Abstract
Background
Individuals with cystic fibrosis (CF) and pancreatic insufficiency (PI) are at risk for fat-soluble vitamin deficiency, including vitamin A. Recent evidence suggests current practices of vitamin A intake results in elevated serum retinol.
Methods
Serum retinol was assessed in 78 subjects (8 to 25 years) with CF and PI by high performance liquid chromatography, and compared to the U.S. National Health and Nutrition Examination Survey (NHANES) data of subjects of similar age and gender. Vitamin A intake, anthropometry and FEV1 were measured, and their relationship to serum retinol status was assessed.
Results
Median (range) serum retinol was 80 µg/dL (33 to 208) in subjects with CF; 58% were above the NHANES reference range (30 to 72 µg/dL). Total vitamin A intake from diet and supplements was high (608+431% Recommended Dietary Allowance). Serum retinol was not correlated with vitamin A intake, age or gender, and was inversely correlated with weight and height z scores (r = −0.28, p<0.05) in the subjects with CF.
Conclusions
Both vitamin A intake and serum retinol were elevated in subjects with CF and PI, corroborating recent evidence of elevated serum retinol in preadolescent children with CF. These findings indicate the need for further study of dosing and monitoring care practices of vitamin A, to ensure adequacy and to avoid toxicity.
Keywords: cystic fibrosis, children, vitamin A, toxicity, serum retinol
Introduction
Cystic Fibrosis (CF) and pancreatic insufficiency (PI) predispose patients to fat and fat-soluble vitamin malabsorption, despite pancreatic enzyme replacement. Historically, individuals with CF have been prone to vitamin A deficiency (1). A recent study reported unexpectedly high serum retinol concentrations and raised the question of excessive supplementation. Current patterns of vitamin A intake and supplementation may increase the risk for vitamin A toxicity (2). The goal of this study was to document the vitamin A status and to verify or refute these unexpected findings with a broader age range cohort of children and young adults with CF and PI, and compare the findings to age- and sex-matched reference data from the U.S. National Health and Nutrition Examination Survey (NHANES) (3).
Materials and Methods
Children and young adults (8 to 25 years old) with CF and PI were recruited from a pediatric and adult CF Center. Inclusion criteria included diagnosis of CF and PI by standard methods (2). Subjects with FEV1<40% predicted and those with other major medical illnesses that affect growth were excluded.
Serum retinol (ug/dL) was collected by research personnel at The Children’s Hospital of Philadelphia Clinical Translational Research Center and analyzed by high performance liquid chromatography (HPLC) (Clinical Nutrition Research Unit Nutrition Assessment Laboratory, University of California, Davis) under research protocols with quality assurance measures. Serum retinol levels of subjects with CF were compared with NHANES 1999–2002 (3) serum reference ranges (5th to 95th percentiles) from age-equivalent white subjects (88% of which were non-hispanic whites), using HPLC (Division of Environmental Health Services, Centers for Disease Control and Prevention, Atlanta, GA.) and with similar research protocol and quality assurance measures.
Height and weight were measured using standard techniques (4), with a stadiometer accurate to 0.1 cm (Holtain, Crymych, UK) and a digital scale accurate to 0.1 kg (Scaletronix, White Plains, NY). All measurements were obtained in triplicate and the mean used in the analyses. Z scores for height (HAZ), weight (WAZ), and body mass index (BMI; kg/m2, BMIZ) were computed (5). Pulmonary function was evaluated by standard methods for spirometry (6;7). FEV1 percent predicted was calculated using the Wang et al (8) and Hankinson et al (9) equations.
Research dieticians determined dietary intake from 3-day weighed food records. Vitamin A intake, as food- and supplement-based, pre-formed and total retinol, were reported in retinol activity equivalents per day (µg RAE/d). The vitamin A intake of subjects with CF was compared with Dietary Reference Intake (DRI) (10) Recommended Dietary Allowance (RDA) and Tolerable Upper Intake Levels (UL) which are age and gender based, and with the CF Foundation Recommendations, which are age-based (11;12).
All variables were tested for normality and skewness. Group comparisons were tested using Student’s t-test or Wilcoxon rank sum test for normally or non-normally distributed variables, respectively. Data were presented as mean ± standard deviation; median values and range were used for the serum retinol due to non-normality. Pearson correlation coefficients or Spearman rank correlations were performed to test for significant associations between variables as appropriate. Statistical significance was defined as a p-value< 0.05. All analyses were performed with STATA release 8.2 (STATA Corporation, College Station, TX).
The protocol was approved by the Committee for the Protection of Human Subjects of the Institutional Review Board at the Children’s Hospital of Philadelphia and the Hospital of the University of Pennsylvania. Informed written consent was obtained from each subject’s parent or legal guardian if under 18 years, and from subjects 18 years or older written assent was obtained from subjects 8 to18 years of age.
Results
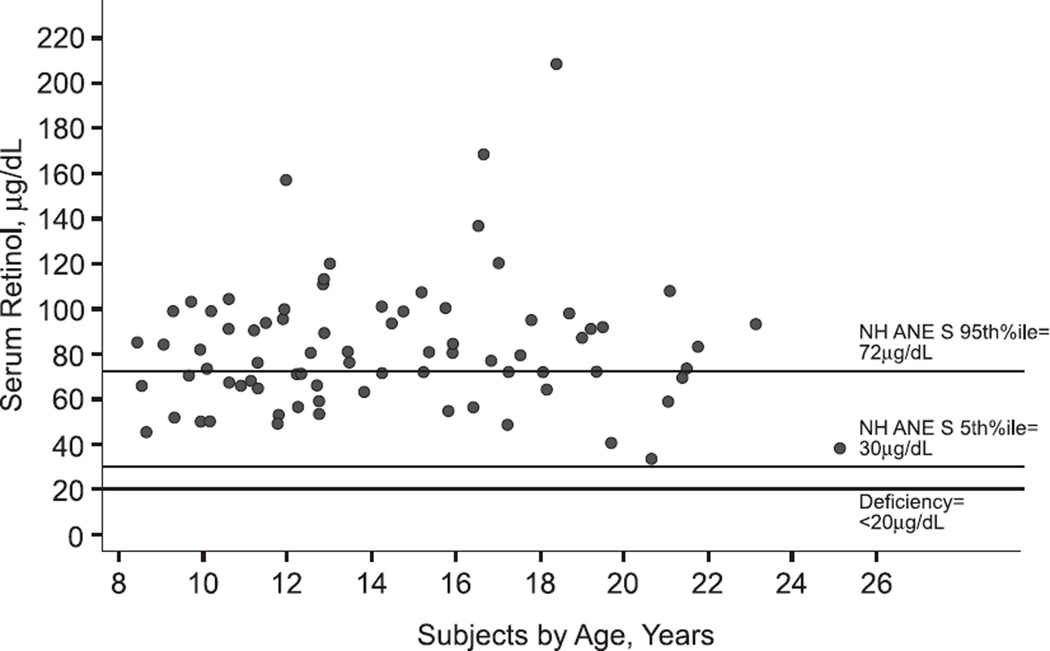
Serum retinol, anthropometry and FEV1 were available for 78 subjects (Table 1). Seven subjects (9%) participated in both the previously cited pre-adolescent study (2) and the current study. Dietary intake data were available for 53 subjects. In comparison to subjects with complete dietary data, the subjects without dietary data had lower %FEV1 (81 ± 13 versus 89 ± 16; p=0.04). Forty-two percent of subjects had serum retinol within and 58% had concentrations above the NHANES reference range of 30 to 72 µg/dL (Figure 1); none of the subjects with CF and PI had a serum retinol below the lower reference range value of 30 µg/dL.
Table 1.
Clinical Characteristics of the 78 Subjects with Cystic Fibrosis and Pancreatic Insufficiency
| Characteristic | Mean ± SD (Range) |
|---|---|
| Age, years | 14.5 ± 4.0 (8.4, 25.2) |
| Gender, % male | 48 |
| Weight for age z-score | −0.54 ± 1.06 (−3.67, 2.15) |
| Height for age z-score | −0.59 ± 0.98 (−2.63, 1.99) |
| BMI for age z-score | −0.26 ± 0.90 (−2.73, 1.97) |
| FEV1, % predicted | 86 ± 15 (47, 118) |
| Serum Retinol, µg/dL | 82 ± 29 |
| Median, µg/dL1 | 80 (33, 208) |
Median value (range) presented given due to non-normality
Figure 1.
Total vitamin A intake (mean ± sd) from food and supplement (Table 2) was 608 ± 431 % RDA, with 67% from supplements. Total preformed retinol intake was 564 ± 409 % RDA, with 86% of subjects exceeding the UL (10) and 73% of subjects exceeding the CF Foundation Recommendations (11).
Table 2.
Energy and Vitamin A intake for the Subjects with Cystic Fibrosis and Pancreatic Insufficiency (n=53)
| Dietary Intake | Mean ± SD | Median | Range |
|---|---|---|---|
| Energy intake, kcal/d | 2,786 ± 827 | 2,616 | 1,617 to 5,293 |
| Vitamin A Intake | |||
| Food | |||
| Preformed Retinol | |||
| µg RAE/d | 1,107 ± 1,410 | 852 | 120 to 10,499 |
| µg RAE/kg/d | 26 ± 25 | 21 | 3 to 172 |
| %RDA1 | 167 ± 204 | 132 | 17 to 1,500 |
| Total Vitamin A | |||
| µg RAE/d | 1,279 ± 1,510 | 1,012 | 150 to 11,330 |
| %RDA | 216 ± 252 | 169 | 25 to 1,888 |
| Supplemental | |||
| Preformed Retinol | |||
| µg RAE/d | 2,580 ± 1,905 | 2,700 | 0 to 9,188 |
| µg RAE/kg/d | 61 ± 56 | 54 | 0 to 350 |
| %RDA | 397 ± 362 | 333 | 0 to 2,297 |
| Total Vitamin A | |||
| µg RAE/d | 2,692 ± 1,908 | 2,700 | 0 to 9,188 |
| %RDA | 415 ± 364 | 375 | 0 to 2,297 |
| Combined Food and Supplement | |||
| Preformed Retinol | |||
| µg RAE/day | 3,687 ± 2,223 | 3,332 | 437 to 12,479 |
| µg RAE/kg/d | 87 ± 61 | 78 | 9 to 386 |
| %RDA | 564 ± 409 | 485 | 73 to 2,538 |
| % CF Recommendations2 | 123 + 74 | 111 | 15 to 416 |
| Total Vitamin A | |||
| µg RAE/day | 3,972 ± 2,359 | 3,614 | 503 to 13,850 |
| %RDA | 608 ± 431 | 521 | 84 to 2,631 |
| % CF Recommendations | 132 ± 79 | 120 | 17 to 462 |
Recommended Dietary Intake is age and gender based, and ranges from 400 to 900 µg RAE/day (10).
For the subject sample with dietary data, serum retinol was inversely correlated with HAZ and WAZ (−0.28, p<0.04; −0.28, P<0.05), and not associated with FEV1, age, gender, or vitamin A intake (food, supplement-based, and/or total). When the study subjects were divided into groups as within or above the NHANES serum retinol reference ranges, there were no group differences in anthropometry, %FEV1, and food and supplement-based (pre-formed or total) vitamin A intake.
Discussion
These results showed that under current patterns of care, 58% of subjects with CF and PI had elevated serum retinol concentrations using NHANES reference data for comparison. None of these subjects were vitamin A deficient based on serum retinol status.
Elevated serum retinol was previously documented in a group of pre-adolescent children with CF and PI (2) from a study involving13 US CF Centers. Mean serum retinol was 52 ± 13 µg/dL (range: 26 to 98), significantly higher than the age-similar NHANES reference group mean of 37 ± 10 µg/dL. In the pre-adolescent group, 47% of subjects had serum retinol concentrations >95th percentile, compared with 58% in the current study. Our present study corroborates these findings in a broader age range, raising further concerns about elevated serum retinol concentrations, high vitamin A intake, and the risk for vitamin A toxicity in the CF population.
The term “vitamin A” refers to a family of compounds important for cellular integrity, growth, and immune function, that is comprised of preformed retinoids and pro-vitamin A carotenoids. Retinoids are fat-soluble, animal-derived compounds that include retinol, retinal, and retinoic acids. Ingested retinoids are solubilized and esterified into retinyl esters (RE) in the intestine, circulated as both bound RE and unbound retinol, and stored in the liver as retinol. While the serum retinol concentration is homeostatically maintained, hepatic stores accumulate indefinitely with increased ingestion. Conversely, pro-vitamin A carotenoids are water-soluble, plant-derived compounds that include carotene, lutein, and xanthophylls. They are less bio-available than retinoids and amounts ingested in excess of needs are not stored but are excreted.
The amount of vitamin A contained in a given food or supplement is described using either international units (IU) or microgram retinol activity equivalents (µg RAE). The former is an outdated unit that does not account for discrepant bioavailability and bioactivity of the different forms of vitamin A. The RAE is the current standard unit which incorporates these differences and allows direct comparison among all different forms of vitamin A. Conversion equations:one µgRAE = 1 µg retinol = 12 µg β-carotene from food= 24 µg other provitamin A carotenoids. One IU retinol = 0.3 µg RAE = 3.6 µg β-carotene from fruits and vegetables = 7.2 µg other pro-vitamin A carotenoids (13).
In addition to the form of vitamin A, the specific preparation of vitamin A supplement is relevant when considering the potential for toxicity (10). Some vitamin A formulas used in the CF population are now biochemically engineered to increase the water-solubility of retinoids to allow for easier absorption in the setting of pancreatic insufficiency. These preparations are helpful when there is ongoing malabsorption but may also confer more risk for the development of elevated serum retinol and potential vitamin A toxicity in this population. A meta-analysis of 259 case reports of chronic hypervitaminosis A in children and adults without CF suggested relatively smaller doses (200 µg RAE/kg/day) of water-miscible, emulsified, and solid forms of retinol supplements ingested over shorter time intervals (weeks to months) induced similar toxicity as oil-based preparations given in larger doses (2,000 µg RAE/kg/day) over months to years (14). Water-miscible preformed vitamin A supplements were used by 58% of our subjects.
The 2002 CF Nutrition Consensus Report (11) made age-specific vitamin A supplement-based intake recommendations; children between four and eight years of age were recommended to take between 5,000 and 10,000 IU/day, and children greater than eight years of age and adults were to take 10,000 IU/day. Further, both the pediatric and adult consensus reports (11;12) recommend monitoring of vitamin A status by serum retinol concentration determination at the time of diagnosis, and annually afterwards. The commonly used CF-specific vitamin products contain water-miscible vitamin A, between 897 to 2,520 µg RAE per dosage unit, with 40 to100% as preformed retinol (2). Preformed vitamin A is more bioavailable, more readily stored, and not subject to the same feedback control as provitamin A carotenoids. Therefore, knowledge of each specific vitamin A formulation is important to the proper evaluation and monitoring of vitamin A supplementation. In the current study, many individuals with CF took many different vitamin supplement products, and some used more than one type of vitamin A supplement per day. In the current study, 27 different vitamin A supplement products were used, and 11% of subjects used more than one type of vitamin A supplement per day; one subject used three different vitamin A-containing supplements per day.
Vitamin A toxicity primarily affects the bone and liver. The bone fracture rate was increased by 64% among non-CF Swedish men with serum retinol above 76 µg/dL compared to those with concentrations between 62 and 67 µg/dL (15). Mean serum retinol in the current study was similar to this higher fracture risk group. Vitamin A intake and bone toxicity has been reported with preformed vitamin A intakes as low as 1500 ug RAE/day in non-CF subjects (16). Preformed retinol intake between 1,500 to >14,000 ug RAE/day over one to 30 years was associated with a spectrum of liver abnormalities (10;17). Pre-existing/coincident liver disease may alter the threshold for vitamin A associated toxicity (10;18). This is an important clinical concern for patients with CF, since CF associated liver disease is usually diagnosed in children and adolescents (19).
Hepatic vitamin A status of subjects with CF has been investigated in a limited number of studies in the past. The liver is the main storage site for vitamin A, and vitamin A status has previously been described in subjects with CF, examining both serum and hepatic vitamin A status. A 1972 study (20) reported significantly higher hepatic and lower serum retinol concentrations in 12 vitamin A supplemented subjects with CF (8 to 23 years old) compared to healthy controls. Another study found normal hepatic retinol concentrations in 15 vitamin A supplemented Swedish subjects with CF (8 to 34 years old) compared to healthy controls (19); hepatic retinyl palmitate decreased with age, serum retinol was 36 ± 17 µg/dL (range 2 to 57 µg/dL), and 33% of subjects had values less than 30 µg/dL. In contrast to this study, we found normal and high serum retinol levels in subjects of comparable age range, and no subjects with low levels. Similar to the Swedish study (19) however, we did not find an association between serum retinol and age.
The limitations of this study included its cross-sectional design, and the fact that the vitamin A analysis was secondary within the bone health in people with cystic fibrosis study. Currently there is no well-accepted non-invasive marker for the assessment of vitamin A excess. Serum retinol is tightly regulated, decreases with depleted hepatic stores (21), and is likely a better bio-marker of vitamin A deficiency rather than excess. Serum retinol increases with long-term supplement use, and intake of foods high in preformed vitamin A. Serum retinol elevations may reflect increased hepatic stores; however, the threshold value to indicate elevated hepatic stores is not known. Retinyl esters have been noted to be elevated with excess vitamin A intake or increased liver stores (22); these were not available in the current study. Retinol binding protein usually parallels the serum retinol level; both of these biomarkers of vitamin A status can be depressed in the setting of inflammation, and thus is not an optimal indicator of vitamin A status in diseases such as CF, with a chronic inflammation component. This is particularly concerning when serum retinol is elevated in these subjects with CF, as it may underestimate degree of vitamin A excess.
In summary, these findings raise concern about current vitamin A supplementation practices and products. Children and young adults had similar findings of high vitamin A intake and elevated serum retinol concentrations as compared to pre-adolescent children with CF and PI. Routine use of preformed, water-miscible vitamin A supplements may result in elevated serum retinol concentrations and may increase the risk for hypervitaminosis A in this population. It is unknown if CF-related liver or bone disease alters the risk for hypervitaminosis A. Serum retinol should be routinely monitored at least on an annual basis, as recommended by the 2002 and 2004 Pediatric and Adult Consensus Reports on Nutrition for Patients with CF (11;12). We suggest adjusting the dose accordingly to the results in order to maintain normal serum retinol Further studies of vitamin A status are warranted, including determination of serum retinyl esters, and possibly even liver biopsy to determine hepatic vitamin A stores. Furthermore, the development of non-invasive methods to measure hepatic and total body stores of vitamin A would enhance the ability to monitor vitamin A supplementation. A better understanding of the relationship between serum and tissue vitamin A is needed for patients with CF. In the meanwhile clinicians should be aware of the composition of different vitamin A supplement preparations and dosing equivalents taken by their patients.
Acknowledgements
We would like to thank our participating children and families, The Children’s Hospital of Philadelphia, Hospital of the University of Philadelphia, the Cystic Fibrosis Center who made this study possible, Rita Herskovitz, Kate Temme, the Clinical Translational Research Center, and Nutrition and Growth Lab staff at the Children’s Hospital of Philadelphia. We would like to thank the Clinical Nutrition Research Unit at the University of California at Davis for the HPLC and serum retinol determination.
Funding Sources:
The Cystic Fibrosis Foundation was the primary source of funding for this study. The Children’s Hospital of Philadelphia Clinical Translational Research Center (UL1-RR0241340) and Nutrition Center supported the collection of data; the University of California at Davis Clinical Nutrition Research Unit (NIH-NIDDK 35747) performed the serum retinol analyses. Dr. Rose Graham-Maar’s manuscript preparation contribution was supported in part by a T-32 (HL 07433) training grant.
Abbreviations
- BMI
Body mass index
- %FEV1
Forced expiratory volume at 1 second, percent predicted value
- CHOP
The Children’s Hospital of Philadelphia
- CF
Cystic fibrosis
- DRI
Dietary Reference Intake
- HAZ
Height for age Z score
- HPLC
High performance liquid chromatography
- NHANES
National Health and Nutritional Examination Survey
- PI
Pancreatic insufficiency
- RDA
Recommended Dietary Allowance, mg/d or %
- RAE
Retinol activity equivalents, µg
- UL
Tolerable upper limit of intake, mg/d
- WAZ
Weight for age Z score
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement:
None of the authors of the manuscript reported any financial or other potential conflict of interest in relation to this manuscript.
Reference List
- 1.Solomons NW, Wagonfeld JB, Rieger C, Jacob RA, Bolt M, Horst JV, et al. Some biochemical indices of nutrition in treated cystic fibrosis patients. Am J Clin Nutr. 1981 Apr;34(4):462–474. doi: 10.1093/ajcn/34.4.462. [DOI] [PubMed] [Google Scholar]
- 2.Graham-Maar R, Schall J, Zemel BS, Stallings VA. Elevated Vitamin A Intake and Serum Retinol in Preadolescent Children with Cystic fibrosis. Am J Clin Nutr. 2006;84(1):174–182. doi: 10.1093/ajcn/84.1.174. [DOI] [PubMed] [Google Scholar]
- 3.NHANES 1999–2000 Data Files, National Health and Nutrition Survey Data. Hyattsville, MD: Public Health Survey, Center for Disease Control and Prevention; 2004. [Google Scholar]
- 4.Lohman T, Roche AR, Martorell R. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics; 1988. [Google Scholar]
- 5.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Adv Data. 2000 Jun 8;(314):1–27. [PubMed] [Google Scholar]
- 6.Gardner RM. Report of Snowbird workshop on standardization of spirometry. Am Rev Resp Dis. 1979;119:831–838. doi: 10.1164/arrd.1979.119.5.831. [DOI] [PubMed] [Google Scholar]
- 7.Morris A, Kanner RE, Crapo R, Gardner RM. Clinical Pulmonary Function Testing: A Manual of Uniform Laboratory Procedures. 2nd ed. Salt Lake City, UT: Intermountain Thoracic Society; 1984. [Google Scholar]
- 8.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG Jr. Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol. 1993;15(2):75–88. doi: 10.1002/ppul.1950150204. [DOI] [PubMed] [Google Scholar]
- 9.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999 Jan;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 10.Institute of Medicine. Dietary reference intakes for vitamin, A, vitamin K, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, vanadium, and zinc. Washington DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- 11.Borowitz D, Baker RD, Stallings V. Consensus report on nutrition for pediatric patients with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2002 Sep;35(3):246–259. doi: 10.1097/00005176-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Yankaskas JR, Marshall BC, Sufian B, Simon RH, Rodman D. Cystic fibrosis adult care: consensus conference report. Chest. 2004 Jan;125(1 Suppl):1S–39S. doi: 10.1378/chest.125.1_suppl.1s. [DOI] [PubMed] [Google Scholar]
- 13.Shills M, Shike M, Caballero B, Cousins RJ, editors. Modern Nutrition in Health and Disease. Tenth ed. Baltimore: Lippincott, Williams & Wilkins; 2006. Appendix A-1b. Factors and formulas used in intervconverting units of vitamin A and carotenoids; p. 1847. [Google Scholar]
- 14.Myhre AM, Carlsen MH, Bohn SK, Wold HL, Laake P, Blomhoff R. Watermiscible, emulsified, and solid forms of retinol supplements are more toxic than oil-based preparations. Am J Clin Nutr. 2003 Dec;78(6):1152–1159. doi: 10.1093/ajcn/78.6.1152. [DOI] [PubMed] [Google Scholar]
- 15.Michaelsson K, Lithell H, Vessby B, Melhus H. Serum retinol levels and the risk of fracture. N Engl J Med. 2003 Jan 23;348(4):287–294. doi: 10.1056/NEJMoa021171. [DOI] [PubMed] [Google Scholar]
- 16.Melhus H, Michaelsson K, Kindmark A, Bergstrom R, Holmberg L, Mallmin H, et al. Excessive dietary intake of vitamin A is associated with reduced bone mineral density and increased risk for hip fracture. Ann Intern Med. 1998 Nov 15;129(10):770–778. doi: 10.7326/0003-4819-129-10-199811150-00003. [DOI] [PubMed] [Google Scholar]
- 17.Shills M, Shike M, Ross A, Caballero B, Cousins RJ. Modern Nutrition in Health and Disease. Tenth ed. Baltimore: 2006. Vitamin A and carotenoids. [Google Scholar]
- 18.Hatoff DE, Gertler SL, Miyai K, Parker BA, Weiss JB. Hypervitaminosis A unmasked by acute viral hepatitis. Gastroenterology. 1982 Jan;82(1):124–128. [PubMed] [Google Scholar]
- 19.Lindblad A, Diczfalusy U, Hultcrantz R, Thorell A, Strandvik B. Vitamin A concentration in the liver decreases with age in patients with cystic fibrosis. J Pediatr Gastroenterol Nutr. 1997 Mar;24(3):264–270. doi: 10.1097/00005176-199703000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Underwood BA, Denning CR. Blood and liver concentrations of vitamins A and E in children with cystic fibrosis of the pancreas. Pediatr Res. 1972 Jan;6(1):26–31. doi: 10.1203/00006450-197201000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Olson JA. Serum levels of vitamin A and carotenoids as reflectors of nutritional status. J Natl Cancer Inst. 1984 Dec;73(6):1439–1444. [PubMed] [Google Scholar]
- 22.Olson JA, Shils ME, Shike M. Modern Nutrition in Health and Disease. Tenth ed. Philadelphia: Lea & Febiger; 1994. Vitamin A, retinoids and carotenoids; pp. 287–307. [Google Scholar]