Abstract
Although most signaling responses initiated by tumor necrosis factor-α (TNF-α) occur in a Ca2+-independent fashion, TNF-α receptor signaling augments Ca2+ entry induced by Gαq/11 G-protein coupled receptors (GPCRs) in endothelial cells and increases transendothelial permeability. The signaling events involved in GPCR-induced Ca2+ influx have been characterized and involve store-operated Ca2+ entry facilitated by the Ca2+ permeable ion channel, transient receptor potential canonical 4 (TRPC4). Little is known about the mechanisms by which TNF-α receptor signaling augments GPCR-induced Ca2+ entry. T NF-α R eceptor U biquitous S ignaling and S caffolding protein (TRUSS) is a tumor necrosis factor receptor-1 (TNF-R1)-associated protein whose gene name is TRPC4-associated protein (TRPC4AP). The goal of our study was to test the hypothesis that TRUSS serves to link TNF-R1 and GPCR-signaling pathways at the level of TRPC4 by: (i) determining if TRUSS and TNF-R1 interact with TRPC4, and (ii) investigating the role of TRUSS, TNF-R1, and TRPC4 in GPCR-induced Ca2+ signaling. Here, we show that TRUSS and TNF-R1 interact with a sub-family of TRPC channels (TRPC1, 4, and 5). In addition, we show that TRUSS and TNF-R1 function together with TRPC4 to elevate endoplasmic reticulum Ca2+ filling in the context of reduced endoplasmic reticulum Ca2+ storage initiated by G-protein coupled m1 muscarinic acetylcholine receptor (m1AchR) signaling. Together, these findings suggest that TNF-R1, TRUSS, and TRPC4 augment Ca2+ loading of endoplasmic reticulum Ca2+ stores in the context of m1AchR stimulation and provide new insights into the mechanisms that connect TNF-R1 to GPCR-induced Ca2+ signaling.
Tumor necrosis factor-α (TNF-α) is a pro-inflammatory cytokine involved in innate and adaptive immunity, inflammation, apoptosis, and cell survival. Although a limited repertoire of TNF-α-induced responses are initiated by TNF-receptor-2 (Peschon et al., 1998; Wang et al., 2009a,b), most are mediated by tumor necrosis factor receptor-1 (TNF-R1), which signals the activation of nuclear factor-κB (NF-κB), mitogen-activated protein kinases (MAPK), Akt, and apoptosis (Locksley et al., 2001; Chen and Goeddel, 2002). TNF-R1 signaling events are activated in a Ca2+-independent fashion. However, TNF-α signaling does affect Ca2+ entry and signaling by Gαq/11-linked G-protein coupled receptors (GPCRs). Studies initially reported by Amrani et al. (1995, 1996) showed that incubation of tracheal smooth muscle cells with TNF-α augmented bradykinin and histamine-induced Ca2+ influx leading to increased smooth muscle cell proliferation and tracheal smooth muscle contraction. Similarly, Tiruppathi et al. (2001) revealed a fundamentally similar signaling response in human umbilical vein endothelial cells that were incubated with TNF-α for 2 h prior to stimulation with the PAR-1 agonist, thrombin. Thus, while TNF-α signaling occurs independently of changes in intracellular Ca2+, it promotes Ca2+ entry and signaling by some Gαq/11-linked GPCRs.
GPCR-stimulated Ca2+ entry in diverse non-excitable cells utilizes Gαq/11-dependent activation of phospholipase C-β, which leads to the generation of diacylglycerol and inositol 1,4,5-trisphosphate (IP3) (Tiruppathi et al., 2002b; Bird et al., 2004). Binding of IP3 by IP3 receptors located on the endoplasmic reticulum stimulates Ca2+ release into the cytosol, which in turn promotes store-operated Ca2+ entry via STIM1, a sensor of endoplasmic reticulum Ca2+ depletion, and Orai1, a STIM1-linked Ca2+ channel located on the plasma membrane (Liou et al., 2005; Roos et al., 2005; Feske et al., 2006; Vig et al., 2006; Putney, 2007). In addition, several members of the mammalian transient receptor potential canonical (TRPC) family have been implicated in store-operated Ca2+ entry in non-excitable cells, including TRPC1, 4, and 5 (Liu et al., 2007; Worley et al., 2007; Yuan et al., 2007). Of these, targeted disruption of the trpc4 gene in mice has been shown to inhibit store-operated Ca2+ entry in endothelial cells (Freichel et al., 2001; Tiruppathi et al., 2002a). Despite the fundamental importance of these events in endothelial barrier disruption in acute lung injury and smooth muscle contraction in airway hyper-responsiveness, little is known about the molecular mechanisms that link TNF-R1 signaling and GPCR-induced Ca2+ signaling.
TNF-R1 ubiquitous scaffolding signaling protein (TRUSS) was originally identified in a two-hybrid screen as a TNF-R1-associated protein and has since been shown to be a component of TNF-R1 signaling complexes involved in NF-κB and JNK activation (Soond et al., 2003, 2006; Rual et al., 2005). The gene name for TRUSS is TRPC4-associated protein (TRPC4AP). In view of the role of TRPC4 in promoting store-operated Ca2+ entry in endothelial cells, together with the effect of TNF-α signaling in augmenting this response, we investigated the potential role of TRUSS in coupling TNF-R1 to the augmentation in GPCR-induced Ca2+ responses. The goals of our study were: (i) to determine if TRUSS and TNF-R1 interact with TRPC channels, and (ii) to evaluate if TRUSS and TNF-R1, along with TRPC channels, influence GPCR-induced Ca2+ signaling. These goals were addressed using an HEK293 cell model since Ca2+ signaling has been extensively studied in this cell line and because of their suitability for studies aimed at manipulating the expression of genes of interest. In addition, we addressed these goals in the context of Ca2+ signaling induced by the G-protein coupledm1muscarinic acetylcholine receptor (m1AcR). As we will show, TRUSS and TNF-R1 interact with a subset of TRPC channels and serve to promote the filling of endoplasmic reticulum Ca2+ stores. Together, these findings suggest that TRUSS and TNF-R1 work together to affect Gαq/11-linked GPCR-induced Ca2+ signaling.
Methods
Cell culture and transfection
HEK293 cells, purchased from ATCC (Manassas, VA), were cultured at 37°C in 5% CO2, in Dulbecco’s modified Eagle’s medium plus 10% heat-inactivated fetal bovine serum supplemented with 2mM l-glutamine, 100 U/ml penicillin, 100µg/ ml streptomycin. Twenty-four to 48 h before transfection, 3–6 × 105 cells/well were plated onto poly-d-lysine-coated, six-well culture dishes. Transfections were accomplished with 5 µl of Lipofectamine 2000 (Invitrogen Life Technology, Carlsbad, CA) per well according to the manufacturer’s instructions. Generally, cells were transfected with the following amounts of pcDNA3.1 plasmids: 1 µg mTRUSS, 0.5 µg h-Flag-TNF-R1, 0.25 µg HATRPC1, -C4 or -C5, and 1µg HA-TRPC3, -C6, -C7. One microgram of m1AchR and m3AchR in pCMV were transfected per well. Transfections were controlled for DNA amounts by adding empty pcDNA3.1. Lipid with DNA complexes were incubated with cells in 2 ml/well Optimem (Gibco Invitrogen, Carlsbad, CA) for 4–6 h before addition of an equal amount of DMEM containing 20% FBS. Co-immunoprecipitation and intracellular Ca2+ measurements were carried out 24–48 h post-transfection.
Co-immunoprecipitation and Western blotting
HEK293 cells were plated at a density of 0.5–1.0 × 106 cells per well, 24 h before transfection as described above. The cells were lysed with lysis buffer (20mM Tris/HCl buffer, pH 8.0, containing 150mMNaCl, 0.5% (w/v) deoxycholate, 1% (v/v) Triton-X 100 and a final concentration of the protease inhibitors: 5µg/ml leupeptin, 5 µg/ml aprotinin, 1mM NaF, and 1mM Na3VO4), then passed 10 times through a 20-gauge needle and 5 times through a 26-gauge needle. Insoluble nuclear material was then removed cleared centrifugation at 2 × 104g for 10 min to obtain a whole cell lysate (WCL). Immunoprecipitations were conducted by adding 2 µg of anti-HA (Roche Diagnostics Corp., Indianapolis, IN), anti-TRUSSC terminal (Soond et al., 2003), 1.5 µg goat anti-TNF-R1 (R&D Systems, Minneapolis, MN) antibodies or an equivalent amount of murine, rabbit, or goat non-immune IgG (Santa Cruz Biotechnology, Santa Cruz, CA) together with Protein G PLUS agarose beads (20–50 µl) (Santa Cruz) to 400–500 µg of WCL protein. The tubes were rotated overnight at 4°C and the agarose-complexes washed three times with 400 µl of lysis buffer containing 150–500mM NaCl. Finally, the agarose beads were resuspended in 2 × Laemmli sample buffer, boiled for 5 min, resolved by SDS–PAGE and the bound proteins were detected by Western blotting with anti-TRUSS C-terminal, anti-FLAG-M2 (Sigma-Aldrich, St. Louis, MO), or anti-HA (Covance, Emoryville, CA) antibodies. To control for the level of protein expression, 15 µg of WCL protein, obtained prior to immunoprecipitation, was included on each gel.
Intracellular Ca2+ measurements
Transfected HEK293 cells were removed from poly-d-lysine-coated plates with 0.25% (w/v) trypsin–EDTA and pelleted by centrifugation at 1,000g for 10 min. Supernatants were removed and the cells were resuspended in 3ml of Ca2+ buffer (10mM HEPES, pH 7.5, containing 135mM NaCl, 5mM KCl, 1mM CaCl2, 1mM MgCl2, 5.6mM glucose, and 0.1% (w/v) BSA). The cells were loaded in Ca2+ buffer with 0.5–0.75 µM Fura-2 AM for 20 min at room temperature, de-esterified in 5ml of fresh Ca2+ buffer for 30 min at room temperature, and collected centrifugation at 2 × 103g for 8–10 min using a modification of a previously described procedure (Lussier et al., 2005). The buffer was removed and the cells were resuspended in 2ml of fresh Ca2+ buffer before analysis in a QM-6/2003 fluorometer (Photon Technology International Inc., Birmingham, NJ). Generally, baseline measurements were collected and, when indicated, EGTA was added to a final concentration of 625 µM before stimulation with a final concentration of 50µM carbachol (Cch) or 1.25 µM ionomycin. For store-operated Ca2+ entry experiments, CaCl2 was added to a final concentration of 2.5 mM.
To quantify the Ca2+ content of the endoplasmic reticulum, appropriately transfected HEK293 cells were loaded with 1.5 µM Mag-Fura-2 AM in Ca2+ buffer for 45 min at 37°C as described (Hofer and Machen, 1993; Hofer et al., 1995; Hofer and Schulz, 1996). Cells were washed with Ca2+ buffer, pelleted and buffer was aspirated from the cells. Immediately prior to analysis, the cells were permeabilized with digitonin (10µg/ml for 5–10 min) in intracellular KCl buffer (10mMHEPES, pH 7.25 containing 125mM KCl, 25mM NaCl, 0.15mM MgCl2, and 170 nM free Ca2+). Fresh KCl buffer was added and cells were immediately analyzed. As a control, cells were loaded with Fura-2AMand examined in parallel. For these experiments, baseline data were collected for 1.5 min prior to the addition of ionomycin (1.25 µM). Data were collected for an additional 1.5 min after the addition of ionomycin.
The area under the curve was determined by summing the fluorescence-ratio (F340/F380) value for each point collected within the indicated phase of the response. This value was then subtracted by the average baseline value multiplied by the number of time points analyzed in the response phase. All samples within an experiment were normalized to the pcD control. The mean of each condition was calculated and a one-way ANOVA with Newman–Keuls multiple comparison post-test was performed on at least three independent experiments.
Results
TRUSS interacts with TRPC1, -C4, and -C5 but not TRPC3, -C6, or -C7
To determine if TRUSS interacts with TRPC channels, we co-transfected HEK293 cells with constructs encoding untagged TRUSS and HA-tagged TRPC channels. The cells were then lysed, immunoprecipitated with anti-TRUSS antibody, separated by SDS–PAGE and co-immunoprecipitating TRPC channels were detected by immunoblotting with anti-HA antibody. Figure 1A (upper parts) shows that TRPC1, 4, and 5 were specifically co-immunoprecipitated with TRUSS, whereas TRPC3, 6, and 7 were not. To confirm these findings we performed the experiments in the reverse direction. Figure 1B (upper parts) shows that TRUSS co-immunoprecipitated with TRPC1, 4, and 5, but not TRPC3, 6, or 7 (Fig. 1B, lower parts). To verify specificity, all immunoprecipitations were performed in parallel with species-matched non-immune IgG (Fig. 1A,B). These data confirm that TRUSS associates specifically with the TRPC1, 4, and 5 subfamily of ion channels, while interactions with TRPC3, 6, and 7 were not detected above that seen with the non-immune IgG control.
Figure 1.
TRUSS (TRPC4AP) interacts with TRPC1, -C4, and -C5, but not TRPC3, -C6, or -C7 in intact cells. A: TRUSS was expressed with HA-tagged TRPC1, -C4, -C5 (top two panels), -C3, -C6, or -C7 (bottom two panels) in HEK293 cells, immunoprecipitated with anti-TRUSS antibody (TR), or rabbit non-immune IgG (rIgG) and immunoblotted with anti-HA (upper panels) or anti-TRUSS antibodies (lower panels). B: TRUSS was expressed with HA-tagged TRPC1, -C4, -C5 (top two panels), -C3, -C6, or -C7 (bottom two panels) in HEK293 cells, immunoprecipitated with anti-HA antibody (HA) or non-immune IgG (mIgG) and immunoblotted with anti-TRUSS (upper panels) or anti-HA antibodies (lower panels).
TNF-R1 associates with TRPC ion channels
We initially cloned TRUSS through its association with TNF-R1 (Soond et al., 2003, 2006). Since TRUSS associates with a sub-family of TRPC channels, and because TNF-R1 ligation augments GPCR-induced Ca2+ entry (Amrani et al., 1995; Tiruppathi et al., 2001), we used a co-immunoprecipitation approach to determine if HA-tagged TRPC channels also interact with FLAG-tagged TNF-R1. Figure 2 shows that TRPC1, 4, and 5 co-immunoprecipitated with TNF-R1, whereas TRPC6 did not. While TNF-R1 expression was below the level of detection in the WCL, it was enriched to equivalent levels upon immunoprecipitation. Control immunoprecipitations with non-immune goat IgG were performed in parallel and confirmed the specificity of the immunoprecipitations (Fig. 2). Thus, the TRPC1, 4, and 5 sub-family of TRPC channels are also capable of forming heteromeric protein complexes with TNF-R1.
Figure 2.
TNF-R1 interacts with TRPC1, -C4, and -C5, but not TRPC6. TNF-R1 was co-expressed with HA-tagged TRPC1, -C4, -C5, or C6 in HEK293 cells and TNF-R1 was immunoprecipitated as discussed in Methods Section with a goat-anti-TNF-R1 antibody or non-immune IgG (IgG). The immunoprecipitates and post-nuclear supernatants (PNS) were separated by SDS–PAGE and subjected to Western blotting with anti-HA (upper panels) or anti-FLAG antibodies (lower panels).
m1AchR-induced reduction in endoplasmic reticulum store Ca2+ content is reversed by co-expression of TNF-R1, TRUSS, and TRPC1 or 4
Next, we investigated the roles of TRUSS, TNF-R1, and TRPC channels in GPCR-induced increases in Ca2+ entry. Cch, a well-characterized agonist for muscarinic acetylcholine receptors (mAchR), signals via Gαq/11 to generate IP3 from phosphatidylinositol-4,5-bisphosphate. IP3 initiates a rapid, transient increase in cytoplasmic Ca2+ following Ca2+ release from the endoplasmic reticulum Ca2+ stores. In turn, the depletion of endoplasmic reticulum Ca2+ stores initiates store-operated Ca2+ entry by Ca2+ channels located at the plasma membrane (Parekh and Putney, 2005). To investigate the function of TRUSS, TNF-R1, and TRPC channels in m1AchR-induced Ca2+ entry, we initially measured changes in [Ca2+]i in response to stimulation of HEK293 cells with the m1AchR agonist, Cch. Since the HEK293 cells responded poorly to Cch stimulation, we transfected HEK293 cells with an m1AchR expression construct. Under these conditions, Cch-induced an increase in [Ca2+]i that was observed in m1AchR-transfected cells but not in cells transfected with the control empty pcDNA3.1 (pcD) (Fig. 3A) indicating that the transfected receptor was functional. As the endoplasmic reticulum is a pivotal organelle for Ca2+ signaling, we next applied the Ca2+ ionophore, ionomycin, to m1AchR and control pcD-transfected cells to pharmacologically induce maximal release of Ca2+ from the endoplasmic reticulum and thereby assess how m1AchR expression affected endoplasmic reticulum Ca2+ content. Figure 3B,C shows that expression of m1AchR significantly decreased ionomycin-elicited Ca2+ release (40.3 ± 4.3%, P < 0.0001) compared to pcD control, suggesting that the transfected m1AchR was capable of signaling a reduction in endoplasmic reticulum Ca2+ store content. To confirm this finding, we loaded cells with Mag-Fura 2AM to directly measure endoplasmic reticulum Ca2+ content in m1AchR versus control pcD cells (Hofer and Machen, 1993; Hofer et al., 1995; Hofer and Schulz, 1996). Figure 3D shows that when Mag-Fura 2 was released by permeabilization with digitonin, control cells transfected with vector only (pcD) had a higher basal level of [Ca2+]i compared to cells transfected with m1AchR, indicating that the expression of m1AchR reduced the Ca2+ content of the endoplasmic reticulum. Figure 3D,E also shows that the change in fluorescence after the addition of ionomycin was significantly decreased by 32.6 ± 4.8% (P < 0.001) in cells expressing m1AchR compared to control cells transfected with empty vector (pcD), consistent with the measurements made using Fura-2 (Fig. 3B,C). The mechanism of signaling by the transfected m1AchR is not known and could involve ligand-independent signaling as has been reported for overexpressed TNF-R1 (Wajant et al., 2003). Alternatively, it is possible that the depletion of endoplasmic reticulum Ca2+ stores in m1AchR-expressing cells is initiated by m1AchR partial agonists, such as choline, that are present in tissue culture medium (Carriere and El-Fakahany, 2000). Consistent with either possibility, we also observed that m1AchR-transfected HEK293 cells exhibited increased extracellular regulated kinase (ERK)-phosphorylation (Fig. 3F). Collectively, these experiments identify a novel m1AchR-induced effect on endoplasmic reticulum Ca2+ storage and provide a system with which we evaluated the functions of TRUSS, TNF-R1, and TRPC channels in GPRC-induced Ca2+ signaling.
Figure 3.
A: Transfection of m1AchR into HEK293 cells increases intracellular Ca2+ ([Ca2+]i) following stimulation with carbachol (50 µM). B: Expressionofm1AchRdecreasesionomycin-sensitiveendoplasmic reticulum Ca2+ stores. C: Quantification of (B) where bars represent the mean areas under the curves, normalized to pcD ± SEM; ***P < 0.0001. D: Confirmation that expression of m1AchR decreases the Ca2+ content of endoplasmic reticulum Ca2+ stores as measured with Mag-Fura 2. E: Quantification of (D) showing the change in fluorescence after stimulation with ionomycin, normalized to pcD. Data shown are the mean ± SEM; ***P < 0.0001. F: Transfection of m1AchR into HEK293 cells promotes ERK phosphorylation. HEK293 were transfected with empty vector (pcD), or m1AchR (m1) for 24 h. Total ERK and phospho-ERK were quantified by Western blotting of whole cell lysates.
Enforced expression of TNF-R1 has previously been shown to activate signaling in the absence of TNF-α ligand (Wajant et al., 2003). Therefore, we determined the role of TRUSS, TNF-R1, and TRPC on the m1AchR signaling-induced reduction in endoplasmic reticulum Ca2+ content by enforced expression of m1AchR in combinations with TNF-R1, TRUSS, and TRPC4. Figure 4A,B shows that co-expression of m1AchR singly with TRPC1 (C1), TRPC4 (C4), TRUSS (TR), or TNF-R1 (R1) did not alter the m1AchR (m1)-induced reduction in endoplasmic reticulum Ca2+ content. Similarly, co-expression of m1AchR with TRPC4 or the related TRPC1 (C1) plus TRUSS did not alter ionomycin-elicited endoplasmic reticulum Ca2+ release (Fig. 4C,D). However, when TNF-R1 was co-expressed with TRPC1, TRPC4, or TRUSS, the m1AchR-induced reduction in endoplasmic reticulum Ca2+ content was significantly reversed (P < 0.05, Fig. 4C,D). Maximal reversal of m1AchR-dependent endoplasmic reticulum Ca2+ storage depletion was achieved when m1AcR was co-expressed together with either TNF-R1 and TRUSS, or with the combination of TNF-R1, TRPC4 (or TRPC1), and TRUSS, (P < 0.0001) (Fig. 4E,F). As a negative control, we co-expressed combinations of m1AchR, TNF-R1, TRUSS, and TRPC4 with the unrelated vesicular stomatitis virus glycoprotein (VSV-G) and found that the expression of this protein with m1AchR, TRUSS, and TRPC4 did not elevate the intracellular Ca2+ content above that of m1AchR alone (Fig. 4G,H). In addition, VSV-G did not act synergistically with TNF-R1 to elevate endoplasmic reticulum Ca2+ storage in the presence of m1AchR (Fig. 4G,H) suggesting that m1AchR specifically signals a reduction in endoplasmic reticulum Ca2+ content and that TRPC1, TRPC4, and TRUSS act together with TNF-R1 to reverse this response and promote endoplasmic reticulum Ca2+ filling.
Figure 4.
m1AchR decreases basal endoplasmic reticulum Ca2+ and co-expression of TNF-R1, TRUSS, and TRPC1 or TRPC4 reverses this response. The fluorescence ratios (340/380) of Fura-2 AM-loaded HEK293 cells expressing the indicated combinations of pcD, m1AchR, TRUSS, TRPC4, TNF-R1, or VSV-G was determined. Parts (A,C,E,G) show representative fluorescence 340/380 ratio measurements of the indicated transfection conditions. Parts (B,D,F,H) show quantification of the ionomycin responses in Panels (A,C,E,G) where bars represent the mean area under the curves, normalized to pcD ± SEM. B: Comparison to pcD, ***P < 0.0001; (D) comparison to m1AchR, ***P < 0.0001; **P < 0.01, and *P < 0.05; (F) comparison to m1AchR, ***P < 0.0001; (H) comparison to pcD, ***P < 0.0001.
To verify: (i) the specificity of the reduced Ca2+ content induced by m1AchR expression, and (ii) the specificity of TNF-R1, TRUSS, and TRPC channels in elevating endoplasmic reticulum Ca2+ store content, we examined ionomycin responses to the related Gαq/11 coupled muscarinic acetylcholine receptor, m3AchR. Ionomycin evoked maximal responses in all cells expressing pcD and combinations of TRPC, TRUSS, and TNF-R1 (Fig. 5A,D). m1AchR expression resulted in the expected reduction in endoplasmic reticulum Ca2+ store content (Fig. 5B,D) and TNF-R1 together with TRUSS and TRPC4 co-expression significantly relieved the m1AchR-dependent reduction in endoplasmic reticulum Ca2+ store content (P < 0.01) (Fig. 5B,D). In contrast, endoplasmic reticulum Ca2+ store content was not significantly altered in m3AchR-expressing cells compared to pcD control conditions. In addition, co-expression of TNF-R1, with TRUSS and TRPCs did not significantly change these values (P > 0.05) (Fig. 5C,D). Dose response experiments were performed to ensure that the alteration in endoplasmic reticulum Ca2+ content observed between m1AchR and m3AchR expression were not due to differences in receptor expression levels. Figure 5E shows that even at the lowest concentration of m1AchR plasmid (0.25 µg), m1AchR elicited an ~40% reduction in endoplasmic reticulum Ca2+ content. In contrast, expression of m3AchR, between 0.25 and 2 µg, did not alter endoplasmic reticulum Ca2+ content compared to pcD control cells. Attempts to quantify the level of expression of the transfected receptor by Western blotting were unsuccessful. These data suggest that expression of m1AchR, but not the m3AchR, activates a distinct signal that reduces endoplasmic reticulum Ca2+ store content. In the absence of m1AchR, that is, when endoplasmic reticulum Ca2+ stores are at their normal filling capacity, TNF-R1, TRUSS, and TRPCs do not further enhance endoplasmic reticulum Ca2+ storage. Taken together, these findings suggest that TRUSS, TNF-R1, and TRPC4 (or TRPC1) interact to form a signaling complex that stimulates increased endoplasmic reticulum Ca2+ content in response to m1AchR signaling.
Figure 5.
m1AchR, but not m3AchR, reduces basal endoplasmic reticulum Ca2+ levels and reveals TNF-R1, TRUSS, TRPC-dependent enhanced endoplasmic reticulum Ca2+ storage. Representative fluorescence ratios (340/380) of Fura-2-loaded HEK293 cells expressing combinations of TRUSS, TRPC4, or TNF-R1 was determined with: (A) pcD, (B) m1AchR, or (C) m3AchR. D: Quantification of the ionomycin-induced responses from panels (A–C). Bars represent the mean area under the curve, normalized to pcD control ± SEM, aP > 0.05 compared to pcD; bP < 0.0001 compared to pcD; cP < 0.01 compared to m1AchR. E: Quantification of the ionomycin-induced endoplasmic reticulum Ca2+ release from Fura-2 AM loaded cells transfected with 0.25–2.0 µg of m1AchR or m3AchR plasmid. Bars represent the mean area under the curve, normalized to pcD (dotted line) ± SEM.
To further explore this notion, we investigated the ability of TNF-R1, TRUSS, and TRPC4 to form heteromeric signaling complexes. In addition, since these molecules contribute to the reversal of the m1AchR-induced reduction in endoplasmic reticulum Ca2+ content, we questioned if expression of the m1AchR affected complex formation. To address these questions, we first transfected HEK293 cells with constructs encoding FLAG-tagged TNF-R1, and HA-tagged TRPC4 in the presence and absence of untagged TRUSS. Complex formation was detected by immunoprecipitation with anti-TNF-R1 antibody followed by Western blotting with anti-HA, anti-TRUSS, and anti-FLAG antibodies. Figure 6A shows that a heteromeric protein complex composed of TNF-R1, TRPC4, and TRUSS was formed in cells transfected with constructs encoding all three proteins. However, in the absence of transfected TRUSS, complex formation between TNF-R1 and TRPC4 was still detected suggesting that the bimolecular interaction between each partner contributes to the formation of the trimolecular TNF-R1–TRPC4–TRUSS complex. Figure 6B shows that expression of TNF-R1, TRPC4, and TRUSS in the presence of m1AcR did not affect these interactions.
Figure 6.
TRUSS, TNF-R1, and TRPC4 associate to form a trimolecular complex in the presence and absence of m1AchR. A: HEK 293 cells were co-transfected with FLAG-tagged TNF-R1 and HA-tagged TRPC4 in the presence and absence of TRUSS. Cell lysates were immunoprecipitated with anti-TNF-R1 antibody and TNF-R1-associated TRPC4 and TRUSS detected by immunoblotting with anti-HA and anti-TRUSS C-terminal antibodies, respectively. B: Complex formation between TNF-R1, TRPC4, and TRUSS was determined in the presence of transfected m1AchR using the same approach to that shown in panel (A).
M1AchR impairs store-operated Ca2+ entry while expression of TNF-R1, TRUSS, and TRPCs enhances store-operated Ca2+ entry
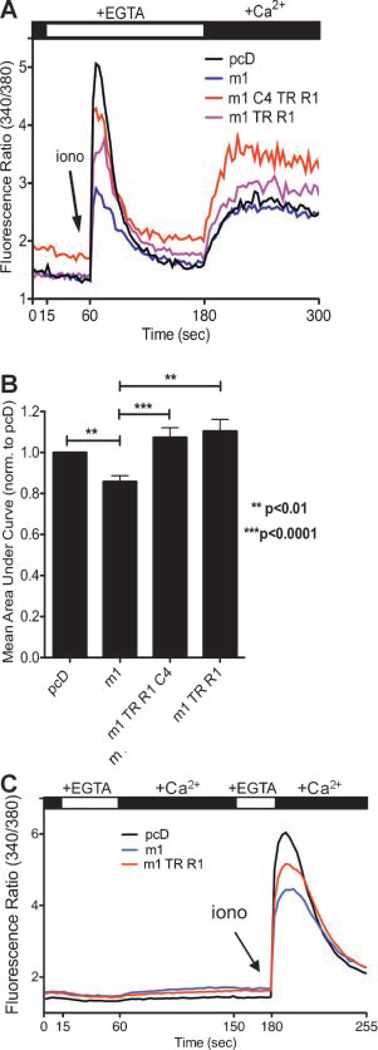
Brandman et al. (2007) have shown that basal store-operated Ca2+ entry feeds Ca2+ into the endoplasmic reticulum to maintain Ca2+ homeostasis. Recently, Sternfeld et al. (2007) reported that acetylcholine application acts through mAchRs to reduce store-operated Ca2+ entry in HEK293 cells. To determine if store-operated Ca2+ entry is altered among cells expressing: (i) control pcD (ii) m1AchR alone, or the combination of m1AchR with either (iii) TRUSS, TNF-R1, and TRPC4 or (iv) TRUSS and TNF-R1, we first depleted endoplasmic reticulum Ca2+ stores with ionomycin (1.25 µM) in Ca2+ free buffer-containing EGTA and then added Ca2+ back to the extracellular medium to reveal and quantify store-operated Ca2+ entry, as described (Kwan et al., 1990). Figure 7A,B shows that m1AchR-expressing cells exhibited a significant decrease (P < 0.01) in store-operated Ca2+ entry compared to pcD control cells. These data confirm the findings of Sternfeld et al. (2007) and support a model wherein the m1AchR-dependent decrease in basal store-operated Ca2+ entry contributes to the observed reduction in endoplasmic reticulum Ca2+ content. Next, we investigated the role of store-operated Ca2+ entry in reversing the reduction in endoplasmic reticulum Ca2+ content. We observed that transfection of m1AchR (m1), TRUSS (TR), TNF-R1 (R1), and TRPC4 (C4) or m1AchR, TRUSS, and TNF-R1 resulted in a significant increase (P < 0.0001 and P < 0.01, respectively) in store-operated Ca2+ entry compared to cells expressing m1AchR alone (Fig. 7A,B). These data suggest that in the context of m1AchR signaling, TNF-R1, TRUSS, and TRPCs elevate store-operated Ca2+ entry to promote endoplasmic reticulum Ca2+ filling.
Figure 7.
m1AchR impairs store-operated Ca2+ entry in HEK293 cells, while co-expression of TNF-R1, TRUSS, and TRPC4 enhances store-operated Ca2+ entry. A: Representative fluorescence ratios (340/380) of Fura-2 AM-loaded HEK293 expressing the indicated proteins. B: Quantification of responses from panel (A). Bars represent the mean area under the curve normalized to pcD ± SEM; **P < 0.01 and ***P < 0.0001. C: The fluorescence ratios (340/380) of Fura-2 AM loaded HEK293 cells expressing pcD, m1AchR (m1) or the combination of m1AchR (m1) TRUSS (TR), and TNF-R1 (R1).
We performed control experiments to determine if the cells exhibited general alterations in Ca2+ permeability that were not attributable to store-operated Ca2+ entry. In Figure 7C extracellular Ca2+ was acutely removed by the addition of EGTA, then extracellular Ca2+ was added back to evaluate if Ca2+ permeability was altered among the conditions examined. Figure 6C shows that none of the conditions (pcD, m1AcR alone or m1AchR (m1), TRUSS (TR), and TNF-R1 (R1)-transfected cells) resulted in changes in Ca2+ permeability following this protocol of extracellular Ca2+ depletion and readdition. The subsequent ionomycin application demonstrates that the cells were properly transfected and Fura-2 AM loaded. These data suggest that the transfection conditions do not alter basal Ca2+ permeability, and further support the interpretation that alterations in store-operated Ca2+ entry contribute to the changes in endoplasmic reticulum Ca2+ storage observed with m1AchR, TNF-R1, TRUSS, and TRPC4.
Discussion
Synergistic interactions between the pro-inflammatory cytokine TNF-α and the GPCR-agonists bradykinin and thrombin have been shown to play an important role in breaches in lung microvascular endothelial cell barrier function and airway smooth muscle cell hyperplasia and contraction through augmentation of GPCR-induced store-operated Ca2+ entry (Amrani et al., 1995; Tiruppathi et al., 2001). Little is known about how TNF-α signaling, acting through the receptor TNF-R1, intersects with GPCR-induced Ca2+ entry. In this study, we investigated: (i) the ability of TNF-R1 and its signaling adaptor TRUSS to interact with members of the Ca2+-permeable TRPC family; and (ii) the consequences of TRUSS, TNF-R1, and TRPC4 expression on GPCR-induced filling of endoplasmic reticulum Ca2+ stores and store-operated Ca2+ entry using m1 muscarinic acetylcholine receptor (m1AchR)-induced endoplasmic reticulum Ca2+ depeletion as a model. For the first time, we show that both TRUSS and TNF-R1 specifically associate with the TRPC1, 4, and 5 sub-family of TRPC channels. In addition, we show TNF-R1, TRUSS, and TRPC channels influence Ca2+ homeostasis by altering store-operated Ca2+ entry that contributes to setting the basal Ca2+ concentration within the endoplasmic reticulum (Brandman et al., 2007).
Using a transient expression and co-immunoprecipitation approach, TRUSS was found to form heteromeric protein complexes with TRPC1, 4, and 5, but not with TRPC3, 6, or 7. In addition, TRPC1, 4, and 5 were found to specifically associate with TNF-R1. This pattern of specificity among protein interaction partners is noteworthy as the endoplasmic reticulum Ca2+ sensor and master regulator of store-operated Ca2+ entry, STIM1, also interacts with TRPC1, 4, and 5, but not with TRPC3, 6, or 7 (Huang et al., 2006). Interestingly, TNF-R1, TRUSS, and TRPC4 were found to associate and form a trimolecular heteromeric complex. However, the formation of this complex was not affected by the presence of the m1AchR. Collectively, these findings suggest that distinct protein binding partners between these two sub-families of TRPCs may contribute to functional regulation of the channels. Our data also suggest that TRUSS, TNF-R1, and a sub-family of TRPC channels may function together to control biological Ca2+ responses.
We investigated the role of TRUSS, TNF-R1, and TRPC channels in GPCR-induced Ca2+ signaling using the m1AchR as a representative Gαq/11-coupled GPCR. In the presence of m1AchR-induced endoplasmic reticulum Ca2+ depletion, TNF-R1 appeared to be an essential factor to confer an increase in endoplasmic reticulum Ca2+ storage since increased endoplasmic reticulum Ca2+ storage was only observed with TNF-R1 co-expression with either TRUSS, TRPC1, TRPC4, or both, but not in the absence of TNF-R1. While this system is potentially confounded by the expression of endogenous TRUSS and TRPC channels (Riccio et al., 2002 and K.E. Mace, J.L. Terry Powers, and D.W.H. Riches, unpublished observations), we propose a model where protein complexes composed of TNF-R1, TRUSS, and TRPC1 or 4 mediate an increase in store-operated Ca2+ entry in the presence of an m1AchR-dependent reduction in basal endoplasmic reticulum Ca2+ content. Our data are consistent with such a model where the transfection of exogenous components shifts the balance of protein complex formation to promote the observed alterations in Ca2+ signaling. Intriguingly, we also found that neither endoplasmic reticulum Ca2+ storage capacity nor store-operated Ca2+ entry was enhanced by TNF-R1, TRUSS, and TRPC expression in the absence of m1AchR (data not shown), suggesting that the ability of TNF-R1, TRUSS, and TRPCs to alter Ca2+ signaling may be context dependent. The dependency on m1AchR for revealing this phenomenon might contribute to the differential susceptibility of various cell types to increases in Ca2+ signaling after exposure to TNF-α. However, our data do not exclude the possibility that m1AchR activates a common signaling pathway to alter store-operated Ca2+ entry. Thus, exclusive reliance on m1AchR activation for the TNF-R1, TRUSS, and TRPC-dependent enhancement of store-operated Ca2+ entry and subsequent endoplasmic reticulum Ca2+ homeostasis might not be necessary.
We have also demonstrated that reductions in store-operated Ca2+ entry by m1AchR expression correlated with decreased endoplasmic reticulum Ca2+ storage capacity. These data are interesting considering recent findings that link basal store-operated Ca2+ entry with homeostatic maintenance of endoplasmic reticulum Ca2+ content, a phenomenon dependent on STIM2, which serves as a sensitive sensor to activate Ca2+ influx upon small decreases in endoplasmic reticulum Ca2+ (Brandman et al., 2007). Our data substantiate recent findings showing that acetylcholine impairs store-operated Ca2+ entry in HEK293 through a mechanism involving the activation of m1AchR (Sternfeld et al., 2007). Our work expands on this to suggest that endoplasmic reticulum homeostasis is directly influenced by m1AchR-mediated changes in store-operated Ca2+ entry. It is unclear how m1AchR expression impairs store-operated Ca2+ entry and Ca2+ accumulation in the endoplasmic reticulum. One possibility is that mere over-expression of m1AchR is sufficient to promote signaling in a ligand-independent fashion, as has been seen with TNF-R1 (Wajant et al., 2003). Alternatively, transfected m1AcR could bind a ligand present in the tissue medium, which in turn, promotes receptor signaling. For example, choline, a component of tissue culture medium used in our study, has been shown to activate signaling by m1AchR (Carriere and El-Fakahany, 2000). Regardless of the mechanism, our data, together with those of Sternfeld et al. (2007) suggest that ligand-induced activation of endogenous m1AchR might have the capacity to alter both store-operated Ca2+ entry and endoplasmic reticulum Ca2+ content.
In a broader setting, our findings may also have potentially important implications for Alzheimer’s disease. Impaired Ca2+ signaling in neurons is a hallmark of Alzheimer’s disease and proteins linked to this disease either promote over-filling or depletion of endoplasmic reticulum Ca2+ stores (Ito et al., 1994; Bezprozvanny and Mattson, 2008). In addition, cholinergic neurodegeneration occurs early in Alzheimer’s disease and attempts to improve cholinergic neuronal function has spawned a significant interest in generating therapeutic m1AchR agonistic drugs (Langmead et al., 2008). Our finding that TNF-R1, TRUSS, and TRPC proteins increase endoplasmic reticulum Ca2+ storage raise the intriguing possibility that disruption of TRUSS, TNF-R1, and TRPC signaling may contribute to the impaired Ca2+ signaling seen in patients with Alzheimer’s disease. In support of this possibility, a recent genome-wide association study revealed an association between several single nucleotide polymorphisms in the coding sequence of TRUSS and increased susceptibility to the development of late-onset Alzheimer’s disease (Poduslo et al., 2009a,b). Furthermore, TNF-R1 deficient mice have been shown to be resistant to the development of neurodegeneration in an amyloid-dependent model of Alzheimer’s disease (He et al., 2007). Together with the results of our study, these findings suggest that links between TNF-R1 and Ca2+ signaling may have broad significance in health and disease.
In summary, the findings presented herein suggest that TRUSS and TRPC1, 4, and 5 are essential components of a signaling complex that serves to link TNF-R1 signaling to increased loading of endoplasmic reticulum Ca2+ stores due to increased store-operated Ca2+ entry in the context of m1AchR-receptor signaling. Future studies in mice bearing a targeted disruption of the TRUSS gene are expected to provide novel insights into the physiologic role of TRUSS in the TNF-R1-induced augmentation of GPCR-stimulated Ca2+ entry.
Acknowledgments
We greatly appreciate the technical expertise of Linda Remigio and Ben Edelman. We also wish to thank Dr. Mark Dell’Acqua, University of Colorado School of Medicine, Aurora, CO for providing the m1AchR and m3AchR expression plasmids. This work was supported by United States Public Health Service grants HL55549, HL68628 from the National Heart Lung and Blood Institute and AI70941 from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health. Kimberly E. Mace was supported in part by T32 training grant AI07405 from the National Institute of Allergy and Infectious Diseases.
Abbreviations
- TNF-α
tumor necrosis factor-α
- TNF-R1
tumor necrosis factor receptor-1
- TRUSS
TNF-R1 ubiquitous scaffolding signaling protein
- TRPC
transient receptor potential canonical
- TRPC4AP
TRPC4-associated protein
- mAchR
muscarinic acetylcholine receptor
- Cch
carbachol
- ERK
extracellular regulated kinase
- IP3
inositol 1, 4, 5-trisphosphate
- IP3R
IP3 receptor
- pcD
pcDNA3.1 transfection control
- VSV-G
vesicular stomatitis virus glycoprotein
- NF-κB
nuclear factor-κB
- JNK
c-Jun-NH2-terminal kinase
- [Ca2+]i
concentration of intracellular Ca2+.
Literature Cited
- Amrani Y, Martinet N, Bronner C. Potentiation by tumour necrosis factor-alpha of calcium signals induced by bradykinin and carbachol in human tracheal smooth muscle cells. Br J Pharmacol. 1995;114:4–5. doi: 10.1111/j.1476-5381.1995.tb14896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrani Y, Panettieri RA, Jr, Frossard N, Bronner C. Activation of the TNF alpha-p55 receptor induces myocyte proliferation and modulates agonist-evoked calcium transients in cultured human tracheal smooth muscle cells. Am J Respir Cell Mol Biol. 1996;15:55–63. doi: 10.1165/ajrcmb.15.1.8679222. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I, Mattson MP. Neuronal calcium mishandling and the pathogenesis of Alzheimer’s disease. Trends Neurosci. 2008;31:454–463. doi: 10.1016/j.tins.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird GS, Aziz O, Lievremont JP, Wedel BJ, Trebak M, Vazquez G, Putney JW., Jr Mechanisms of phospholipase C-regulated calcium entry. Curr Mol Med. 2004;4:291–301. doi: 10.2174/1566524043360681. [DOI] [PubMed] [Google Scholar]
- Brandman O, Liou J, Park WS, Meyer T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell. 2007;131:1327–1339. doi: 10.1016/j.cell.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriere JL, El-Fakahany EE. Choline is a full agonist in inducing activation of neuronal nitric oxide synthase via the muscarinic M1 receptor. Pharmacology. 2000;60:82–89. doi: 10.1159/000028351. [DOI] [PubMed] [Google Scholar]
- Chen G, Goeddel DV. TNF-R1 signaling: A beautiful pathway. Science. 2002;296:1634–1635. doi: 10.1126/science.1071924. [DOI] [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- Freichel M, Suh SH, Pfeifer A, Schweig U, Trost C, Weissgerber P, Biel M, Philipp S, Freise D, Droogmans G, Hofmann F, Flockerzi V, Nilius B. Lack of an endothelial store-operated Ca2+ current impairs agonist-dependent vasorelaxation in TRP4−/− mice. Nat Cell Biol. 2001;3:121–127. doi: 10.1038/35055019. [DOI] [PubMed] [Google Scholar]
- He P, Zhong Z, Lindholm K, Berning L, Lee W, Lemere C, Staufenbiel M, Li R, Shen Y. Deletion of tumor necrosis factor death receptor inhibits amyloid beta generation and prevents learning and memory deficits in Alzheimer’s mice. J Cell Biol. 2007;178:829–841. doi: 10.1083/jcb.200705042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer AM, Machen TE. Technique for in situ measurement of calcium in intracellular inositol 1,4,5-trisphosphate-sensitive stores using the fluorescent indicator mag-fura-2. Proc Natl Acad Sci USA. 1993;90:2598–2602. doi: 10.1073/pnas.90.7.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer AM, Schulz I. Quantification of intraluminal free [Ca] in the agonist-sensitive internal calcium store using compartmentalized fluorescent indicators: Some considerations. Cell Calcium. 1996;20:235–242. doi: 10.1016/s0143-4160(96)90029-9. [DOI] [PubMed] [Google Scholar]
- Hofer AM, Schlue WR, Curci S, Machen TE. Spatial distribution and quantitation of free luminal [Ca] within the InsP3-sensitive internal store of individual BHK-21 cells: Ion dependence of InsP3-induced Ca release and reloading. FASEB J. 1995;9:788–798. doi: 10.1096/fasebj.9.9.7601343. [DOI] [PubMed] [Google Scholar]
- Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, Worley PF. STIM1 carboxyl-terminus activates native SOC, I(crac) and TRPC1 channels. Nat Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- Ito E, Oka K, Etcheberrigaray R, Nelson TJ, McPhie DL, Tofel-Grehl B, Gibson GE, Alkon DL. Internal Ca2+ mobilization is altered in fibroblasts from patients with Alzheimer disease. Proc Natl Acad Sci USA. 1994;91:534–538. doi: 10.1073/pnas.91.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan CY, Takemura H, Obie JF, Thastrup O, Putney JW., Jr Effects of MeCh, thapsigargin, and La3+ on plasmalemmal and intracellular Ca2+ transport in lacrimal acinar cells. Am J Physiol. 1990;258:C1006–C1015. doi: 10.1152/ajpcell.1990.258.6.C1006. [DOI] [PubMed] [Google Scholar]
- Langmead CJ, Watson J, Reavill C. Muscarinic acetylcholine receptors as CNS drug targets. Pharmacol Ther. 2008;117:232–243. doi: 10.1016/j.pharmthera.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Cheng KT, Bandyopadhyay BC, Pani B, Dietrich A, Paria BC, Swaim WD, Beech D, Yildrim E, Singh BB, Birnbaumer L, Ambudkar IS. Attenuation of store-operated Ca2+ current impairs salivary gland fluid secretion in TRPC1(−/−) mice. Proc Natl Acad Sci USA. 2007;104:17542–17547. doi: 10.1073/pnas.0701254104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: Integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- Lussier MP, Cayouette S, Lepage PK, Bernier CL, Francoeur N, St-Hilaire M, Pinard M, Boulay G. MxA, a member;1;1; of the dynamin superfamily, interacts with the ankyrin-like repeat domain of TRPC. J Biol Chem. 2005;280:19393–19400. doi: 10.1074/jbc.M500391200. [DOI] [PubMed] [Google Scholar]
- Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- Peschon JJ, Torrance DS, Stocking KL, Glaccum MB, Otten C, Willis CR, Charrier K, Morrissey PJ, Ware CB, Mohler KM. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J Immunol. 1998;160:943–952. [PubMed] [Google Scholar]
- Poduslo SE, Huang R, Huang J. The frequency of the TRPC4AP haplotype in Alzheimer’s patients. Neurosci Lett. 2009a;450:344–346. doi: 10.1016/j.neulet.2008.11.050. [DOI] [PubMed] [Google Scholar]
- Poduslo SE, Huang R, Huang J, Smith S. Genome screen of late-onset Alzheimer’s extended pedigrees identifies TRPC4AP by haplotype analysis. Am J Med Genet B Neuropsychiatr Genet. 2009b;150:50–55. doi: 10.1002/ajmg.b.30767. [DOI] [PubMed] [Google Scholar]
- Putney JW., Jr Recent breakthroughs in the molecular mechanism of capacitative calcium entry (with thoughts on how we got here) Cell Calcium. 2007;42:103–110. doi: 10.1016/j.ceca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio A, Medhurst AD, Mattei C, Kelsell RE, Calver AR, Randall AD, Benham CD, Pangalos MN. mRNA distribution analysis of human TRPC family in CNS and peripheral tissues. Brain Res Mol Brain Res. 2002;109:95–104. doi: 10.1016/s0169-328x(02)00527-2. [DOI] [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rual JF, Venkatesan K, Hao T, Hirozane-Kishikawa T, Dricot A, Li N, Berriz GF, Gibbons FD, Dreze M, Ayivi-Guedehoussou N, Klitgord N, Simon C, Boxem M, Milstein S, Rosenberg J, Goldberg DS, Zhang LV, Wong SL, Franklin G, Li S, Albala JS, Lim J, Fraughton C, Llamosas E, Cevik S, Bex C, Lamesch P, Sikorski RS, Vandenhaute J, Zoghbi HY, Smolyar A, Bosak S, Sequerra R, Doucette-Stamm L, Cusick ME, Hill DE, Roth FP, Vidal M. Towards a proteome-scale map of the human protein-protein interaction network. Nature. 2005;437:1173–1178. doi: 10.1038/nature04209. [DOI] [PubMed] [Google Scholar]
- Soond SM, Terry JL, Colbert JD, Riches DW. TRUSS, a novel tumor necrosis factor receptor 1 scaffolding protein that mediates activation of the transcription factor NF-kappaB. Mol Cell Biol. 2003;23:8334–8344. doi: 10.1128/MCB.23.22.8334-8344.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soond SM, Terry JL, Riches DW. TRUSS, a tumor necrosis factor receptor-1-interacting protein, activates c-Jun NH(2)-terminal kinase and transcription factor AP-1. FEBS Lett. 2006;580:4591–4596. doi: 10.1016/j.febslet.2006.06.098. [DOI] [PubMed] [Google Scholar]
- Sternfeld L, Dudenhoffer M, Ludes A, Heinze D, Anderie I, Krause E. Activation of muscarinic receptors reduces store-operated Ca2+ entry in HEK293 cells. Cell Signal. 2007;19:1457–1464. doi: 10.1016/j.cellsig.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Tiruppathi C, Naqvi T, Sandoval R, Mehta D, Malik AB. Synergistic effects of tumor necrosis factor-alpha and thrombin in increasing endothelial permeability. Am J Physiol Lung Cell Mol Physiol. 2001;281:L958–L968. doi: 10.1152/ajplung.2001.281.4.L958. [DOI] [PubMed] [Google Scholar]
- Tiruppathi C, Freichel M, Vogel SM, Paria BC, Mehta D, Flockerzi V, Malik AB. Impairment of store-operated Ca2+ entry in TRPC4(−/−)mice interferes with increase in lung microvascular permeability. Circ Res. 2002a;91:70–76. doi: 10.1161/01.res.0000023391.40106.a8. [DOI] [PubMed] [Google Scholar]
- Tiruppathi C, Minshall RD, Paria BC, Vogel SM, Malik AB. Role ofCa2+ signaling in the regulation of endothelial permeability. Vascul Pharmacol. 2002b;39:173–185. doi: 10.1016/s1537-1891(03)00007-7. [DOI] [PubMed] [Google Scholar]
- Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang M, Abarbanell AM, Weil BR, Herrmann JL, Tan J, Novotny NM, Coffey AC, Meldrum DR. MEK mediates the novel cross talk betweenTNFR2and TGF-EGFR in enhancing vascular endothelial growth factor (VEGF) secretion from human mesenchymal stem cells. Surgery. 2009a;146:198–205. doi: 10.1016/j.surg.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Wang Y, Weil BR, Herrmann JL, Abarbanell AM, Tan J, Markel TA, Kelly ML, Meldrum DR. MEK, p38, and PI-3K mediate cross talk between EGFR and TNFR in enhancing hepatocyte growth factor production from human mesenchymal stem cells. Am J Physiol Cell Physiol. 2009b;297:C1284–C1293. doi: 10.1152/ajpcell.00183.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley PF, Zeng W, Huang GN, Yuan JP, Kim JY, Lee MG, Muallem S. TRPC channels as STIM1-regulated store-operated channels. Cell Calcium. 2007;42:205–211. doi: 10.1016/j.ceca.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol. 2007;9:636–645. doi: 10.1038/ncb1590. [DOI] [PMC free article] [PubMed] [Google Scholar]