Abstract
The IL-6/signal transducer and activator of transcription 3 (STAT3) pathway is a critical signaling pathway for colitis-associated colorectal cancer (CAC). Peroxisome proliferator-activated receptor (PPAR)-δ, a lipid nuclear receptor, up-regulates IL-6. 15-Lipoxygenase-1 (15-LOX-1), which is crucial to production of lipid signaling mediators to terminate inflammation, down-regulates PPAR-δ. 15-LOX-1 effects on IL-6/STAT3 signaling and CAC tumorigenesis have not been determined. We report that intestinally targeted transgenic 15-LOX-1 expression in mice inhibited azoxymethane- and dextran sodium sulfate–induced CAC, IL-6 expression, STAT3 phosphorylation, and IL-6/STAT3 downstream target (Notch3 and MUC1) expression. 15-LOX-1 down-regulation was associated with IL-6 up-regulation in human colon cancer mucosa. Reexpression of 15-LOX-1 in human colon cancer cells suppressed IL-6 mRNA expression, STAT3 phosphorylation, IL-6 promoter activity, and PPAR-δ mRNA and protein expression. PPAR-δ overexpression in colonic epithelial cells promoted CAC tumorigenesis in mice and increased IL-6 expression and STAT3 phosphorylation, whereas concomitant 15-LOX-1 expression in colonic epithelial cells (15-LOX-1-PPAR-δ-Gut mice) suppressed these effects: the number of tumors per mouse (mean ± sem) was 4.22 ± 0.68 in wild-type littermates, 6.67 ± 0.83 in PPAR-δ-Gut mice (P = 0.026), and 2.25 ± 0.25 in 15-LOX-1-PPAR-δ-Gut mice (P = 0.0006). Identification of 15-LOX-1 suppression of PPAR-δ to inhibit IL-6/STAT3 signaling-driven CAC tumorigenesis provides mechanistic insights that can be used to molecularly target CAC.—Mao, F., Xu, M., Zuo, X., Yu, J., Xu, W., Moussalli, M. J., Elias, E., Li, H. S., Watowich, S. S., Shureiqi, I. 15-Lipoxygenase-1 suppression of colitis-associated colon cancer through inhibition of the IL-6/STAT3 signaling pathway.
Keywords: 15-LOX-1, PPAR-δ, CAC, IL-6 expression, STAT3 phosphorylation
Inflammatory bowel disease (IBD) affects an estimated 1–1.5 million Americans (1). IBD is associated with increased mortality risk because of colitis-associated colorectal cancer (CAC) (2, 3), which is responsible for an estimated 15% of deaths in patients with IBD (4). Whereas sporadic colorectal cancer arises in easily detectable colonic polyps, CAC arises in colitis-associated dysplasia that can be difficult to detect endoscopically, even with random colonic biopsies (5). New strategies are needed to prevent CAC and reduce its devastating morbidity and mortality in patients with IBD.
IL-6 is a major proinflammatory cytokine that plays an important role in CAC. IL-6 is up-regulated in patients with ulcerative colitis and contributes to colonic tumorigenesis in these patients (6–11). IL-6 binds to its membrane-bound receptor to activate signal transducer and activator of transcription 3 (STAT3) (11, 12). In cells without membrane-bound IL-6 receptors, IL-6 can bind to the soluble IL-6 receptor to form a complex that subsequently binds to another membrane receptor, gp130, to activate STAT3 (11, 12). STAT3 activation drives protumorigenic signaling (13) through transcription of various protumorigenic genes that promote malignant transformation (14) by enhancing cell survival, proliferation, tumor-mediated immunosuppression, angiogenesis, and invasion (15). Identification of IL-6/STAT3 signaling regulators is important not only to better understand the molecular mechanisms of colorectal tumorigenesis, including CAC, but more importantly, to identify new therapeutic and preventive molecular targets.
Human 15-lipoxygenase-1 (15-LOX-1) is a critical enzyme for production of lipid signaling mediators to actively terminate inflammation (e.g., lipoxins from arachidonic acid, resolvins from docosahexaenoic acid) (16, 17). 15-LOX-1 expression inhibits polymorphonuclear cell-mediated tissue destruction in rabbits (18) and glomerulonephritis in rats (19). However, studies of 12/15-LOX, the mouse homolog of human 15-LOX-1 with additional 12-LOX hybrid enzymatic activity, have demonstrated a complex relationship between 12/15-LOX and inflammation, suggesting both proinflammatory and anti-inflammatory roles (20). Therefore, the anti-inflammatory role of 15-LOX-1 has been questioned (21). Nonetheless, indirect evidence suggests that 15-LOX-1 inhibits CAC. For example, 15-LOX pharmaceutical inhibition exacerbated colitis in mice (22), and the major lipid product of 15-LOX-1, 13-hydroxyoctadecadienoic acid (13-S-HODE), suppresses peroxisome proliferator-activated receptor (PPAR)-δ, a lipid transcriptional receptor (23, 24) that promotes colitis and CAC through activation of various proinflammatory tumorigenic pathways, including IL-6 (25). Furthermore, 15-LOX-1 suppresses NF-κB signaling in intestinal epithelial cells (26), which promotes protumorigenic signaling via various downstream targets, including IL-6 (11, 12). Nevertheless, the direct effects of 15-LOX-1 on IL-6/STAT3 signaling and CAC remain poorly defined.
To gain further mechanistic insight into the regulation of IL-6/STAT3 signaling during CAC tumorigenesis, we examined the effects of 15-LOX-1 expression in colonic epithelial cells, which is commonly lost during colonic tumorigenesis (27), on CAC tumorigenesis, especially in relation to IL-6/STAT3 signaling.
MATERIALS AND METHODS
Cells, antibodies, and reagents
The human colorectal cancer cell lines HCT116 and LoVo were obtained from the American Type Culture Collection (Manassas, VA, USA).
The following commercially available monoclonal or polyclonal antibodies were used: anti-STAT3 (cat. no. 9132) and anti-phospho-STAT3 (anti-p-STAT3) (Tyr705; cat. no. 9145) from Cell Signaling Technology (Danvers, MA, USA), anti-IL-6 (cat. no. sc-1265R) and anti-human PPAR-δ (cat. no. sc-7197) from Santa Cruz Biotechnology (Dallas, TX, USA), anti-Ki-67 (cat. no. RM-9106) from Neomarkers (Fremont, CA, USA), and anti-mouse PPAR-δ (cat. no. ab8937) from Abcam (Cambridge, MA, USA). Polyclonal antibody against human 15-LOX-1 was obtained as described previously (23).
Modified Ad-hTert-15-LOX-1 (Ad-15-LOX-1), the control modified Ad-hTert-luciferase (Ad-luciferase), and the control modified Ad-hTert-GFP (Ad-GFP) adenoviral vectors were developed as described previously (24, 28). The probes for quantitative real-time RT-PCR (qRT-PCR) of human 15-LOX-1, IL-6, and PPAR-δ and mouse mucin 1 (MUC1), Notch3, IL-6, and PPAR-δ were purchased from Life Technologies (Grand Island, NY, USA). Mouse recombinant IL-6 (cat. no. 406-ML-005) was purchased from R&D Systems (Minneapolis, MN, USA). The Vectastain Elite ABC Kit (cat. no. PK-6101) and the Liquid DAB Substrate Chromogen System (cat. no. K346889-2) for immunohistochemistry staining were purchased from Vector Laboratories (Burlingame, CA, USA) and Dako (Carpinteria, CA, USA), respectively. The pGL3-IL-6 promoter vector and its control vector pGL3 were gifts from Dr. Chen Dong (University of Texas MD Anderson Cancer Center). Other reagents or chemicals were obtained as specified.
Genetic mouse models
We generated 15-LOX-1-Gut and PPAR-δ-Gut mice via pronuclear injection of a villin promoter-driven expression construct containing either human 15-LOX-1 cDNA or mouse PPAR-δ cDNA to target gene expression to mouse intestinal epithelial cells as described previously (26, 29). 15-LOX-1-Gut mice were bred with PPAR-δ-Gut mice to generate double heterozygous mice that carried both 15-LOX-1 and PPAR-δ genes (PPAR-δ-Gut(−/+)/15-LOX-1-Gut(−/+)), and then double heterozygous mice were bred to generate double homozygous mice (PPAR-δ-Gut(+/+)/15-LOX-1-Gut(+/+)). Mice were housed and bred in an animal facility accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care at MD Anderson Cancer Center. The mice were maintained on a 12-h-light/12-h-dark cycle. Experiments were conducted according to protocols approved by the MD Anderson Institutional Animal Care and Use Committee. The mice were treated in accordance with the U.S. Public Health Service Guide for the Care and Use of Laboratory Animals. For analgesia, buprenorphine was administered at a dose of 0.5–2.5 mg/kg of body weight subcutaneously every 6–12 hours.
Mouse genotyping
Genomic DNA was extracted from mouse tail snips and processed for measurement of genomic human 15-LOX-1 cDNA or mouse PPAR-δ cDNA by qPCR as previously described (26, 29).
Clinical samples
After institutional review board approvals were obtained, paired normal and cancerous colorectal biopsy specimens were obtained from colorectal cancer patients, as described previously (27).
Induction of CAC in mice
For induction of CAC, mice 6–8 weeks of age were injected intraperitoneally with either azoxymethane (AOM; Sigma-Aldrich, St. Louis, MO, USA) at 7.5 mg/kg. The mice received 3 cycles of 1.2% dextran sodium sulfate (DSS) (molecular weight, 36,000–50,000; MP Biomedicals, Santa Ana, CA, USA) in drinking water for 7 days, followed by 14 days of regular drinking water. Then the mice were killed, and colon tissue from each mouse was used for RNA, protein, and immunohistochemistry staining analyses. The entire intestinal tract was removed, washed in cold PBS, and processed to assess the tumor burden, as described previously (26, 29, 30).
Isolation of primary mouse colonic crypt cells and incubation with mouse recombinant IL-6
Primary mouse colonic crypt cells were isolated by enzymatic digestion with Dispase (BD Biosciences, Rockville, MD, USA) and collagenase (Sigma-Aldrich) as previously described (30). Isolated crypt cells were cultured with mouse recombinant IL-6 (50 ng/ml; R&D Systems) in RPMI 1640 medium with 1% bovine serum albumin for the indicated times and processed for RNA and protein expression analyses.
Cell culture and adenovirus infection
Colorectal cancer cells were cultured in McCoy medium (for HCT116 cells) or RPMI 1640 medium (for LoVo cells) supplemented with 10% fetal bovine serum, 2 mM l-glutamine, penicillin, and streptomycin (Life Technologies) in 5% CO2 at 37°C.
Caco-2 colon cancer cells stably transfected with 15-LOX-1 shRNA or a control vector were cultured for 14 days after confluence to induce intestinal cell differentiation as described previously (31). At the end of 14 days, cells were treated with either caffeic acid (2.2 µM; Cayman Chemical, Ann Arbor, MI, USA) or 13-S-HODE (13.5 µM; Cayman Chemical) as described previously (32) alone or in combination with IL-6 (50 ng/ml) 40 minutes prior to cell harvesting for protein expression analyses by Western blotting.
The cells were infected with either Ad-15-LOX-1 or Ad-luciferase at a ratio of 200 adenovirus particles per cell for HCT116 cells and 400 adenovirus particles per cell for LoVo cells in the specified cell culture medium supplemented with 1% fetal bovine serum. All in vitro studies were started at 50–70% cell density to avoid the effects of confluence. The cells were collected 48 hours after infection for RNA analysis and 72 hours for protein analysis.
Total RNA extraction and qRT-PCR
Total RNA was isolated using TRIzol reagent (Molecular Research Center, Cincinnati, OH, USA). RNA samples were quantified, and 500 ng of total RNA was reverse transcribed into cDNA using the iScript kit (Bio-Rad Laboratories, Hercules, CA, USA). qRT-PCR analyses were performed using the 7300 Real-Time PCR system (Applied Biosystems, Foster City, CA, USA) as previously described (33). Relative RNA expression levels were calculated using a comparative threshold cycle method (ddCt) (34).
Protein extraction and Western blot analysis
Cells were homogenized in lysis buffer [0.5% Nonidet P-40, 20 mM 3-(N-morpholino)propanesulfonic acid (pH 7.0), 2 mM EGTA, 5 mM EDTA, 30 mM sodium fluoride, 40 mM β-glycerophosphate, 2 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, and 1× complete protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN, USA)]. Fifty microgram samples of each protein were separated onto a 7.5% SDS polyacrylamide gel, and after electrophoresis, the proteins were transferred to nitrocellulose membranes. The membranes were blocked with 5% milk for 1 hour at room temperature and hybridized at 4°C overnight with anti-PPAR-δ, anti-15-LOX-1, anti-STAT3, or anti-STAT3 phosphorylated at Tyr-705 [p-STAT3(705)]. On the next day, the blots were hybridized with the secondary antibody for 1 hour at room temperature. The blots were analyzed using enhanced chemiluminescence (GE Healthcare, Piscataway, NJ, USA). ImageJ software (National Institutes of Health, Bethesda, MD, USA) was used to measure band densities of scanned blot images.
Analysis of IL-6 promoter activity
The IL-6 promoter reporter construct pGL3-IL-6 was made by PCR cloning the 1175 bp IL-6 promoter sequence upstream of the transcriptional start site into the SacI/HindIII sites of the pGL-3 vector (Promega, Madison, WI, USA). HCT116 and LoVo cells were seeded in 24-well plates at 80–90% confluence and then infected with either Ad-15-LOX-1 or Ad-GFP at a ratio of 200 adenovirus particles per cell for HCT116 cells or 400 adenovirus particles per cell for LoVo cells in the specified cell culture medium supplemented with 1% fetal bovine serum. Twenty-four hours later, the cells were transfected with either the pGL3-IL-6 promoter luciferase vector or the control pGL3 vector with the pSV-β-galactosidase vector (Promega) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). Luciferase activity was measured 24 hours after the second transfection, as described previously (23).
Immunohistochemistry staining
Three micrometer thick sections were cut from paraffin-embedded tissue blocks and deparaffinized in xylene. Antigen retrieval was performed by using sodium citrate, pH 6.0, at subboiling temperature for 40 minutes, and the slides were allowed to cool before incubation with the primary antibody. Sections were stained for p-STAT3, IL-6, or Ki-67 as described previously (33).
Colitis induction and PPAR-δ modulator treatment
PPAR-δ antagonist GSK3787 and PPAR-δ agonist GW501516 were synthesized at the Chemistry Synthetic Core and the Institute for Applied Cancer Science at MD Anderson Cancer Center. The authenticity of the compounds was confirmed by liquid chromatography-tandem mass spectrometry. The GSK3787 (50 mg/kg), GW501516 (50 mg/kg), and control diets were prepared at Harlan Laboratories (Madison, WI, USA). Wild-type (WT) and PPAR-δ-Gut mice were fed the GSK3787, GW501516, or control diet for 2 weeks and then for 1 more week in combination with 1.2% DSS-containing drinking water to induce colitis. The mice were killed at the end of the third week. Colon crypts were digested and harvested for RNA and protein expression level measurements.
Statistical analyses
We used the t test for 2-group comparisons. Comparisons of single-factor experimental conditions for continuous outcome measures were performed using 1-way ANOVA, and Bonferroni adjustments were used for all multiple comparisons. We analyzed data involving simultaneous consideration of 2 factors using 2-way ANOVA. We used log-transformation for analyses of non-normally distributed data. We used Poisson regression for tumor count analyses as previously described (30).
RESULTS
15-LOX-1 intestinal transgenic expression suppressed CAC and proliferation of colonic crypt cells in mice
To study the effect of 15-LOX-1 on CAC, we tested the effects of 15-LOX-1 expression on AOM/DSS-induced colonic tumorigenesis by using our humanized 15-LOX-1 transgenic mouse model (26). Two mouse lines of different genetic backgrounds—FVB/N (15-LOX-1-Gut-FVB) and C57BL/6 (15-LOX-1-Gut-B6)—were tested to ensure that observed effects were independent of genetic background. For 15-LOX-1-Gut-FVB mice, the number of tumors per mouse was 8.11 ± 2.93 (mean ± sem) for WT littermates, 5.00 ± 1.88 for 15-LOX-1-Gut heterozygotes, and 3.44 ± 1.42 for 15-LOX-1-Gut homozygotes (Fig. 1A, B). For 15-LOX-1-Gut-B6 mice, the number (mean ± sem) of tumors per mouse was 7.67 ± 0.93 for WT littermates, 4.64 ± 1.64 for 15-LOX-1-Gut heterozygotes, and 4.01 ± 0.86 for 15-LOX-1-Gut homozygotes (Fig. 1C, D).
Figure 1.

Effects of 15-LOX-1 transgenic expression on colonic tumorigenesis induced by AOM and DSS. A–D) FVB/N (FVB) and C57BL/6 (B6) mice with (15-LOX-1-Gut) and without (WT) 15-LOX-1 transgenic expression were treated with AOM and DSS to induce colitis-associated colon tumors. Mice were then killed and examined for tumor formation. A, C) Scatter plots of tumor incidence. Horizontal bars represent means. B, D) Representative photographs of dissected colons of mice after treatment. Magnification ×10. E–G) Proliferative zone lengths in the small intestine and colon in the mice. E) Representative photographs of Ki-67 immunohistochemistry staining. F, G) Proliferative zone lengths (means ± sd). H) 15-LOX-1 mRNA relative expression levels measured by qRT-PCR in normal colonic epithelial cells and paired tumors of the15-LOX-1-Gut mice. I) 15-LOX-1 protein expression levels measured by Western blot in representative normal colonic epithelial cells and paired tumors of the 15-LOX-1-Gut mice. J) Densitometric analyses of the protein bands in I.
Because crypt proliferative zone expansion significantly contributes to CAC tumorigenesis (35), we examined whether 15-LOX-1 would affect this protumorigenic mechanism. The crypt proliferative zone, measured by Ki-67 immunohistochemistry, was significantly smaller in 15-LOX-1-Gut mice than in their WT littermates in both the small intestine and the colon (Fig. 1E–G). We also examined the effect of CAC tumorigenesis on expression of transgenic 15-LOX-1 in colonic epithelial cells. In all 34 tested mice that developed AOM/DSS-induced tumors, 15-LOX-1 mRNA relative expression levels were lower in tumors than in normal tissue. In heterozygous 15-LOX-1-Gut mice, 15-LOX-1 mRNA relative expression levels (means ± sd) were 1.076 ± 0.22 in nonmalignant colonic epithelial cells and 0.52 ± 0.18 in tumor cells. In homozygous 15-LOX-1-Gut mice, 15-LOX-1 mRNA relative expression levels (means ± sd) were 2.24 ± 0.5 in nonmalignant colonic epithelial cells and 0.85 ± 0.34 in tumor cells (Fig. 1H). 15-LOX-1 protein expression levels in tumor cells were also lower than in nonmalignant colonic epithelial cells in heterozygote and homozygote 15-LOX-1-Gut mice (Fig. 1I, J).
15-LOX-1 inhibited IL-6/STAT3 signaling in mice
We next investigated whether 15-LOX-1 modulated IL-6/p-STAT3 signaling to suppress CAC in mice. IL-6 expression (mean ± sd) was significantly higher in tumorous colonic crypts than in normal colonic crypts in WT mice, heterozygote 15-LOX-1 Gut mice, and homozygote 15-LOX-1 Gut mice (Supplemental Table S1). 15-LOX-1 dramatically inhibited IL-6 expression in both normal and tumor crypts (Fig. 2A). The difference in expression levels (tumor - normal) was higher in WT mice (mean ± sd, 23.59 ± 2.9) than in 15-LOX-1-Gut heterozygote mice (12.38 ± 3.08) and 15-LOX-1-Gut homozygote mice (6.15 ± 1.14; P = 0.0004).
Figure 2.

15-LOX-1 transgenic expression inhibited IL-6/STAT3 signaling. A) Effects of 15-LOX-1 on IL-6 expression. FVB/N mice were treated with AOM and DSS to induce colitis-associated colon tumors. The mice were then killed, and crypts of normal and colonic tumor tissues were isolated. IL-6 mRNA expression was measured by qRT-PCR. Values are means ± sd. *P < 0.0001 compared with WT normal crypts. B–E) Effects of 15-LOX-1 on STAT3(705) phosphorylation and IL-6/STAT3 target gene expression. Crypt samples were obtained as described for A. B) Protein levels of p-STAT3(705) and total STAT3 were measured by Western blotting. C) Densitometric analyses of the protein bands in B. The data are presented as ratio of p-STAT3(705) to total STAT3. D, E) mRNA expression levels of the STAT3 target genes MUC1 and Notch3 measured by qRT-PCR. Values are means ± sd. *P < 0.01 compared with WT normal crypts. F, G) Effects of 15-LOX-1 on IL-6-induced p-STAT3(705) expression. F) Colonic crypts from FVB/N WT and 15-LOX-1-Gut(+/+) mice were isolated and cultured with mouse recombinant IL-6 (50 ng/ml) for the indicated times. Protein levels of p-STAT3(705) and total STAT3 were measured by Western blotting. G) Densitometric analyses of the protein bands in F.
Next, we tested the effects of 15-LOX-1 on the levels of the p-STAT3(705) and STAT3 target genes MUC1 and Notch3. In WT mice, p-STAT3(705) levels were higher in tumorous colonic crypts than in normal colonic crypts. In 15-LOX-1-Gut heterozygote and homozygote mice, p-STAT3(705) levels were markedly lower in normal and tumor crypts than the levels in WT mice. However, total STAT3 levels were similar for all mouse groups (Fig. 2B, C). MUC1 expression levels were higher in tumorous colonic crypts than in normal colonic crypts in WT mice, 15-LOX-1-Gut heterozygotes, and 15-LOX-1-Gut homozygotes (Fig. 2D and Supplemental Table S2). Notch3 expression levels were also higher in tumorous colonic crypts than in normal colonic crypts in WT mice, but similar in 15-LOX-1-Gut heterozygotes and 15-LOX-1-Gut homozygotes (Fig. 2E and Supplemental Table S3). 15-LOX-1 inhibited MUC1 and Notch3 expression in both normal and tumor crypts compared with expression in WT mice (Fig. 2D, E).
Because IL-6 is commonly secreted by macrophages during colitis (36), we also tested the effects of 15-LOX-1 on STAT3 phosphorylation induced by paracrine IL-6 signaling. We treated isolated colonic epithelial cells ex vivo with exogenous mouse recombinant IL-6 (50 ng/ml). In cells from WT mice, IL-6 increased p-STAT3(705) levels within 10 minutes, and the levels were further increased at 20 minutes. In contrast, in cells from 15-LOX-1-Gut mice, IL-6-induced STAT3(705) phosphorylation was markedly inhibited (Fig. 2F, G).
We confirmed by immunohistochemistry staining that AOM/DSS-induced CAC markedly increased IL-6 protein expression and p-STAT3(705) levels in colonic epithelial cells in tumors of WT mice (Fig. 3). 15-LOX-1 transgenic expression inhibited IL-6 protein up-regulation and STAT3(705) phosphorylation in tumorous colonic mucosa (Fig. 3).
Figure 3.
15-LOX-1 inhibited IL-6 up-regulation (A) and STAT3(705) phosphorylation (B) in colitis-associated colorectal tumorigenesis. 15-LOX-1-Gut and WT littermate mice were treated with AOM and DSS to induce colitis-associated colon tumors (T). IL-6 and p-STAT3(705) protein expression levels were measured by immunohistochemistry staining. N, nonmalignant tissue.
15-LOX-1 regulated IL-6/STAT3 signaling in human colon cancer cells by inhibiting expression of PPAR-δ
To determine the translational relevance of our observation of repression of IL-6/STAT3 signaling by transgenic 15-LOX-1 in mice, we examined the effects of 15-LOX-1 on IL-6/STAT3 signaling in human colon cancer cells. First, we measured IL-6 and 15-LOX-1 mRNA expression in paired normal and tumor biopsy tissue samples from patients with colorectal cancer. IL-6 relative expression levels were higher in colorectal tumor tissue (mean ± sem, 416.7 ± 350) than in paired normal mucosa (3.57 ± 0.91; Fig. 4A). In contrast, 15-LOX-1 relative expression levels were lower in tumor tissue (4.64 ± 2.39) than in paired normal mucosa (16.63 ± 5.78; Fig. 4B).
Figure 4.
15-LOX-1 inhibited IL-6 up-regulation and STAT3(705) phosphorylation in human colon cancer cells. A, B) IL-6 and 15-LOX-1 expression levels measured by qRT-PCR in paired human normal and colorectal tumor tissue samples. Values shown are means ± sd of triplicate measurements for each patient. C–H) 15-LOX-1 inhibited IL-6 up-regulation and STAT3(705) phosphorylation in colorectal cancer cells. The cells were infected with either modified Ad-hTert-15-LOX-1 (Ad-15-LOX-1) or modified Ad-hTert-luciferase (Ad-luciferase) at a ratio of 200 adenovirus particles per cell for HCT116 cells and 400 adenovirus particles per cell for LoVo cells. Cells were harvested 48 hours after infection for measurement of IL-6 mRNA expression by qRT-PCR (C, D) and 72 hours after infection for measurement of p-STAT3(705) protein expression by Western blotting (E, F). G, H) Densitometric analyses of the protein bands in E and F. The data are presented as ratio of p-STAT3(705) to total STAT3. I–L) Effects of 15-LOX-1 protein expression and enzymatic activity on IL-6/STAT3 signaling. Caco-2 cells stably transfected with either nontargeted shRNA (control shRNA) or 15-LOX-1 shRNA were cultured for 14 days after confluence to induce differentiation and then harvested and analyzed for 15-LOX-1 mRNA expression by qRT-PCR (I) and protein expression by Western blotting (J). Control shRNA and 15-LOX-1 shRNA cells were cultured as described for I for 14 days and then treated with caffeic acid (2.2 µM), 13-S-HODE (13.5 µM), or solvent control as indicated with or without IL-6 (50 ng/ml) for 40 min before cell harvesting for protein expression analyses by Western blotting (K). L) Densitometric analyses of the protein bands in J. M, N) 15-LOX-1 inhibited IL-6 transcription in colorectal cancer cells. HCT116 and LoVo cells were transfected with either Ad-15-LOX-1 or Ad-GFP combined with the IL-6 promoter luciferase vector. O–T) 15-LOX-1 inhibited PPAR-δ mRNA and protein expression. HCT116 and LoVo cells were treated as described for C–H. O, P) PPAR-δ mRNA expression was measured by qRT-PCR 48 hours after infection. Q, R) PPAR-δ protein expression was measured by Western blotting 72 hours after infection. S, T) Densitometric analyses of the protein bands in Q and R.
15-LOX-1 expression is silenced in colon cancer cell lines (31). We found that 15-LOX-1 reexpression by Ad-15-LOX-1 in the HCT116 and LoVo cell lines significantly reduced IL-6 mRNA and p-STAT3(705) levels compared with the levels in cells transfected with the control Ad-luciferase (Fig. 4C–H).
We further investigated the mechanistic relevance of 15-LOX-1 protein expression and enzymatic activity to IL-6/STAT3 signaling suppression in colon cancer cells by using the 15-LOX-1 knockdown experimental system of Caco-2 cells. The Caco-2 human colon cancer cell line spontaneously expresses 15-LOX-1 when undergoing intestinal cell differentiation in extended cultures (37). We used Caco-2 cells that are stably transfected with either a control shRNA or 15-LOX-1 shRNA (31) to specifically knock down 15-LOX-1 expression. 15-LOX-1 shRNA stable transfection significantly down-regulated 15-LOX-1 expression (Fig. 4I, J). The addition of IL-6 to Caco-2 cell culture medium increased p-STAT3 levels in cells that expressed 15-LOX-1; 15-LOX-1 down-regulation by shRNA strongly augmented the IL-6-mediated increases in p-STAT3 levels (Fig. 4K). Inhibition of 15-LOX-1 enzymatic activity by 2.2 µM caffeic acid, a concentration that specifically inhibits 15-LOX-1 (38), also enhanced the IL-6-induced increases in p-STAT3 levels (Fig. 4K). Furthermore, the addition of 13-S-HODE, the main enzymatic product of 15-LOX-1, to cells with 15-LOX-1 down-regulation reduced the IL-6-induced increases in p-STAT3 levels (Fig. 4K).
To identify the mechanisms by which 15-LOX-1 suppressed IL-6 expression, we investigated whether 15-LOX-1 regulated IL-6/STAT3 signaling at the transcriptional level. We infected HCT116 and LoVo cells with either Ad-15-LOX-1 or a control Ad-GFP vector in combination with an IL-6 promoter luciferase vector. 15-LOX-1 inhibited IL-6 promoter activity in both HCT116 and LoVo cells (Fig. 4M, N).
We also measured the effects of 15-LOX-1 on PPAR-δ, because PPAR-δ is a transcriptional factor that 13-S-HODE can bind to (23) and because PPAR-δ up-regulates IL-6 expression and thereby promotes colitis (25). Reexpression of 15-LOX-1 in HCT116 and LoVo cells via Ad-15-LOX-1 significantly reduced PPAR-δ expression at both the mRNA level and the protein level (Fig. 4O–T).
15-LOX-1 suppressed CAC in mice by decreasing PPAR-δ expression and thereby inhibiting IL-6/STAT3 signaling
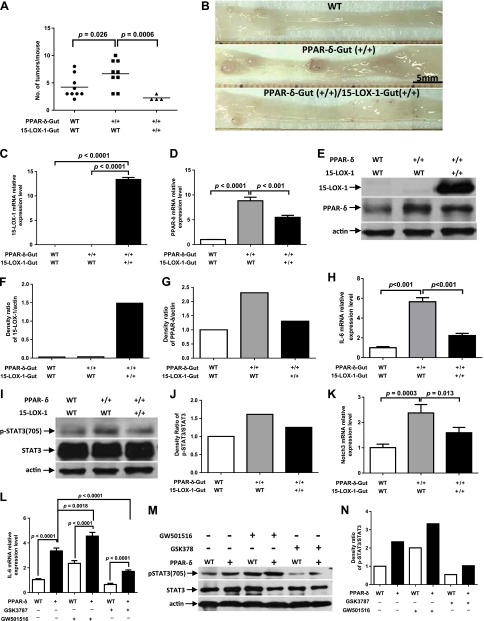
To determine the mechanistic significance of 15-LOX-1-induced downregulation of PPAR-δ, which suppressed IL-6/STAT3 signaling and CAC in vivo, we developed a novel mouse model in which transgenic 15-LOX-1 and PPAR-δ overexpression were targeted to intestinal epithelial cells. The number of tumors per mouse (mean ± se) was 4.22 ± 0.68 in WT littermates, 6.67 ± 0.83 for PPAR-δ-Gut mice, and 2.25 ± 0.25 in PPAR-δ-15-LOX-1-Gut mice (Fig. 5A, B).
Figure 5.
Effects of 15-LOX-1 on PPAR-δ-mediated promotion of CAC tumorigenesis and IL-6/STAT3 signaling. A, B) Indicated genetic strains of mice were treated with AOM and DSS to induce CAC tumors. The mice were then killed and examined for tumor formation. A) Scatter plot of tumor incidence. B) Representative photographs taken at ×10 magnification. C–G) 15-LOX-1 decreased PPAR-δ expression in murine colonic crypts. Mice were treated as described for A and B, and crypts of normal colonic tissues were isolated. 15-LOX-1 and PPAR-δ mRNA relative expression levels were measured by qRT-PCR (C, D), and protein levels were measured by Western blotting (E). F, G) Densitometric analyses of the protein bands in E. H–K) Effects of 15-LOX-1 on PPAR-δ-mediated activation of IL-6/STAT3 signaling and target gene expression. Mice were treated as described for A and B. Colonic crypt samples were obtained as for C–G. H, K) IL-6 and Notch3 mRNA relative expression levels were measured by qRT-PCR. I) Protein expression levels of p-STAT3(705) and total STAT3 were measured by Western blotting. J) Densitometric analyses of the protein bands in I. L–N) Effects of PPAR-δ expression and activation on IL-6/STAT3 signaling. WT and PPAR-δ-Gut (PPAR-δ+) mice were fed a control diet or a GW501516 (50 mg/kg) or GSK3787 (50 mg/kg) diet for 2 weeks and then treated with 1.2% DSS for 1 wk. The mice were then killed, and colon crypts were isolated. L) IL-6 mRNA relative expression levels were measured by qRT-PCR. M) Protein expression levels of p-STAT3(705) and total STAT3 were measured by Western blotting. N) Densitometric analyses of the protein bands in M.
Transgenic 15-LOX-1-Gut expression in PPAR-δ-15-LOX-1-Gut mice, which was confirmed by 15-LOX-1 mRNA and protein expression measurements (Fig. 5C, E), inhibited PPAR-δ overexpression at the mRNA and protein levels (Fig. 5D, E). 15-LOX-1 down-regulation of exogenous villin promoter-expressed PPAR-δ suggests posttranscriptional regulation of PPAR-δ expression by 15-LOX-1 in agreement with our prior observations (23). PPAR-δ mRNA relative expression levels (mean ± sd) were significantly higher in PPAR-δ-Gut mice (8.81 ± 0.72) than in PPAR-δ-15-LOX-1-Gut mice (5.48 ± 0.4; Fig. 5D). IL-6 mRNA relative expression levels (means ± sd) were significantly higher in PPAR-δ-Gut mice (5.65 ± 0.42) than in WT littermates (1.0 ± 0.1); however, the IL-6 level was reduced to 2.24 ± 0.22 in PPAR-δ-15-LOX-1-Gut mice (Fig. 5H). PPAR-δ overexpression increased STAT3(705) phosphorylation in PPAR-δ-Gut mice, and 15-LOX-1 in PPAR-δ-15-LOX-1-Gut mice inhibited this increase (Fig. 5I, J). The levels (means ± sd) of the IL-6/STAT3 signaling target Notch3 were significantly higher in PPAR-δ-Gut mice (2.38 ± 0.33) than in WT littermates (1.0 ± 0.14); however, Notch3 levels were reduced to 1.59 ± 0.21 in PPAR-δ-15-LOX-1-Gut mice (Fig. 5K).
To determine whether PPAR-δ directly modulates IL-6 expression and signaling during colitis induction, we fed PPAR-δ-Gut mice and their WT littermates a control diet or a diet containing GW501516 (a PPAR-δ agonist) (39) or GSK3787 (a PPAR-δ antagonist) (40). PPAR-δ overexpression increased IL-6 expression and STAT3 phosphorylation levels (Fig. 5L–N). GW501516 increased IL-6 expression and STAT3 phosphorylation levels in PPAR-δ-Gut mice more than in WT mice (Fig. 5L–N). GSK3787, in contrast, reduced IL-6 expression and STAT3 phosphorylation levels (Fig. 5L–N).
DISCUSSION
We found in the studies reported here that 15-LOX-1 inhibited IL-6/STAT3 signaling and CAC by down-regulating PPAR-δ. These findings demonstrate a novel mechanism of regulation of IL-6/STAT3 signaling in CAC tumorigenesis.
15-LOX-1 intestinal transgenic expression in mice was sufficient to suppress CAC and colonic crypt proliferative zone expansion. 15-LOX-1 transgenic expression in colonic epithelial cells significantly reduced colorectal tumorigenesis in 2 mouse strains of different genetic backgrounds, FVB/N and C57BL/6, confirming that the observed effects were not mouse strain specific. These findings demonstrate for the first time the ability of 15-LOX-1 expression in colonic epithelial cells to suppress CAC. This suppression of CAC was associated with suppression of crypt proliferative zone expansion as a mechanism to promote CAC tumorigenesis (35). These findings help define the role of 15-LOX-1 in CAC tumorigenesis. Although several indirect lines of evidence support an anti-inflammatory role for 15-LOX-1, as discussed in the introduction (19, 22, 41, 42), this role has remained poorly understood, because studies using 12/15-LOX mouse modeling suggested that 12/15-LOX plays both proinflammatory and anti-inflammatory roles (20). Our current report is the first, to our knowledge, to establish the specific role of 15-LOX-1 in inhibiting CAC.
CAC induction was associated with down-regulation of 15-LOX-1 in all tested mice that developed tumors. These findings further confirm that colorectal cancer tumorigenesis requires 15-LOX-1 down-regulation. Furthermore, these findings are consistent with our previously reported observation that 15-LOX-1 was down-regulated in all tumors produced in 15-LOX-1-Gut mice following treatment with AOM, a model that represents sporadic colon cancer (26). These experimental observations are also in agreement with studies on human colorectal cancer tumorigenesis that showed a very high prevalence (approaching 100%) of loss of 15-LOX-1 expression in human colorectal cancer (43, 44). Our very intriguing findings in 15-LOX-1-Gut mice are unlikely secondary to tumorigenesis-associated inactivation of the villin promoter that drives 15-LOX-1 transgenic expression in 15-LOX-1-Gut mice, because the villin promoter remains activated in immature intestinal cells during colorectal cancer tumorigenesis (45). The alternative and more likely explanation is that 15-LOX-1 down-regulation is necessary to drive tumorigenesis, because tumors developed only when 15-LOX-1 was down-regulated. These findings underscore the significance of 15-LOX-1 down-regulation not only in sporadic colon cancer but also in CAC.
We found that 15-LOX-1 expression was sufficient to inhibit IL-6/STAT3 signaling in CAC. IL-6/STAT3 signaling is a major protumorigenic pathway for CAC (6–12, 46) and strongly promotes malignant transformation (14, 15, 47, 48). The specific effects of 15-LOX-1 on this signaling pathway had remained unknown. Our novel findings showed that 15-LOX-1 transgenic expression in colonic epithelial cells suppressed IL-6 up-regulation and subsequent STAT3 phosphorylation during CAC induction in mice. These effects were dependent on 15-LOX-1 transgenic expression levels (i.e., heterozygote vs. homozygote).
MUC1 and Notch3, important downstream protumorigenic targets of IL-6/STAT3, were suppressed by transgenic 15-LOX-1 expression. MUC1 is a mucin-like glycoprotein that is transcriptionally up-regulated by IL-6/STAT3 in human cancer cells (49, 50). MUC1 acts as an oncoprotein, promoting malignant transformation including CAC tumorigenesis (51) by activating critical protumorigenic mechanisms (e.g., aberrant B-catenin signaling) (52, 53). Notch3 is a transmembrane receptor protein and critical signaling component that regulates stem cell survival and differentiation (54). Notch3 promotes tumorigenesis of various human cancers, including colorectal cancer (55, 56). Notch3 promoter contains a STAT3 regulatory transcription binding site (57). Our results showed that 15-LOX-1 markedly inhibited Notch3, whereas PPAR-δ up-regulated it. These Notch3 modulations corresponded to inhibition of IL-6/STAT3 by 15-LOX-1 and up-regulation of Notch3 by IL-6/STAT3. These novel findings establish for the first time a link between 15-LOX-1 and regulation of MUC1 and Notch3 and suggest a signaling pathway by which 15-LOX-1, through modulation of PPAR-δ and subsequently IL-6/STAT3, regulates these important protumorigenic proteins.
15-LOX-1 regulation of IL-6/STAT3 signaling is not limited to mouse models but also occurs in human colon cancer cells. In clinical samples from patients with colorectal cancer, we found that 15-LOX-1 down-regulation was associated with IL-6 up-regulation. We evaluated the mechanistic importance of this inverse association using human colon cancer cell lines. We found that restoring 15-LOX-1 expression in these cell lines down-regulated IL-6 expression and inhibited STAT3 phosphorylation. Furthermore, using the complementary approach of down-regulating 15-LOX-1 expression or inhibiting its enzymatic activity when 15-LOX-1 was spontaneously expressed during intestinal cell differentiation augmented the IL-6-induced enhancement of STAT3 phosphorylation. Replacement of 13-S-HODE, the main product of 15-LOX-1, in cells with down-regulated 15-LOX-1 expression inhibited IL-6 from increasing STAT3 phosphorylation. These findings from complementary experimental models demonstrate that enzymatically active 15-LOX-1 expression suppressed IL-6/STAT3 signaling. We further demonstrated that the down-regulation of IL-6 occurred at the transcriptional level, as 15-LOX-1 reexpression repressed IL-6 promoter transcriptional activation. This transcriptional repression occurred via PPAR-δ down-regulation, because 15-LOX-1 reexpression in the human colon cancer cells repressed PPAR-δ expression at the mRNA and protein levels. These observations demonstrate for the first time a link between 15-LOX-1 down-regulation of PPAR-δ and repression of IL-6/STAT3 signaling.
15-LOX-1 down-regulation of PPAR-δ to suppress IL-6/STAT3 signaling is important mechanistically to CAC inhibition. We generated a new mouse model—15-LOX-1–PPAR-δ-Gut mice—with combined transgenic 15-LOX-1 and PPAR-δ targeted intestinal expression via the villin promoter to mechanistically examine in vivo the impact of our newly identified pathway on CAC. PPAR-δ intestinal epithelial overexpression significantly increased CAC induction via AOM/DSS, in agreement with the recently reported studies by others showing that PPAR-δ promoted colitis and that PPAR-δ genetic deletion in mice reduced IL-6 expression (25). In the current study, we demonstrated for the first time that PPAR-δ overexpression increased IL-6/p-STAT3 signaling during colitis induction in vivo, and these effects were enhanced by the PPAR-δ agonist GW501516 and inhibited by the PPAR-δ antagonist GSK3787. These findings establish PPAR-δ’s role in enhancing IL-6/p-STAT signaling and thereby promoting colitis and CAC. Furthermore, our findings are unique in showing for the first time in a representative genetic model that 15-LOX-1 transgenic expression significantly reduced PPAR-δ expression and the promotion of CAC by PPAR-δ. Furthermore, in this novel model, we delineated the downstream mechanisms for 15-LOX-1-induced inhibition of PPAR-δ promotion of CAC via suppression of IL-6 expression, STAT3 phosphorylation, and Notch3 expression. These novel in vivo findings support the conceptual model in which 15-LOX-1 down-regulates PPAR-δ and thereby suppresses IL-6 expression and STAT3 phosphorylation and subsequently expression of important protumorigenic genes such as Notch3 and MUC1 (Fig. 6).
Figure 6.
Conceptual model of the effects of 15-LOX-1 on the PPAR-δ–IL-6/STAT3 signaling pathway in relation to colorectal tumorigenesis.
Our study’s novel mechanistic insights provide the opportunity to identify important molecular targets (e.g., 15-LOX-1 and PPAR-δ) for treatment and prevention of IBD-related colon cancers.
Supplementary Material
Acknowledgments
The authors thank Dr. Rui Tian for technical assistance. This study was supported by U.S. National Institutes of Health (NIH) National Cancer Institute Grants R01CA137213 and R01CA142969 (to I.S.), and an institutional research grant from The University of Texas MD Anderson Cancer Center (to I.S.). The study was also supported by Cancer Center Support Grant CA016672 from the NIH National Cancer Institute, the MD Anderson Cancer Center Genetically Engineered Mouse Facility, DNA Analysis Facility, Chemistry Synthetic Core, and the Research Animal Support Facility–Smithville. The authors declare no conflicts of interest.
Glossary
- AOM
azoxymethane
- CAC
colitis-associated colorectal cancer
- DSS
dextran sodium sulfate
- HODE
hydroxyoctadecadienoic acid
- IBD
inflammatory bowel disease
- LOX
lipoxygenase
- MUC
mucin
- PPAR
peroxisome proliferator-activated receptor
- p-STAT3
phospho-STAT3
- qRT-PCR
quantitative real-time RT-PCR
- STAT3
signal transducer and activator of transcription 3
- WT
wild-type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Kappelman M. D., Rifas-Shiman S. L., Porter C. Q., Ollendorf D. A., Sandler R. S., Galanko J. A., Finkelstein J. A. (2008) Direct health care costs of Crohn’s disease and ulcerative colitis in US children and adults. Gastroenterology 135, 1907–1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ekbom A., Helmick C., Zack M., Adami H. O. (1990) Ulcerative colitis and colorectal cancer. A population-based study. N. Engl. J. Med. 323, 1228–1233 [DOI] [PubMed] [Google Scholar]
- 3.Itzkowitz S. H., Yio X. (2004) Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 287, G7–G17 [DOI] [PubMed] [Google Scholar]
- 4.Breynaert C., Vermeire S., Rutgeerts P., Van Assche G. (2008) Dysplasia and colorectal cancer in inflammatory bowel disease: a result of inflammation or an intrinsic risk? Acta Gastroenterol. Belg. 71, 367–372 [PubMed] [Google Scholar]
- 5.van Schaik F. D. M., Offerhaus G. J. A., Schipper M. E. I., Siersema P. D., Vleggaar F. P., Oldenburg B. (2009) Endoscopic and pathological aspects of colitis-associated dysplasia. Nat Rev Gastroenterol Hepatol 6, 671–678 [DOI] [PubMed] [Google Scholar]
- 6.Bollrath J., Phesse T. J., von Burstin V. A., Putoczki T., Bennecke M., Bateman T., Nebelsiek T., Lundgren-May T., Canli O., Schwitalla S., Matthews V., Schmid R. M., Kirchner T., Arkan M. C., Ernst M., Greten F. R. (2009) gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell 15, 91–102 [DOI] [PubMed] [Google Scholar]
- 7.Grivennikov S., Karin E., Terzic J., Mucida D., Yu G.-Y., Vallabhapurapu S., Scheller J., Rose-John S., Cheroutre H., Eckmann L., Karin M. (2009) IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 15, 103–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kathiria A. S., Neumann W. L., Rhees J., Hotchkiss E., Cheng Y., Genta R. M., Meltzer S. J., Souza R. F., Theiss A. L. (2012) Prohibitin attenuates colitis-associated tumorigenesis in mice by modulating p53 and STAT3 apoptotic responses. Cancer Res. 72, 5778–5789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y., de Haar C., Chen M., Deuring J., Gerrits M. M., Smits R., Xia B., Kuipers E. J., van der Woude C. J. (2010) Disease-related expression of the IL6/STAT3/SOCS3 signalling pathway in ulcerative colitis and ulcerative colitis-related carcinogenesis. Gut 59, 227–235 [DOI] [PubMed] [Google Scholar]
- 10.Liang J., Nagahashi M., Kim E. Y., Harikumar K. B., Yamada A., Huang W. C., Hait N. C., Allegood J. C., Price M. M., Avni D., Takabe K., Kordula T., Milstien S., Spiegel S. (2013) Sphingosine-1-phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis-associated cancer. Cancer Cell 23, 107–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neurath M. F., Finotto S. (2011) IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine Growth Factor Rev. 22, 83–89 [DOI] [PubMed] [Google Scholar]
- 12.Naugler W. E., Karin M. (2008) The wolf in sheep’s clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol. Med. 14, 109–119 [DOI] [PubMed] [Google Scholar]
- 13.Li N., Grivennikov S. I., Karin M. (2011) The unholy trinity: inflammation, cytokines, and STAT3 shape the cancer microenvironment. Cancer Cell 19, 429–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frank D. A. (2007) STAT3 as a central mediator of neoplastic cellular transformation. Cancer Lett. 251, 199–210 [DOI] [PubMed] [Google Scholar]
- 15.Yu H., Kortylewski M., Pardoll D. (2007) Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat. Rev. Immunol. 7, 41–51 [DOI] [PubMed] [Google Scholar]
- 16.Serhan C. N. (2007) Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu. Rev. Immunol. 25, 101–137 [DOI] [PubMed] [Google Scholar]
- 17.Serhan C. N., Savill J. (2005) Resolution of inflammation: the beginning programs the end. Nat. Immunol. 6, 1191–1197 [DOI] [PubMed] [Google Scholar]
- 18.Serhan C. N., Jain A., Marleau S., Clish C., Kantarci A., Behbehani B., Colgan S. P., Stahl G. L., Merched A., Petasis N. A., Chan L., Van Dyke T. E. (2003) Reduced inflammation and tissue damage in transgenic rabbits overexpressing 15-lipoxygenase and endogenous anti-inflammatory lipid mediators. J. Immunol. 171, 6856–6865 [DOI] [PubMed] [Google Scholar]
- 19.Munger K. A., Montero A., Fukunaga M., Uda S., Yura T., Imai E., Kaneda Y., Valdivielso J. M., Badr K. F. (1999) Transfection of rat kidney with human 15-lipoxygenase suppresses inflammation and preserves function in experimental glomerulonephritis. Proc. Natl. Acad. Sci. USA 96, 13375–13380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kühn H., O’Donnell V. B. (2006) Inflammation and immune regulation by 12/15-lipoxygenases. Prog. Lipid Res. 45, 334–356 [DOI] [PubMed] [Google Scholar]
- 21.Klil-Drori A. J., Ariel A. (2013) 15-Lipoxygenases in cancer: a double-edged sword? Prostaglandins Other Lipid Mediat. 106, 16–22 [DOI] [PubMed] [Google Scholar]
- 22.Mangino M. J., Brounts L., Harms B., Heise C. (2006) Lipoxin biosynthesis in inflammatory bowel disease. Prostaglandins Other Lipid Mediat. 79, 84–92 [DOI] [PubMed] [Google Scholar]
- 23.Shureiqi I., Jiang W., Zuo X., Wu Y., Stimmel J. B., Leesnitzer L. M., Morris J. S., Fan H. Z., Fischer S. M., Lippman S. M. (2003) The 15-lipoxygenase-1 product 13-S-hydroxyoctadecadienoic acid down-regulates PPAR-delta to induce apoptosis in colorectal cancer cells. Proc. Natl. Acad. Sci. USA 100, 9968–9973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zuo X., Wu Y., Morris J. S., Stimmel J. B., Leesnitzer L. M., Fischer S. M., Lippman S. M., Shureiqi I. (2006) Oxidative metabolism of linoleic acid modulates PPAR-beta/delta suppression of PPAR-gamma activity. Oncogene 25, 1225–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang, D., Fu, L., Ning, W., Guo, L., Sun, X., Dey, S. K., Chaturvedi, R., Wilson, K. T., DuBois,R. N. (2014) Peroxisome proliferator-activated receptor δ promotes colonic inflammation and tumor growth. Proc. Natl. Acad. Sci. USA 111, 7084–7089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zuo X., Peng Z., Wu Y., Moussalli M. J., Yang X. L., Wang Y., Parker-Thornburg J., Morris J. S., Broaddus R. R., Fischer S. M., Shureiqi I. (2012) Effects of gut-targeted 15-LOX-1 transgene expression on colonic tumorigenesis in mice. J. Natl. Cancer Inst. 104, 709–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shureiqi I., Chen D., Day R. S., Zuo X., Hochman F. L., Ross W. A., Cole R. A., Moy O., Morris J. S., Xiao L., Newman R. A., Yang P., Lippman S. M. (2010) Profiling lipoxygenase metabolism in specific steps of colorectal tumorigenesis. Cancer Prev. Res. (Phila.) 3, 829–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu, Y., Fang, B., Yang, X. Q., Wang, L., Chen, D., Krasnykh, V., Carter, B. Z., Morris, J. S.,Shureiqi, I. (2008) Therapeutic molecular targeting of 15-lipoxygenase-1 in colon cancer. Molec. Therapy 16, 886–892 [DOI] [PMC free article] [PubMed]
- 29.Zuo X., Xu M., Yu J., Wu Y., Moussalli M. J., Manyam G. C., Lee S. I., Liang S., Gagea M., Morris J. S., Broaddus R. R., Shureiqi I. (2014) Potentiation of colon cancer susceptibility in mice by colonic epithelial PPAR-δ/β overexpression. J. Natl. Cancer Inst. 106, dju052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zuo X., Peng Z., Moussalli M. J., Morris J. S., Broaddus R. R., Fischer S. M., Shureiqi I. (2009) Targeted genetic disruption of peroxisome proliferator-activated receptor-delta and colonic tumorigenesis. J. Natl. Cancer Inst. 101, 762–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moussalli M. J., Wu Y., Zuo X., Yang X. L., Wistuba I. I., Raso M. G., Morris J. S., Bowser J. L., Minna J. D., Lotan R., Shureiqi I. (2011) Mechanistic contribution of ubiquitous 15-lipoxygenase-1 expression loss in cancer cells to terminal cell differentiation evasion. Cancer Prev. Res. (Phila.) 4, 1961–1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shureiqi I., Chen D., Lotan R., Yang P., Newman R. A., Fischer S. M., Lippman S. M. (2000) 15-Lipoxygenase-1 mediates nonsteroidal anti-inflammatory drug-induced apoptosis independently of cyclooxygenase-2 in colon cancer cells. Cancer Res. 60, 6846–6850 [PubMed] [Google Scholar]
- 33.Shureiqi I., Wu Y., Chen D., Yang X. L., Guan B., Morris J. S., Yang P., Newman R. A., Broaddus R., Hamilton S. R., Lynch P., Levin B., Fischer S. M., Lippman S. M. (2005) The critical role of 15-lipoxygenase-1 in colorectal epithelial cell terminal differentiation and tumorigenesis. Cancer Res. 65, 11486–11492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfaffl M. W. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee, G., Goretsky, T., Managlia, E., Dirisina, R., Singh, A. P., Brown, J. B., May, R., Yang, G. Y., Ragheb, J. W., Evers, B. M., Weber, C. R., Turner, J. R., He, X. C., Katzman, R. B., Li, L.,Barrett, T. A. (2010) Phosphoinositide 3-kinase signaling mediates β-catenin activation in intestinal epithelial stem and progenitor cells in colitis. Gastroenterology 139, 869–881 [DOI] [PMC free article] [PubMed]
- 36.Matsumoto S., Hara T., Mitsuyama K., Yamamoto M., Tsuruta O., Sata M., Scheller J., Rose-John S., Kado S., Takada T. (2010) Essential roles of IL-6 trans-signaling in colonic epithelial cells, induced by the IL-6/soluble-IL-6 receptor derived from lamina propria macrophages, on the development of colitis-associated premalignant cancer in a murine model. J. Immunol. 184, 1543–1551 [DOI] [PubMed] [Google Scholar]
- 37.Matsumoto H., Erickson R. H., Gum J. R., Yoshioka M., Gum E., Kim Y. S. (1990) Biosynthesis of alkaline phosphatase during differentiation of the human colon cancer cell line Caco-2. Gastroenterology 98, 1199–1207 [DOI] [PubMed] [Google Scholar]
- 38.Shureiqi I., Chen D., Lee J. J., Yang P., Newman R. A., Brenner D. E., Lotan R., Fischer S. M., Lippman S. M. (2000) 15-LOX-1: a novel molecular target of nonsteroidal anti-inflammatory drug-induced apoptosis in colorectal cancer cells. J. Natl. Cancer Inst. 92, 1136–1142 [DOI] [PubMed] [Google Scholar]
- 39.Gupta R. A., Wang D., Katkuri S., Wang H., Dey S. K., DuBois R. N. (2004) Activation of nuclear hormone receptor peroxisome proliferator-activated receptor-delta accelerates intestinal adenoma growth. Nat. Med. 10, 245–247 [DOI] [PubMed] [Google Scholar]
- 40.Palkar P. S., Borland M. G., Naruhn S., Ferry C. H., Lee C., Sk U. H., Sharma A. K., Amin S., Murray I. A., Anderson C. R., Perdew G. H., Gonzalez F. J., Müller R., Peters J. M. (2010) Cellular and pharmacological selectivity of the peroxisome proliferator-activated receptor-beta/delta antagonist GSK3787. Mol. Pharmacol. 78, 419–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Su C. G., Wen X., Bailey S. T., Jiang W., Rangwala S. M., Keilbaugh S. A., Flanigan A., Murthy S., Lazar M. A., Wu G. D. (1999) A novel therapy for colitis utilizing PPAR-gamma ligands to inhibit the epithelial inflammatory response. J. Clin. Invest. 104, 383–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanaka T., Kohno H., Yoshitani S., Takashima S., Okumura A., Murakami A., Hosokawa M. (2001) Ligands for peroxisome proliferator-activated receptors alpha and gamma inhibit chemically induced colitis and formation of aberrant crypt foci in rats. Cancer Res. 61, 2424–2428 [PubMed] [Google Scholar]
- 43.Shureiqi I., Wojno K. J., Poore J. A., Reddy R. G., Moussalli M. J., Spindler S. A., Greenson J. K., Normolle D., Hasan A. A., Lawrence T. S., Brenner D. E. (1999) Decreased 13-S-hydroxyoctadecadienoic acid levels and 15-lipoxygenase-1 expression in human colon cancers. Carcinogenesis 20, 1985–1995 [DOI] [PubMed] [Google Scholar]
- 44.Yuri M., Sasahira T., Nakai K., Ishimaru S., Ohmori H., Kuniyasu H. (2007) Reversal of expression of 15-lipoxygenase-1 to cyclooxygenase-2 is associated with development of colonic cancer. Histopathology 51, 520–527 [DOI] [PubMed] [Google Scholar]
- 45.Pinto D., Robine S., Jaisser F., El Marjou F. E., Louvard D. (1999) Regulatory sequences of the mouse villin gene that efficiently drive transgenic expression in immature and differentiated epithelial cells of small and large intestines. J. Biol. Chem. 274, 6476–6482 [DOI] [PubMed] [Google Scholar]
- 46.Knüpfer H., Preiss R. (2010) Serum interleukin-6 levels in colorectal cancer patients—a summary of published results. Int. J. Colorectal Dis. 25, 135–140 [DOI] [PubMed] [Google Scholar]
- 47.Grivennikov S. I., Karin M. (2010) Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 21, 11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lerner I., Hermano E., Zcharia E., Rodkin D., Bulvik R., Doviner V., Rubinstein A. M., Ishai-Michaeli R., Atzmon R., Sherman Y., Meirovitz A., Peretz T., Vlodavsky I., Elkin M. (2011) Heparanase powers a chronic inflammatory circuit that promotes colitis-associated tumorigenesis in mice. J. Clin. Invest. 121, 1709–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gaemers I. C., Vos H. L., Volders H. H., van der Valk S. W., Hilkens J. (2001) A stat-responsive element in the promoter of the episialin/MUC1 gene is involved in its overexpression in carcinoma cells. J. Biol. Chem. 276, 6191–6199 [DOI] [PubMed] [Google Scholar]
- 50.Ahmad, R., Rajabi, H., Kosugi, M., Joshi, M. D., Alam, M., Vasir, B., Kawano, T., Kharbanda, S.,Kufe, D. (2011) MUC1-C Oncoprotein Promotes STAT3 Activation in an Autoinductive Regulatory Loop. Sci. Signal 4, ra9, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beatty P. L., Plevy S. E., Sepulveda A. R., Finn O. J. (2007) Cutting edge: transgenic expression of human MUC1 in IL-10-/- mice accelerates inflammatory bowel disease and progression to colon cancer. J. Immunol. 179, 735–739 [DOI] [PubMed] [Google Scholar]
- 52.Li Y., Liu D., Chen D., Kharbanda S., Kufe D. (2003) Human DF3/MUC1 carcinoma-associated protein functions as an oncogene. Oncogene 22, 6107–6110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang L., Chen D., Liu D., Yin L., Kharbanda S., Kufe D. (2005) MUC1 oncoprotein blocks glycogen synthase kinase 3β-mediated phosphorylation and degradation of β-catenin. Cancer Res. 65, 10413–10422 [DOI] [PubMed] [Google Scholar]
- 54.Artavanis-Tsakonas S., Rand M. D., Lake R. J. (1999) Notch signaling: cell fate control and signal integration in development. Science 284, 770–776 [DOI] [PubMed] [Google Scholar]
- 55.Serafin V., Persano L., Moserle L., Esposito G., Ghisi M., Curtarello M., Bonanno L., Masiero M., Ribatti D., Stürzl M., Naschberger E., Croner R. S., Jubb A. M., Harris A. L., Koeppen H., Amadori A., Indraccolo S. (2011) Notch3 signalling promotes tumour growth in colorectal cancer. J. Pathol. 224, 448–460 [DOI] [PubMed] [Google Scholar]
- 56.Pastò A., Serafin V., Pilotto G., Lago C., Bellio C., Trusolino L., Bertotti A., Hoey T., Plateroti M., Esposito G., Pinazza M., Agostini M., Nitti D., Amadori A., Indraccolo S. (2014) NOTCH3 signaling regulates MUSASHI-1 expression in metastatic colorectal cancer cells. Cancer Res. 74, 2106–2118 [DOI] [PubMed] [Google Scholar]
- 57.NOTCH3 Gene. (2014) Weizmann Institute of Science, GeneCards Database, http://genecards/genecards/cgi-bin/carddisp.pl?gene=NOTCH3&search=NOTCH3
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.