Abstract
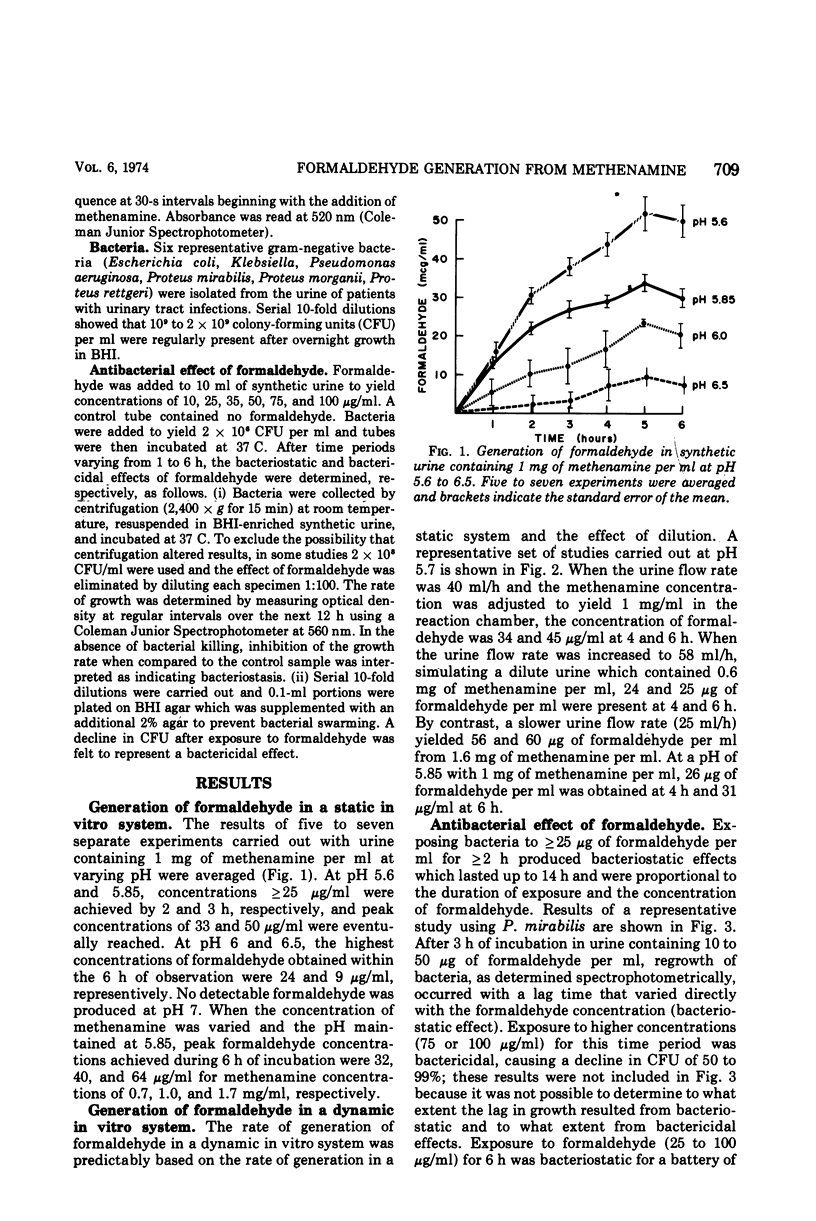
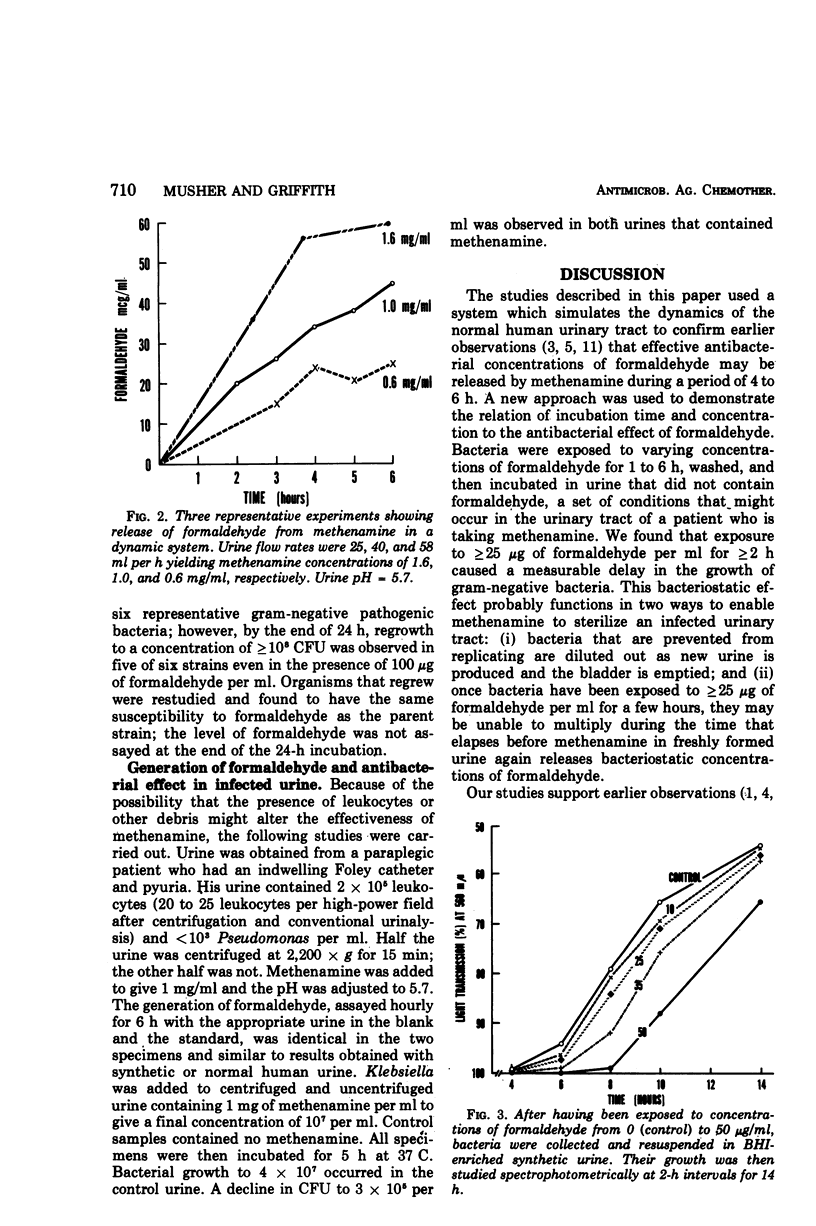
Using an in vitro system that simulates the dynamics of the urinary tract, we have shown that concentrations of formaldehyde ≥ 25 μg/ml can be achieved in urine containing ≥ 0.6 mg of methenamine per ml at pH ≤ 5.7 or ≥ 1 mg/ml at pH ≤ 5.85. Exposure to this concentration of formaldehyde for 2 h produced a measurable antibacterial effect. These studies suggest that an effective bacteriostatic level of formaldehyde is likely to be achieved with currently used dosages of methenamine when the urine pH is less than 5.7 to 5.85.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Jackson J., Stamey T. A. The Riker method for determining formaldehyde in the presence of methenamine. Invest Urol. 1971 Sep;9(2):124–129. [PubMed] [Google Scholar]
- Miller H., Phillips E. Antibacterial correlates of urine drug levels of hexamethylenetetramine and formaldehyde. Invest Urol. 1970 Jul;8(1):21–33. [PubMed] [Google Scholar]
- Musher D. M., Griffith D. P., Tyler M., Woelfel A. Potentiation of the antibacterial effect of methenamine by acetohydroxamic acid. Antimicrob Agents Chemother. 1974 Feb;5(2):101–105. doi: 10.1128/aac.5.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson W. G., Peacock M., Nordin B. E. Activity products in stone-forming and non-stone-forming urine. Clin Sci. 1968 Jun;34(3):579–594. [PubMed] [Google Scholar]


