Abstract
Mutations in the rhodopsin gene cause retinal degeneration and clinical phenotypes including retinitis pigmentosa (RP) and congenital stationary night blindness. Effective gene therapies have been difficult to develop, however, because generating precise levels of rhodopsin expression is critical; overexpression causes toxicity, and underexpression would result in incomplete rescue. Current gene delivery strategies routinely use cDNA-based vectors for gene targeting; however, inclusion of noncoding components of genomic DNA (gDNA) such as introns may help promote more endogenous regulation of gene expression. Here we test the hypothesis that inclusion of genomic sequences from the rhodopsin gene can improve the efficacy of rhodopsin gene therapy in the rhodopsin knockout (RKO) mouse model of RP. We utilize our compacted DNA nanoparticles (NPs), which have the ability to transfer larger and more complex genetic constructs, to deliver murine rhodopsin cDNA or gDNA. We show functional and structural improvements in RKO eyes for up to 8 months after NP-mediated gDNA but not cDNA delivery. Importantly, in addition to improvements in rod function, we observe significant preservation of cone function at time points when cones in the RKO model are degenerated. These results suggest that inclusion of native expression elements, such as introns, can significantly enhance gene expression and therapeutic efficacy and may become an essential option in the array of available gene delivery tools.— Han, Z., Banworth, M. J., Makkia, R., Conley, S. M., Al-Ubaidi, M. R., Cooper, M. J., Naash, M. I. Genomic DNA nanoparticles rescue rhodopsin-associated retinitis pigmentosa phenotype.
Keywords: gene therapy, retinal gene therapy, cDNA, vector engineering
Mutations in the gene for the rod photopigment rhodopsin are a common cause of RP, a hereditary blinding disease for which there is no proven therapy (1). Because conventional treatments are limited, exploration of alternative approaches is warranted, and given the monogenic nature of rhodopsin-associated RP, a logical choice is gene replacement therapy. Various viral vector systems have successfully addressed numerous technical gene transfer landmarks, yet the established limitations in the payload and intraocular immune response to various viral antigens remain concerns (2–6), so we have been exploring alternative nonviral delivery strategies.
Clinically applicable rhodopsin gene therapy will likely include a combination of gene knockdown (to eliminate toxic dominant alleles) and gene replacement. The predominant gene replacement strategy (both for rhodopsin and other genes) utilizes cDNA (7, 8), but having a delivery strategy that yields properly regulated gene expression levels is especially critical because overexpression of rhodopsin is detrimental to rods (9, 10) and underexpression will yield incomplete rescue (11). Various DNA elements such as introns, enhancers, and scaffold/matrix attachment regions (S/MARs) have been widely tested for their ability to improve gene expression (12–14). Some introns have functions independent of the gene in which they reside, for example, they can function as exons for other genes (15, 16), or they can be processed into critical noncoding RNAs (17). However, more relevant to our work, introns are well known to be able to play an important role in regulating gene expression. For example, early experiments clearly showed that inclusion of an intron dramatically increased production of mature RNA (and subsequent protein expression) in SV40 (18). The ability of introns to promote optimal gene expression has since been replicated in several systems, including mammals (e.g., see Brinster et al.) (19). Since then, the role of introns has only become more complicated, and effects vary widely depending on the intron, gene, and position in question. Various specific cellular functions have been shown to be affected by introns including polyadenylation, transcription, translational efficiency (20), and mRNA stability (14). Our goal is to deliver as much of the endogenous rhodopsin genomic sequence as possible (i.e., including promoter region, 5′UTR, and introns) to promote better regulation of gene expression and thus improve efficacy after gene transfer.
One practical difficulty with incorporating extensive genomic sequences is the capacity of the delivery vehicle; for example, traditional adeno-associated viruses are limited to ∼5 kbp. To overcome this, we utilize large-capacity compacted DNA NPs composed of polylysine peptides and polyethylene glycol (CK30-PEG). CK30-PEG NPs adopt varying shapes depending on the lysine counterion used at the time of compaction; we most commonly use rod-shaped NPs formulated with acetate as the counterion (12, 21, 22). These NPs have been successfully tested with plasmids of multiple sizes; thus far the maximum tested in the eye has been ∼15 kbp (22), and plasmids up to 20 kbp have been successfully used in the lung (23). Because widely varying plasmid sizes are used (depending on gene and vector content), the relationship between plasmid size and NP size has been evaluated. Previous work showed that plasmids ranging from 5.3 to 9.7 kbp (very close to the plasmid sizes used in this study) yield rod-shaped particles of ∼8 nm in minor diameter, and a larger 20 kbp plasmid yielded a minor NP diameter of ∼11 nm. The length of these rod-shaped particles has been shown to be proportional to the plasmid size, but importantly, this difference in NP length made no difference to the efficiency of gene expression (when normalized to moles of plasmid delivered) (23).
We have extensively evaluated these NPs in the eye. They efficiently transfect both photoreceptors and the retinal pigment epithelium (RPE) and can mediate persistent (up to 2 yr) gene expression (12, 24). This gene expression promotes phenotypic improvement in various retinal and neurodegenerative models (Abca4−/− and Rpe65−/− mouse and a rat Parkinson’s model) (22, 25, 26). The nanoparticles are also safe and well tolerated in the eye after intravitreal and subretinal delivery; we observed no induction of systemic or local immune response (21, 27, 28) and no signs of ectopic expression in extraocular tissues such as the brain (29). We hypothesized that delivery of rhodopsin gDNA using these NPs would promote more endogenous gene regulation and improved therapeutic efficacy in the rhodopsin knockout (RKO) mouse model of RP (11) compared with that achieved with cDNA.
MATERIALS AND METHODS
Study approvals
The RKO mice were kindly provided by Dr. Janis Lem (Tufts Medical Center, Boston, MA, USA) (11). All experiments were approved by the local Institutional Animal Care and Use Committee and conformed to the guidelines in the National Institutes of Health Guide for the Care and Use of Laboratory Animals (http://grants.nih.gov/grants/olaw/Guide-for-the-care-and-use-of-laboratory-animals.pdf) and the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Research (Rockville, MD, USA).
Vector construction
As shown in Supplemental Fig. 1A, 2 expression cassettes were generated for this project, Rho-g (genomic) and Rho-c (cDNA), and were cloned into the pEPI backbone (Supplemental Fig. 1B) (22, 30). The vectors are described in the Results section, but both expression cassettes contain the 5′ flanking region of the mouse rhodopsin gene corresponding to the MOP500 promoter (−385 to +86) and exons 1–5 (up to the EcoRI restriction site). Rho-g also contains all 4 full-length introns. Neither expression cassette contains any of the well-characterized native polyA signals, as they all follow the EcoRI site (31). The resulting pEPI-Rho-c vector is 7.0 kbp, and the pEPI-Rho-g vector is 10.6 kbp. Vector identity was confirmed by restriction digest and sequence analysis.
Nanoparticle formulation
Endotoxin free Rho-c and Rho-g were amplified by Aldevron (Fargo, ND, USA) and compacted into DNA NPs, hereafter referred to as NP-c and -g, respectively. DNA NPs were formulated as described previously (23, 32). Briefly, a 30-mer lysine peptide with an N-terminal cysteine conjugated via a maleimide linkage to 10 kDa polyethylene glycol (CK30-PEG10k, acetate counterion) was generated and then mixed with naked DNA to formulate NPs comprised essentially of a single molecule of DNA per NP. After solvent exchange (to saline) and concentration (to 4.3 mg/ml of DNA), NPs underwent a standard panel of quality control tests including turbidity and saline sedimentation analyses (colloidal stability), transmission electron microscopy (NP size and shape), serum stability test (protection of DNA from nucleases), endotoxin measurements, and gel analysis (DNA integrity) as described previously (33).
Subretinal injections and animal husbandry
All mice [RKO and wild-type (WT)] used in this study were in the C57BL/6 background. Animals were maintained in the breeding colony under cyclic light (30 lux, 12 hour light-dark) conditions. Subretinal injections were performed at postnatal day 3, as previously described (21). Postnatal day 3 RKO mice were anesthetized by placement on ice for 2 minutes. The eyelid was cut, and the cornea was punctured using a 30-gauge needle. A 35-gauge blunt-end needle attached to a 10 μl Nanofil syringe (World Precision Instruments, Sarasota, FL, USA) was inserted into the puncture site with visualization aided by use of an operating microscope (Carl Zeiss Surgical, Incorporated, Thornwood, NY, USA). Nanoparticles (0.3μl, 4.3 µg/µl) or saline (vehicle) were delivered into the subretinal space, usually in the superior temporal quadrant. Because the retina is not fully developed at this age, some injected material is likely released into the vitreous, although the site of injection is subretinal. After injection, the needle was left in place for a few seconds to allow full treatment delivery before being slowly withdrawn. The eyelid was returned to its original position, a drop of Triple Antibiotic (Equate, Wal-Mart, Bentonville, AR, USA) ointment was applied. Mice were warmed on a 37°C bed until fully awake.
Quantitative real-time PCR
Relative quantitative RT-PCR was performed as described previously (22, 29). Trizol reagent (Life Technologies, Grand Island, NY, USA) was used to extract total retinal RNA according to the manufacturer’s instructions. DNase digestion was performed to remove any remaining nanoparticle or gDNA and cDNAs were prepared by reverse transcription using Oligo dT and Superscript III reverse transcriptase (Life Technologies). No RT controls (reactions that contain everything except reverse transcriptase) were included in all experiments as negative controls, and no gene expression was detected in these samples. Quantitative RT-PCR was performed using Sybr green and the CFX96 real-time system (Bio-Rad, Hercules, CA, USA). Each sample was analyzed in triplicate in each plate using primers against mouse rhodopsin and the housekeeping gene β-actin. Relative gene expression was determined according to the ΔcT method where (ΔcT = rho cT-β-actin cT). Four to six eyes were evaluated for each treatment/age and shown are means ± sem.
Immunofluorescence labeling and Western blotting
Tissue fixation and sectioning were performed as previously described (22, 29). Briefly, eyes were enucleated and fixed with phosphate-buffered saline containing 4% paraformaldehyde at 4°C, dissected to remove the cornea and lens, then sequentially immersed in 10, 20, and 30% (w/v) sucrose. Eyecups were embedded in M1 medium (Thermo Fisher Scientific, Waltham, MA, USA) and frozen sections (10 µm thickness along vertical meridian) were cut with a cryostat (Leica, Buffalo Grove, IL, USA). The entire eye was sectioned, and every tenth section was collected. For immunohistochemistry, sections were blocked, and then incubated overnight with monoclonal anti-rhodopsin 1D4 (kindly provided by Dr. Robert. S. Molday, University of British of Columbia, Vancouver, BC, Canada). After incubation with appropriate fluorescent secondary antibodies, slides were mounted using Prolong Gold with DAPI (Life Technologies). Imaging was performed using a spinning disk confocal microscope (BX62 Olympus, Tokyo, Japan) and Slidebook, version 4 software. Western blotting was performed using standard protocols as previously described (22, 29). Blots were imaged and densitometrically analyzed using ImageLab 5.0 (Bio-Rad).
Northern blots
For Northern blots, total retinal RNAs were extracted using Trizol reagent (Life Technologies). EtBr-containing (50 µg/ml) gel-loading buffer (NorthernMax Formaldehyde Load Dye, Life Technology) was added to the sample prior to denaturing at 70°C for 10 minutes. Total RNA (30 µg/lane for treated samples and 5 µg/lane for control samples) from each sample mixture was loaded into 1% agarose formaldehyde gel. The 18s and 28s rRNA bands were used to normalize total RNA loading following electrophoresis in 1× MOPS buffer (Life Technology). Subsequently gels were transferred to a positively charged nylon membrane (BrightStar-Plus, Ambion, Austin, TX, USA). Northern blots were prehybridized in NorthernMax Prehybridization/Hybridization buffer for 4 hours and hybridized overnight at 42°C with α-[32P]-deoxycytidine triphosphate using random priming (Ready-To-Go DNA labeling Beads, GE Healthcare UK Limited, Amersham, United Kingdom) labeled probe for rhodopsin. After hybridization, membranes were washed in 0.2× saline-sodium citrate, 0.1% SDS at 45°C and exposed to Kodax X-ray photographic film (Eastman Kodak, Rochester, NY, USA) for varying lengths of time at −70°C.
Light and electron microscopy and morphometric analysis
Eyes were enucleated, fixed, and sectioned along the vertical meridian as described previously (22). Following initial fixation in 2%/2% paraformaldehyde/glutaraldehyde and removal of the cornea and lens, eyecups were postfixed in 1% OsO4 at room temperature. After rinsing, eyecups were embedded in Spurr’s resin (Ted Pella Incorporated, Redding, CA, USA). Semithin (0.75 μm) sections were stained with 1% toluidine blue in 1% sodium borate, and central retinal sections (containing the optic nerve head) were used for morphometry. The number of cells in a 435 µm wide section of outer nuclear layer ONL (the width of a ×20 image) was counted at increasing distances from the optic nerve head by an observer blinded to treatment group. One to three animals per group were analyzed. Ultrathin electron microscopysections cut along the superior-inferior plane were poststained with uranyl acetate and lead citrate and imaged at ×75,000 using a JEOL 100CX electron microscope (JEOL, Tokyo, Japan).
ERG analysis
Full-field ERG was performed as previously reported (21, 22). For testing scotopic responses, a stimulus intensity of 1.89 log cd s m−2 (logarithm of scotopic candela seconds per meter squared) was applied to the dark-adapted (overnight), dilated eyes in a Ganzfeld (UTAS-3000; LKC Corporation, Gaithersburg, MD, USA). For testing photopic responses, mice were adapted to a 1.46 log cd m−2 light for 5 minutes, and then a light intensity of 1.89 log cd s m−2 was applied to the eye. At least 5–10 animals were examined per group. Data are presented as means ± sem.
Statistical analysis
For quantitative RT-PCR, values are expressed as percent of WT, and NP-g and NP-c groups were compared using 2-way ANOVA with Bonferroni’s post hoc test. For ERG data, NP-injected groups were compared with age-matched uninjected RKO controls, and P values are from 2-way ANOVA with Bonferroni’s post hoc test. Means ± sem are presented. Graphpad Prism (La Jolla, CA, USA) was used for analysis.
RESULTS
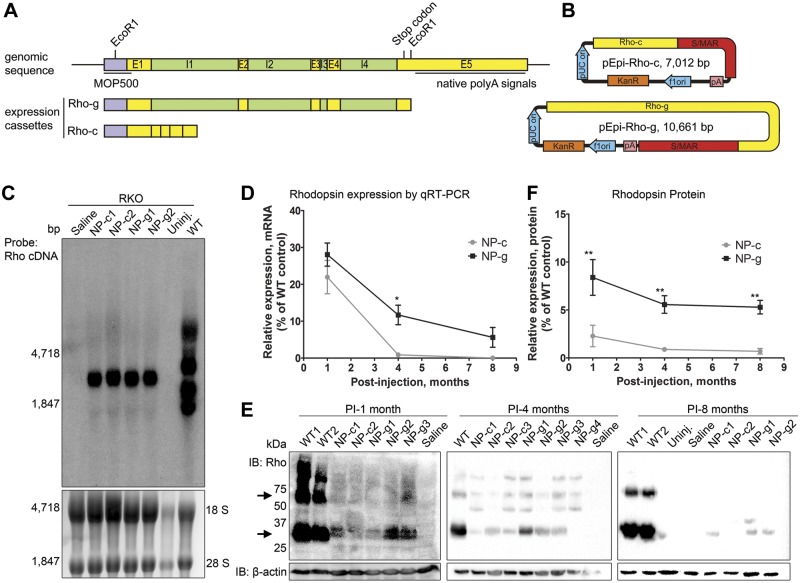
To assess the efficacy of nanoparticle-mediated rhodopsin gene transfer, Rho-g (genomic) and Rho-c (cDNA) (Fig. 1A), were cloned into the pEPI backbone, which contains an S/MAR at the 3′ end of the expression cassette followed by an SV40 polyA signal (Fig. 1B). Both expression cassettes contain: 1) the immediate 5′ flanking region of the mouse rhodopsin gene corresponding to the well-characterized MOP500 promoter (22, 34), 2) full-length exons 1–4, and 3) the first 245 bp of exon 5. The first 111 bp of exon 5 are translated, and the remainder is a short 3′ flanking sequence. Rho-g also contains all 4 full-length introns found in the genomic sequence. Neither contains the native rhodopsin polyA signals (31). The resulting pEPI-Rho-c (7.0 kbp) and pEPI-Rho-g (10.6 kbp) were compacted into NPs (NP-c and NP-g) and 1 µl (4.3 mg/ml) was subretinally injected into RKO eyes at postnatal day 3. Uncompacted DNA does not transfect photoreceptors well (22) and therefore was not included in this study. Controls included saline-injected and uninjected RKO animals.
Figure 1.
NP-g drives better rhodopsin expression than NP-c in RKO retinas. A and B) Illustration of method of construction of pEPI-Rho-c and pEPI-Rho-g vectors. E1-5: exon 1-5, I1-4: intron 1-4. A) Top illustrates native rhodopsin gene, bottom shows the 2 expression cassettes we constructed (Rho-c and Rho-g). EcoRI was used to cut the rhodopsin sequence out. B) Schematic of vectors, pA: SV40 PolyA signal. C) Northern blotting was performed on total RNA at 1 month PI (NP-c and NP-g 1–2 represent 2 different preps of 6 pooled retinas each) and probed with [α-[32P]] rhodopsin cDNA. 18S and 28S RNA loading controls are shown below. D) Rhodopsin mRNA levels in retinas were normalized to β-actin and plotted as percent of age-matched WT levels. E) Representative Western blots from retinal extracts probed with rhodopsin/β-actin. NP-c1-2, NP-g1-4, WT1-2, represent individual retinas. Arrows show rhodopsin monomer/dimer. D) Densitometric quantification (mean ± sem) of Western blots; band densities were normalized to β-actin and expressed as a percentage of levels found in uninjected WT mice. n = 2–5 eyes per group. *P < 0.05, **P < 0.01, for comparison between NP-c and NP-g.
To assess the transcripts produced from the NPs, Northern blots on RNAs from treated eyes were performed at postinjection (PI) 1 mo using α-[32P] rhodopsin cDNA as a probe (all methodological details can be found in the supporting information). As expected due to the presence of multiple polyA signals in the native rhodopsin gene, the WT retina exhibits several transcripts, ranging in size from ∼1.7 to ∼5.1 kbp (31) (Fig. 1C). We observed that RNAs prepared from both NP-c- and NP-g-treated eyes demonstrated a similarly sized transcript, confirming that the introns in NP-g were properly spliced. The size of the transcript observed suggests that the S/MAR is part of the transcript, consistent with the polyA signal following, rather than preceding the S/MAR (Fig. 1B). To quantify rhodopsin expression levels, we conducted quantitative RT-PCR and Western blot from retinas at 1, 4, and 8 months PI (Fig. 1D–F). NP-c and NP-g yielded rhodopsin message levels ∼20 and ∼30% of WT at 1 month PI, which dropped to ∼3 and ∼12%, respectively, at 4 months PI (Fig. 1D). At 8 months PI, rhodopsin message levels were ∼8% of WT in NP-g treated eyes, but no expression was detected in the NP-c eyes at this time point (Fig. 1D). At all time points, mean expression levels were higher in NP-g eyes than NP-c eyes, and this difference was statistically significant at 4 months PI (P < 0.05).
Western blots of retinal extracts collected at 1, 4, and 8 months PI were probed with anti-rhodopsin mAB 1D4 antibody or actin (as a loading control). Representative results from multiple NP-injected mice at each time point are shown in Fig. 1E (labels 1–3 represent individual mice) and quantified in Fig. 1F. Rhodopsin protein levels from retinas injected with NP-c were significantly lower than those from retinas injected with NP-g at all time points (Fig. 1F): ∼3% vs. ∼12% WT at 1 month PI and ∼2% (NP-c) vs. ∼8% (NP-g) at 4 months PI for NP-c vs. NP-g. At 8 mo PI, NP-g generated ∼5% of WT rhodopsin protein levels, and rhodopsin protein in NP-c-treated eyes was largely undetectable (although a few samples showed a very low level of rhodopsin protein). No rhodopsin message or protein was detected in uninjected RKO or saline-treated animals.
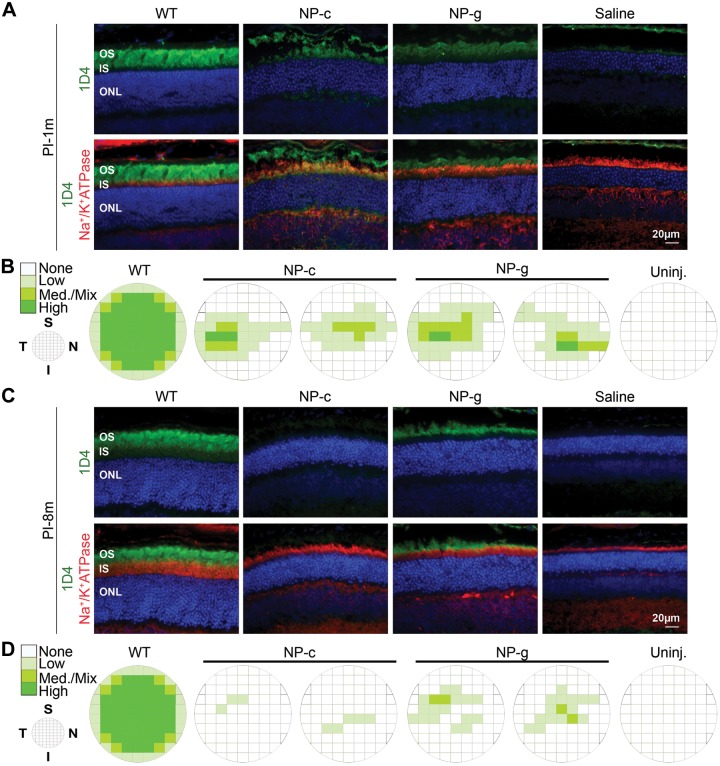
Although biochemical assessments are used to quantify total protein levels, a critical issue for gene therapy studies is understanding to what extent the NP-generated protein is distributed throughout the retina. Therefore to assess the rhodopsin distribution in NP-c- and NP-g-treated animals at 1 (Fig. 2A, B) and 8 mo PI (Fig. 2C, D), frozen retinal sections cut along the inferior-superior plane were taken every ∼200 μm from the nasal to the temporal side of the eye and labeled for rhodopsin (mAB 1D4, green) and Na+/K+ ATPase (red), a plasma membrane protein used as an inner segment marker. Rhodopsin levels in adjacent frames across each section were qualitatively graded as none, low, medium/mixed, or high by an observer blinded to treatment group. Intensity of expression throughout the eye correlates with intensity of the green coloring in the schematics in Fig. 2B, D (as described elsewhere) (22). Shown in Fig. 2A, C are representative images captured from the temporal central region (near the site of injection) demonstrating that rhodopsin expression is localized to the photoreceptors and in the outer segment (OS) as expected. At 1 month PI, some rhodopsin expression from NP-c-treated animals is occasionally found in the inner segment, possibly because there is insufficient protein to generate OS structures.
Figure 2.
Distribution of transduced photoreceptors in NP-g and NP-c treated eyes. Retinal cryosections at PI-1m (A and B) and PI-8m (C and D) were collected every ∼200 μm throughout the eye along the superior-inferior plane and were labeled with rhodopsin (green), Na+/K+ ATPase (red) antibodies, and DAPI (blue). In each section, adjacent fields were graded for level of expression by an observer blinded to type of transferred NP. A and C) Representative images from the temporal central region. Schematics (B, D) depict distribution of transferred rhodopsin throughout the eyes. n = 2–3 eyes per group. I, inferior; IS, inner segment; Med., medium; N, nasal; ONL, outer nuclear layer; PI-1m, 1 month PI; PI-8m, 8 months PI; S, superior; T, temporal; Uninj., uninjected. Scale bar, 20μm.
At 1 mo PI, eyes injected with NP-c exhibited rhodopsin expression in ∼23% of fields and eyes injected with NP-g exhibited rhodopsin expression in ∼30% of fields (2 representative eyes from each treatment group are shown in Fig. 2B). Expression was most pronounced in the central retina, with less expression in the far superior/inferior regions. Consistent with our data showing that overall rhodopsin protein levels were decreased at later time points, at 8 months PI rhodopsin distribution was also restricted compared with 1 mo PI. Eyes injected with NP-g exhibited rhodopsin expression in ∼10% of fields, and 1D4 labeling was hardly detectable in the NP-c-treated eyes (Fig. 2D). No immunofluorescence was detected in saline treated or uninjected eyes.
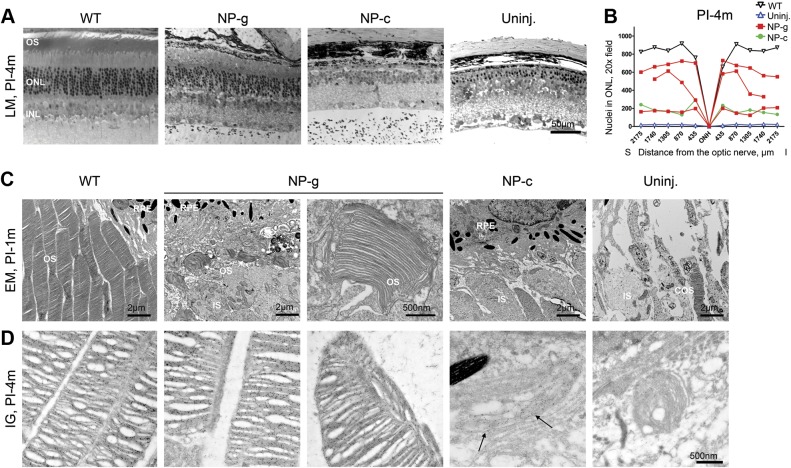
We next asked whether the observed NP-mediated rhodopsin expression mediated structural improvements in photoreceptors. Eyes were enucleated, fixed, and sectioned along the superior-inferior plane (Fig. 3A shows representative sections from the central region). Because the absence of rhodopsin leads to progressive degeneration of photoreceptors, we conducted morphometric analysis on treated eyes/controls at 4 mo PI. We used central retinal sections collected along the superior-inferior plane containing the optic nerve head, and counted the number of cells in a 435 μm section (the width of a ×20 image) of outer nuclear layer (ONL). We found that the number of nuclei in the ONL in NP-g-treated mice was markedly improved in 2/3 eyes in the central retina compared with uninjected eyes (Fig. 3B, each treated eye is shown individually in the graph), although improvement did not meet WT levels. Consistent with the observed lower rhodopsin expression levels in NP-c-treated eyes, the number of nuclei in the NP-c-treated eye at 4 months PI was only slightly improved compared with uninjected RKO mice (Fig. 3A, B).
Figure 3.
NP-g-mediated rhodopsin promotes structural and functional improvement in RKO mice. A) Retinal sections collected from the indicated groups demonstrating overall retinal structure. B) ONL nuclei were counted along the vertical meridian at increasing distances from the ONH and plotted from PI-4m (A) samples (WT, n = 2, all others represent individual eyes). C) EM at PI-1m of the OS region, top row captured at ×4000, bottom row at ×25,000. Red arrows show small rod OSs, blue arrowhead shows a COS. D) Immunogold labeling of rhodopsin was followed by EM at PI-4m. Black arrows show minor labeling in NP-c-treated eyes with appreciable rhodopsin in NP-g-treated eyes. COS, cone outer segment; I, inferior; INL, inner nuclear layer; LM, light microscope; ONH, optic nerve; ONL, outer nuclear layer; OS, outer segment; PI-1m, 1 month PI; PI-4m, 4 months PI; RPE, retinal pigment epithelium; S, superior; Uninj., uninjected.
RKO mice do not elaborate rod OSs (although at early time points cone OS are present), so to assess the ability of NPs to initiate OS formation, we conducted EM at 1 month PI (Fig. 3C). Images were captured from the central region. At 1 mo PI, OS structures were formed in 2/2 NP-g-treated eyes but not the NP-c-treated (0/2) eyes [Fig. 3C shows a low (top row) and higher magnification image (bottom row) from 2 different NP-g and NP-c eyes]. The OS structures observed in NP-g-treated eyes at 1 month PI were variable. In one eye, the OSs were quite short (red arrows) although they did make flattened discs. In the other NP-g eye, the OS layer was even more well organized, with OSs that looked almost like WT, although shorter, but were properly packed and aligned (Fig 3C, left NP-g). No rod OSs were observed in uninjected RKO eyes, although cone OSs (blue arrowhead Fig. 3C) were present. Because it can be difficult to visualize the small OSs we observed, several additional images from each treated eye are shown in Supplemental Fig. 1.
To determine whether these OS structures persisted at later ages and to assess how rhodopsin was distributed within them, we next conducted immunogold labeling/EM at 4 months PI. At this time point, OS structures were present in 2/3 NP-g-treated eyes (Fig. 3D), and these structures did contain rhodopsin. As at 1 mo PI, the OSs in NP-g-treated eyes at 4 months PI exhibited well-stacked discs. Small amounts of rhodopsin-labeled membrane were detected in the NP-c-treated eye (arrows, Fig. 3D), but no identifiable OS structures (i.e., discs/rims enclosed by plasma membrane) were observed. No specific rhodopsin immunolabeling was detected in uninjected eyes, although some degenerating cone OSs were observed. Combined, these data indicate that NP-g is capable of preventing or retarding photoreceptor cell death in the RKO model and of supporting OS formation.
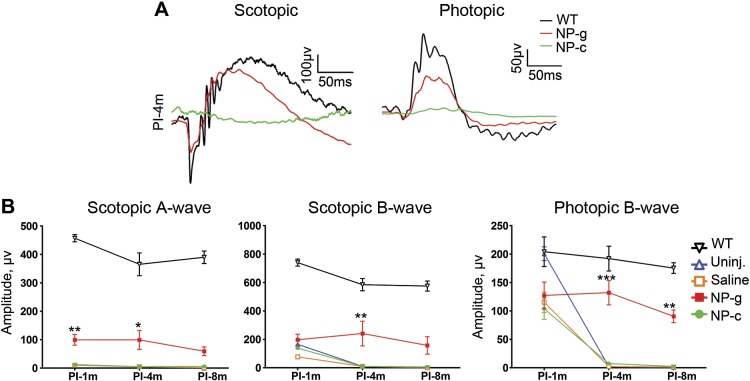
To assess whether improvements in ONL thickness and OS morphology were reflected in improved retinal function, full-field scotopic and photopic electroretinography (ERG) was performed at 1, 4, and 8 months PI in NP-c- and/or -g-treated and control animals, respectively (Fig. 4). Consistent with immunofluorescence and histology, NP-c-treated eyes did not exhibit any improvement in scotopic ERG amplitudes at any time points compared with uninjected or saline-injected animals. In contrast, NP-g-treated animals exhibited nicely shaped scotopic and photopic waveforms (Fig. 4A). This improvement was reflected in quantitation of maximum scotopic a- and b-wave amplitudes, both of which were significantly improved (compared with uninjected) at multiple time points (Fig. 4B, left and middle). The benefit was most marked at 4 months PI, consistent with the good OS structure we observed at that time point, although the improvements did not reach WT levels.
Figure 4.
NP-g-mediated rhodopsin promotes functional improvement in RKO mice. Full-field ERGs were performed. Shown are representative PI-4m scotopic and photopic traces (A), with maximum amplitudes plotted in B (mean ± sem, n = 5–10/group).PI-1m, 1 month PI; PI-4m, 4 months PI; PI-8m, 8 months PI; Uninj., uninjected. *P < 0.05, **P < 0.01, ***P < 0.001 for comparisons between NP-g and uninjected by 2-way ANOVA.
Photopic ERGs in all injected cohorts (NP-c, saline, and NP-g, Fig. 4B) were lower than uninjected RKO and WT mice at 1 mo PI, likely reflecting the effects of the injection procedure. However, by 4 months PI, the remaining cones in the RKO mice have degenerated, and photopic ERG amplitudes are close to 0. Neither saline nor NP-c mediated any improvements in photopic ERG amplitudes at 4 or 8 mo PI (Fig. 4B, right), but excitingly, NP-g mediated significant improvements in photopic ERG amplitudes at both 4 and 8 months PI (compared with uninjected/saline-injected controls).
DISCUSSION
Here we present data indicating that NP-g and NP-c are taken up and expressed by photoreceptors but only NP-g mediates long-term rhodopsin protein expression in the eye. Likewise, phenotypic rescue of the RKO model, specifically leading to retardation of retinal degeneration, development of OS structures, and improvements in scotopic and photopic ERG amplitudes is provided by NP-g. These results suggest that CK30-PEG NPs are an effective delivery strategy for photoreceptors, but that the vector content is critical for therapeutic efficacy.
Our data indicate that inclusion of introns and other genomic elements (such as the 5′UTR) are beneficial for rhodopsin gene expression. Introns have been shown to promote improved gene expression by affecting transcription, polyadenylation, and nuclear export as well as translation (14, 20). Though we do not empirically test these possibilities here, insight into the mechanisms responsible for the improved efficacy of NP-c versus NP-g can be gleaned from our data. We observe that NP-c and NP-g transfect a similar swath of retina and generate similar message levels at early time points, but protein expression from NP-g is higher than from NP-c. This difference is even more striking considering that one-third fewer molecules of NP-g are actually delivered than NP-c (i.e., 4.3 µg DNA was injected but the NP-g plasmid is larger than the NP-c, resulting in fewer plasmid copies). Improved protein but not message in NP-g versus NP-c at 1 month PI suggests that in this case inclusion of introns affected translational rather than transcriptional efficiency. A key difference between transcripts from intron-less vs. the intron-containing DNAs is the presence of the exon junction complex (EJC) on transcripts that have undergone splicing. Several components of this complex remain attached to the transcript after splicing has completed and have been shown to promote export of the transcript to the cytoplasm in multiple systems as well as promote proper localization of mRNAs once in the cytoplasm in Drosophila (35). Improved translational efficiency for intron-containing DNA constructs has also been shown to be tied to components of the EJC that remain attached to the mRNA after export from the nucleus. Importantly, this translational benefit could be recapitulated by artificially attaching several EJC components to an intron-less expression construct (36). These data clearly show that the presence of EJC components on mRNAs can promote improved gene expression; however, for very large genes, inclusion of all introns may be more challenging. Some work has shown, however, that inclusion of merely some (or one) intron, usually in the 5′ rather than 3′ position, can confer these regulatory benefits (20), so it may not be necessary to include all genomic material to promote improved gene expression (compared with cDNA).
At 4 months PI, we observe that both message and protein levels are lower in NP-c versus NP-g. This suggests that either some transcriptional benefits are also conferred by the inclusion of introns in NP-g or that the NP-c vector is more susceptible to DNA silencing/degradation than NP-g, possibilities that cannot be distinguished here. Although we have shown that vectors delivered via CK30-PEG NPs continue generating excellent expression in the RPE for several years (12, 25), we have routinely observed decreased NP-mediated gene expression in photoreceptors over time (21, 22). To help address this, studies are ongoing in our group to assess and distinguish between potential mechanisms of vector loss and DNA silencing.
Although NP-g yielded higher rhodopsin protein levels than NP-c, they did not meet WT levels. Part of this is clearly due to incomplete transfection of the retina: at 1 mo PI, only ∼30% of the retina was transfected with NP-g, suggesting that improving distribution of expression is a key future goal. Message levels from NP-g-treated eyes at 1 mo PI agree quite well with the distribution studies (i.e., transcript levels from NP-g were ∼30% of WT), suggesting that each transfected cell may express, on average, WT levels of rhodopsin transcript. However, protein levels were significantly lower (∼8% of WT), suggesting that although translation of the NP-g transcript was higher than from NP-c, it was not as high as that from the endogenous rhodopsin message. The primary difference between the NP-g message and the endogenous rhodopsin message lies in the 3′ tail: the endogenous allele features a long 3′ UTR comprising 5 different native polyA signals, and the NP-g message features a short 3′UTR, S/MAR, and SV40 polyA signal. Splicing and polyadenylation have been shown to influence each other (reviewed elsewhere) (37). Thus, inclusion of the native 3′UTR in constructs may improve gene expression, and we may test this in future.
Although here our focus was on comparing constructs containing native rhodopsin genomic elements to constructs without those elements, both our gDNA vector and our cDNA vector also contained an S/MAR region. Previously we observed that inclusion of S/MARs improved the levels and duration of exogenous gene expression in the Rpe65−/− model (12), and we have since included S/MARs in additional vectors (22). Although they lack distinct sequence motifs, S/MARs contain ∼70% AT-rich regions (38), which have a high affinity for the nuclear matrix (39). When located directly downstream of the expression cassette, S/MARs can help promote transcription (40, 41), in part thought to be due to localization of the DNA to an actively transcribed region of the nucleus and their ability to serve as an anchoring point for components needed for transcription. In theory, S/MARs and introns could have additive benefits in terms of gene expression, with S/MARs acting primarily at the DNA level in the nucleus and introns have downstream effects on transcription and translation. However, the lack of significant rhodopsin expression from the NP-c in the RKO model suggests that the S/MAR alone is insufficient and that different cell types (RPE and photoreceptors) may have different susceptibility to DNA elements in the expression vector.
The magnitude of improvement we observe in scotopic ERG in NP-g-treated animals exceeds that predicted based on protein levels (i.e., NP-g protein levels at 4 months PI are ∼6% of WT and scotopic a-wave amplitudes are ∼27% of WT), suggesting that matching WT protein levels may not be required for rescue. This observation is consistent with our previous observation showing that expression of ∼10% of WT levels of ABCA4 mediated correction of the delayed dark adaptation phenotype in the Abca4−/− mouse model of Stargardts (22). It is also consistent with the phenotype in the rhodopsin heterozygote mouse; though it does not exhibit normal ERG function, the ERG responses are reduced by only a small amount at early ages (11, 42). Furthermore, the magnitude of improvement was greater for photopic responses than scotopic (e.g., at 4 months PI NP-g photopic ERG amplitudes were not significantly different from WT). This differential preservation of cone vs. rod function highlights the differential mechanisms that underlie rod versus cone vision loss in the RKO model. Specifically, rod vision loss is due to the absence of rhodopsin, and cone vision loss is due to the degeneration of cone cells as a consequence of rod cell loss. Thus the quantity of rhodopsin expressed, although not sufficient to mediate full rod functional improvement, is sufficient to retard cone degeneration, likely because the continuing presence of rod inner segments and cell bodies provides a more favorable environment for cone survival. From a clinical standpoint, preservation of cones and cone function is exciting as these cells mediate our central and high acuity vision, and in fact secondary cone loss is the cause of eventual central vision loss in RP patients.
Our results demonstrate that NP-g mediates improved gene expression and phenotypic rescue compared with the cDNA-containing construct and highlights the potential clinical significance of this technology for treating blindness in RP models, particularly when used in combination with knockdown technology to combat dominant mutations [e.g., see O’Reilly et al. (43)]. We show that inclusion of native genomic elements improves phenotypic outcomes. Although this may vary from gene to gene, these data add to the wealth of other research on the value of introns and genomic elements and suggest that the time may have come to rethink using cDNA as the standard in gene delivery cassettes. The quantity of intronic material in many eukaryotic genes may still be too large to practically deliver in a gene replacement vector; however, our data coupled with that of others (14) suggest that inclusion of even some native introns may promote improved gene expression and clinically relevant therapeutic efficacy.
Supplementary Material
Acknowledgments
The authors thank Miles Merwin and Junjing Guo (both at the University of Oklahoma Health Sciences Center) for their technical assistance. This work was supported by the U.S. National Institutes of Health National Eye Institute (R21EY024059-ZH, EY018656-MIN and EY22778-MIN, EY018137-MRA), the Oklahoma Center for the Advancement of Science and Technology (Z.H., M.I.N., S.M.C.), the Knights Templar Eye Foundation (Z.H., S.M.C.), the Carolina Center of Cancer Nanotechnology Excellence and the North Carolina Translational and Clinical Sciences (Z.H.), and the Research to Prevent Blindness to the University of North Carolina Department Of Ophthalmology. M.J.C. is an employee of Copernicus Therapeutics and holds stock in the company. The remaining authors declare no conflicts of interest.
Glossary
- CK30-PEG
polylysine conjugated to polyethylene glycol
- EJC
exon-junction complex
- ERG
electroretinography
- gDNA
genomic DNA
- NP
nanoparticle
- NP-c
CK30PEG compacted nanoparticle carrying the pEPI rhodopsin cDNA plasmid
- NP-g
CK30PEG compacted nanoparticle carrying the pEPI rhodopsin gDNA plasmid
- OS
outer segment
- PI
postinjection
- RKO
rhodopsin knockout
- RP
retinitis pigmentosa
- RPE
retinal pigment epithelium
- S/MAR
scaffold/matrix attachment region
- WT
wild-type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Wilson J. H., Wensel T. G. (2003) The nature of dominant mutations of rhodopsin and implications for gene therapy. Mol. Neurobiol. 28, 149–158 [DOI] [PubMed] [Google Scholar]
- 2.Amado D., Mingozzi F., Hui D., Bennicelli J. L., Wei Z., Chen Y., Bote E., Grant R. L., Golden J. A., Narfstrom K., Syed N. A., Orlin S. E., High K. A., Maguire A. M., Bennett J. (2010) Safety and efficacy of subretinal readministration of a viral vector in large animals to treat congenital blindness. Sci. Transl. Med. 2, 21ra16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith A. J., Bainbridge J. W., Ali R. R. (2009) Prospects for retinal gene replacement therapy. Trends Genet. 25, 156–165 [DOI] [PubMed] [Google Scholar]
- 4.Annear M. J., Bartoe J. T., Barker S. E., Smith A. J., Curran P. G., Bainbridge J. W., Ali R. R., Petersen-Jones S. M. (2011) Gene therapy in the second eye of RPE65-deficient dogs improves retinal function. Gene Ther. 18, 53–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Millington-Ward S., Chadderton N., O’Reilly M., Palfi A., Goldmann T., Kilty C., Humphries M., Wolfrum U., Bennett J., Humphries P., Kenna P. F., Farrar G. J. (2011) Suppression and replacement gene therapy for autosomal dominant disease in a murine model of dominant retinitis pigmentosa. Mol. Ther. 19, 642–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chadderton N., Millington-Ward S., Palfi A., O’Reilly M., Tuohy G., Humphries M. M., Li T., Humphries P., Kenna P. F., Farrar G. J. (2009) Improved retinal function in a mouse model of dominant retinitis pigmentosa following AAV-delivered gene therapy. Mol. Ther. 17, 593–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palfi A., Millington-Ward S., Chadderton N., O’Reilly M., Goldmann T., Humphries M. M., Li T., Wolfrum U., Humphries P., Kenna P. F., Farrar G. J. (2010) Adeno-associated virus-mediated rhodopsin replacement provides therapeutic benefit in mice with a targeted disruption of the rhodopsin gene. Hum. Gene Ther. 21, 311–323 [DOI] [PubMed] [Google Scholar]
- 8.Jacobson S. G., Acland G. M., Aguirre G. D., Aleman T. S., Schwartz S. B., Cideciyan A. V., Zeiss C. J., Komaromy A. M., Kaushal S., Roman A. J., Windsor E. A., Sumaroka A., Pearce-Kelling S. E., Conlon T. J., Chiodo V. A., Boye S. L., Flotte T. R., Maguire A. M., Bennett J., Hauswirth W. W. (2006) Safety of recombinant adeno-associated virus type 2-RPE65 vector delivered by ocular subretinal injection. Mol. Ther. 13, 1074–1084 [DOI] [PubMed] [Google Scholar]
- 9.Wen X. H., Shen L., Brush R. S., Michaud N., Al-Ubaidi M. R., Gurevich V. V., Hamm H. E., Lem J., Dibenedetto E., Anderson R. E., Makino C. L. (2009) Overexpression of rhodopsin alters the structure and photoresponse of rod photoreceptors. Biophys. J. 96, 939–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan E., Wang Q., Quiambao A. B., Xu X., Qtaishat N. M., Peachey N. S., Lem J., Fliesler S. J., Pepperberg D. R., Naash M. I., Al-Ubaidi M. R. (2001) The relationship between opsin overexpression and photoreceptor degeneration. Invest. Ophthalmol. Vis. Sci. 42, 589–600 [PubMed] [Google Scholar]
- 11.Lem J., Krasnoperova N. V., Calvert P. D., Kosaras B., Cameron D. A., Nicolò M., Makino C. L., Sidman R. L. (1999) Morphological, physiological, and biochemical changes in rhodopsin knockout mice. Proc. Natl. Acad. Sci. USA 96, 736–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koirala A., Makkia R. S., Conley S. M., Cooper M. J., Naash M. I. (2013) S/MAR-containing DNA nanoparticles promote persistent RPE gene expression and improvement in RPE65-associated LCA. Hum. Mol. Genet. 22, 1632–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicoud M., Kong J., Iqball S., Kan O., Naylor S., Gouras P., Allikmets R., Binley K. (2007) Development of photoreceptor-specific promoters and their utility to investigate EIAV lentiviral vector mediated gene transfer to photoreceptors. J. Gene Med. 9, 1015–1023 [DOI] [PubMed] [Google Scholar]
- 14.Nott A., Meislin S. H., Moore M. J. (2003) A quantitative analysis of intron effects on mammalian gene expression. RNA 9, 607–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amy C. M., Williams-Ahlf B., Naggert J., Smith S. (1992) Intron-exon organization of the gene for the multifunctional animal fatty acid synthase. Proc. Natl. Acad. Sci. USA 89, 1105–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farabaugh P. J. (1993) Alternative readings of the genetic code. Cell 74, 591–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rearick D., Prakash A., McSweeny A., Shepard S. S., Fedorova L., Fedorov A. (2011) Critical association of ncRNA with introns. Nucleic Acids Res. 39, 2357–2366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gruss P., Lai C. J., Dhar R., Khoury G. (1979) Splicing as a requirement for biogenesis of functional 16S mRNA of simian virus 40. Proc. Natl. Acad. Sci. USA 76, 4317–4321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brinster R. L., Allen J. M., Behringer R. R., Gelinas R. E., Palmiter R. D. (1988) Introns increase transcriptional efficiency in transgenic mice. Proc. Natl. Acad. Sci. USA 85, 836–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsumoto K., Wassarman K. M., Wolffe A. P. (1998) Nuclear history of a pre-mRNA determines the translational activity of cytoplasmic mRNA. EMBO J. 17, 2107–2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai X., Conley S. M., Nash Z., Fliesler S. J., Cooper M. J., Naash M. I. (2010) Gene delivery to mitotic and postmitotic photoreceptors via compacted DNA nanoparticles results in improved phenotype in a mouse model of retinitis pigmentosa. FASEB J. 24, 1178–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han Z., Conley S. M., Makkia R. S., Cooper M. J., Naash M. I. (2012) DNA nanoparticle-mediated ABCA4 delivery rescues Stargardt dystrophy in mice. J. Clin. Invest. 122, 3221–3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fink T. L., Klepcyk P. J., Oette S. M., Gedeon C. R., Hyatt S. L., Kowalczyk T. H., Moen R. C., Cooper M. J. (2006) Plasmid size up to 20 kbp does not limit effective in vivo lung gene transfer using compacted DNA nanoparticles. Gene Ther. 13, 1048–1051 [DOI] [PubMed] [Google Scholar]
- 24.Farjo R., Skaggs J., Quiambao A. B., Cooper M. J., Naash M. I. (2006) Efficient non-viral ocular gene transfer with compacted DNA nanoparticles. PLoS ONE 1, e38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koirala A., Conley S. M., Makkia R., Liu Z., Cooper M. J., Sparrow J. R., Naash M. I. (2013) Persistence of non-viral vector mediated RPE65 expression: case for viability as a gene transfer therapy for RPE-based diseases. J. Control. Release 172, 745–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yurek D. M., Flectcher A. M., Kowalczyk T. H., Padegimas L., Cooper M. J. (2009) Compacted DNA nanoparticle gene transfer of GDNF to the rat striatum enhances the survival of grafted fetal dopamine neurons. Cell Transplant. 18, 1183–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding X. Q., Quiambao A. B., Fitzgerald J. B., Cooper M. J., Conley S. M., Naash M. I. (2009) Ocular delivery of compacted DNA-nanoparticles does not elicit toxicity in the mouse retina. PLoS ONE 4, e7410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han Z., Koirala A., Makkia R., Cooper M. J., Naash M. I. (2012) Direct gene transfer with compacted DNA nanoparticles in retinal pigment epithelial cells: expression, repeat delivery and lack of toxicity. Nanomedicine (Lond.) 7, 521–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han Z., Conley S. M., Makkia R., Guo J., Cooper M. J., Naash M. I. (2012) Comparative analysis of DNA nanoparticles and AAVs for ocular gene delivery. PLoS ONE 7, e52189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piechaczek C., Fetzer C., Baiker A., Bode J., Lipps H. J. (1999) A vector based on the SV40 origin of replication and chromosomal S/MARs replicates episomally in CHO cells. Nucleic Acids Res. 27, 426–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Ubaidi M. R., Pittler S. J., Champagne M. S., Triantafyllos J. T., McGinnis J. F., Baehr W. (1990) Mouse opsin. Gene structure and molecular basis of multiple transcripts. J. Biol. Chem. 265, 20563–20569 [PubMed] [Google Scholar]
- 32.Ziady A. G., Gedeon C. R., Miller T., Quan W., Payne J. M., Hyatt S. L., Fink T. L., Muhammad O., Oette S., Kowalczyk T., Pasumarthy M. K., Moen R. C., Cooper M. J., Davis P. B. (2003) Transfection of airway epithelium by stable PEGylated poly-L-lysine DNA nanoparticles in vivo. Mol. Ther. 8, 936–947 [DOI] [PubMed] [Google Scholar]
- 33.Liu G., Li D., Pasumarthy M. K., Kowalczyk T. H., Gedeon C. R., Hyatt S. L., Payne J. M., Miller T. J., Brunovskis P., Fink T. L., Muhammad O., Moen R. C., Hanson R. W., Cooper M. J. (2003) Nanoparticles of compacted DNA transfect postmitotic cells. J. Biol. Chem. 278, 32578–32586 [DOI] [PubMed] [Google Scholar]
- 34.Flannery J. G., Zolotukhin S., Vaquero M. I., LaVail M. M., Muzyczka N., Hauswirth W. W. (1997) Efficient photoreceptor-targeted gene expression in vivo by recombinant adeno-associated virus. Proc. Natl. Acad. Sci. USA 94, 6916–6921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hachet O., Ephrussi A. (2001) Drosophila Y14 shuttles to the posterior of the oocyte and is required for oskar mRNA transport. Curr. Biol. 11, 1666–1674 [DOI] [PubMed] [Google Scholar]
- 36.Nott A., Le Hir H., Moore M. J. (2004) Splicing enhances translation in mammalian cells: an additional function of the exon junction complex. Genes Dev. 18, 210–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Proudfoot N. J., Furger A., Dye M. J. (2002) Integrating mRNA processing with transcription. Cell 108, 501–512 [DOI] [PubMed] [Google Scholar]
- 38.Jackson D. A., Juranek S., Lipps H. J. (2006) Designing nonviral vectors for efficient gene transfer and long-term gene expression. Mol. Ther. 14, 613–626 [DOI] [PubMed] [Google Scholar]
- 39.Heng H. H., Goetze S., Ye C. J., Liu G., Stevens J. B., Bremer S. W., Wykes S. M., Bode J., Krawetz S. A. (2004) Chromatin loops are selectively anchored using scaffold/matrix-attachment regions. J. Cell Sci. 117, 999–1008 [DOI] [PubMed] [Google Scholar]
- 40.Stehle I. M., Scinteie M. F., Baiker A., Jenke A. C., Lipps H. J. (2003) Exploiting a minimal system to study the epigenetic control of DNA replication: the interplay between transcription and replication. Chromosome Res. 11, 413–421 [DOI] [PubMed] [Google Scholar]
- 41.Bode J., Benham C., Knopp A., Mielke C. (2000) Transcriptional augmentation: modulation of gene expression by scaffold/matrix-attached regions (S/MAR elements). Crit. Rev. Eukaryot. Gene Expr. 10, 73–90 [PubMed] [Google Scholar]
- 42.Liang Y., Fotiadis D., Maeda T., Maeda A., Modzelewska A., Filipek S., Saperstein D. A., Engel A., Palczewski K. (2004) Rhodopsin signaling and organization in heterozygote rhodopsin knockout mice. J. Biol. Chem. 279, 48189–48196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Reilly M., Palfi A., Chadderton N., Millington-Ward S., Ader M., Cronin T., Tuohy T., Auricchio A., Hildinger M., Tivnan A., McNally N., Humphries M. M., Kiang A. S., Humphries P., Kenna P. F., Farrar G. J. (2007) RNA interference-mediated suppression and replacement of human rhodopsin in vivo. Am. J. Hum. Genet. 81, 127–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.