Abstract
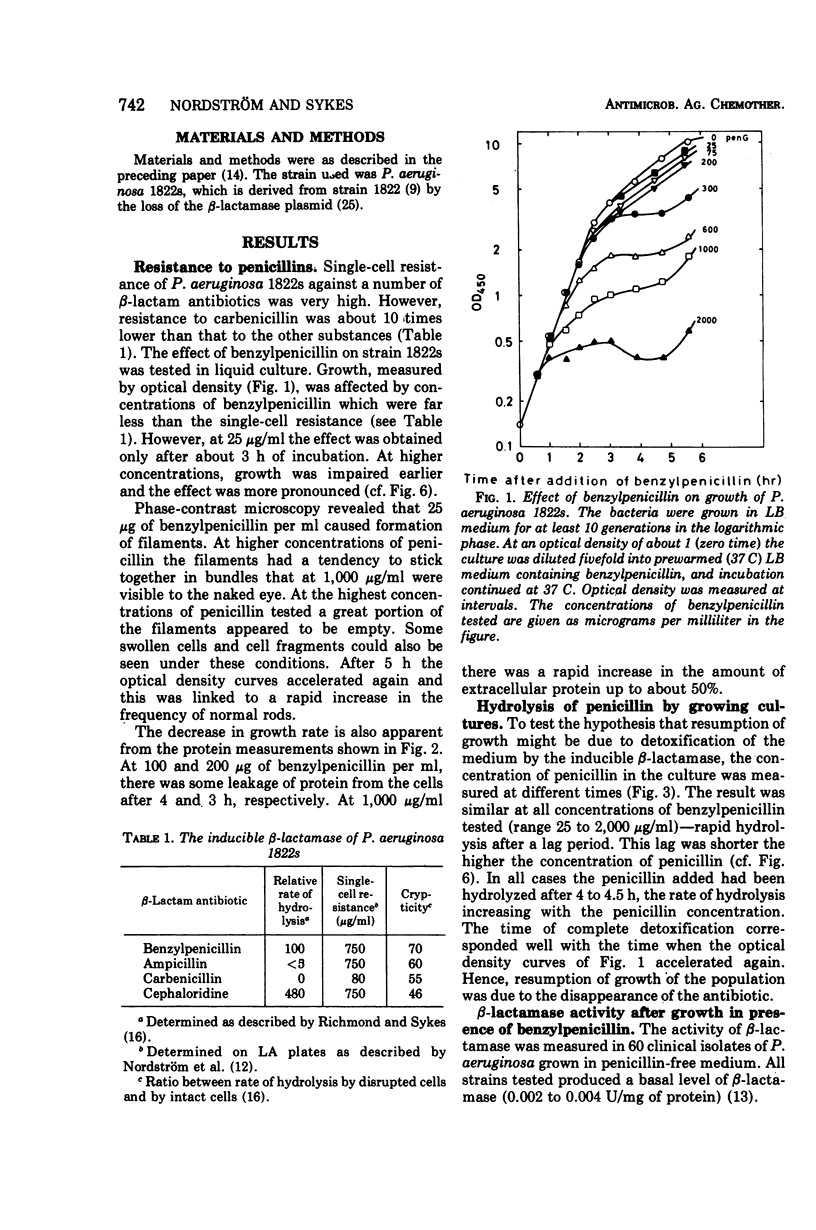
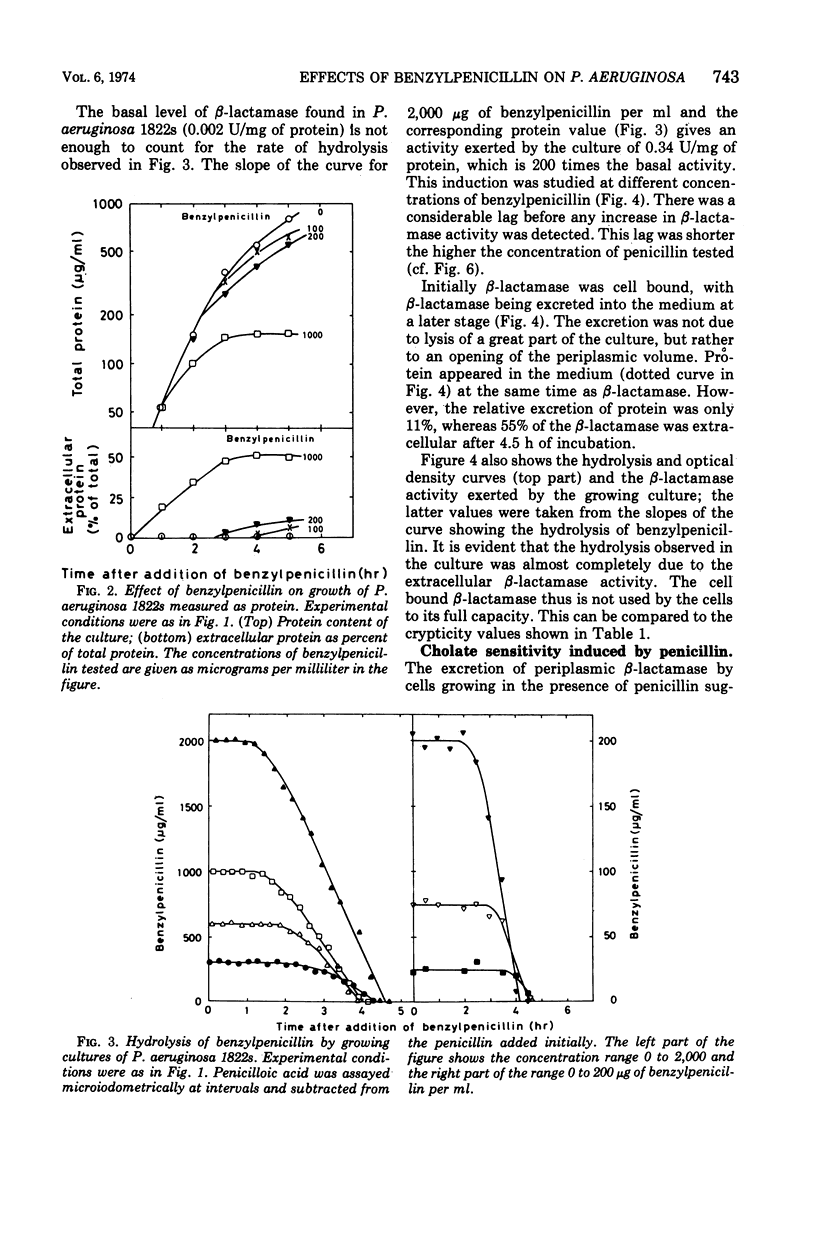
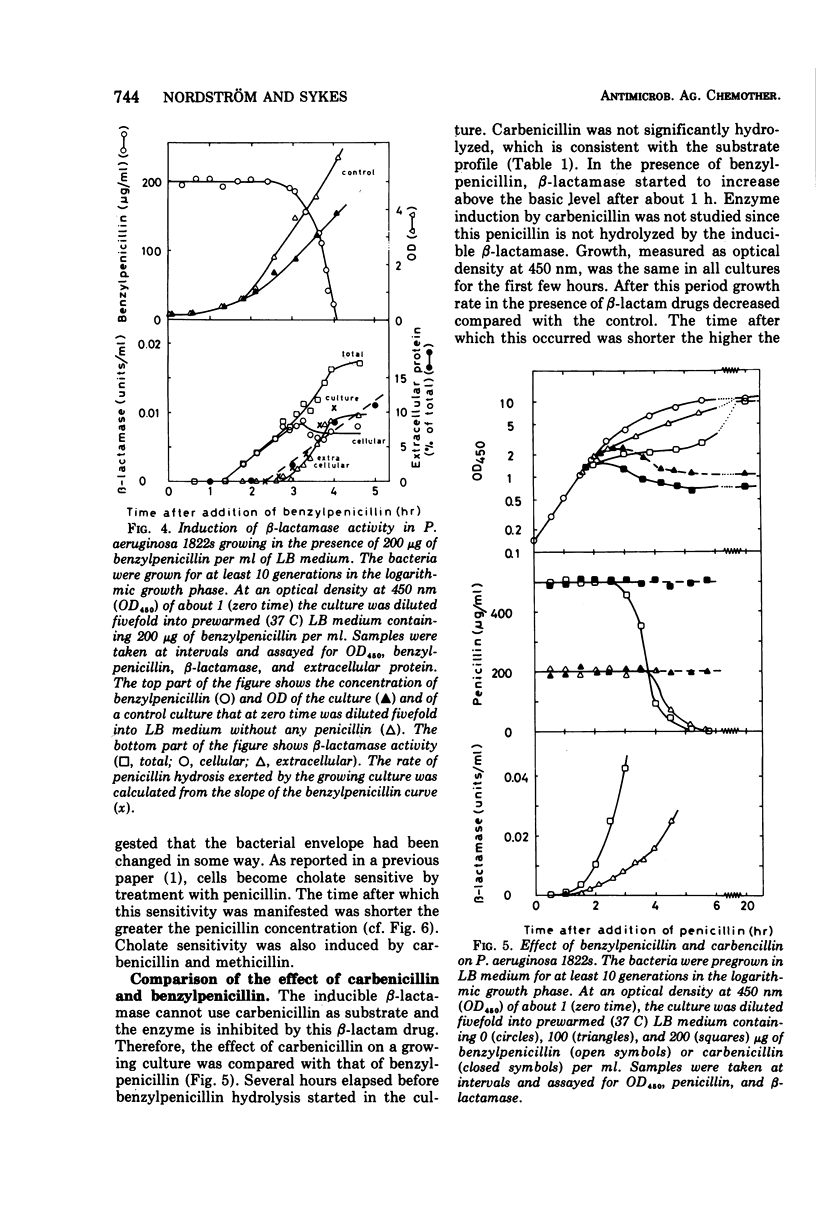
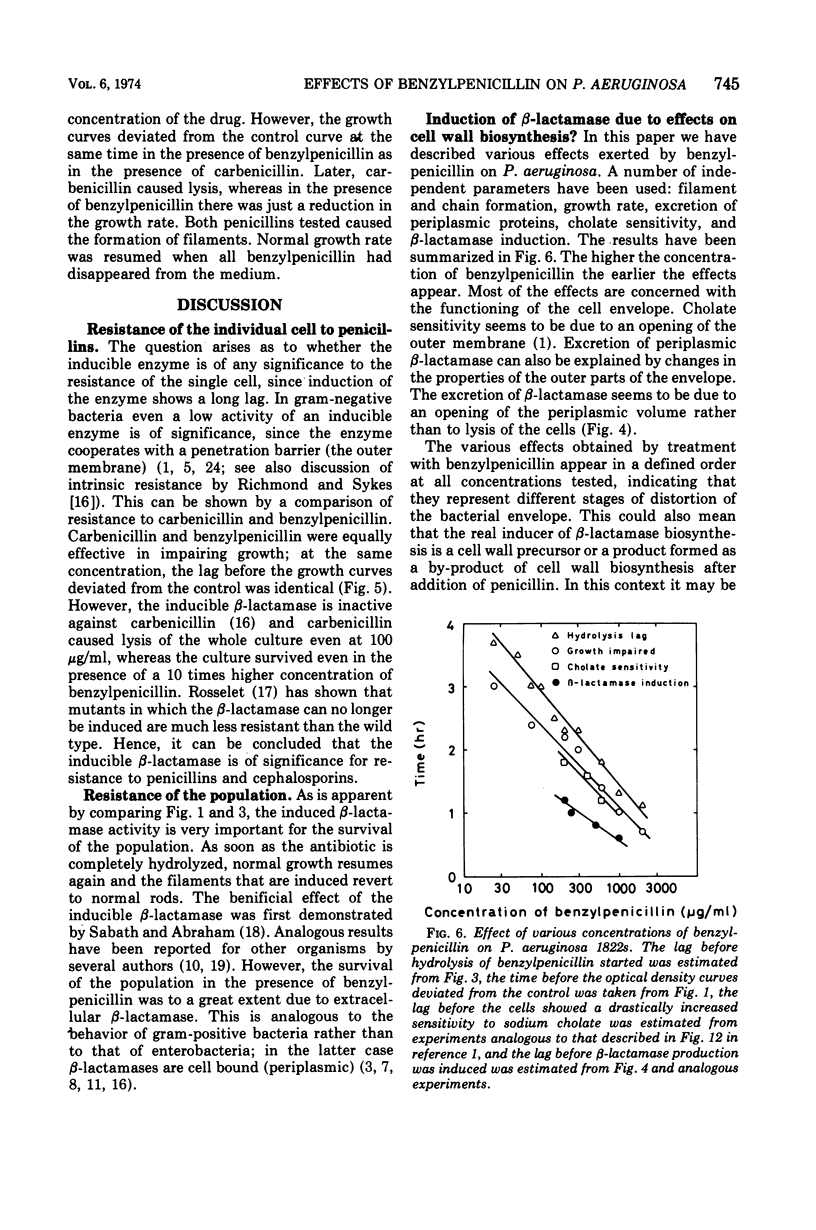
Pseudomonas aeruginosa produces a low basal level of β-lactamase (0.002 to 0.004 IU/mg of protein when benzylpenicillin is used as substrate). The β-lactamase specific activity can be increased several hundredfold by growing the bacteria in the presence of β-lactam antibiotics. This induction was studied in Pseudomonas aeruginosa 1822s. The single-cell resistance to benzylpenicillin was 750 μg/ml. In liquid culture all concentrations of benzylpenicillin tested (25 to 2,000 μg/ml) affected the bacteria similarly: β-lactamase formation was induced, the cells became cholate sensitive, growth rate decreased, filaments were formed, and β-lactamase was excreted. The effect appeared earlier the higher the concentration of the antibiotic. Most of the effects obtained are concerned with the functioning of the outer membrane. The excretion of β-lactamase seems to be due to an opening of the periplasmic volume rather than to lysis of the cells. Carbenicillin gave the same effects as benzylpenicillin at the same concentrations; the 10-fold lower resistance to carbenicillin than to benzylpenicillin can be explained by the inability of the inducible β-lactamase to hydrolyze carbenicillin. The induced β-lactamase was first cell bound and to a great extent located in the periplasmic volume, but later it was excreted into the medium. This extracellular activity was responsible for the detoxification of the medium. This is analogous to the behavior of gram-positive bacteria rather than to that of Enterobacteriaceae.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burman L. G., Nordström K., Bloom G. D. Murein and the outer penetration barrier of Escherichia coli K-12, Proteus mirabilis, and Pseudomonas aeruginosa. J Bacteriol. 1972 Dec;112(3):1364–1374. doi: 10.1128/jb.112.3.1364-1374.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citri N., Pollock M. R. The biochemistry and function of beta-lactamase (penicillinase). Adv Enzymol Relat Areas Mol Biol. 1966;28:237–323. doi: 10.1002/9780470122730.ch4. [DOI] [PubMed] [Google Scholar]
- Datta N., Richmond M. H. The purification and properties of a penicillinase whose synthesis is mediated by an R-factor in Escherichia coli. Biochem J. 1966 Jan;98(1):204–209. doi: 10.1042/bj0980204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber N., Friedman J. Beta-lactamase and the resistance of Pseudomonas aeruginosa to various penicillins and cephalosporins. J Gen Microbiol. 1970 Dec;64(3):343–352. doi: 10.1099/00221287-64-3-343. [DOI] [PubMed] [Google Scholar]
- Hamilton-Miller J. M. Damaging effects of ethylenediaminetetra-acetate and penicillins on permeability barriers in Gram-negative bacteria. Biochem J. 1966 Sep;100(3):675–682. doi: 10.1042/bj1000675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstadt Ozer J., Lowery D. L., Saz A. K. Derepression of beta-lactamase (penicillinase in Bacillus cereus by peptidoglycans. J Bacteriol. 1970 Apr;102(1):52–63. doi: 10.1128/jb.102.1.52-63.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAGO M., MIGLIACCI A., ABRAHAM E. P. PRODUCTION OF A CEPHALOSPORINASE BY PSEUDOMONAS PYOCYANEA. Nature. 1963 Jul 27;199:375–375. doi: 10.1038/199375a0. [DOI] [PubMed] [Google Scholar]
- Lindqvist R. C., Nordström K. Resistance of Escherichia coli to penicillins. VII. Purification and characterization of a penicillinase mediated by the R factor R1. J Bacteriol. 1970 Jan;101(1):232–239. doi: 10.1128/jb.101.1.232-239.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linström E. B., Boman H. G., Steele B. B. Resistance of Escherichia coli to penicillins. VI. Purification and characterization of the chromosomally mediated penicillinase present in ampA-containing strains. J Bacteriol. 1970 Jan;101(1):218–231. doi: 10.1128/jb.101.1.218-231.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowbury E. J., Lilly H. A., Kidson A., Ayliffe G. A., Jones R. J. Sensitivity of Pseudomonas aeruginosa to antibiotics: emergence of strains highly resistant to carbenicillin. Lancet. 1969 Aug 30;2(7618):448–452. doi: 10.1016/s0140-6736(69)90163-9. [DOI] [PubMed] [Google Scholar]
- Neu H. C. The surface localization of penicillinases in Escherichia coli and Salmonella typhimurium. Biochem Biophys Res Commun. 1968 Jul 26;32(2):258–263. doi: 10.1016/0006-291x(68)90378-1. [DOI] [PubMed] [Google Scholar]
- Nordström K., Eriksson-Grennberg K. G., Boman H. G. Resistance of Escherichia coli to penicillins. 3. AmpB, a locus affecting episomally and chromosomally mediated resistance to ampicillin and chlorampheincol. Genet Res. 1968 Oct;12(2):157–168. doi: 10.1017/s0016672300011770. [DOI] [PubMed] [Google Scholar]
- Nordström K., Sykes R. B. Induction kinetics of beta-lactamase biosynthesis in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1974 Dec;6(6):734–740. doi: 10.1128/aac.6.6.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond M. H., Sykes R. B. The beta-lactamases of gram-negative bacteria and their possible physiological role. Adv Microb Physiol. 1973;9:31–88. doi: 10.1016/s0065-2911(08)60376-8. [DOI] [PubMed] [Google Scholar]
- Rosselet A., Zimmermann W. Mutants of Pseudomonas aeruginosa with impaired -lactamase inducibility and increased sensitivity to -lactam antibiotics. J Gen Microbiol. 1973 Jun;76(2):455–457. doi: 10.1099/00221287-76-2-455. [DOI] [PubMed] [Google Scholar]
- SABATH L. D., ABRAHAM E. P. SYNERGISTIC ACTION OF PENICILLINS AND CEPHALOSPORINS AGAINST PSEUDOMONAS PYOCYANEA. Nature. 1964 Dec 12;204:1066–1069. doi: 10.1038/2041066a0. [DOI] [PubMed] [Google Scholar]
- SABATH L. D., FINLAND M. Inactivation of methicillin, oxacillin and ancillin by Staphylococcus aureus. Proc Soc Exp Biol Med. 1962 Dec;111:547–550. doi: 10.3181/00379727-111-27850. [DOI] [PubMed] [Google Scholar]
- SMITH J. T. Penicillinase and ampicillin resistance in a strain of Escherichia coli. J Gen Microbiol. 1963 Feb;30:299–306. doi: 10.1099/00221287-30-2-299. [DOI] [PubMed] [Google Scholar]
- Sabath L. D., Finland M. Resistance of penicillins and cephalosporins to beta-lactamases from Gram-negative bacilli: some correlations with antibacterial activity. Ann N Y Acad Sci. 1967 Sep 27;145(2):237–247. doi: 10.1111/j.1749-6632.1967.tb50222.x. [DOI] [PubMed] [Google Scholar]
- Sabath L. D., Jago M., Abraham E. P. Cephalosporinase and penicillinase activities of a beta-lactamase from Pseudomonas pyocyanea. Biochem J. 1965 Sep;96(3):739–752. doi: 10.1042/bj0960739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes R. B., Richmond M. H. Intergeneric transfer of a beta-lactamase gene between Ps. aeruginosa and E. coli. Nature. 1970 Jun 6;226(5249):952–954. doi: 10.1038/226952a0. [DOI] [PubMed] [Google Scholar]



