Abstract
Redox homeostasis is essential for basal signaling of several physiological processes, but a unilateral shift towards an ‘oxidative’ or ‘reductive’ trait will alter intracellular redox milieu. Typically, such an event influences the structure and the native function of a cell or an organelle. Numerous experimental research and clinical trials over the last 6 decades have demonstrated that enhanced oxygen-derived free radicals constitutes a major stimuli to trigger damage in several human diseases, including cardiovascular complications supporting the theory of oxidative stress (OS). However, until our key discovery, the dynamic interrelationship between “Reductive Stress (RS)” and cardiac health has been obscured by overwhelming OS studies (Rajasekaran et al., 2007). Notably, this seminal finding spurred considerable interest in investigations of other mechanistic insights, and thus far the results indicate a similar or stronger role for RS, than that of OS. In addition, from our own findings we strongly believe that constitutive activation of pathways that enable sustained generation of reducing equivalents glutathione (GSH), reduced nicotinamide adenine dinucleotide phosphate (NADPH) will cause RS and impair the basal cellular signaling mechanisms operating through harmless pro-oxidative events, in turn, disrupting single and/or a combination of key cellular processes such as growth, maturation, differentiation, survival, death etc., that govern healthy cell physiology. Here, we have discussed the role of RS as a causal or contributing factor in relevant pathophysiology of a major cardiac disease of human origin.
Redox Homeostasis vs. Redox Stress
Intracellular stress is known as an abnormal state or a stimulus that challenges physiological function of an organelle, cell, organ or organism as a whole. Based on the source and nature, stress exerts physiological or pathological consequences. Profound changes in the intracellular redox milieu are broadly termed as redox stress. Under basal state of redox milieu known as “redox homeostasis (RH)”, a biological pas de deux involving oxidants and reductants is essential to regulate many fundamental biological processes including, but not limited to, cellular signaling pathways, chromatin remodeling, transcriptional and post-transcriptional activity, protein folding/conformation, mitochondrial biogenesis and membrane permeability [1-4]. Thus, interference in the balance of reactive oxygen species (ROS; oxidants) and reductants (antioxidants) can dis-equilibrate RH and derange normal cellular life processes. Though ROS and reactive nitrogen species (RNS) candidates, such as superoxide, hydroxyl radicals, hydrogen peroxide, nitric oxide and peroxynitrite, are indispensable to support cellular vitality, when in excess can accumulate oxidative stress (OS), causing oxidation of lipids, proteins, and DNA, leading to a multitude of pathological conditions including myocardial infarction, vascular abnormality, neurodegenerative diseases and accelerated aging [5-9]. Depending on the chemistry and concentration of molecules that preserve intracellular RH, cellular stress can be classified as (a) oxidative (b) reductive and (c) nitrosative. Usually, OS is defined as the shift of balance between cellular oxidative and reductive potential toward oxidative one that are caused both by excessive generation of highly reactive free radicals from the mitochondria or other ROS generating sources and an impairment of cytoprotective defense mechanisms that scavenge ROS generated during normal physiological and/or pathological processes. In contrast, an increase in reducing equivalents including, but not restricted to, GSH, NADH, NADPH, cysteine etc. in conjunction with immense activation of antioxidant system and suppressed oxidative activity is referred to as reductive stress (RS). In otherwords, the imbalance between oxidants and antioxidants in favor of the latter forms the core of the definition for “reductive stress”. Typically, RS is likely to be elicited from the intrinsic signals that enable cellular defense through a pro-oxidative or optimal-oxidative setting (ie.) the body’s cytoprotective defense system turning against itself. Since the body’s own defense system itself attacks the system which it is supposed to shield, we feel that RS could be a potent threat and thus, in a given context, RS could be as deleterious as and/or more deleterious than the OS. To better understand the latter case, an analogy could be drawn as to what would happen to civilians (cellular system) if the law maker (reductive potential, the cop) turns a law breaker (stressor). The redox regulation of cellular response to acute versus chronic stress is illustrated in Figure 1.
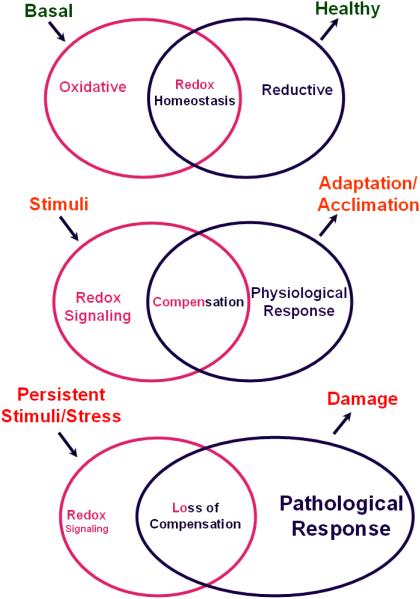
Figure 1. Cellular fitness associated with redox State.
(A) Basal State: Under normal physiology, an equilibrium is maintained between the generation of reactive oxygen/nitrogen species (ROS/RNS) (oxidative species) and counteracting reductant molecules that quenches free radical/oxidant species which is broadly termed as “redox homeostasis (RH)”. (B) Defensive State: An acute stress stimuli induces intracellular redox signals evoking compensatory responses which moderately adjusts the concentrations of oxidants and reductants and helps maintaining a normal physiology. This is a state of adaptation or acclimation. (C) Stress: When the stress signal is sustained, the redox signaling might go awry and abnormal reflecting in loss of compensatory responsiveness leading to pathological consequences.
Over 6 decades of research strongly support that a shift in the redox state towards OS as one of the leading causes for various pathological processes and diseases in humans [5,7,8, 10-13]. Specifically, studies using animals have demonstrated that either activation of enzymes that generate ROS, such as NADPH oxidases and xanthine oxidase, or inhibition of enzymatic pathways counterbalancing ROS production [such as superoxide dismutase (SOD), catalase, glutathione peroxidase (GPx), and the thioredoxin/thioredoxin-reductase (Trx/TrxR system)] foster pathological cardiac remodeling and end-stage heart failure [14]. Nevertheless, the cause and consequences of RS has neither thoroughly been studied using appropriate in vitro and/or in vivo models, nor reviewed in the context of human health. Here, we intend to describe the role of RS in cardio-pathophysiology through analyzing the available reports since our first discovery of reductive stress in a major human cardiac disease.
Reductive Stress
RS was first introduced by Albert Wendel, to denote the excessive accumulation of reduced cofactor, NADH that can facilitate reduction of chelated ferric iron [15-17]. Later, Ghyczy and Boros suggested that RS could be defined as an abnormal increase in pathological processes – associated electron pressure or reducing power or high energy reducing electrons (NADH) accompanied by failure of mechanisms to quench the rise in electron pressure [16]. Alternatively, the simplest definition for RS could be that a shift of RH with excessive levels of reducing bio-equivalents. Our laboratory reported a breakthrough finding that RS (in the form of increased GSH and NADPH production) coupled with increased antioxidative pathway enzymes and decreased oxidative stress biomarkers could be genetically and causally linked to cardiac hypertrophy and mutant protein aggregation cardiomyopathy [18]. Subsequently, overabundance of reducing power in various experimental models and humans is increasingly linked with several complications, such as lipid membrane damages [19], triacyl glycerol deposition and mitochondrial stress [20], mitochondrial dysfunction and cytotoxicity [21], cardiac ischemic injury [22], risk of Alzheimer`s disease [23] and several others.
Reductive stress induces protein aggregation cardiomyopathy
It is well known that the equilibrium between molecular duos, such as the oxidation-reduction ratio of glutathione (GSH/GSSG), nicotinamide adenine dinucleotide phosphate (NADPH/NADP) or that of cysteine to its oxidized form cystine (disulfide), tend to regulate various signaling processes including a number of transcription factors responsible for controlling redox genes and thereby maintaining intracellular homeostasis [1, 24-26]. However, reductant/antioxidative stress remains to be an under-represented phenomenon in the fields of biochemistry and medicine since the cause (for RS) and consequences (of RS) are not familiar. In the recent past, we have found a transcriptional link that likely provokes or contributes to RS in vivo and in vitro [18, 27]. In a mouse model of human heart disease named mutant protein aggregation cardiomyopathy (MPAC), we demonstrated that increased activity of glutathione-6-phosphate dehydrogenase (G6PD) enhanced the levels of NADPH, a major cofactor/substrate for multiple enzymes such as glutathione reductase (GSR), NADPH oxidases and nitric oxide synthase (NOS), and GSH, a ubiquitously present intracellular redox controller. In addition, the exemplified production of two most abundant antioxidants is linked with activation of antioxidative pathway enzymes such as G6PD, GSR, GPx and catalase further bolstering the reductive environment. This oxido-redox shift towards “reductive side” facilitated a diminution of oxidative stress modifications of lipids and proteins and all of which was strictly correlated to protein aggregation related pathophysiology and heart failure [18]. Notably, this clue of toxic gain-of-function mutation causing excessive reducing equivalents and pathological remodelling of heart may also reconcile conflicting data and unanticipated outcomes from clinical trials assessing antioxidant therapies in patients with different cardiovascular diseases including heart failure. While some of the beneficial effects of drugs antagonizing the beta-adrenergic and the angiotensin II systems have been recognized for their ability to partially quench ROS [2, 14], the direct suppression of ROS-generating enzymes (i.e. superoxide dismutase, xanthine oxidase etc.) has produced rather debilitating consequences [28]. This raises an important question as to whether dysregulation of redox homeostasis towards any extreme (either reductive or oxidative) condition in a living cell is a biochemical hallmark of protein misfolding and/or aggregation diseases. Secondly, are there more intermediates and/or pathways involved in dis-equilibrating the redox balance towards “reductive side” other than the mutant protein reported in our study? Thus we strongly believe that the “reductionist” approaches are expected to deliver a more comprehensive understanding to counter the maladaptive protein folding mechanisms prevailing in human diseases including heart failure, neurodegeneration etc., and thus pressing the need for additional studies along these lines.
It is important to note that a sole increase in GSH cannot be strictly referred to as “Reductive Stress”. In our model pertaining to reductive stress, GSH measurement was used as one of the indices. It was combined with the measurement of other duo NADPH/NADP; battery of antioxidant enzymes and oxidative stress markers [18]. In this context, latest advancements in the chemistry and biology of per- and polysulfies are worth noting. Recent evidences indicate that hydrogen sulfide (H2S), a gasotransmitter physiologically promotes the formation of persulfide groups and S-sulfhydrates the proteins (ie. converting cysteine -SH groups to -SSH). Large number of proteins are shown to be sulfhydrated both basally as well as in the inducible state in diverse cell types triggering perturbations in biological functions [29-32]. A precise role for polysulfides in critical cellular processes and signal transduction has been documented viz. (i) GAPDH and actin sulfhydration in liver impairing GAPDH activity and cytoskeletal rearrangements respectively [30] (ii) activating transient receptor potential ankyrin 1 (TRPA1) in brain [31] and activation of TRPA1 promotes cell cycle progression and differentiation of cardiac fibroblasts augmenting cardiovascular stress [33] (iii) inhibiting protein tyrosine phosphatase 1B (PTP1B) thus promoting protein kinase-like endoplasmic reticulum (ER) kinase (PERK) activity and controlling translation as a ER stress response [29] (iv) sulfhydrating the p65 subunit of NF-κB at cysteine-38 and augmenting anti-apopotosis gene transcription in macrophage cultures and liver tissue [32]. Very recently, it has been demonstrated that endogenous generation of reactive persulfides and polysulfides could scavenge hydrogen peroxide effectively than GSH thus endows antioxidant and cytoprotective functions [34, 35]. Thus any one and/or combination of these polysulfide based mechanisms could also trigger the collapse of cascade of homeostatic signaling events leading to RS. Future studies warrant the inclusion of per- and polysulfides based indices in conjunction with the existing gold standards to approach and explain the concept of redox based disturbance, including “reductive stress”.
Bio-signature for RS
In a subsequent study, we attempted to build a bio-signature of RS based on abnormally and differentially expressed genes globally, by using one of the most effective approaches viz. genomic mining [27]. Primarily, we identified that genes and/or pathways related to de novo synthesis and recycling of GSH and NADPH were profoundly altered at the onset and during the progression of hR120GCryAB cardiomyopathy, which was consistent with our early findings [27]. In addition, we found several genes involved in protein synthesis and mitochondrial electron transport chain pathways were overexpressed and downregulated respectively, at both early and late compensatory stages [27]. The same study also identified dysregulation of genes involved in targeting cytosolic protein aggregates for degradation when there is a redox equilibrium tilt towards RS. This is worth noting, since alterations in components associated with folding/aggregation network could overburden the machinery that governs quality control pathways conferring either novel or secondary toxic functions that dictate disease severity. Notably, the endoplasmic reticulum (ER), a key cellular organelle which promotes folding and maturation of proteins operates uniquely under oxidizing condition compared with the cytosol [36, 37]. In otherwords, a pro-oxidative setting is a prerequisite for an efficient ER function and any disturbance (for example: high reductants) would presumably impair the folding and subsequent removal, resulting in extended accrual of unassembled proteins with marred protein quality and function. This study provided a snapshot of perturbations at the global genome level and a robust platform for the understanding of RS in a mutant protein cardiomyopathy model with respect to aggregation in vivo. Nevertheless, the study is still in its early days and many fundamental questions need to be addressed. For example, (a) what is the balance between protein folding and degradation and (b) what are all the cellular compartments that could be overburdened due to aberrant processing of folded proteins from ER, which is subsequently disposed by proteolytic events occurring in but not limited to cytosolic proteasomes, lysosomes, intramembrane system and endoplasmic reticulum (ER) in the context of RS [38-41].
Causal Mechanisms and Transcriptional Regulation of Reductive Stress
In the process of understanding how and why reductive stress occur and also important to gain insight into the long-term effect of RS, our subsequent research discovered that sustained activation of nuclear erythroid-2 like factor-2 (Nrf2), transcriptional regulator of antioxidant genes significantly contributes to RS in the TG mouse heart expressing human mutant CryAB that is associated with protein aggregation cardiomyopathy (MPAC) [42]. The findings of this study are summarized in Figure 2. A majority of the transcription factors are redox-sensitive and their dysregulation affects the induction of key targets that might contribute to the pathogenic changes in the intracellular redox milieu. Of interest, Nrf2, a basic leucine zipper protein, is a redox-sensitive master transcriptional regulator of several antioxidant genes [43, 44]. Under basal physiological setting, interaction with Keap1 promotes rapid ubiquitination of Nrf2 and its subsequent proteasome degradation [45, 46]. In response to oxidative/electrophilic stress, Nrf2 dissociates from Keap1 and translocate to the nucleus and induce transcription of the antioxidant response element (ARE)-containing genes. It has been implicated that Nrf2 protects vital organs from various OS related diseases such as acute respiratory distress syndrome, pulmonary fibrosis, hyperoxic injury, hepatocellular necrosis, hepatotoxicity, cancer, and neuronal dysfunction using in vitro and in vivo Nrf2 knockout models [47-51]. Thus far, Keap1-Nrf2 interaction is considered to be the primary regulatory point for the overall response to redox stress, which is also supported by a recent study in cardiovascular system [52, 53]. Independent in vitro studies from our laboratory points out that genetic knock-down of Keap1, a repressor of Nrf2 function, up-regulates the transcription of most of the antioxidant genes and thereby constantly upholds the intracellular glutathione (GSH) levels (>2.0 fold), which is likely to cause ‘reductive state’ in an acute setting or ‘reductive stress’ in a chronic condition in the cardiomyocytes (unpublished data). Thus we believe the cardiac pathology/heart failure phenotype is preceded by relentless activation of Nrf2 that is developed in two stages, initially in response to ROS generation (~3 months), and later, as a consequence of Keap1 dysfunction (6 months). This raises an important point as to how a process that commenced as a reparative signaling response for mutant protein stress, turned out to be over-compensatory and “toxic RS” jeopardizing protein turnover of Nrf2. Hypothetically, oligomerization of the mutant protein and formation of mysterious aggregates could trigger ROS facilitating the dissociation of the Keap1-Nrf2 complex. On the other hand, interactions between oligomers/aggregates and Keap1 may play a key role in stabilization/activation of Nrf2-antioxidant signaling leading to RS in the hR120GCryAB-TG mouse heart [42].
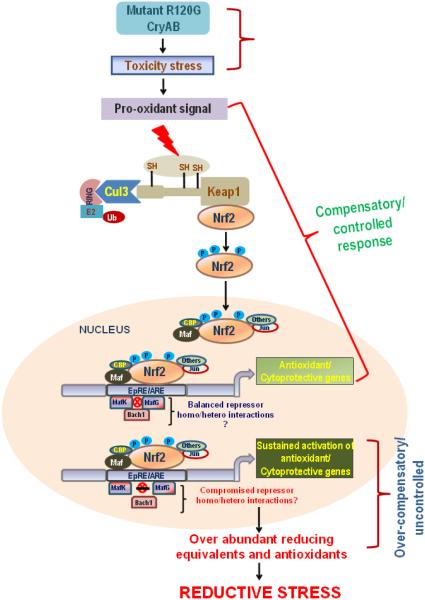
Figure 2. Transcriptional regulation of Reductive Stress.
Cells in general, respond to oxidative stress by activating Nrf2, a master transcription factor that controls battery of antioxidant genes. Mutant R120G-CryAB protein mice displayed uncontrolled hyper-activation of Nrf2, with an over-amplification of Nrf2-dependent antioxidant genes. Though induction of Nrf2 has been shown to be promising in many acute models, the sustained and chronic amplification of Nrf2 pathway, however was not beneficial but for resulting in damaging hypertrophy. This forms the unique aspect of our model explaining that a protracted Nrf2 activation could over-compensate for the original stress due to lack of CryAB protein resulting in the observed “reductive stress”.
We also propose other mechanisms for the sustained Nrf2 activation observed in hR120G cryAB mutant model viz. (i) autoregulation of Nrf2 wherein Nrf2 can directly bind to its own promoter and keeps activating it in a positive feedback manner [54] (ii) a persistent activation of kinase signaling pathways such as protein kinase C (PKC) and PKR-like endoplasmic reticulum kinase (PERK) that phosphorylates Nrf2 is also likely due to mutant cryAB stress, in turn resulting in disruption of Nrf2-Keap1 association [55, 56] (iii) perturbations in levels and/or function of Cullin 3 (Cul3), a scaffold protein that forms the E3 ligase complex with Ring Box1 (Rbx1)-dependent ubiquitin-protein ligase complex [57, 58] thus letting Nrf2 to evade proteasomal degradation and tilting the balance towards Nrf2 (iv) overproduction of p62 that can compete with and occupy Nrf2 binding site on Keap1 thus freeing and stabilizing Nrf2 [59] (v) a defective and/or compromised fine tuning mechanism such as microRNA (miR)-mediated gene silencing of Nrf2 as shown by Narasimhan et al., in non-cardiac cells [60, 61]. Currently studies are underway in our laboratory along these lines to investigate the reason as to why Nrf2 is activated persistently in hR120G cryAB reductive stress model.
Typically, downstream of Nrf2 activation, ARE dependent transcription is also determined by several factors. Particularly, the basic leucine zipper (bZIP) family of transcription factors viz. Jun, Fos, and Maf families, widely undergo dimerization (homo and hetero) to activate or repress gene transcription [62]. With Musculoaponeurotic fibrosarcoma oncogene homolog-K (MafK) lacking transactivation domain, it must interact with other Mafs, bZIP proteins such as Nrf2 and Bric-a-Bac, Tramtrack, Broad-complex (BTB) and cap‘n’collar homolog 1 (Bach1) to regulate transcription [63, 64]. Nrf2 being one such member of bZIP family also readily engage in dimerizing interactions [25, 65]. Typically, during stress Nrf2 is tethered and translocates to nucleus, heterodimerizes with small maf transcription factors such as MafK. Complexation of Nrf2 with MafK has been shown to activate target genes [46]. On the other hand, either homodimerization of small Mafs or its heterodimerization with Bach1 that contains a transcription repressing BTB/POZ domain has been shown to inhibit the transcription of target genes [63, 66, 67]. In addition, cross talk between Nrf2 and nuclear factor of kappa light polypeptide gene enhancer in B cells (NF-κB) under oxidative stress is documented with NF-κB p65 subunit promoting the recruitment and interaction of histone deacetylase (HDAC3) with either CREB binding protein (CBP) or MafK causing hypoacetylation and repression of Nrf2-ARE transactivation [68]. Thus, we believe a sustained Nrf2 activation that is followed by one or more of these events such as heterodimerization of Bach1-MafK or homodimerization of small Mafs or p65 dependent HDAC3 recruitment that act as repressors for Nrf2/ARE transcription could presumably be compromised in the nucleus of hR120GCryAB-TG cardiac tissue leading to persistent activation of Nrf2/antioxidant pathways [25, 65, 68] (Fig. 2). Notably, emerging science indicates that antioxidant supplementation has failed to establish a beneficial effect on cardiovascular disease morbidity and mortality [69-75]. Thus, research of this kind are unique in elucidating the conflicting dichotomy of too much “oxidant evil vs antioxidant good” theory further urging the need to employ appropriate model system and expand the understanding of Nrf2 signaling in heart tissue and cardiovascular pathogenesis.
Rescue of Redox Homeostasis Prevents Reductive Stress Induced Protein Aggregation Disease
A definitive role for Nrf2 in the RS associated hypertrophic process seen in MPAC mouse model was established recently [42, 76] and the significance of this finding is pictorially represented in Figure 3. Genetic manipulation of myocardial Nrf2 levels was achieved by crossing the hR120GCryAB-TG mice in the Nrf2 knockout background and obtained transgenics with reduced Nrf2 (Nrf2+/−) levels. The impact of diminution of Nrf2 on the time-dependent development of hypertrophy in the context of mutant-CryAB overexpression was scrutinized. Results indicated that Nrf2 deficiency normalized the excessive GSH levels observed in the TG mice in association with preserved cardiac function reflecting in retraction of premature death [76]. Moreover, the insufficiency of Nrf2 reduced the expression of genes involved in GSH metabolic and antioxidative enzymes, such as glutamate-cysteine ligase, catalytic subunit (GCLC), glutamate-cysteine ligase, modifier subunit (GCLM), NAD(P)H dehydrogenase, qunione 1 (NQO1), catalase, G6PD, and GSR, which in turn stifle the RS. These events were accompanied by reversal of 4-hydroxy-nonenol adducts to basal level, indicating the loss of redox regulation due to excessive reducing milieu. Subsequent to restrained RS, and in the setting of establishing redox equilibrium in the myocardium, the formation of mutant protein aggregation and over-ubiquitination of proteins was also diminished in all cardiac fractions of TG:Nrf2+/− mice. In addition, sequestration of Grp78, the ER-resident chaperone, compromised ER function and prompted ER stress in TG mice. Interestingly, this effect was partially rescued in TG:Nrf2+/− mice [76]. Thus the pathological phenotype observed in hR120GCryAB-TG versus the normal phenotype exhibited in hR120GCryAB-TG x Nrf2-KO crossed mice, indicates that this model serve as an excellent tool to study the relationship of cardiac hypertrophy and “Reductive stress”, a less-studied spectrum of ROS pathology. The key observations from the transgenic animal model were recapitulated in HL1 cardiomyocytes transfected with mutant-CryAB plasmids. Overall this study revealed the sinister consequences of overcompensation by Nrf2 in the context of cardiac remodeling and highlighted the “double-edged sword” attribute of Nrf2, i.e.- protective upon transient activation in response to stress, but injurious upon persistent activation. Although trapping of Keap1 by the aggregation of mutant protein (hR120GCryAB) appears to be crucial for activation of Nrf2 beyond biological levels, it is obvious that a definite degree of protein oxidation seems to be essential for key protein quality control mechanisms, echoing the eminence of redox equilibrium in cellular homeostasis. It is thus clear, that any effort at influencing the redox milieu through Nrf2 for therapeutic purposes will require careful optimization in analyzing dose dependent effects to prevent destruction. However, this will need to be preceded by detailed analyses of the regulatory mechanisms controlling its intracellular levels in cardiac and other systems, which are underway in our laboratory. Although our claim that Keap1/Nrf2/ARE pathway could play a vital role in RS is entirely based on partially understood mechanisms and some preliminary findings (unpublished from our laboratory), we are also certain that many pathways and intermediates could fuel RS in cardiac diseases.
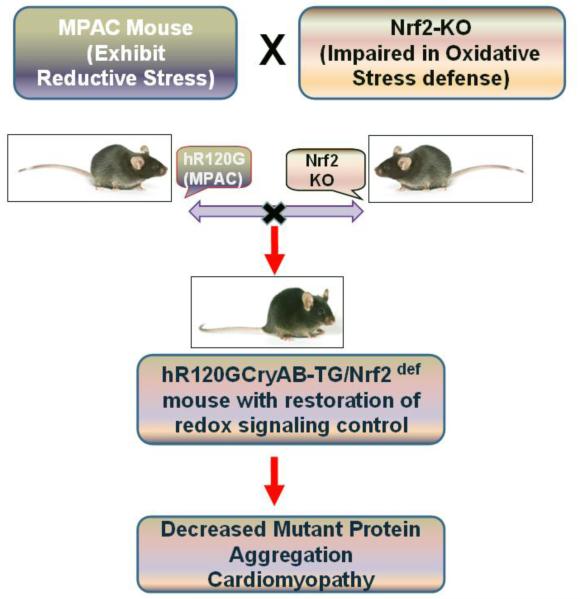
Fig 3. Limiting Reductive Stress protects myocardium.
Genetic disruption of Nrf2 and its diminution in RS hearts was achieved by intercrossing the hR120G-CryAB-TG (Reductive Stress) and Nrf2-Knockout (Nrf2-deficient). The hR120G-CryAB-TG:Nrf2def mice exhibited homeostatic redox state, decreased protein aggregation and delayed cardiac pathology. Survival of these mice was significantly improved with no mortality at the age of 75 weeks when compared to 90% of the RS mice exhibited premature sudden cardiac death at the age of 40 weeks. This signifies that sustained Nrf2 activation could hamper the natural cellular adaptation further pressing to revisit “oxidants evil/antioxidants angel” theory.
Other Studies Highlighting the Paradoxical Outcome of Super-boosting Reductive Power
In a classical study as early as 1993, it has been demonstrated that treatment of DTT, a strong reducing agent, led to accumulation of misfolded proteins in the ER resulting in abnormal regulation of Ire1 transmembrane kinase/nuclease ultimately leading to aberrant Unfolded Protein Response (UPR) in S. cerevisiae [77]. This suggests that induction of reductive state could affect the intracellular signal transduction pathway mediating homeostatic adaptation to stress related to ER which might have greater bearing in ER stress related-disorders such as cardiac diseases, diabetes, neurodegeneration etc. In another study, Schulz and colleagues reported that treatment of antioxidants such as N-acetylcysteine (NAC, a precursor of reduced GSH), abolished the 2-Deoxy-D-Glucose (2-DG)-induced caloric restriction associated life span extension in C. elegans [78]. Thus, this study lends further support to the idea that boosting of reducing power could forestall a beneficial outcome, in this case, life span extension. Contrasting to the widely accepted concept of Hsp induction serving as a protective mechanism against oxidative stress, Zhang et al [79] demonstrated that cardiomyocyte-specific overexpression of Hsp27 induces reductive stress in the heart that is reflected by an increase in the levels of GSH and GSH-peroxidase along with decreased ROS resulting in cardiac hypertrophy and reduced lifespan. In addition, they demonstrated that Hsp-induced cardiomyopathy could be prevented by partial inhibition of GSH-peroxidase.
Very recently, another study cautioned against blunting NADPH oxidase (Nox), an important enzyme involved in cellular ROS generation [22]. In this study, they demonstrated that loss-of-function for Nox4 in a mouse model displayed altered redox homeostasis with increased GSH, NADPH and decreased ROS which was associated with unexpected and poor recovery response to ischemia/reperfusion injury than the gain-of-function Nox4 mouse. Thus, this study clearly demonstrated that inducing cellular reductive power (by loss-of-function Nox4 mouse) could greatly compromise the myocardial energetics and aggravate the cardiac dysfunction during the essential myocardial-reperfusion management strategies. Studies focusing on RS in human subjects are emerging. In a recent study, Margaritelis et al., [80] reported the existence of a wide heterogeneity in exercise-induced redox response with increased reductive stress instead of the expected oxidative stress in human subjects. This study suggests that the individuals who responded with reductive stress after exercise may not be benefited from exercise to the same extent as that of the subjects who responded with oxidative stress. In another research trial with human subjects it has been demonstrated that young and healthy carriers of Apo ε4 allele suffer from reductive stress. Typically, carrying an Apo ε4 allele is considered as the major genetic risk factor for Alzhemier Disease (AD) and in this context, this new study provide a key clue that RS could be involved in the progression of AD [23]. These are some of the classical and relevant studies that highlight the paradoxical outcome of super-boosting reductants impairing the signaling mechanisms that depend on physiological level of ROS and redox-sensitive transcription factors and emphasizing the complexity of developing therapies that affect the intricately connected redox states.
Conclusions
As a firm believer of the fact that “Too much of anything is not good”, boosting the biological system with too many reductants may not be a healthy fix. This is clearly evident from the growing body of scientific discoveries that demonstrates the dark side of antioxidant supplementations [69, 70, 73-75]. Thus, as scientific researchers we are convinced that this would be the right time to revisit the theory of “redox mechanisms” and to investigate if abrogating OS beyond a certain level for an extended time (chronic) would induce pathological mechanisms, in a given cell or an organ system, that are uncontrollable. In short, we need to know “How much is too much” on either side of the spectrum i.e. how much quenching oxidants is beneficial and how much boosting reductants is good? A concise schema of the importance of redox switch in cardiac pathogenesis is illustrated in Figure 4. With research into RS still in its infancy, we emphasize that our RS concepts bear potential for broad reach within the context of heart, as cardiomyocytes have unique structure and function that can undergo pathological remodeling in response to multiple kinds of stress. Further, arming ourselves with deepened understanding of the role of RS in pathophysiology of major cardiac diseases will certainly position us to optimize the response and effectively manage the myocardial related stress and its impact on overall cardiac health. From the advancements we have made thus far in the field of RS, we believe that "as much as it is necessary to study the oxidative capacity of the cell, concept of reductive power in the context of a pathology should also be viewed as a "bombardier beetle" that when disturbed can spray noxious reactive chemicals". Importantly, these scientific ideas may be consistent in different organ systems in a given context.
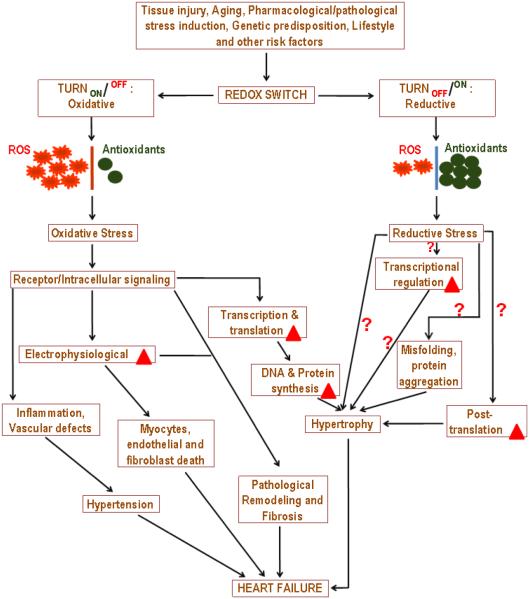
Fig 4. Redox Switch – Cause and Consequences.
Though the concept of redox based events in the biological system is long known, the understanding as to how “redox switch” precisely operates and regulated still remains a work-in-progress. Any type of cell/tissue injury (wounding), pharmacological agents, chronic diseases, genetic predisposition, nutrition, life style, aging and other unknown risk factors could “switch” on redox signals either temporarily or permanently. In the context of cardiovascular disease, turning ‘ON’ the oxidative switch continuously is expected to elicit oxidative stress causing vascular inflammation, endothelial dysfunction, impaired nitric oxide (NO) signaling and vascular tone, cardiomyocytes apoptosis, hyper-contractility, impaired electrophysiology and remodeling of cardiomyocytes, atrial fibrillation, myocardial infarction, cardiac hypertrophy and heart failure. Contrary to the expectation, an uninterrupted supply of “reductive power” is also proven to be deleterious causing impairment in transcriptional regulation, folding/unfolding of proteins, ER stress response, autophagic response/protein quality control and pathological cardiac remodeling. As illustrated in the schematic cartoon, the information known for RS is relatively very minimal in relation to OS. Bold red triangle indicates changes/alterations.
Highlights.
A role for “reductive stress” in cardiac pathophysiology is explained.
Mutant protein expression induces “reductive stress”.
Sustained activation of Nrf2 signaling might be a causal mechanism for “reductive stress”.
Reductive stress is under-represented phenomena which should be explored in detail.
Acknowledgements
The studies discussed in the review supported by multiple awards/research funds from the NHLBI (R01HL118067), NIA (1R03AG042860-01), the AHA (BGIA-0865015F), University of Utah center for Aging (Pilot grant-2009), the Division of Cardiovascular Medicine/Department of Medicine, University of Utah and the start-up funds (for NSR) by Department of Pathology, the University of Alabama at Birmingham, AL. We thank Dr. Victor M. Darley-Usmar, Dr. Jianhua Zhang and Dr. Christopher Davidson for critically reading the review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Wang K, Zhang T, Dong Q, Nice EC, Huang C, Wei Y. Redox homeostasis: the linchpin in stem cell self-renewal and differentiation. Cell death & disease. 2013;4:e537. doi: 10.1038/cddis.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sunggip C, Kitajima N, Nishida M. Redox control of cardiovascular homeostasis by angiotensin II. Current pharmaceutical design. 2013;19:3022–3032. doi: 10.2174/1381612811319170008. [DOI] [PubMed] [Google Scholar]
- [3].Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cellular signalling. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Haddad JJ. Oxygen homeostasis, thiol equilibrium and redox regulation of signalling transcription factors in the alveolar epithelium. Cellular signalling. 2002;14:799–810. doi: 10.1016/s0898-6568(02)00022-0. [DOI] [PubMed] [Google Scholar]
- [5].Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury: Part I: basic mechanisms and in vivo monitoring of ROS. Circulation. 2003;108:1912–1916. doi: 10.1161/01.CIR.0000093660.86242.BB. [DOI] [PubMed] [Google Scholar]
- [6].Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury: Part II: animal and human studies. Circulation. 2003;108:2034–2040. doi: 10.1161/01.CIR.0000093661.90582.c4. [DOI] [PubMed] [Google Scholar]
- [7].Jay D, Hitomi H, Griendling KK. Oxidative stress and diabetic cardiovascular complications. Free radical biology & medicine. 2006;40:183–192. doi: 10.1016/j.freeradbiomed.2005.06.018. [DOI] [PubMed] [Google Scholar]
- [8].Schnabel R, Blankenberg S. Oxidative stress in cardiovascular disease: successful translation from bench to bedside? Circulation. 2007;116:1338–1340. doi: 10.1161/CIRCULATIONAHA.107.728394. [DOI] [PubMed] [Google Scholar]
- [9].Takano H, Zou Y, Hasegawa H, Akazawa H, Nagai T, Komuro I. Oxidative stress-induced signal transduction pathways in cardiac myocytes: involvement of ROS in heart diseases. Antioxidants & redox signaling. 2003;5:789–794. doi: 10.1089/152308603770380098. [DOI] [PubMed] [Google Scholar]
- [10].Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free radical biology & medicine. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Popolo A, Autore G, Pinto A, Marzocco S. Oxidative stress in patients with cardiovascular disease and chronic renal failure. Free radical research. 2013;47:346–356. doi: 10.3109/10715762.2013.779373. [DOI] [PubMed] [Google Scholar]
- [12].Griendling KK, Alexander RW. Oxidative stress and cardiovascular disease. Circulation. 1997;96:3264–3265. [PubMed] [Google Scholar]
- [13].Halliwell B. Oxidative stress and cancer: have we moved forward? The Biochemical journal. 2007;401:1–11. doi: 10.1042/BJ20061131. [DOI] [PubMed] [Google Scholar]
- [14].Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. The Journal of clinical investigation. 2005;115:500–508. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wendel A. Measurement of in vivo lipid peroxidation and toxicological significance. Free radical biology & medicine. 1987;3:355–358. doi: 10.1016/s0891-5849(87)80047-3. [DOI] [PubMed] [Google Scholar]
- [16].Ghyczy M, Boros M. Electrophilic methyl groups present in the diet ameliorate pathological states induced by reductive and oxidative stress: a hypothesis. The British journal of nutrition. 2001;85:409–414. doi: 10.1079/bjn2000274. [DOI] [PubMed] [Google Scholar]
- [17].Jaeschke H, Kleinwaechter C, Wendel A. NADH-dependent reductive stress and ferritin-bound iron in allyl alcohol-induced lipid peroxidation in vivo: the protective effect of vitamin E. Chemico-biological interactions. 1992;81:57–68. doi: 10.1016/0009-2797(92)90026-h. [DOI] [PubMed] [Google Scholar]
- [18].Rajasekaran NS, Connell P, Christians ES, Yan LJ, Taylor RP, Orosz A, Zhang XQ, Stevenson TJ, Peshock RM, Leopold JA, Barry WH, Loscalzo J, Odelberg SJ, Benjamin IJ. Human alpha B-crystallin mutation causes oxido-reductive stress and protein aggregation cardiomyopathy in mice. Cell. 2007;130:427–439. doi: 10.1016/j.cell.2007.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Torreggiani A, Chatgilialoglu C, Ferreri C, Melchiorre M, Atrian S, Capdevila M. Non-enzymatic modifications in metallothioneins connected to lipid membrane damages: structural and biomimetic studies under reductive radical stress. Journal of proteomics. 2013;92:204–215. doi: 10.1016/j.jprot.2013.02.005. [DOI] [PubMed] [Google Scholar]
- [20].He Q, Wang M, Petucci C, Gardell SJ, Han X. Rotenone induces reductive stress and triacylglycerol deposition in C2C12 cells. The international journal of biochemistry & cell biology. 2013;45:2749–2755. doi: 10.1016/j.biocel.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhang H, Rajasekaran NS, Orosz A, Xiao X, Rechsteiner M, Benjamin IJ. Selective degradation of aggregate-prone CryAB mutants by HSPB1 is mediated by ubiquitin-proteasome pathways. Journal of molecular and cellular cardiology. 2012;49:918–930. doi: 10.1016/j.yjmcc.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yu Q, Lee CF, Wang W, Karamanlidis G, Kuroda J, Matsushima S, Sadoshima J, Tian R. Elimination of NADPH oxidase activity promotes reductive stress and sensitizes the heart to ischemic injury. Journal of the American Heart Association. 2014;3:e000555. doi: 10.1161/JAHA.113.000555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Badia MC, Giraldo E, Dasi F, Alonso D, Lainez JM, Lloret A, Vina J. Reductive stress in young healthy individuals at risk of Alzheimer disease. Free radical biology & medicine. 2013;63:274–279. doi: 10.1016/j.freeradbiomed.2013.05.003. [DOI] [PubMed] [Google Scholar]
- [24].Liu H, Colavitti R, Rovira, Finkel T. Redox-dependent transcriptional regulation. Circulation research. 2005;97:967–974. doi: 10.1161/01.RES.0000188210.72062.10. [DOI] [PubMed] [Google Scholar]
- [25].Ishikawa M, Numazawa S, Yoshida T. Redox regulation of the transcriptional repressor Bach1. Free radical biology & medicine. 2005;38:1344–1352. doi: 10.1016/j.freeradbiomed.2005.01.021. [DOI] [PubMed] [Google Scholar]
- [26].Shaikhali J, Noren L, de Dios Barajas-Lopez J, Srivastava V, Konig J, Sauer UH, Wingsle G, Dietz KJ, Strand A. Redox-mediated mechanisms regulate DNA binding activity of the G-group of basic region leucine zipper (bZIP) transcription factors in Arabidopsis. The Journal of biological chemistry. 2012;287:27510–27525. doi: 10.1074/jbc.M112.361394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rajasekaran NS, Firpo MA, Milash BA, Weiss RB, Benjamin IJ. Global expression profiling identifies a novel biosignature for protein aggregation R120GCryAB cardiomyopathy in mice. Physiological genomics. 2008;35:165–172. doi: 10.1152/physiolgenomics.00297.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dimmeler S, Zeiher AM. A "reductionist" view of cardiomyopathy. Cell. 2007;130:401–402. doi: 10.1016/j.cell.2007.07.028. [DOI] [PubMed] [Google Scholar]
- [29].Krishnan N, Fu C, Pappin DJ, Tonks NK. H2S-Induced sulfhydration of the phosphatase PTP1B and its role in the endoplasmic reticulum stress response. Science signaling. 2011;4:ra86. doi: 10.1126/scisignal.2002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mustafa AK, Gadalla MM, Sen N, Kim S, Mu W, Gazi SK, Barrow RK, Yang G, Wang R, Snyder SH. H2S signals through protein S-sulfhydration. Science signaling. 2009;2:ra72. doi: 10.1126/scisignal.2000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ogawa H, Takahashi K, Miura S, Imagawa T, Saito S, Tominaga M, Ohta T. H(2)S functions as a nociceptive messenger through transient receptor potential ankyrin 1 (TRPA1) activation. Neuroscience. 2012;218:335–343. doi: 10.1016/j.neuroscience.2012.05.044. [DOI] [PubMed] [Google Scholar]
- [32].Sen N, Paul BD, Gadalla MM, Mustafa AK, Sen T, Xu R, Kim S, Snyder SH. Hydrogen sulfide-linked sulfhydration of NF-kappaB mediates its antiapoptotic actions. Molecular cell. 2012;45:13–24. doi: 10.1016/j.molcel.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Oguri G, Nakajima T, Yamamoto Y, Takano N, Tanaka T, Kikuchi H, Morita T, Nakamura F, Yamasoba T, Komuro I. Effects of methylglyoxal on human cardiac fibroblast: Roles of transient receptor potential ankyrin 1 (TRPA1)channels. American journal of physiology. Heart and circulatory physiology. 2014 doi: 10.1152/ajpheart.01021.2013. ajpheart 01021 02013. [DOI] [PubMed] [Google Scholar]
- [34].Ida T, Sawa T, Ihara H, Tsuchiya Y, Watanabe Y, Kumagai Y, Suematsu M, Motohashi H, Fujii S, Matsunaga T, Yamamoto M, Ono K, Devarie-Baez NO, Xian M, Fukuto JM, Akaike T. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:7606–7611. doi: 10.1073/pnas.1321232111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Nishida M, Sawa T, Kitajima N, Ono K, Inoue H, Ihara H, Motohashi H, Yamamoto M, Suematsu M, Kurose H, van der Vliet A, Freeman BA, Shibata T, Uchida K, Kumagai Y, Akaike T. Hydrogen sulfide anion regulates redox signaling via electrophile sulfhydration. Nature chemical biology. 2012;8:714–724. doi: 10.1038/nchembio.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Enyedi B, Varnai P, Geiszt M. Redox state of the endoplasmic reticulum is controlled by Ero1L-alpha and intraluminal calcium. Antioxidants & redox signaling. 2010;13:721–729. doi: 10.1089/ars.2009.2880. [DOI] [PubMed] [Google Scholar]
- [37].Hwang C, Sinskey AJ, Lodish HF. Oxidized redox state of glutathione in the endoplasmic reticulum. Science. 1992;257:1496–1502. doi: 10.1126/science.1523409. [DOI] [PubMed] [Google Scholar]
- [38].Laurindo FR, Pescatore LA, Fernandes Dde C. Protein disulfide isomerase in redox cell signaling and homeostasis. Free radical biology & medicine. 2012;52:1954–1969. doi: 10.1016/j.freeradbiomed.2012.02.037. [DOI] [PubMed] [Google Scholar]
- [39].Wassler M, Fries E. Proteolytic cleavage of haptoglobin occurs in a subcompartment of the endoplasmic reticulum: evidence from membrane fusion in vitro. The Journal of cell biology. 1993;123:285–291. doi: 10.1083/jcb.123.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lemberg MK, Martoglio B. Requirements for signal peptide peptidase-catalyzed intramembrane proteolysis. Molecular cell. 2002;10:735–744. doi: 10.1016/s1097-2765(02)00655-x. [DOI] [PubMed] [Google Scholar]
- [41].Kowarik M, Kung S, Martoglio B, Helenius A. Protein folding during cotranslational translocation in the endoplasmic reticulum. Molecular cell. 2002;10:769–778. doi: 10.1016/s1097-2765(02)00685-8. [DOI] [PubMed] [Google Scholar]
- [42].Rajasekaran NS, Varadharaj S, Khanderao GD, Davidson CJ, Kannan S, Firpo MA, Zweier JL, Benjamin IJ. Sustained activation of nuclear erythroid 2-related factor 2/antioxidant response element signaling promotes reductive stress in the human mutant protein aggregation cardiomyopathy in mice. Antioxidants & redox signaling. 2011;14:957–971. doi: 10.1089/ars.2010.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kobayashi M, Li L, Iwamoto N, Nakajima-Takagi Y, Kaneko H, Nakayama Y, Eguchi M, Wada Y, Kumagai Y, Yamamoto M. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Molecular and cellular biology. 2009;29:493–502. doi: 10.1128/MCB.01080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kwak MK, Wakabayashi N, Greenlaw JL, Yamamoto M, Kensler TW. Antioxidants enhance mammalian proteasome expression through the Keap1-Nrf2 signaling pathway. Molecular and cellular biology. 2003;23:8786–8794. doi: 10.1128/MCB.23.23.8786-8794.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].McMahon M, Itoh K, Yamamoto M, Hayes JD. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. The Journal of biological chemistry. 2003;278:21592–21600. doi: 10.1074/jbc.M300931200. [DOI] [PubMed] [Google Scholar]
- [46].Kobayashi M, Yamamoto M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Advances in enzyme regulation. 2006;46:113–140. doi: 10.1016/j.advenzreg.2006.01.007. [DOI] [PubMed] [Google Scholar]
- [47].Shibata T, Ohta T, Tong KI, Kokubu A, Odogawa R, Tsuta K, Asamura H, Yamamoto M, Hirohashi S. Cancer related mutations in NRF2 impair its recognition by Keap1-Cul3 E3 ligase and promote malignancy. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:13568–13573. doi: 10.1073/pnas.0806268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hosoya T, Maruyama A, Kang MI, Kawatani Y, Shibata T, Uchida K, Warabi E, Noguchi N, Itoh K, Yamamoto M. Differential responses of the Nrf2-Keap1 system to laminar and oscillatory shear stresses in endothelial cells. The Journal of biological chemistry. 2005;280:27244–27250. doi: 10.1074/jbc.M502551200. [DOI] [PubMed] [Google Scholar]
- [49].Taguchi K, Fujikawa N, Komatsu M, Ishii T, Unno M, Akaike T, Motohashi H, Yamamoto M. Keap1 degradation by autophagy for the maintenance of redox homeostasis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:13561–13566. doi: 10.1073/pnas.1121572109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ke B, Shen XD, Zhang Y, Ji H, Gao F, Yue S, Kamo N, Zhai Y, Yamamoto M, Busuttil RW, Kupiec-Weglinski JW. KEAP1-NRF2 complex in ischemia-induced hepatocellular damage of mouse liver transplants. Journal of hepatology. 2013;59:1200–1207. doi: 10.1016/j.jhep.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Taguchi K, Motohashi H, Yamamoto M. Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution. Genes to cells : devoted to molecular & cellular mechanisms. 2011;16:123–140. doi: 10.1111/j.1365-2443.2010.01473.x. [DOI] [PubMed] [Google Scholar]
- [52].Li J, Ichikawa T, Villacorta L, Janicki JS, Brower GL, Yamamoto M, Cui T. Nrf2 protects against maladaptive cardiac responses to hemodynamic stress, Arteriosclerosis, thrombosis. and vascular biology. 2009;29:1843–1850. doi: 10.1161/ATVBAHA.109.189480. [DOI] [PubMed] [Google Scholar]
- [53].Brewer AC, Mustafi SB, Murray TV, Rajasekaran NS, Benjamin IJ. Reductive stress linked to small HSPs, G6PD, and Nrf2 pathways in heart disease. Antioxidants & redox signaling. 2013;18:1114–1127. doi: 10.1089/ars.2012.4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kwak MK, Itoh K, Yamamoto M, Kensler TW. Enhanced expression of the transcription factor Nrf2 by cancer chemopreventive agents: role of antioxidant response element-like sequences in the nrf2 promoter. Molecular and cellular biology. 2002;22:2883–2892. doi: 10.1128/MCB.22.9.2883-2892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Molecular and cellular biology. 2003;23:7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Huang HC, Nguyen T, Pickett CB. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. The Journal of biological chemistry. 2002;277:42769–42774. doi: 10.1074/jbc.M206911200. [DOI] [PubMed] [Google Scholar]
- [57].Nguyen T, Sherratt PJ, Huang HC, Yang CS, Pickett CB. Increased protein stability as a mechanism that enhances Nrf2-mediated transcriptional activation of the antioxidant response element. Degradation of Nrf2 by the 26 S proteasome. The Journal of biological chemistry. 2003;278:4536–4541. doi: 10.1074/jbc.M207293200. [DOI] [PubMed] [Google Scholar]
- [58].Stewart D, Killeen E, Naquin R, Alam S, Alam J. Degradation of transcription factor Nrf2 via the ubiquitin-proteasome pathway and stabilization by cadmium. The Journal of biological chemistry. 2003;278:2396–2402. doi: 10.1074/jbc.M209195200. [DOI] [PubMed] [Google Scholar]
- [59].Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, Sou YS, Ueno I, Sakamoto A, Tong KI, Kim M, Nishito Y, Iemura S, Natsume T, Ueno T, Kominami E, Motohashi H, Tanaka K, Yamamoto M. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nature cell biology. 2010;12:213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- [60].Narasimhan M, Patel D, Vedpathak D, Rathinam M, Henderson G, Mahimainathan L. Identification of novel microRNAs in post-transcriptional control of Nrf2 expression and redox homeostasis in neuronal, SH-SY5Y cells. PloS one. 2012;7:e51111. doi: 10.1371/journal.pone.0051111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Narasimhan M, Riar AK, Rathinam ML, Vedpathak D, Henderson G, Mahimainathan L. Hydrogen peroxide responsive miR153 targets Nrf2/ARE cytoprotection in paraquat induced dopaminergic neurotoxicity. Toxicology letters. 2014;228:179–191. doi: 10.1016/j.toxlet.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Newman JR, Keating AE. Comprehensive identification of human bZIP interactions with coiled-coil arrays. Science. 2003;300:2097–2101. doi: 10.1126/science.1084648. [DOI] [PubMed] [Google Scholar]
- [63].Blank V. Small Maf proteins in mammalian gene control: mere dimerization partners or dynamic transcriptional regulators? Journal of molecular biology. 2008;376:913–925. doi: 10.1016/j.jmb.2007.11.074. [DOI] [PubMed] [Google Scholar]
- [64].Eggler AL, Liu G, Pezzuto JM, van Breemen RB, Mesecar AD. Modifying specific cysteines of the electrophile-sensing human Keap1 protein is insufficient to disrupt binding to the Nrf2 domain Neh2. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:10070–10075. doi: 10.1073/pnas.0502402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Oyake T, Itoh K, Motohashi H, Hayashi N, Hoshino H, Nishizawa M, Yamamoto M, Igarashi K. Bach proteins belong to a novel family of BTB-basic leucine zipper transcription factors that interact with MafK and regulate transcription through the NF-E2 site. Molecular and cellular biology. 1996;16:6083–6095. doi: 10.1128/mcb.16.11.6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Blank V, Andrews NC. The Maf transcription factors: regulators of differentiation. Trends in biochemical sciences. 1997;22:437–441. doi: 10.1016/s0968-0004(97)01105-5. [DOI] [PubMed] [Google Scholar]
- [67].Igarashi K, Sun J. The heme-Bach1 pathway in the regulation of oxidative stress response and erythroid differentiation. Antioxidants & redox signaling. 2006;8:107–118. doi: 10.1089/ars.2006.8.107. [DOI] [PubMed] [Google Scholar]
- [68].Liu GH, Qu J, Shen X. NF-kappaB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochimica et biophysica acta. 2008;1783:713–727. doi: 10.1016/j.bbamcr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- [69].Aro A. Antioxidant supplementation and risk of chronic disease. Forum of nutrition. 2003;56:361–363. [PubMed] [Google Scholar]
- [70].Green AC, Hughes MC, Ibiebele TI, Williams GM, van der Pols JC, Ortonne JP. Antioxidant supplementation and risk of skin cancers. The Journal of nutrition. 2008;138:978. doi: 10.1093/jn/138.5.978. author reply 979. [DOI] [PubMed] [Google Scholar]
- [71].Plummer M, Vivas J, Lopez G, Bravo JC, Peraza S, Carillo E, Cano E, Castro D, Andrade O, Sanchez V, Garcia R, Buiatti E, Aebischer C, Franceschi S, Oliver W, Munoz N. Chemoprevention of precancerous gastric lesions with antioxidant vitamin supplementation: a randomized trial in a high-risk population. Journal of the National Cancer Institute. 2007;99:137–146. doi: 10.1093/jnci/djk017. [DOI] [PubMed] [Google Scholar]
- [72].Miller ER, 3rd, Appel LJ, Levander OA, Levine DM. The effect of antioxidant vitamin supplementation on traditional cardiovascular risk factors. Journal of cardiovascular risk. 1997;4:19–24. doi: 10.1177/174182679700400104. [DOI] [PubMed] [Google Scholar]
- [73].Czernichow S, Vergnaud AC, Galan P, Arnaud J, Favier A, Faure H, Huxley R, Hercberg S, Ahluwalia N. Effects of long-term antioxidant supplementation and association of serum antioxidant concentrations with risk of metabolic syndrome in adults. The American journal of clinical nutrition. 2009;90:329–335. doi: 10.3945/ajcn.2009.27635. [DOI] [PubMed] [Google Scholar]
- [74].Mennen LI, de Courcy GP, Guilland JC, Ducros V, Bertrais S, Nicolas JP, Maurel M, Zarebska M, Favier A, Franchisseur C, Hercberg S, Galan P. Homocysteine, cardiovascular disease risk factors, and habitual diet in the French Supplementation with Antioxidant Vitamins and Minerals Study. The American journal of clinical nutrition. 2002;76:1279–1289. doi: 10.1093/ajcn/76.6.1279. [DOI] [PubMed] [Google Scholar]
- [75].Goudev A, Kyurkchiev S, Gergova V, Karshelova E, Georgiev D, Atar D, Kehayov I, Nachev C. Reduced concentrations of soluble adhesion molecules after antioxidant supplementation in postmenopausal women with high cardiovascular risk profiles--a randomized double-blind study. Cardiology. 2000;94:227–232. doi: 10.1159/000047322. [DOI] [PubMed] [Google Scholar]
- [76].Kannan S, Muthusamy VR, Whitehead KJ, Wang L, Gomes AV, Litwin SE, Kensler TW, Abel ED, Hoidal JR, Rajasekaran NS. Nrf2 deficiency prevents reductive stress-induced hypertrophic cardiomyopathy. Cardiovascular research. 2013;100:63–73. doi: 10.1093/cvr/cvt150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Cox JS, Shamu CE, Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell. 1993;73:1197–1206. doi: 10.1016/0092-8674(93)90648-a. [DOI] [PubMed] [Google Scholar]
- [78].Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell metabolism. 2007;6:280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- [79].Zhang X, Min X, Li C, Benjamin IJ, Qian B, Zhang X, Ding Z, Gao X, Yao Y, Ma Y, Cheng Y, Liu L. Involvement of reductive stress in the cardiomyopathy in transgenic mice with cardiac-specific overexpression of heat shock protein 27. Hypertension. 2010;55:1412–1417. doi: 10.1161/HYPERTENSIONAHA.109.147066. [DOI] [PubMed] [Google Scholar]
- [80].Margaritelis NV, Kyparos A, Paschalis V, Theodorou AA, Panayiotou G, Zafeiridis A, Dipla K, Nikolaidis MG, Vrabas IS. Reductive stress after exercise: The issue of redox individuality. Redox biology. 2014;2:520–528. doi: 10.1016/j.redox.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]