Abstract
Background
The incidence of thrombotic events increases during aging, but the mechanisms are not well understood. To investigate the prothrombotic role of oxidative stress during aging, we tested the hypothesis that aged mice overexpressing the antioxidant enzyme glutathione peroxidase-1 (Gpx1) are protected from experimental thrombosis.
Methods and Results
Susceptibility to carotid artery thrombosis was first examined in wild-type C57BL/6J mice. After photochemical injury of the carotid artery, the time to stable occlusion was significantly shorter in 12- and 18-month-old mice compared with 4-month-old mice (P<0.01). Unlike wild-type mice, transgenic mice overexpressing Gpx1 (Gpx1 Tg) did not exhibit shortened times to occlusion of the carotid artery at 12 or 18 months of age. Wild-type mice also exhibited increased susceptibility to venous thrombosis after inferior vena cava ligation at 12 or 18 months of age (P<0.05 versus 4 months of age). Gpx1 Tg mice were protected from this aging-related enhanced susceptibility to venous thrombosis. Age-dependent platelet hyperactivation, evidenced by increased hydrogen peroxide, fibrinogen binding, and activation of fibrinogen receptor αIIbβ3, was observed in thrombin-activated platelets from wild-type but not Gpx1 Tg mice (P<0.05). Enhanced platelet activation responses in aged mice were also prevented by polyethylene glycol-catalase or apocynin, an inhibitor of NADPH oxidase. Aged mice displayed increased intraplatelet expression of p47phox and superoxide dismutase-1, suggesting a mechanistic pathway for increased hydrogen peroxide generation.
Conclusions
Our findings demonstrate that hydrogen peroxide is a key mediator of platelet hyperactivity and enhanced thrombotic susceptibility in aged mice.
Keywords: aging, blood platelets, thrombosis
Thrombotic events such as stroke, myocardial infarction, deep vein thrombosis, and pulmonary embolism are common causes of morbidity and mortality in the elderly.1–3 Despite the well-established clinical associations between aging and thrombosis, surprisingly little is known about the mechanisms by which aging increases susceptibility to thrombotic events.
Several lines of evidence indicate that aging is accompanied by a generalized increase in oxidative stress and that increased generation of reactive oxygen species may contribute to cardiovascular events, including thrombosis. A marked increase in oxidative stress has been observed with aging in multiple tissues in humans4,5 and experimental animals.6,7 Increased oxidative stress has been mechanistically implicated in several of the cardiovascular consequences that are also associated with aging such as myocardial dysfunction,8,9 myocardial ischemia/reperfusion injury,10 hypertension,11 and endothelial dysfunction.12,13 Moreover, there is abundant evidence that reactive oxygen species regulate several components of thrombotic processes,14–16 including platelet activation. 17–19 To date, however, no studies have directly investigated the mechanistic contribution of reactive oxygen species to aging-related thrombosis.
The increased reactive oxygen species production associated with aging has been attributed in part to decreased expression and activity of antioxidant enzymes such as glutathione peroxidase,20–23 which reduces and detoxifies peroxides like hydrogen peroxide (H2O2). Glutathione peroxidase-1 (Gpx1) is the most abundant and widely expressed isoform of glutathione peroxidase in most tissues. A prospective human study demonstrated that the risk of cardiovascular events was inversely associated with increasing quartiles of erythrocyte Gpx1 activity.24 This observation suggests that peroxides may contribute to arterial thrombotic vascular events and that Gpx1 may be protective. It remains unclear, however, whether peroxides or Gpx1 plays a role in the increased thrombotic susceptibility of aging.
In the present study, we compared thrombotic responses to arterial or venous injury in young versus aged mice and examined the protective effects of overexpression of Gpx1. Our findings suggest that, in aging, peroxides mediate enhanced arterial and venous thrombosis and platelet hyperactivity. Our data also suggest that strategies to lower platelet H2O2 levels abrogate the enhanced activation of platelets from aged mice, providing mechanistic insights and a potential therapeutic approach to prevent aging-related thrombosis.
Methods
Mice
C57BL/6 mice were purchased from Jackson Laboratory at the age of 3 months and maintained in the animal care facilities of the University of Iowa. Gpx1 transgenic (Tg) mice were obtained from Dr Yi Shi Ho25 and bred at least 15 generations with C57BL/6 mice before study. Genotyping for the Gpx1 transgene was performed with real-time polymerase chain reaction (PCR).26 All animal protocols were approved by the University of Iowa Animal Care and Use Committee. Both male and female mice were included in the study. Mice at 4, 12, and 18 months of age were used for studies. These age groups were chosen in accordance with accepted principles for experiments on the biology of aging in mice.27 Because the mean life span of C57BL/6 mice is ≈27 to 29 months, the 4-, 12-, and 18-month-old mice are roughly equivalent to young adult (18–20 years of age), middle-aged (≈45–50 years of age), and older (>70 years of age) humans, respectively.
Carotid Artery Thrombosis
Carotid artery thrombosis was induced by photochemical injury as described previously.28 Mice were anesthetized with sodium pentobarbital (70–90 mg/kg IP) and ventilated mechanically with room air and supplemental oxygen. To induce photochemical injury to the endothelial layer, the right common carotid artery was dissected free and transilluminated continuously with a 1.5-mV, 540-nm green laser (Melles Griot, Carlsbad, CA) from a distance of 6 cm, and rose bengal (35 mg/kg) was injected via a femoral vein catheter. Blood flow was monitored continuously for 90 minutes or until stable occlusion occurred, at which time the experiment was terminated. Stable occlusion was defined as the time at which blood flow remained absent for ≥10 minutes.
Inferior Vena Cava Thrombosis
Susceptibility to thrombosis in the venous system was measured as described previously with minor modifications.29 Briefly, mice were anesthetized with ketamine/xylazine (87.5 mg/kg ketamine and 12.5 mg/kg xylazine IP). A midline laparotomy was made, and the inferior vena cava was exposed directly via blunt dissection. The inferior vena cava was ligated inferiorly to the left renal vein with a 6-0 silk suture, and mice were allowed to recover. Two days later, the inferior vena cava was harvested for measurement of the length and weight of thrombus.
Platelet Activation
Washed platelets were isolated and resuspended in modified Tyrode buffer (134 mmol/L NaCl, 2.9 mmol/L KCl, 2.9 mmol/L CaCl2, 0.34 mmol/L Na2HPO4, 12 mmol/L NaHCO3, 20 mmol/L HEPES, 1.0 mmol/L MgCl2, 5.0 mmol/L glucose, 0.05% [wt/vol] fatty acid–free BSA, pH 7.35) as described previously.30 To assess platelet activation, washed platelets were activated with human thrombin (0.5 U/ mL; Hematological Technologies, Essex Junction, VT) for 2 minutes at 37°C; incubated for 10 minutes with FITC-conjugated sheep anti-human fibrinogen antibody (Novus Biologicals, Littleton, CO), FITC-conjugated rat anti-mouse CD62P antibody for P-selectin (BD Biosciences), or PE-conjugated JON/A for activated αIIbβ3 (Emfret Analytics, Würzburg, Germany); fixed in 1% paraformaldehyde; diluted; and analyzed on a Becton Dickinson (San Diego, CA) FACScan flow cytometer. In some experiments, platelets were incubated with polyethylene glycol (PEG)-catalase (500 U/mL), apocynin (600 µmol/L), or N-nitro-l-arginine methyl ester (L-NAME; 100 µmol/L) for 15 minutes before stimulation with thrombin.
Measurement of Intracellular H2O2 in Platelets
Levels of platelet-derived intracellular H2O2 were measured as described previously with minor modifications.31 Platelets were prein-cubated with 10 µmol/L 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA; Molecular Probes, Gottingen, Germany) in either the presence or absence of PEG-catalase (500 U/mL) for 15 minutes. Platelets were then activated with 0.5 U/mL human thrombin, and the PEG-catalase–inhibitable fluorescent signal formed as a result of oxidation of H2DCF-DA was measured by flow cytometry.
Real-time PCR
Levels of mRNA for Gpx1, Nox1, Nox2, Nox4, p47phox, Sod1, cata-lase, and 18S were measured by quantitative real-time PCR as described previously.30 Total RNA was isolated from washed platelets with Trizol reagent (Invitrogen, Carlsbad, CA). Reverse-transcribed cDNA was incubated with TaqMan Universal PCR mix, PCR primers, and 6-carboxy fluorescein-labeled probes (Applied Biosystems) at 50°C for 2 minutes and then at 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. The comparative threshold cycle (ΔΔCT) method was used for quantification with values normalized to 18S and expressed relative to levels in 4-month-old wild-type (WT) mice. Validation experiments were performed to confirm equal amplification efficiency for all primer sets. Platelet purity was confirmed by 2 methods: complete blood counting and real-time PCR with primers for CD45, a marker for leukocytes. All samples used for real-time PCR had undetectable levels of CD45 mRNA, and the leukocyte and red blood cell counts were equivalent to background.
Platelet Count
Blood was collected by retro-orbital bleeding into a 20-µL EDTA-coated glass capillary tube and immediately diluted 1:10 into PBS with 5% BSA and analyzed by a laser-based Bayer Advia 120 whole-blood analyzer.
Statistical Analysis
One-way ANOVA with the Tukey test for multiple comparisons was used to compare occlusion time, baseline blood flow, platelet counts, platelet activation responses, and mRNA levels in C57BL/6 or WT mice of different ages. Two-way ANOVA with the Tukey test for multiple comparisons was used to compare time to occlusion, thrombus length and weight, H2O2 level, platelet activation response, platelet count, and baseline blood flow in Gpx1 Tg mice and WT littermate control mice. The paired Student t test was performed to compare platelet activation responses before and after treatment with inhibitors. All data were normally distributed except for the data in Figure 1; therefore, 1-way ANOVA was performed on log-transformed values for this data set. Statistical significance was defined as a value of P<0.05. All the values are reported as mean±SE.
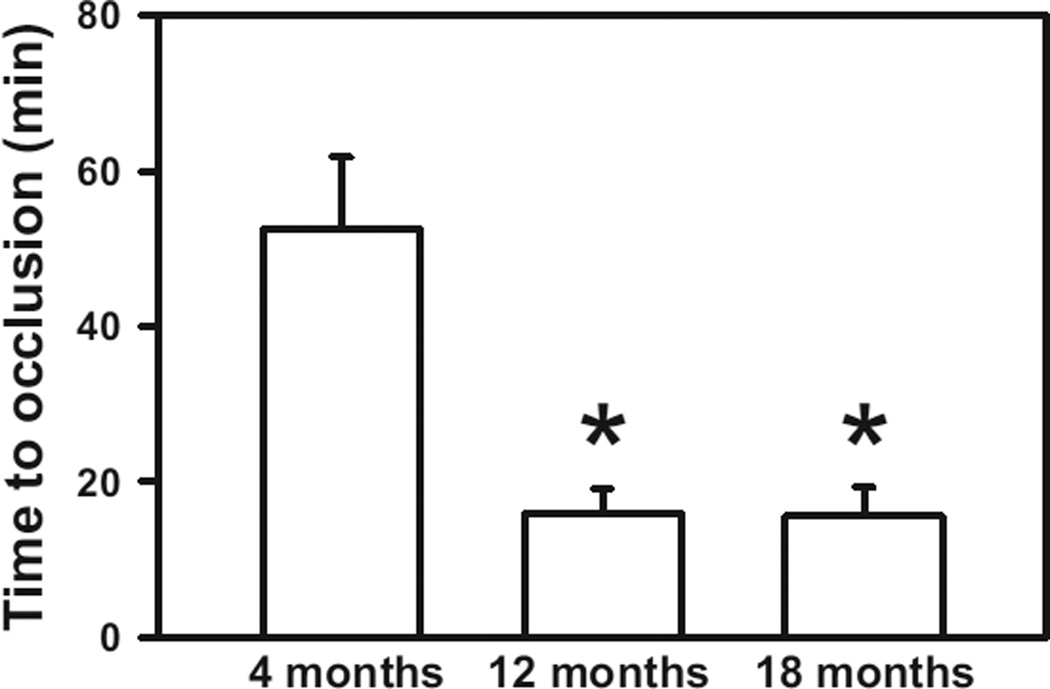
Figure 1.
Susceptibility to carotid artery thrombosis increases with age. The time to stable occlusion of the carotid artery after photochemical injury was measured in 4-, 12-, and 18-month-old wild-type C57BL/6 mice. Seven to 12 mice were studied in each group. Values are mean±SE. *P<0.05 vs 4-month-old mice by 1-wayANOVA.
Results
Susceptibility to Carotid Artery Thrombosis Is Increased in C57BL/6 Mice With Advancing Age
We first examined the relationship between age and susceptibility to arterial thrombosis in WT C57BL/6 mice (Figure 1). After photochemical injury of the carotid artery, the time to stable occlusion in 4-month-old mice was 52.5±9.3 minutes, whereas the time to develop stable occlusion was significantly shorter for both 12- and 18-month-old mice (15.9±3.2 and 15.6±3.7 minutes, respectively; P<0.01). No differences in baseline carotid artery blood flow were observed among 4-, 12-, and 18-month-old mice (Table 1). Platelet count did not differ significantly between 4- and 12-month-old mice but was ≈30% higher in 18-month-old mice (P<0.05 versus 4- and 12-month-old mice). These findings demonstrate that C57BL/6 mice exhibit increased susceptibility to carotid artery thrombosis with aging.
Table 1.
Baseline Carotid Artery Blood Flow and Platelet Count in C57BL/6 Mice
| Age Group | |||
|---|---|---|---|
| 4 mo | 12 mo | 18 mo | |
| Baseline blood flow, mL/min | 0.24±0.03 | 0.22±0.02 | 0.20±0.05 |
| Platelet count, × 1000/µL | 1393±66 | 1445±77 | 1835±146* |
Eight to 12 mice were studied in each group.
P<0.05 versus 4-month-old mice by 1 -way ANOVA.
Overexpression of Gpx1 Protects Aged Mice From Accelerated Arterial and Venous Thrombosis
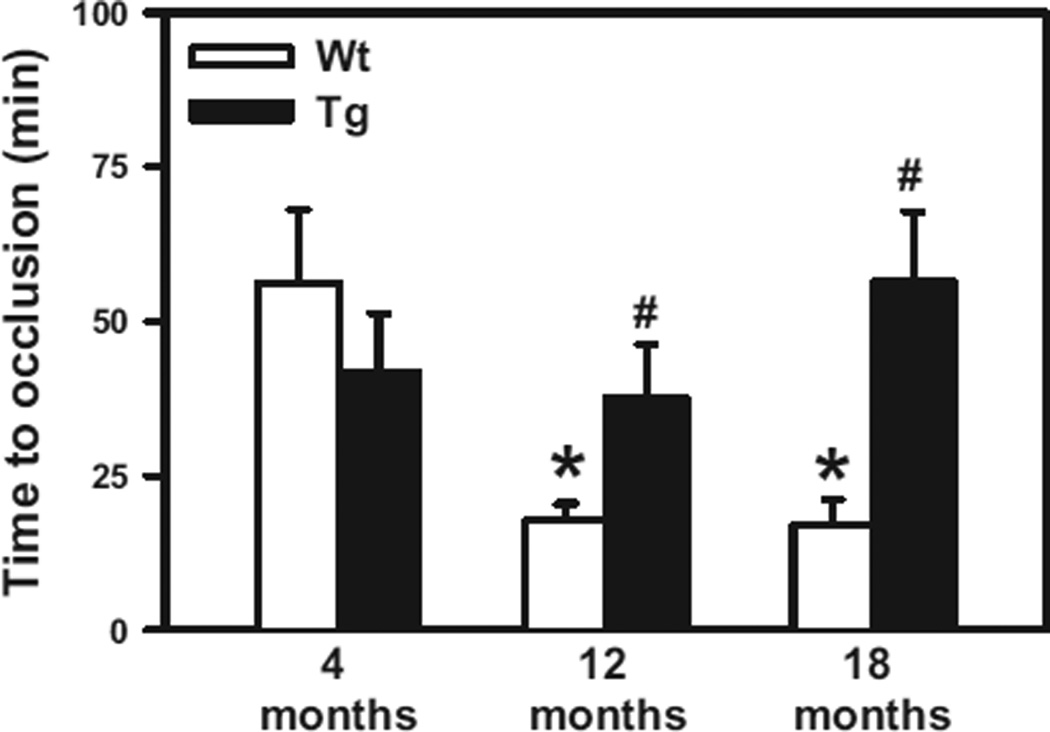
We next examined the role of oxidative stress in accelerated carotid artery thrombosis in aging using Gpx1 Tg mice and their WT littermates at 4, 12, and 18 months of age (Figure 2). Consistent with the findings in C57BL/6 mice, the time to develop stable occlusion of the carotid artery was significantly shorter in 12- and 18-month-old WT mice compared with 4-month-old WT mice (17.7±2.8, 16.9±4.2, and 56.2±1 minutes, respectively; P<0.05). At 4 months of age, the time to stable occlusion was similar in WT and Gpx1 Tg mice (56.2±10 and 41.7±9.6 minutes, respectively; P=0.35), suggesting that, at a young age, overexpression of Gpx1 does not influence susceptibility to arterial thrombosis. However, the 12- and 18-month-old Gpx1 Tg mice were protected from the age-induced shortening of the time to stable occlusion (37.6±8.6 and 56.3±11.4 minutes, respectively, compared with age-matched WT littermates; P<0.05). Of note, the times to stable occlusion in the 12- and 18-month-old Gpx1 Tg mice did not differ significantly from that of 4-month-old Gpx1 Tg mice (P=0.9 and P=0.4, respectively). There was a significant overall interaction between age and genotype by 2-way ANOVA (P<0.017), which reflects the lack of effect of the Gpx1 Tg genotype on stable occlusion in 4-month-old mice.
Figure 2.
Overexpression of glutathione peroxidase-1 (Gpx1) protects against age-associated increased susceptibility to carotid artery thrombosis. The time to stable occlusion of the carotid artery after photochemical injury was measured in 4-, 12-, and 18-month-old Gpx1 transgenic (Tg) mice or wild-type (WT) littermate controls. Five to 10 mice were studied in each group. Values are mean±SE. *P<0.05 vs 4-month-old WT mice; #P<0.05 vs age-matched WT littermates by 2-way ANOVA.
No significant differences in baseline carotid artery blood flow were observed between 4-, 12-, and 18-month-old WT or Gpx1 Tg mice (Table 2). Platelet counts were again found to be higher in 18-month-old WT or Gpx1 Tg mice compared with 4- or 12-month-old mice of the same genotype (P<0.05). There were no significant differences in platelet count between WT and Gpx1 Tg mice at any age.
Table 2.
Baseline Carotid Artery Blood Flow and Platelet Count in WT and Gpx1 Tg Littermates
| Age Group | |||
|---|---|---|---|
| 4 mo | 12 mo | 18 mo | |
| Baseline blood flow, mL/min | |||
| WT | 0.23±0.04 | 0.21±0.02 | 0.19±0.05 |
| Gpx1 Tg | 0.22±0.02 | 0.21±0.02 | 0.18±0.03 |
| Platelet count, × 1000/µL | |||
| WT | 1360±53 | 1474±61 | 1747±112* |
| Gpx1 Tg | 1286±47 | 1437±104 | 1880±72* |
Tg indicates transgenic; and WT, wild-type. Six to 11 mice were studied in each group.
P<0.05 versus 4- and 12-month-old mice by 2-way ANOVA.
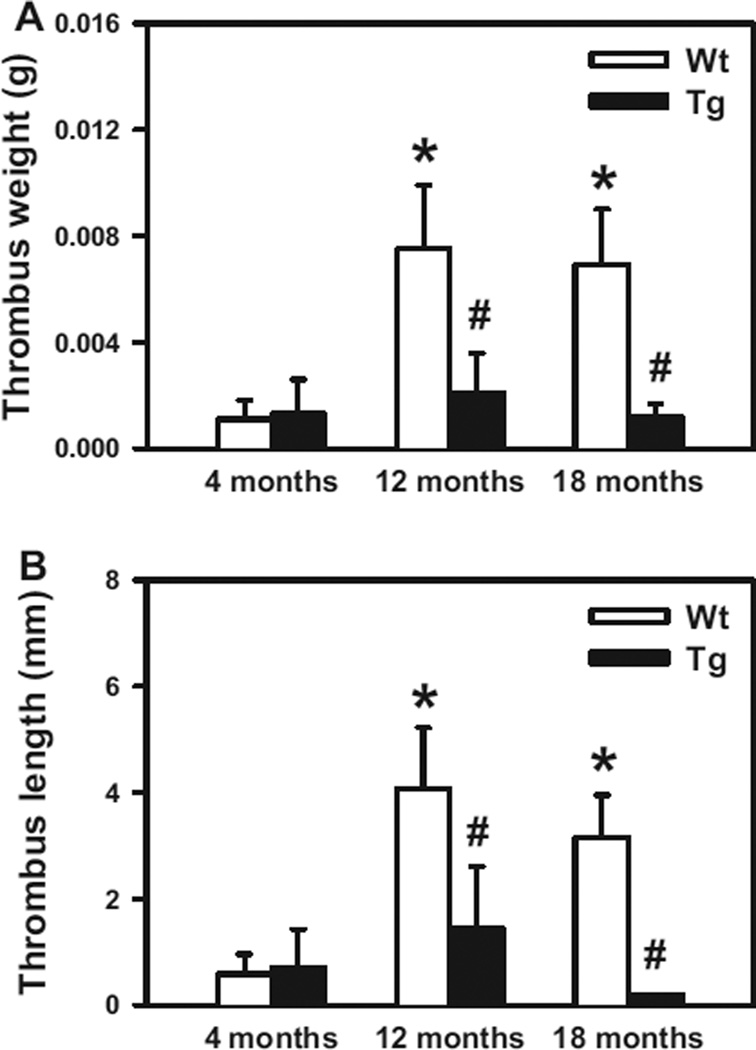
We next performed inferior vena cava ligation to examine whether Gpx1 overexpression provides protection from stasis-induced venous thrombosis (Figure 3). At 4 months of age, WT and Gpx1 Tg mice developed thrombi of similar length and weight (P>0.8). This observation suggests that overexpression of Gpx1 at a young age does not affect susceptibility to venous thrombosis. In contrast, the 12- and 18-month-old WT mice developed significantly larger and heavier inferior vena cava thrombi compared with 4-month-old WT mice (P<0.05). Overexpression of Gpx1 protected against this age-dependent effect, as evidenced by the significantly smaller thrombi developed in 12- and 18-month-old GPx1 Tg mice compared with age-matched WT littermates (P<0.05). Collectively, these findings demonstrate that mice develop increased susceptibility to both arterial and venous thrombosis with advancing age and that overexpression of Gpx1 protects against these adverse effects.
Figure 3.
Overexpression of glutathione peroxidase-1 (Gpx1) protects against age-associated increased susceptibility to venous thrombosis. The weight (A) and length (B) of thrombi that developed in the inferior vena cava 48 hours after ligation were measured in 4-, 12-, or 18-month-old wild-type (WT) or Gpx1 transgenic (Tg) mice. Ten to 11 mice were studied in each group. Values are mean±SE. *P<0.05 vs 4-month-old WT mice; #P<0.05 vs age-matched WT littermates by 2-way ANOVA.
Platelets From Aged Mice Exhibit Increased Platelet Adhesion During Thrombus Formation In Vivo
Because H2O2 has been reported to contribute to platelet activation responses,32,33 we hypothesized that the protective effect of overexpression of Gpx1 may be mediated through an effect on platelets. To address this hypothesis, platelets isolated from 4- or 18-month-old mice were differentially fluorescently labeled and infused into 4-week-old recipient mice. Thrombus formation in mesenteric arterioles was initiated with 5% ferric chloride, and the relative adhesion of young versus old platelets was quantified in real time by intravital microscopy. The results (Figure IA and IC and Movie I in the online-only Data Supplement) demonstrate that platelets from 18-month-old mice exhibit increased adhesion during thrombus formation in vivo compared with platelets from 4-month-old mice (P=0.017). There was also a trend toward decreased adhesion of infused platelets from aged (18-month-old) Gpx1 Tg mice compared with platelets from 18-month-old WT mice (Figure IB and ID and Movie II in the online-only Data Supplement).
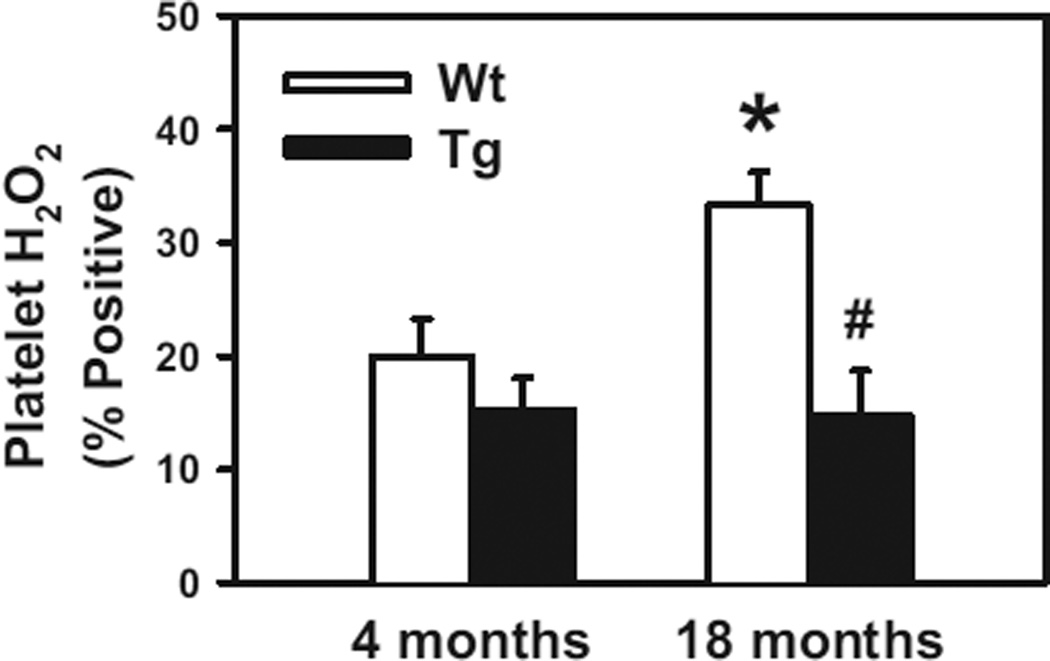
Activated Platelets From Aged Mice Generate Increased Levels of H2O2
To further investigate the role of H2O2 in platelet activation during aging, platelets were stimulated with thrombin in vitro. We found that the intracellular concentration of H2O2 in thrombin-stimulated platelets was significantly higher in 18-month-old WT mice than in 4-month-old WT mice (P<0.05; Figure 4). In contrast, platelet H2O2 levels in 18-month-old Gpx1 Tg mice were similar to those in 4-month-old Gpx1 Tg mice (P>0.8). These data suggest that aging produces an elevation in levels of H2O2 within activated platelets and that overexpression of Gpx1 protects against this response. To confirm that Gpx1 is overexpressed in platelets from Gpx1 Tg mice, we measured levels of Gpx1 mRNA and Gpx1 protein in isolated platelets by quantitative PCR and Western blotting, respectively. Regardless of age, Gpx1 Tg mice had a large increase in both Gpx1 mRNA and Gpx1 protein levels in platelets compared with WT littermates (P<0.05; Figure IIA-IIC in the online-only Data Supplement).
Figure 4.
Accumulation of H2O2 is increased in platelets from aged mice, and glutathione peroxidase-1 (Gpx1) overexpression is protective. Platelets from 4- or 18-month-old wild-type (WT) or Gpx1 transgenic (Tg) mice were activated with thrombin (0.5 U/mL), and then levels of intracellular H2O2 were measured as the polyethylene glycol-catalase-inhibitable fluorescent signal formed as a result of oxidation of 2’,7’-dichlorodihydrofluorescein diacetate by H2O2. Platelets from 7 to 8 mice were studied in each group. Values are mean±SE. *P<0.05 vs 4-month-old WT mice; #P<0.05 vs age-matched WT littermates by 2-way ANOVA.
Aging Is Associated With H2O2-Dependent Inside-out Platelet Activation
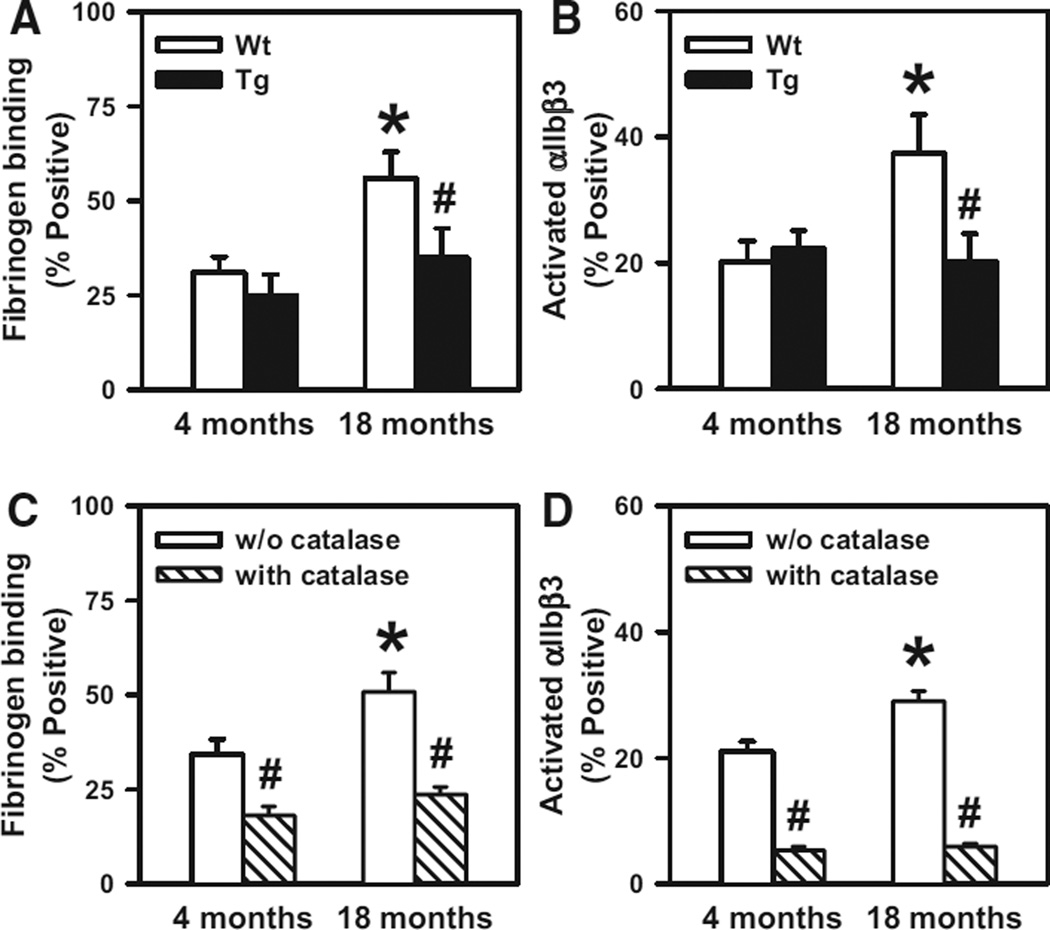
To determine whether increased generation of platelet H2O2 causes enhanced platelet activation in aged mice, we next examined surface P-selectin expression, fibrinogen binding, and integrin αIIbβ3 activation in thrombin-stimulated platelets from 4- and 18-month-old mice. No effects of advancing age or Gpx1 overexpression on P-selectin expression were observed (Figure III in the online-only Data Supplement), which suggests that the increased levels of platelet H2O2 in aged mice do not influence platelet granule release. In contrast, we observed an age-dependent increase in fibrinogen binding and αIIbβ3 activation in platelets from WT mice (P<0.05; Figure 5A and 5B). The increases in fibrinogen binding and αIIbβ3 activation were absent in 18-month-old Gpx1 Tg mice (P<0.05), suggesting a peroxide-dependent mechanism. To confirm the role of H2O2 versus other peroxides that are reduced by Gpx1, platelets from 4- and 18-month-old WT mice were treated with PEG-catalase, which selectively reduces H2O2. We found that PEG-catalase attenuated thrombin-stimulated fibrinogen binding and αIIbβ3 activation at both 4 and 18 months of age (P<0.05) and completely abolished the age-dependent increased platelet activation in mice (Figure 5C and 5D). Taken together, these findings suggest that H2O2 is a critical mediator of increased inside-out activation of integrin αIIbβ3 (the major receptor for fibrinogen), leading to hyperactivation of platelets in aged mice.
Figure 5.
Aging is associated with increased fibrinogen binding and activation of αIIbβ3, which are prevented with glutathione peroxidase-1 (Gpx1) overexpression or polyethylene glycol (PEG)-catalase treatment. Fibrinogen binding (A) and activation of αIIbβ3 (B) were examined in thrombin-activated platelets from either wild-type (WT) or Gpx1 transgenic (Tg) mice at 4 or 18 months of age. Platelets from 4- or 18-month-old WT mice were treated with or without PEG-catalase (500 U/mL), and fibrinogen binding (C) and activation of αIIbβ3 (D) were measured after thrombin activation. Platelets from 10 to 11 mice were studied in each group. Values are mean±SE. *P<0.05 vs 4-month-old WT mice; #P<0.05 vs age-matched WT littermates by 2-way ANOVA for A and B and by paired f test for C and D.
A Potential Mechanistic Pathway Leading to H2O2-Mediated Platelet Hyperactivation
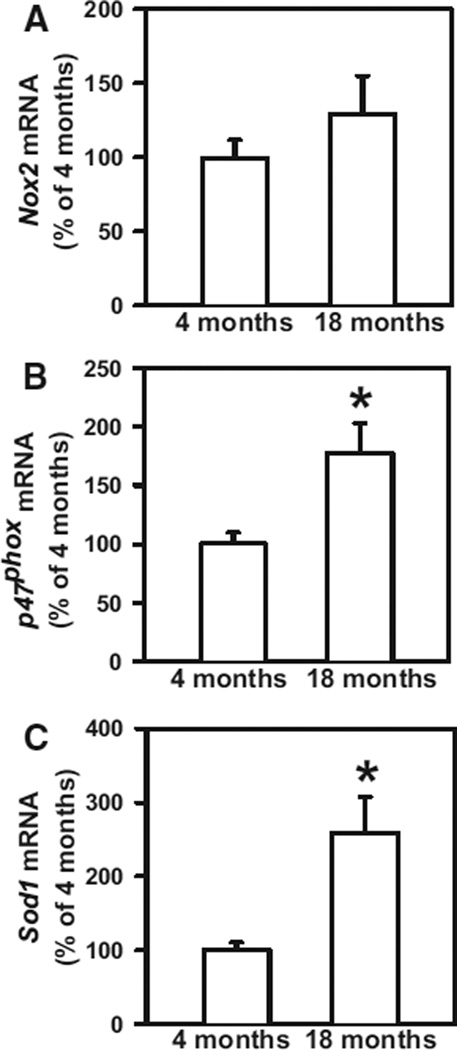
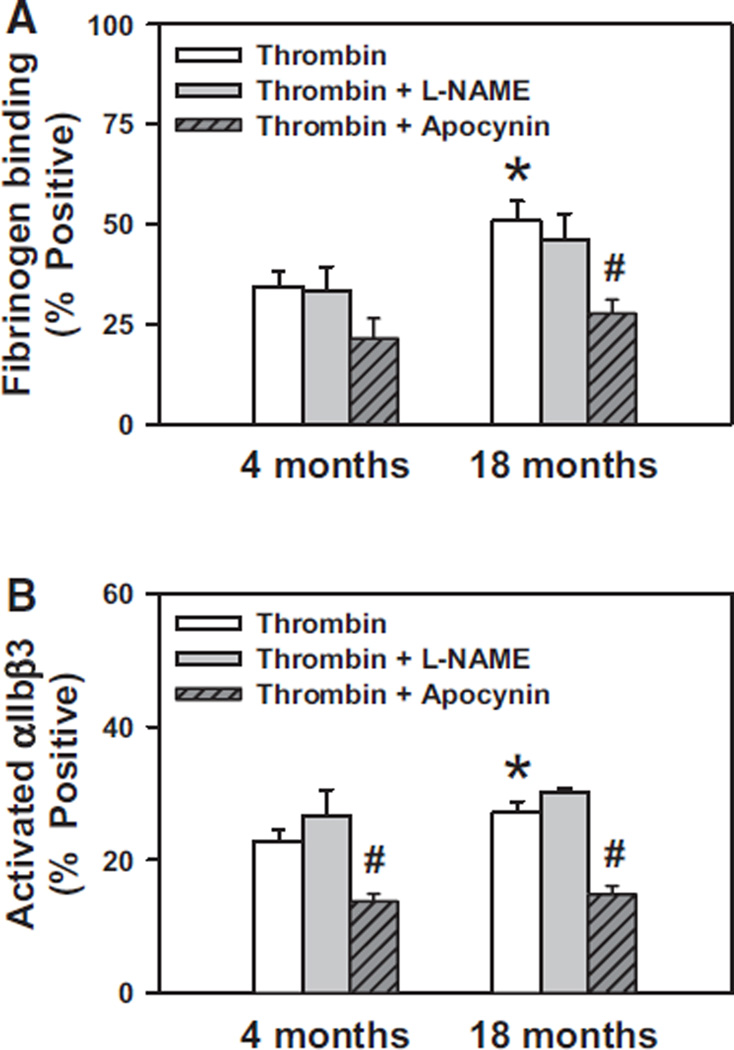
To identify potential mechanisms of H2O2-mediated platelet hyperactivation, we performed quantitative real-time PCR to measure mRNA levels of NADPH oxidase subunits and superoxide dismutase-1 (Sod1) in platelets. We found that platelets express significant levels of mRNA for the NADPH oxidase catalytic subunit Nox2 (Figure 6A) but do not express detectable levels of Nox1 or Nox4 mRNA at either 4 or 18 months of age (data not shown). We also observed a significant increase in platelet mRNA levels of the NADPH oxidase regulatory subunit p47phox (P<0.05; Figure 6B) in 18-month-old mice compared with 4-month-old mice. Furthermore, we observed an increase in Sod1 mRNA in platelets from aged mice (P<0.05; Figure 6C). These findings suggest a potential mechanistic pathway involving NADPH oxidase and SOD1 for increased generation of H2O2 in platelets from aged mice. To further assess the potential role of NADPH oxidase in aging-related platelet hyperactivation, we treated platelets from 4- and 18-month-old WT mice with the NADPH oxidase inhibitor apocynin. We found that apocynin inhibited the increase in both fibrinogen binding and activation of αIIbβ3 in platelets from 18-month-old mice (Figure 7A and 7B). In contrast, no inhibition of platelet activation was observed in the presence of the nitric oxide (NO) synthase inhibitor L-NAME, which suggests that NO synthase is not a source of reactive oxygen species leading to platelet hyperactivation in aging.
Figure 6.
P47phox and Sod1 are upregulated in platelets from aged mice. Platelet mRNA levels of Nox2 (A), p47Phox (B), and Sod1 (C) were measured by real-time polymerase chain reaction. Values were normalized to 18S mRNA and are expressed as percent of the control values observed in 4-month-old wild-type mice. Values are mean±SE. Platelets from 9 to 15 mice were studied in each group. *P<0.05 vs 4-month-old mice by 1-way ANOVA.
Figure 7.
Activation of platelets from aged mice is mediated in part by NADPH oxidase. A, Platelets from 4- or 18-month-old wild-type (WT) mice were treated with or without N-nitro-l-arginine methyl ester (L-NAME; 100 µmol/L) or apocynin (600 µmol/L) followed by activation with thrombin (0.5 U/mL), and then fibrinogen binding was measured by flow cytometry. B, Platelets from 4- or 18-month-old WT mice were treated as in A, and activation of αIIbβ3 was measured by flow cytometry. Values are mean±SE. Platelets from 7 to 8 mice were studied in each group. *P<0.05 vs 4-month-old mice without apocynin by 1-way ANOVA; #P<0.05 vs age-matched mice without apocynin by paired t test.
Discussion
In the present study, we examined the role of H2O2 in the mechanism of increased susceptibility to arterial and venous thrombosis with aging. The main findings from this study are the following: (1) With advancing age, C57BL/6 mice become increasingly susceptible to both arterial and venous thrombosis; (2) overexpression of the peroxide-reducing enzyme Gpx1 protects against the accelerated thrombosis of aging; and (3) platelet activation responses are increased in aging, and platelet hyperresponsiveness is mediated by H2O2 Taken together, these findings suggest a mechanism in which increased H2O2 production leads to platelet hyperactivity and enhanced susceptibility to thrombosis in aging.
One advantage of animal models of thrombosis, as opposed to human association studies, is that the contribution of aging can be assessed independently of other cardiovascular risk factors such as hypercholesterolemia, hypertension, obesity, and diabetes mellitus.34 In accordance with accepted principles for experiments on the biology of aging in mice,27 we used a systematic study design with a control group of 4-month-old mice to avoid potential confounding from studying younger mice still in the developmental stage. We included 2 experimental groups of 12- and 18-month-old mice. Finally, we examined susceptibility to both arterial and venous thrombosis. In agreement with a previous study,29 we demonstrated increased susceptibility to venous thrombosis in mice at 12 and 18 months of age compared with 4 months of age. We also observed increased susceptibility to carotid artery thrombosis in mice at 12 and 18 months of age using the rose bengal photochemical injury method of experimental thrombosis. In a prior study, Stämpfli and colleagues34 did not observe increased susceptibility to rose bengal-induced carotid artery thrombosis in mice at 15 or 24 months of age compared with mice at 11 weeks of age. Potential reasons for the discrepant results between our study and that of Stämpfli et al include their use of mice <4 months of age as the control group, a higher dose of rose bengal to initiate carotid artery injury, and a protocol that did not include mechanical ventilation to avoid acidosis and altered carotid artery blood flow.28 Overall, the consistent effects of age on both venous and arterial thrombosis in our study suggest that this murine model is an appropriate one for mechanistic studies of aging-associated thrombosis.
One novel finding of our study is that aged mice overex-pressing Gpx1 were protected from enhanced thrombotic susceptibility, which implicates H2O2 and/or lipid peroxides in the prothrombotic phenotype of aging. Previous studies in humans have suggested that prothrombotic conditions such as diabetes mellitus are associated with increased platelet H2O2 production and enhanced platelet activation responses.35,36 In concordance with these previous studies in humans, we found that intraplatelet H2O2 levels were elevated in activated platelets from aged mice with increased thrombotic susceptibility. We also found that platelets from aged mice exhibited increased adhesion to injured mesenteric arterioles in an in vivo model of thrombosis and enhanced activation responses to thrombin in vitro, in conjunction with increased accumulation of H2O2. Platelet hyperactivation was overcome not only by overexpression of Gpx1 but also by treatment with PEG-catalase, which implicates platelet H2O2 rather than lipid peroxides in the mechanism of enhanced platelet activation with aging.
We explored several possible mechanisms for the increased accumulation of H2O2 in activated platelets from aged mice. We first considered decreased Gpx1 expression in platelets as a potential mechanism because decreased expression of Gpx1 has been reported to be associated with advancing age in several other tissues20,21,23,37 and because decreased erythrocyte Gpx1 activity is associated with increased cardiovascular risk in patients with coronary artery disease.24 However, we did not observe any differences in platelet Gpx1 mRNA or protein levels between young and old mice (Figure II in the online-only Data Supplement). We also did not observe a decrease in the expression of catalase mRNA in platelets from aged mice (data not shown). These findings suggest that the increase in platelet H2O2 in aged mice is not caused by decreased expression of Gpx1 or catalase.
We next considered the potential roles of NADPH oxidase and superoxide dismutase as enzymatic sources of elevated H2O2 in platelets from aged mice. NADPH oxidase-dependent generation of superoxide has been reported to regulate platelet integrin αIIbβ3 activation.31 Additionally, platelets from patients with chronic granulomatous disease resulting from a deficiency of the NADPH oxidase catalytic subunit Nox2 have almost complete suppression of platelet superoxide production.38,39 Because H2O2 can be generated from superoxide by superoxide dismutase, we hypothesized that increased generation of H2O2 in platelets from aged mice results from increased NADPH oxidase-dependent production of superoxide that is subsequently converted to H2O2 by superoxide dismutase. In agreement with this hypothesis, we found that preincubation of platelets from aged mice with the NADPH oxidase inhibitor apocynin resulted in a significant decrease in thrombin-induced activation of αIIbβ3 and surface fibrinogen binding. We also observed increased expression of mRNA for the NADPH oxidase regulatory subunit p47Phox, as well as Sod1, in platelets from aged mice. Furthermore, we considered the possibility that platelet NADPH oxidase might directly generate H2O2 because isoforms of NADPH oxidase that contain the Nox4 catalytic subunit can generate significant amounts of H2O2.40,41 We consider this to be unlikely, however, because we did not detect any significant expression of Nox4 mRNA in platelets from young or old mice. Unlike Nox4-containing NADPH oxidases, Nox2-containing NADPH oxidases usually generate superoxide with little or no direct generation of H2O241 Future studies using mice deficient in p47phox or Nox2 might provide additional mechanistic insights into the role of platelet NADPH oxidases in platelet hyperactivity and thrombosis.
Interestingly, previous work by Freedman and colleagues42 and Jin et al43 has demonstrated that deficiency of plasma glutathione peroxidase (glutathione peroxidase-3, Gpx3) causes platelet hyperactivation in both humans and mice. The proposed mechanism was that deficiency of Gpx3 may lead to increased extracellular levels of H2O2, resulting in decreased bioavailability of NO and decreased NO-mediated inhibition of platelet activation. Our findings suggest that regulation of intracellular H2O2 by Gpx1 also affects platelet activation responses. The protective effect of Gpx1 is likely independent of platelet-derived NO because we did not observe any inhibition of platelet activation in the presence of the NO synthase inhibitor L-NAME (Figure 7). Although our data suggest a direct, NO-independent effect of aging on H2O2-mediated platelet activation, we recognize that aging is also associated with increased oxidative stress in the vessel wall and that decreased endothelium-derived NO may contribute in part to increased thrombotic susceptibility. In addition, other vascular cell-derived thrombotic and inflammatory factors such as plasminogen activator inhibitor-1, P-selectin, and tissue factor29 also may play a role in the mechanism by which increased oxidative stress enhances susceptibility to thrombosis during aging.
Given the well-established role of platelets in arterial thrombosis as opposed to venous thrombosis, it is perhaps surprising that we observed similar effects of aging and Gpx1 overexpression in experimental models of both arterial and venous thrombosis. However, the paradigm that platelets are more important in arterial than venous thrombosis has been challenged by recent work in mouse models44,45 and by human studies in which aspirin has proven to have benefit in the prevention of recurrent venous thromboembolism.46,47 Our work presented here is consistent with these recent findings and suggests a possible mechanism for enhanced platelet-dependent thrombosis in both arterial and venous systems during aging.
Finally, although there was a modest but significant increase in the platelet count in mice at 18 months of age compared with 4 or 12 months of age (Tables 1 and 2), it is unlikely that this difference contributed appreciably to the increased thrombotic susceptibility with aging for 2 reasons: First, the platelet count was significantly elevated only at 18 months of age, whereas increased susceptibility to arterial and venous thrombosis was apparent at both 12 and 18 months of age; and second, a similar elevation of the platelet count was observed in WT and Gpx1 Tg mice at 18 months of age (Table 2), and Gpx1 Tg mice were nevertheless protected from platelet hyperactivation and thrombosis. We previously reported that a similar increase in platelet count was not associated with increased thrombotic susceptibility in mice with hyperhomo-cysteinemia.48 We conclude therefore that the prothrombotic phenotype of aged mice is likely to be mediated by platelet hyperactivity rather than thrombocytosis.
Conclusions
We demonstrate here that aged mice develop increased susceptibility to both arterial and venous thrombosis and that H2O2-mediated platelet hyperactivation is a likely mechanism leading to this prothrombotic phenotype. These findings suggest that therapeutic strategies targeted toward lowering platelet H2O2 levels may have the potential to decrease thrombotic complications of aging. One potential strategy is to identify drugs that increase platelet glutathione peroxidase or catalase activity, perhaps by upregulating or allosterically increasing their activity. Another approach might be to develop inhibitors of the platelet NADPH oxidase/SOD1 pathway. Inhibition of SOD1 may prove to be problematic, however, because SOD1 inhibitors might be expected not only to decrease H2O2 but also to increase superoxide, which also may produce platelet hyperactivation. Targeting NADPH oxidase may prove to be more a promising approach because several small-molecule and peptide-based inhibitors of Nox2-containing NADPH oxidases subunits are currently in development.49,50
Supplementary Material
CLINICAL PERSPECTIVE.
Aging is associated with an increased incidence of thrombotic events such as stroke, myocardial infarction, deep vein thrombosis, and pulmonary embolism. However, the mechanisms by which aging increases susceptibility to thrombosis remain elusive. One potential causal link between aging and thrombotic susceptibility may be oxidative stress, which increases with aging and is hypothesized to play a pivotal role in cardiovascular disease. The objective of this study was to use a mouse model to investigate the mechanistic role of oxidative stress in aging-related thrombosis. To do so, we tested the specific hypothesis that aged mice overexpressing an antioxidant enzyme, glutathione peroxidase-1, are protected from experimental thrombosis. Our data demonstrate that susceptibility to both arterial and venous thrombosis is enhanced with aging by a mechanism involving hydrogen peroxide and that peroxide-mediated hyperactivation of platelets in aged mice may be particularly important for this prothrombotic phenotype. These findings suggest that targeted approaches to prevent the accumulation of peroxide in activated platelets may represent an alternative to general antioxidant therapy as a therapeutic strategy to prevent thrombotic complications of aging.
Acknowledgments
We thank Li Wang for her help in measuring platelet count in whole-blood samples. We also thank Kristina W Thiel for assistance in manuscript preparation and editing.
Sources of Funding
This work was supported by American Heart Association Beginning Grant-in-Aid 0860052Z to Dr Dayal, American Heart Association Scientist Development Grant SDG7520015 to Dr Chauhan, and by National Institutes of Health grants HL063943 and NS024621 (to Dr Lentz) and HL106495 (to Dr Motto).
Footnotes
Disclosures
None.
References
- 1.Wolf PA, Lewis A. Conner Lecture: contributions of epidemiology to the prevention of stroke. Circulation. 1993;88(pt 1):2471–2478. doi: 10.1161/01.cir.88.5.2471. [DOI] [PubMed] [Google Scholar]
- 2.Kelly-Hayes M, Beiser A, Kase CS, Scaramucci A, D’Agostino RB, Wolf PA. The influence of gender and age on disability following ischemic stroke: the Framingham study. J Stroke.Cerebrovasc Dis. 2003;12:119–126. doi: 10.1016/S1052-3057(03)00042-9. [DOI] [PubMed] [Google Scholar]
- 3.Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ3rd. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998;158:585–593. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- 4.Kasapoglu M, Ozben T. Alterations of antioxidant enzymes and oxidative stress markers in aging. Exp Gerontol. 2001;36:209–220. doi: 10.1016/s0531-5565(00)00198-4. [DOI] [PubMed] [Google Scholar]
- 5.Kawamoto EM, Munhoz CD, Glezer I, Bahia VS, Caramelli P, Nitrini R, Gorjão R, Curi R, Scavone C, Marcourakis T. Oxidative state in platelets and erythrocytes in aging and Alzheimer’s disease. Neurobiol Aging. 2005;26:857–864. doi: 10.1016/j.neurobiolaging.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Collins AR, Lyon CJ, Xia X, Liu JZ, Tangirala RK, Yin F, Boyadjian R, Bikineyeva A, Praticò D, Harrison DG, Hsueh WA. Age-accelerated atherosclerosis correlates with failure to upregulate antioxidant genes. Circ Res. 2009;104:e42–e54. doi: 10.1161/CIRCRESAHA.108.188771. [DOI] [PubMed] [Google Scholar]
- 7.Jacobson A, Yan C, Gao Q, Rincon-Skinner T, Rivera A, Edwards J, Huang A, Kaley G, Sun D. Aging enhances pressure-induced arterial superoxide formation. Am J Physiol Heart Circ Physiol. 2007;293:H1344–H1350. doi: 10.1152/ajpheart.00413.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oudot A, Martin C, Busseuil D, Vergely C, Demaison L, Ro-chette L. NADPH oxidases are in part responsible for increased cardiovascular superoxide production during aging. Free Radio Biol Med. 2006;40:2214–2222. doi: 10.1016/j.freeradbiomed.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 9.Yang B, Larson DF, Watson RR. Modulation of iNOS activity in age-related cardiac dysfunction. Life Sci. 2004;75:655–667. doi: 10.1016/j.lfs.2003.09.076. [DOI] [PubMed] [Google Scholar]
- 10.Liu P, Xu B, Cavalieri TA, Hock CE. Attenuation of antioxidative capacity enhances reperfusion injury in aged rat myocardium after MI/R. Am J Physiol Heart Circ Physiol. 2004;287:H2719–H2727. doi: 10.1152/ajpheart.00317.2004. [DOI] [PubMed] [Google Scholar]
- 11.Chou TC, Yen MH, Li CY, Ding YA. Alterations of nitric oxide synthase expression with aging and hypertension in rats. Hypertension. 1998;31:643–648. doi: 10.1161/01.hyp.31.2.643. [DOI] [PubMed] [Google Scholar]
- 12.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. 2007;100:1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- 13.Santhanam L, Lim HK, Lim HK, Miriel V, Brown T, Patel M, Balanson S, Ryoo S, Anderson M, Irani K, Khanday F, Di Costanzo L, Nyhan D, Hare JM, Christianson DW, Rivers R, Shoukas A, Berkowitz DE. Inducible NO synthase dependent S-nitrosylation and activation of arginasel contribute to age-related endothelial dysfunction. Circ Res. 2007;101:692–702. doi: 10.1161/CIRCRESAHA.107.157727. [DOI] [PubMed] [Google Scholar]
- 14.Herkert O, Diebold I, Brandes RP, Hess J, Busse R, Görlach A. NADPH oxidase mediates tissue factor-dependent surface procoagulant activity by thrombin in human vascular smooth muscle cells. Circulation. 2002;105:2030–2036. doi: 10.1161/01.cir.0000014611.28864.1e. [DOI] [PubMed] [Google Scholar]
- 15.Wood MJ, Helena Prieto J, Komives EA. Structural and functional consequences of methionine oxidation in thrombomodulin. Biochim Biophys Acta. 2005;1703:141–147. doi: 10.1016/j.bbapap.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Fu X, Chen J, Gallagher R, Zheng Y, Chung DW, López JA. Shear stress-induced unfolding of VWF accelerates oxidation of key methionine residues in the A1A2A3 region. Blood. 2011;118:5283–5291. doi: 10.1182/blood-2011-01-331074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Handin RI, Karabin R, Boxer GJ. Enhancement of platelet function by superoxide anion. J Clin Invest. 1977;59:959–965. doi: 10.1172/JCI108718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chlopicki S, Olszanecki R, Janiszewski M, Laurindo FR, Panz T, Mied-zobrodzki J. Functional role of NADPH oxidase in activation of platelets. Antioxid Redox Signal. 2004;6:691–698. doi: 10.1089/1523086041361640. [DOI] [PubMed] [Google Scholar]
- 19.Bakdash N, Williams MS. Spatially distinct production of reactive oxygen species regulates platelet activation. Free Radic Biol Med. 2008;45:158–166. doi: 10.1016/j.freeradbiomed.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 20.Hamden K, Carreau S, Ellouz F, Masmoudi H, El FA. Protective effect of 17beta-estradiol on oxidative stress and liver dysfunction in aged male rats. J Physiol Biochem. 2007;63:195–201. doi: 10.1007/BF03165782. [DOI] [PubMed] [Google Scholar]
- 21.Rey C, Véricel E, Némoz G, Chen W, Chapuy P, Lagarde M. Purification and characterization of glutathione peroxidase from human blood platelets: age-related changes in the enzyme. Biochim Biophys Acta. 1994;1226:219–224. doi: 10.1016/0925-4439(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 22.Mariani E, Mangialasche F, Feliziani FT, Cecchetti R, Malavolta M, Bastiani P, Baglioni M, Dedoussis G, Fulop T, Herbein G, Jajte J, Monti D, Rink L, Mocchegiani E, Mecocci P. Effects of zinc supplementation on antioxidant enzyme activities in healthy old subjects. Exp Gerontol. 2008;43:445–451. doi: 10.1016/j.exger.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Kishido T, Unno K, Yoshida H, Choba D, Fukutomi R, Asahina S, Iguchi K, Oku N, Hoshino M. Decline in glutathione peroxidase activity is a reason for brain senescence: consumption of green tea catechin prevents the decline in its activity and protein oxidative damage in ageing mouse brain. Biogerontology. 2007;8:423–430. doi: 10.1007/s10522-007-9085-7. [DOI] [PubMed] [Google Scholar]
- 24.Blankenberg S, Rupprecht HJ, Bickel C, Torzewski M, Hafner G, Tiret L, Smieja M, Cambien F, Meyer J, Lackner KJ. AtheroGene Investigators. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. N Engl J Med. 2003;349:1605–1613. doi: 10.1056/NEJMoa030535. [DOI] [PubMed] [Google Scholar]
- 25.Cheng WH, Ho YS, Ross DA, Han Y, Combs GF, Jr, Lei XG. Overex-pression of cellular glutathione peroxidase does not affect expression of plasma glutathione peroxidase or phospholipid hydroperoxide glutathione peroxidase in mice offered diets adequate or deficient in selenium. J Nutr. 1997;127:675–680. doi: 10.1093/jn/127.5.675. [DOI] [PubMed] [Google Scholar]
- 26.Chrissobolis S, Didion SP, Kinzenbaw DA, Schrader LI, Dayal S, Lentz SR, Faraci FM. Glutathione peroxidase-1 plays a major role in protecting against angiotensin II-induced vascular dysfunction. Hypertension. 2008;51:872–877. doi: 10.1161/HYPERTENSIONAHA.107.103572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller RA, Nadon NL. Principles of animal use for gerontological research. J Gerontol A Biol Sci Med Sci. 2000;55:B117–B123. doi: 10.1093/gerona/55.3.B117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson KM, Lynch CM, Faraci FM, Lentz SR. Effect of mechanical ventilation on carotid artery thrombosis induced by photochemical injury in mice. J Thromb Haemost. 2003;1:2669–2674. doi: 10.1111/j.1538-7836.2003.00482.x. [DOI] [PubMed] [Google Scholar]
- 29.McDonald AP, Meier TR, Hawley AE, Thibert JN, Farris DM, Wrobleski SK, Henke PK, Wakefield TW, Myers DD., Jr Aging is associated with impaired thrombus resolution in a mouse model of stasis induced thrombosis. Thromb Res. 2010;125:72–78. doi: 10.1016/j.thromres.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Dayal S, Wilson KM, Leo L, Arning E, Bottiglieri T, Lentz SR. Enhanced susceptibility to arterial thrombosis in a murine model of hyperhomocys-teinemia. Blood. 2006;108:2237–2243. doi: 10.1182/blood-2006-02-005991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Begonja AJ, Gambaryan S, Geiger J, Aktas B, Pozgajova M, Nieswandt B, Walter U. Platelet NAD(P)H-oxidase-generated ROS production regulates alphaIIbbeta3-integrin activation independent of the NO/cGMP pathway. Blood. 2005;106:2757–2760. doi: 10.1182/blood-2005-03-1047. [DOI] [PubMed] [Google Scholar]
- 32.Pignatelli P, Pulcinelli FM, Lenti L, Gazzaniga PP, Violi F. Hydrogen peroxide is involved in collagen-induced platelet activation. Blood. 1998;91:484–490. [PubMed] [Google Scholar]
- 33.Pignatelli P, Pulcinelli FM, Lenti L, Gazzaniga PP, Violi F. Vitamin E inhibits collagen-induced platelet activation by blunting hydrogen peroxide. Arterioscler Thromb Vasc Biol. 1999;19:2542–2547. doi: 10.1161/01.atv.19.10.2542. [DOI] [PubMed] [Google Scholar]
- 34.Stämpfli SF, Akhmedov A, Gebhard C, Lohmann C, Holy EW, Rozenberg I, Spescha R, Shi Y, Lüscher TF, Tanner FC, Camici GG. Aging induces endothelial dysfunction while sparing arterial thrombosis. Arterioscler Thromb Vasc Biol. 2010;30:1960–1967. doi: 10.1161/ATVBAHA.110.206920. [DOI] [PubMed] [Google Scholar]
- 35.Alexandru N, Constantin A, Popov D. Carbonylation of platelet proteins occurs as consequence of oxidative stress and thrombin activation, and is stimulated by ageing and type 2 diabetes. Clin Chem Lab Med. 2008;46:528–536. doi: 10.1515/CCLM.2008.104. [DOI] [PubMed] [Google Scholar]
- 36.Leoncini G, Signorello MG, Piana A, Carrubba M, Armani U. Hyperactivity and increased hydrogen peroxide formation in platelets of NIDDM patients. Thromb Res. 1997;86:153–160. doi: 10.1016/s0049-3848(97)00058-3. [DOI] [PubMed] [Google Scholar]
- 37.He T, Joyner MJ, Katusic ZS. Aging decreases expression and activity of glutathione peroxidase-1 in human endothelial progenitor cells. Microvasc Res. 2009;78:447–452. doi: 10.1016/j.mvr.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pignatelli P, Carnevale R, Di Santo S, Bartimoccia S, Sanguigni V, Lenti L, Finocchi A, Mendolicchio L, Soresina AR, Plebani A, Violi F. Inherited human gp91phox deficiency is associated with impaired isopros-tane formation and platelet dysfunction. Arterioscler Thromb Vasc Biol. 2011;31:423–434. doi: 10.1161/ATVBAHA.110.217885. [DOI] [PubMed] [Google Scholar]
- 39.Pignatelli P, Sanguigni V, Lenti L, Ferro D, Finocchi A, Rossi P, Violi F. gp91phox-dependent expression of platelet CD40 ligand. Circulation. 2004;110:1326–1329. doi: 10.1161/01.CIR.0000134963.77201.55. [DOI] [PubMed] [Google Scholar]
- 40.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 41.Takac I, Schröder K, Zhang L, Lardy B, Anilkumar N, Lambeth JD, Shah AM, Morel F, Brandes RP. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J Biol Chem. 2011;286:13304–13313. doi: 10.1074/jbc.M110.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Freedman JE, Loscalzo J, Benoit SE, Valeri CR, Barnard MR, Michelson AD. Decreased platelet inhibition by nitric oxide in two brothers with a history of arterial thrombosis. J Clin Invest. 1996;97:979–987. doi: 10.1172/JCI118522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin RC, Mahoney CE, Coleman Anderson L, Ottaviano F, Croce K, Leopold JA, Zhang YY, Tang SS, Handy DE, Loscalzo J. Glutathione peroxidase-3 deficiency promotes platelet-dependent thrombosis in vivo. Circulation. 2011;123:1963–1973. doi: 10.1161/CIRCULATIONAHA.110.000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brill A, Fuchs TA, Chauhan AK, Yang JJ, De Meyer SF, Köllnberger M, Wakefield TW, Lämmle B, Massberg S, Wagner DD. von Willebrand factor-mediated platelet adhesion is critical for deep vein thrombosis in mouse models. Blood. 2011;117:1400–1407. doi: 10.1182/blood-2010-05-287623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.von Brühl ML, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M, Khandoga A, Tirniceriu A, Coletti R, Köllnberger M, Byrne RA, Laitinen I, Walch A, Brill A, Pfeiler S, Manukyan D, Braun S, Lange P, Riegger J, Ware J, Eckart A, Haidari S, Rudelius M, Schulz C, Echtler K, Brink-mann V, Schwaiger M, Preissner KT, Wagner DD, Mackman N, Engel-mann B, Massberg S. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209:819–835. doi: 10.1084/jem.20112322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brighton TA, Eikelboom JW, Mann K, Mister R, Gallus A, Ockelford P, Gibbs H, Hague W, Xavier D, Diaz R, Kirby A, Simes J. ASPIRE Investigators Low-dose aspirin for preventing recurrent venous thromboembolism. NEngl J Med. 2012;367:1979–1987. doi: 10.1056/NEJMoa1210384. [DOI] [PubMed] [Google Scholar]
- 47.Becattini C, Agnelli G, Schenone A, Eichinger S, Bucherini E, Silingardi M, Bianchi M, Moia M, Ageno W, Vandelli MR, Grandone E, Prandoni P. WARFASA Investigators. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med. 2012;366:1959–1967. doi: 10.1056/NEJMoa1114238. [DOI] [PubMed] [Google Scholar]
- 48.Dayal S, Chauhan AK, Jensen M, Leo L, Lynch CM, Faraci FM, Kru-ger WD, Lentz SR. Paradoxical absence of a prothrombotic phe-notype in a mouse model of severe hyperhomocysteinemia. Blood. 2012;119:3176–3183. doi: 10.1182/blood-2011-09-380568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.El-Benna J, Dang PM, Périanin A. Towards specific NADPH oxidase inhibition by small synthetic peptides. Cell Mol Life Sci. 2012;69:2307–2314. doi: 10.1007/s00018-012-1008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Drummond GR, Selemidis S, Griendling KK, Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov. 2011;10:453–471. doi: 10.1038/nrd3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.