Abstract
Context
Endothelial dysfunction has been suggested as a potential mechanism by which ambient air pollution may cause acute cardiovascular events. Recently, plasma nitrite has been developed as a marker of endothelial dysfunction.
Objectives
We examined the changes in plasma nitrite concentration associated with increases in ambient air pollutant concentrations in the previous 7 d.
Materials and methods
We linked up to three measurements of plasma nitrite concentrations obtained from 49 students to 24-h average concentrations of five criteria air pollutants [particle mass<2.5 mm in aerodynamic diameter (PM2.5), carbon monoxide (CO), sulfur dioxide (SO2), nitrogen dioxide (NO2), and ozone (O3)] measured at two monitoring sites closest to Rutgers University campus (6–15 miles) in New Jersey during the years 2006–2009. We examined the change in plasma nitrite associated with each interquartile-range (IQR) increase in pollutant concentration in the previous 24 h and six preceding 24- h periods, using linear mixed models.
Results
IQR increases in mean PM2.5 (7.0 μg/m3) and CO (161.7 parts per billion) concentrations in the first 24 h before the plasma nitrite measurement were associated with increased plasma nitrite concentrations (PM2.5: 15.5 nanomolar; 95% confidence interval (CI): 2.4, 28.5; CO: 15.6 nanomolar; 95% CI: 2.4, 28.9). Increased plasma nitrite associated with IQR increases in O3 and SO2 concentrations over longer lags were observed.
Discussion and conclusion
Rapid increases in plasma nitrite following exposure to ambient air pollutants support the hypothesis that ambient air pollution is associated with inducible nitric oxide synthase-mediated systemic inflammation in humans.
Keywords: Air pollution, ambient air pollutants, plasma nitrite concentration
Introduction
A growing body of literature has consistently confirmed an acute association between increased ambient air pollutant concentrations and increased hospital admissions and deaths due to cardiovascular (CV) diseases (Bell et al., 2008; Chang et al., 2013; Dominici et al., 2006; Hsieh et al., 2013; Mann et al., 2002; Samet et al., 2000; Wellenius et al., 2005a,b; Yang et al., 2013; Zhang et al., 2014). Elevated levels of pollutants such as ambient particulate matter<2.5 μm in aerodynamic diameter (PM2.5), ozone (O3), sulfur dioxide (SO2), nitrogen dioxide (NO2) and elemental carbon have been acutely associated with adverse CV events (Brook et al., 2004, 2010; Peters et al., 2001; Rich et al., 2005, 2006a,b; Nuvolone et al., 2011; Wellenius et al., 2005a,b, 2006). For instance, we and others have observed significantly increased risk of ventricular arrhythmia, paroxysmal atrial fibrillation, myocardial infarction, congestive heart failure, and stroke, associated with increases in at least one of the above-mentioned criteria air pollutants in the 24 h before the CV event (Nuvolone et al., 2011; Peters et al., 2001; Rich et al., 2005, 2006a,b; Wellenius et al., 2005a,b, 2006).
Adverse CV events associated with elevated levels of air pollutants can be observed rapidly following an increase in pollutant concentrations or exposure (Bhaskaran et al., 2011; Burgan et al., 2010; Peters et al., 2004, 2013; Rich et al., 2005, 2006a). Although several mechanisms may operate concurrently in response to inhalation of pollutants, many epidemiological and clinical studies have suggested a role for endothelial dysfunction and impaired vascular reactivity in mediating acute and chronic CV outcomes among healthy and susceptible populations such as those with type 2 diabetes mellitus (Dales et al., 2007; Liu et al., 2007; O'Neill et al., 2005; Rundell et al., 2007; Schneider et al., 2008). These studies demonstrated a 0.5–17% decrease in flow-mediated dilation of the brachial artery (BAFMD) and suggested that acute exposures to ambient air pollutants may produce endothelial dysfunction, or vascular smooth muscle dysfunction. These effects may be either via reduction of endothelial production of nitric oxide (NO) or via excess depletion of NO due to production of oxidant radicals resulting from particle-associated inflammation and oxidative stress (Ghio et al., 2000; Sorensen et al., 2003; Sullivan et al., 2003).
Recently, venous plasma nitrite, the main oxidation product of nitric oxide has been used as a marker of NO production following a physiological endothelial stimulus such as reactive hyperemia of the forearm (Allen et al., 2005; Lauer et al., 2001). Allen et al. demonstrated the utility of plasma nitrite in discriminating individuals with peripheral vascular disease from individuals without vascular disease by measuring a relative change in this marker following exercise (Allen et al., 2009). In this article, we sought to examine the changes in plasma nitrite associated with ambient increases in concentrations of five criteria air pollutant, namely PM2.5, carbon monoxide (CO), SO2, NO2 and O3 in the 7 d prior to nitrite measurement. We hypothesized that increases in mean ambient air pollutant levels may be associated with a decrease in the endothelial production of NO and thus nitrite, consistent with decreases in flow-mediated dilation of the brachial artery (Dales et al., 2007; Liu et al., 2007; O'Neill et al., 2005; Rundell et al., 2007).
Methods
Data source
We used data from a double-blind crossover study conducted at the Environmental and Occupational Health Sciences Institute (EOHSI) in Piscataway, NJ (Kipen et al., 2011). The primary study examined acute mechanisms possibly leading to adverse CV outcomes, associated with 2-h exposures to two fresh pollution aerosols, namely diesel exhaust (DE) or secondary organic aerosol (SOA), relative to filtered clean air (CA), in a panel of college students. The current secondary data analysis focuses on baseline measures obtained on one of the markers of endothelial function, i.e. plasma nitrite concentration obtained prior to the exposure to air pollutants. The study was approved by the Rutgers-Robert Wood Johnson Medical School Institutional Review Board.
Study subjects
Healthy, non-smoking men and women between the ages of 18–30 years were recruited from the Busch campus of Rutgers University in Piscataway during the years 2005–2009. Potential candidates were screened using a telephone questionnaire to determine their eligibility for participation. Candidates were excluded if they indicated presence of CV disease, cancer, stroke, hypertension, pulmonary diseases including asthma, endocrine disease, and kidney/liver disease. Eighty-two eligible candidates gave a written informed consent, following which they underwent a history and physical examination including standard laboratory evaluation, electrocardiogram, and spirometry at the Clinical Center of EOHSI. Based on the physician's review, 10 subjects were excluded from participation for safety and comfort reasons and of the 72 subjects who were invited to participate in the study, 9 withdrew from the study due to personal or scheduling reasons prior to commencing exposures, resulting in a sample of 63 subjects. Although the primary study enrolled 63 subjects, data on plasma nitrite were available on only 49 (77.7% of the total study sample) subjects, because the plasma nitrite measurement was introduced to the primary study design 8 months after its initiation. Thus, the final study sample comprised of 49 subjects.
Plasma nitrite measurements
Each subject underwent measurement of markers of endothelial function including plasma nitrite 1 d per week over 3 consecutive weeks. For a majority of the subjects, visits were scheduled on the same day of the week. All outcomes were evaluated upon arrival for each exposure session, at baseline, i.e. prior to the exposure to DE, SOA, or CA, in a controlled environmental facility, as well as immediately following a 2-h exposure to DE, SOA and CA, as reported previously for other outcomes in this population (Kipen et al., 2011). However, the current analysis focused only on the baseline measurements obtained prior to the exposure. On each day of testing, subjects reported to the Clinical Center at 8:00 AM following an overnight fast beginning at 12:00 midnight the day before testing. They were instructed to abstain from caffeinated/alcoholic beverages, vigorous exercise less than 12 h prior to the testing, aspirin containing medications and other anti-inflammatory medications such as non-steroidal anti-inflammatory drugs 2 weeks prior to the testing. After verifying the self-reported adherence to these instructions, a pre-exposure blood sample (4.5 ml) was collected in a vacuum tube containing EDTA as an anticoagulant using standard phlebotomy procedure. Samples were immediately transported on ice to a laboratory three floors above and centrifuged for 15 min at a speed of 1600 × g at 4 °C. Plasma was aliquotted into 1.5-ml tubes. Samples were then shock frozen in liquid nitrogen and were stored at −80 °C. The NO content of plasma samples was measured by chemical reduction-linked chemiluminescence using a Sievers 280 NO analyzer (Sievers Instruments, Boulder, CO). Reduction of nitrite to NO was achieved with the use of a KI and concentrated acetic acid mixture. Nitrite concentration was reported in nanomolar (nM). The final sample of 49 subjects comprised 128 observations where data on plasma nitrite concentration were available on all three visits from 31 subjects, on two visits from 17 subjects, and on one visit from 1 subject.
Air pollutants
Hourly ambient concentrations of five criteria air pollutants, namely, PM2.5, CO, SO2, NO2 and O3 were measured by New Jersey monitoring stations sponsored by the US Environmental Protection Agency and retrieved for the study period (US EPA). The study period during which plasma nitrite measurements were made, extended from 1 August 2006 to 31 May 2009. Thus, there were 24 840 h and 1035 d in the study period. Since the subjects either lived on the Campus or in close proximity to the campus at the time of their study participation, the pollutant-specific monitor closest to the campus was selected to represent subjects' exposure to ambient air pollutants during the study. One monitor located in Perth Amboy (~15 miles from the EOHSI) measured CO and SO2 whereas a monitor located in East Brunswick (~6 miles from EOHSI) measured O3, NO2 and PM2.5. We identified pollutant concentrations for the seven 24-h periods (i.e. 168 h) before each clinic visit start time (8 AM). These data were then linked to the plasma nitrite measurements by date and hour of the day. We calculated seven moving averages of pollutant concentrations as follows: mean of hours 0–23 (lag day 0), mean of hours 24–47 (lag day 1), mean of hours 48–71 (lag day 2), mean of hours 72–95 (lag day 3), mean of hours 96–119 (lag day 4), mean of hours 120–143 (lag day 5) and mean of hours 144–167 (lag day 6). If greater than 25% of the total hours of pollutant concentrations were missing, we set the mean for that lag period to missing.
Statistical analyses
We summarized the demographic and clinical characteristics of the study subjects using means and standard deviations or percentages. Next, we calculated means, standard deviations and interquartile ranges (IQR) to summarize pollutant concentrations and weather parameters such as temperature and relative humidity. Linear mixed models examined the change in plasma nitrite concentration associated with increases in the pollutant concentrations over seven lagged hour intervals (0–23, 24–47, 48–71, 72–95, 96–119, 120–143 and 144–167 h). The changes in the plasma nitrite measurements associated with increases in the pollutant concentrations obtained using the linear mixed models were based on a cross-sectional study and the estimates did not imply a temporal shift in plasma nitrite measurements. A random effect for each subject induced a compound symmetry between observations taken on the same subject. Confounding effects of ambient temperature, relative humidity, day of the week, calendar month and year were examined. Hourly temperature and relative humidity measurements made at the Newark International Airport approximately 27 miles from EOHSI were retrieved from National Climatic Data Center (NCDC). However, final analyses only adjusted for temperature in the first 24 h prior to the plasma nitrite measurement (i.e. mean of hourly temperature recorded from 0 to 23 h, lag day 0) in a linear fashion, calendar month, and year. This was because relative humidity levels and day of the week were not found to be independently associated with plasma nitrite, thus, did not meet the criteria to be a confounder. Scatter plots and Akaike's Information Criterion were used to confirm the suitability of these models. The changes in plasma nitrite concentration associated with changes in pollutants and their 95% confidence intervals (CI's) obtained from the models were scaled to the IQR of the 24-h/daily mean pollutant concentrations observed during the study period.
To assess the stability and robustness of our main analyses, we performed three sensitivity analyses. First, we adjusted for lagged temperature using the same lags as the pollutant to examine if the changes in plasma nitrite using this method were different from the models that adjusted for the effect of mean temperature from 0 to 23 h. For example, we adjusted for mean temperature over lag day 1 (24–47 h) in the model examining the change in plasma nitrite associated with pollutant concentration on lag day 1. Second, we redefined the pollutant lags using a cumulative method (mean of hours 0–23, mean of hours 0–47, mean of hours 0–71, mean of hours 0–95, mean of hours 0–119, mean of hours 0–143, and mean of hours 0–167) to assess if the pollutant concentrations had cumulative effects on plasma nitrite. Third, we fit two pollutant models for those pollutants only where increased concentrations in specific lags were associated with an increase in plasma nitrite in the single pollutant model. This was done to evaluate whether each pollutant effect observed in the single pollutant model was independent of other pollutants. All analyses were conducted using the SAS software (Version 9.3 SAS Institute Inc., Cary, NC).
Results
Study subjects
Table 1 provides demographic characteristics of the study subjects. Approximately 80% of the subjects were white males. The mean age was 21.2 [±Standard Deviation=3.0] years. Standard clinical characteristics including systolic and diastolic blood pressures, body mass index and lipid profile were within a normal range.
Table 1.
Demographic and clinical characteristics of study subjects (N = 49).
| Characteristic | Mean±standard deviation or N (%), wherever applicable |
|---|---|
| Age (in years) | 21.2±3.0 |
| Systolic BP (in mm Hg) | 115.3±12.0 |
| Diastolic BP (in mm Hg) | 69.4±8.6 |
| Weight (kg) | 73.0±16.0 |
| Body mass index (kg/m2) | 24.7±4.6 |
| Serum cholesterol (mg/dL) | 155.2±32.0 |
| HDL cholesterol (mg/dL) | 51.2±13.1 |
| LDL cholesterol (mg/dL) | 82.2±28.9 |
| Serum triglyceride (mg/dL) | 109.3±64.2 |
| Gender | |
| Males | 38 (77.6%) |
| Females | 11 (22.4%) |
| Race | |
| White | 39 (79.6%) |
| Black | 1 (2%) |
| Asian | 9 (18.4%) |
| Ethnicitya | |
| Hispanic | 5 (10.4%) |
| Non-Hispanic | 43 (89.6%) |
Missing data on one subject.
Air pollutant and meteorological measurements
The distributions of air pollutant concentrations and meteorological characteristics during the study period measured at the selected monitoring sites, averaged daily, are summarized in Table 2. We scaled all our effect estimates by the daily IQR's presented in Table 2. Table 3 shows Spearman correlation coefficients for individual pollutants and meteorological measurements. Daily PM2.5 concentrations were moderately correlated with CO (r = 0.47) and SO2 (r = 0.50). Daily NO2 concentrations were moderately correlated with CO (r = 0.46), with SO2 (r = 0.47) and with O3 (r = −0.66).
Table 2.
Distribution of daily pollutant concentrations and meteorological characteristics for the study period of August 2006 to May 2009 in the Middlesex County, New Jersey.
| Distribution of pollutant/weather parameter |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | No. of non-missing days | No. of missing days | Inter quartile range (IQR) | Mean±SD | Min | 25th percentile | 50th percentile | 75th percentile | Max |
| PM2.5 (μg/m3)a | 989 | 46 | 7.0 | 8.2±6.0 | 0.2 | 3.9 | 6.6 | 10.9 | 39.2 |
| NO2 (ppb)a | 1027 | 8 | 9.6 | 13.0±7.1 | 0.8 | 7.5 | 11.5 | 17.1 | 39.0 |
| CO (ppm)a | 999 | 36 | 0.16 | 0.35±0.13 | 0.004 | 0.26 | 0.33 | 0.42 | 0.93 |
| SO2 (ppb)a | 1016 | 19 | 2.2 | 2.40±2.03 | 0 | 1.0 | 1.9 | 3.2 | 13.8 |
| O3 (ppb)a | 1027 | 8 | 17.1 | 25.3±12.0 | 2 | 16.1 | 24.8 | 33.2 | 67.7 |
| Temperature (Deg C) | 1035 | 0 | 16.1 | 12.4±9.5 | −11.5 | 4.7 | 12.5 | 20.8 | 33.1 |
| Relative humidity (%) | 1035 | 0 | 20.9 | 58.0±14.4 | 17.7 | 47.3 | 57.5 | 68.2 | 93.0 |
PM2.5, particulate matter less than 2.5 μm in aerodynamic diameter;
ppb, parts per billion; ppm, parts per million.
Table 3.
Spearman correlation coefficients for daily pollutant concentrations and meteorological characteristics.
| Pollutant/weather | PM2.5 | CO | O3 | SO2 | NO2 | Temperature |
|---|---|---|---|---|---|---|
| PM2.5 | ||||||
| CO | 0.47 | |||||
| O3 | 0.05 | −0.21 | ||||
| SO2 | 0.50 | 0.29 | −0.22 | |||
| NO2 | 0.29 | 0.46 | −0.66 | 0.47 | ||
| Temperature | 0.24 | 0.16 | 0.51 | −0.24 | −0.45 | |
| Relative Humidity | 0.17 | 0.28 | −0.28 | −0.13 | 0.10 | 0.19 |
Main analyses
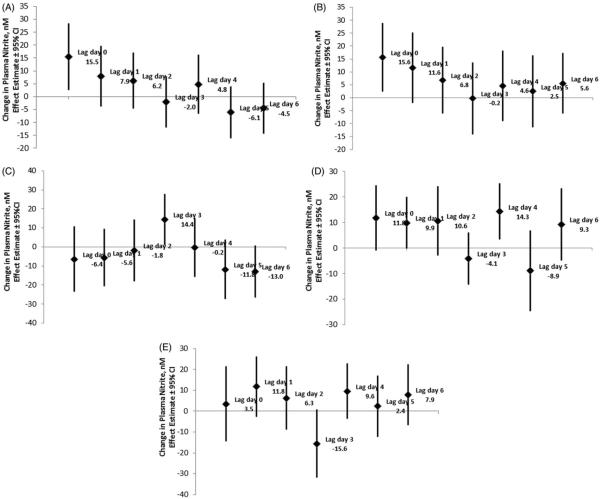
The absolute changes in the plasma nitrite concentration associated with each IQR increase in the mean pollutant concentrations are presented in Table 4 and Figure 1.
Table 4.
Changes in plasma nitrite concentration associated with an interquartile range increase in the mean pollutant concentrations in single pollutant mixed-effects models.
| PM2.5 (μg/m3) IQR: 7.0 μg/m3 |
Ozone (ppb) IQR: 17.1 ppb |
NO2 (ppb) IQR: 9.6 ppb |
SO2 (ppb) IQR: 2.2 ppb |
CO (ppb) IQR: 161.7 ppb |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lag period (in hours) | Change in plasma nitritea (95%CI) | p Value | Change in plasma nitritea (95%CI) | p Value | Change in plasma nitritea (95%CI) | p Value | Change in plasma nitritea (95%CI) | p Value | Change in plasma nitritea (95%CI) | p Value |
| Main analyses | ||||||||||
| 0–23 | 15.5 (2.4, 28.5) | 0.021 | −6.4 (−23.5, 10.8) | 0.46 | 3.5 (−14.5, 21.4) | 0.70 | 11.8 (−1.0, 24.5) | 0.070 | 15.6 (2.4, 28.9) | 0.021 |
| 24–47 | 7.9 (−3.7, 19.6) | 0.18 | −5.6 (−20.9, 9.6) | 0.47 | 11.8 (−2.6, 26.2) | 0.11 | 9.9 (−0.2, 20.0) | 0.054 | 11.6 (−2.0, 25.3) | 0.095 |
| 48–71 | 6.2 (−4.8, 17.1) | 0.27 | −1.8 (−18.1, 14.4) | 0.82 | 6.3 (−8.8, 21.4) | 0.41 | 10.6 (−2.9, 24.2) | 0.12 | 6.8 (−6.1, 19.7) | 0.30 |
| 72–95 | −2.0 (−11.9, 7.9) | 0.69 | 14.4 (1.0, 27.7) | 0.035 | −15.6 (−31.9, 0.70) | 0.060 | −4.1 (−14.3, 6.1) | 0.43 | −0.2 (−14.0, 13.7) | 0.98 |
| 96–119 | 4.8 (−6.7, 16.3) | 0.41 | −0.2 (−15.7, 15.3) | 0.98 | 9.6 (−3.7, 22.9) | 0.15 | 14.3 (3.4, 25.3) | 0.011 | 4.6 (−9.0, 18.2) | 0.50 |
| 120–143 | −6.1 (−16.1, 3.9) | 0.23 | −11.8 (−27.5, 3.8) | 0.14 | 2.4 (−12.3, 17.1) | 0.75 | −8.9 (−24.9, 7.0) | 0.27 | 2.5 (−11.4, 16.4) | 0.72 |
| 144–167 | −4.5 (−14.4, 5.4) | 0.37 | −13.0 (−26.8, 0.7) | 0.062 | 7.9 (−6.7, 22.5) | 0.28 | 9.3 (−4.8, 23.4) | 0.19 | 5.6 (−6.1, 17.3) | 0.35 |
| Cumulative method of defining pollutant lagsb | ||||||||||
| 0–23 | 15.5 (2.4, 28.5) | 0.021 | −6.4 (−23.5, 10.8) | 0.46 | 3.5 (−14.5, 21.4) | 0.70 | 11.8 (−1.0, 24.5) | 0.070 | 15.6 (2.4, 28.9) | 0.021 |
| 0–47 | 16.8 (2.8, 30.8) | 0.019 | −11.2 (−31.8, 9.4) | 0.28 | 13.7 (−5.6, 32.9) | 0.16 | 17.0 (3.6, 30.4) | 0.014 | 16.0 (2.9, 29.1) | 0.017 |
| 0–167 | 2.4 (−11.5, 16.2) | 0.73 | −10.9 (−38.4, 16.7) | 0.44 | 11.8 (−6.2, 29.9) | 0.20 | 19.2 (0.7, 37.6) | 0.042 | 13.8 (0.4, 27.3) | 0.044 |
This estimate was based on a cross-sectional study and it does not imply a temporal shift in the plasma nitrite measurements.
The findings from only three pollutant intervals 0–23 h, 0–47 h and 0–167 h have been presented as the time lags defined by the cumulative method are highly correlated.
Figure 1.
(A) Absolute change (and 95% confidence interval) in plasma nitrite concentration associated with an interquartile range increase in the mean PM2.5 concentration lag hours. (B) Absolute change (and 95% confidence interval) in plasma nitrite concentration associated with an interquartile range increase in the mean CO concentration lag hours. (C) Absolute change (and 95% confidence interval) in plasma nitrite concentration associated with an interquartile range increase in the mean O3 concentration lag hours. (D) Absolute change (and 95% confidence interval) in plasma nitrite concentration associated with an interquartile range increase in the mean SO2 concentration lag hours. (E) Absolute change (and 95% confidence interval) in plasma nitrite concentration associated with an interquartile range increase in the mean NO2 concentration lag hours.
Plasma nitrite concentration increased significantly by 15.5 nM (95% CI: 2.4, 28.5 nM) and 15.6 nM (95% CI: 2.4, 28.9 nM) with each IQR increase in the mean PM2.5 and the CO concentration over lag day 0 (0–23 h), respectively (Table 4 and Figure 1A and B). A delayed increase by 14.4 nM (95% CI: 1.0, 27.7 nM) in plasma nitrite was also observed with each IQR increase in the mean O3 concentration over lag day 3 (Table 4 and Figure 1C). A haphazard pattern of increase as well as decrease in plasma nitrite was observed associated with each IQR increase in the mean SO2 concentration over lag days 0–6 (Table 4 and Figure 1D). An increase in plasma nitrite was observed with each IQR increase in the mean SO2 concentration from lag day 0 till lag day 2, followed by a slight decrease over lag day 3 [−4.1 nM (−14.3, 6.1 nM)]. There was an increase of 14.3 nM (95% CI: 3.4, 25.3 nM) on lag day 4 followed by a decrease observed on lag day 5. Similarly, a trend toward an increase in plasma nitrite observed from lag day 0 (0–23 h) till lag day 2 (48–71 h), peaking on lag day 1 (23–47 h) was observed with each IQR increase in the mean NO2 concentration. This was followed by a decrease of −15.6 nM (95% CI: −31.9, 0.70 nM) in plasma nitrite over lag day 3 (Table 4 and Figure 1E). However, none of these patterns reached statistical significance.
Sensitivity analyses
When we adjusted for lagged temperature using the same lags as the pollutant, the changes in the plasma nitrite were comparable to those produced in the main analyses (data not shown). For instance, plasma nitrite increased by 7.9 nM (95% CI: −3.7, 19.6) and by 7.3 nM (95% CI: −4.8, 19.3) with each IQR increase in mean PM2.5 concentration over lag day 1 when the models were adjusted for mean temperature over lag day 0 (main analyses) and lag day 1 (sensitivity analyses), respectively. When pollutant lags were redefined using a cumulative method, the risk estimates were in agreement with the main analyses (Table 4). The greatest increase in plasma nitrite was observed with each IQR increase in mean PM2.5, CO and SO2 over 0–47 h, where the changes seen in 0–47 h were primarily driven by the changes observed in the first 24 h. When we fit two pollutant models for those pollutants where increased concentrations in specific lags were associated with an increase in plasma nitrite in the single pollutant model, we found that the effect estimates obtained from the two pollutant models were in agreement with the effect estimates obtained in the single pollutant models (Table 5). For example, when SO2 concentration over 0–23 h was included in the model examining the change in plasma nitrite associated with PM2.5 concentration over 0–23 h, it was observed that plasma nitrite showed a similar increase of 12.8 nM (95% CI: −1.2, 26.7) compared to the increase observed in a single pollutant model (15.5 nM; 95% CI: 2.4, 28.5). Similar results were obtained for other pollutants (Table 5).
Table 5.
Effect of pollutants on plasma nitrite concentration: two pollutant models.
| Lag period (in hrs) | Single pollutant model estimates (95% CI) | Two pollutant model # 1 estimates (95% CI) | Two pollutant model # 2 estimates (95% CI) | Two pollutant model # 3 estimates (95% CI) | Two pollutant model # 4 estimates (95% CI) | Two pollutant model # 5 estimates (95% CI) | Two pollutant model # 6 estimates (95% CI) |
|---|---|---|---|---|---|---|---|
| 0–23 | |||||||
| PM2.5 | 15.5 (2.4, 28.5) | 12.8 (−1.2, 26.7) | 11.0 (−4.0, 25.9) | 16.1 (3.0, 29.1) | |||
| SO2 | 11.8 (−1.0, 24.5) | 9.3 (−5.4, 24.0) | 8.7 (−4.1, 21.6) | 12.1 (−0.9, 25.1) | |||
| CO | 15.6 (2.4, 28.9) | 10.7 (−5.1, 26.5) | 13.5 (−0.02, 27.0) | 15.5 (1.6, 29.3) | |||
| O3 | −6.4 (−23.5, 10.8) | −12.2 (−30.8, 6.4) | −6.0 (−22.8, 10.7) | −0.04 (−17.0, 16.9) | |||
| 72–95 | |||||||
| PM2.5 | −2.0 (−11.9, 7.9) | 2.6 (−9.4, 14.5) | −1.1 (−12.6, 10.5) | −5.8 (−16.2, 4.5) | |||
| SO2 | −4.1 (−14.3, 6.1) | −8.4 (−21.4, 4.6) | −4.4 (−15.0, 6.2) | −6.0 (−16.2, 4.1) | |||
| CO | −0.2 (−14.0, 13.7) | −1.9 (−18.6, 14.7) | 1.3 (−13.0, 15.6) | 2.8 (−11.2, 16.9) | |||
| O3 | 14.4 (1.0, 27.7) | 16.9 (1.6, 32.3) | 15.5 (2.0, 29.0) | 13.6 (−0.7, 27.9) | |||
| 96–119 | |||||||
| PM2.5 | 4.8 (−6.7, 16.3) | −3.6 (−17.6, 10.5) | 5.1 (−8.6, 18.9) | 4.9 (−6.7, 16.5) | |||
| SO2 | 14.3 (3.4, 25.3) | 16.0 (1.6, 30.5) | 15.6 (3.1, 28.1) | 14.5 (3.4, 25.6) | |||
| CO | 4.6 (−9.0, 18.2) | −0.4 (−17.4, 16.6) | −4.0 (−19.1, 11.0) | 5.2 (−9.1, 19.5) | |||
| O3 | −0.2 (−15.7, 15.3) | −1.3 (−18.9, 16.3) | 1.4 (−13.8, 16.7) | 2.2 (−14.1, 18.4) | |||
Discussion
Our study demonstrates that plasma nitrite concentration increased significantly by approximately 15 nM in response to increased PM2.5 and carbon monoxide exposure in the previous 24 h (lag day 0). We also observed an increase in plasma nitrite concentration associated with increases in the mean ozone and sulfur dioxide concentrations over a longer time frame (lag days 3 and 4, respectively). Since nitrite () is a major oxidation product of NO in plasma (Lauer et al., 2002), the increase in plasma nitrite within a short period of time following exposure to air pollutants confirms NO generation via one or more pathways. We speculate that these observed increases result from an inflammatory response to the exposure.
NO is synthesized in various mammalian cells via nitric oxide synthase (NOS) (Lauer et al., 2002; Nussler & Billiar, 1993). There are three main NOS isoforms: neuronal NOS (type I, nNOS), inducible NOS (type II, iNOS), and endothelial NOS (type III, eNOS) (Griffith & Stuehr, 1995). All NOSs synthesize NO from arginine, oxygen and nicotinamide adenine dinucleotide phosphate (NADPH). Both eNOS and nNOS are inducibly activated but constitutively produced, while iNOS is induced in its synthesis by inflammatory activation (Guo et al., 1995; Heo et al., 2008; Kleeberger et al., 2001; Nussler & Billiar, 1993). In addition, iNOS produces NO at a higher flux rate than both eNOS and nNOS and, once synthesized, remains constitutively active.
Systemic inflammation has been suggested as a mechanism to explain the link between air pollution and adverse CV events such as arrhythmia and myocardial infarction in healthy individuals and in vulnerable populations such as those with diabetes (Chuang et al., 2007; Eeden et al., 2012; Fang et al., 2012; Panasevich et al., 2009; Pope et al., 2004; Rich et al., 2012; Schneider et al., 2010; Zeka et al., 2006). Gaseous and particulate air pollutants may enter the lung and activate pulmonary neural reflexes as well as induce inflammation at both the local and systemic level (Brook et al., 2010). Prior studies have reported that the cytokines associated with systemic inflammation such as interleukin-1ß (IL-1ß), interleukin-6, tumor necrosis factor-α (TNF-α), intercellular adhesion molecules, and C-reactive protein were significantly increased with exposure to ambient carbon black, NO2, PM2.5, PM10 and O3 (Chuang et al., 2007; Eeden et al., 2012; Fang et al., 2012; Panasevich et al., 2009; Pope et al., 2004; Zeka et al., 2006). Therefore, it is reasonable to propose that exposure to air pollutants may acutely result in increased iNOS levels. Previous work in an animal model has shown iNOS induction as a result of intratracheal exposure to 0.5–5 mg of particulate matter (Ulrich et al., 2002). In addition to iNOS stimulation, eNOS stimulation as an injury response following endothelial membrane damage by pollutants may also be responsible for NO production (Li et al., 2010), although in diabetics a decrease in BAFMD suggested reduced endothelial production of NO (Schneider et al., 2008). However, the time frame of our observations is more consistent with a protein synthetic response (0–23 h) than with an acute induction, making inflammatory activation the more probable pathway. Another finding that further supports the inflammatory pathway is the delayed increase in plasma nitrite associated with an increase in the mean O3 and SO2 concentrations on lag day 3 and 4, respectively. This is in line with a delayed increase in the markers of systemic inflammation on lag day 2 in association with an increase in PM2.5 concentration in diabetics shown by Schneider et al. (2010).
In addition to its role in inflammation, NO is responsible for several functions within the vasculature including vasorelaxation, inhibition of platelet adhesion and aggregation, inhibition of neutrophil chemotaxis, and signal transduction in the peripheral nervous systems (Nussler & Billiar, 1993). Inflammation through the generation of reactive oxygen species can oppose these functions by increasing the oxidation of NO. Therefore, inflammatory processes have the paradoxical position of both contributing to NO production and opposing its physiological function within the vasculature. This depressant effect of systemic inflammation on endothelial function has been previously described in humans (Hingorani et al., 2000). Of the four studies that reported a reduction in flow-mediated dilation following ambient air pollutant exposures (Dales et al., 2007; O'Neill et al., 2005; Rundell et al., 2007; Schneider et al., 2008), at least one report (O'Neill et al., 2005) supported inflammatory pathway activation as an underlying mechanism responsible for the observed decrease in endothelial function, although this was in a clinical setting of reactive hyperemia and changes were within 60 s of a vascular stimulus, localized to the upper extremity, rather than a systemic measurement. Thus, measurement of circulating nitrite has a complex relationship to air pollution as an inflammatory response can either increase the levels, by increasing iNOS activity, or decrease them by increasing oxidation of NO to nitrate. Indeed, our original hypothesis on the basis of prior studies that showed decreased BAFMD was that air pollution would decrease circulating nitrite (Dales et al., 2007; O'Neill et al., 2005; Rundell et al., 2007; Schneider et al., 2008). Our results are best rationalized by increased inflammatory production of nitrogen oxides. Further studies will allow more precise descriptions for the role(s) played by NO and oxidant production in mediation of vascular health effects of air pollution. Our study findings suggest the importance of other analyses that can be conducted using controlled exposure study data. For example, the analysis of data obtained from controlled exposure studies could include an assessment of whether increased ambient pollutant concentrations in the hours and days prior to the experimental visit are associated with the pre-exposure outcome measurement, the change in outcome from pre to post-exposure, or possibly a modification of the controlled exposure's effect on the outcome. For controlled exposure studies with exposure levels near ambient background levels, such analyses may provide important information as to why a controlled exposure did or did not elicit the hypothesized response.
Our study has several strengths. The within subject repeated measure study design reduced the chance of confounding by time-independent covariates. It was possible to explore the changes in plasma nitrite associated with pollutants using time lags extending up to the preceding 7 d due to the availability of continuously recorded hourly pollutant concentrations. While interpreting the results from this study, it is important to note a few limitations. First, exposure misclassification is likely to occur as ambient measurements of air pollutants, rather than personal exposure measurements were used to approximate each subject's exposure. This exposure misclassification along with the classical error of measurement device resulted in underestimation of the changes in plasma nitrite associated with increases in air pollutant concentrations. Moreover, a monitor closest to our campus was assigned to each subject regardless of the time spent in that area by each subject. This was done because we did not have information on subjects' exact location in the few days prior to their study participation and assumed that they spent most of their time on the campus. However, it is possible that subjects may have stayed at their parental residence or any other location away from the campus at one or more times during their study participation. However, this misclassification is likely to be non-differential with respect to the plasma nitrite measurement, likely resulting in underestimation air pollutants effects. Second, the study estimated the changes in plasma nitrite associated with increases in five pollutants averaged over seven time lags. It must be acknowledged that these are a substantial number of comparisons where one may expect several significant results due to chance alone. However, we did not make inferences based on statistical significance alone, but based on the patterns of changes in the plasma nitrite observed across lag times. Therefore, Bonferroni correction to account for multiple comparisons may be overaggressive in this situation and hence, was not performed.
Conclusions
Rapid increases in plasma nitrite following exposure to ambient air pollutants generally support the hypothesis that ambient air pollution is associated with iNOS-mediated systemic inflammation in humans. Our findings demonstrate the utility of plasma nitrite as an indicator of acute changes in ambient air pollutant concentrations. This is complicated by the expected air pollution induced reductions in endothelial production of NO/nitrite, leaving open the question of how the endothelial decrease and pulmonary/systemic increase in NO/nitrite will balance out for a systemic measurement. To our knowledge, this is the first study addressing the effects of short-term ambient air pollutant concentrations on plasma nitrite. Future studies are warranted to examine the changes from baseline in plasma nitrite following a hyperemic stimulus, as a marker of nitric oxide bioavailability. These changes in nitrite, or their physiological correlates such as BAFMD, can then be associated with changes in ambient air pollutants allowing separation of local versus systemic effects of air pollution.
Acknowledgements
We would like to thank the staff at the Computational Chemodynamics Laboratory at the Environmental and Occupational Health Sciences Institute for their help in the acquisition of the ambient air pollutant measurements and weather data used in the analysis. We acknowledge the generous participation of our volunteer subjects, and the dedication and expertise of the research staff at EOHSI who made these experiments possible.
Declaration of interest This work was supported by NIEHS Center grant P30ES0205022, EPA STAR grant R832144, and grant HL086621. Dr Ohman Strickland reports grants from Environmental Protection Agency, grants from NIEHS, during the conduct of the study; Drs Gandhi, Kipen, Rich and Gow report no declaration of interest.
References
- Allen JD, Cobb FR, Gow AJ. Regional and whole body markers of nitric oxide production following hyperemic stimuli. Free Radic Biol Med. 2005;38:1164–9. doi: 10.1016/j.freeradbiomed.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Allen JD, Miller EM, Schwark E, et al. Plasma nitrite response and arterial reactivity differentiate vascular health and performance. Nitric Oxide. 2009;20:231–7. doi: 10.1016/j.niox.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML, Ebisu K, Peng R, et al. Seasonal and regional short-term effects of fine particles on hospital admissions in 202 US counties, 1999–2005. Am J Epidemiol. 2008;168:1301–10. doi: 10.1093/aje/kwn252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskaran K, Hajat S, Armstrong B, et al. The effects of hourly differences in air pollution on the risk of myocardial infarction: case crossover analysis of the MINAP database. Br Med J. 2011;343:d5531. doi: 10.1136/bmj.d5531. doi: 10.1136/bmj.d5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Franklin B, Cascio W, et al. Air pollution and cardiovascular disease: a statement for healthcare professionals from the expert panel on population and prevention science of the American Heart Association. Circulation. 2004;109:2655–71. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- Brook RD, Rajgopalan S, Pope CA, III, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–78. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Burgan O, Smargiassi A, Perron S, et al. Cardiovascular effects of sub-daily levels of ambient fine particles: a systematic review. Environ Health. 2010;9:26. doi: 10.1186/1476-069X-9-26. doi: 10.1186/1476-069X-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Kuo C, Liou S. Fine particulate air pollution and hospital admissions for myocardial infarction in a subtropical city: Taipei, Taiwan. J Toxicol Environm Health, A, Curr Iss. 2013;76:440–8. doi: 10.1080/15287394.2013.771559. [DOI] [PubMed] [Google Scholar]
- Chuang KJ, Chan C, Su T, et al. The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am J Respir Crit Care Med. 2007;176:370–6. doi: 10.1164/rccm.200611-1627OC. [DOI] [PubMed] [Google Scholar]
- Dales R, Liu L, Szyszkowicz M, et al. Particulate air pollution and vascular reactivity: the bus stop study. Int Arch Occup Environ Health. 2007;81:159–64. doi: 10.1007/s00420-007-0199-7. [DOI] [PubMed] [Google Scholar]
- Dominici F, Peng R, Bell M, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. J Am Med Assoc. 2006;295:1127–34. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeden SV, Leipsic J, Paul Man SF, et al. The relationship between lun inflammation and cardiovascular disease. Am J Respir Crit Care Med. 2012;186:11–16. doi: 10.1164/rccm.201203-0455PP. [DOI] [PubMed] [Google Scholar]
- Fang SC, Mehta A, Alexeeff S. Residential black carbon exposure and circulating markers of systemic inflammation in elderly males: the normative aging study. Environ Health Perspect. 2012;120:674–80. doi: 10.1289/ehp.1103982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghio A, Kim C, Devlin R. Concentrated ambient air particles induce mild pulmonary inflammation in healthy human volunteers. Am J Resp Crit Care. 2000;162:981–8. doi: 10.1164/ajrccm.162.3.9911115. [DOI] [PubMed] [Google Scholar]
- Griffith OW, Stuehr DJ. Nitric oxide synthases: properties and catalytic mechanism. Annu Rev Physiol. 1995;57:707–36. doi: 10.1146/annurev.ph.57.030195.003423. [DOI] [PubMed] [Google Scholar]
- Guo FH, Raeve H, Rice T, et al. Continuous nitric oxide synthesis by inducible nitric oxide synthase in normal human airway epithelium in vivo. Proc Natl Acad Sci USA. 1995;92:7809–13. doi: 10.1073/pnas.92.17.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo SK, Yun HJ, Noh EK, et al. LPS induces inflammatory responses in human aortic vascular smooth muscle cells via Toll-like receptor 4 expression and nitric oxide production. Immunol Lett. 2008;120:57–64. doi: 10.1016/j.imlet.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Hingorani AD, Cross J, Kharbanda R, et al. Acute systemic inflammation impairs endothelium-dependent dilatation in humans. Circulation. 2000;102:994–9. doi: 10.1161/01.cir.102.9.994. [DOI] [PubMed] [Google Scholar]
- Hsieh YL, Tsai S, Yang C. Fine particulate air pollution and hospital admissions for congestive heart failure: a case-crossover study in Taipei. Inhal Toxicol. 2013;25:455–60. doi: 10.3109/08958378.2013.804609. [DOI] [PubMed] [Google Scholar]
- Kipen HM, Gandhi S, Rich DQ, et al. Acute decreases in proteasome pathway activity after inhalation of fresh diesel exhaust or secondary organic aerosol. Environm Health Perspect. 2011;119:658–63. doi: 10.1289/ehp.1002784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleeberger SR, Reddy S, Zhang L, et al. Toll-like receptor 4 mediates ozone-induced murine lung hyperpermeability via inducible nitric oxide synthase. Am J Physiol Lung Cell Mol Physiol. 2001;280:L326–33. doi: 10.1152/ajplung.2001.280.2.L326. [DOI] [PubMed] [Google Scholar]
- Lauer T, Kleinbongard P, Kelm M. Indexes of NO bioavailability in human blood. News Physiol Sci. 2002;17:251–5. doi: 10.1152/nips.01405.2002. [DOI] [PubMed] [Google Scholar]
- Lauer T, Preik M, Rassaf T, et al. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc Natl Acad Sci USA. 2001;98:12814–19. doi: 10.1073/pnas.221381098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Li R, Meng Z. Sulfur dioxide upregulates the aortic nitric oxide pathway in rats. Eur J Pharmacol. 2010;645:143–50. doi: 10.1016/j.ejphar.2010.07.034. [DOI] [PubMed] [Google Scholar]
- Liu L, Ruddy T, Dalipaj M, et al. Influence of personal exposure to particulate air pollution on cardiovascular physiology and bio-markers of inflammation and oxidative stress in subjects with diabetes. J Occup Environ Med. 2007;49:258–65. doi: 10.1097/JOM.0b013e31803220ef. [DOI] [PubMed] [Google Scholar]
- Mann JK, Tager I, Lurmann F, et al. Air pollution and hospital admissions for ischemic heart disease in persons with congestive heart failure or Arrhythmia. Environ Health Perspect. 2002;110:1247–52. doi: 10.1289/ehp.021101247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Climatic Data Centre (NCDC) National Oceanic Atmospheric Administration (NOAA) [last accessed: 10 Jan 2014]; Available from: http://www.ncdc.noaa.gov/oa/climate/climatedata.html#hourly.
- Nussler AK, Billiar TR. Inflammation, immunoregulation and inducible nitric oxide synthase. J Leukocyte Biol. 1993;54:171–8. [PubMed] [Google Scholar]
- Nuvolone D, Balzi D, Chini M, et al. Short-term association between ambient air pollution and risk of hospitalization for acute myocardial infarction: results of the cardiovascular risk and air pollution in tuscany (RISCAT) Study. Am J Epidemiol. 2011;174:63–71. doi: 10.1093/aje/kwr046. [DOI] [PubMed] [Google Scholar]
- O'Neill MS, Veves A, Zanobetti A, et al. Diabetes enhances vulnerability to particulate air pollution-associated impairment in vascular reactivity and endothelial function. Circulation. 2005;111:2913–20. doi: 10.1161/CIRCULATIONAHA.104.517110. [DOI] [PubMed] [Google Scholar]
- Panasevich S, Leander K, Rosenlund M, et al. Associations of long- and short-term air pollution exposure with markers of inflammation and coagulation in a population sample. Occup Environ Med. 2009;66:747–53. doi: 10.1136/oem.2008.043471. [DOI] [PubMed] [Google Scholar]
- Peters A, Dockery D, Muller J, et al. Increased particulate air pollution and the triggering of myocardial infarction. Circulation. 2001;103:2810–15. doi: 10.1161/01.cir.103.23.2810. [DOI] [PubMed] [Google Scholar]
- Peters A, Von klot S, Heier M, et al. Exposure to traffic and onset of myocardial infarction. N Engl J Med. 2004;351:1721–30. doi: 10.1056/NEJMoa040203. [DOI] [PubMed] [Google Scholar]
- Peters A, von Klot S, Mittleman MA, et al. Triggering of acute myocardial infarction by different means of transportation. Eur J Prev Cardiol. 2013;20:750–8. doi: 10.1177/2047487312446672. [DOI] [PubMed] [Google Scholar]
- Pope CA, III, Hansen M, Long R, et al. Ambient particulate air pollution, heart rate variability, and blood markers of inflammation in a panel of elderly subjects. Environ Health Perspect. 2004;12:339–45. doi: 10.1289/ehp.6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich DQ, Kim M, Turner JR, et al. Association of ventricular arrhythmias detected by implantable cardioverter defibrillator and ambient air pollutants in the St. Louis, Missouri metropolitan area. Occup Environ Med. 2006a;63:591–6. doi: 10.1136/oem.2005.023457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich DQ, Mittleman M, Link M, et al. Increased risk of paroxysmal atrial fibrillation episodes associated with acute increases in ambient air pollution. Environ Health Perspect. 2006b;114:120–3. doi: 10.1289/ehp.8371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich DQ, Schwartz J, Mittleman M, et al. Association of short-term ambient air pollution concentrations and ventricular arrhythmias. Am J Epidemiol. 2005;161:1123–32. doi: 10.1093/aje/kwi143. [DOI] [PubMed] [Google Scholar]
- Rich DQ, Kipen HM, Huang W, et al. Association between changes in air pollution levels during the Beijing Olympics and biomarkers of inflammation and thrombosis in healthy young adults. J Am Med Assoc. 2012;307:2068–78. doi: 10.1001/jama.2012.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundell KW, Hoffman J, Caviston R, et al. Inhalation of ultrafine and fine particulate matter disrupts systemic vascular function. Inhal Toxicol. 2007;19:133–40. doi: 10.1080/08958370601051727. [DOI] [PubMed] [Google Scholar]
- Samet JM, Dominici F, Curriero F, et al. Fine particulate air pollution and mortality in 20 U.S. cities, 1987–1994. N Engl J Med. 2000;343:1742–9. doi: 10.1056/NEJM200012143432401. [DOI] [PubMed] [Google Scholar]
- Schneider A, Neas L, Herbst M, et al. Endothelial dysfunction: associations with exposure to ambient fine particles in diabetic individuals. Environ Health Perspect. 2008;116:1666–74. doi: 10.1289/ehp.11666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Neas L, Graff D, et al. Association of cardiac and vascular changes with ambient PM2.5 in diabetic individuals. Particle Fibre Toxicol. 2010;7:14. doi: 10.1186/1743-8977-7-14. doi: 10.1186/1743-8977-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen M, Daneshvar B, Hansen M, et al. Personal PM2.5 exposure and markers of oxidative stress in blood. Environ Health Perspect. 2003;111:161–6. doi: 10.1289/ehp.111-1241344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan J, Ishikawa N, Sheppard L, et al. Exposure to ambient fine particulate matter and primary cardiac arrest among persons with and without clinically recognized heart disease. Am J Epidemiol. 2003;157:501–9. doi: 10.1093/aje/kwg015. [DOI] [PubMed] [Google Scholar]
- Ulrich MM, Alink GM, Kumarathasan P, et al. Health effects and time course of particulate matter on the cardiopulmonary system in rats with lung inflammation. J Toxicol Environ Health A. 2002;65:1571–95. doi: 10.1080/00984100290071676. [DOI] [PubMed] [Google Scholar]
- US Environmental Protection Agency [last accessed: 5 Dec 2013];Air quality system data mart [Internet database] Available from: http://www.epa.gov/ttn/airs/aqsdatamart.
- Wellenius GA, Bateson T, Mittleman A, et al. Particulate air pollution and the rate of hospitalization for congestive heart failure among medicare beneficiaries in Pittsburgh, Pennsylvania. Am J Epidemiol. 2005a;161:1030–6. doi: 10.1093/aje/kwi135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellenius GA, Schwartz J, Mittleman M. Air pollution and hospital admissions for ischemic and hemorrhagic stroke among medicare beneficiaries. Stroke. 2005b;36:2549–53. doi: 10.1161/01.STR.0000189687.78760.47. [DOI] [PubMed] [Google Scholar]
- Wellenius GA, Schwartz J, Mittleman M. Particulate air pollution and hospital admissions for congestive heart failure in seven United States Cities. Am J Cardiol. 2006;97:404–8. doi: 10.1016/j.amjcard.2005.08.061. [DOI] [PubMed] [Google Scholar]
- Yang Y, Li R, Li W, et al. The Association between ambient air pollution and daily mortality in Beijing after the 2008 Olympics: a time series study. PLoS One. 2013;8:e76759. doi: 10.1371/journal.pone.0076759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Chen X, Xue X, et al. Long-term exposure to high particulate matter pollution and cardiovascular mortality: a 12-year cohort study in four cities in northern China. Environ Int. 2014;62:41–7. doi: 10.1016/j.envint.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Zeka A, Sullivan J, Vokonas P, et al. Inflammatory markers and particulate air pollution: characterizing the pathway to disease. Int J Epidemiol. 2006;35:1347–54. doi: 10.1093/ije/dyl132. [DOI] [PubMed] [Google Scholar]