Summary
Background
The bile acid derivative 6-ethylchenodeoxycholic acid (obeticholic acid) is a potent activator of the farnesoid X nuclear receptor that reduces liver fat and fibrosis in animal models of fatty liver disease. We assessed the efficacy of obeticholic acid in adult patients with non-alcoholic steatohepatitis.
Methods
We did a multicentre, double-blind, placebo-controlled, parallel group, randomised clinical trial at medical centres in the USA in patients with non-cirrhotic, non-alcoholic steatohepatitis to assess treatment with obeticholic acid given orally (25 mg daily) or placebo for 72 weeks. Patients were randomly assigned 1:1 using a computer-generated, centrally administered procedure, stratified by clinical centre and diabetes status. The primary outcome measure was improvement in centrally scored liver histology defined as a decrease in non-alcoholic fatty liver disease activity score by at least 2 points without worsening of fibrosis from baseline to the end of treatment. A planned interim analysis of change in alanine aminotransferase at 24 weeks undertaken before end-of-treatment (72 weeks) biopsies supported the decision to continue the trial (relative change in alanine aminotransferase −24%, 95% CI −45 to −3). A planned interim analysis of the primary outcome showed improved efficacy of obeticholic acid (p=0·0024) and supported a decision not to do end-of-treatment biopsies and end treatment early in 64 patients, but to continue the trial to obtain the 24-week post-treatment measures. Analyses were done by intention-to-treat. This trial was registered with ClinicalTrials.gov, number NCT01265498.
Findings
Between March 16, 2011, and Dec 3, 2012, 141 patients were randomly assigned to receive obeticholic acid and 142 to placebo. 50 (45%) of 110 patients in the obeticholic acid group who were meant to have biopsies at baseline and 72 weeks had improved liver histology compared with 23 (21%) of 109 such patients in the placebo group (relative risk 1·9, 95% CI 1·3 to 2·8; p=0·0002). 33 (23%) of 141 patients in the obeticholic acid developed pruritus compared with nine (6%) of 142 in the placebo group.
Interpretation
Obeticholic acid improved the histological features of non-alcoholic steatohepatitis, but its long-term benefits and safety need further clarification.
Funding
National Institute of Diabetes and Digestive and Kidney Diseases, Intercept Pharmaceuticals.
Introduction
Non-alcoholic steatohepatitis is an increasingly common cause of chronic liver disease worldwide and it is associated with increased liver-related mortality and hepatocellular carcinoma, even in the absence of cirrhosis.1–3 Non-alcoholic steatohepatitis progresses to cirrhosis in 15–20% of affected individuals and is a rising indication for liver transplantation4 but at present there are no approved therapies.
Obesity, diabetes, and insulin resistance (especially in adipose tissue) are all associated with non-alcoholic steatohepatitis and probably contribute to its pathogenesis. 5,6 Consequently, dietary changes and lifestyle modification to achieve weight reduction and improve insulin sensitivity are recommended.7,8 The long-term effectiveness of these interventions is debatable because many patients are unable to initiate or maintain dietary and lifestyle changes,7,9 underscoring the need for pharmacological therapy. Vitamin E and thiazolidinediones are the best studied drugs for the treatment of non-alcoholic steatohepatitis.10 Although both improve liver histology in patients without diabetes, their effects in patients with diabetes are unknown. Moreover, thiazolidinediones are associated with weight gain and other adverse outcomes, and the long-term efficacy and safety of vitamin E also remain uncertain.11,12
Over the last decade, lipophilic bile acids have emerged as potent modulators of metabolism and insulin sensitivity.13,14 When bound to the farnesoid X nuclear receptor, lipophilic bile acids promote insulin sensitivity and decrease hepatic gluconeogenesis and circulating triglycerides.15 These beneficial effects are mediated by decreased hepatic lipid synthesis and enhanced peripheral clearance of VLDL.16–18 Farnesoid X nuclear receptor activation also increases the expression of hepatic scavenger receptors (SRB1), which accelerates reverse cholesterol transport by increasing the clearance of HDL. Based on these metabolic effects, pharma cological activation of farnesoid X nuclear receptor has been proposed as a target for the treatment of non-alcoholic steatohepatitis.19
6-ethylchenodeoxycholic acid (obeticholic acid), a synthetic variant of the natural bile acid chenode oxycholic acid, is a potent activator of farnesoid X nuclear receptor. In pre-clinical studies, it improved hepatic steatosis, fibrosis, and portal hypertension.20–22 In a small group of patients with type 2 diabetes and suspected non-alcoholic fatty liver disease, obeticholic acid improved insulin sensitivity and reduced serum alanine aminotransferase concen trations.23 The less lipophilic bile acid ursodeoxycholic acid binds negligibly to farnesoid X nuclear receptor and in a randomised clinical trial it did not show efficacy in non-alcoholic steatohepatitis.24
With this background, we designed and undertook a clinical trial to assess the efficacy of obeticholic acid in adult patients with non-alcoholic steatohepatitis.
Methods
Study design and participants
The Farnesoid X Receptor Ligand Obeticholic Acid in NASH Treatment (FLINT) trial was a multicentre, randomised trial of 72 weeks of obeticholic acid versus placebo in patients with biopsy evidence of non-alcoholic steatohepatitis. Between March 16, 2011, and Dec 3, 2012, we enrolled patients at eight participating medical centres in the USA (appendix). Details of the trial were approved by local institutional review boards and a central data safety and monitoring board (DSMB) appointed by the National Institute of Diabetes and Digestive and Kidney Diseases, and all patients provided written informed consent. The study was done by site investigators and data were analysed by the data coordinating centre at Johns Hopkins University.
Patients enrolled in the study satisfied the following inclusion criteria: 18 years or older at the time of screening, histological evidence of definite or borderline non-alcoholic steatohepatitis based upon a liver biopsy obtained 90 days or less before randomisation, and a histological non-alcoholic fatty liver disease (NAFLD) activity score of 4 or more with a score of 1 or more in each component of the score25 (steatosis scored 0–3, ballooning 0–2, and lobular inflammation 0–3). Grading and staging of biopsies for the purposes of enrolment were done by the Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) pathologist at the site of enrolment. Patients were excluded for the presence of cirrhosis, other causes of liver disease, substantial alcohol consumption (>20 g/day for women or >30 g/day for men), or other confounding conditions (appendix).
Randomisation and masking
We randomly assigned (1:1) patients meeting eligibility criteria to oral obeticholic acid, 25 mg once-daily, or placebo using a computer-generated, centrally admin istered procedure, stratified by clinical centre and diabetes status, and blocked by calendar date. Obeticholic acid and placebo were provided as identical tablets in identical containers labelled with code numbers. Treatment was assigned centrally using a web-based application. Patients, investigators, clinical site staff, and pathologists were masked to treatment assignment.
Procedures
After randomisation, patients returned for study visits at weeks 2, 4, and 12, and then every 12 weeks until completion of treatment at week 72, and then 24 weeks later. Blood samples were obtained at these visits for routine biochemical tests and assessment of fasting concentrations of lipids, glucose, and insulin. Bodyweight, height, and waist and hip circumferences were measured at the initial assessment and designated interim times. The Medical Outcomes Study 36-Item Short-Form Health Survey (version 2.0) (SF-36v2) was administered for the assessment of quality of life at the initial assessment and treatment completion. All patients received standardised recommendations on healthy eating habits, weight reduction, exercise, and the management of hypertension, hypercholesterolaemia, and diabetes when indicated. We recorded all protocol violations as they occurred. There were no protocol violations, either accidental or unintentional, that increased risk, decreased benefits, assigned study medications in error, or enrolled patients without non-alcoholic steatohepatitis confirmed by a NASH CRN pathologist at the clinical centre. Baseline and end-of-treatment liver biopsies were centrally assessed by the NASH CRN Pathology Committee members as a group for consensus scoring of each component of the NAFLD activity score, determine fibrosis stage, and assign a diagnosis of non-alcoholic steatohepatitis, borderline non-alcoholic steatohepatitis, or not non-alcoholic steatohepatitis. Study pathologists were masked to treatment assignment and the slides assessed centrally were different cuts than the slides used to determine enrolment eligibility.
Outcomes
The primary outcome measure was improvement in centrally scored liver histology defined as a decrease in NAFLD activity score by at least 2 points without worsening of fibrosis from baseline to the end of treatment. Worsening of fibrosis was defined as any numerical increase in the stage. Secondary histological outcomes included resolution of non-alcoholic steatohepatitis, change in NAFLD activity score, and changes in the individual scores for hepatocellular ballooning, steatosis, lobular and portal inflammation, and fibrosis. Improvement in fibrosis was defined as any numerical decrease in the stage. Fibrosis stages 1a, 1b, and 1c were considered stage 1 for the purposes of analysis. Other secondary outcomes included changes from baseline to 72 weeks in serum aminotransferase and γ-glutamyl transpeptidase concentrations, fasting homoeostasis model of assessment of insulin resistance (HOMA-IR), anthropometric measures (weight, body-mass index, waist-to-hip ratio, waist circumference), and health-related quality-of-life scores.
Because there were no human data on the efficacy and safety of obeticholic acid as a treatment for non-alcoholic steatohepatitis, an interim analysis of a surrogate outcome, the change in alanine aminotransferase concentrations at 24 weeks, was done 65 weeks into the trial. This analysis was completed before any end-of-treatment liver biopsies were done to avoid unnecessary biopsies and drug exposure if treatment appeared to have no effect. The DSMB advised continuation of the trial as planned because the pre-specified interim criterion of a relative change in alanine aminotransferase of −20% or less in the lower 95% CI was met (−24%, 95% CI −45 to −3) (appendix). Also, because serum cholesterol concentrations increased more in the obeticholic acid-treated patients than in the placebo-treated patients, a more aggressive approach to lipid management was adopted based on a DSMB recommendation. Patients were referred to their primary care provider for treatment of fasting LDL cholesterol more than 3·36 mmol/L if non-diabetic or more than 2·59 mmol/L if diabetic or with risks for atherosclerosis, or fasting serum triglycerides more than 2·26 mmol/L. To avoid unnecessary biopsies, there was one planned interim analysis of the primary histological outcome measure when about 50% (140 of the planned 280 patients) had completed their end-of-treatment biopsies. Based on the Lan-DeMets method with O’Brien-Fleming boundaries,26 the criteria for superiority (p=0·0031) of obeticholic acid for the primary outcome were met (primary outcome was met in 35 [43%] of 82 in the obeticholic acid group vs 17 [21%] of 82 in the placebo group; p=0·0024). Crossing the superiority boundary led to a DSMB recommendation and resulting decision not to biopsy the final 64 patients. Because of concerns about persisting changes in cholesterol concentrations in patients receiving obeticholic acid, the DSMB also recommended discontinuing treatment, but continuing the study to the final 24-week post-treatment assessment and the patients were managed accordingly. Effect estimates and precision for the primary outcome measure were done as specified in the original protocol; no adjustments were made for potential statistical bias due to the two interim monitoring analyses (the vanguard futility analysis using alanine aminotransferase as a surrogate outcome measure and the interim efficacy analysis using the histological primary outcome).
Statistical analysis
The primary intention-to-treat analysis of patients with histological improvement excluded the final 64 patients who did not have a biopsy. All patients were included in the analyses of secondary non-histological outcomes and safety issues. The primary outcome and binary secondary outcomes were analysed using the Mantel-Haenszel test for binary outcomes stratified by clinical centre and diabetes status; continuous secondary outcomes were analysed using ANCOVA models relating change in the continuous outcome from baseline to 72 weeks to treatment group and to the baseline value of the outcome. The planned sample size was 280 patients with equal assignment to two groups (140 per group). The study was powered at 90% to detect a 1·5 times increase in the rate of histological improvement assuming 10% loss to follow-up, 39% improvement rate in the placebo group, and a two-sided type 1 error of 5%. Statistical analyses were done with SAS (SAS Institute 2011, Base SAS 9·3 Procedures Guide) and Stata (StataCorp 2013, Stata Statistical Software: release 13). The trial was registered with ClinicalTrials.gov, number NCT01265498.
Role of the funding source
The FLINT trial protocol was written by a subcommittee and approved by the steering committee of the NASH CRN (appendix). JT and MLVN had full access to the data; BAN-T, RL, AJS, JEL, MLVN, JMC, JT, EMB, and DEK were responsible for submitting the manuscript. Data analyses were reviewed by the study investigators and the DSMB. The manuscript was written by a subcommittee and approved by the members of the steering committee, who assume responsibility for the conduct of the trial and integrity of the data, the overall content of the manuscript, and the decision to submit it for publication. Partial funding for the trial, obeticholic acid, and an identical placebo were provided by Intercept Pharmaceuticals under a Collaborative Research and Development Agreement with the National Institute of Diabetes and Digestive and Kidney Diseases. Intercept Pharmaceuticals provided comments on the study protocol but was not involved with the study design, data analyses, and interpretation, or writing and submission of the manuscript.
Results
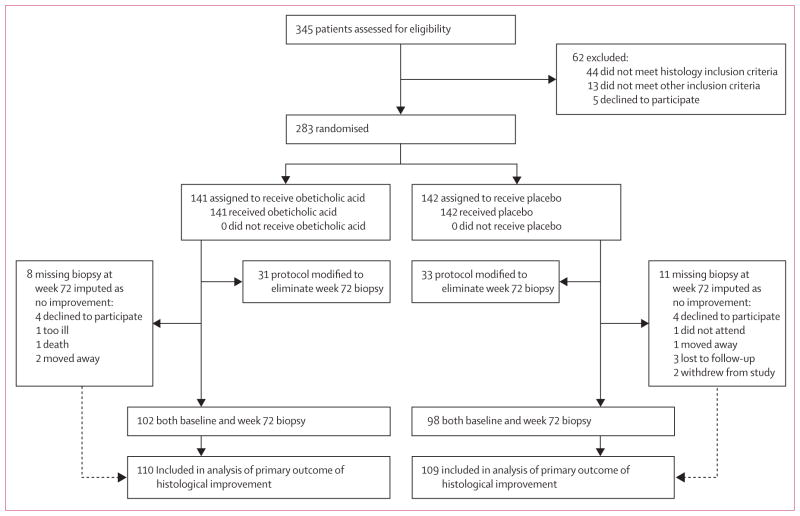
283 patients with histologically proven non-alcoholic steatohepatitis or borderline non-alcoholic steatohepatitis based on the site pathologist reading of the liver biopsy were randomly assigned to receive obeticholic acid (n=141) or placebo (n=142) (figure 1). 15 (5%) patients had minor protocol deviations, mainly from timing targets (entry liver biopsies outside the stated 90-day window by up to 11 days, and baseline laboratory results outside stated time windows, but never after randomisation). The baseline demographic, clinical, laboratory, and histological characteristics of the two treatment groups were similar (table 1). Subsequent central review of the initial liver biopsies indicated that of 282 patients (one patient had missing baseline histology), 225 (80%) had definite non-alcoholic steatohepatitis at study entry. The overall mean NAFLD activity score on central review was 5·2 and ranged from 3 to 8. Of 282 patients, stage 3 fibrosis was present in 63 (22%) and cirrhosis in 2 (1%) on central review. All patients received their assigned treatment.
Figure 1.
Trial profile
Table 1.
Baseline characteristics of the study population
| Obeticholic acid (n=141) | Placebo (n=142) | |
|---|---|---|
|
Demographics
| ||
| Age (years) | 52 (11) | 51 (12) |
| Male | 43 (30%) | 53 (37%) |
| Race | ||
| Asian | 6 (4%) | 10 (7%) |
| Black or African-American | 2 (1%) | 4 (3%) |
| White | 123 (87%) | 111 (78%) |
| Other | 10 (7%) | 17 (12%) |
| Ethnic origin | ||
| Hispanic | 22 (16%) | 21 (15%) |
|
| ||
|
SF-36 Quality of life
| ||
| Physical component summary | 45 (11) | 44 (11) |
| Mental component summary | 48 (12) | 48 (12) |
|
| ||
|
Liver enzymes
| ||
| Alanine aminotransferase (U/L) | 83 (49) | 82 (51) |
| Aspartate aminotransferase (U/L) | 64 (38) | 58 (34) |
| Alkaline phosphatase (U/L) | 82 (29) | 81 (25) |
| γ-glutamyl transpeptidase (U/L) | 78 (85) | 76 (97) |
| Total bilirubin (μmol/L) | 11·5 (5·9) | 11·3 (7·5) |
|
| ||
|
Lipids
| ||
| Total cholesterol (mmol/L) | 4·9 (1·2) | 4·8 (1·2) |
| HDL cholesterol (mmol/L) | 1·1 (0·3) | 1·1 (0·4) |
| LDL cholesterol (mmol/L) | 2·9 (1·0) | 2·9 (1·1) |
| Trigylcerides (mmol/L) | 2·2 (1·5) | 2·0 (1·7) |
|
| ||
|
Haematology
| ||
| Haemoglobin (g/L) | 140 (15) | 140 (14) |
| Haematocrit (proportion of 1·0) | 0·41 (0·04) | 0·41 (0·04) |
| Mean corpuscular volume (fL) | 88·7 (4·8) | 89·0 (5·3) |
| White blood cell count (×109 per L) | 7·3 (1·9) | 6·9 (2·3) |
| Platelet count (×109 per L) | 237 (59) | 237 (65) |
|
| ||
|
Chemistries
| ||
| Bicarbonate (mmol/L) | 25·9 (2·5) | 26·2 (2·6) |
| Calcium (mmol/L) | 2·4 (0·1) | 2·4 (0·1) |
| Phosphate (mmol/L) | 1·1 (0·2) | 1·1 (0·2) |
| Creatinine (μmol/L) | 71 (18) | 70 (16) |
| Uric acid (μmol/L) | 375 (89) | 366 (86) |
| Albumin (g/L) | 43 (4) | 43 (4) |
| Total protein (g/L) | 73 (5) | 74 (5) |
|
| ||
|
Other laboratory results
| ||
| Prothrombin time (s) | 11·7 (2·1) | 11·7 (2·2) |
| International normalised ratio | 1·01 (0·08) | 1·00 (0·07) |
|
| ||
|
Metabolic factors
| ||
| Fasting serum glucose (mmol/L) | 6·5 (1·8) | 6·4 (2·2) |
| Insulin (pmol/L) | 201 (226) | 138 (129) |
| HOMA-IR (glucose [mmol/L] × insulin [pmol/L]/22·5) | 61 (74) | 40 (42) |
| Glycated haemoglobin A1c (mmol/mol) | 48 (12) | 47 (11) |
| Weight (kg) | 100 (23) | 96 (18) |
| Body-mass index (kg/m2) | 35 (7) | 34 (6) |
| Waist circumference (cm) | 112 (15) | 109 (14) |
| Waist-to-hip ratio | 0·96 (0·07) | 0·95 (0·09) |
| Systolic blood pressure (mm Hg) | 132 (17) | 132 (15) |
| Diastolic blood pressure (mm Hg) | 77 (11) | 78 (10) |
|
| ||
|
Comorbidities
| ||
| Hyperlipidaemia* | 87 (62%) | 86 (61%) |
| Hypertension | 87 (62%) | 85 (60%) |
| Cardiovascular disease | 7 (5%) | 8 (6%) |
| Diabetes | 75 (53%) | 74 (52%) |
|
| ||
|
Concomitant medications in the past 6 months
| ||
| Antilipidaemic | 72 (51%) | 64 (45%) |
| Cardiovascular | 97 (69%) | 92 (65%) |
| Antidiabetic | 67 (48%) | 73 (51%) |
| Metformin | 55 (39%) | 62 (44%) |
| Pioglitazone | 1 (1%) | 6 (4%) |
| Vitamin E | 29 (21%) | 32 (23%) |
| Thiazolidinedione | 3 (2%) | 5 (4%) |
| Aspirin (81 mg) | 37 (26%) | 33 (23%) |
|
| ||
|
Liver histology findings
| ||
| Definite steatohepatitis | 114 (81%) | 111 (79%) |
| Fibrosis stage† | 1·9 (1·1) | 1·8 (1·0) |
| Total NAFLD activity score‡ | 5·3 (1·3) | 5·1 (1·3) |
| Hepatocellular ballooning score | 1·4 (0·7) | 1·3 (0·7) |
| Steatosis score | 2·1 (0·8) | 2·0 (0·8) |
| Lobular inflammation score | 1·8 (0·7) | 1·8 (0·7) |
| Portal inflammation score§ | 1·2 (0·6) | 1·1 (0·6) |
| Biopsy length (mm) | 21 (10) | 21 (10) |
Data are n (%) or mean (SD). HOMA-IR=homoeostasis model assessment–estimated insulin resistance.
History of cholesterol or triglyceride elevations as determined by the site investigator.
Fibrosis was assessed on a scale of 0–4, with higher scores showing more severe fibrosis.
Total non-alcoholic fatty liver disease (NAFLD) activity was assessed on a scale of 0–8, with higher scores indicating more severe disease; the components of this measure are steatosis (assessed on a scale of 0–3), lobular inflammation (assessed on a scale of 0–3), and hepatocellular ballooning (assessed on a scale of 0–2).
Portal inflammation was assessed on a scale of 0–2 with higher scores showing more severe inflammation.
The primary intention-to-treat analysis of the proportion of patients with histological improvement was done on 219 patients (110 in the obeticholic acid group and 109 in the placebo group), which excluded the 64 patients not eligible for a biopsy (31 were assigned to obeticholic acid and 33 to placebo). This analysis represented the patients who were eligible for a 72-week biopsy before the decision was made to stop post-treatment biopsies and treatment (figure 1). Biopsy-eligible patients who were not biopsied were counted as not improved (eight in the obeticholic acid group and 11 in the placebo group).
50 (45%) of 110 patients in the obeticholic acid group had improved liver histology (2-point or greater improvement in NAFLD activity score without worsening of fibrosis) compared with 23 (21%) of 109 patients in the placebo group (relative risk 1·9, 95% CI 1·3–2·8; p=0·0002) (table 2). These results did not change after prespecified sensitivity analyses with adjustment for confounders (including weight loss) and multiple imputation for eight patients treated with obeticholic acid and 11 treated with placebo who had missing data on the primary outcome (appendix). Post-hoc subgroup analysis showed no difference in the treatment effect for the primary outcome between baseline demographic, clinical, or previous treatment subgroups (appendix).
Table 2.
Changes in histological features of the liver after 72 weeks of treatment
| Obeticholic acid | Placebo | Relative risks or mean changes from baseline* (95% CI) (obeticholic acid vs placebo) | p value* | |
|---|---|---|---|---|
|
Primary outcome†
| ||||
| Number of patients at risk‡ | 110 | 109 | ||
| Patients with improvement | 50 (45%) | 23 (21%) | 1·9 (1·3 to 2·8) | 0·0002 |
|
| ||||
|
Changes from baseline in histological features
| ||||
| Number of patients with biopsy specimens at baseline and 72 weeks | 102 | 98 | ||
| Resolution§ of definite non-alcoholic steatohepatitis | 22 (22%) | 13 (13%) | 1·5 (0·9 to 2·6) | 0·08 |
| Fibrosis¶ | ||||
| Patients with improvement | 36 (35%) | 19 (19%) | 1·8 (1·1 to 2·7) | 0·004 |
| Change in score | −0·2 (1·0) | 0·1 (0·9) | −0·3 (−0·6 to −0·1) | 0·01 |
| Total NAFLD activity score | ||||
| Change in score | −1·7 (1·8) | −0·7 (1·8) | −0·9 (−1·3 to −0·5) | <0·0001 |
| Hepatocellular ballooning | ||||
| Patients with improvement | 47 (46%) | 30 (31%) | 1·5 (1·0 to 2·1) | 0·03 |
| Change in score | −0·5 (0·9) | −0·2 (0·9) | −0·2 (−0·5 to 0·0) | 0·03 |
| Steatosis | ||||
| Patients with improvement | 62 (61%) | 37 (38%) | 1·7 (1·2 to 2·3) | 0·001 |
| Change in score | −0·8 (1·0) | −0·4 (0·8) | −0·4 (−0·6 to −0·2) | 0·0004 |
| Lobular inflammation | ||||
| Patients with improvement | 54 (53%) | 34 (35%) | 1·6 (1·1 to 2·2) | 0·006 |
| Change in score | −0·5 (0·8) | −0·2 (0·9) | −0·3 (−0·5 to −0·1) | 0·0006 |
| Portal inflammation|| | ||||
| Patients with improvement | 12 (12%) | 13 (13%) | 1·0 (0·6 to 1·7) | 0·90 |
| Change in score | 0·2 (0·7) | 0·2 (0·7) | 0·0 (−0·1 to 0·2) | 0·59 |
Data are n (%) or mean (SD).
p values and relative benefit were calculated with the Cochran-Mantel-Haenszel chi-square test, stratified by clinic and diabetes status, for binary outcomes; p values and mean changes from baseline were calculated using ANCOVA, regressing change from baseline to 72 weeks on treatment group and baseline value of the outcome, for outcome scores.
The primary outcome was an improvement in histological findings, which required a decrease of 2 or more points in the total non-alcoholic fatty liver disease (NAFLD) activity score and no worsening in the fibrosis score; 11 patients in the placebo group and eight in the obeticholic acid group had missing histological data at week 72, and the results for these patients were imputed as a lack of improvement; NAFLD activity score was assessed on a scale of 0–8, with higher scores showing more severe disease (the components of this measure are steatosis [assessed on a scale of 0–3], lobular inflammation [assessed on a scale of 0–3], and hepatocellular ballooning [assessed on a scale of 0–2]).
Number of randomly assigned patients with observed or expected week 72 visit before protocol modified on Jan 6, 2014, to eliminate week 72 biopsy.
Resolution defined as either not NAFLD, or NAFLD but not non-alcoholic steatohepatitis on week 72 biopsy.
Fibrosis was assessed on a scale of 0–4, with higher scores showing more severe fibrosis.
Portal inflammation was assessed on a scale of 0–2, with higher scores showing more severe inflammation.
More patients assigned to obeticholic acid compared with placebo had improvement in fibrosis, hepato cellular ballooning, steatosis, and lobular inflammation (table 2). The mean change in the NAFLD activity score was greater in patients treated with obeticholic acid than placebo (change from baseline=−1·7 vs −0·7; p<0·0001). Despite these improvements in the individual histological features of non-alcoholic steatohepatitis, the proportion of patients with resolution of non-alcoholic steatohepatitis (ie, change from baseline diagnosis to not non-alcoholic steatohepatitis) did not differ in patients treated with obeticholic acid compared with placebo (22 [22%] of 102 vs 13 [13%] of 98; p=0·08) (table 2).
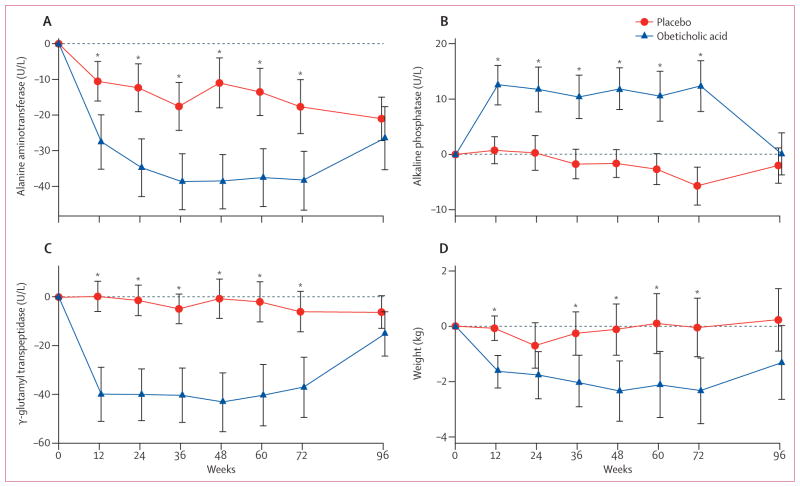
All patients who completed their final on-treatment study visit and the visit 24 weeks after stopping treatment (including those without a final biopsy due to early treatment termination) were included in the group comparisons of non-histological secondary outcomes. Significant reductions in serum alanine aminotransferase and aspartate aminotransferase concentrations developed over the first 36 weeks of treatment with obeticholic acid and were sustained for the duration of treatment (figure 2, appendix). By contrast, serum alkaline phosphatase concentrations increased, although γ-glutamyl transpeptidase concentrations (another indicator of cholestasis) decreased. These changes in liver enzyme concentrations reversed after obeticholic acid was stopped and at 24 weeks after treatment discontinuation there were no significant differences between the two groups (figure 2, appendix).
Figure 2. Changes from baseline in liver enzymes and bodyweight according to treatment group.
Mean values of changes from baseline during treatment with obeticholic acid (141 patients) or placebo (142 patients) for up to 72 weeks followed by a 24-week post-treatment period are shown. Error bars show 95% CIs. *p<0·05; p values were derived from linear regression modelling change as a function of treatment group and the baseline value of the outcome. Data are included from patients whose treatment was terminated early according to protocol design and their serum biochemical test results obtained 24 weeks after stopping treatment are included with the 96-week mean values. (A) Alanine aminotransferase concentrations decreased during treatment with obeticholic acid, reaching a reduced baseline 36 weeks after initiating treatment, whereas concentrations in patients treated with placebo remained unchanged. Alanine aminotransferase concentrations in the obeticholic acid group reverted back to being indistinguishable from placebo 24 weeks after treatment discontinuation. (B) Serum alkaline phosphatase concentrations increased whereas (C) serum γ-glutamyl transpeptidase decreased early in the course of treatment with obeticholic acid. (D) Bodyweight decreased throughout treatment with a rebound back toward baseline after treatment discontinuation.
Compared with placebo, treatment with obeticholic acid was associated with weight loss and a small decrease in systolic blood pressure (figure 2, table 3). Fasting serum insulin concentrations were higher and the homoeostasis model of assessment (HOMA) indicated greater hepatic insulin resistance with obeticholic acid treatment compared with placebo at week 72 (table 3). The changes in HOMA and weight reverted towards baseline after treatment was stopped in the obeticholic acid group but not in the placebo group (appendix).
Table 3.
Changes in liver enzymes, biochemical concentrations, metabolic factors, and quality of life from baseline to 72 weeks
| Change from baseline to 72 weeks (mean [SD])
|
Mean changes from baseline* (obeticholic acid vs placebo) (95% CI) | p value* | ||
|---|---|---|---|---|
| Obeticholic acid (n=126) | Placebo (n=131) | |||
|
Liver enzymes
| ||||
| Alanine aminotransferase (U/L) | −38 (47) | −18 (44) | −20 (−28 to −11) | <0·0001 |
| Asparate aminotransferase (U/L) | −27 (37) | −10 (31) | −12 (−18 to −6) | 0·0001 |
| Alkaline phosphatase (U/L) | 12 (26) | −6 (20) | 18 (13 to 24) | <0·0001 |
| γ-glutamyl transpeptidase (U/L) | −37 (70) | −6 (48) | −24 (−35 to −14) | <0·0001 |
| Total bilirubin (μmol/L) | −1·0 (4·1) | 0·6 (3·7) | −1·5 (−2·4 to −0·5) | 0·002 |
|
| ||||
| Lipids | ||||
|
| ||||
| Total cholesterol (mmol/L) | 0·16 (1·07) | −0·19 (0·96) | 0·38 (0·16 to 0·60) | 0·0009 |
| HDL cholesterol (mmol/L) | −0·02 (0·20) | 0·03 (0·19) | −0·06 (−0·10 to −0·01) | 0·01 |
| LDL cholesterol (mmol/L) | 0·22 (0·90) | −0·22 (0·80) | 0·45 (0·26 to 0·65) | <0·0001 |
| Trigylcerides (mmol/L) | −0·22 (1·27) | −0·08 (1·74) | −0·02 (−0·35 to 0·30) | 0·88 |
|
| ||||
|
Haematology
| ||||
| Haemoglobin (g/L) | 0·6 (9·6) | 0·3 (9·5) | 0·4 (−1·8 to 2·6) | 0·72 |
| Haematocrit—proportion of 1·0 | 0·00 (0·03) | 0·00 (0·03) | 0·0 (−0·01 to 0·01) | 0·71 |
| Mean corpuscular volume (fL) | −0·8 (2·6) | 0·3 (3·5) | −1·1 (−1·8 to −0·4) | 0·002 |
| White blood cell count (109/L) | 0·0 (1·5) | 0·0 (1·1) | 0·1 (−0·2 to 0·4) | 0·40 |
| Platelet count (109/L) | 12 (33) | −4 (46) | 16 (7 to 26) | 0·001 |
|
| ||||
|
Chemistries
| ||||
| Bicarbonate (mmol/L) | −0·7 (3·2) | −0·1 (2·7) | −0·7 (−1·4 to −0·1) | 0·03 |
| Calcium (mmol/L) | 0·01 (0·10) | −0·01 (0·11) | 0·02 (0·00 to 0·04) | 0·04 |
| Phosphate (mmol/L) | 0·01 (0·18) | 0·02 (0·16) | 0·01 (−0·03 to 0·05) | 0·53 |
| Creatinine (μmol/L) | 1·5 (11·3) | −1·1 (9·6) | 2·6 (0·2 to 5·0) | 0·03 |
| Uric acid (μmol/L) | 2 (68) | −11 (56) | 14 (0 to 29) | 0·05 |
| Albumin (g/L) | −0·2 (3·1) | 0·3 (3·1) | −0·5 (−1·2 to 0·2) | 0·13 |
| Total protein (g/L) | 0·2 (4·5) | −0·5 (4·5) | 0·5 (−0·5 to 1·6) | 0·31 |
|
| ||||
|
Other laboratory results
| ||||
| Prothrombin time (s) | −0·1 (2·4) | 0·0 (2·2) | −0·2 (−0·6 to 0·1) | 0·16 |
| International normalised ratio | −0·03 (0·07) | 0·00 (0·08) | −0·02 (−0·04 to −0·01) | 0·002 |
|
| ||||
|
Metabolic factors
| ||||
| Fasting serum glucose (mmol/L) | 0·4 (2·1) | 0·2 (2·3) | 0·3 (−0·2 to 0·8) | 0·26 |
| Insulin (pmol/L) | 29 (159) | 10 (111) | 38 (6 to 69) | 0·02 |
| HOMA-IR (glucose [mmol/L] × insulin [pmol/L]/22·5) | 15 (50) | 4 (29) | 13 (3 to 23) | 0·01 |
| Glycated haemoglobin A1c (mmol/mol) | 0·5 (9·7) | 0·4 (8·3) | 0·4 (−1·7 to 2·6) | 0·71 |
| Weight (kg) | −2·3 (6·7) | 0·0 (6·1) | −2·2 (−3·7 to −0·6) | 0·008 |
| Body-mass index (kg/m2) | −0·7 (2·4) | 0·1 (2·2) | −0·7 (−1·3 to −0·2) | 0·01 |
| Waist circumference (cm) | −1·5 (7·1) | −0·6 (8·7) | −0·4 (−2·2 to 1·5) | 0·70 |
| Waist-to-hip ratio | 0·00 (0·06) | 0·00 (0·06) | 0·00 (−0·01 to 0·02) | 0·57 |
| Systolic blood pressure (mm Hg) | −4 (17) | −1 (16) | −3 (−7 to 0) | 0·05 |
| Diastolic blood pressure (mm Hg) | 0 (11) | 0 (10) | −1 (−4 to 1) | 0·23 |
|
| ||||
|
SF-36 Quality of life
| ||||
| Physical component summary | 0 (7) | −1 (7) | 1 (−1 to 3) | 0·22 |
| Mental component summary | 0 (9) | 1 (9) | 0 (−3 to 2) | 0·65 |
HOMA-IR=homoeostasis model assessment–estimated insulin resistance.
p values and mean changes from baseline were calculated using ANCOVA models, regressing change from baseline to 72 weeks on treatment group and baseline value of the outcome.
Compared with placebo, treatment with obeticholic acid was associated with higher concentrations of total serum cholesterol and LDL cholesterol, and a decrease in HDL cholesterol (appendix). These changes developed within 12 weeks of beginning treatment, diminished in magnitude while on treatment, and were not sustained after treatment discontinuation. There was an early decrease in serum triglycerides at 12 weeks of treatment but the concentrations were not different from placebo at 72 weeks (table 3, appendix).
Clinical adverse events were generally mild to moderate in severity and were similar in the two groups for all symptoms except pruritus (table 4). Pruritus was reported in 33 (23%) of 141 obeticholic acid-treated patients and nine (6%) of 142 placebo-treated patients (p<0·0001). Pruritus was also more severe in the obeticholic acid group, led to the use of antipruritic medications or short periods of withholding treatment in some patients, and treatment discontinuation in one patient. There were no differences in mental or physical quality-of-life measures between treatment groups before, during, or after therapy (tables 1, 3, appendix).
Table 4.
Adverse events
| Obeticholic acid (n=141) | Placebo (n=142) | |
|---|---|---|
| Composite cardiovascular events | ||
| Cardiovascular death or non-fatal myocardial infarction or non-fatal stroke | 3 | 1 |
| Other coronary artery disease or angina | 2 | 2 |
| Other† | 8* | 6 |
|
| ||
| Neurological events (excluding stroke) | ||
| Dizziness or syncope | 3 | 4* |
| Headache | 2 | 5* |
| Neuralgia | 3 | 2 |
| Other‡ | 5 | 10* |
|
| ||
| Renal events | ||
| Urinary tract infection or cystitis | 2 | 3 |
| Kidney stones | 6 | 2 |
| Other§ | 2 | 2 |
|
| ||
| Pruritus | ||
| Grade 1 (mild or localised) | 9 | 6 |
| Grade 2 (intense or widespread) | 21* | 3 |
| Grade 3 (intense or widespread and interfering with activities of daily living) | 3* | 0 |
| Any | 33 | 9 |
|
| ||
| Hepatobiliary events (excluding pruritus) | 1 | 1 |
|
| ||
| Gastrointestinal events | ||
| Abdominal pain | 7 | 9* |
| Dental or tooth pain | 4 | 1 |
| Liver pain post biopsy | 1 | 1 |
| Nausea, vomiting, or diarrhoea | 12 | 12* |
| Constipation | 5 | 1 |
| Dyspepsia | 3 | 1 |
| Pancreatitis | 1 | 2 |
| Other¶ | 9* | 4 |
Includes one or more serious or life-threatening events described in the text.
Congestive heart failure, cardiomyopathy, or arrhythmia.
Neuropathy, ataxia, insomnia, or restlessness.
Incontinence, pain, acute renal failure, or urine colour change.
Small bowel obstruction, dehydration, haemorrhoids, distension, benign polyp removal, oesophageal injury, blood in stool, appendicitis, melaena, diverticulitis, anorexia, or painful bowel movement.
There were severe or life-threatening adverse events in 30 obeticholic acid-treated patients (43 events) and 21 placebo-treated patients (43 events) but in most patients (42 [82%] of 51) the adverse events were judged to be unrelated to therapy. Five severe or life-threatening adverse events in those receiving obeticholic acid that were judged by the masked local physician possibly, probably, or definitely related included three events of pruritus, one of hyperglycaemia, and one of dysarthria and dizziness possibly due to cerebral ischaemia. Four severe or life-threatening events in those receiving placebo that were judged related included gastrointestinal pain, headache, muscle weakness, and vertigo with nausea and vomiting. Two patients died during the study, both receiving obeticholic acid (one from sepsis and congestive heart failure, and the other from cardiac ischaemia or infarction) but neither was considered related to treatment by the site investigator.
Discussion
In this randomised, placebo-controlled, clinical trial, the farnesoid X nuclear receptor agonist obeticholic acid improved the biochemical and histological features of non-alcoholic steatohepatitis compared with placebo in patients without cirrhosis. Importantly, all components of the NAFLD activity score (steatosis, hepatocellular ballooning, and lobular inflammation) and fibrosis improved. The improvement in fibrosis, although small, shows that this therapy might be beneficial in preventing progression to cirrhosis (panel).
A strength of this trial was the assessment of liver biopsies by a panel of expert liver pathologists who arrived at consensus interpretations of the features of non-alcoholic fatty liver disease and the diagnosis of non-alcoholic steatohepatitis for each biopsy. Inherent to this approach is the inability to use a committee assessment to determine enrolment eligibility; instead, each pathologist individually assessed the biopsies using different slides to assess enrolment eligibility. This approach led to unavoidable discordant interpretations in a fraction of cases due to interobserver variability and differences between different sections of the same biopsy, as it did in the PIVENS trial.27
Weaknesses of the trial included lack of detailed tracking of interventions including dose information to treat hyperlipidaemia during the trial. Additionally, smoking history was not captured until midway through the course of the trial.
Although obeticholic acid had an effect on the primary endpoint, it did not cause a significant proportion of patients to cross the threshold from a histological diagnosis of definite or borderline non-alcoholic steatohepatitis to a diagnosis of not non-alcoholic steatohepatitis. One explanation is that the improvement in the NAFLD activity score might reflect a decrease in the severity of disease but not to the point of resolution of non-alcoholic steatohepatitis. Alternatively, reductions in the NAFLD activity score might not fully reflect changes in non-alcoholic steatohepatitis severity because the two histological assessments of this disease are not equivalent. Findings from a previous cross-sectional study reported that the NAFLD activity score correlates more with increases in serum aminotransferase whereas the pathologist’s diagnosis of non-alcoholic steato hepatitis correlates more with features of the metabolic syndrome.28 Long-term outcome studies are needed to determine which histological measures of non-alcoholic fatty liver disease severity have the greatest clinical significance in terms of progression to cirrhosis and its complications.
The effects of obeticholic acid treatment on non-alcoholic steatohepatitis need to be placed in context. In a previous study done by the NASH CRN, improvements in histological features of non-alcoholic steatohepatitis were also reported with vitamin E and pioglitazone.27 The studies had somewhat different inclusion and exclusion criteria and primary endpoints, but the overall histological improvement rates were similar: 50 (45%) of 110 treated with obeticholic acid, 36 (43%) of 84 with vitamin E, and 27 (34%) of 80 with pioglitazone. Improvement in the two placebo groups was also similar (23 [21%] of 109 and 16 [19%] of 83). Finally, mean changes in histological scores were similar in the two trials, with improvements in mean NAFLD activity scores of −1·7 (SD 1·8) for obeticholic acid, −1·9 (2·1) for vitamin E, and −1·9 (1·8) for pioglitazone, compared with −0·7 (1·8) and −0·5 (1·8) in the two placebo groups.
Farnesoid X receptor activation decreases hepatic lipogenesis by down-regulating the transcription factor SREBP1c and increasing SIRT117,18 and these effects could play a part in the beneficial effect of obeticholic acid in non-alcoholic steatohepatitis. However, an important function of farnesoid X receptor activation is to reduce bile acid synthesis by inhibiting the conversion of cholesterol to bile acids, a major mechanism of cholesterol disposal. Blocking the conversion of cholesterol to bile acids could increase serum cholesterol concentrations, which might account for the changes in serum cholesterol concentrations recorded during obeticholic acid treatment. The effect of farnesoid X receptor agonists on cholesterol metabolism is complex because they might also promote reverse cholesterol transport out of tissues. In view of these complexities, cholesterol changes need prospective monitoring and analysis in future studies of obeticholic acid therapy for liver disease.15,29 In this study, the changes in cholesterol peaked in the first 12 weeks and the management of dyslipidaemia might have contributed to the changes towards baseline observed over time.
Obeticholic acid was generally well tolerated. The only adverse event occurring more frequently than with placebo was pruritus. Pruritus was also observed with obeticholic acid treatment in patients with primary biliary cirrhosis.30
Panel: Research in context.
Systematic review
Reported clinical trials for non-alcoholic steatohepatitis were reviewed by searching Medline for English language publications from Jan 1, 1965, to Sept 30, 2014, for “fatty liver”, “NAFLD”, “NASH”, “steatohepatitis”, “farnesoid X receptor”, and “FXR”. A paucity of evidence supports the routine use of any drugs, and at present there are no approved therapies for non-alcoholic steatohepatitis. Non-alcoholic steatohepatitis is a common cause of chronic liver disease and is rapidly increasing as a cause of cirrhosis and hepatocellular cancer. Obesity and insulin resistance are the most common risk factors for non-alcoholic steatohepatitis and clinical trials of thiazolidinediones such as pioglitazone and vitamin E suggest that they are both better than placebo in terms of improving liver histology.27 However, the use of thiazolidinediones is restricted by adverse effects such as weight gain, fluid retention, increased fracture risk (especially in older women), and bladder cancer. Similarly, the long-term safety of vitamin E has not been established and it might increase the risk of prostate cancer.
Bile acids have emerged as key regulators of metabolism via membrane and nuclear receptors, such as farnesoid X receptor. Farnesoid X receptor activation can improve hepatic insulin sensitivity and also decrease steatosis by inhibition of lipogenesis. In animal models, farnesoid X receptor agonists cause regression of atherosclerosis and have indirect anti-fibrotic effects.15,17,21,22 These characteristics make farnesoid X receptor an attractive target for the treatment of non-alcoholic steatohepatitis. Findings from a pilot study of the farnesoid X receptor agonist obeticholic acid in diabetic patients with suspected non-alcoholic fatty liver disease showed an improvement in insulin sensitivity, weight loss, and a decrease in some, but not all, liver enzymes.23 These results formed the basis for our phase 2b randomised controlled trial of obeticholic acid for non-alcoholic steatohepatitis.
Interpretation
In our trial, obeticholic acid improved the histological features of non-alcoholic steatohepatitis, including hepatic steatosis, inflammation, hepatocyte ballooning, and fibrosis. Importantly, the trial included a substantial proportion of diabetic patients and also vitamin E non-responders. Despite the improvement in the key features of non-alcoholic steatohepatitis, including fibrosis, these improvements were not enough to reduce the number of patients with a diagnosis of non-alcoholic steatohepatitis. The positive findings are tempered by the observation of pruritus in 23% of patients and an increase in total cholesterol and LDL cholesterol, and a modest decrease in HDL cholesterol. Long-term studies are needed to confirm the beneficial effects of obeticholic acid in patients with non-alcoholic steatohepatitis, and to determine the clinical relevance of the changes in circulating lipids induced by farnesoid X receptor ligands.
Hepatic insulin resistance, estimated using the homoeostasis model of assessment based on fasting glucose and insulin concentrations, increased between baseline and 72 weeks of treatment with obeticholic acid, whereas in a recently published small study in diabetic patients, treatment for 6 weeks was associated with improved insulin responsiveness as measured by the hyperinsulinaemic euglycaemic clamp method.23 These findings suggest that the effects of obeticholic acid on insulin resistance might be transient and reversed by adaptive mechanisms in response to long-term treatment with farnesoid X nuclear receptor agonists.
The benefits of improving the histological features of non-alcoholic steatohepatitis and reducing serum aminotransferase concentrations without suppressing disease activity to the point of resolution need to be shown. Importantly, the improvements with obeticholic acid confirm that farnesoid X nuclear receptor signalling affects lipid metabolism in the liver and can be changed with farnesoid X nuclear receptor ligands in human beings. Treatment with obeticholic acid was associated with pruritus that rarely needed discontinuation, but treatment caused changes in the serum cholesterol pool and insulin resistance that could signal an increased risk of atherogenesis. Future studies of farnesoid X nuclear receptor agonists will need to address the consequences of these changes on cardiovascular outcomes. Thus, obeticholic acid improves the histological features of non-alcoholic steatohepatitis, but its long-term safety requires further clarification.
Acknowledgments
The Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (grants U01DK061718, U01DK061728, U01DK061731, U01DK061732, U01DK061734, U01DK061737, U01DK061738, U01DK061730, U01DK061713). Several clinical centres had support from the National Center for Advancing Translational Sciences (NCATS) for NASH CRN Studies (grants UL1TR000439, UL1TR000436, UL1TR000006, UL1TR000448, UL1TR000100, UL1TR000004, UL1TR000423, and UL1TR000058). Additional support was provided by the Laboratory of Pathology, Intramural Division of the National Cancer Institute. The NASH CRN expresses its gratitude to the patients enrolled in this study, Patricia Robuck for her guidance in trial design, Jay Hoofnagle for his guidance in trial design and data analysis, and Averell Sherker for his assistance in coordinating communications among the NIDDK, the industry sponsor, and the NASH CRN steering committee.
Footnotes
Contributors
BAN-T, RL, AJS, JEL, MFA, NC, AMD, KVK, AM, JMC, JT, EMB, DEK, and ED participated in study design. BAN-T, RL, AJS, MFA, NC, SD, AMD, BH, KVK, AM, NT, EMB, and DEK were responsible for data collection. BAN-T, RL, AJS, JEL, MLVN, MFA, NC, AMD, KVK, AM, JMC, JT, EMB, and DEK participated in data analysis. BAN-T, RL, AJS, JEL, MLVN, MFA, NC, SD, AMD, KVK, AM, NC, JMC, JT, EMB, DEK, and ED participated in data interpretation. BAN-T, RL, AJS, JEL, MFA, NC, SD, AMD, KVK, AM, NT, JMC, EMB, and DEK participated in manuscript review and writing. MLVN and JT were responsible for preparation of the tables and figures.
For the FLINT protocol see http://jhuccs1.us/nash/open/protocols/FLINT/FLINTdocs.htm
Declaration of interests
BAN-T reports personal fees from Genentech/Roche, Nimbus Discovery, Boehringer Ingelheim, and Bristol-Myers Squibb. RL has research grants from Merck, Gilead, KineMed, Promedior, and Daiichi Sankyo, and has served as a consultant to Janssen, Merck, Gilead, Galmed, Siemens, and Genentech. AJS reports grants from Conatus, Gilead, Ikaria, Salix, Takeda, Astellas, Novartis, and Galectin; reports royalties from UpToDate; is a consulting advisor to Abbott, Genentech, Gilead, Ikaria, Merck, Norgine, Roche, Salix, Takeda, Nimbus, Nitto Denko, and Bristol-Myers Squibb; and is consultant with no financial conflicts for Genfit, Echosens, Immuron, Intercept, Novartis, Galectin, and Sequana. MFA reports grants from Gilead, Genfit, Immuron, Tobira, and Mochida, and consulting from Islet Sciences and TaiwanJ Pharmaceuticals. NC reports grants from Intercept, Gilead, Galectin, and Enterome and personal fees for consulting from Lilly, Merck, Aegerion, Boehringer Ingelheim, Janssen, Tobira, Mochida, AbbVie, Salix, and Nimbus. AMD reports grants from Shire, Metabolon, and Gilead and personal fees from AstraZeneca, Genentech, Japan Tobacco, and NuSI Foundation. KVK reports grants, personal fees, and nonfinancial support from BMS, Boehringer Ingelheim, Gilead, Janssen, Merck, Novartis, and Vertex; grants from Intercept and Mochida; personal fees and non-financial support from AbbVie; and personal fees from Evidera, Trio Health, and Tekmira. EB reports personal fees from Pfizer, Rottapharm, European Society of Pathology, and Synageva. All other authors declare no competing interests.
Contributor Information
Prof. Brent A Neuschwander-Tetri, Saint Louis University, St Louis, MO, USA.
Rohit Loomba, University of California San Diego, La Jolla, CA, USA.
Prof. Arun J Sanyal, Virginia Commonwealth University, Richmond, VA, USA.
Prof. Joel E Lavine, Columbia University, New York, NY, USA.
Mark L Van Natta, Johns Hopkins University, Baltimore, MD, USA.
Manal F Abdelmalek, Duke University, Durham, NC, USA.
Prof. Naga Chalasani, Indiana University, Indianapolis, IN, USA.
Srinivasan Dasarathy, Case Western Reserve University, Cleveland, OH, USA.
Prof. Anna Mae Diehl, Duke University, Durham, NC, USA.
Bilal Hameed, University of California San Francisco, San Francisco, CA USA.
Prof. Kris V Kowdley, Virginia Mason Medical Center, Seattle, WA, USA.
Prof. Arthur McCullough, Cleveland Clinic, Cleveland, OH, USA.
Prof. Norah Terrault, University of California San Francisco, San Francisco, CA USA.
Prof. Jeanne M Clark, Johns Hopkins University, Baltimore, MD, USA.
Prof. James Tonascia, Johns Hopkins University, Baltimore, MD, USA.
Prof. Elizabeth M Brunt, Washington University, St Louis, MO, USA.
David E Kleiner, The National Cancer Institute, Bethesda, MD, USA.
Edward Doo, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, USA.
References
- 1.Marrero JA, Fontana RJ, Su GI, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2002;36:1349–54. doi: 10.1053/jhep.2002.36939. [DOI] [PubMed] [Google Scholar]
- 2.Page JM, Harrison SA. NASH and HCC. Clin Liver Dis. 2009;13:631–47. doi: 10.1016/j.cld.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Sanyal A, Poklepovic A, Moyneur E, Barghout V. Population-based risk factors and resource utilization for HCC: US perspective. Curr Med Res Opin. 2010;26:2183–91. doi: 10.1185/03007995.2010.506375. [DOI] [PubMed] [Google Scholar]
- 4.Agopian VG, Kaldas FM, Hong JC, et al. Liver transplantation for nonalcoholic steatohepatitis: the new epidemic. Ann Surg. 2012;256:624–33. doi: 10.1097/SLA.0b013e31826b4b7e. [DOI] [PubMed] [Google Scholar]
- 5.Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology. 2012;142:711–25. e6. doi: 10.1053/j.gastro.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Lomonaco R, Ortiz-Lopez C, Orsak B, et al. Effect of adipose tissue insulin resistance on metabolic parameters and liver histology in obese patients with nonalcoholic fatty liver disease. Hepatology. 2012;55:1389–97. doi: 10.1002/hep.25539. [DOI] [PubMed] [Google Scholar]
- 7.Bellentani S, Dalle Grave R, Suppini A, Marchesini G Fatty Liver Italian Network. Behavior therapy for nonalcoholic fatty liver disease: the need for a multidisciplinary approach. Hepatology. 2008;47:746–54. doi: 10.1002/hep.22009. [DOI] [PubMed] [Google Scholar]
- 8.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–23. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 9.Musso G, Gambino R, Cassader M, Pagano G. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52:79–104. doi: 10.1002/hep.23623. [DOI] [PubMed] [Google Scholar]
- 10.Ratziu V. Pharmacological agents for NASH. Nat Rev Gastroenterol Hepatol. 2013;10:676–85. doi: 10.1038/nrgastro.2013.193. [DOI] [PubMed] [Google Scholar]
- 11.Yau H, Rivera K, Lomonaco R, Cusi K. The future of thiazolidinedione therapy in the management of type 2 diabetes mellitus. Curr Diab Rep. 2013;13:329–41. doi: 10.1007/s11892-013-0378-8. [DOI] [PubMed] [Google Scholar]
- 12.Bjelakovic G, Nikolova D, Gluud C. Meta-regression analyses, meta-analyses, and trial sequential analyses of the effects of supplementation with beta-carotene, vitamin A, and vitamin E singly or in different combinations on all-cause mortality: do we have evidence for lack of harm? PLoS One. 2013;8:e74558. doi: 10.1371/journal.pone.0074558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trauner M, Claudel T, Fickert P, Moustafa T, Wagner M. Bile acids as regulators of hepatic lipid and glucose metabolism. Dig Dis. 2010;28:220–24. doi: 10.1159/000282091. [DOI] [PubMed] [Google Scholar]
- 14.Karpen SJ. Do therapeutic bile acids hit the sweet spot of glucose metabolism in NAFLD? Gastroenterology. 2013;145:508–10. doi: 10.1053/j.gastro.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Porez G, Prawitt J, Gross B, Staels B. Bile acid receptors as targets for the treatment of dyslipidemia and cardiovascular disease. J Lipid Res. 2012;53:1723–37. doi: 10.1194/jlr.R024794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Modica S, Gadaleta RM, Moschetta A. Deciphering the nuclear bile acid receptor FXR paradigm. Nucl Recept Signal. 2010;8:e005. doi: 10.1621/nrs.08005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calkin AC, Tontonoz P. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat Rev Mol Cell Biol. 2012;13:213–24. doi: 10.1038/nrm3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Aguiar Vallim TQ, Tarling EJ, Edwards PA. Pleiotropic roles of bile acids in metabolism. Cell Metab. 2013;17:657–69. doi: 10.1016/j.cmet.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cariou B. The farnesoid X receptor (FXR) as a new target in non-alcoholic steatohepatitis. Diabetes Metab. 2008;34:685–91. doi: 10.1016/S1262-3636(08)74605-6. [DOI] [PubMed] [Google Scholar]
- 20.Cipriani S, Mencarelli A, Palladino G, Fiorucci S. FXR activation reverses insulin resistance and lipid abnormalities and protects against liver steatosis in Zucker (fa/fa) obese rats. J Lipid Res. 2010;51:771–84. doi: 10.1194/jlr.M001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fickert P, Fuchsbichler A, Moustafa T, et al. Farnesoid X receptor critically determines the fibrotic response in mice but is expressed to a low extent in human hepatic stellate cells and periductal myofibroblasts. Am J Pathol. 2009;175:2392–405. doi: 10.2353/ajpath.2009.090114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verbeke L, Farre R, Trebicka J, et al. Obeticholic acid, a farnesoid X receptor agonist, improves portal hypertension by two distinct pathways in cirrhotic rats. Hepatology. 2014;59:2286–98. doi: 10.1002/hep.26939. [DOI] [PubMed] [Google Scholar]
- 23.Mudaliar S, Henry RR, Sanyal AJ, et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145:574–82. doi: 10.1053/j.gastro.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 24.Lindor KD, Kowdley KV, Heathcote EJ, et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004;39:770–78. doi: 10.1002/hep.20092. [DOI] [PubMed] [Google Scholar]
- 25.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 26.Reboussin DM, DeMets DL, Kim KM, Lan KK. Computations for group sequential boundaries using the Lan-DeMets spending function method. Control Clin Trials. 2000;21:190–207. doi: 10.1016/s0197-2456(00)00057-x. [DOI] [PubMed] [Google Scholar]
- 27.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–85. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brunt EM, Kleiner DE, Wilson LA, Belt P, Neuschwander-Tetri BA for the NASH Clinical Research Network (CRN) Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology. 2011;53:810–20. doi: 10.1002/hep.24127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hageman J, Herrema H, Groen AK, Kuipers F. A role of the bile salt receptor FXR in atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:1519–28. doi: 10.1161/ATVBAHA.109.197897. [DOI] [PubMed] [Google Scholar]
- 30.Kowdley KV, Jones D, Luketic VA, et al. An international study evaluating the farnesoid X receptor agonist obeticholic acid as monotherapy in PBC. J Hepatol. 2011;54 (suppl 1):S13. [Google Scholar]