Abstract
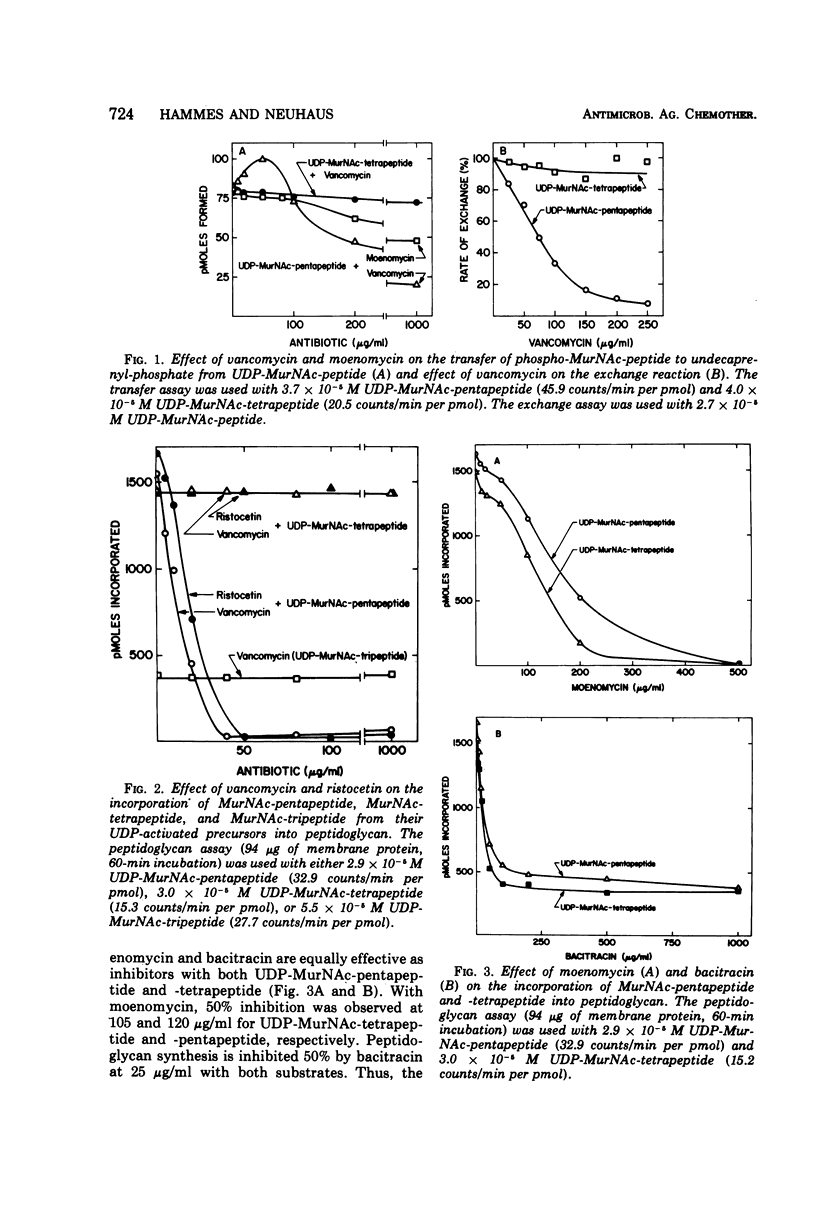
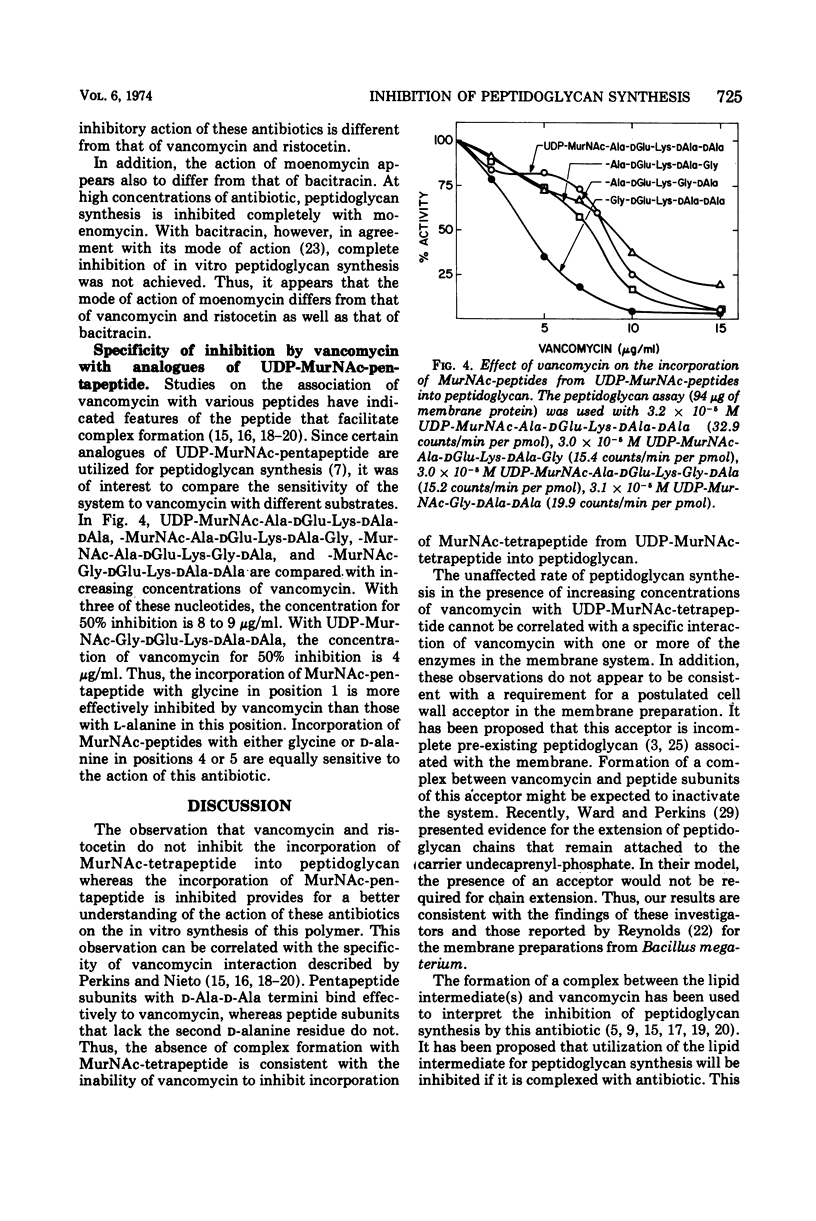
Vancomycin inhibits the synthesis of peptidoglycan in membrane preparations from Gaffkya homari with uridine diphosphate-N-acetylmuramyl (UDP-Mur-NAc)-pentapeptide as substrate, but not with either UDP-MurNAc-tetrapeptide or UDP-MurNAc-tripeptide. These results are correlated with the specificity studies described by Perkins and Nieto for complex formation between the antibiotic and the peptide subunit. It is concluded that the formation of a complex between vancomycin and a postulated cell wall acceptor or between vancomycin and the enzymes involved in peptidoglycan synthesis does not contribute to the inhibitory action of this antibiotic. The mechanism of vancomycin action on peptidoglycan synthesis is clearly different from that of moenomycin and bacitracin. In the presence of these antibiotics, peptidoglycan synthesis is inhibited with both UDP-MurNAc-pentapeptide and -tetrapeptide as substrates. In addition, these results provide additional insight into the mechanism of phospho-MurNAc-pentapeptide translocase. For example, enhancement of the transfer of phospho-MurNAc-peptide from UDP-MurNAc-peptide to undecaprenyl-phosphate at low concentrations of vancomycin is observed with UDP-MurNAc-pentapeptide and not with -tetrapeptide. Complexation of vancomycin with undecaprenyl-diphosphate-MurNAc-pentapeptide, resulting in an ineffective intermediate, would increase the rate of transfer by preventing the reassociation of undecaprenyl-diphosphate-MurNAc-pentapeptide with the enzyme.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON J. S., MATSUHASHI M., HASKIN M. A., STROMINGER J. L. LIPID-PHOSPHOACETYLMURAMYL-PENTAPEPTIDE AND LIPID-PHOSPHODISACCHARIDE-PENTAPEPTIDE: PRESUMED MEMBRANE TRANSPORT INTERMEDIATES IN CELL WALL SYNTHESIS. Proc Natl Acad Sci U S A. 1965 Apr;53:881–889. doi: 10.1073/pnas.53.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. S., Matsuhashi M., Haskin M. A., Strominger J. L. Biosythesis of the peptidoglycan of bacterial cell walls. II. Phospholipid carriers in the reaction sequence. J Biol Chem. 1967 Jul 10;242(13):3180–3190. [PubMed] [Google Scholar]
- Anderson J. S., Meadow P. M., Haskin M. A., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. I. Utilization of uridine diphosphate acetylmuramyl pentapeptide and uridine diphosphate acetylglucosamine for peptidoglycan synthesis by particulate enzymes from Staphylococcus aureus and Micrococcus lysodeikticus. Arch Biochem Biophys. 1966 Sep 26;116(1):487–515. doi: 10.1016/0003-9861(66)90056-7. [DOI] [PubMed] [Google Scholar]
- Best G. K., Durham N. N. Vancomycin adsorption to Bacillus subtilis cell walls. Arch Biochem Biophys. 1965 Sep;111(3):685–691. doi: 10.1016/0003-9861(65)90250-x. [DOI] [PubMed] [Google Scholar]
- Chatterjee A. N., Ward J. B., Perkins H. R. Synthesis of mucopeptide by L-form membranes. Nature. 1967 Jun 24;214(5095):1311–1314. doi: 10.1038/2141311a0. [DOI] [PubMed] [Google Scholar]
- Hammes W. P., Neuhaus F. C. Biosynthesis of peptidoglycan in Gaffkya homari: role of the peptide subunit of uridine diphosphate-N-acetylmuramyl-pentapeptide. J Bacteriol. 1974 Oct;120(1):210–218. doi: 10.1128/jb.120.1.210-218.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes W. P., Neuhaus F. C. On the specificity of phospho-N-acetylmuramyl-pentapeptide translocase. The peptide subunit of uridine diphosphate-N-actylmuramyl-pentapeptide. J Biol Chem. 1974 May 25;249(10):3140–3150. [PubMed] [Google Scholar]
- Huber G., Nesemann G. Moenomycin, an inhibitor of cell wall synthesis. Biochem Biophys Res Commun. 1968 Jan 11;30(1):7–13. doi: 10.1016/0006-291x(68)90704-3. [DOI] [PubMed] [Google Scholar]
- Izaki K., Matsuhashi M., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. 8. Peptidoglycan transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reaction in strains of Escherichia coli. J Biol Chem. 1968 Jun 10;243(11):3180–3192. [PubMed] [Google Scholar]
- Lugtenberg E. J., v Schijndel-van Dam A., van Bellegem T. H. In vivo and in vitro action of new antibiotics interfering with the utilization of N-acetyl-glucosamine-N-acetyl-muramyl-pentapeptide. J Bacteriol. 1971 Oct;108(1):20–29. doi: 10.1128/jb.108.1.20-29.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirelman D., Bracha R., Sharon N. Role of the penicillin-sensitive transpeptidation reaction in attachment of newly synthesized peptidoglycan to cell walls of Micrococcus luteus. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3355–3359. doi: 10.1073/pnas.69.11.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto M., Perkins H. R. Modifications of the acyl-D-alanyl-D-alanine terminus affecting complex-formation with vancomycin. Biochem J. 1971 Aug;123(5):789–803. doi: 10.1042/bj1230789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins H. R., Nieto M. The chemical basis for the action of the vancomycin group of antibiotics. Ann N Y Acad Sci. 1974 May 10;235(0):348–363. doi: 10.1111/j.1749-6632.1974.tb43276.x. [DOI] [PubMed] [Google Scholar]
- Perkins H. R., Nieto M. The preparation of iodinated vancomycin and its distribution in bacteria treated with the antibiotic. Biochem J. 1970 Jan;116(1):83–92. doi: 10.1042/bj1160083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins H. R. Specificity of combination between mucopeptide precursors and vancomycin or ristocetin. Biochem J. 1969 Jan;111(2):195–205. doi: 10.1042/bj1110195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds P. E. Peptidoglycan synthesis in bacilli. II. Characteristics of protoplast membrane preparations. Biochim Biophys Acta. 1971 May 18;237(2):255–272. doi: 10.1016/0304-4165(71)90316-3. [DOI] [PubMed] [Google Scholar]
- STRUVE W. G., NEUHAUS F. C. EVIDENCE FOR AN INITIAL ACCEPTOR OF UDP-NAC-MURAMYL-PENTAPEPTIDE IN THE SYNTHESIS OF BACTERIAL MUCOPEPTIDE. Biochem Biophys Res Commun. 1965 Jan 4;18:6–12. doi: 10.1016/0006-291x(65)90873-9. [DOI] [PubMed] [Google Scholar]
- Siewert G., Strominger J. L. Bacitracin: an inhibitor of the dephosphorylation of lipid pyrophosphate, an intermediate in the biosynthesis of the peptidoglycan of bacterial cell walls. Proc Natl Acad Sci U S A. 1967 Mar;57(3):767–773. doi: 10.1073/pnas.57.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. K., Neuhaus R. C. Reversal of the vancomycin inhibition of peptidoglycan synthesis by cell walls. J Bacteriol. 1968 Aug;96(2):374–382. doi: 10.1128/jb.96.2.374-382.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struve W. G., Sinha R. K., Neuhaus F. C. On the initial stage in peptidoglycan synthesis. Phospho-N-acetylmuramyl-pentapeptide translocase (uridine monophosphate). Biochemistry. 1966 Jan;5(1):82–93. doi: 10.1021/bi00865a012. [DOI] [PubMed] [Google Scholar]
- Ward J. B., Perkins H. R. The direction of glycan synthesis in a bacterial peptidoglycan. Biochem J. 1973 Dec;135(4):721–728. doi: 10.1042/bj1350721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. B. The synthesis of peptidoglycan in an autolysin-deficient mutant of Bacillus licheniformis N.C.T.C. 6346 and the effect of beta-lactam antibiotics, bacitracin and vancomycin. Biochem J. 1974 Jul;141(1):227–241. doi: 10.1042/bj1410227. [DOI] [PMC free article] [PubMed] [Google Scholar]



