revealed by genome-wide expression profiling. Genome Biol. 2008;9:R17. doi: 10.1186/gb-2008-9-1-r17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DS. The Th1 and Th2 concept in atopic allergic disease. Chem. Immunol. 2000;78:50–61. doi: 10.1159/000058816. [DOI] [PubMed] [Google Scholar]
- Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N. Engl. J. Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- Sabin EA, Kopf MA, Pearce EJ. Schistosoma mansoni egg-induced early IL-4 production is dependent upon IL-5 and eosinophils. J. Exp. Med. 1996;184:1871–1878. doi: 10.1084/jem.184.5.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeij JP, Boyle JP, Grigg ME, Arrizabalaga G, Boothroyd JC. Bioluminescence imaging of Toxoplasma gondii infection in living mice reveals dramatic differences between strains. Infect. Immun. 2005;73:695–702. doi: 10.1128/IAI.73.2.695-702.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satpathy AT, Briseno CG, Lee JS, Ng D, Manieri NA, KC W, Wu X, Thomas SR, Lee WL, Turkoz M, McDonald KG, Meredith MM, Song C, Guidos CJ, Newberry RD, Ouyang W, Murphy TL, Stappenbeck TS, Gommerman JL, Nussenzweig MC, Colonna M, Kopan R, Murphy KM. Notch2-dependent classical dendritic cells orchestrate intestinal immunity to attaching-and-effacing bacterial pathogens. Nat. Immunol. 2013;14:937–948. doi: 10.1038/ni.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satpathy AT, KC W, Albring JC, Edelson BT, Kretzer NM, Bhattacharya D, Murphy TL, Murphy KM. Zbtb46 expression distinguishes classical dendritic cells and their committed progenitors from other immune lineages. J. Exp. Med. 2012a;209:1135–1152. doi: 10.1084/jem.20120030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satpathy AT, Wu X, Albring JC, Murphy KM. Re(de)fining the dendritic cell lineage. Nat. Immunol. 2012b;13:1145–1154. doi: 10.1038/ni.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlitzer A, McGovern N, Teo P, Zelante T, Atarashi K, Low D, Ho AW, See P, Shin A, Wasan PS, Hoeffel G, Malleret B, Heiseke A, Chew S, Jardine L, Purvis HA, Hilkens CM, Tam J, Poidinger M, Stanley ER, Krug AB, Renia L, Sivasankar B, Ng LG, Collin M, Ricciardi-Castagnoli P, Honda K, Haniffa M, Ginhoux F. IRF4 Transcription Factor-Dependent CD11b(+) Dendritic Cells in Human and Mouse Control Mucosal IL-17 Cytokine Responses. Immunity. 2013;38:970–983. doi: 10.1016/j.immuni.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraml BU, Hildner K, Ise W, Lee WL, Smith WA, Solomon B, Sahota G, Sim J, Mukasa R, Cemerski S, Hatton RD, Stormo GD, Weaver CT, Russell JH, Murphy TL, Murphy KM. The AP-1 transcription factor Batf controls T(H)17 differentiation. Nature. 2009;460:405–409. doi: 10.1038/nature08114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segre JA, Bauer C, Fuchs E. Klf4 is a transcription factor required for establishing the barrier function of the skin. Nat. Genet. 1999;22:356–360. doi: 10.1038/11926. [DOI] [PubMed] [Google Scholar]
- Spits H, Couwenberg F, Bakker AQ, Weijer K, Uittenbogaart CH. Id2 and Id3 inhibit development of CD34(+) stem cells into predendritic cell (pre-DC)2 but not into pre-DC1. Evidence for a lymphoid origin of pre-DC2. J Exp. Med. 2000;192:1775–1784. doi: 10.1084/jem.192.12.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tailor P, Tamura T, Morse HC, Ozato K. The BXH2 mutation in IRF8 differentially impairs dendritic cell subset development in the mouse. Blood. 2008;111:1942–1945. doi: 10.1182/blood-2007-07-100750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry RL, Miller SD. Molecular control of monocyte development. Cell Immunol. 2014 doi: 10.1016/j.cellimm.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torti N, Walton SM, Murphy KM, Oxenius A. Batf3 transcription factor-dependent DC subsets in murine CMV infection: differential impact on T-cell priming and memory inflation. Eur. J. Immunol. 2011;41:2612–2618. doi: 10.1002/eji.201041075. [DOI] [PubMed] [Google Scholar]
- Tussiwand R, Lee WL, Murphy TL, Mashayekhi M, Wumesh KC, Albring JC, Satpathy AT, Rotondo JA, Edelson BT, Kretzer NM, Wu X, Weiss LA, Glasmacher E, Li P, Liao W, Behnke M, Lam SS, Aurthur CT, Leonard WJ, Singh H, Stallings CL, Sibley LD, Schreiber RD, Murphy KM. Compensatory dendritic cell development mediated by BATF-IRF interactions. Nature. 2012;490:502–507. doi: 10.1038/nature11531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander LB, Khan AA, Hackney JA, Agrawal S, Lesch J, Zhou M, Lee WP, Park S, Xu M, DeVoss J, Spooner CJ, Chalouni C, Delamarre L, Mellman I, Singh H. Transcriptional programming of dendritic cells for enhanced MHC class II antigen presentation. Nat. Immunol. 2014;15:161–167. doi: 10.1038/ni.2795. [DOI] [PubMed] [Google Scholar]
- Wang Y, Szretter KJ, Vermi W, Gilfillan S, Rossini C, Cella M, Barrow AD, Diamond MS, Colonna M. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat. Immunol. 2012;13:753–760. doi: 10.1038/ni.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JW, Tjota MY, Clay BS, Vander LB, Bandukwala HS, Hrusch CL, Decker DC, Blaine KM, Fixsen BR, Singh H, Sciammas R, Sperling AI. Transcription factor IRF4 drives dendritic cells to promote Th2 differentiation. Nat. Commun. 2013;4:2990. doi: 10.1038/ncomms3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka S. Induction of pluripotent stem cells from mouse fibroblasts by four transcription factors. Cell Prolif. 2008;41(Suppl 1):51–56. doi: 10.1111/j.1365-2184.2008.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Hayashi M. Role of Kruppel-like factor 4 and its binding proteins in vascular disease. J. Atheroscler. Thromb. 2014;21:402–413. doi: 10.5551/jat.23044. [DOI] [PubMed] [Google Scholar]
- Zheng H, Pritchard DM, Yang X, Bennett E, Liu G, Liu C, Ai W. KLF4 gene expression is inhibited by the notch signaling pathway that controls goblet cell differentiation in mouse gastrointestinal tract. Am. J. Physiol Gastrointest. Liver Physiol. 2009;296:G490–G498. doi: 10.1152/ajpgi.90393.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Ho AW, Schlitzer A, Tang Y, Wong KH, Wong FH, Chua YL, Angeli V, Mortellaro A, Ginhoux F, Kemeny DM. GM-CSF-licensed CD11b+ lung dendritic cells orchestrate Th2 immunity to Blomia tropicalis. J. Immunol. 2014;193:496–509. doi: 10.4049/jimmunol.1303138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.