Abstract
Transforming growth factor (TGF-β) derived from bone fuels melanoma bone metastases by inducing tumor secretion of pro-metastatic factors that act on bone cells to change the skeletal microenvironment. Halofuginone is a plant alkaloid derivative that blocks TGF-β signaling with antiangiogenic and antiproliferative properties. Here, we demonstrate for the first time that halofuginone therapy decreases development and progression of bone metastasis caused by melanoma cells through inhibition of TGF-β signaling.
Halofuginone treatment of human melanoma cells inhibited cell proliferation, phosphorylation of SMAD proteins in response to TGF-β, and TGF-β-induced SMAD-driven transcription. In addition, halofuginone reduced expression of TGF-β target genes that enhance bone metastases, including PTHrP, CTGF, CXCR4, and IL11. Also, cell apoptosis was increased in response to halofuginone.
In nude mice inoculated with 1205Lu melanoma cells, a preventive protocol with halofuginone inhibited bone metastasis. The beneficial effects of halofuginone treatment were comparable to those observed with other anti-TGF-β strategies, including systemic administration of SD208, a small molecule inhibitor of TGF-β receptor I kinase, or forced overexpression of Smad7, a negative regulator of TGF-β signaling. Furthermore, mice with established bone metastases treated with halofuginone had significantly less osteolysis than mice receiving placebo assessed by radiographys. Thus, halofuginone is also effective in reducing the progression of melanoma bone metastases. Moreover, halofuginone treatment reduced melanoma metastasis to the brain, showing the potential of this novel treatment against cancer metastasis.
Keywords: Halofuginone, bone metastases, melanoma bone metastases, TGF-β signaling, TGF-β inhibitors
Introduction
Melanoma, a malignant tumor that originates in melanocytes, is the sixth most common cancer in the United States (1). The majority of patients with melanoma initially present with localized disease; however in advanced melanoma, cancer cells metastasize to lungs, liver, brain, and frequently to bone (2). Once melanoma metastasizes to bone it is incurable. The metastases cause severe pain, fractures, hypercalcemia, nerve compression syndromes, and paralysis (3, 4). Melanoma cells in bone secrete multiple factors that induce osteoclastic bone destruction to release and activate growth factors stored in mineralized bone matrix, which in turn stimulate tumor growth and further bone destruction (5). Transforming growth factor-beta (TGF-β) is the major bone-derived factor responsible for driving a feed-forward cycle of cancer metastasis in bone. TGF-β, stored in the bone matrix, is released and activated during osteoclastic bone resorption, and further induces tumor production of osteolytic and prometastatic factors, such as parathyroid hormone-related protein (PTHrP) and interleukin 11 (IL-11), which stimulate bone destruction, connective tissue growth factor (CTGF) and vascular endothelial growth factor (VEGF) regulating angiogenesis, and the chemokine receptor CXCR4, which causes tumors to home to bone (6, 7). Although TGF-β is a tumor suppressor in normal epithelial cells, it becomes a pro-metastatic factor in advanced cancer, where it activates the epithelial-mesenchymal transition, tumor cell invasion, angiogenesis and immunosuppression. Disruption of TGF-β signaling or neutralization of TGF-β-regulated genes in cancer cells decreases bone metastases in preclinical models (8–11).
Melanoma, like other human malignant tumors, secretes large amounts of TGF-β, which in turn promotes resistance to the TGF-β growth inhibitory effects (12–14) and metastatic progression (15, 16). Elevated plasma concentrations of TGF-β are associated with melanoma progression, and high levels of TGF-β2 expression in malignant melanoma correlate with tumor invasion in patients (17, 18). Preclinical studies showed that treatment with the TβRI kinase inhibitor, SD208, caused a reduction in osteolytic bone metastasis and TGF-β-regulated metastatic genes in mice with melanoma and bone metastases (10). Overexpression of the TGF-β-signaling inhibitor Smad7 in 1205Lu melanoma cells prevented metastasis to bone in mice, and delayed the establishment and growth of melanoma bone metastases (9). However, the in vitro and animal studies described above have not yet led to clinical application. Indeed, the treatment of metastatic melanoma has not changed substantially in more than a decade, and new strategies are urgently needed.
Halofuginone is a modified plant alkaloid first identified as an inhibitor of fibrosis that decreases type I collagen synthesis (19, 20); it was tested in a recently completed phase II clinical trial in patients with AIDS-related Kaposi’s Sarcoma (KS) and showed efficacy to reduce KS lesions (21). Halofuginone inhibits TGF-β-dependent phosphorylation of Smad2/3 proteins and increases Smad7 expression (22), We hypothesize that it could be effective against metastatic melanoma. Findings from extensive studies in animal models suggest that halofuginone might be effective for the treatment of cancer and fibrotic diseases. Halofuginone decreased growth of bladder carcinoma (23), glioma (24), prostate cancer (25), hepatocellular carcinoma (26), Wilms tumor (27), and malignancies of brain (28), and pancreas (29).
Here, we investigated the potential of halofuginone to reduce bone metastases from melanoma in a murine model. Our findings suggest that halofuginone is a promising agent to decrease metastasis of melanoma to bone.
Materials and Methods
Cell culture & reagents
Human 501mel and 888mel cells were obtained from and validated by the American Type Culture Collection [ATCC] by DNA profiling (Manassas, VA). They were grown in RPMI medium supplemented with 10% FBS and antibiotics. Human melanoma cell lines 1205Lu and WM852, derived from WM793 by serial passage through athymic mice and selection of cells metastatic to the lungs, and nodular melanoma metastases, respectively, were a kind gift from Dr M. Herlyn, Wistar Institute (Philadelphia, PA), and have been previously described (10, 30). They were grown in W489 medium comprised of three parts MDCB153 and one part L15 with 4% FBS and antibiotics. The Smad7 overexpressing clone (1205Lu-Smad7) was described previously (31). Halofuginone (dl-trans-7-bromo-6-chloro-3-[3-(3-hydroxy-2piperidyl) acetonyl]-4(3H)-quinazolinone hydrobromide) was a gift from Intervet Innovation GmbH (Schwabenheim, Germany). Stock solutions for in vitro studies of 2mg/ml were prepared by dissolving halofuginone in a lactic acid buffer (0.44M, pH 4.3), and stored at −20°C (22). For in vivo experiments, halofuginone (1 or 5µg/0.1ml) was resuspended in PBS. SD208 was obtained from Scios, Inc. (San Francisco, CA). Recombinant human TGF-β1 was purchased from R&D Systems, Inc. (Minneapolis, MN).
Dual-luciferase assay
Melanoma cells were transfected with pGL3-luc constructs (Promega, Madison, WI) expressing firefly luciferase either constitutively or under the control of a TGF-β-induced reporter (CAGA)9–luc (32) and with a phRL-CMV plasmid constitutively expressing renilla luciferase (Promega) for normalization. Twenty-four hrs later, cells were pretreated with halofuginone for 4hrs, followed by TGF-β1 (5ng/ml) for 24hrs. Cells were lysed using Passive Lysis buffer (Promega). Luciferase activities were determined using Dual-Luciferase Reporter Assay System (Promega).
Proliferation assay
Cell proliferation was assessed using a colorimetric assay (MTT assay). 1205Lu cells were treated with increasing concentrations of halofuginone (50–300nM) TGF-β (5ng/ml). Next, MTT reagent (20µl, 5mg/ml) was added to each well and incubated for 5hrs at 37C followed by a stop buffer (100µl of HCL 0.01M-10% SDS) to lyse the cells. Absorbance was measured at 570nm using a Synergy™HT spectrophotometer (Biotek, Winooski, VT).
Apoptosis assay
Cell apoptosis was evaluated using Annexin V-EGFP apoptosis detection kit (BioVision, Milpitas, CA). Melanoma 1205Lu cells were treated with increasing concentrations of halofuginone (50–300nM) for 24, 48 or 72hrs. Melanoma 1205Lu cells treated with H2O2 (8mM) overnight, served as a positive control. Cells were trypsinized and counted, and 5×105 cells were collected by centrifugation and resuspended in 500µl of binding buffer. Next, 5µl of Annexin V-EGFP and 5µl of propidium iodide (PI) were added and incubated at room temperature for 5min in the dark. Cells were quantified using a FACSCalibur flow cytometer (BD Biosciences). Experiments were performed in triplicate and for each sample, 15,000-events were collected and analyzed using FlowJo software (Ashland, OR).
Western blot analysis
Cells were pretreated with halofuginone (200nM) for 4hrs followed by TGF-β1 (5ng/ml) for 24hrs. Samples were run on 10% SDS-PAGE minigels and transferred onto a Hybond™-P membrane (GE Healthcare Life Sciences, Waukesham WI). Membranes were blocked in TBS-T-milk (5%) for 1hr, incubated overnight with primary antibody and for 1hr with secondary antibody. Protein detection was performed using Western-Chemiluminescent HRPsubstrate (Millipore, Billerica, MA). Antibodies against Phospho-Smad2 (Ser465/467), Phospho-Smad3 (Ser423/425), Smad2 and Smad3 were purchased from Cell Signaling, Danvers, MA; anti β-actin antibody (Cell Signaling) was used for normalization.
Gene expression analysis: RNA extraction & Real time PCR
1205Lu cells were treated with halofuginone (200nM) for 4hrs followed by TGF-β1 (5ng/ml) for 24hrs. Total RNA was isolated using RNeasy kit (Qiagen, Valencia, CA) and reverse-transcribed using Superscript II (Invitrogen). Resulting cDNAs were processed for real time PCR using QuantiTect SYBR Green PCR kit (Qiagen) and analyzed using a MyiQ™ Single-color-Real-Time PCR detection system (Biorad) for 40 cycles (95°C for 15sec/58°C for 30sec/72°C for 30sec) after an initial 15min incubation at 95°C. Primers were optimized for real-time PCR (amplification efficiency 100±5%). Primer sequences used for the analysis were: PTHrP (sense, 5′-ACTCGCTCTGCCTGGTTAGA-3′; antisense, 5′-GGAGGTGTCAGACAGGTGGT-3′); CTGF (sense, 5′-GCTACCACATTTCCTACCTAGAAATCA-3′; antisense, 5′-GACAGTCCGTCAAAACAGATTGTT-3′); CXCR4 (sense, 5′-CCGTGGCAAACTGGTACTTT-3′; antisense, 5′-GACGCCAACATAGACCACCT-3′); IL-11 (sense, 5′-TGAAGACTCGGCTGTGACC-3′; antisense, 5′- CCTCACGGAAGGACTGTCTC-3′). Target gene expression was normalized using housekeeping gene ribosomal protein L32 (RPL32). Samples were analyzed in triplicate and data analyzed using the ΔΔCt method (33).
Animal experiments
Animal experiments were performed at the University of Virginia in Charlottesville, Virginia (UVA). Animal protocols were approved by the Institutional Animal Care and Use Committee and were done in accordance with national and international guidelines.
Intracardiac inoculation of tumor was performed as previously described (34). Briefly, 1205Lu melanoma cells were resuspended to a final concentration of 105 cells in 100µL in PBS. Tumor inoculation into the left cardiac ventricle was done on anesthetized mice positioned ventral side up. Development of osteolytic lesions in mice was followed weekly by radiography using a Faxitron MX-20 with digital camera (Faxitron X-ray Corporation, Wheeling, IL). Lesion area was quantified using the image analysis system MetaMorph software (Universal Imaging Corporation, Downingtown, PA).
Experimental design
Prevention protocol
Female nude mice were divided into 6 groups (n=15 per group) identified as 1) PBS (vehicle control), 2) halofuginone 1µg/mouse/day, 3) halofuginone 5µg/mouse/day, 4) methylcellulose (1%) and 5) SD208 (60mg/kg/day), according with the respective treatment. Groups 1 to 5 were inoculated with 1205Lu melanoma cells into the left ventricle. Group 6, identified as Smad7, received 1205Lu-Smad7 cells, a stable clone that overexpresses the inhibitory protein Smad7, previously described (31). All treatments were initiated two days prior to melanoma cell inoculation. PBS and halofuginone treatments (100µL) were administered daily by intraperitoneal (i.p.) injection. SD208 and its methylcellulose vehicle were administered daily by oral gavage. For survival studies, mice were monitored daily and euthanized when they presented signs of tumor-associated morbidity such as severe loss of body weight, breathing troubles or paralysis.
Therapeutic Protocol
Female nude mice were divided into 3 groups (n=15 per group) and inoculated in the left ventricle with 1205Lu cells. Osteolytic lesion progression in the hindlimbs of the mice was followed using X-ray. Treatment with PBS (100µl) or halofuginone (1 or 5µg/mouse/day) i.p. was initiated after osteolytic lesions were detected (about 13 1days after the intracardiac inoculation) and continued until the end of the study.
Bone histology & histomorphometry
Hindlimbs were fixed in formalin (10%) for 48h and decalcified in EDTA (10%) for 2weeks and embedded in paraffin for sectioning. Longitudinal, mid-sagittal sections 3.5mm in thickness were cut from the tibia and femur using an automated Microm HM 355S microtome (Thermo Fisher Scientific) and stained with hematoxylin and eosin, or for TRAP activity to identify osteoclasts. All sections were visualized on a Leica DM LB compound microscope (Leica Microsystems, Bannockburn, IL) with a Q-Imaging Micropublisher Cooled CCD color digital camera (Vashaw Scientific Inc., Washington, DC). Images were captured and analyzed using BIOQUANT OSTEO software (Image Analysis Corporation, Nashville, TN). Tumor burden per bone was defined as area of tibial and femoral bone occupied by cancer cells. Osteoclast number at the tumor/bone interface (OC/mm bone surface) was measured on TRAP-stained slides of femur and tibia at 40× magnification.
Brain and soft tissue histology
In the prevention protocol, brain were collected from 5 mice/group and fixed in 10% buffered formalin. Each brain was cut coronally into four quarters and stained for Ki-67, a tumor cell proliferation marker, according to the manufacturer’s instructions (Abcam, Cambridge, MA). We counted clusters of Ki-67-positive tumor cells per total area in the cerebral cortex.
Statistical analysis
Differences between groups were determined by one-way ANOVA or two-way ANOVA followed by Bonferroni’s post-test. Kaplan-Meier survival curve data were analyzed by log-rank test. All the results were analyzed using GraphPad Prism v4.0 software (La Jolla, CA), expressed as mean SEM, and P< 0.05 was considered significant.
Results
Halofuginone inhibits TGF-β signaling in 1205Lu human melanoma cells
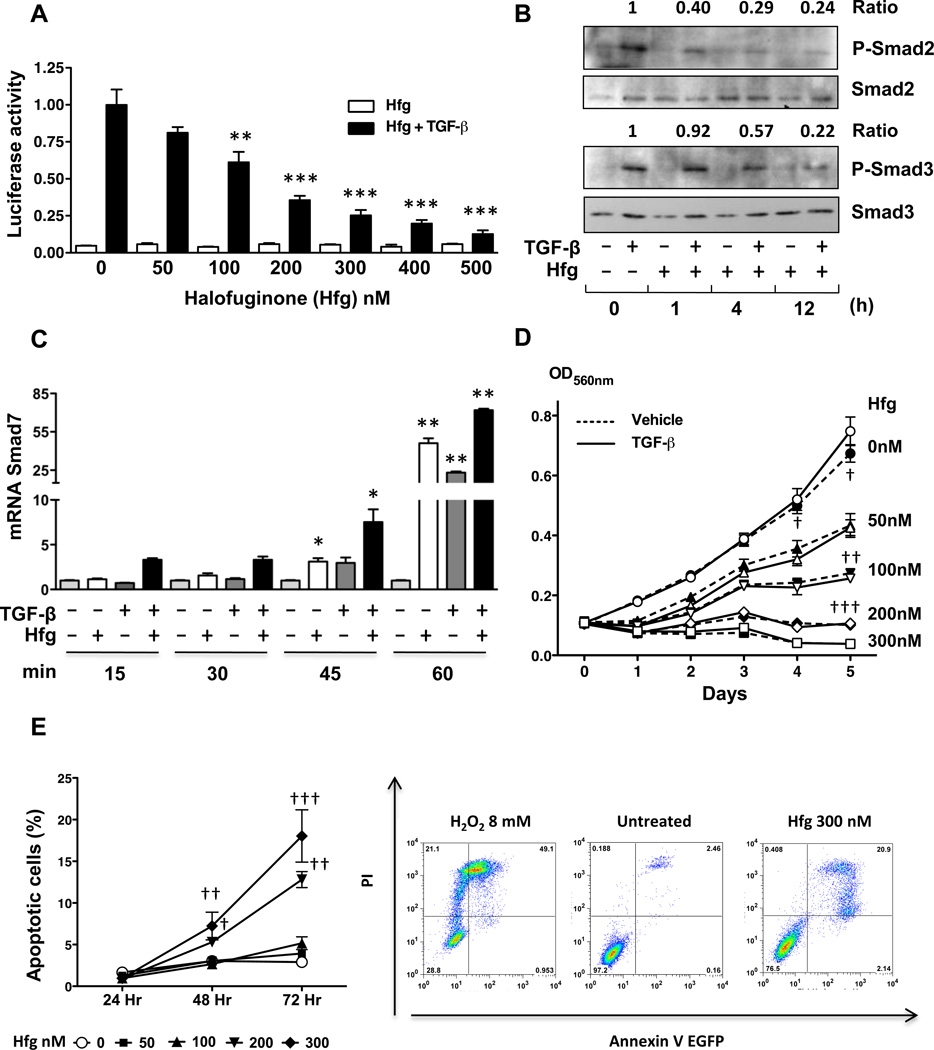
The effect of halofuginone on TGF-β-signaling was examined using 1205Lu human melanoma cells transfected with TGF-β-induced reporter (CAGA)9–luc (32), and treated with increasing concentrations of halofuginone in the presence or absence of TGF-β for 24hrs. As shown in Fig. 1A, halofuginone dose-dependently inhibited TGF-β-induced promoter activity. Next, we determined by Western blotting the effect of halofuginone pretreatment of 1205Lu cells at three different time points (1, 4 and 12hrs) on the phosphorylation of Smad2/3 protein in response to TGF-β. Halofuginone pretreatment by 4 and 12hrs followed by 30 mins of TGF-β treatment decreased the levels of phosphoSmad2 and phosphoSmad3 levels, while total Smad2/3 levels remained unchanged (Fig. 1B). Halofuginone can induce the mRNA expression of the TGF-β-signaling inhibitor Smad7 in epithelial and hepatocellular carcinoma cells (22). Thus, we studied the ability of halofuginone to induce expression of Smad7mRNA in melanoma cells. As shown on Fig. 1C, halofuginone pretreatment of human 1205Lu cells for 4hrs followed by TGF-β treatment for 15 to 60min significantly increased expression of Smad7 mRNA, whereas the higher Smad7 mRNA expression was observed with TGF-β treatment for 45min or longer in the presence of halofuginone.
Figure 1. Halofuginone inhibits TGF-β signaling in 1205Lu human melanoma cells.
A. 1205Lu cells were transfected with a (CAGA)9-luc vector together with a pRL-TK Renilla luciferase vector, then treated with Halofuginone (Hfg) alone or with TGF-β 5ng/ml for 24hrs. Cells were lyzed and dual-luciferase activity was measured. B. 1205Lu cells were treated with 200nM Hfg for 1, 4, 12h and lyzed 30min after addition of 5ng/ml TGF-β. Protein lysates were analyzed for total and phosphorylated Smad 2&3 by Western blot. Ratio P-Smad2–3/Smad2–3 is indicated. C. 1205Lu cells were treated with 200nM Hfg for 4h followed by 5ng/ml TGF-β for 15, 30, 45 or 60min. Smad7 mRNA was assayed by quantitative RT-PCR. D. MTT assay of 1205Lu cells treated with increasing concentrations of Hfg alone or in the presence of TGF-β 5ng/ml (continuous line). E. Left, quantification of percent of 1205Lu cells annexin V+-PI+ after being treated with Hfg (50–300 nM) for 24, 48, 72 hrs. Right, representative dot plots of positive control, untreated & cells treated with Hfg 300 nM for 72 hrs. *P<0.05, ** P<0.01, ***P<0.001 vs control using one-way ANOVA. †† P<0.01, ††† P<0.001 vs control using two-way ANOVA with a Bonferroni’s post test.
Halofuginone reduces proliferation and induces apoptosis of 1205Lu human melanoma cells
Halofuginone (50nM or 100nM) significantly reduced the proliferation of 1205Lu cells when compared to untreated cells (Fig. 1D). Treatment with higher doses of halofuginone (200–300nM) completely prevented cell proliferation. Presence or absence of TGF-β did not change the effect of halofuginone on the proliferation of 1205Lu (Fig. 1D).
To determine whether halofuginone induce apoptosis in 1205Lu melanoma cells, cells where incubated with increasing concentrations of halofuginone (50–300nM) and a flow cytometric annexin V-PI assay was performed after 24, 48 and 72hrs. Halofuginone 200 and 300nM treatments for 48 and 72hrs, significantly induced cell death (Fig. 1E).
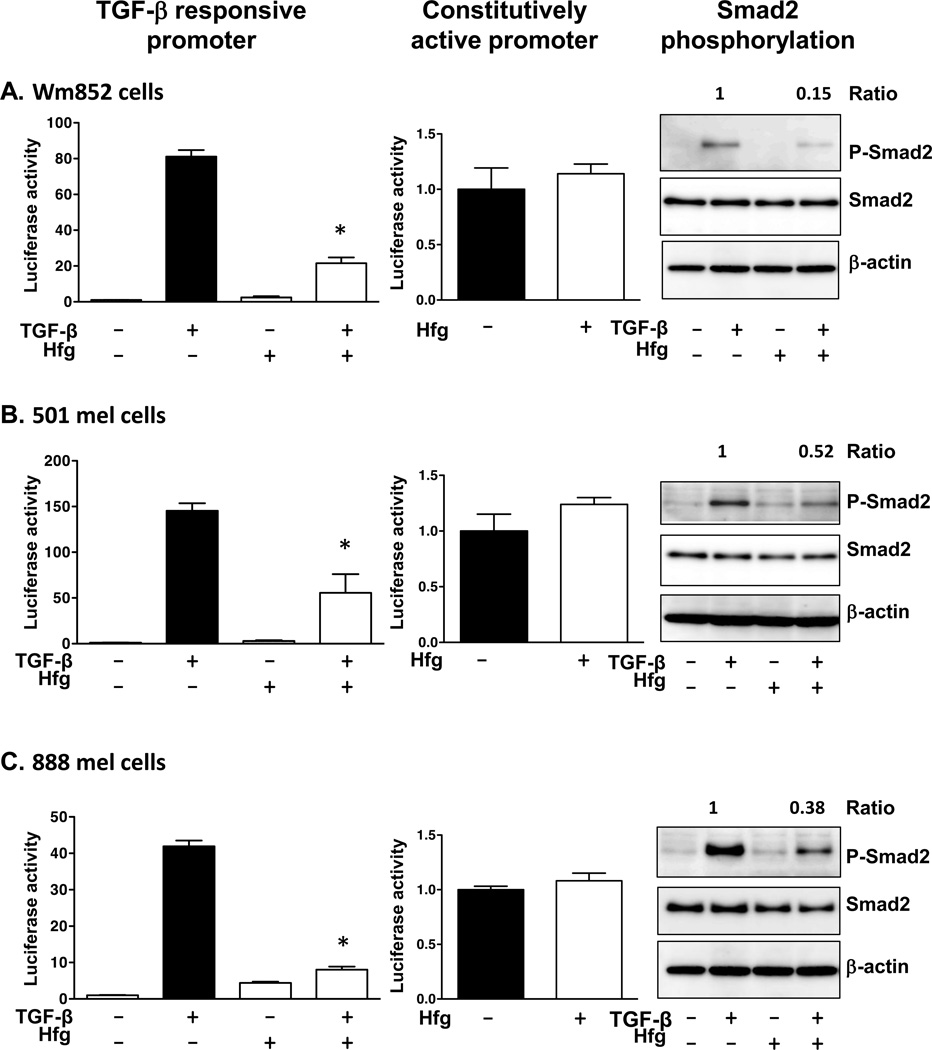
Halofuginone blocks TGF-β-signaling in multiple human melanoma cell lines
We tested the effects of halofuginone in three additional TGF-β-responsive human melanoma cell lines: WM852, 501mel and 888mel (13). Halofuginone (200nM) treatment for 24hrs significantly inhibited TGF-β-induced activity of the (CAGA)9 promoter in all the melanoma lines analyzed (Fig. 2). In contrast, halofuginone had no effect on cells transfected with a constitutively active promoter, showing that at the concentration tested halofuginone was not having nonspecific transcriptional effects. Furthermore, TGF-β-induced Smad2 phosphorylation was decreased by halofuginone treatment in all three-cell lines (Fig. 2).
Figure 2. Halofuginone reduces TGF-β-signaling in human melanoma cell lines.
Wm852 (A), 501mel (B) and 888mel cells (C) were co-tranfected with a TGF-β-induced reporter (CAGA)9–luc or constitutively activated CMV-promoter, and phRL-CMV Renilla luciferase vector. Cells were treated with Hfg (200nM) alone or with TGF-β (5ng/ml) for 24hrs before measuring luciferase activity. Western blot analysis for melanoma cells treated for 4hrs with Hfg (200nM) followed by TGF-β-treatment (5ng/ml) for 30min. Ratio P-Smad2/Smad2 is indicated. *P<0.05 vs TGF-β-treated cells using one-way ANOVA with Bonferroni’s post test.
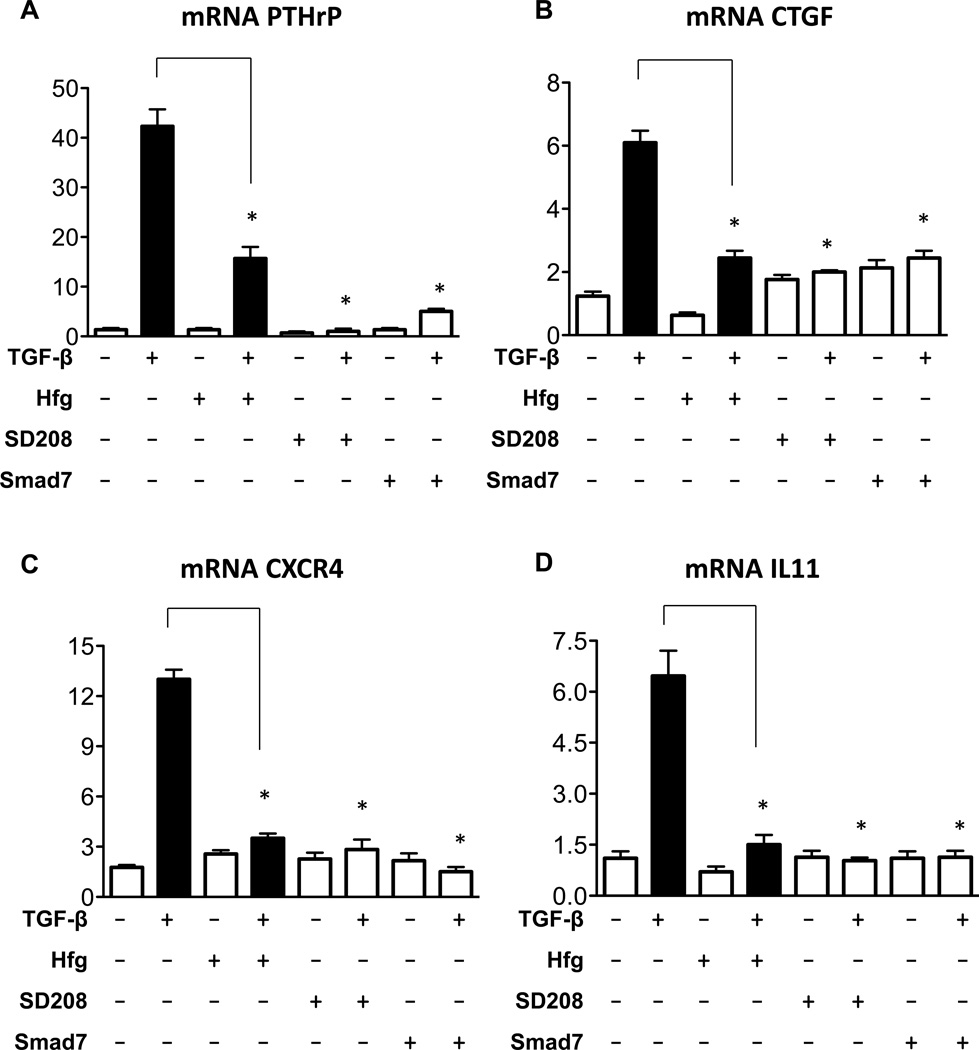
Halofuginone reduces mRNA expression of TGF-β-regulated bone metastasis specific genes
Previous work identified a group of TGF-β-induced genes that promote bone metastasis in breast cancer cells (6) as well as in melanoma cells (9, 10). We examined whether halofuginone can modulate the expression of bone metastasis specific genes in 1205Lu melanoma cells. TGF-β treatment of 1205Lu cells resulted in the induction of mRNA levels of PTHrP, CTGF, CXCR4 and IL11 (Fig. 3). Meanwhile cells treated with TGF-β in the presence of halofuginone showed a reduction of mRNA levels of bone metastasis genes compared to TGF-β alone (Fig. 3). Halofuginone-mediated downregulation of the expression of TGF-β-regulated metastatic genes was similar to that of other TGF-β inhibitors including the small molecule inhibitor of TGF-β receptor I kinase, SD208, or overexpression of the Smad7 inhibitor in 1205Lu-Smad7 cells (Fig 3).
Figure 3. Halofuginone reduces mRNA expression of TGF-β regulated pro-metastatic genes.
Human 1205Lu melanoma cells or Smad7 overexpressing cells (1205Lu-Smad7) were preincubated with Hfg (200nM) or SD208 (1mM) for 4hrs prior stimulation with TGF-β (5ng/ml) for 24hrs. Total RNA was extracted and gene expression was measured using quantitative RT-PCR. *P<0.01 vs TGF-β using one-way ANOVA with Bonferroni’s post test.
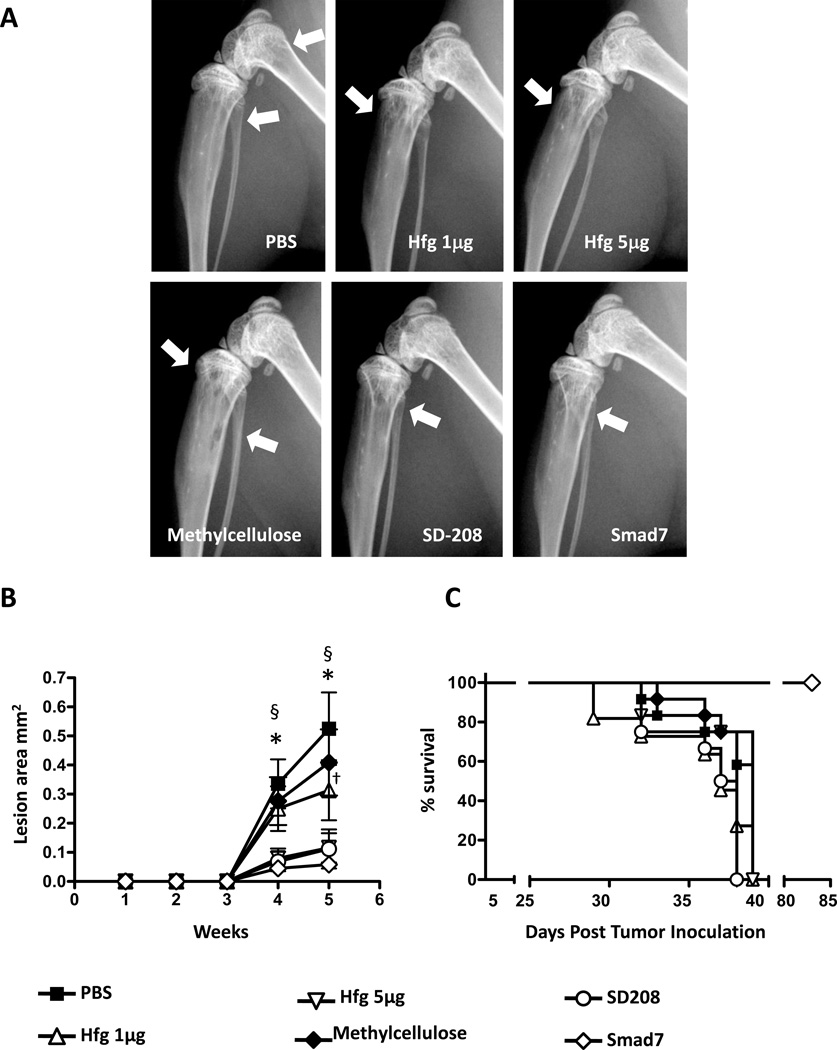
Preventive treatment with Halofuginone reduces osteolytic metastases
To investigate the ability of halofuginone to prevent melanoma bone metastases in vivo, we used nude mice inoculated with 1205Lu melanoma cells into the cardiac left ventricle (9). Two doses of halofuginone (1 or 5µg/mouse/day) were evaluated in this prevention protocol. Drug treatment started two days prior to intracardiac inoculation of cells and was continued daily throughout the experiment. To compare the efficacy of halofuginone with another inhibitor of the TGF-β-signaling pathway and bone metastasis, mice were treated with SD208 (60mg/kg/day, p.o.) as well as mice receiving the methylcellulose vehicle. In addition, mice were inoculated with 1205Lu-Smad7 cells that overexpressed the TGF-β-signaling inhibitor, Smad7, as a further control. Figure 4(A–B) shows representative X-ray images of hindlimbs of mice inoculated with 1205Lu cells, the different treatments, and quantification of osteolytic area. Preventive treatment with halofuginone significantly reduced osteolytic lesion area compared to animals treated with PBS in a dose dependent manner Figure 4(A–B). As previously reported (10) treatment with SD208 significantly decreased osteolytic lesions compared to vehicle-treated mice to a level comparable to 5µg of halofuginone. Similarly, overexpression of Smad7 protein protected the mice from developing melanoma bone metastasis, as described previously (9). Treatments with halofuginone or SD208 did not increase the overall survival of the mice (Fig 4C). However, mice inoculated with 1205Lu cells overexpressing Smad7 had significantly improved survival compared to controls (Fig. 4C).
Figure 4. Preventive treatment with Halofuginone reduces osteolytic melanoma bone metastases.
A. Representative radiography of the hindlimbs of female mice (4 weeks old, n=12) after intracardiac inoculation of 1205LU cells. Treatment was started 2 days before intracardiac inoculation and continued daily. B. Osteolytic lesion area (mm2) was measured on radiographs of hindlimbs of mice with bone metastasis. C. Kaplan-Meyer analysis of mouse survival. *P<0.05, Hfg 1µg/5µg or Smad7 vs PBS; §, P<0.05; SD208 vs Methylcellulose; †, P<0.05, Hfg 5µg vs Hfg 1µg, using two-way ANOVA with Bonferroni’s post test.
Halofuginone reduces the progression of established osteolytic bone metastases
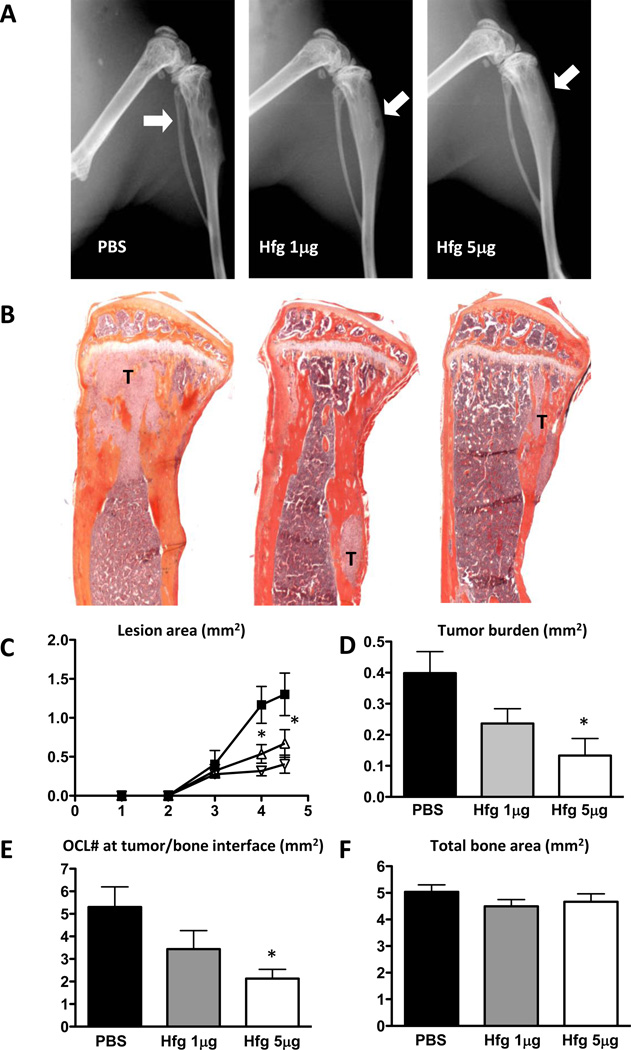
Next we tested the ability of Halofuginone to reduce osteolytic metastasis in a therapeutic protocol. Halofuginone was administered after osteolytic lesions were detected on radiographs, 13 days after tumor cell inoculation. Nude mice were then inoculated i.p. daily with PBS or halofuginone (1 or 5µg/mouse/day). Animals were euthanized at the same time point in order to compare tumor burden between PBS-and halofuginone-treated groups. Both doses of halofuginone significantly reduced the area of osteolytic lesions measured on radiographs (Fig. 5A, C). Histomorphometric analysis confirmed that halofuginone reduced significantly tumor area (Fig. 5B, D). Because TGF-β-stimulated cancer cells increase osteoclast differentiation and bone resorption, we measured the number of osteoclasts at the tumor-bone interface. Mice treated with halofuginone (5g/day) had a significantly lower osteoclast number at the tumor-bone interface compared to PBS-treated mice (Fig. 5E). Halofuginone treatment did not have any effect on the total bone area when compared to mice treated with PBS (Fig. 5F). These results suggest that halofuginone inhibits melanoma bone metastases through reduction of osteoclasts in addition to its action on tumor cells.
Figure 5. Halofuginone therapeutic treatment decreases osteolytic melanoma bone metastases.
A. Representative X-ray images from hindlimbs of mice 4weeks post inoculation with 1205Lu cells. Hfg treatment initiated 13 days after cell inoculation, once osteolytic lesion was detected. Arrows indicate osteolytic lesions. B. Representative histology of tibias with tumor indicated by letter T. C. Quantification of osteolytic lesion area. D. Tumor burden area measured by quantitative histomorphometry. E. Osteoclast number per bone surface was counted in TRAP stained sections of hindlimbs of mice. F. Total bone area analyzed by quantitative histomorphometry. *P<0.05 vs PBS using one-way ANOVA with Bonferroni’s post test.
Halofuginone decreases brain metastasis of 1205Lu melanoma cells
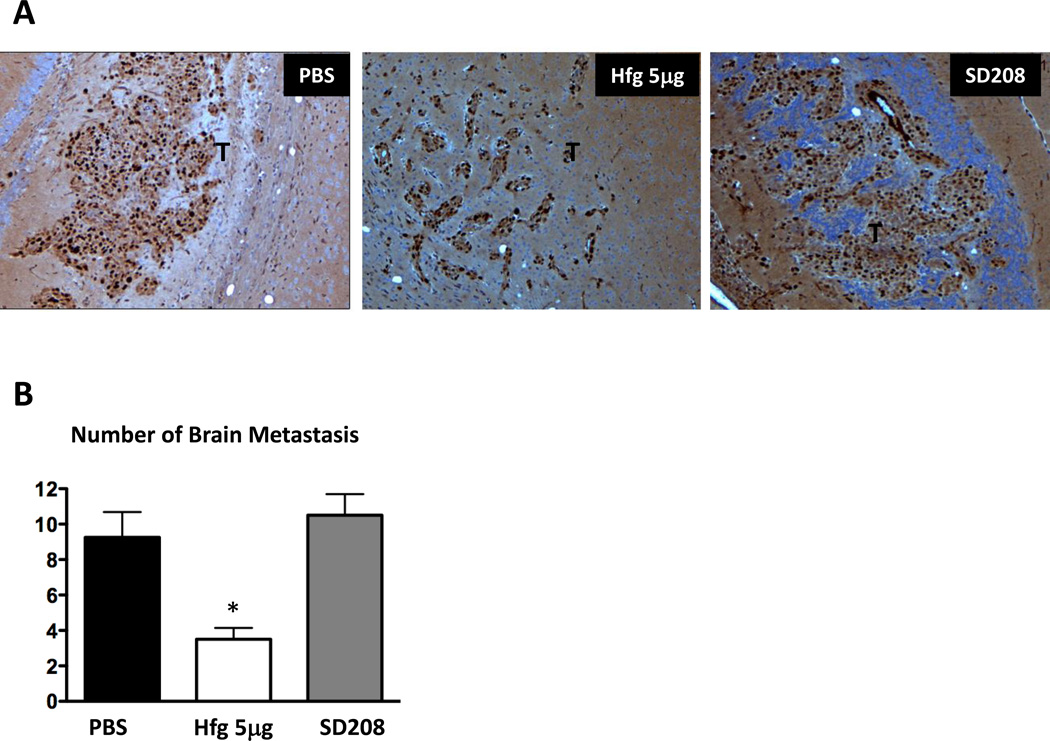
1205Lu melanoma cells metastasize preferentially to bone, but can also metastasize to other organs, including adrenal glands, lungs, liver, skin, and brain after inoculation into the cardiac left ventricle (9). We visualized 1205Lu cells on brain tissue sections using Ki-67 immunostaining, a marker of proliferating tumor cells. Mice were treated with or without halofuginone (5µg/mouse/day) or SD208 (60mg/kg/day) in a preventive protocol. We counted the number of tumor cell clusters per total area.
Halofuginone treatment significantly reduced the number of brain metastases compared to mice treated with vehicle (Fig. 6). In contrast, SD208 treatment did not reduce the number of metastases to the brain, consistent with previous findings that SD208 effects were specific to bone (10).
Figure 6. Halofuginone decreases melanoma brain metastases.
A. Representative sections of Ki-67 immunohistostaining from brains of mice treated with PBS, with Halofuginone (5µg/day) or SD208 (60mg/kg/day) in a preventive protocol. Ki-67 is a marker of tumor cell proliferation. Tumor is indicated by letter T. B, Quantification of the number of brain metastasis per total area (n=5). *P<0.05 vs PBS using a One-way ANOVA with Bonferroni’s post test.
Discussion
Bone metastases are frequent complications of cancer, occurring in up to 80 percent of patients with advanced breast or prostate cancer, and in approximately 50 percent of patients with advanced malignant melanoma (4, 35). TGF-β is a multifunctional cytokine with an established pro-metastatic role in advanced cancer (36, 37). Blockade of TGF-β function can interrupt multiple events important for the establishment and maintenance of tumors (38, 39).
Previously, we showed that TGF-β plays a key role in the establishment and progression of melanoma bone metastasis. The overexpression of TGF-β-signaling inhibitor Smad7 (9) or the systemic administration of SD208, the small molecule inhibitor of TβRI kinase (10), inhibited TGF-β-signaling, thereby reducing expression of TGF-β-regulated metastatic genes and delaying the progression of melanoma-bone metastasis. In this work, we searched for a pharmacological way to induce the inhibitory molecule Smad7 and focused on halofuginone, a plant alkaloid derivative that blocks TGF-β-signaling in epithelial cells by inhibiting the phosphorylation and activation of Smad2 and Smad3, and inducing Smad7 expression (22). Effects of the compound on tumor metastasis to bone have not previously been reported. Here we show for the first time that halofuginone is effective in an animal model of bone metastasis produced by melanoma.
Inhibition of TGF-β-signaling by halofuginone in melanoma cells was demonstrated as follows: 1) dose-dependent reduction of a TGF-β-responsive reporter (CAGA)9-luc ; 2) inhibition of Smad2/3 phosphorylation induced by TGF-β; 3) induction of Smad7, an inhibitor of TGF-β-signaling, by halofuginone alone and enhanced in the presence of TGF-β; and 4) inhibition of TGF-β-regulated genes PTHrP, CTGF, CXCR4 and IL11 that encode for metastatic and osteolytic factors required for the establishment and development of bone metastasis (6). Halofuginone inhibited cell proliferation and induced apoptosis in vitro in a dose- and time-dependent manner. In contrast to myeloma and leukemia cells where halofuginone induced apoptosis after 48hrs of treatment (40, 41), the effect of halofuginone on 1205Lu melanoma cells was mainly observed after 72hrs. This is consistent with the fact that melanoma 1205Lu cells are often considered more resistant to apoptosis than other cancer cell lines (42, 43). High concentrations of halofuginone did not induce more than 20% of cell death suggesting that apoptosis is not the sole mechanism of action of halofuginone, yet it may contribute together with inhibition of TGF-β signaling to the experimental therapeutic efficacy of halofuginone against bone metastases.
In a mouse model of bone metastasis, systemic halofuginone treatment reduced the establishment of melanoma bone metastases, significantly reducing osteolytic lesion area. Halofuginone inhibitory effects on skeletal metastasis of melanoma cells were similar to those of SD208 or Smad7 protein overexpression by a stable cell clone (9, 10). Halofuginone treatment did not improve mouse survival, although in this setting, there was no significant effect on the survival of mice using SD208 treatment. Notably, mice inoculated with cancer cells overexpressing Smad7 protein survived for more than two months, as seen in past studies (9), compared to mice treated with halofuginone or SD208, that started dying after 5weeks. These results suggest that the effect of halofuginone on bone metastases may not be due entirely to Smad7, or that pronounced overexpression of Smad7 in excess of that achieved with halofuginone may have independent effects on tumor metastases. Halofuginone has systemic effects and could induce Smad7 in the host as well as the tumor, whereas Smad7 overexpression in the tumor cells alone may result in tumor cell specific alterations, which may explain differences in survival.
Indeed, the precise mechanism of action of halofuginone still remains unclear and could involve factors other than Smad7. Roffe et al. demonstrated that halofuginone modulates the PI3K/Akt and MAPK/ERK pathways causing the inhibition of Smad3 phosphorylation (44). In mouse models of pancreatic fibrosis and acute promyelocytic leukemia, halofuginone treatment increased N-terminal phosphorylation of c-jun kinase, inhibiting TGF-β-signaling and cell proliferation (40, 45). In recent studies, halofuginone activated the amino acid starvation response pathway in vivo, and altered differentiation of Th17 cells (46). Whether halofuginone activates this starvation response in melanoma cells and whether this can affect the development of bone metastases has not yet been studied. Furthermore, Th17 cells have been implicated in tumor immunology and may be a target for cancer therapy. Increased infiltration of IL-17+ T cells correlated with poor survival in patients having hepatocellular carcinoma (47), and IL-17 from T cells protects breast cancer cells from apoptosis and increases metastases (48). However, our experimental model used nude mice lacking T cells. We would need to use a syngeneic model to determine the effect of halofuginone on Th17 and the development of bone metastases.
A highlight of our findings with halofuginone was its ability to reduce tumor growth and tumor progression when bone metastatic lesions were already established. Current therapies for the treatment of bone metastases (e.g., bisphosphonates, anti-RANKL antibody) improve the skeletal morbidity but do not cure or cause regression of the established bone metastases, so more effective therapies are needed. Halofuginone treatment reduced the number of osteoclasts at the tumor-bone interface, which could be due to decreased expression of pro-osteolytic genes by cancer cells treated with halofuginone, or to a direct effect of halofuginone on osteoclast and their precursors. TGF-β signaling also stimulates osteoclastogenesis, and inhibitors of TGF-β decrease bone resorption and osteoclast number in normal bone (49). Thus, halofuginone may be beneficial alone or in combination with other bone-targeted anti-resorptive therapy.
Halofuginone also reduced melanoma metastasis to the brain, showing that its effects are not limited to bone and bone metastases, unlike the effects of other inhibitors of TGF-β signaling. In mice, disruption of TGF-β signaling with a truncated form of TGF-β type II receptor in breast cancer cells, with overexpression of Smad7 in 1205Lu melanoma cells, or with SD208, a specific inhibitor of TGF-β type I receptor kinase domain, did not have anti-cancer effects outside of bone. Thus, the anti-cancer effect of halofuginone cannot be explained solely by inhibition of TGF-β-signaling pathway.
In summary, halofuginone inhibits TGF-β-signaling and TGF-β-regulated pro-metastatic genes in vitro. Systemic administration of halofuginone in a mouse model reduced the establishment of osteolytic bone metastases by melanoma cells. More importantly, halofuginone therapy reduced the progression of established osteolytic bone metastasis in mice, demonstrating its potential as a therapeutic agent. Halofuginone is a novel treatment for metastases that could rapidly be brought to the clinic for the treatment of patients with malignant melanoma.
Acknowledgments
The authors are thankful to Dr. Hunter Heath III for his valuable comments to improve the quality of the paper.
Financial support: This work was supported by NIH grants R01CA69158, R01DK067333, and R01DK065837; the Mary Kay Ash Foundation, the V-Foundation, Aurbach Endowment of the University of Virginia, Jerry W. and Peggy S. Throgmartin Endowment of Indiana University and Indiana Economic Development Fund (to T.A.G.) as well as a grant from the Susan Komen Foundation (to P.J. and T.A.G.), and by Ligue Nationale Contre le Cancer (Equipe Labellisée LIGUE EL2011-AM), INCa (PLBIO08-126), a donation from Emile and Henriette Goutière, and institutional funding from Institut Curie, INSERM and CNRS (to A.M.), and Association pour la Recherche contre le Cancer (to DJ).
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Institute N-C-. [cited 2012 April 11];Statistics for Specific Cancers: Melanoma. 2011 Available from: http://seer.cancer.gov/statfacts/html/melan.html.
- 2.Kretschmer L, Beckmann I, Thoms KM, Mitteldorf C, Bertsch HP, Neumann C. Factors predicting the risk of in-transit recurrence after sentinel lymphonodectomy in patients with cutaneous malignant melanoma. Ann Surg Oncol. 2006;13:1105–1112. doi: 10.1245/ASO.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 3.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 4.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 5.Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat Rev Cancer. 2011;11:411–425. doi: 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 7.Sethi N, Kang Y. Unravelling the complexity of metastasis - molecular understanding and targeted therapies. Nat Rev Cancer. 2011;11:735–748. doi: 10.1038/nrc3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yin JJ, Selander K, Chirgwin JM, Dallas M, Grubbs BG, Wieser R, et al. TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Invest. 1999;103:197–206. doi: 10.1172/JCI3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Javelaud D, Mohammad KS, McKenna CR, Fournier P, Luciani F, Niewolna M, et al. Stable overexpression of Smad7 in human melanoma cells impairs bone metastasis. Cancer Res. 2007;67:2317–2324. doi: 10.1158/0008-5472.CAN-06-3950. [DOI] [PubMed] [Google Scholar]
- 10.Mohammad KS, Javelaud D, Fournier PG, Niewolna M, McKenna CR, Peng XH, et al. TGF-beta-RI kinase inhibitor SD-208 reduces the development and progression of melanoma bone metastases. Cancer Res. 2011;71:175–184. doi: 10.1158/0008-5472.CAN-10-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganapathy V, Ge R, Grazioli A, Xie W, Banach-Petrosky W, Kang Y, et al. Targeting the Transforming Growth Factor-beta pathway inhibits human basal-like breast cancer metastasis. Mol Cancer. 2010;9:122. doi: 10.1186/1476-4598-9-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krasagakis K, Garbe C, Schrier PI, Orfanos CE. Paracrine and autocrine regulation of human melanocyte and melanoma cell growth by transforming growth factor beta in vitro. Anticancer Res. 1994;14:2565–2571. [PubMed] [Google Scholar]
- 13.Rodeck U, Bossler A, Graeven U, Fox FE, Nowell PC, Knabbe C, et al. Transforming growth factor beta production and responsiveness in normal human melanocytes and melanoma cells. Cancer Res. 1994;54:575–581. [PubMed] [Google Scholar]
- 14.Rodeck U, Nishiyama T, Mauviel A. Independent regulation of growth and SMAD-mediated transcription by transforming growth factor beta in human melanoma cells. Cancer research. 1999;59:547–550. [PubMed] [Google Scholar]
- 15.Berking C, Takemoto R, Schaider H, Showe L, Satyamoorthy K, Robbins P, et al. Transforming growth factor-beta1 increases survival of human melanoma through stroma remodeling. Cancer Res. 2001;61:8306–8316. [PubMed] [Google Scholar]
- 16.Javelaud D, Alexaki VI, Mauviel A. Transforming growth factor-beta in cutaneous melanoma. Pigment cell & melanoma research. 2008;21:123–132. doi: 10.1111/j.1755-148X.2008.00450.x. [DOI] [PubMed] [Google Scholar]
- 17.Moretti S, Pinzi C, Berti E, Spallanzani A, Chiarugi A, Boddi V, et al. In situ expression of transforming growth factor beta is associated with melanoma progression and correlates with Ki67, HLA-DR and beta 3 integrin expression. Melanoma Res. 1997;7:313–321. doi: 10.1097/00008390-199708000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Reed JA, McNutt NS, Prieto VG, Albino AP. Expression of transforming growth factor-beta 2 in malignant melanoma correlates with the depth of tumor invasion. Implications for tumor progression. Am J Pathol. 1994;145:97–104. [PMC free article] [PubMed] [Google Scholar]
- 19.Granot I, Halevy O, Hurwitz S, Pines M. Halofuginone: an inhibitor of collagen type I synthesis. Biochim Biophys Acta. 1993;1156:107–112. doi: 10.1016/0304-4165(93)90123-p. [DOI] [PubMed] [Google Scholar]
- 20.Pines M, Knopov V, Genina O, Lavelin I, Nagler A. Halofuginone, a specific inhibitor of collagen type I synthesis, prevents dimethylnitrosamine-induced liver cirrhosis. J Hepatol. 1997;27:391–398. doi: 10.1016/s0168-8278(97)80186-9. [DOI] [PubMed] [Google Scholar]
- 21.Koon HB, Fingleton B, Lee JY, Geyer JT, Cesarman E, Parise RA, et al. Phase II AIDS Malignancy Consortium trial of topical halofuginone in AIDS-related Kaposi sarcoma. J Acquir Immune Defic Syndr. 2011;56:64–68. doi: 10.1097/QAI.0b013e3181fc0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xavier S, Piek E, Fujii M, Javelaud D, Mauviel A, Flanders KC, et al. Amelioration of radiation-induced fibrosis: inhibition of transforming growth factor-beta signaling by halofuginone. J Biol Chem. 2004;279:15167–15176. doi: 10.1074/jbc.M309798200. [DOI] [PubMed] [Google Scholar]
- 23.Elkin M, Miao HQ, Nagler A, Aingorn E, Reich R, Hemo I, et al. Halofuginone: a potent inhibitor of critical steps in angiogenesis progression. FASEB J. 2000;14:2477–2485. doi: 10.1096/fj.00-0292com. [DOI] [PubMed] [Google Scholar]
- 24.Abramovitch R, Dafni H, Neeman M, Nagler A, Pines M. Inhibition of neovascularization and tumor growth, and facilitation of wound repair, by halofuginone, an inhibitor of collagen type I synthesis. Neoplasia. 1999;1:321–329. doi: 10.1038/sj.neo.7900043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gavish Z, Pinthus JH, Barak V, Ramon J, Nagler A, Eshhar Z, et al. Growth inhibition of prostate cancer xenografts by halofuginone. Prostate. 2002;51:73–83. doi: 10.1002/pros.10059. [DOI] [PubMed] [Google Scholar]
- 26.Nagler A, Ohana M, Shibolet O, Shapira MY, Alper R, Vlodavsky I, et al. Suppression of hepatocellular carcinoma growth in mice by the alkaloid coccidiostat halofuginone. Eur J Cancer. 2004;40:1397–1403. doi: 10.1016/j.ejca.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 27.Pinthus JH, Sheffer Y, Nagler A, Fridman E, Mor Y, Genina O, et al. Inhibition of Wilms tumor xenograft progression by halofuginone is accompanied by activation of WT-1 gene expression. J Urol. 2005;174:1527–1531. doi: 10.1097/01.ju.0000179218.16587.d2. [DOI] [PubMed] [Google Scholar]
- 28.Abramovitch R, Itzik A, Harel H, Nagler A, Vlodavsky I, Siegal T. Halofuginone inhibits angiogenesis and growth in implanted metastatic rat brain tumor model--an MRI study. Neoplasia. 2004;6:480–489. doi: 10.1593/neo.03520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spector I, Honig H, Kawada N, Nagler A, Genin O, Pines M. Inhibition of pancreatic stellate cell activation by halofuginone prevents pancreatic xenograft tumor development. Pancreas. 2010;39:1008–1015. doi: 10.1097/MPA.0b013e3181da8aa3. [DOI] [PubMed] [Google Scholar]
- 30.Rodeck U, Nishiyama T, Mauviel A. Independent regulation of growth and SMAD-mediated transcription by transforming growth factor beta in human melanoma cells. Cancer Res. 1999;59:547–550. [PubMed] [Google Scholar]
- 31.Javelaud D, Delmas V, Moller M, Sextius P, Andre J, Menashi S, et al. Stable overexpression of Smad7 in human melanoma cells inhibits their tumorigenicity in vitro and in vivo. Oncogene. 2005;24:7624–7629. doi: 10.1038/sj.onc.1208900. [DOI] [PubMed] [Google Scholar]
- 32.Dennler S, Itoh S, Vivien D, ten Dijke P, Huet S, Gauthier JM. Direct binding of Smad3 and Smad4 to critical TGF beta-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J. 1998;17:3091–3100. doi: 10.1093/emboj/17.11.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan JS, Wang D, Stewart CN., Jr Statistical methods for efficiency adjusted real-time PCR quantification. Biotechnol J. 2008;3:112–123. doi: 10.1002/biot.200700169. [DOI] [PubMed] [Google Scholar]
- 34.Guise TA, Mundy GR. Physiological and pathological roles of parathyroid hormone-related peptide. Curr Opin Nephrol Hypertens. 1996;5:307–315. doi: 10.1097/00041552-199607000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 36.Kang Y, He W, Tulley S, Gupta GP, Serganova I, Chen CR, et al. Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proc Natl Acad Sci U S A. 2005;102:13909–13914. doi: 10.1073/pnas.0506517102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Javelaud D, Alexaki VI, Dennler S, Mohammad KS, Guise TA, Mauviel A. TGF-beta/SMAD/GLI2 signaling axis in cancer progression and metastasis. Cancer Res. 2011;71:5606–5610. doi: 10.1158/0008-5472.CAN-11-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Juarez P, Guise TA. TGF-beta in cancer and bone: implications for treatment of bone metastases. Bone. 2011;48:23–29. doi: 10.1016/j.bone.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 39.Hawinkels LJ, Ten Dijke P. Exploring anti-TGF-beta therapies in cancer and fibrosis. Growth Factors. 2011;29:140–152. doi: 10.3109/08977194.2011.595411. [DOI] [PubMed] [Google Scholar]
- 40.de Figueiredo-Pontes LL, Assis PA, Santana-Lemos BA, Jacomo RH, Lima AS, Garcia AB, et al. Halofuginone has anti-proliferative effects in acute promyelocytic leukemia by modulating the transforming growth factor beta signaling pathway. PLoS ONE. 2011;6:e26713. doi: 10.1371/journal.pone.0026713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leiba M, Jakubikova J, Klippel S, Mitsiades CS, Hideshima T, Tai YT, et al. Halofuginone inhibits multiple myeloma growth in vitro and in vivo and enhances cytotoxicity of conventional and novel agents. British journal of haematology. 2012;157:718–731. doi: 10.1111/j.1365-2141.2012.09120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phipps LE, Hino S, Muschel RJ. Targeting cell spreading: a method of sensitizing metastatic tumor cells to TRAIL-induced apoptosis. Molecular cancer research : MCR. 2011;9:249–258. doi: 10.1158/1541-7786.MCR-11-0021. [DOI] [PubMed] [Google Scholar]
- 43.Satyamoorthy K, Chehab NH, Waterman MJ, Lien MC, El-Deiry WS, Herlyn M, et al. Aberrant regulation and function of wild-type p53 in radioresistant melanoma cells. Cell growth & differentiation : the molecular biology journal of the American Association for Cancer Research. 2000;11:467–474. [PubMed] [Google Scholar]
- 44.Roffe S, Hagai Y, Pines M, Halevy O. Halofuginone inhibits Smad3 phosphorylation via the PI3K/Akt and MAPK/ERK pathways in muscle cells: effect on myotube fusion. Exp Cell Res. 2010;316:1061–1069. doi: 10.1016/j.yexcr.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 45.Zion O, Genin O, Kawada N, Yoshizato K, Roffe S, Nagler A, et al. Inhibition of transforming growth factor beta signaling by halofuginone as a modality for pancreas fibrosis prevention. Pancreas. 2009;38:427–435. doi: 10.1097/MPA.0b013e3181967670. [DOI] [PubMed] [Google Scholar]
- 46.Sundrud MS, Koralov SB, Feuerer M, Calado DP, Kozhaya AE, Rhule-Smith A, et al. Halofuginone inhibits TH17 cell differentiation by activating the amino acid starvation response. Science. 2009;324:1334–1338. doi: 10.1126/science.1172638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang JP, Yan J, Xu J, Pang XH, Chen MS, Li L, et al. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol. 2009;50:980–989. doi: 10.1016/j.jhep.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 48.Nam JS, Terabe M, Kang MJ, Chae H, Voong N, Yang YA, et al. Transforming growth factor beta subverts the immune system into directly promoting tumor growth through interleukin-17. Cancer Res. 2008;68:3915–3923. doi: 10.1158/0008-5472.CAN-08-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mohammad KS, Chen CG, Balooch G, Stebbins E, McKenna CR, Davis H, et al. Pharmacologic inhibition of the TGF-beta type I receptor kinase has anabolic and anti-catabolic effects on bone. PLoS ONE. 2009;4:e5275. doi: 10.1371/journal.pone.0005275. [DOI] [PMC free article] [PubMed] [Google Scholar]