Abstract
Newer approaches are needed for the treatment of relapsed and refractory acute lymphoblastic leukemia (ALL). Asparaginase-based regimens are active in the treatment of pediatric ALL and may be important in salvage therapy for adult patients. We conducted a pilot trial combining methotrexate, vincristine, PEGylated-asparaginase, and dexamethasone (MOpAD) in adults with relapsed or refractory ALL. We added tyrosine kinase inhibitors in patients with Philadelphia chromosome positive (Ph+) ALL and rituximab in patients with CD20 positive B-cell ALL. Among 37 patients treated (median age 42 years; median 2 prior therapies), the complete remission (CR) rate was 28% and an overall response rate (ORR) was 39%. The median CR duration was 4.3 months. Patients with Ph+ ALL had CR and ORR of 50% and 67%, respectively and the CR and ORR in patients with T-cell leukemia were 45% and 56%, respectively. The median survival in patients with CR/CRp was 10.4 versus 3.4 months in nonresponders (P =0.02). The most common grade 3 or 4 nonhematologic toxicities were elevations in bilirubin and transaminases, nausea, peripheral neuropathy, and hyperglycemia, which were managed with supportive care, dose adjustments, and interruptions.
Introduction
There have been significant advances in the treatment of newly diagnosed adult acute lymphoblastic leukemia (ALL), with complete response rates of 75 to 80% and cure rates of 30 to 40% [1,2]. But, relapses do occur and effective salvage therapy is needed. Patients receiving salvage therapy for their first relapse have CR rates of only 31 to 44% and 1-year survival rates of 22 to 24% [3–5]. Beyond first salvage, the outcomes are even worse, with a median survival of 3 months or less [6]. The addition of monoclonal antibodies in CD20-positive ALL [7,8] and tyrosine kinase inhibitors (TKIs) in Philadelphia chromosome positive disease [8,9] have improved outcomes in adults, but have not matched corresponding cure rates achieved in childhood ALL [1,10]. Agents such as asparaginase are mainstays of multidrug regimens in chiIdhood ALL, but incorporation of these agents into adult regimens has been met with only incremental progress [1,11]. Safely employing asparaginase into salvage therapy programs for adult ALL is desirable, since it offers a new mechanism of action, is nonmyelosuppressive, and unlikely to be cross-resistant with many of the prior induction therapies.
The combination of methotrexate and asparaginase has been shown to be synergistic, but the sequence of administration of this combination is important [12–15]. Giving asparaginase before methotrexate inhibits the polyglutamination that is needed for methotrexate activity [12–15]. Giving asparaginase after the methotrexate leads to enhanced efficacy and can provide “rescue” from toxicity related to prolonged methotrexate levels [16,17]. A combination of methotrexate, vincristine, L-asparaginase, and dexamethasone (MOAD) was previously studied. In single-arm trial in newly diagnosed adults with ALL and a median age of 38 (range, 15–73), MOAD demonstrated a CR rate of 76% with a median CR duration of over 12 months [18]. One-third of patients achieving CR remained in remission for over 5 years. Toxicities included myelosuppression, elevation of liver enzymes, pancreatitis, and thrombosis [18].
A new, PEGylated, formulation of L-asparaginase has been developed exhibiting more favorable pharmacokinetic properties and better tolerance [19,20]. The new formulation has a longer half-life, allows less frequent dosing, and may be associated with lower rates of allergic reactions and development of neutralizing antibodies. We conducted a phase II trial investigating the safety and efficacy of methotrexate, vincristine, PEG-L-asparaginase, and dexamethasone (MOpAD) in adults with relapsed and refractory ALL. Building on previous studies, we allowed the incorporation of monoclonal antibodies and tyrosine kinase inhibitors to this asparaginase-containing, nonanthracycline-based regimen when appropriate.
Methods
Eligibility criteria
This open-label, prospective phase II study (Protocol 2008-0267) was approved by the Institutional Review Board of MD Anderson Cancer Center, and all patients provided written informed consent according to institutional guidelines. The study was conducted in concordance with the declaration of Helsinki.
Patients ≥1 year of age and Zubrod performance status ≤3, with previously treated ALL (including Burkitt’s leukemia/lymphoma) or lymphoblastic lymphoma were eligible for enrollment. Other eligibility criteria included adequate hepatic (serum bilirubin ≤3 mg/dL) and renal (creatinine ≤3.0 mg/dL) functions, and the ability to sign informed consent. Pregnant patients and those with a known history of allergic reaction, serious pancreatitis, hemorrhagic or thrombotic event related to PEG-L-asparaginase were excluded.
Treatment plan
A treatment cycle was defined as a minimum of 28 days and at least two cycles were administered before determining failure, in the absence of rapidly proliferating disease. Responding patients could receive up to six cycles of therapy on study.
The treatment dosing was as follows: methotrexate 200 mg/m2 intravenously (IV) on Days 1 and 15 (reduced by 50% for creatinine clearance 10–50 mL/min); vincristine 1.4 mg/m2 IV (maximum dose 2 mg) on Days 1, 8, and 15 (reduced dose to 1 mg for pre-existing neuropathy and/or bilirubin 2–3 mg/dL, hold for bilirubin >3 mg/dL); PEG-L-asparaginase 2,500 IU/m2 IV on Days 2 and 16 (no capping of dose; decrease by 50% if direct bilirubin between 2 and 3 mg/dL; hold for bilirubin ≥3 mg/dL, clinically serious pancreatitis, thrombosis not controlled with anticoagulation, or DIC); and dexamethasone 40 mg IV or orally (PO) daily on Days 1 to 4 and 15 to 18. After an amendment due to toxicity, the maximum dose of PEG-L-asparaginase was limited to 3,750 units per dose. Patients whose leukemia was CD20 positive (≥20% by flow cytometry or positive by immunohistochemistry) received rituximab 375 mg/m2 IV on Days 1 and 15 during the first four cycles. After an amendment, patients with BCR-ABL (Philadelphia chromosome) positive ALL could be treated with: imatinib 400 to 800 mg PO daily, or dasatinib 70 to 100 mg PO daily, or nilotinib 200 to 400 mg PO daily, in combination with the chemotherapy. Intrathecal chemotherapy for prophylaxis or active CNS disease was allowed. Use of hydroxyurea or additional glucocorticoids to control proliferative disease was allowed before starting therapy and for up 7 days (per cycle) during cycles 1 to 3 only. Amylase, lipase, PT, PTT, fibrinogen, and AST were checked before each dose of PEG-L-asparaginase. Supportive care was continued in all patients per institutional guidelines. Myeloid growth factors (G-CSF or GM-CSF) were permitted, if in the best interest of the patient and were temporally separated from methotrexate administration by 24 h. Prophylactic broad spectrum antibiotics were permitted, and all patients received levofloxacin, fluconazole, and valacyclovir (or their equivalent) while on study. As per our standard practice, patients were advised to hold azoles the day before and day of vincristine administration to reduce risk of toxicity.
Response criteria
A CR was defined as normalization of peripheral blood and bone marrow (BM) with <5% blasts, a peripheral absolute neutrophil count (ANC) ≥1 × 109/L, and a platelet count ≥100 x 109/L. A partial response (PR) was defined as the same blood count recovery as CR with a decrease in BM blasts of at least 50% and not more than 6 to 25% of abnormal cells in the BM. CRp was defined the same as CR, but without recovery of platelet count ≥100 × 109/L. CRp did not require freedom from platelet transfusion. MLFS (morphologic leukemia-free state) was defined as CR but without recovery of ANC ≥1 × 109/L and without platelet count ≥100 × 109/L. The duration of response was measured from the time of response (CR or PR) were first met until recurrent or progressive disease. Overall survival was measured from the time of initiation on study until death.
Statistical analysis
The primary objective of this phase II trial is to evaluate the efficacy of the combination therapy and the primary endpoint is complete response (CR). The method of Thall et al. [21] was employed to perform continuous interim futility and safety monitoring. Dynamic futility boundaries were employed depending on salvage status, targeting a CR rate of ≥33% in patients with less than two salvage therapies, and a CR rate of ≥15% in patients with primary refractory disease or ≥2 salvage therapies. Continuous variables were summarized by descriptive statistics such as median and range. Categorical variables were tabulated with frequency and percentage. The overall response rate (ORR), complete response (CR) rate, partial response rate (PR), etc. were estimated for all evaluable patients and by each subgroup separately. Survival and response duration were evaluated using the Kaplan-Meier method for all patients and by subgroup, respectively. Safety data was summarized by category, severity, and frequency.
Results
Patient characteristics
A total of 37 patients (43% female) were treated. Their median age was 42 years (range, 22–69). One patient refused to complete treatment or evaluation; therefore, 36 patients received treatment on study and were evaluable for response. The baseline characteristics of the patients are outlined in Table I. Patients had received a median of two prior therapies (range, 1–6). For patients who were treated with MOpAD in the second-salvage setting and beyond, their response to the immediate prior therapy was: refractory disease in 72%, CR in 20%, and PR in 8%. Prior therapies included: HyperCVAD-based chemotherapy in 37 (100%), rituximab-based therapy in 13 (35%), nelarabine-based therapy in 10 (27%), asparaginase-based therapy in 8 (22%), and tyrosine kinase inhibitor-based therapy in 6 (16%).
TABLE I.
Patient Characteristics
| Characteristic | All patients (N =37) |
|---|---|
| Median age, yrs (range) | 42 (22–69) |
| Female sex, N (%) | 16 (43) |
| Median WBC [×109/L] (range) | 4.8 (0.1–264.5) |
| % PB blasts, median (range) | 1.3 (0–11.9) |
| Median Hb [×109/L] (range) | 9.8 (7.8–14.5) |
| Median platelets [×109/L] (range) | 30 (5–194) |
| % Marrow blasts, median (range) | 79 (1–100) |
| Diagnosis | |
| Pre-B ALL | 24 (65) |
| Pre-T ALL | 8 (22) |
| T-lymphoblastic lymphoma | 4 (11) |
| CML-LBP | 1 (3) |
| Philadelphia chromosome positive | 6 (16) |
| Salvage status | |
| First salvage | 12 (32) |
| CR1D <12 mo | 10 (83) |
| CR1D not reported | 2 (17) |
| Second salvage | 14 (38) |
| Third or greater salvage | 11 (30) |
N: number of patients; WBC: white blood cell; PB: peripheral blood; Hb: hemoglobin; ALL: acute lymphoblastic leukemia; CML-LBP: chronic myelogenous leukemia-lymphoid blast phase; CR1D: Duration of first complete remission.
Efficacy
A summary of responses overall and by disease subtype is shown in Table II. Patients completed a median of 1 course of therapy (range 1–5). Among the 36 evaluable patients, the overall response rate (ORR, CR +CRp +PR) was 39%. Of the six patients with Philadelphia chromosome-positive disease, including one with CML-LBP and five with Philadelphia positive ALL, the ORR was 67%. Three of these patients received a concomitant TKI (dasatinib in 2, nilotinib in 1). The response rate among these three patients was 100%: two CR and one CRp. Eight patients with CD20+ pre-B-ALL received rituximab in combination with MOpAD, resulting in three CR/CRp (38%). The ORR by salvage status is outlined in Table II. Of the 13 patients who achieved a CR or CRp, 6 (46%) were able to proceed to stem cell transplant.
TABLE II.
Responses (N =36 Evaluable)
| N | ORR (%) | CR (%) | CRp (%) | PR (%) | MLF (%) | |
|---|---|---|---|---|---|---|
| All patients | 36 | 14 (39) | 10 (28) | 3 (8) | 1 (3) | 3 (8) |
| B-ALL | 24 | 7 (29) | 4 (17) | 3 (13) | 3 (13) | |
| T-ALL | 8 | 3 (38) | 3 (38) | |||
| T-LL | 3 | 3 (100) | 2 (67) | 1 (33) | ||
| CML-LBP | 1 | 1 (100) | 1 (100) | |||
| Philadelphia positive | 6 | 4 (67) | 3 (50) | 1 (17) | ||
| First salvage | 11 | 4 (36) | 3 (27) | 1 (9) | 1 (9) | |
| Second salvage | 14 | 4 (29) | 4 (29) | 2 (14) | ||
| Third+ salvage | 11 | 5 (45) | 2 (18) | 3 (27) |
N: number of patients; CR: complete remission; CRp: CR with incomplete platelet recovery; PR: partial remission; MLF: morphologic leukemia free; ORR: overall response rate, equals CR +CRp +PR; ALL: acute lymphoblastic leukemia; LL: lymphoblastic lymphoma; CML-LBP: chronic myelogenous leukemia-lymphoid blast phase.
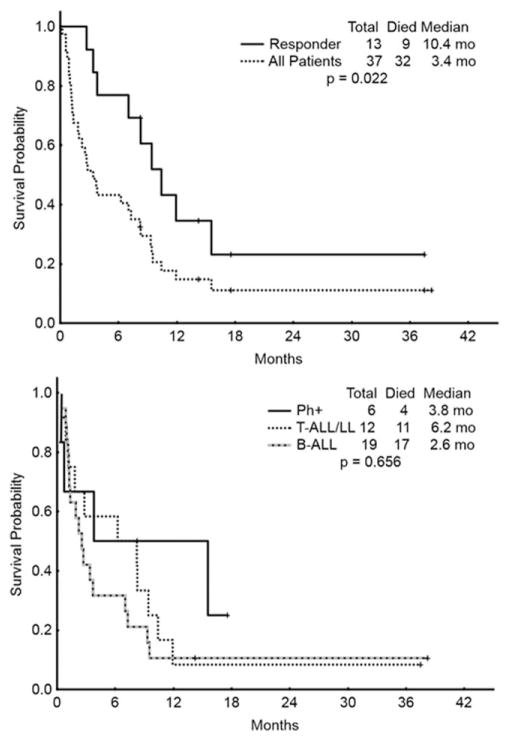
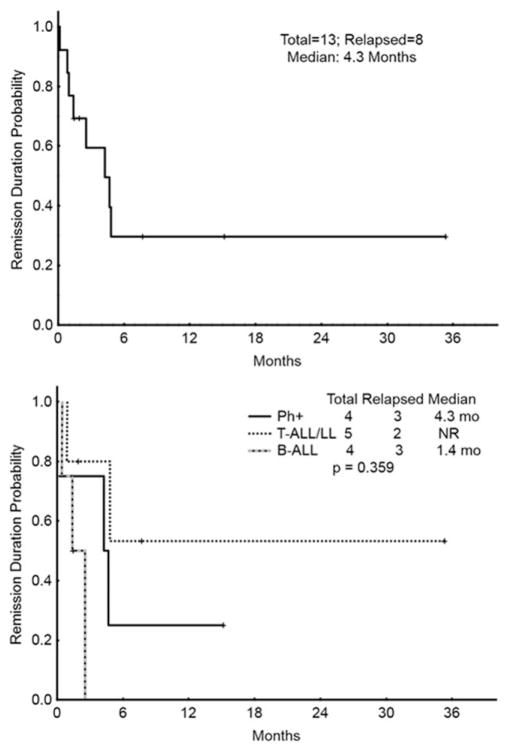
The overall survival for the entire population, for responding patients (CR/CRp/PR), and for histologic subgroups is depicted in Fig. 1. The median survival for all patients was 3.4 months, compared with 10.4 months for responders. The CR duration for all patients achieving a CR/CRp is shown in Fig. 2. CR durations for subgroups are also shown. The median CR duration was 4.3 months overall, 4.3 months in patients with Philadelphia chromosome positive leukemia, and not reached in patients with T-cell disease (Fig. 2).
Figure 1.
Overall survival (OS). Top figure represents OS for all patients and those who achieved a response (CR or CRp). Bottom figure represents the OS among subgroups: Philadelphia positive B-ALL (Ph+), T-cell acute lymphoblastic leukemia/lymphoma (T-ALL/LL), and Philadelphia negative B-ALL (B-ALL). mo =Months.
Figure 2.
Remission duration (CRd). Top figure represents CRd for all patients who achieved a response (CR or CRp). Bottom figure represents the CRd among subgroups: Philadelphia positive B-ALL (Ph+), T-cell acute lymphoblastic leukemia/lymphoma (T-ALL/LL), and Philadelphia negative B-ALL (B-ALL). mo =Months.
Safety and tolerability
All patients who received any therapy were eligible for toxicity evaluation. Given the patient population and treatment, myelosuppression was universal. Nonhematologic, possibly-related adverse events are outlined by frequency and grade in Table III. With appropriate dose-modifications and interruptions for toxicity, the regimen had acceptable tolerability. Nineteen of the 37 patients (51%) did not receive the Day-15 peg-asparaginase and/or vincristine during Cycle 1 for various reasons: abnormal bilirubin/transaminases (42%), mucositis (11%), infection (11%), elevated amylase or lipase (11%), death (11%), peripheral neuropathy (5%), disease progression in CNS (5%), decreased fibrinogen (5%), or thrombosis (5%). Elevations in liver function tests (AST/ALT, bilirubin) were mostly transient and responded to interruptions or delays in therapy. There was one case of abnormal liver function which progressed to liver failure and death. Similarly, elevation in amylase and lipase were transient and responded to interruption in therapy. There was one case of clinically significant pancreatitis, treated with medical management with improvement. Although a decrease in fibrinogen was common (70%) after administration of peg-asparaginase, the incidence of thrombosis was only 14%.
TABLE III.
Possibly Related Nonhematologic Toxicities (N =37 Evaluable Patients)
| Toxicity | All grades, N (%) | Grade 3/4, N (%) |
|---|---|---|
| Elevated AST/ALT | 34 (92) | 8 (22) |
| Elevated bilirubin | 31 (84) | 15 (41) |
| Decreased fibrinogen | 26 (70) | 13 (35) |
| Nausea | 11 (30) | |
| Neuropathy | 10 (27) | 2 (6) |
| Elevated amylase/lipase | 9 (24) | 4 (11) |
| Mucositis | 8 (22) | 3 (8) |
| Hyperglycemia | 8 (22) | 8 (22) |
| Diarrhea | 7 (19) | |
| Thrombosis | 5 (14) | 3 (8) |
| Vomiting | 4 (11) | |
| Pleural effusion | 4 (11) |
Neutropenic fever and/or infections occurred in 26 (70%) patients on the trial. There were 40 episodes of infection among these 26 patients: 20 with a proven bacterial infection, 6 with pneumonia of unknown pathogen, 5 with neutropenic fever of unknown origin, 4 with proven fungal infection (3 Candida, 1 Aspergillus), 2 with proven viral infection (influenza, herpes simplex virus), and 3 with other infections (rectal abscess, osteomyelitis, and one at outside hospital with no further information).
There were 14 deaths on study: 9 patients with bacteremia, pneumonia, or other infection, 1 sudden death of unknown etiology, and 4 patients who died outside of our institution with no further information available. Two of these patients were in a CR/CRp at the time death, while the rest had progressive disease or no response. The 30-day mortality rate was 19% (7 of 37).
Discussion
Newer therapeutic strategies using existing drugs have been effective in the treatment of relapsed and refractory ALL in adults. Agents such as L-asparaginase, which are widely used in the treatment of pediatric ALL, can be applied in adult practice after careful consideration of existing comorbidities, organ function, and the implementation of dose- and schedule-modification. In the current phase II pilot study of MOpAD, we build on previous work exploiting the schedule-dependent synergy of asparaginase and methotrexate in a nonanthracycline based program. Along with leveraging the longer half-life and lower immunogenicity of pegylated asparaginase in this trial, we also pilot the use of tyrosine kinase inhibitors in Philadelphia positive ALL, as well as the addition of rituximab in patients with CD20 positive ALL. In a refractory, heavily pretreated population, we demonstrated significant clinical activity and feasibility of the MOpAD regimen, with notable benefit in specific subgroups.
Wiernik et al. first studied the combination of methotrexate, vincristine, L-asparaginase, and dexamethasone as initial therapy in patients with newly diagnosed ALL [18]. This was based on preclinical data suggesting schedule-dependent efficacy. They reported a complete response rate of 76% in 55 previously untreated patients with a median age of 38 years [18]. The median CR duration was 12+ months, and the 3-year relapse free survival (RFS) rate was 35%. This compares to a CR rate of 28% overall (38% in T-ALL and 50% in Philadelphia positive disease) in the current study in heavily pre-treated patients with a median of two prior therapies. Importantly, the regimen reported by Wiernik et al. was somewhat different than the current protocol. Their regimen consisted of: an “induction/consolidation” with up to 12 cycles of methotrexate (100 mg/m2 IV with successive 50% dose escalation up to 225 mg/m2), vincristine, and L-asparaginase given every 10 days along with daily dexamethasone; followed by “cytoreduction” using high-dose methotrexate (100 mg/kg with stepwise 25% dose-escalation for subsequent doses) with leucovorin rescue, vincristine, and dexamethasone; and then maintenance therapy. The higher-intensity, prolonged therapy in a previously untreated population likely accounts for the higher rates of response and prolonged survival.
In a more comparable study, Aguayo et al. studied the combination of MOAP, using methotrexate 100 mg/m2 IV on Days 1 and 15; vincristine 1.4 mg/m2 (maximum 2 mg) IV on Days 1, 7, and 14; prednisone 200 mg orally daily on Days 1 to 5 and 14 to 19; and, similar to the current study, peg-asparaginase 2500 IU/m2 IV on Days 1 and 14 given 4 to 6 h after the methotrexate [22]. In 32 patients with relapsed or refractory ALL and a median age of 34 years, they reported a CR rate of 22% [22]. Unlike the current study, the PEG-L-asparaginase was capped at 3,750 IU and given on the same day, but 4 to 6 h after the methotrexate. We used a higher dose of methotrexate and chose dexamethasone over prednisone, based on data suggesting its superiority in ALL and its association with lower rates of CNS disease [23–27]. Our CR/CRi rate of 36% compares favorably to Aguayo et al. [22]. The CR rates among patients in first salvage (33% for MOAP vs. 27% for MOpAD) and ≥2nd salvage (15% for MOAP vs. 24% for MOpAD) were similar.
We found significant activity in several subgroups that highlights heterogeneity of ALL and may encourage further studies with MOpAD in these diseases. Among patients with Philadelphia chromosome positive disease (N =6), MOpAD produced a CR/CRp rate of 67% (100% among a subset of those who received a concomitant TKI), and a CR duration of 4.3 months (compared with a CR rate of 0% reported by Aguayo et al.). The combination with tyrosine kinase inhibitors was well tolerated and feasible. Although this represented a small cohort of patients, our experience suggests promising evidence for activity and the need for further studies.
In patients with CD20+ disease, we added the anti-CD20 monoclonal antibody, rituximab. Of eight patients with CD20 (+) B-ALL who were treated with rituximab combined with MOpAD, three had a CR/CRp (38%). This compares with two CR/CRps (15%) in 13 patients with B-ALL who did not receive rituximab. Monoclonal antibodies could be safely added to MOpAD and may potentially improve outcomes. The regimen also demonstrated significant activity in patients with relapsed and refractory T-cell acute lymphoblastic leukemia and lymphoma with overall response rates of 38% and 100%, respectively and median CR duration that was not reached. This observation supports previous data suggesting a trend toward better outcomes for T-ALL with asparaginase-based regimens [21]. Confirmation of this preliminary signal of activity could translate into an important salvage regimen for adults with T-cell ALL.
Toxicity was an important concern on the trial, particularly with the use of asparaginase in adults with multiply pretreated ALL. Myelosuppression occurred in all patients and was commonly associated with admissions for neutropenic fever and infection. There were nine (24%) deaths on study due to infections. This compares with 19% (6 of 32) infectious deaths reported by Aguayo et al. [22] in a salvage cohort and 13% (7 of 55) reported by Wiernik et al. [18] in an untreated cohort. Besides myelosuppression, the most common toxicities involved transient elevations in liver transaminases and bilirubin. Similar to Wiernik et al. [18], we noted one case of liver function abnormality that ultimately progressed to liver failure and death. Amylase and/or lipase elevations were seen in about a quarter of patients, similar to the 22% incidence reported by Aguayo et al. However, this was associated with clinically apparent pancreatitis in only one patient (3%), in contrast to an incidence of 11% in a large CALGB experience [28] with unpegylated L-asparaginase. Hyperglycemia was common with our use of dexamethasone, but was actually lower than reported by Aguayo et al. [22] (22% vs. 41%), who utilized prednisone in their regimen. Due to its effect on the production of coagulation factors in the liver, asparaginase has been associated with coagulopathy and increased risk of thrombosis. Grade 3 or 4 hypofibrinogenemia was seen in about a third of patients on the study, and associated with a 14% incidence of any and 8% incidence of serious thrombosis. Larson et al. [28] similarly reported a 21% incidence of severe hypofibrinogenemia and 6% incidence of clots. In contrast, Aguayo et al. [22] reported a 19% incidence of decreased fibrinogen, but no clinically significant clots. The higher incidence of coagulopathy in our cohort could be accounted for by differences in patients across studies and the higher dose-intensity of peg-asparaginase (no capping of dose) in our study. Because of the higher rates of liver enzyme abnormalities, coagulopathy, and omissions of the Day-15 dose, we subsequently modified our protocol to limit the maximum dose of peg-asparaginse to 3,750 units per dose. Severe hypersensitivity reactions have been seen in up to 15% of ALL patients treated with un-pegylated L-asparaginase [28]. However, no clinically evident hypersensitivity reactions were seen in our study with the use of peg-asparaginase. Our use of high-dose steroids (dexamethasone) as part of our treatment and on the same days of peg-asparaginase treatment may have contributed to the lack of clinically evident reactions and could certainly mask a silent hypersensitivity.
The outcomes of patients with relapsed ALL are poor. In the first-salvage setting, large groups have reported CR rates of 31 to 44%, with a median OS of 5 to 6.3 months and a 5-year OS of 3 to 7% [3–5]. Achieving a CR and proceeding to an allogeneic stem cell transplant in this setting is associated with the best outcomes. Indeed, 53% and 25% of patients were able to proceed for an allogeneic SCT in the French [4] and MD Anderson Cancer Center [5] experiences, respectively. In patients beyond first salvage, the outcomes are even more difficult. In a cohort of patients receiving second salvage therapy for ALL, O’Brien et al. [6] report a CR rate of 18% and a median OS of only 3 months. Only 8% were able to go on to an allogeneic stem cell transplant. In the current study, in patients with a median of two prior therapies, we demonstrated a CR rate of 28% overall and 29% in the second salvage setting. The median OS was 3.4 months overall, but 10.4 months in responding patients. In subgroups of patients with Philadelphia positive and T-cell disease, the outcomes were even better. Among responders, 43% of patients were able to go on to a stem cell transplant. The similar response rates among early and late salvage patients may again be explained by lack of overlapping resistance to asparaginase and prior chemotherapies. In conclusion, MOpAD is an active and tolerable regimen for the treatment of relapsed ALL. Suggestion of significant activity in patients with T-ALL and with a tyrosine kinase inhibitor in patients with Philadelphia positive ALL warrants further study with MOpAD in these subgroups.
Acknowledgments
Contract grant sponsor: Sigma Tau Pharmaceuticals, Inc. and National Institutes of Health through MD Anderson’s Cancer Center; Contract grant number: CA016672.
Footnotes
Author Contributions
T.M.K., H.K., J.C., and G.B. coordinated the study and performed the research. T.M.K., G.B., X.W., M.B., and S.P. collected, analyzed, and interpreted the data. T.M.K., H.K., D.A.T., S.O.B., Z.E., F.R., E.J., N.P., N.D., G.G.M., J.C., and G.B. contributed patients for the study. T.M.K. and G.B. wrote the manuscript with input from H.K., D.A.T., S.O.B., Z.E., F.R., E.J., X.W., N.P., N.D., G.G.M., and J.C. All authors approved the final manuscript.
Conflict of interest: G.B. has received research funding from Sigma Tau Pharmaceuticals, Inc.
References
- 1.Faderl S, O’Brien S, Pui CH, et al. Adult acute lymphoblastic leukemia: Concepts and strategies. Cancer. 2010;116:1165–1176. doi: 10.1002/cncr.24862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pui CH, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet. 2008;371:1030–1043. doi: 10.1016/S0140-6736(08)60457-2. [DOI] [PubMed] [Google Scholar]
- 3.Fielding AK, Richards SM, Chopra R, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ ECOG 2993 study. Blood. 2007;109:944–950. doi: 10.1182/blood-2006-05-018192. [DOI] [PubMed] [Google Scholar]
- 4.Tavernier E, Boiron JM, Huguet F, et al. Outcome of treatment after first relapse in adults with acute lymphoblastic leukemia initially treated by the LALA-94 trial. Leukemia. 2007;21:1907–1914. doi: 10.1038/sj.leu.2404824. [DOI] [PubMed] [Google Scholar]
- 5.Thomas DA, Kantarjian H, Smith TL, et al. Primary refractory and relapsed adult acute lymphoblastic leukemia: characteristics, treatment results, and prognosis with salvage therapy. Cancer. 1999;86:1216–1230. doi: 10.1002/(sici)1097-0142(19991001)86:7<1216::aid-cncr17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 6.O’Brien S, Thomas D, Ravandi F, et al. Outcome of adults with acute lymphocytic leukemia after second salvage therapy. Cancer. 2008;113:3186–3191. doi: 10.1002/cncr.23919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas DA, Faderl S, O’Brien S, et al. Chemoimmunotherapy with hyper-CVAD plus rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma or acute lymphoblastic leukemia. Cancer. 2006;106:1569–1580. doi: 10.1002/cncr.21776. [DOI] [PubMed] [Google Scholar]
- 8.Ravandi F, O’Brien S, Thomas D, et al. First report of phase 2 study of dasatinib with hyper-CVAD for the frontline treatment of patients with Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia. Blood. 2010;116:2070–2077. doi: 10.1182/blood-2009-12-261586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas DA, Faderl S, Cortes J, et al. Treatment of Philadelphia chromosome-positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood. 2004;103:4396–4407. doi: 10.1182/blood-2003-08-2958. [DOI] [PubMed] [Google Scholar]
- 10.Pui CH, Mullighan CG, Evans WE, et al. Pediatric acute lymphoblastic leukemia: Where are we going and how do we get there? Blood. 2012;120:1165–1174. doi: 10.1182/blood-2012-05-378943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rytting M. Peg-asparaginase for acute lymphoblastic leukemia. Expert Opin Biol Ther. 2010;10:833–839. doi: 10.1517/14712591003769808. [DOI] [PubMed] [Google Scholar]
- 12.Capizzi RL. Schedule-dependent synergism and antagonism between methotrexate and asparaginase. Biochem Pharmacol. 1974;23:151–161. [Google Scholar]
- 13.Capizzi RL. Asparaginase-methotrexate in combination chemotherapy: Schedule-dependent differential effects on normal versus neoplastic cells. Cancer Treat Rep. 1981;65(Suppl 4):115–121. [PubMed] [Google Scholar]
- 14.Sur P, Fernandes DJ, Kute TE, et al. L-asparaginase-induced modulation of methotrexate polyglutamylation in murine leukemia L5178Y. Cancer Res. 1987;47:1313–1318. [PubMed] [Google Scholar]
- 15.Vadlamudi S, Krishna B, Reddy VV, et al. Schedule-dependent therapeutic synergism for L-asparaginase and methotrexate in leukemic (L5178Y) mice. Cancer Res. 1973;33:2014–2019. [PubMed] [Google Scholar]
- 16.Jolivet J, Cole DE, Holcenberg JS, et al. Prevention of methotrexate cytotoxicity by asparaginase inhibition of methotrexate polyglutamate formation. Cancer Res. 1985;45:217–220. [PubMed] [Google Scholar]
- 17.Lobel JS, O’Brien RT, McIntosh S, et al. Methotrexate and asparaginase combination chemotherapy in refractory acute lymphoblastic leukemia of childhood. Cancer. 1979;43:1089–1094. doi: 10.1002/1097-0142(197903)43:3<1089::aid-cncr2820430346>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 18.Wiernik PH, Dutcher JP, Paietta E, et al. Long-term follow-up of treatment and potential cure of adult acute lymphocytic leukemia with MOAD: A non-anthracycline containing regimen. Leukemia. 1993;7:1236–1241. [PubMed] [Google Scholar]
- 19.van den Berg H. Asparaginase revisited. Leuk Lymphoma. 2011;52:168–178. doi: 10.3109/10428194.2010.537796. [DOI] [PubMed] [Google Scholar]
- 20.Wetzler M, Sanford BL, Kurtzberg J, et al. Effective asparagine depletion with pegylated asparaginase results in improved outcomes in adult acute lymphoblastic leukemia: Cancer and Leukemia Group B Study 9511. Blood. 2007;109:4164–4167. doi: 10.1182/blood-2006-09-045351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thall PF, Simon RM, Estey EH. New statistical strategy for monitoring safety and efficacy in single-arm clinical trials. J Clin Oncol. 1996;14:296–303. doi: 10.1200/JCO.1996.14.1.296. [DOI] [PubMed] [Google Scholar]
- 22.Aguayo A, Cortes J, Thomas D, et al. Combination therapy with methotrexate, vincristine, polyethylene-glycol conjugated-asparaginase, and prednisone in the treatment of patients with refractory or recurrent acute lymphoblastic leukemia. Cancer. 1999;86:1203–1209. doi: 10.1002/(sici)1097-0142(19991001)86:7<1203::aid-cncr15>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Bostrom BC, Sensel MR, Sather HN, et al. Dexamethasone versus prednisone and daily oral versus weekly intravenous mercaptopurine for patients with standard-risk acute lymphoblastic leukemia: a report from the Children’s Cancer Group. Blood. 2003;101:3809–3817. doi: 10.1182/blood-2002-08-2454. [DOI] [PubMed] [Google Scholar]
- 24.Ito C, Evans WE, McNinch L, et al. Comparative cytotoxicity of dexamethasone and prednisolone in childhood acute lymphoblastic leukemia. J Clin Oncol. 1996;14:2370–2376. doi: 10.1200/JCO.1996.14.8.2370. [DOI] [PubMed] [Google Scholar]
- 25.Jones B, Freeman AI, Shuster JJ, et al. Lower incidence of meningeal leukemia when prednisone is replaced by dexamethasone in the treatment of acute lymphocytic leukemia. Med Pediatr Oncol. 1991;19:269–275. doi: 10.1002/mpo.2950190411. [DOI] [PubMed] [Google Scholar]
- 26.Kaspers GJ, Veerman AJ, Popp-Snijders C, et al. Comparison of the antileukemic activity in vitro of dexamethasone and prednisolone in childhood acute lymphoblastic leukemia. Med Pediatr Oncol. 1996;27:114–121. doi: 10.1002/(SICI)1096-911X(199608)27:2<114::AID-MPO8>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 27.Silverman LB, Gelber RD, Dalton VK, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91-01. Blood. 2001;97:1211–1218. doi: 10.1182/blood.v97.5.1211. [DOI] [PubMed] [Google Scholar]
- 28.Larson RA, Fretzin MH, Dodge RK, et al. Hypersensitivity reactions to L-asparaginase do not impact on the remission duration of adults with acute lymphoblastic leukemia. Leukemia. 1998;12:660–665. doi: 10.1038/sj.leu.2401007. [DOI] [PubMed] [Google Scholar]