Abstract
Objectives
As no single HIV prevention program has eliminated HIV transmission, there is growing interest in the effectiveness of “combined” prevention programming. To compare HIV infection among persons injecting in the initial programs environment (IPE) in New York City (self-initiated risk reduction, methadone, education/outreach, and HIV testing) to HIV infection among persons injecting in a combined programs environment (CPE) (above programs plus large-scale syringe exchange). To identify potential behavioral mechanisms through which combined programs are effective.
Methods
Subjects were recruited from the Beth Israel drug detoxification program. A risk behavior questionnaire was administered and HIV testing conducted. Subjects who injected only between 1984 and 1994 (IPE) were compared to subjects who injected only between 1995 and 2008 (CPE).
Results
261 IPE subjects and 1153 CPE subjects were recruited. HIV infection was significantly lower among the CPE subjects compared to IPE subjects: prevalence 6% versus 21%, estimated incidence 0.3/100 person-years versus 4/100 person-years (both p < 0.001). The percentage of subjects at risk of acquiring HIV through receptive syringe sharing was similar across CPE and IPE subjects (30% versus 33%). The percentage of subjects at risk of transmitting HIV through injection-related behaviors (who were both HIV seropositive and reported passing on used needles/syringes), was much lower among the CPE subjects than among the IPE subjects (1% versus 10%, p < 0.001).
Conclusions
Combined prevention programs can greatly reduce HIV transmission. Reducing distributive sharing by HIV seropositive injecting drug users (IDUs) may be a critical component in reducing HIV transmission in high seroprevalence settings.
Keywords: Syringe exchange, HIV prevention programs, IDUs, New York City, Prevention for positives
1. Introduction
1.1. Combined HIV prevention programs for injecting drug users (IDUs)
No single HIV prevention intervention has been able to eliminate risk behavior in any population at high risk of HIV, and no single intervention has been equally effective with all members of a high-risk population. These limitations on individual interventions have generated increased interest in the potential effectiveness of “combined” systems of prevention programs targeted to a single population at risk (Coates et al., 2008).
The concept of “combined” prevention programs for injecting drug users (IDUs) has not been precisely defined. The Technical Guide for Countries to Set Targets for the Universal Access to HIV Prevention, Treatment and Care for Injecting Drug Users (http://www.who.int/hiv/idu/target_setting/en/index.html) describes what may be considered a fully comprehensive set of programs, including needle and syringe programs, opiate substitution therapy, voluntary HIV counseling and testing, anti-retroviral therapy, sexually transmitted infection prevention and treatment, condom programming for IDUs and partners, targeted information, education and communication for IDUs and their partners, hepatitis diagnosis, treatment and vaccination, and tuberculosis prevention, diagnosis and treatment (Donoghoe et al., 2008). Whether all of these components are needed to control HIV epidemics among IDUs has not been determined, and this is a critical question for HIV prevention among IDUs in resource-limited settings.
If combined interventions—particularly community outreach and legal access to sterile injection equipment—are implemented when HIV prevalence is low, it is often possible to keep HIV prevalence below 5% indefinitely (Committee on the Prevention of HIV Infection among Injecting Drug Users in High Risk Countries, 2006; Des Jarlais et al., 1995). Combined interventions face a more difficult challenge if they are not implemented until after HIV has reached high prevalence levels (30% or greater) in an IDU population. With large numbers of IDUs at risk of acquiring HIV and large numbers of IDUs at risk of transmitting HIV, even moderate levels of risk behavior may generate substantial HIV incidence (4–6/100 person-years) (Des Jarlais et al., 1998a).
There is evidence from many areas, including Amsterdam (Van Den Berg et al., 2007), France (Desenclos, 2005), New York (Des Jarlais et al., 2005), Chicago (Ouellet and Huo, 2009) and Baltimore, Philadelphia and Vancouver that implementation of “combined” prevention programs is associated with large reductions in HIV prevalence among IDUs and reductions in incidence to 1/100 person-years or less (Des Jarlais, 2009). There are, however, a number of important questions with respect to combined prevention programs that still need resolution, including (1) can combined prevention programs eliminate HIV among IDUs? (2) what are the limitations of current combined prevention programs, and (3) what are the mechanisms through which combined prevention programs work. This last question is of particular importance for potentially decreasing the time needed for combined programs to be effective and for implemented effective combined programs in limited-resource settings. The great majority of new HIV infections among IDUs are currently occurring in low and middle income countries (UNAIDS/WHO, 2007).
1.2. Brief history of HIV prevention for IDUs in New York City
In this report, we examine these questions through new analyses of data from IDUs in New York City. With over 63,000 cases of AIDS among IDUs (Li et al., 2008), New York City has experienced the world’s largest IDU HIV epidemic. HIV entered the IDU population in the mid-1970s, spread rapidly during the late 1970s, and then stabilized at over 50% prevalence during the 1980s (Des Jarlais et al., 1995).
Implementation of HIV prevention programs for IDUs occurred in three stages in New York:
Pre-AIDS awareness. Methadone maintenance was developed in the late 1960s and then expanded to approximately 36,000 treatment “slots” (for an IDU population of 150,000–200,000), prior to the introduction of HIV into the IDU population (Newman, 1977). While being in methadone treatment was associated with a lower likelihood of HIV infection, the availability of methadone treatment did not prevent the rapid transmission of HIV among IDUs in the city.
Initial HIV prevention programs. The first specific to AIDS prevention programs were self-organized efforts among IDUs to educate themselves about AIDS and to increase the use of clean needles (Des Jarlais and Hopkins, 1985; Des Jarlais et al., 1985) and AIDS education within drug abuse treatment programs. These were followed by a variety of additional HIV prevention programs, including community outreach and voluntary HIV counseling and testing in the mid-1980s. Small scale activist operated syringe exchange programs were implemented in the late 1980s.
Combined HIV prevention programs. Syringe exchange programs received legal authorization and public funding in late 1992. Syringe exchange expanded from approximately 250,000 syringes exchanged annually in 1990–1992 to 2,500,000 syringes exchanged annually in 1996–1998. HIV incidence among IDUs declined from 3.55/100 person-years at risk (PYAR) from 1990 to 1992, to 2.63/100 PYAR from 1993 to 1995, to 1.05/100 PYAR from 1996 to 1998, and to 0.77/100 PYAR from 1999 to 2002 (p < 0.001) (Des Jarlais et al., 2005). The prescription requirement for pharmacy sales of syringes was removed in 2001, and pharmacists were permitted to sell syringes to drug users, and highly active anti-retroviral treatment (HAART) became available to large numbers of IDUs in the early 2000s.
Comparison of HIV infection and risk behaviors among persons who injected only in the “initial prevention programs” environment with persons who injected only in the “combined prevention programs” environment would not only provide a strong contrast of trends in HIV infection in the two environments, but may also provide insight into how combined programs “work,” and into the long-term outcomes of combined HIV prevention programs for IDUs.
2. Methods
2.1. Data collection procedures
The data reported here are derived from ongoing analyses of data collected from IDUs entering the Beth Israel Medical Center drug detoxification program in New York City. The methods for this “risk factors” study have been previously described in detail (Des Jarlais et al., 1989), so only a summary will be presented here. The Beth Israel detoxification program serves the city as a whole, and approximately half of its patients live in Manhattan, one quarter in Brooklyn, one fifth in the Bronx, and the remainder (i.e., 5%) live elsewhere. Patients enter the program voluntarily.
Research staff visited the general admission wards of the program in a preset order and examined all intake records of a specific ward to construct lists of patients admitted within the prior 3 days. All of the patients on the list for the specific ward are then asked to participate in the study. Among patients approached by our interviewers, willingness to participate was more than 95%. After all of the patients admitted to a specific ward in the 3-day period have been asked to participate and interviews have been conducted among those who agreed to participate, the interviewer moved to the next ward in the preset order. Because there is no relationship between assigning patients to wards and the order that the staff rotate through the wards, these procedures should produce an unbiased sample of persons entering the detoxification program.
A structured questionnaire covering demographics, drug use, sexual risk behavior, and use of HIV prevention services was administered by a trained interviewer. Participants were included in the analyses presented here if they had injected illicit drugs in the 6 months prior to the interview. Most HIV risk behavior questions referred to the 6 months prior to the interview. Male-with-male sexual (MSM) behavior was assessed by asking males about sex with other males in the 5 years prior to the interview. The questionnaire included date of birth and age when illicit drugs were first injected to determine the year of first injection.
After completing the interview, the participant was seen by an HIV counselor for pretest counseling and specimen collection. HIV testing was conducted at the New York City Department of Health Laboratory by using a commercial, enzyme-linked, immunosorbent assays (EIA) test with Western blot confirmation (BioRad Genetic Systems HIV-1-2+0 EIA and HIV-1 Western Blot, BioRad Laboratories, Hercules, CA).
2.2. Research design
Serial cross-sectional data have been collected in the project since 1990. The conceptual sampling frame has been IDUs who entered the Beth Israel detoxification program in a given year. Individuals might enter the program multiple times during their drug use careers. To maintain the conceptual sampling frame, we did not permit an individual to participate in the study more than once in a given year. However, we did permit individuals to participate in the study in different years. This rule was necessary to maintain the sampling frame. If individuals were to be excluded from contributing data in a given year because they had participated previously, then severe bias in the data from later years would be created, such as bias toward persons with shorter drug use histories and less likelihood of HIV infection.
2.3. Statistical comparisons of HIV infection among IDUs in different risk environments
As noted above, it is possible to divide the implementation of HIV prevention programs for IDUs into three phases: pre-AIDS awareness methadone treatment, initial HIV prevention programs and combined HIV prevention programs. We used 1984 as a start date for the initial HIV prevention programs because there was evidence that IDUs were aware of AIDS and organizing to reduce their risks by that time (Des Jarlais et al., 1985; Des Jarlais and Hopkins, 1985). Funded community outreach and HIV counseling and testing programs were implemented very shortly thereafter. We used 1995 as a cut-off date separating the initial prevention programs from the combined programs because 1995 was the mid-point in the rapid expansion of the syringe exchange programs, and 1995 also marked the beginning of a sharp decline in HIV prevalence among IDUs in the city. Using 1995 as a cut-off date also showed a marked change in the relationships between hepatitis C virus and herpes simplex virus 2 with HIV among IDUs in the city (Des Jarlais et al., 2009). We did repeat our analyses using plus/minus 1 year for the cut-off dates, which did not change the patterns of statistical significance in our results.
The primary comparison was on HIV status and risk behavior between persons who injected only in the “initial prevention programs” environment versus persons who injected only in the “combined prevention programs” environment. “Persons injecting only in the initial prevention programs” environment was operationally defined as data collected from 1990 through 1994 from persons who began injecting between January 1, 1984 and December 31, 1994. “Persons injecting only in the combined prevention programs” environment was operationally defined as data collected from persons who began injecting after January 1, 1995, with data collected from January 1995 through August 2008. Thus, no individual subject would be included as injecting in both the “initial prevention programs” environment and the “combined prevention programs” environments.
It is important to note that data were collected as part of the overall research project but were not included in the comparison. First, data from persons who began injecting prior to 1984 were not included. Many, if not most, of the HIV infections in this group would have occurred prior to implementation of even the initial prevention programs, and thus would not contribute to understanding the differences between the initial and combined prevention programs. Second, data collected after 1995 from persons who began injecting in the initial prevention program environment (1984–1994) also were not included. Most of the HIV infections in post-1995 data from persons who began injecting between 1984 and 1995 would have occurred prior to implementation of the combined prevention program environment. It would not be possible, however, to allocate HIV infections in post-1995 as occurring prior to or after 1995. Thus, including data collected after 1995 from persons who began injecting prior to 1995 would also detract from understanding the differences between the initial prevention and combined prevention environments.
For convenience in terminology, we refer to persons who injected only in the initial programs environment (IPE) IDUs and persons who injected only in the combined programs environment (CPE) IDUs.
2.4. Ethics review
The study was approved by the Beth Israel and National Development and Research Institutes institutional review boards.
3. Results
3.1. Demographic and drug use characteristics
Table 1 presents selected demographic and drug use characteristics of the 261 IDUs who began injecting in 1984 or later and were recruited from 1990–1994 (IPE IDUs) and 1153 IDUs who began injecting in 1995 or later and were recruited in 1995–2008 (CPE IDUs). The IPE subjects were more likely to be female, and were younger. There were differences in the drugs injected, though heroin was the most commonly injected drug in both groups, and 79% of IPE group and 82% of CPE reported injecting daily. More of the CPE subjects reported intranasal heroin use, while more of the IPE subjects reported smoking crack cocaine. The mean length of injection history was 4.2 years (SD = 2.7) for the IPE IDUs and 3.5 years (SD = 2.9) for the CPE IDUs; however, 47% of IPE IDUs and 32% of CPE IDUs injected for 5 years or more, so that both samples included substantial percentages of subjects who were past “new injectors” status.
Table 1.
Demographic and drug use characteristics of IPE and CPE IDUs.
| IPE IDUs (n = 261) N (%) |
CPE IDUs (n = 1153) N (%) |
|
|---|---|---|
| Gender | ||
| Non-MSM males | 166 (64) | 817 (71) |
| MSM males | 15 (6) | 60 (5) |
| Females | 80 (31) | 276 (24) |
| Race/ethnicity | ||
| White | 74 (28) | 333 (30) |
| Black | 27 (10) | 137 (12) |
| Hispanic | 154 (59) | 639 (58) |
| Other | 5 (2) | 44 (4) |
| Age* | ||
| <40 years of age | 247 (95) | 988 (86) |
| 40 or older | 14 (5) | 165 (14) |
| Injection drug | ||
| Heroin* | 225 (86) | 1074 (93) |
| Cocaine* | 166 (64) | 439 (38) |
| Speedball* | 198 (76) | 509 (44) |
| Non-injection drug | ||
| Intranasal heroin* | 100 (38) | 519 (45) |
| Crack cocaine* | 126 (48) | 403 (35) |
| Daily injection | 205 (79) | 944 (82) |
| Avg. age at first injection† | 25 (6) | 28 (8) |
| Avg. years of injection† | 4.2 (3) | 3.5 (3) |
The comparison is for each demographic/drug use characteristic across IPE and CPE periods.
Significant difference by chi-square test (p < 0.01).
Significant difference by t-test (p < 0.01).
3.2. HIV prevalence
Table 2 shows HIV prevalence for the total IPE and CPE subjects and by demographic characteristics. HIV prevalence was much higher among the IPE versus CPE subjects (21% versus 6%). The IPE subjects had significantly higher HIV prevalence in all demographic subgroups. The odds ratios for HIV prevalence among IPE versus CPE subjects were relatively consistent across the different demographic subgroups.
Table 2.
HIV prevalence among IPE IDUs and CPE IDUs.
| HIV prevalence IPE IDUs n/N (%) |
HIV prevalence CPE IDUs n/N (%) |
CPE to IPE OR (95% CI) |
|
|---|---|---|---|
| Total (ref = IPE) | 55/261 (21%) | 66/1153 (6%) | 0.23 (0.15–0.34) |
| Gender/sexual behavior (ref = IPE non-MSM males) | |||
| Non-MSM males | 25/166 (15%) | 29/817 (4%) | 0.21 (0.12–0.37) |
| MSM males | 5/15 (33%) | 12/60 (18%) | 0.50 (0.14–1.74) |
| Females | 25/80 (31%) | 25/276 (9%) | 0.22 (0.12–0.41) |
| Race/ethnicity (ref = IPE Whites) | |||
| Whites | 10/74 (14%) | 9/333 (3%) | 0.18 (0.07–0.46) |
| Blacks | 8/27 (30%) | 20/137 (15%) | 0.41 (0.16–1.05) |
| Hispanics | 36/154 (23%) | 35/639 (5%) | 0.19 (0.12–0.32) |
| Other | 1/5 (20%) | 2/44 (5%) | 0.19 (0.01–2.59) |
| Age (ref = IPE <40) | |||
| <40 | 50/247 (20%) | 45/988 (5%) | 0.19 (0.12–0.29) |
| 40 and older | 5/14 (36%) | 21/165 (13%) | 0.26 (0.08–0.86) |
The CPE subjects were interviewed over the 13-year period from 1995 through 2008. To assess whether there might have been changes in HIV prevalence among CPE subjects during this time, we compared HIV prevalence among CPE subjects with <4 years of injecting who were interviewed between 1995 and 2001 to CPE subjects with <4 years of injecting who were interviewed from 2002 through 2008. HIV prevalence in the former group was 4% and was 6% in the latter group, p = 0.46 by chi-square test. Thus, there did not appear to be any trends over time in HIV infection among the CPE subjects.
3.3. Estimated HIV incidence
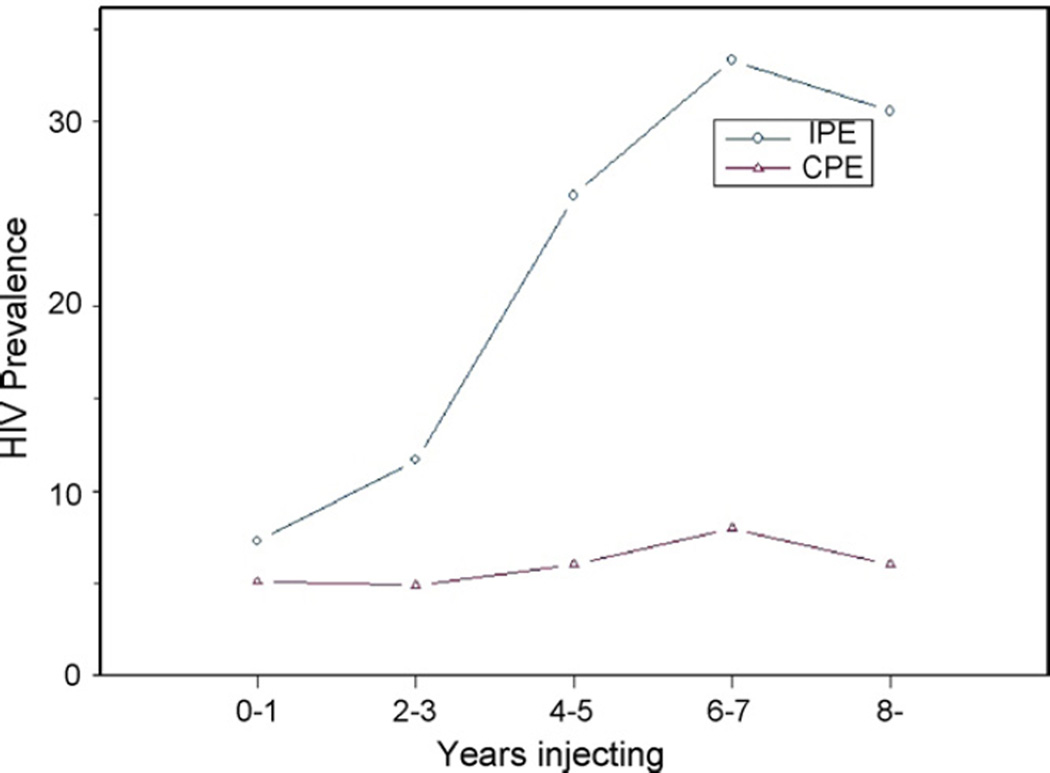
Fig. 1 shows HIV prevalence by years of injecting for IPE and CPE subjects. The IPE subjects show a marked increase in HIV prevalence by duration of injecting (p for trend <0.001 by Cochran–Armitage test). In contrast, there was no significant increase in HIV seroprevalence by duration of injecting among the CPE subjects, despite the large sample size (N= 1153, p = 0.28 by Cochrane–Armitage test.). There was not a significant difference in HIV prevalence between the IPE and CPE subjects among the “very new” injectors, persons injecting for 0–1 year, but meaningful differences emerged as early as 2–3 years after beginning to inject. We fitted linear regression lines to the relationships between HIV prevalence by duration of injecting for the IPE and CPE subjects, while controlling for race/ethnicity and gender/MSM behavior. (Because age and years injecting were highly correlated, however, it was not possible to include both of these variables in the same models.) For the IPE subjects, the slope for HIV prevalence by duration of injecting was 0.068 (95% CI: 0.051–0.084) per 2-year injecting period (or 0.04 per injecting year). For the CPE subjects, the slope was 0.007 (95% CI: −0.002 to 0.011) per 2-year injecting period (or 0.003 per injecting year). The difference in slopes (injecting years by period interaction) was highly significant, p < 0.0001.
Fig. 1.
Prevalence in IPE and CPE IDUs by years injecting.
Slopes of HIV prevalence by time at risk are often used as estimates of HIV incidence in the population (Ghys et al., 2006). The slopes in Fig. 1 would correspond to estimated incidence rates of 4/100 person-years (95% CI: 2.9–4.7) among the IPE subjects and 0.3/100 (95% CI: 0.1–0.6) person-years among the CPE subjects (note the complete separation in the 95% CIs).
3.4. HIV risk behaviors
We examined HIV risk behaviors among the IPE and CPE subjects to assess how differences in risk behaviors might explain the differences in HIV infection. Table 3 compares injecting and sexual risk behaviors (for the 6 months prior to the interview) of the IPE and CPE subjects. The only significant difference was the lower rate of distributive sharing among the CPE subjects.
Table 3.
Sexual and injection risk behaviors among IPE and CPE IDUs in New York City.
| Risk behaviors | IPE IDUs n (%) |
CPE IDUs n (%) |
CPE to IPE OR (95% CI) |
CPE to IPE AOR (95% CI)a |
|---|---|---|---|---|
| Unprotected sex w/primary partner of opposite sex | 129/261 (49) | 564/1153 (49) | 0.98 (0.75–1.28) | 1.05 (0.80–1.39) |
| Unprotected sex w/casual partner of opposite sex | 39/261 (15) | 166/1153 (14) | 0.96 (0.66–1.40) | 0.94 (0.64–1.38) |
| Unprotected sex w/commercial partner of opposite sex | 9/261 (4) | 59/1153 (5) | 1.51 (0.74–3.09) | 2.00 (0.94–4.22) |
| Receptive sharing of used needles/syringes | 95/261 (36) | 394/1153 (34) | 0.91 (0.69–1.20) | 1.03 (0.77–1.38) |
| Distributive sharing of used needles/syringes | 137/261 (53) | 360/1153 (31) | 0.41 (0.31–0.54) | 0.45 (0.34–0.60) |
Odds ratios adjusted for age as a continuous variable, gender, and race/ethnicity.
We also examined risk behaviors by HIV serostatus. This permits distinguishing between risk of HIV acquisition and risk of HIV transmission. HIV seronegative subjects who engaged in unprotected sex or receptive syringe sharing (injecting with a needle/syringe used by someone else) would put themselves at risk of acquiring HIV, while HIV seropositive subjects who engaged in unprotected sex or distributive syringe sharing (passing on a used needle/syringe to someone else) would be at risk of transmitting HIV to others. Table 4 compares different risks for HIV acquisition among HIV seronegatives and risk of HIV transmission among HIV seropositives between the IPE and CPE subjects. (The denominators for the percentages in Table 3 are subjects of the same HIV serostatus.) There were no significant differences in sexual risks or in the risk of HIV acquisition through receptive syringe sharing by HIV seronegatives. There was a highly significant difference in the risk of HIV transmission through distributive syringe sharing by HIV seropositives.
Table 4.
Potential HIV acquisition and transmission behaviors among IPE and CPE IDUs in New York City.
| Potential acquisition and transmission behaviors | IPE IDUs n (%) |
CPE IDUs n (%) |
CPE to IPE OR (95% CI) |
CPE to IPE AOR (95% CI)a |
|---|---|---|---|---|
| Any unprotected sex among HIV seronegatives | 130/206 (63) | 677/1087 (62) | 1.00 (0.71–1.31) | 1.05 (0.76–0.99) |
| Any unprotected sex among HIV seropositives | 28/55 (51) | 24/66 (36) | 0.55 (0.27–1.14) | 0.78 (0.35–1.73) |
| Receptive sharing among HIV seronegatives | 77/206 (37) | 375/1086 (35) | 0.88 (0.65–1.20) | 1.00 (0.73–1.38) |
| Distributive sharing among HIV seropositives | 26/55 (47) | 11/66 (17) | 0.22 (0.10–0.52) | 0.22 (0.09–0.54) |
Odds ratios adjusted for age as a continuous variable, gender, and race/ethnicity.
We also calculated the IPE and CPE risks for HIV acquisition and transmission at a “total sample/population” level. For example, the percentage of IPE subjects at risk of HIV acquisition through receptive syringe sharing level would be HIV seronegatives reporting receptive syringe sharing (numerator) divided by the combined number of HIV seronegatives and HIV seropositives in the total IPE sample (denominator). These total sample results for HIV acquisition and transmission risks would reflect risks in the underlying IPE and CPE IDU populations, and are presented in Table 5. There was a higher rate of unprotected sex by HIV seronegatives among the CPE subjects. The CPE subjects, however, had much lower percentages of subjects at risk for transmitting HIV through unprotected sex (11% of total IPE sample versus only 2% of total CPE sample) and through distributive sharing (10% of total IPE sample versus only 1% of total CPE sample). In addition, note the very low odds ratios and narrow 95% confidence limits for these population transmission risk comparisons between IPE and CPE IDUs.
Table 5.
Potential HIV acquisition and transmission behaviors among IPE and CPE IDUs in New York City.
| Potential acquisition and transmission behaviors | IPE IDUs n (%) |
CPE IDUs n (%) |
CPE to IPE OR (95% CI) |
CPE to IPE AOR (95% CI)a |
|---|---|---|---|---|
| Any unprotected sex by HIV seronegatives among all IDUs | 130/261 (50) | 677/1153 (59) | 1.43 (1.10–1.88) | 1.56 (1.18–2.07) |
| Any unprotected sex by HIV seropositives among all IDUs | 28/261 (11) | 24/1153 (2) | 0.18 (0.10–0.31) | 0.19 (0.11–0.34) |
| Receptive sharing by HIV seronegatives among all IDUs | 77/261 (30) | 375/1153 (33) | 1.15 (0.86–1.54) | 1.30 (0.96–1.77) |
| Distributive sharing by HIV seropositives among all IDUs | 26/261 (10) | 11/1153 (1) | 0.09 (0.04–0.18) | 0.08 (0.04–0.17) |
Odds ratios adjusted for age as a continuous variable, gender, and race/ethnicity.
3.5. Individual utilization of prevention services
We collected individual-level data on the use of three different prevention services for our IPE and CPE IDUs. Among the IPE IDUs, 54% reported having ever been in methadone maintenance treatment, 66% reported having been tested for HIV (at any time prior to enrollment in the study), and 39% reported obtaining syringes from a “guaranteed sterile source” (syringe exchange, pharmacy or healthcare provider) in the 6 months prior to the interview. Among the CPE IDUs, 46% reported ever having been in methadone maintenance, 91% reported having previously been tested for HIV, and 66% reported obtaining syringes from a “guaranteed sterile” source in the 6 months prior to the interview. (All differences had p < 0.05 by chi-square tests.) The higher percentages with HIV testing and obtaining syringes from guaranteed sterile sources reflect expansion of these services after 1995.
We used multiple logistic regression analysis to assess whether participation in the three prevention services was associated with either receptive sharing among HIV seronegatives (which would put them at risk for acquiring HIV) or distributive sharing among HIV seropositives (which would put them at risk for transmitting HIV). The models used the demographic and drug use characteristics presented in Table 1 as control variables and also included a term for the IPE versus CPE variable. Having participated in methadone maintenance treatment and having previously been tested for HIV were not associated with receptive sharing among the HIV seronegatives (data not presented, available from the first author). However, obtaining syringes from a “guaranteed sterile source” (exchange, pharmacy or healthcare provider) approached significance as a predictor of receptive sharing among seronegatives, AOR= 0.77, 95% CI: 0.59–1.00). We examined the relationship between obtaining syringes from a guaranteed sterile source and receptive sharing separately for the IPE IDUs and the CPE IDUs. Obtaining syringes from a guaranteed sterile source was not significantly associated with receptive sharing among the IPE IDUs (AOR = 1.13, 95% CI: 0.60–2.11), but was significantly associated with receptive sharing among the CPE IDUs (AOR = 0.68, 95% CI: 0.51–0.92, p < 0.05).
Participation in methadone maintenance, previous HIV testing, and obtaining syringes from a guaranteed sterile source were not associated with distributive sharing among HIV seropositives, either among the combined IPE and CPE IDUs or in either IPE IDUs or CPE IDUs separately (data not presented, available from the first author). It should be noted that we had relatively few HIV seropositives who reported distributive sharing, particularly among the CPE IDUs (only 11/66), so that we did not have adequate statistical power for identifying variables with anything less than very strong associations. Additionally, the HIV testing variable had a highly skewed distribution (91% of the CPE IDUs reported previous HIV testing). In the combined IPE and CPE model, however, distributive sharing was much less common among the CPE IDUs, AOR= 0.25, 95% CI: 0.086–0.71, with control for demographic and drug use variables in Table 1 and use of methadone maintenance, obtaining syringes from a guaranteed sterile source and for previous HIV testing.
4. Discussion
4.1. Consistency with other NYC incidence studies
Before considering the implications of the findings, it is important to note the consistency of the incidence estimates developed here with other incidence studies in New York City. The incidence estimates developed here were for IDUs injecting only within specified time frames and there are no other studies that use the same samples. However, we utilized the STAHRS testing to estimate incidence among all IDUs entering the Beth Israel detoxification program from 1990 to 1993. Although that estimate included IDUs who began injecting prior to the beginning of the IPE as well as IDUs who injected only in the IPE, the STAHRS incidence estimate—3.6/100 person-years—was almost identical to the incidence estimate for IPE IDUs in the data reported here—4.0/100 person-years.
We are aware of three cohort studies of “new injectors” (persons injecting for 5 years or less) conducted in New York City after 1995, two reported in (Des Jarlais et al., 2003) and one in (Garfein et al., 2007). There would be considerable overlap between these three new injector cohorts and the CPE IDUs in this report, though the CPE IDUs included a considerable number of persons with injecting histories of 5–12 years. There was again close agreement among the incidence estimates: 0/100 person-years, 0.8/100 person-years and 0/100 person-years in the three cohort studies and 0.3/100 person-years for the IPE IDUs in this report. Thus, evidence from other incidence studies conducted in New York are strongly supportive of the incidence estimates developed here and the very large difference in HIV incidence between the IPE and the CPE IDUs.
4.2. Novel finding
That HIV infection was much lower among IDUs who injected only in the combined prevention programs environment than among IDUs who injected only in the initial prevention programs environment is consistent with both previous research in NewYork (Des Jarlais et al., 2005), as well as research on combined prevention programs from Amsterdam (Van Den Berg et al., 2007), Chicago (Ouellet and Huo, 2009) and from Baltimore, Philadelphia, and Van-couver [personal communications from S. Metha, D. Metzger and E. Wood, summarized in ref. Des Jarlais (2009)].
In these New York data, however, the low rate of HIV infection among the CPE IDUs was not associated with fewer IDUs reporting traditional “risk behavior” (receptive syringe sharing among HIV seronegatives) but rather with many fewer IDUs reporting transmission behavior (distributive sharing by HIV seropostives). There has been relatively little study of “prevention for positives” (programs to reduce transmission behavior among HIV seropositives) among IDUs (Crepaz et al., 2006; Latkin et al., 2008; Metsch et al., 2007). Research on HIV among IDUs has traditionally focused on behavior that puts an individual IDU at risk for acquiring HIV. For example, in their analysis of combined HIV prevention programs for IDUs in Amsterdam, Van Den Berg et al. (2007) concluded that participation in both syringe exchange and methadone maintenance treatment was necessary to obtain protection again becoming infected with HIV. The most recent US Institute of Medicine report on HIV prevention for IDUs (Committee on the Prevention of HIV Infection among Injecting Drug Users in High Risk Countries, 2006), does not address reducing transmission behavior by HIV seropositives. None of the nine meta-analyses of HIV prevention for IDUs used reducing transmission behavior as an outcome measure of interventions (Semaan et al., 2009).
We were not able to identify factors associated with distributive sharing among HIV seropositive CPE IDUs. We were limited by the modest numbers of HIV seropositive CPE IDUs (66) and the very modest number of these subjects who reported distributive sharing (11). Additionally, the HIV testing variable was highly skewed among the HIV seropositive CPE IDUs, with 91% reporting previous HIV testing. We would hypothesize that very low rates of distributive sharing among HIV seropositives is most likely in environments in which: (1) there is relatively good legal access to sterile injection equipment, so that there would be minimal economic and logistical pressure for sharing, (2) a very large percentage of IDUs have been tested for HIV, so that the great majority know their HIV status, and (3) the level of stigmatization of HIV is sufficiently low that IDUs will openly discuss HIV/AIDS and many seropositives may be willing to altruistically reduce transmission behavior, even if this means disclosing their HIV status.
4.3. Long-term outcomes of combined prevention programs in New York
As the CPE IDUs, who began injecting in 1995 or later, will eventually replace IDUs who began injecting before 1995 in the New York IDU population, HIV among the CPE IDUs provides the best prediction for future patterns of HIV among IDUs in the city. One of the critical questions for combined prevention programs is whether they can eliminate HIV among IDUs. The combined prevention programs in New York clearly have not eliminated HIV among IDUs in the city. In a previous study of IDUs who began injecting in 1995 or later in New York, HIV was strongly associated with herpes simplex virus 2, a biomarker for sexual risk behavior (OR = 10.71) and not associated with hepatitis C virus, a biomarker for injecting risk behavior (OR = 1.04). Studies from Baltimore (Strathdee and Sherman, 2003) and San Francisco (Kral et al., 2001) also found that sexual transmission of HIV became more important than injecting related transmission after implementation of large-scale syringe exchange programs. Continuing sexual transmission of HIV may be one of the most important weaknesses of current combined prevention programs for IDUs.
4.4. Limitations
Several limitations of this study should be noted. Some subjects may have lived and injected drugs outside New York City for periods of time, so that the conditions within the city did not reflect their chances of becoming infected with HIV. In both the “initial prevention program environment” and the “combined prevention program environment” different prevention programs were scaled up at different rates, and no program ever reached 100% of IDUs in either environment. There is also the likelihood of cumulative effects for some interventions, e.g., as more IDUs were contacted through community outreach in the mid-to-late 1980s, the likelihood of new social norms against sharing syringes would increase. Thus, any specific dates for the beginning and end of these risk environments will be somewhat arbitrary. Nevertheless, there clearly were changes in the HIV risk environment for IDUs in New York with the implementation of the initial programs in the mid-1980s and the large-scale expansion of the syringe exchange programs in the mid-1990s. Repeating the analyses with plus/minus one year for the cut-off dates did not change any of the statistically significant findings.
There were differences in non-injecting drug use between the IPE and CPE IDUs, including differences in crack cocaine use and intranasal heroin use. We did statistically control for these in our analyses, but the relationships of non-injecting drug use and HIV status may be sufficiently complex that standard multivariate statistical control is not adequate to capture the complexities in the associations.
We were not able to quantify how deaths due to AIDS or how provision of highly active anti-retroviral therapy (HAART) might have affected the patterns of HIV infection between IPE and CPE IDUs. (Approximately two-thirds of IDUs diagnosed with AIDS through December 31, 1995 had died. HAART treatment did not reach large numbers of active IDUs until after 2000.) Both of these factors, however, would tend to reduce differences in HIV infection between the IPE and CPE groups rather than create artificial differences.
We were also not able to assess the extent to which CPE IDUs engaged in risk behavior with IPE IDUs who began injecting prior to 1995. In our previous research (Des Jarlais et al., 2004), we found that after large-scale implementation of the exchanges, most IDUs who shared syringes restricted this behavior to within small social networks. If CPE IDUs confined their risk behaviors within the CPE group, with its low HIV prevalence, this form of restricted mixing would have provided important protection against HIV transmission.
The study is also from a single site, and includes only subjects whose drug use was sufficient that they were voluntarily entering drug abuse treatment. Data from this site, however, has historically been very consistent with other IDU samples in New York City, including IDUs recruited from community settings (Des Jarlais et al., 1998b, 2000a,b, 2007a,b.
5. Summary and conclusions
Large-scale implementation of syringe exchange programs in the mid-1990s created an environment of “combined” prevention programs for IDUs in New York City. IDUs who injected in this environment had much lower rates of HIV infection than IDUs who had injected in the previous “initial HIV prevention programs” environment. The low prevalence and low estimated incidence among the CPE IDUs were clearly associated with very low rates of HIV transmission behavior among seropositive IDUs rather than very low rates of acquisition risk behavior among HIV seronegative IDUs. There has been relatively little study of “prevention for positives” (programs to reduce transmission behavior among HIV seropositives) among IDUs (Cooper et al., 2009; Crepaz et al., 2006; Kalichman et al., 2001; Latkin et al., 2008; Metsch et al., 2007).
Indeed, the HIV prevention research among IDUs has been so dominated by acquisition risk behavior (receptive sharing) as the outcome measure, that transmission risk behavior by HIV seropositives is rarely reported as an outcome measure (Committee on the Prevention of HIV Infection among Injecting Drug Users in High Risk Countries, 2006; Semaan et al., 2009). We would suggest that reducing transmission behavior receive much more attention in epidemiological and intervention research on HIV among IDUs, and that future studies should at the very least report on both transmission risk behavior among HIV seropositives and acquisition risk behavior among HIV seronegatives.
Acknowledgments
Role of funding source
Funding for this study was provided by the NIH Grant # DA 03574 and 2 P30 DA 11041 but the NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Conflict of interest
All authors declare that they have no conflicts of interest.
Contributors
DC Des Jarlais conceived of the study and supervised all aspects of its implementation. K. Arasteh assisted with the study and completed the analyses. H. Hagan, C. McKnight, D. Perlman and S. Friedman assisted with the study and writing and reading drafts of the paper. All authors helped to conceptualize ideas, interpret findings, and review drafts of the manuscript.
References
- Coates T, Richter L, Caceres C. Behavioural strategies to reduce HIV transmission: how to make them work better. Lancet. 2008;372:669–684. doi: 10.1016/S0140-6736(08)60886-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D, Pantaleo G, Mastro T, Abdool-Karim Q, editors. Current Opinion in HIV and AIDS Journal. 2009;4(4):1–345. doi: 10.1097/COH.0b013e32832c92ac. [DOI] [PubMed] [Google Scholar]
- Committee on the Prevention of HIV Infection among Injecting Drug Users in High Risk Countries. Preventing HIV Infection among Injecting Drug Users in High Risk Countries: an Assessment of the Evidence. Institute of Medicine, Washington; 2006. [Google Scholar]
- Crepaz N, Lyles C, Wolitski R, Passin W, Rama S, Herbst J, Purcell D, Malow R, Stall R. Do prevention interventions reduce HIV risk behaviours among people living with HIV? A meta-analytic review of controlled trials. AIDS. 2006;20:143–157. doi: 10.1097/01.aids.0000196166.48518.a0. [DOI] [PubMed] [Google Scholar]
- Des Jarlais D. Can HIV be eliminated among injecting drug users?. Proceedings of the 2009 National HIV Prevention Conference; Atlanta, GA. 2009. [Google Scholar]
- Des Jarlais D, Arasteh K, McKnight C, Hagan H, Perlman D, Friedman S. Using hepatitis C virus and herpes simplex virus-2 to track HIV among injecting drug users in New York City. Drug Alcohol Depend. 2009;101:88–91. doi: 10.1016/j.drugalcdep.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Des Jarlais D, Arasteh K, Perlis T, Hagan H, Abdul-Quader A, Heckathorn D, McKnight C, Bramson H, Nemeth C, Torian L, Friedman S. Convergence of HIV seroprevalence among injecting and non-injecting drug users in New York City: a new stage in a very large HIV epidemic. AIDS. 2007a;21:231–235. doi: 10.1097/QAD.0b013e3280114a15. [DOI] [PubMed] [Google Scholar]
- Des Jarlais D, Arasteh K, Perlis T, Hagan H, Heckathorn D, McKnight C, Bramson HSRF. The transition from injection to non-injection drug use: long-term outcomes among heroin and cocaine users in New York City. Addiction. 2007b;102:778–785. doi: 10.1111/j.1360-0443.2007.01764.x. [DOI] [PubMed] [Google Scholar]
- Des Jarlais D, Hagan H, Friedman S, Friedmann P, Goldberg D, Frischer M, Green S, Tunving K, Ljungberg B, Wodak A, Ross M, Purchase D, Millson M, Myers T. Maintaining low HIV seroprevalence in populations of injecting drug users. JAMA. 1995;274:1226–1231. doi: 10.1001/jama.274.15.1226. [DOI] [PubMed] [Google Scholar]
- Des Jarlais DC, Choopanya K, Millson P, Friedmann P, Friedman S. The structure of stable seroprevalence HIV-1 epidemics among injecting drug users. In: Stimson G, Des Jarlais DC, Ball A, editors. Drug Injecting and HIV Infection: Global Dimensions and Local Response. London: UCL Press Limited; 1998a. [Google Scholar]
- Des Jarlais DC, Diaz R, Perlis T, Vlahov D, Maslow C, Latka M, Rockwell R, Edwards V, Friedman S, Monterroso E, Williams I, Garfein R. Variability in the incidence of HIV, HBV, and HCV infection among young injecting drug users in New York City. Am. J. Epidemiol. 2003;157:467–471. doi: 10.1093/aje/kwf222. [DOI] [PubMed] [Google Scholar]
- Des Jarlais DC, Friedman SR, Hopkins W. Risk reduction for the acquired immunodeficiency syndrome among intravenous drug users. Ann. Intern. Med. 1985;103:755–759. doi: 10.7326/0003-4819-103-5-755. [DOI] [PubMed] [Google Scholar]
- Des Jarlais DC, Friedman SR, Novick DM, Sotheran JL, Thomas P, Yancovitz S, Mildvan D, Weber J, Kreek MJ, Maslansky R, Bartelme S, Spira T, Marmor M. HIV-1 infection among intravenous drug users in Manhattan, New York City, from 1977 through 1987. JAMA. 1989;261:1008–1012. doi: 10.1001/jama.261.7.1008. [DOI] [PubMed] [Google Scholar]
- Des Jarlais DC, Hopkins W. “Free” needles for intravenous drug users at risk for AIDS: current developments in New York City. N. Engl. J. Med. 1985;313:1476. doi: 10.1056/NEJM198512053132311. [DOI] [PubMed] [Google Scholar]
- Des Jarlais DC, Marmor M, Friedmann P, Aviles E, Deren S, Torian LV, Glebatis D, Murrill C, Monterroso EM, Friedman SR. HIV incidence among injecting drug users in New York City, 1992–1997: evidence for a declining epidemic. Am. J. Public Health. 2000a;90:352–359. doi: 10.2105/ajph.90.3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Jarlais DC, Perlis T, Arasteh K, Hagan H, Milliken J, Braine N, Yancovitz S, Mildvan D, Perlman D, Maslow C, Friedman SR. “Informed altruism” and “partner restriction” in the reduction of HIV infection in injecting drug users entering detoxification treatment in New York City, 1990–2001. J. Acquir. Immune Defic. Syndr. 2004;35:158–166. doi: 10.1097/00126334-200402010-00010. [DOI] [PubMed] [Google Scholar]
- Des Jarlais DC, Perlis T, Arasteh K, Torian LV, Beatrice S, Milliken J, Mildvan D, Yancovitz S, Friedman S. HIV incidence among injection drug users in New York City, 1990 to 2002: use of serologic test algorithm to assess expansion of HIV prevention services. Am. J. Public Health. 2005;95:1439–1444. doi: 10.2105/AJPH.2003.036517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Jarlais DC, Perlis T, Friedman SR, Chapman T, Kwok J, Rockwell R, Paone D, Milliken J, Monterroso E. Behavioral risk reduction in a declining HIV epidemic: injection drug users in New York City, 1990–1997. Am. J. Public Health. 2000b;90:1112–1116. doi: 10.2105/ajph.90.7.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Jarlais DC, Perlis T, Friedman SR, Deren S, Chapman TF, Sotheran JL, Tortu S, Beardsley M, Paone D, Torian LV, Beatrice ST, DeBernardo E, Monterroso E, Marmor M. Declining seroprevalence in a very large HIV epidemic: injecting drug users in New York City, 1991 to 1996. Am. J. Public Health. 1998b;88:1801–1806. doi: 10.2105/ajph.88.12.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desenclos J. Surveillance of infectious diseases: principles and organisation in France in 2005. Med. Malpract. Infect. 2005;35:232–244. doi: 10.1016/j.medmal.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Donoghoe M, Verster A, Pervilhac C, Williams P. Setting targets for universal access to HIV prevention, treatment and care for injecting drug users (IDUs): towards consensus and improved guidance. Int. J. Drug Policy. 2008;19S:S5–S14. doi: 10.1016/j.drugpo.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Garfein R, Sweartzendruber A, QOuellet L, Kapadia F, Hudson S, Thiede H, Strathdee S, Williams I, Bailey S, Hagan H, Golub E, Kerndt P, Hanson D, Latka M, Team DS. Methods to recruit and retain a cohort of young-adult injection drug users for the third collaborative Injection Drug Users Study/Drug Users Intervention Trial (CIDUS III/DUIT) Drug Alcohol Depend. 2007;91:S4–S17. doi: 10.1016/j.drugalcdep.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Ghys P, Kufa E, George M. Measuring trends in prevalence and incidence of HIV infection in countries with generalised epidemics. Sex Trans. Dis. 2006;82:i52–i56. doi: 10.1136/sti.2005.016428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalichman S, Rompa D, Cage M, DiFonzo K, Simpson D, Austin J, Luke W, Buckles J, Kyomugisha F, Benotsch E, Pinkerton S, Graham J. Effectiveness of an intervention to reduce HIV transmission risks in HIV-positive people. Am. J. Prev. Med. 2001;21:84–92. doi: 10.1016/s0749-3797(01)00324-5. [DOI] [PubMed] [Google Scholar]
- Kral AH, Bluthenthal RN, Lorvick J, Gee L, Bacchetti P, Edlin BR. Sexual transmission of HIV-1 among injection drug users in San Francisco, USA: risk-factor analysis. Lancet. 2001;357:1397–1401. doi: 10.1016/S0140-6736(00)04562-1. [DOI] [PubMed] [Google Scholar]
- Latkin C, Buchanan A, Metsch L, Knight K, Latka M, Mizuno Y, Knowlton A. Predictors of sharing injection equipment by HIV-seropositive injection drug users. J. Acquir. Immune Defic. Syndr. 2008;49:447–450. doi: 10.1097/qai.0b013e31818a6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Kennedy J, Kelley D, Sun Y, Maduro G, Curry A, Das T. New York City Department of Health and Mental Hygiene, Statistical Analysis and Reporting Unit and Research and Surveillance Unit of the Bureau of Vital Statistics. New York: 2008. Summary of Vital Statistics 2007: The City of New York; pp. 1–94. [Google Scholar]
- Metsch L, Pereyra M, Purcell D, Latkin C, Malow R, Gómez C, Latka M, Team IS. Correlates of lending needles/syringes among HIV-seropositive injection drug users. J. Acquir. Immune Defic. Syndr. 2007;46:572–579. doi: 10.1097/QAI.0b013e3181576818. [DOI] [PubMed] [Google Scholar]
- Newman R. Methadone Treatment in Narcotic Addiction. San Diego: Academic Press Inc; 1977. [Google Scholar]
- Ouellet L, Huo D. Declines in HIV prevalence and incidence among injection drug users in Chicago, 1988–2007. Proceedings of the 2009 National HIV Prevention Conference; Atlanta, GA. 2009. [Google Scholar]
- Semaan S, Johnson W, Arasteh K, Des Jarlais D. What do 9 meta-analyses of HIV risk reduction interventions with drug users tell us?. Proceedings of the National HIV Prevention Conference; Atlanta, GA. 2009. [Google Scholar]
- Strathdee SA, Sherman SG. The role of sexual transmission of HIV infection among injection and non-injection drug users. J. Urban Health. 2003;80:iii7–iii14. doi: 10.1093/jurban/jtg078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNAIDS/WHO. AIDS Epidemic Update: December 2006. Geneva: Joint United Nations Programme on HIV/AIDS; 2007. [Google Scholar]
- Van Den Berg C, Smit C, Van Brussel G, Coutinho R, Prins M, Cohort A. Full participation in harm reduction programmes is associated with decreased risk for human immunodeficiency virus and hepatitis C virus: evidence from the Amsterdam Cohort Studies among drug users. Addiction. 2007;102:1454–1462. doi: 10.1111/j.1360-0443.2007.01912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]