Abstract
Retinoids comprise a group of compounds each composed of three basic parts: a trimethylated cyclohexene ring that is a bulky hydrophobic group, a conjugated tetraene side chain that functions as a linker unit, and a polar carbon-oxygen functional group. Biochemical conversion of carotenoid or other retinoids to retinoic acid (RA) is essential for normal regulation of a wide range of biological processes including development, differentiation, proliferation, and apoptosis. Retinoids regulate various physiological outputs by binding to nuclear receptors called retinoic acid receptors (RARs) and retinoid X receptors (RXRs), which themselves are DNA-binding transcriptional regulators. The functional response of RA and their receptors are modulated by a host of coactivators and corepressors. Retinoids are essential in the development and function of several organ systems; however, deregulated retinoid signaling can contribute to serious diseases. Several natural and synthetic retinoids are in clinical use or undergoing trials for treating specific diseases including cancer. In this review, we provide a broad overview on the importance of retinoids in development and various diseases, highlighting various retinoids in the drug discovery process, ranging all the way from retinoid chemistry to clinical uses and imaging.
GRAPHICAL ABSTRACT
1. Introduction
Vitamin A or retinol is a fat-soluble compound derived from [beta]-carotene found in plants and retinyl esters from animal sources. It is stored in the liver and adipose tissue as retinyl esters and circulates in the blood in a protein complex containing retinol binding protein and transthyretin. Retinoic acid, the main derivative of vitamin A, binds to nuclear receptors, retinoic acid receptor (RAR) and retinoic X receptor (RXR) to regulation gene transcription. RA plays an important role in tissue development and differentiation. This review focuses on the role of RA in development and diseases, and the development of synthetic RA compounds for therapy and molecular imaging.
2. Synthesis, chemistry and analysis of RA
Retinoids comprise a group of compounds related to vitamin A, including its natural and synthetic analogues, which contain four isoprenoid units joined in a head-to-tail fashion. The basic structure of a retinoid consists of three parts: a trimethylated cyclohexene ring that is a bulky hydrophobic group, a conjugated tetraene side chain that serves as a linker unit, and a polar carbon-oxygen functional group, typically carboxylic acid as shown in Figure 1.
Figure 1.
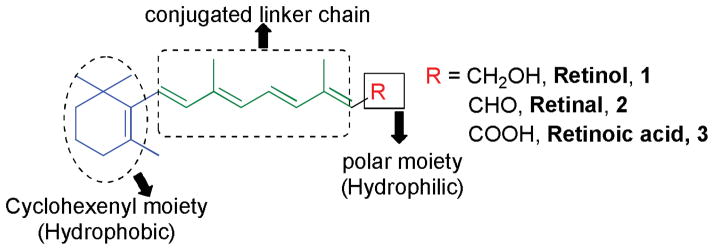
Basic structure of retinoids.
Retinoids are unstable due to the presence of conjugated double bonds and easily undergo oxidation and/or isomerization in the presence of oxidants, light or excessive heat. Retinoids that contain alcoholic and carboxylic groups are soluble in methanol and ethanol, whereas esterified long-chain fatty acid is only slightly soluble in alcohol but highly soluble in hexane.
Numbering for the retinoids is practiced in either of two ways, starting the numbering from the cyclohexenyl group as shown in Figure 2A, or by designating 1 for the carbon atom bonded to the functional group, as shown in Figure 2B.
Figure 2.
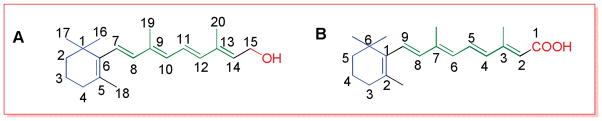
Numbering of retinoids.
Animals are unable to synthesize vitamin A de novo, and therefore it must be obtained through the diet. Dietary intake in the form of retinol, retinyl ester, or beta-carotene are good sources of vitamin A. Carotenoids, the main precursor of fat soluble vitamin A, including α/β-carotene are converted into retinal or apocarotenoids and subsequently to retinoids.1
Unlike other most biological compounds, carotenoids and retinoids have conjugated polyene systems that absorb light in the visible and ultraviolet spectrums, around 450 nm and 325–380 nm, respectively. Historically, colorimetric assays were widely used for the analysis of vitamin A and carotenoids. In this methodology, the polyene system of a retinoid or carotenoid is protonated by a strong acid such as antimony trichloride or trifluoroacetic acid in organic solvent to give a highly intense characteristic transient blue color, which provides a selective and very sensitive assay for vitamin A analysis.2, 3 The reagents used in this method are corrosive and noxious, which is one reason these methods are rarely used today. Still, absorbance spectroscopy remains a major tool for quantification of retinoids and carotenoids. Today, high performance liquid chromatography (HPLC) equipped with absorbance detectors and photodiode-array detectors have become the standard means for quantitative and qualitative analysis of retinoids and carotenoids. Fluorescence spectroscopy, which offers greater specificity than absorbance spectroscopy for analysis of retinol and retinyl esters in biological samples, is another widely used technique. For assignment of cis- and trans-isomers of retinoids, nuclear magnetic resonance (NMR) has been very helpful. Similarly, infrared spectroscopy (IR), though not as important as ultraviolet (UV) and visible light spectroscopy, provides useful information in structural analysis of retinoids. Typically, a combination of techniques including HPLC with NMR and mass spectroscopy (MS) are now used for separation, identification, and quantitation of retinoids.4
3. Retinoid and carotenoid metabolism
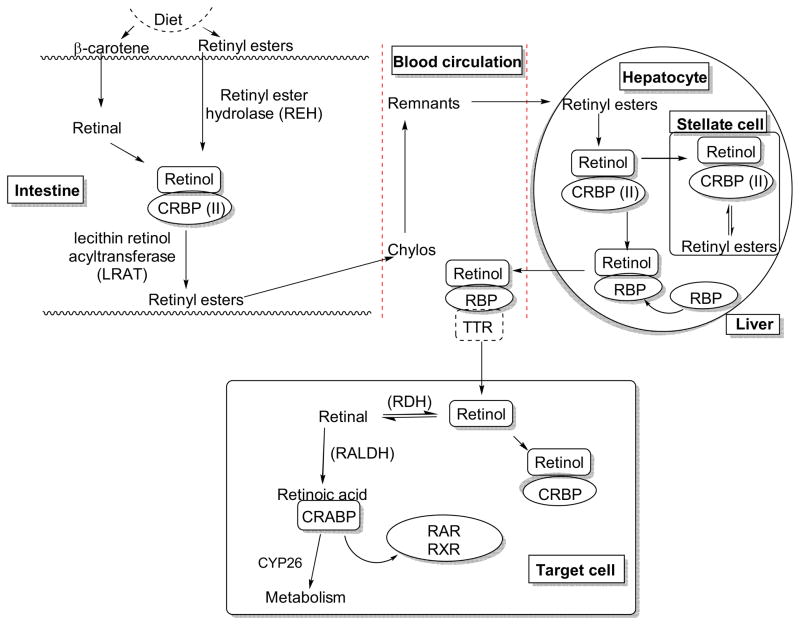
Carotenoids or other retinoids are biochemically converted to retinoic acid (RA), which is essential for modulating a wide range of biological processes including development, differentiation, proliferation, and apoptosis. The bioconversion takes place successively (Figure 3), first in the intestine, then the liver, and finally in target cells with the support of various binding proteins including cellular retinol-binding proteins (CRBPs), retinol-binding proteins (RBPs), and cellular retinoic acid binding proteins (CRABPs).5 β-Carotene and retinyl esters derived from plants or animals, respectively, are good sources of retinol. β-Carotenes are converted to retinal that binds to the cellular retinol-binding protein (CRBP)II. Binding to CRBPII prevents the oxidation of retinal to RA in the intestine and facilitates the reduction into the retinol in support of microsomal retinal reductase.6 The retinyl esters from animal source can undergo hydrolysis to give retinol. The retinol binds with CRBPII to give the complex, which acts as a substrate for the conversion of retinol to retinyl esters in the presence of lecithin-retinol acyltransferase (LRAT) enzyme.7,8 Most of the retinyl esters are incorporated into the chylomicron, which is then secreted into the lymph and subsequently into general circulation where elimination of triacylglycerol takes place with the help of lipoprotein lipase.9,10 This results in the formation of chylomicron remnants, which are taken up via endocytosis by hepatocytes in the liver. Retinyl ester undergoes hydrolysis to give retinol. The free retinol binds with the retinol-binding protein (RBP) and is secreted into plasma where it binds with transthyretin (TTR).11 Binding to TTR prevents the elimination of retinol by the kidney. At the same time, large numbers of unesterified retinols are transported to another type of liver cell called as “stellate cell”. The retinols are converted to retinyl ester in the presence of LRAT and are packed in cytoplasmic lipid droplets.12,13 The retinol-RBP-TTR complex in plasma is taken up by target cells via the RBP receptor, a trans-membrane protein also called “stimulated by retinoic acid 6” (STRA6).14 After entering the target cell, free retinol either binds with CRBP or undergoes oxidation to give retinal, an aldehyde, in the presence of retinol dehydrogenase (RDH). The retinal is subsequently converted to RA by retinaldehyde dehydrogenase (RALDH).15 The RA binding with cellular RA binding protein (CRABP) allows it to enter the nucleus where it can bind, and serve as ligand, with various nuclear receptors including RAR and RXR, to mediate genomic and non-genomic mechanisms. At the same time RA can be converted into oxidized metabolites such as 4-hydroxyretinoic acid and 4-oxoretinoic acid by the cytochrome P450 enzyme (CYP26).16,17,18
Figure 3.
Biochemical conversion of carotenoids and retinoids to form retinoic acid.5,16,17,18
4. Retinoid receptors and their genomic actions
4.1. Genomic action of retinoids
Retinoids regulate various biological activities by binding RARs and RXRs. Ligand binding to the receptor causes conformational changes that modulate receptor complex function. In addition, these receptor complexes have a range of additional co-activators and co-repressors that modulate receptor activity. The composition of receptor complex is complicated by the presence of distinct subtypes and isoforms. Both RAR and RXR have three subtypes α, β, and γ, each with different isoforms. RARα and RARγ both have two isoforms 1 (RARα1, RARγ1) and 2 (RARα2, RARγ2) whereas RARβ has five isoforms (RARβ1–4, and 1′). In the case of RXR, all subtypes have two isoforms 1 and 2.19 RAR can be activated by both all-trans and 9-cis RA, whereas RXR is only activated by 9-cis RA. Heterodimerization of retinoid receptors, one RAR and one RXR, is essential for the biological activity, although it should be noted that RXRs can heterodimerize with numerous other non-RA associated nuclear receptors to mediate alternative signaling pathways. Availability of several subtypes and isoforms enables the generation of a large group of distinct receptor dimers. As is typical for DNA-binding nuclear receptors, the retinoid receptors have a basic modular domain design. Domains A-F (Figure 4) can be isolated and studied as distinct units without loss of their function.20,21,22
Figure 4.

A/B encodes the least conserved N-terminal domain, which contains an autonomous transcriptional activation function, called AF-1. Domain C is highly conserved and contains two zinc finger modules responsible for DNA binding, and therefore it is also called the DNA-binding domain. Domains C and E are separated by a flexible hinge region called domain D. Domain D is also essential for interaction with certain nuclear receptor coregulators. Domain E is a multifunctional domain, comprising the ligand-binding domain (LBD) and activation function-2 (AF-2) domain. This domain is responsible for ligand binding, receptor dimerization, and interaction with other coactivators. When the ligand (retinoid) binds to the LBD, a conformational change in the receptor complex occurs. AF-2 structure includes an amphipathic α-helix that also modulates the conformational change. While unliganded receptor is complexed with transcriptional co-repressors, the conformational changes caused by ligand binding releases these negative regulators and stimulates the recruitment of distinct coactivators. The C-terminal F domain is present in some but not all receptors, and can participate in the action of AF-2.
Receptor dimerization occurs through the interaction of the LBD on each receptor. It is the heterodimeric receptor that is responsible for binding to DNA at specific retinoid acid response elements (RARE) with high affinity. In the presence of ligands (retinoids), the holo-receptor binds to the RARE and activates the histone acetyl transferase (HAT) activity. As a result, histone proteins are acetylated, helping to open the chromatin and activate the transcription machinery. On the other hand, in absence of ligands, the apo receptor pair binds with the RARE in a complex with corepressors and histone deacetylase (HDAC) activity. As a result, histone deacetylation prevails causing chromatin condensation and gene silencing.23
4.2. Non-genomic actions of retinoids
As described above, retinoids mediate various genomic transcriptional effects via RA nuclear receptors, but they also have non-genomic effects including retinoylation (RA acylation), a post-translational modification of proteins occurring in many eukaryotic cells. Not all target cells for RA contain cellular RA binding proteins. One example is the human acute myeloid leukemia HL-60 cell line. It has been reported that retinoylation in HL-60 occurs through the covalent bonding of RA to perform post-translational modification of proteins. It is believed that RA is first converted into an activated intermediate retinoyl-CoA.24
In another study, it was reported that N-(4-hydroxyphenyl)retinamide (4-HPR) increases the level of retinoylation. It was found that the combination of 4-HPR and RA has a synergistic effect on the differentiation of HL-60 cells and the retinoylation process. RA also mediated retinoylation on proteins of tumoural leydig (MLTC-1) cells.25,26 Similarly, when treated with RA, undifferentiated and squamous-differentiated normal human epidermal keratinocytes were found to undergo retinoylation. Retinoylation occurred mainly on the cytokeratin proteins.27
5. RA signaling in normal development
The requirement for retinoids during embryogenesis has been long appreciated, since vitamin A is an essential dietary requirement. Yet for understanding mechanism, it is perplexing that loss of signaling or hyper-activation of the pathway can generate similar phenotypes. For example, excess RA causes many of the same embryonic developmental defects seen with vitamin A deficiency (VAD), which is why vitamin A intake levels should be monitored carefully during pregnancy. Because RA is teratogenic, loss-of-function assays are essential to understand the normal function of the RA-dependent pathways controlling embryonic patterning, growth, and organogenesis. A major tool for this purpose is the targeted knockout of receptors in mouse models. However, interpreting the knockout phenotypes is complicated by partial or total compensation from the related receptor subtypes. For this reason, a valuable approach for studying RA requirements during embryogenesis has been VAD animal models. Complete VAD effectively and specifically eliminates retinoid signaling, since the ligand (in contrast to the receptors) is non-redundant. The developing embryo normally metabolizes RA from maternal stores of Vitamin A. In a VAD model, the mother remains healthy as long as her diet is supplemented with RA. However, RA is not passed on to the embryo, which develops in the absence of retinoids. Both rodent and avian models have been used effectively, although a quail model developed by Zile and colleagues is arguably the best characterized.28 The VAD quail survives until around day four, and clearly demonstrates the requirements for RA signaling in the formation of the nervous and cardiovascular systems.29 Importantly, the VAD quail is completely rescued if RA is added in ovo or to cultured embryos prior to the 5 somite stage, demonstrating specificity for the ligand, and showing that retinoid signaling becomes essential only after this stage of development. The requirements for RA are conserved in mammalian models, shown for example by abnormalities in rats30 or pigs31 born from mothers on a VAD diet. VAD animal models have documented clear requirements for RA signaling in a host of organ systems, including the development of the brain, heart, and kidney.
RA contributes to the anteroposterior patterning of the neural plate.32 The neural plate is initially anterior or forebrain-like. RA contributes to the induction of more posterior domains.33 Hindbrain segments called rhombomeres are transient structures that reflect the evolutionarily conserved segmented nature of positional information, including the ordered pattern of Hox gene expression. In the VAD quail model, there is a failure in the development of posterior regions of the hindbrain (rhombomeres 4–7).34 RA also has an important role in the development of the spinal cord and somites, as VAD quail embryos have fewer spinal cord neurons and smaller somites.35 Eye morphogenesis and differentiation also requires RA signaling. During early stages of eye development, RA activates retinoid receptors present in the perioptic mesenchyme to regulate the development of the anterior eye segment and closure of the optic fissure. RA/RAR regulates the transcription factor Pitx2 that is essential for the morphogenesis of anterior eye segment, and closure of the optic fissure.36 Similarly, RA promotes the differentiation of the neural retina at later stages of development.37
Vitamin A also plays many regulatory roles in the development of mammalian heart, including heart tube patterning and looping, chamber and outflow tract septation, ventricular trabeculation, cardiomyocyte differentiation, and coronary vessel development.38,39,40 VAD models have shown the importance of RA in kidney and urinary tract development, including causing embryonic renal hypoplasia. Similarly, rat fetuses that are VAD can display ectopic kidneys, renal fusion and failure of the renal pelvis and calyx to undergo dilatation, and other genitourinary tract defects. Moreover, vitamin A is required for the normal development of diaphragm, lung and upper respiratory tract and airways, somites and skeleton, pancreas, and limb.41 The VAD quail embryo develops with a significant defect in primitive (yolk sac) erythropoiesis, at least in part due to the failure to properly regulate the GATA2 transcription factor.42
Clearly, RA does not function alone in controlling the development of these organ systems. There is a large literature linking RA functions with other signaling pathways during vertebrate development. For example, the RA signaling pathway interacts with the fibroblast growth factor (FGF),43 Wnt, Nodal,44 bone morphogenetic protein (BMP), and sonic hedgehog (Shh)45,46 signaling cascades, which together are essential for the regulation of somitogenesis, heart development, patterning of the central nervous system (CNS), and optic, limb, and pharyngeal differentiation.47,48,49,50,51,52
Despite the value of VAD models, they are limited in terms of understanding temporal and spatial regulation by RA. The RARs are widely expressed in development, and although complicated by functional redundancies and the range of isoforms, mouse knockout models have been generated for each of the RAR/RXR genes and used to describe both developmental and tissue-specific functions for the pathway. This has been reviewed extensively in the literature,53 and the phenotypes described largely support the notion that different heterodimer pairs mediate the developmental functions of RA signaling during development. In coming years, combinations of conditional somatic alleles, for example targeting specific isoforms in defined cell types, will be needed to probe fully the molecular mechanisms by which receptors mediate the myriad developmental functions for the RA signaling pathway during embryogenesis.
6. Retinoids in Diseases
Though RA and it’s analogs are essential for normal regulation of a wide range of biological processes, while deregulated retinoid signaling can contribute to serious diseases. In this section, we will briefly describe, how aberrant signaling of RA causes diseases, and how synthetic retinoids are used to treat some diseases.
6.1. Retinoids in cancer and control of differentiation
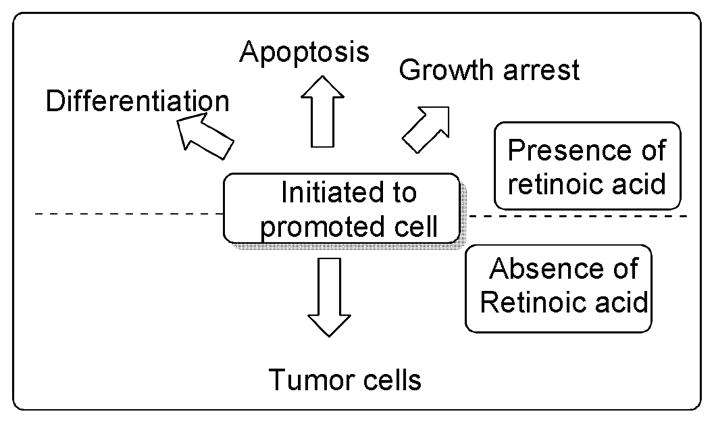
Retinoids can induce differentiation and/or apoptosis in tumor cells and show anti-proliferative and anti-oxidant activity, and therefore have great potential as chemotherapeutic or chemopreventive agents. Depending on the target cells, they can mediate growth inhibitory roles at various stages of carcinogenesis. The use of retinoids for treating cancer was spurred by the critical discovery that the chromosomal translocation t(15:17), a cytogenetic hallmark of acute promyelocytic leukemia (APL), leads to the expression of an oncoprotein consisting of a retinoic acid receptor α(RARα) fused to a previously unknown protein called PML.54,55 The hematopoietic cells expressing RAR-PML are blocked for differentiation. Retinoid or rexinoids act as strong inducers of cell differentiation to overcome this block, and pharmacological doses of RA are used clinically to activate the RA-APL fusion protein, leading to cell cycle arrest and terminal differentiation of leukemic cells. The affect on cell-cycle arrest at G1 is also mediated by increased expression of the p21/WAF1 gene, which is a potential mediator of p53 mediated tumor suppression.56 This success story provides a paradigm for differentiation therapy by RA. In addition, retinoids and rexinoids (rexinoids compounds are chemically related to vitamin A, that bind to the retinoid X receptor but not the retinoic acid receptor), alone or in combination with chemotherapeutic agents, are able to induce apoptosis in various other types of cancer cells.57,58
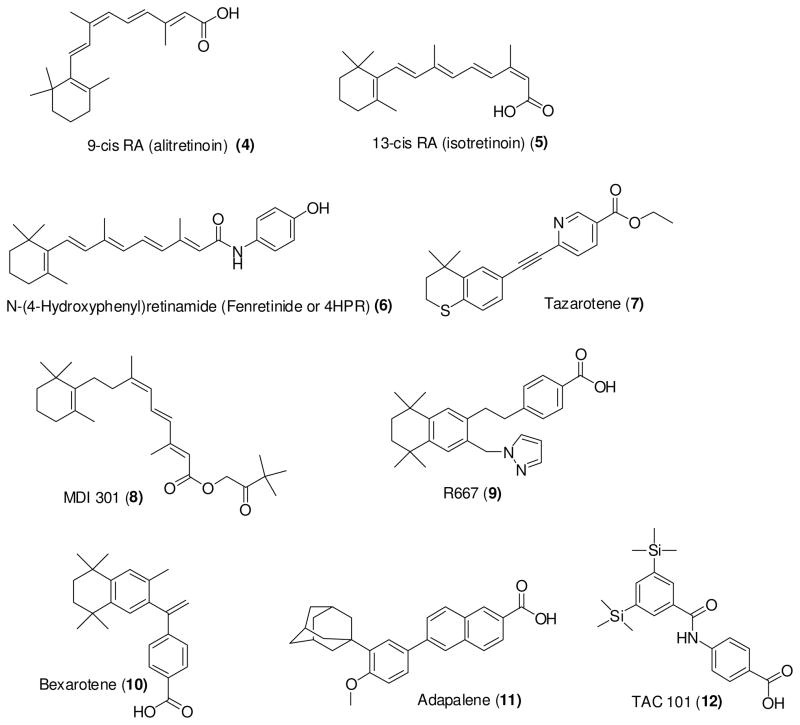
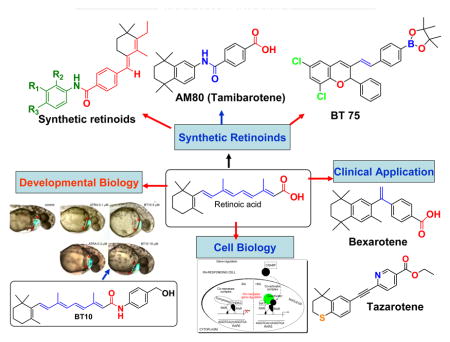
All-trans retinoic acid (RA, tretinoin), the most abundant natural retinoid, has been developed for the treatment of various types of cancers including lymphoma, leukemia, cervical cancer, lung cancer, kidney cancer, neuroblastoma, and glioblastoma. As described above, RA activates only RARs whereas 9-cis RA (alitretinoin) can activate both RAR and RXR. 9-cis RA has been approved by the US Food Drug Administration (FDA) for the topical treatment of cutaneous lesions of Kaposi’s sarcoma59 and has also been studied for the prevention of mammary and prostate cancer.60,61 Similarly, 13-cis RA (isotretinoin) has been in clinical trial for various types of cancers including thyroid cancer, lung cancer, prostate cancer, head-and-neck cancer. Moreover, there are several synthetic retinoids that are in clinical trial for the treatment of different types of cancer. Fenretinide (or 4HPR), tazarotene, MDI 301, R667, Bexarotene, Adapalene, and TAC101 (as shown in Figure 6) are examples of synthetic retinoids that have been tested in clinical trials. 5,62,70, 104,109,120 Fenretinide, first synthesized in 1960s, is one of the most promising clinically tested retinoids.104
Figure 6.
Structure of natural or synthetic retinoids previously developed for clinical testing.
6.2. Retinoids in metabolic disease
Metabolic disease occurs when there is a disruption in the normal metabolism that regulates the conversion of food into energy used for cellular and physiological processes. Genetic defects that affect expression of key enzymes, transporter proteins, or even channel proteins can be responsible for causing metabolic diseases. Major metabolic disorders that are linked with retinoids are obesity, diabetes, and the metabolic syndrome.
Multiple studies have documented that RA regulates adipogenesis.63,64,65,66 RA acts as a high affinity ligand for the nuclear receptor peroxisome proliferation-activated receptor β/δ (PPARβ/δ). As PPARβ/δ receptor is a master regulator of lipid metabolism and glucose homeostasis, activation of these receptors increases lipid catabolism in adipose tissue and skeletal muscle to prevent obesity. In one study, obese mice that were treated with RA showed an induction of PPARβ/δ and RAR target genes correlating with weight loss and improved insulin responsiveness.67 RA precursors, including β-carotene, β-apo-8′-cartotenal, retinol, and retinal are reported to inhibit adipocyte differentiation. 68 Moreover, the RARγ selective retinoid ER36009 has been found to reduce adipose tissue when applied topically in rats.69
Similarly, the compound AM80 (Tamibarotene) has been reported as a potential anti-diabetic agent. It was found to inhibit the development of type 1 diabetes in the non-obese diabetic (NOD) mouse model.70 RA has also been shown to mediate immune-modulatory effects for the prevention of type I diabetes. Treatment with RA induces T regulatory (Treg) cell-dependent immune tolerance by suppressing both CD4+ and CD8+ T effector cells, while promoting Treg cell expansion.71
6.3. Retinoids in the eye
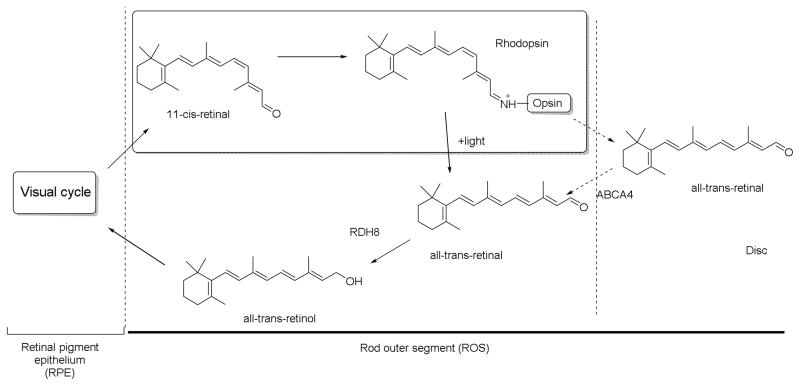
As discussed previously, retinoid signaling has an important role in the development of the nervous system including the eye. Studies in the mouse showed a requirement for RA signaling in the normal development of the ventral retina and optic nerve through its activities in the neural crest cell-derived periocular mesenchyme, dependent on the activities of various retinoid receptors RARα/β/γ and RXRα/β/γ. RA-synthesizing enzyme (RALDH) and RA-degrading enzymes (CYP26) also have critical roles in embryonic eye development.72 Subsequently, vitamin A is essential throughout life for maintaining normal vision as shown in Figure 8. The visual cycle occurs in photoreceptor cells and the adjacent retinal pigment epithelium (RPE). Rhodopsin in rod photoreceptors and cone pigment in the cone photoreceptors are the visual pigments that respond to light.73,74 In rods, the chromophore 11-cis-retinal binds to opsin to form rhodopsin. Photoisomerization of 11-cis-retinal changes it to an active conformation, all-trans-retinylidene, also known as Meta II. It recruits and binds to intracellular G proteins that facilitate various signaling cascades responsible for transducing vision.75
Figure 8.
Involvement of retinoids in the visual cycle.77
For the visual cycle to function, a continuous supply of 11-cis-retinal is necessary. After binding of 11-cis retinal with opsin, it undergoes photoisomerization to give 11-cis-retinylidene that is further changed to all-trans-retinylidene and finally to all-trans-retinal. All-trans-retinals are then dissociated from the opsin. Most of these products diffuse into the cytoplasm, while those dissociated into the disc lumen are also transferred into cytoplasm by the ATP-binding cassette transporter A4 (ABCA4).76 In cytoplasm, all-trans-retinal is reduced to all-trans-retinol, catalyzed by the NADPH-dependent all-trans-retinol dehydrogenases (RDH), RDH8 and RDH12. The all-trans-retinol diffuses into the RPE. In the RPE, all-trans-retinol is esterified into retinyl esters, as catalyzed by lecithin:retinol acyl transferase (LRAT), and aggregates into lipophilic droplets called retinosomes. These retinyl esters are converted to 11-cis-retinol by retinoid isomerase RPE65, and are further oxidized back to 11-cis-retinal by various enzymes including RDH5, RDH11, and other RDHs to be ready for next visual cycle.77
6.4. Retinoids in neurobiology and aging
Retinoids have essential functions in the CNS throughout life. In addition to controlling development of the hindbrain and the anterior spinal cord, retinoids regulate interneuron and motoneuron development along the dorsoventral axis. In fish and amphibian models, the formation of appropriate numbers of primary neurons is controlled by RA signaling. In vertebrates, various proneural genes, which regulate the process of primary neurogenesis, are regulated by RA signaling.78
The wide distribution of different RA receptors throughout the adult brain strongly suggests that retinoid signaling is necessary for neuronal plasticity, neurogenesis, and for cognitive and motor controlling functions. Neuronal plasticity in the brain is the capacity to rearrange the pattern of different neuronal connections that are essential for learning, memory, and promoting rapid response to damage and distress. These processes require synaptic turnover, neurite remodeling, neurogenesis, and changes in synaptic strength, which is measured by long-term potentiation (LTP) and long-term depression (LTD). Because such dynamic processes require transcriptional activation and inactivation of an array of genes in the brain, it is not surprising that neural functions including LTP and LTD are under the control of RA signaling. Individuals with vitamin A deficiency may show defective performances in spatial learning and memory tasks. This can occur in aged individuals who display decreased RA signaling in the brain. Supplementation of diet with RA can help reverse age-related decreases in hippocampal LTP and other brain functions.79
In a related sense, vitamin A and its precursor β-carotene may impact neurodegenerative diseases including Alzheimer’s disease (AD) and Parkinson’s disease (PD). They inhibit the formation of amyloid-β (Aβ) fibrils in a dose-dependant manner. Furthermore, they destabilize the preformation of Aβ fibrils in vitro.80 Oligomerization of Aβ40 and Aβ42 can also be prevented by vitamin A and β-carotene.81 One study showed that 9-cis-RA could provide neuroprotection in CNS injury in a transient focal ischemia model due to the activation of bone morphogenetic proteins (BMPs). Retinoid-dependent expression of BMP7 was found to protect neurons against injuries caused or induced by neurotoxins, free radicals, and ischemia in vitro and in vivo.82 Such reports support possibilities for retinoids and their derivatives as therapeutic agents for AD and other neurological diseases.
Various natural and synthetic retinoids have been studied for their anti-aging effects. Most of the studies have focused on patients with the photoaged skin, caused by chronic UV exposure. Tretinoin is the most potent and widely investigated retinoid for treatment of photoaging. Retinol, retinal, RA, adapalene, tazarotene, and aromatic compounds such as etretinate, acitretin, and seletinoid G (Figure 9) have been studied for anti-aging activity.83
Figure 9.
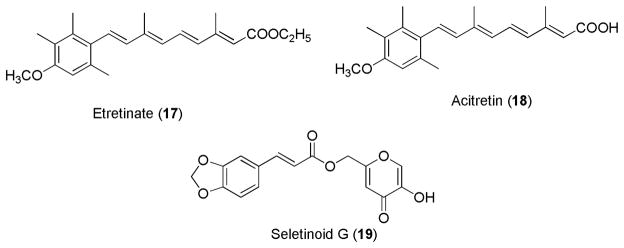
Aromatic retinoids for anti-aging activity.
6.5. Retinoids in Kidney
Retinoic acid is necessary for renal development and even mild gestational vitamin A deficiency can affect nephron endowment. 84, 85, 86 This was first demonstrated by feeding pregnant rats a vitamin A-deficient diet that caused reduced vitamin A levels in the fetal circulation leading to a significant reduction in nephron number and severe renal defects in the newborn rats.84 Retinoic acid stimulates ureteric bud branching in embryonic kidneys placed in metanephric organ culture,87 and this effect on ureteric bud morphogenesis was thought to be mediated by RAR regulation of Ret expression.88 Despite compelling evidence for a central role for retinoids in nephrogenesis, it remains unclear if retinoids or RAR/RXR signaling are involved in renal function after birth. Using a RARE reporter mouse, endogenous levels of renal RAR activity were found in collecting duct cells, which are cells derived from the ureteric bud, but not other renal cell types, raising the question of whether vitamin A modulates solute and water homeostasis 89 Retinoids have been found to have beneficial effects in some renal diseases 90 including glomerulosclerosis;91 however, the cellular mechanisms responsible for these renoprotective effects remain unclear. In a rat model of polycystic kidney disease (PKD), RXR-related genes that are involved in cell proliferation and organ morphogenesis were found to be aberrantly expressed early in disease, suggesting that aberrant RXR signaling may be involve in PKD cystogenesis. Interestingly, retinoic acids activate the human promoter for PKD1, the gene when mutated accounts for 85% of the clinical cases.92 Recent evidence shows that retinoids have anti-fibrotic and cytoprotective effects in the kidney,93, 94 supporting the need for the development of synthetic retinoid derivatives for the treatment of renal disease.
7. Drug discovery and use of Retinoids for treatment of diseases
As described above, several natural and synthetic retinoids have moved to clinical trials for various types of cancer, while several other classes of synthetic retinoids continue to be investigated at the pre-clinical level for anti-cancer activities. A major strategy to develop various synthetic retinoid derivatives with the potential to impart different biological activities is to systematically modify the hydrophobic, linker, or hydrophilic moieties of RA.
Recently, a series of acitretin analogs (Figure 10) was reported to have anti-proliferative activity. In this study, the modification was performed on the lipophilic part of RA using various functional moieties. Among the evaluated derivatives, the compounds 20, 21, 22, and 23 exhibited strong anti-proliferative activity in a dose-dependent manner. Compound 20 containing three methoxy groups in the aromatic ring was compound displaying the most potent anti-proliferative activity.95
Figure 10.
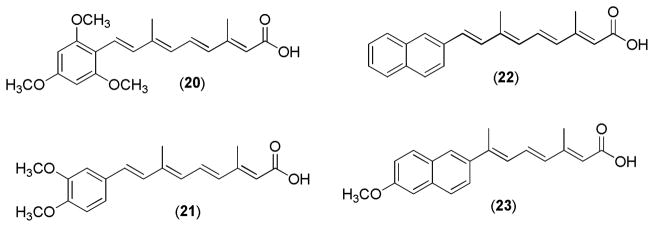
Acitretin derivatives.
Similarly, modification at the C-4 position of all-trans RA led to the discovery of potent novel retinoic acid metabolism blocking agents (RAMBAs). In this case, the C-4 position of all-trans RA was modified with various nucleophilic ligands. Compound 24 (Figure 11, VN/14-1) had inhibitory activity, superior to all-trans RA, on growth of the human breast cancer MCF-7 cell line in nude mice. The compound was administered subcutaneously to the mice following implantation of human breast tumor MCF-7 xenografts.96
Figure 11.
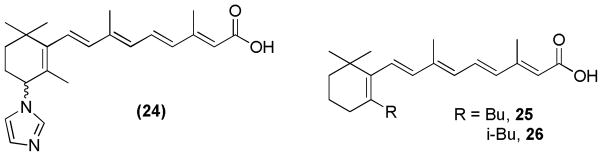
Retinoid derivatives
Modifications at position-5 of the lipophilic moiety (cyclohexene ring) have also been tested with various substituents. Such modified analogs of all-trans RA and 9-cis RA were evaluated for their anti-proliferative and pro-differentiative activities with the human leukemia HL-60 cell line. The all-trans analogs 25 and 26 (Figure 11) were found to be potent whereas none of the 9-cis analogs showed strong activity.97
Modifications on the cyclohexene ring were also used to derive various acyclic demethyl geranylgeranoic acids (GGAs) as retinoid analogs. 3-Demethyl derivative 27 (Figure 12) showed the most significant anti-proliferative activity using the HL-60 cell line, and induced apoptosis more potently than 9-cis RA.98
Figure 12.
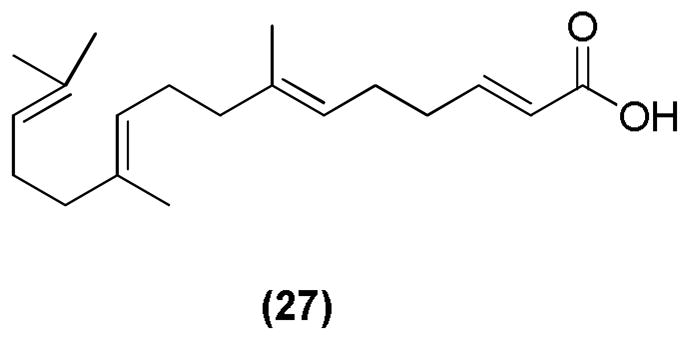
Acyclic demethyl geranylgeranoic acid (GGA)
In an attempt to modify the hydrophilic moiety, various ferrocenyl retinoates, such as 28 (Figure 13) were developed. These analogues were tested for their cytotoxicity against various human cancer cell lines including a lung cancer cell line (A549), a liver cancer cell line (BEL7404), and a tongue cancer cell line (Tca). The modified compounds showed better cytotoxicity than the standard 13-cis RA.99
Figure 13.
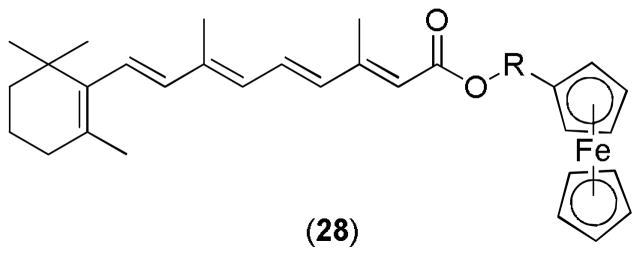
Ferrocenyl retinoate
The combination of all-trans RA and histone deacetylase inhibitors (HDACIs) resulted in various novel mutual products (MPs). The carboxylic acid group of all-trans RA was linked with different HDACIs. These MPs (Figure 14) were tested for growth inhibitory activity against several breast and prostate cancer cell lines. Compounds 29 and 30 performed better than the parental compounds, and showed the most significant growth inhibitory activity against a prostate cancer cell line (PC-3) and a breast cancer cell line (MDA-MB-231), respectively.100
Figure 14.
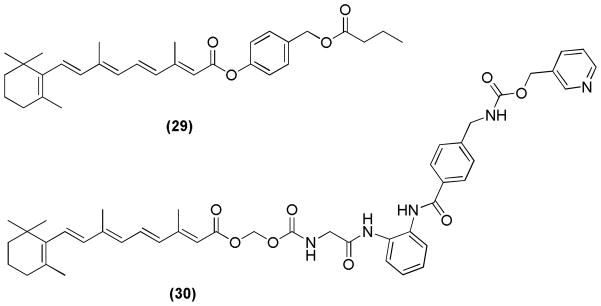
Novel mutual products (MPs)
The substitution of carboxylic acid of all-trans RA was carried out to develop a series of retinoate and retinamide derivatives (Figure 15). These derivatives were tested for their anti-tumor activities in NB4 cells using the MTT proliferation assay. Two compounds 31 and 32 showed higher cytotoxicity relative to other derivatives or all-trans RA.101
Figure 15.
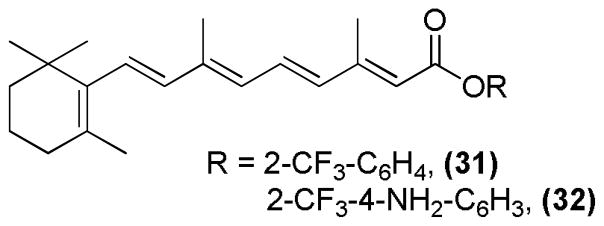
All-trans RA derivatives
Recently, novel indole retinoid derivatives (Figure 16) were reported with anti-cancer activities. Most of the developed compounds showed strong anti-proliferative activity, with compound 33 being the most potent. Compound 33 showed significant activity especially in breast cancer cell lines, including estrogen receptor (ER)-positive and ER-negative cell lines.102
Figure 16.
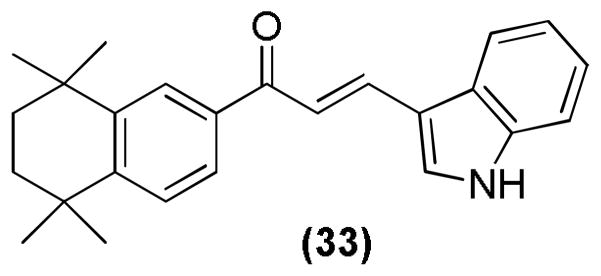
Indole retinoid derivative
A novel class of retinoid-chalcone hybrids has also been developed and studied for their anti-cancer activities (Figure 17). They were tested against the colon cancer cell line (HT-29), which indicated that compound 34 possessed the most significant proliferation inhibitory activity with an IC50 of 0.66 μM.103
Figure 17.
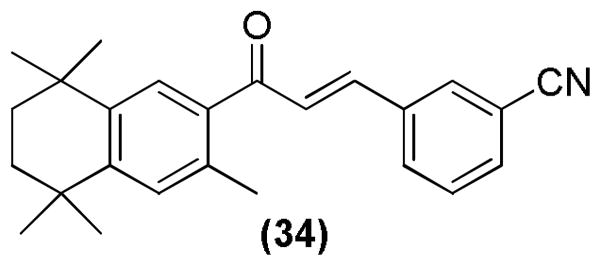
Retinoid-chalcone hybrid
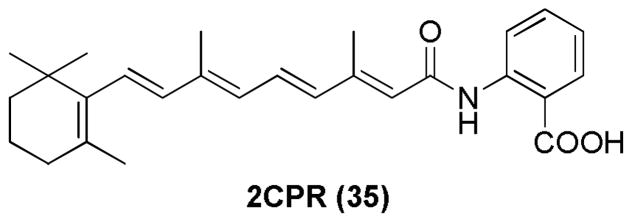
4HPR has been studied for activity against a variety of cancer types. Derivatives of 4HPR have been investigated for their growth inhibitory activity against human lung and head-and-neck cancer cells. This resulted in discovery of a more potent compound 2CPR, 35.104
Similarly, our research group has reported various synthetic derivatives of 4HPR for inhibition of rhabdoid tumors and for targeting a protein that is over-expressed in prostate carcinoma called mitochondria-associated granulocyte–macrophage colony stimulating factor signaling (Magmas). Compound 36 (Figure 19) was found to be a potent Magmas inhibitor.105 Moreover, compound 37 that contains iodo substituted on the phenyl ring showed more potent inhibitory activity (IC50 = 3.14 μM) against rhabdoid tumor cells than the parent compound 4HPR (IC50 = 14.68 μM).106 Compound 38 also showed significant inhibition of rhabdoid tumor cells when compared with the parent compound 4HPR.107
Figure 19.
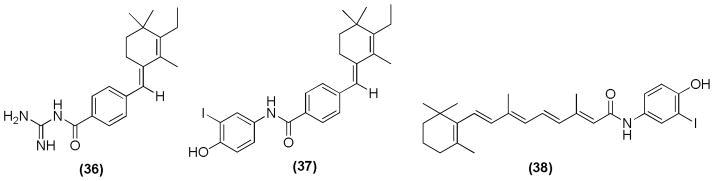
4HPR derivatives as inhibitors of Magmas and rhabdoid tumors.
There have been reports of diaryl sulfide retinoid analogs that can specifically bind to and transactivate RXRs. Compounds bearing 3-methyl on the tetrahydronaphthalene ring did not show any binding and transactivation of the RARs, indicating the importance of 3-methyl group for the RXR specificity. Compound 39 (Figure 20) was found to be the most potent and specific RXR agonist.108
Figure 20.
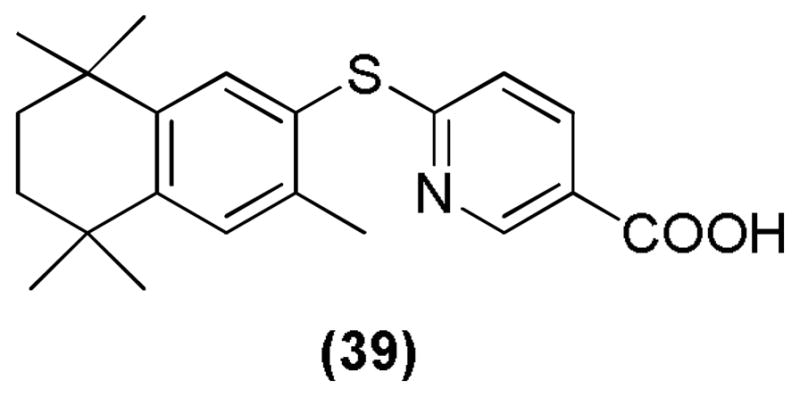
Diaryl sulfide retinoid.
Retinoids AM80 (Tamibarotene, 16), mentioned earlier for its anti-diabetic activity, has also been studied for treatment of AD. For this purpose, AM80 was orally administered to amyloid precursor protein (APP)23 mice, an animal model of AD. The result showed a significant reduction of insoluble Aβ levels, especially Aβ42, in the brain but no effect on the soluble Aβ levels.109 Similarly all-trans RA has been reported as a promising agent for treatment of AD. It was found that treatment of an AD transgenic mouse model with all-trans RA resulted in reduction of Aβ accumulation and tau hyperphosphorylation. Interestingly, there was significant reduction in glial activation and neuronal loss in the brain and improvement in spatial learning and memory.110
Finally, retinoids have also been used for the treatment of acne. Tretinoin (all-trans RA), isotretinoin (13-cis RA), and synthetic retinoids adapalene and tazarotene are FDA approved retinoids for the treatment of acne. Retinaldehyde has shown anti-bacterial effect against the P. acnes organism.111, 112
All these above examples are clearly demonstrated the ability and utility of retinoids as novel therapeutic agents for many life threatening diseases.
8. Retinoids in molecular imaging
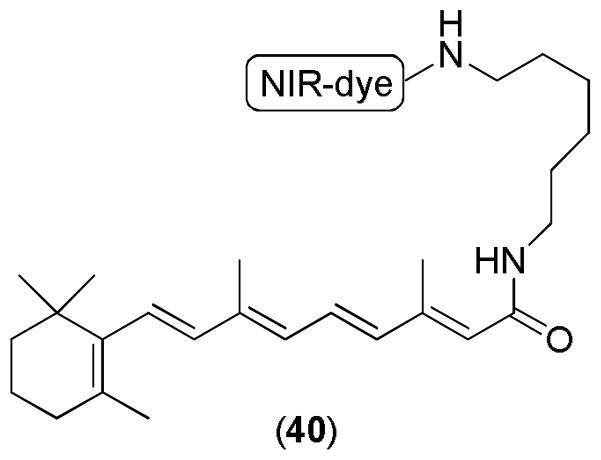
Molecular imaging plays a very important role in the field of drug discovery. It provides a useful tool to track molecules and identify the molecular targets. Fortunately, an imageable RA derivative has been reported. The imageable compound 40 (Figure 21) was developed by the conjugation of IRdye800cw and N-(aminohexyl0retinoicamide) [RA-NH(CH2)6NH2]. This near-infrared (NIR)-labeled agent was used for both in vitro and in vivo imaging studies. It can be used to detect multiple human cancer xenografts. Interestingly, only retinoid labeled dye was able to internalize in human tumor cells as observed by confocal microscopy. This technique has enabled the analysis of biodistribution, pharmacokinetics, and pharmacodynamics of the experimental agent in real-time. It can be applied to determine an appropriate therapeutic dose to avoid the systemic toxicity caused by high doses of RA.113
Figure 21.
An imageable RA derivative.
The development of imaging agents of hybrid compounds containing retinoids and HDAcs would open a new avenue to study the epigenetic biology in cancer and other neurodisorder diseases.
9. Retinoids in stem cell biology and regenerative medicine
Retinoids regulates the stem cell differentiation via transcription activation. Retinoids are able to activate transcription by binding to the nuclear receptors RARα, RARβ, and RARγ. These RARs can form heterodimer with one of the RXRs and can bind with the DNA. This binding facilitates the activation of transcription of RA primary response genes, which are important for stem cell differentiation.114 Stem cells are unspecialized cells that have the ability to self-reproduce and differentiate into functional phenotypes. They can be originated from embryonic, fetal, or adult tissues. Differentiation ability of stem cells into functional cells in the damaged organ has been an important tool in the field of regenerative medicine. Regenerative therapy, emerging field of biomedicine, involves the repair and replacement or regeneration of cells, tissues, or organs. The importance of regenerative therapy has been studied in a wide range of diseases including kidney diseases,115 cardiac/heart diseases,116 skin diseases,117 retinal disorders,118 and neurodegenerative diseases such as AD and PD.119 In another experiments Marder group showed that synthetic retinoids are able to differentiate embryonal carcinoma stem cells to become neurons. 120 Further studies are needed to define the potential for RA in regenerative medicine.
10. Conclusions
Retinoids comprise a group of natural and synthetic molecules, which regulate a variety of essential biological processes during normal development, help maintain homeostasis, and also mediate protection from diseases such as cancer and age-related disorders of the CNS. Genomic functions of the retinoids are mediated via their nuclear DNA-binding receptors, RARs and RXRs, which regulate gene transcription through recruitment of corepressors and coactivators. Retinoids also have notable non-genomic functions through retinoylation. The synthesis, testing, and development of retinoid derivatives as drugs that target RARs and RXRs is currently an active field for chemical biology. Novel RA derivatives may be useful as therapeutics for the treatment of various malignancies, as well as renal, metabolic and CNS diseases, while avoiding the teratogenic affects of RA. Synthesis of new retinoids may also serve as useful reagents for molecular imaging. The challenge to synthetic chemists is how to design and synthesize receptor sub-type and isotype specific ligands. Moreover, there are concerns in regard to how receptor isotypes and sub-types function in particular tissue and organ, and how the concentration of RA is spatial, temporal and kinetic controlled in organ development and disease. As regenerative medicine and stem cell biology are the frontier areas of research and RA signaling pathway plays a major role differentiation, the development of receptor specific ligands may open new avenues for chemical biologists.
Figure 5.
Role of retinoic acid in inhibiting cancer development. 57,58
Figure 7.
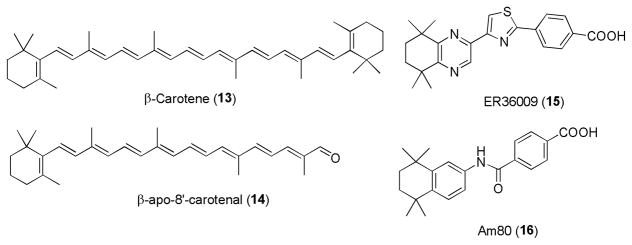
Retinoids for treatment of metabolic disease
Figure 18.
Discovery of a 4HPR derivative
Acknowledgments
B.C.D. is thankful to University of Kansas Medical Center for startup funding. B.C.D. is supported by grants from the NIH (AA020630 and AI093220). DWP is supported by grant from NIH (DK081579). T.E. is supported by grants from the NIH (HL56182, HL111400) and NYSTEM (C028100).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References & Notes
- 1.Barua AB, Furr HC. Mol Biotechnol. 1998;10:167. doi: 10.1007/BF02760863. [DOI] [PubMed] [Google Scholar]
- 2.Carr FH, Price EA. Biochemical J. 1926;20:497. doi: 10.1042/bj0200497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neeld JB, Pearson WN. J Nutr. 1963;79:454. doi: 10.1093/jn/79.4.454. [DOI] [PubMed] [Google Scholar]
- 4.Furr HC. J Nutr. 2004;134:281S. doi: 10.1093/jn/134.1.281S. [DOI] [PubMed] [Google Scholar]
- 5.Bushue N, Wan YJ. Adv Drug Deliv Rev. 2010;62:1285. doi: 10.1016/j.addr.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kakkad BP, Ong DE. J Biol Chem. 1988;263:12916. [PubMed] [Google Scholar]
- 7.MacDonald PN, Ong DE. J Biol Chem. 1988;263:12478. [PubMed] [Google Scholar]
- 8.Harrison EH, Hussain MM. J Nutr. 2001;131:1405. doi: 10.1093/jn/131.5.1405. [DOI] [PubMed] [Google Scholar]
- 9.Green PH, Glickman RM. J Lipid Res. 1981;22:1153. [PubMed] [Google Scholar]
- 10.Blomhoff R, Green MH, Berg T, Norum KR. Science. 1990;250:399. doi: 10.1126/science.2218545. [DOI] [PubMed] [Google Scholar]
- 11.Noy N, Slosberg E, Scarlata S. Biochemistry. 1992;31:11118. doi: 10.1021/bi00160a023. [DOI] [PubMed] [Google Scholar]
- 12.MacDonald PN, Ong DE. Biochem Biophys Res Commun. 1988;156:157. doi: 10.1016/s0006-291x(88)80818-0. [DOI] [PubMed] [Google Scholar]
- 13.Blaner WS, O’Byrne SM, Wongsiriroj N, Kluwe J, D’Ambrosio DM, Jiang H, Schwabe RF, Hillman EM, Piantedosi R, Libien J. Biochim Biophys Acta. 2009;1791:467. doi: 10.1016/j.bbalip.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berry DC, O’Byrne SM, Vreeland AC, Blaner WS, Noy N. Mol Cell Biol. 2012;32:3164. doi: 10.1128/MCB.00505-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duester G. Eur J Biochem. 2000;267:4315. doi: 10.1046/j.1432-1327.2000.01497.x. [DOI] [PubMed] [Google Scholar]
- 16.Blomhoff R, Blomhoff HK. J Neurobiol. 2006;66:606. doi: 10.1002/neu.20242. [DOI] [PubMed] [Google Scholar]
- 17.Napoli JL. Clin Immunol Immunopathol. 1996;80:S52. doi: 10.1006/clin.1996.0142. [DOI] [PubMed] [Google Scholar]
- 18.Theodosiou M, Laudet V, Schubert M. Cell Mol Life Sci. 2010;67:1423. doi: 10.1007/s00018-010-0268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chambon P. FASEB J. 1996;10:940. [PubMed] [Google Scholar]
- 20.Moras D, Gronemeyer H. Curr Opin Cell Biol. 1998;10:384. doi: 10.1016/s0955-0674(98)80015-x. [DOI] [PubMed] [Google Scholar]
- 21.Renaud JP, Moras D. Cell Mol Life Sci. 2000;57:1748. doi: 10.1007/PL00000656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rastinejad F. Curr Opin Struct Biol. 2001;11:33. doi: 10.1016/s0959-440x(00)00165-2. [DOI] [PubMed] [Google Scholar]
- 23.Wei LN. Annu Rev Pharmacol Toxicol. 2003;43:47. doi: 10.1146/annurev.pharmtox.43.100901.140301. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi N, Breitman TR. J Biol Chem. 1989;264:5159. [PubMed] [Google Scholar]
- 25.Takahashi N, Sausville EA, Breitman TR. Clin Cancer Res. 1995;1:637. [PubMed] [Google Scholar]
- 26.Cione E, Tucci P, Senatore V, Ioele G, Genchi G. Mol Cell Biochem. 2005;276:55. doi: 10.1007/s11010-005-2845-2. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi N, Jetten AM, Breitman TR. Biochem Biophys Res Commun. 1991;180:393. doi: 10.1016/s0006-291x(05)81306-3. [DOI] [PubMed] [Google Scholar]
- 28.Dong D, Zile MH. Biochem Biophys Res Commun. 1995;217:1026. doi: 10.1006/bbrc.1995.2872. [DOI] [PubMed] [Google Scholar]
- 29.Zile MH. Exp Biol Med. 2004;229:598. doi: 10.1177/153537020422900703. [DOI] [PubMed] [Google Scholar]
- 30.Li Z, Shen J, Wu WK, Wang X, Liang J, Qiu G, Liu J. PloS One. 2012;1:e46565. doi: 10.1371/journal.pone.0046565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hale F. J Hered. 1933;24:105. [Google Scholar]
- 32.Glover JC, Renaud JS, Rijli FM. J Neurobiol. 2006;66:705. doi: 10.1002/neu.20272. [DOI] [PubMed] [Google Scholar]
- 33.Maden M. J Neurobiol. 2006;66:726. doi: 10.1002/neu.20248. [DOI] [PubMed] [Google Scholar]
- 34.Maden M, Gale E, Kostetskii I, Zile M. Curr Biol. 1996;6:417. doi: 10.1016/s0960-9822(02)00509-2. [DOI] [PubMed] [Google Scholar]
- 35.Diez del Corral R, Olivera-Martinez I, Goriely A, Gale E, Maden M, Storey K. Neuron. 2003;40:65. doi: 10.1016/s0896-6273(03)00565-8. [DOI] [PubMed] [Google Scholar]
- 36.Lupo G, Gestri G, O’Brien M, Denton RM, Chandraratna RA, Ley SV, Harris WA, Wilson SW. Proc Natl Acad Sci U S A. 2011;108:8698. doi: 10.1073/pnas.1103802108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelley MW, Turner JK, Reh TA. Development. 1994;120:2091. doi: 10.1242/dev.120.8.2091. [DOI] [PubMed] [Google Scholar]
- 38.Niederreither K, Vermot J, Messaddeq N, Schuhbaur B, Chambon P, Dollé P. Development. 2001;128:1019. doi: 10.1242/dev.128.7.1019. [DOI] [PubMed] [Google Scholar]
- 39.Collop AH, Broomfield JA, Chandraratna RA, Yong Z, Deimling SJ, Kolker SJ, Weeks DL, Drysdale TA. Dev Biol. 2006;291:96. doi: 10.1016/j.ydbio.2005.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin SC, Dollé P, Ryckebüsch L, Noseda M, Zaffran S, Schneider MD, Niederreither K. Proc Natl Acad Sci U S A. 2010;107:9234. doi: 10.1073/pnas.0910430107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clagett-Dame M, Knutson D. Nutrients. 2011;3:385. doi: 10.3390/nu3040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghatpande S, Ghatpande A, Sher J, Zile MH, Evans T. Blood. 2002;99:2379. doi: 10.1182/blood.v99.7.2379. [DOI] [PubMed] [Google Scholar]
- 43.Stavridis MP, Collins BJ, Storey KG. Development. 2010;137:881. doi: 10.1242/dev.043117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Engberg N, Kahn M, Petersen DR, Hansson M, Serup P. Stem Cells. 2010;28:1498. doi: 10.1002/stem.479. [DOI] [PubMed] [Google Scholar]
- 45.Metallo CM, Ji L, de Pablo JJ, Palecek SP. Stem Cells. 2008;26:372. doi: 10.1634/stemcells.2007-0501. [DOI] [PubMed] [Google Scholar]
- 46.Liu L, Suzuki K, Nakagata N, Mihara K, Matsumaru D, Ogino Y, Yashiro K, Hamada H, Liu Z, Evans SM, Mendelsohn C, Yamada G. Birth Defects Research (Part B) 2012;95:79. doi: 10.1002/bdrb.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duester G. Birth Defects Res C Embryo Today. 2007;81:84. doi: 10.1002/bdrc.20092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sirbu IO, Zhao X, Duester G. Dev Dyn. 2008;237:1627. doi: 10.1002/dvdy.21570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gavalas A, Krumlauf R. Curr Opin Genet Dev. 2000;10:380. doi: 10.1016/s0959-437x(00)00100-3. [DOI] [PubMed] [Google Scholar]
- 50.Maden M, Blentic A, Reijntjes S, Seguin S, Gale E, Graham A. Int J Dev Biol. 2007;51:191. doi: 10.1387/ijdb.062175mm. [DOI] [PubMed] [Google Scholar]
- 51.Lewandoski M, Mackem S. Curr Biol. 2009;19:R558. doi: 10.1016/j.cub.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 52.Wendling O, Dennefeld C, Chambon P, Mark M. Development. 2000;127:1553. doi: 10.1242/dev.127.8.1553. [DOI] [PubMed] [Google Scholar]
- 53.Mark M, Ghyselinck NB, Chambon P. Nucl Recept Signal. 2009;7:e002. doi: 10.1621/nrs.07002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Thé H, Chomienne C, Lanotte M, Degos L, Dejean A. Nature. 1990;347:558. doi: 10.1038/347558a0. [DOI] [PubMed] [Google Scholar]
- 55.Borrow J, Goddard AD, Sheer D, Solomon E. Science. 1990;249:1577. doi: 10.1126/science.2218500. [DOI] [PubMed] [Google Scholar]
- 56.el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. Cell. 1993;75:817. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 57.Altucci L, Gronemeyer H. Nat Rev Cancer. 2001;1:181. doi: 10.1038/35106036. [DOI] [PubMed] [Google Scholar]
- 58.Niles RM. Nutrition. 2000;16:1084. doi: 10.1016/s0899-9007(00)00436-6. [DOI] [PubMed] [Google Scholar]
- 59.Baumann L, Vujevich J, Halem M, Martin LK, Kerdel F, Lazarus M, Pacheco H, Black L, Bryde J. Cutis. 2005;76:69. [PubMed] [Google Scholar]
- 60.Wu K, Kim HT, Rodriquez JL, Munoz-Medellin D, Mohsin SK, Hilsenbeck SG, Lamph WW, Gottardis MM, Shirley MA, Kuhn JG, Green JE, Brown PH. Clin Cancer Res. 2000;6:3696. [PubMed] [Google Scholar]
- 61.Christov KT, Moon RC, Lantvit DD, Boone CW, Steele VE, Lubet RA, Kelloff GJ, Pezzuto JM. Cancer Res. 2002;62:5178. [PubMed] [Google Scholar]
- 62.Altucci L, Leibowitz MD, Ogilvie KM, de Lera AR, Gronemeyer H. Nat Rev Drug Discov. 2007;6:793. doi: 10.1038/nrd2397. [DOI] [PubMed] [Google Scholar]
- 63.Schwarz EJ, Reginato MJ, Shao D, Krakow SL, Lazar MA. Mol Cell Biol. 1997;17:1552. doi: 10.1128/mcb.17.3.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sato M, Hiragun A, Mitsui H. Biochem Biophys Res Commun. 1980;95:1839. doi: 10.1016/s0006-291x(80)80113-6. [DOI] [PubMed] [Google Scholar]
- 65.Pairault J, Quignard-Boulange A, Dugail I, Lasnier F. Exp Cell Res. 1988;177:27. doi: 10.1016/0014-4827(88)90022-5. [DOI] [PubMed] [Google Scholar]
- 66.Kuri-Harcuch W. 1982;23:164. doi: 10.1111/j.1432-0436.1982.tb01279.x. [DOI] [PubMed] [Google Scholar]
- 67.Berry DC, Noy N. Mol Cell Biol. 2009;29:3286. doi: 10.1128/MCB.01742-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kawada T, Kamei Y, Fujita A, Hida Y, Takahashi N, Sugimoto E, Fushiki T. Biofactors. 2000;13:103. doi: 10.1002/biof.5520130117. [DOI] [PubMed] [Google Scholar]
- 69.Sakuta T, Uchiyama T, Kanayama T. Endocrine. 2006;30:113. doi: 10.1385/ENDO:30:1:113. [DOI] [PubMed] [Google Scholar]
- 70.Miwako I, Shudo K. Biol Pharm Bull. 2009;32:157. doi: 10.1248/bpb.32.157. [DOI] [PubMed] [Google Scholar]
- 71.Van YH, Lee WH, Ortiz S, Lee MH, Qin HJ, Liu CP. Diabetes. 2009;58:146. doi: 10.2337/db08-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cvekl A, Wang WL. Exp Eye Res. 2009;89:280. doi: 10.1016/j.exer.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khorana HG. J Biol Chem. 1992;267:1. [PubMed] [Google Scholar]
- 74.Kefalov VJ. J Biol Chem. 2012;287:1635. doi: 10.1074/jbc.R111.303008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.von Lintig J, Kiser PD, Golczak M, Palczewski K. Trends Biochem Sci. 2010;35:400. doi: 10.1016/j.tibs.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Molday RS. J Bioenerg Biomembr. 2007;39:507. doi: 10.1007/s10863-007-9118-6. [DOI] [PubMed] [Google Scholar]
- 77.Palczewski K. Trends Pharmacol Sci. 2010;31:284. doi: 10.1016/j.tips.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maden M. Nat Rev Neurosci. 2002;3:843. doi: 10.1038/nrn963. [DOI] [PubMed] [Google Scholar]
- 79.Etchamendy N, Enderlin V, Marighetto A, Vouimba RM, Pallet V, Jaffard R, Higueret P. J Neurosci. 2001;21:6423. doi: 10.1523/JNEUROSCI.21-16-06423.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ono K, Yoshiike Y, Takashima A, Hasegawa K, Naiki H, Yamada M. Exp Neurol. 2004;189:380. doi: 10.1016/j.expneurol.2004.05.035. [DOI] [PubMed] [Google Scholar]
- 81.Takasaki J, Ono K, Yoshiike Y, Hirohata M, Ikeda T, Morinaga A, Takashima A, Yamada M. J Alzheimers Dis. 2011;27:271. doi: 10.3233/JAD-2011-110455. [DOI] [PubMed] [Google Scholar]
- 82.Shen H, Luo Y, Kuo CC, Deng X, Chang CF, Harvey BK, Hoffer BJ, Wang Y. J Neurosci Res. 2009;87:545. doi: 10.1002/jnr.21865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mukherjee S, Date A, Patravale V, Korting HC, Roeder A, Weindl G. Clin Interv Aging. 2006;1:327. doi: 10.2147/ciia.2006.1.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wilson JG, Warkany J. Am J Anat. 1948;83:357. doi: 10.1002/aja.1000830303. [DOI] [PubMed] [Google Scholar]
- 85.Lelièvre-Pégorier M, Vilar J, Ferrier ML, Moreau E, Freund N, Gilbert T, Merlet-Bénichou C. Kidney Int. 1998;54:1455. doi: 10.1046/j.1523-1755.1998.00151.x. [DOI] [PubMed] [Google Scholar]
- 86.Merlet-Bénichou C, Vilar J, Lelièvre-Pégorier M, Gilbert T. Curr Opin Nephrol Hypertens. 1999;8:39. doi: 10.1097/00041552-199901000-00007. [DOI] [PubMed] [Google Scholar]
- 87.Gilbert T. Nephrol Dial Transplant. 2002;17:78. doi: 10.1093/ndt/17.suppl_9.78. [DOI] [PubMed] [Google Scholar]
- 88.Batourina E, Gim S, Bello N, Shy M, Clagett-Dame M, Srinivas S, Costantini F, Mendelsohn C. Nat Genet. 2001;27:74. doi: 10.1038/83792. [DOI] [PubMed] [Google Scholar]
- 89.Wong YF, Kopp JB, Roberts C, Scambler PJ, Abe Y, Rankin AC, Dutt N, Hendry BM, Xu Q. PloS One. 2011;6:e16770. doi: 10.1371/journal.pone.0016770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou TB, Qin YH, Lei FY, Su LN, Zhao YJ, Huang WF. Exp Mol Pathol. 2011;90:287. doi: 10.1016/j.yexmp.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 91.Zhang J, Pippin JW, Vaughan MR, Krofft RD, Taniguchi Y, Romagnani P, Nelson PJ, Liu ZH, Shankland SJ. Nephron Exp Nephrol. 2012;121:e23. doi: 10.1159/000342808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Islam MR, Puri S, Rodova M, Magenheimer BS, Maser RL, Calvet JP. Am J Physiol Renal Physiol. 2008;295:F1845. doi: 10.1152/ajprenal.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xu Q, Lucio-Cazana J, Kitamura M, Ruan X, Fine LG, Norman JT. Kidney Int. 2004;66:2119. doi: 10.1111/j.1523-1755.2004.66002.x. [DOI] [PubMed] [Google Scholar]
- 94.Kishimoto K, Kinoshita K, Hino S, Yano T, Nagare Y, Shimazu H, Nozaki Y, Sugiyama M, Ikoma S, Funauchi M. Nephron Exp Nephrol. 2011;118:e69. doi: 10.1159/000322409. [DOI] [PubMed] [Google Scholar]
- 95.Magoulas GE, Bariamis SE, Athanassopoulos CM, Haskopoulos A, Dedes PG, Krokidis MG, Karamanos NK, Kletsas D, Papaioannou D, Maroulis G. Eur J Med Chem. 2011;46:721. doi: 10.1016/j.ejmech.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 96.Patel JB, Huynh CK, Handratta VD, Gediya LK, Brodie AM, Goloubeva OG, Clement OO, Nanne IP, Soprano DR, Njar VC. J Med Chem. 2004;47:6716. doi: 10.1021/jm0401457. [DOI] [PubMed] [Google Scholar]
- 97.Wada A, Matsuura N, Mizuguchi Y, Nakagawa K, Ito M, Okano T. Bioorg Med Chem. 2008;16:8471. doi: 10.1016/j.bmc.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 98.Wada A, Wang F, Suhara Y, Yamano Y, Okitsu T, Nakagawa K, Okano T. Bioorg Med Chem. 2010;18:5795. doi: 10.1016/j.bmc.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 99.Long B, Liang S, Xin D, Yang Y, Xiang J. Eur J Med Chem. 2009;44:2572. doi: 10.1016/j.ejmech.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 100.Gediya LK, Khandelwal A, Patel J, Belosay A, Sabnis G, Mehta J, Purushottamachar P, Njar VC. J Med Chem. 2008;51:3895. doi: 10.1021/jm8001839. [DOI] [PubMed] [Google Scholar]
- 101.Shen J, Shi JB, Chen FH, Wang Y, Ruan JJ, Huang Y. Chin Chem Lett. 2009;20:809. [Google Scholar]
- 102.Gurkan-Alp AS, Mumcuoglu M, Andac CA, Dayanc E, Cetin-Atalay R, Buyukbingol E. Eur J Med Chem. 2012;58:346. doi: 10.1016/j.ejmech.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 103.Mizuno CS, Paul S, Suh N, Rimando AM. Bioorg Med Chem Lett. 2010;20:7385. doi: 10.1016/j.bmcl.2010.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sun SY, Yue P, Kelloff GJ, Steele VE, Lippman SM, Hong WK, Lotan R. Cancer Epidemiol Biomarkers Prev. 2001;10:595. [PubMed] [Google Scholar]
- 105.Jubinsky PT, Short MK, Ghanem M, Das BC. Bioorg Med Chem Lett. 2011;21:3479. doi: 10.1016/j.bmcl.2011.03.050. [DOI] [PubMed] [Google Scholar]
- 106.Das BC, Smith ME, Kalpana GV. Bioorg Med Chem Lett. 2008;18:4177. doi: 10.1016/j.bmcl.2008.05.097. [DOI] [PubMed] [Google Scholar]
- 107.Das BC, Smith ME, Kalpana GV. Bioorg Med Chem Lett. 2008;18:3805. doi: 10.1016/j.bmcl.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 108.Beard RL, Colon DF, Song TK, Davies PJA, Kochhar DM, Chandraratna RAS. J Med Chem. 1996;39:3556. doi: 10.1021/jm960386h. [DOI] [PubMed] [Google Scholar]
- 109.Kawahara K, Nishi K, Suenobu M, Ohtsuka K, Maeda A, Nagatomo K, Kuniyasu A, Staufenbiel M, Nakagomi M, Shudo K, Nakayama H. Biol Pharm Bull. 2009;32:1307. doi: 10.1248/bpb.32.1307. [DOI] [PubMed] [Google Scholar]
- 110.Ding Y, Qiao A, Wang Z, Goodwin JS, Lee ES, Block ML, Allsbrook M, McDonald MP, Fan GH. J Neurosci. 2008;28:11622. doi: 10.1523/JNEUROSCI.3153-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Thielitz A, Abdel-Naser MB, Fluhr JW, Zouboulis CC, Gollnick H. J Dtsch Dermatol Ges. 2008;6:1023. doi: 10.1111/j.1610-0387.2008.06741.x. [DOI] [PubMed] [Google Scholar]
- 112.Pechère M, Pechère JC, Siegenthaler G, Germanier L, Saurat JH. Dermatology. 1999;199:29. doi: 10.1159/000051375. [DOI] [PubMed] [Google Scholar]
- 113.Wang W, Qiu X, Zhang F, Sun J, Cameron AG, Wendt JA, Mawad ME, Ke S. Contrast Media Mol Imaging. 2011;6:200. doi: 10.1002/cmmi.419. [DOI] [PubMed] [Google Scholar]
- 114.Gudas LJ, Wagner JA. J Cell Physiol. 2011;226:322. doi: 10.1002/jcp.22417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yokoo T, Fukui A, Kobayashi E. Organogenesis. 2007;3:34. doi: 10.4161/org.3.1.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ptaszek LM, Mansourm M, Ruskin JN, Chien KR. Lancet. 2012;379:933. doi: 10.1016/S0140-6736(12)60075-0. [DOI] [PubMed] [Google Scholar]
- 117.Uitto J. J Invest Dermatol. 2011;131:812. doi: 10.1038/jid.2011.2. [DOI] [PubMed] [Google Scholar]
- 118.Daftarian N, Kiani S, Zahabi A. J Ophthalmic Vis Res. 2010;5:250. [PMC free article] [PubMed] [Google Scholar]
- 119.Peng J, Zeng X. Stem Cell Res Ther. 2011;2:32. doi: 10.1186/scrt73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Christie B, Barnard H, Batsanov A, Bridgens C, Cartmell B, Collings J, Maltman D, Redfern C, Marder T, Przyborski S, Whiting A. Org Biomol Chem. 2008;6:3497–3507. doi: 10.1039/b808574a. [DOI] [PubMed] [Google Scholar]