Abstract
Low nephron endowment secondary to intrauterine growth restriction (IUGR) results in compensatory hypertrophy of the remaining glomeruli, which in turn is associated with hypertension. However, gender differences exist in the response of the kidney to injury, and IUGR female offspring seems protected from an unfavorable outcome. We previously reported differences in gender-specific gene expression in the IUGR kidney as well as increased circulating corticosterone levels following uteroplacental insufficiency (UPI). Vascular endothelial growth factor (VEGF), which is critical for renal development, is an important candidate in the IUGR kidney since its expression can be regulated by sex-steroids and glucocorticoids. We hypothesize that IUGR leads to altered kidney VEGF expression in a gender-specific manner. Following uterine ligation in the pregnant rat, UPI decreases renal VEGF levels in male and female IUGR animals at birth and through postnatal day 21. However, by day 120 of life, IUGR females have increased kidney VEGF expression, not present in the IUGR males. In addition, IUGR males exhibit increased serum testosterone levels as well as proteinuria. These findings are intriguing in light of the difference in glomerular hypertrophy observed: IUGR males show increased glomerular area when compared to IUGR females. In this model characterized by decreased nephron number and adult onset hypertension, UPI decreases renal VEGF expression during nephrogenesis. Our most intriguing finding is the increased renal VEGF levels in adult IUGR females, associated with a more benign phenotype. We suggest that the mechanisms underlying renal disease in response to IUGR are most likely regulated in a gender specific manner.
Keywords: nephron number, glucocorticoids, hypertension, gender specific-effect, VEGFR-2
INTRODUCTION
Intrauterine growth restriction (IUGR) caused by uteroplacental insufficiency (UPI) is a morbidity associated with complications of pregnancy such as preeclampsia (1). A number of epidemiological studies show that IUGR neonates experience impaired renal function, with an increased risk of developing adult morbidities such as hypertension (2–4).
In both humans and several animal models, UPI results in decreased nephron number (5–8). Importantly, low nephron endowment can result in compensatory glomerular hypertrophy of the remaining glomeruli, which is in turn associated with an increased incidence of hypertension in humans (9–11). Similarly, nephron deficit in the rat following UPI also leads to impaired renal function as well as to compensatory hypertrophy (12, 13).
A large number of epidemiological and animal studies point to the fact that gender differences play a very important role in the development of cardiovascular and renal disease following UPI. In most reports, the female IUGR offspring appears to be protected from an unfavorable phenotypic outcome (such as severity of hypertension and renal injury) when compared to the IUGR male (14, 15). However, the molecular mechanisms mediating this particular phenotype are largely still unknown.
In recent years, our laboratory has used a well established animal model of IUGR -induced following bilateral uterine ligation in the pregnant rat dam- characterized by decreased nephron number and adult onset hypertension, in an attempt to unravel the molecular mechanisms involved in altered nephrogenesis and in the development of hypertension (8, 16, 17). Among the molecular mechanisms studied, we have observed that the IUGR kidney exhibits an abnormal glucocorticoid (GC) pathway, with increased circulating corticosterone levels at birth associated with down regulation of key synthetic enzymes expression, including 11 β-hydroxysteroid dehydrogenase type 2 and cyclooxygenase-2. Moreover, these genes exhibited differences in expression that were gender-specific in the IUGR kidney (8, 16).
In this study we further characterize the effect that UPI and increased GC may have upon molecular mechanisms underlying abnormal nephrogenesis. Increased GC can affect vascular endothelial growth factor (VEGF) expression, which constitutes an important candidate in the IUGR kidney (18). VEGF is involved not only in nephrogenesis, where it plays a key role by inducing endothelial cell differentiation, capillary formation and proliferation of tubular epithelia, but also in the development of compensatory glomerular hypertrophy following several disorders including diabetes and nephrectomy (19–22).
However, the gender-specific effects of UPI and elevated corticosterone levels upon kidney VEGF expression in the IUGR rat at different stages of development are unknown. We therefore hypothesize that IUGR and resultant GC overexposure of the offspring leads to altered kidney VEGF expression and that this effect is developmentally regulated and gender-specific.
To test this hypothesis, bilateral uterine artery ligation was performed on day 19 of gestation in Sprague-Dawley rats (term, 21.5 days). UPI in this animal model results in offspring with low birth weight and asymmetrical IUGR (23), with a 25% reduction in glomeruli number and adult onset hypertension (8, 16). In addition, we had previously reported increased serum corticosterone levels at birth in IUGR animals (24). We will now investigate if elevated serum corticosterone levels persist through day 21 of life, a time frame that includes the completion of nephrogenesis (postnatal day 8) through juvenile stages. VEGF is an endothelial cell-specific mitogen that exerts specific biological effects by binding to its respective tyrosine-kinase VEGF receptor 1 (VEGFR-1 or VEGFR-1) and VEGFR-2 (KDR/VEGFR-2), both of which are also expressed in endothelial cells (25). Therefore, levels of VEGF, VEGFR-1 and VEGFR-2 mRNA and protein were quantified from whole kidneys at birth (P0), at 21 days of life (juvenile rat) (P21), and at 120 days of life (adult rat) (P120). Glomerular area was determined in both control and IUGR kidneys at P21 and P120 to assess the presence of glomerular hypertrophy in this animal model. To investigate the presence of glomerular pathology, protein/creatinine ratio was measured in urine at P120. Since sex differences play an important role in adult renal pathophysiology, both testosterone and estradiol levels were also measured in the adult rats. Gender differences were examined in all experiments.
METHODS
Animals
All procedures were approved by the University of Utah Animal Care Committee and are in accordance with the American Physiological Society’s guiding principles (26). These surgical methods have been previously described (8, 23). In this well-established model of asymmetrical growth restriction, IUGR pups are 20–25% lighter than the control animals at birth (IUGR: 4.00 ±.25 vs. control: 5.25 ±.22, p<0.05), with a normal distribution of birth weights within and among litters, and no difference in litter size between control and IUGR groups (27, 28). In brief, on day 19 of gestation, the maternal rats (Sprague-Dawley) were anesthetized with intraperitoneal xylazine (8 mg/kg) and ketamine (40 mg/kg), and both inferior uterine arteries were ligated (IUGR) (n = 12 litters). Control animals received anesthesia (control) (n = 12 litters). Maternal rats recovered within a few hours and had ad lib access to food and water. At term (21.5 days gestation), day 0 (P0) pups were delivered by caesarian section, weighed and decapitated (n= 6 litters IUGR and control, respectively). To minimize litter to litter variation, one pup from each litter was used for all day 0 studies. The selection of the pups was done randomly, without knowledge of size or position in the uterine horn. To study 21-day-old (P21) and 120-day-old (P120) rats, the remaining maternal rats were allowed to deliver spontaneously at term (n= 6 litters IUGR and control, respectively), and litters were randomly culled to 6 pups.
At P21 and P120, 1 male and 1 female pup from each litter was randomly selected, anesthetized and sacrificed. For all ages, both male and female rats were included in the study in equal numbers (n=6 male and 6 female animals, for IUGR and control groups, respectively). Pup gender was determined by dissection and visualization. For all dates, kidneys were quickly harvested and frozen in liquid nitrogen or placed in 10% Formalin for histologic studies.
RNA isolation and Real-time RT-PCR
DNase I treated total RNA (Ambion Inc, Austin TX) was extracted from 30–100 mg of P0, P21 and P120 IUGR and control rat pup kidneys using the NucleoSpin RNA and Virus Purification Kit (BD Biosciences Clontech Palo Alto, CA). Total RNA was quantified using the NanoDrop Spectrometer ND-1000 (NanoDrop Technologies, Wilmington, DE). RNA integrity was confirmed by gel electrophoresis.
Kidney mRNA levels of VEGF, VEGFR-1 and VEGFR-2 were measured at P0, P21 and P120 with the real-time RT-PCR method as previously described (24). cDNA was synthesized using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) from 1.0 μg of DNase treated total RNA. Primers and probes for VEGF exon 1–2, VEGFR-1, VEGFR-2 and GAPDH were designed using Primer Express Software™ (Applied Biosystems, Foster CA). The sequences for GAPDH were: Forward: 5′ CAAGATGGTGAAGGTCGGTGT ; Reverse: 5′ CAAGAGAAGACCTGGT; Probe: 5′ GTCCGATACGCAAATCCG. The sequences for VEGF were forward: 5′ TACCTCCACCATCAAGTG; reverse: 5′ CACTTCATGGTTTCTTC; probe: 5′ CCAGTACCCACGACAGAA. The sequences for VEGFR-2 were forward: 5′ TCTCCTTCCATGTGATCAGGG; reverse: 5′ CATACTCTCCCTCGGTTG; probe: 5′ CCTGAAATTACTGTCCACTTACCCAG. Assay-on-Demand ID# for VEGFR-1 was Rn00570815_m1. Target probes were labeled with fluorescent reporter dye FAM. Reporter dye emission is detected by an automated sequence detector combined with ABI Prism 7900 Sequence Detection System® software (Applied Biosystem, Foster CA). GAPDH was used as an internal control. Relative quantification of PCR products are based upon value differences between the target and GAPDH control using the comparative CT method (29). Cycle parameters were 50°C × 2 minutes, 95°C×10 minutes, and then 40 cycles of 95°C×15 seconds and 60°C×60 seconds. Each sample was run in quadruplicate.
Immunoblotting and Antibodies
Whole kidneys were obtained from P0, P21 and P120 IUGR and control rats. Total protein was isolated by homogenizing 50–100 mg of tissue in RIPA Buffer with EDTA protease inhibitor (Roche, Mannheim, Germany), centrifuged at 10,000 × g for 15 min at 4°C. The supernatants were collected and stored at −80°C until use. Total protein was detected by Western blotting as described previously (16). Antibodies included: VEGF (ab9544 Abcam Inc., Cambridge, MA) 1:500 in 3% BSA; VEGFR-1 (SC-316 Santa Cruz Biotechnology, Santa Cruz, CA) 1:200 in 3% BSA; VEGFR-2 (ab2349 Abcam Inc., Cambridge, MA) 1:500 in 3% BSA; GAPDH (Abcam Inc., Cambridge, MA) 1:2000 in 3% BSA. Secondary antibody included HRP-conjugated anti-rabbit IgG antibody (2118 Cell Signaling Technology, Beverly, MA). Antibody signals were detected with Western Lighting™ ECL (Perkin Elmer Life Sciences, Boston, MA) and quantified using a Kodak Image Station 2000R (Eastman Kodak/SIS, Rochester, NY). GAPDH signal was used as an internal control.
Histology
Formalin-fixed tissue was embedded in paraffin and sectioned at approximately 5 microns. One section was stained with periodic acid-Schiff (PAS), imaged at a final magnification of 100x, and glomerular profile areas measured using ScionImage Software (Scion Corporation, Frederick, MD). At least 15 profiles from each animal were averaged as an index of glomerular size. We have shown previously that this index of glomerular area correlates well with measures of glomerular volume determined through serial section analysis (30, 31).
Serum corticosterone levels
Blood samples were collected following decapitation of IUGR and control animals at day 21 and used to determine serum corticosterone levels by chemiluminescent immunoassay (ARUP laboratories).
Testosterone and estradiol levels
Blood samples were collected following decapitation of IUGR and control rats at day 120 and used to determine serum testosterone and estradiol levels by liquid chromatography/ tandem mass spectrometry (ARUP laboratories).
Total protein/creatinine ratio in urine
Urine samples were collected using a rodent metabolic cage (Nalgene™ Metabolic Cage for Rats, Nalgene Company, Rochester, NY) in IUGR and control rats at P120 (n=6). Briefly, P120 rats were acclimatized in the metabolic cage starting 5 days prior to the collection of urine. We measured each animal weight daily to confirm no weight loss prior to the sample collection. Samples were collected every 24 hours for 5 days and stored at −80°C until processing. We determined urine the total protein/creatinine ratio by spectrophotometry (ARUP laboratories) in a 24 hour sample.
Statistics
All data presented are expressed as mean ± SEM. Western blotting, and real-time RT-PCR were analyzed using ANOVA (Fisher’s protected least significance difference), and Student’s unpaired t-test as applicable (p<0.05 used for statistical significance).
RESULTS
Uteroplacental insufficiency decreases kidney VEGF mRNA and protein levels in newborn and juvenile IUGR rats
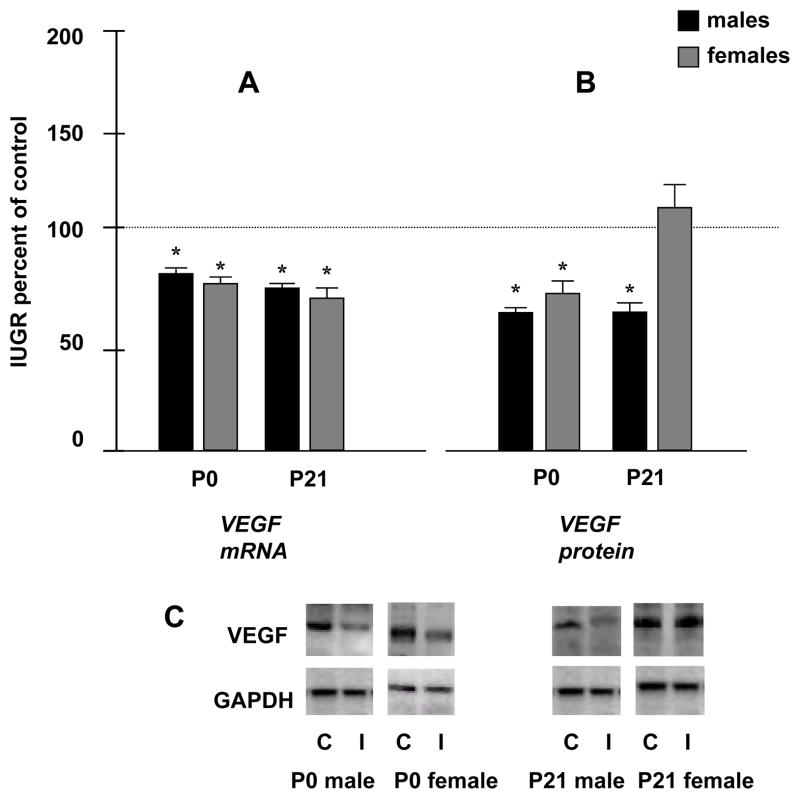
UPI significantly decreased kidney VEGF mRNA expression in both male and female IUGR pups at P0 (81 ± 7* and 77 ± 5* % of control values, respectively). The decrease in kidney VEGF mRNA expression in IUGR pups persisted through P21 in both genders (75 ± 2* and 72 ± 6* % of control values, in males and females, respectively). Consistent with the real-time RT-PCR results, UPI significantly decreased kidney protein levels of VEGF at P0 in both males and females IUGR rats to 65 ± 3* and 72 ± 8* % of control values, respectively. By P21 protein levels of kidney VEGF were significantly decreased in IUGR males to 62 ± 5 %* of control with no significant change observed in the IUGR females (112 ± 10 % of control) (*p<0.05) (fig.1-A, 1-B and 1-C).
Figure 1.
Figure 1. A- Quantification of P0 and P21 renal VEGF mRNA levels in males and females. Results are expressed as mean ± SE % relative to controls (n= 6 litters) (* p< 0.05). Figure 1. B- Quantification of P0 and P21 renal VEGF protein levels. Results are expressed as mean ± SE % relative to controls (n= 6 litters) (*p<0.05). Figure 1. C-The bottom panels are representative Western blots. GAPDH is used as internal control. C, Control; I, IUGR.
Uteroplacental insufficiency increases kidney VEGF mRNA and protein levels in adult female IUGR rats
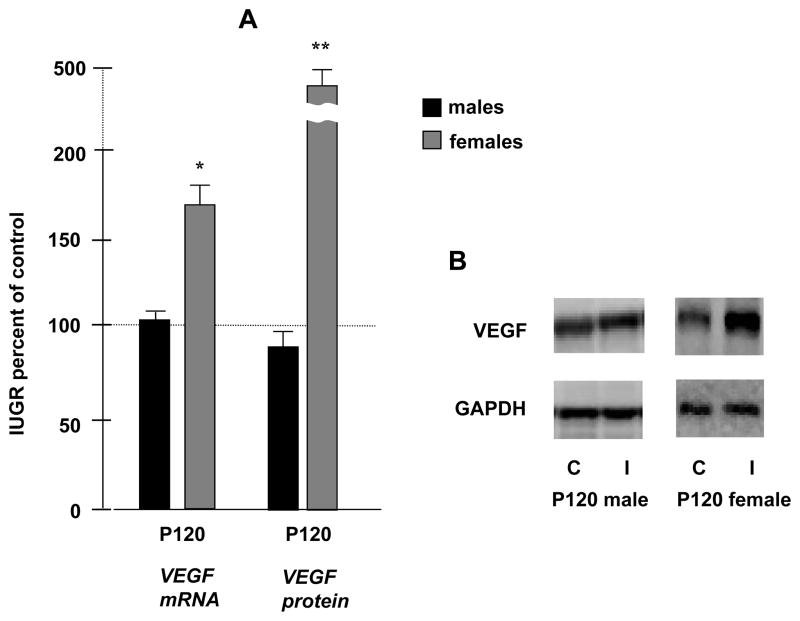
Interestingly, in contrast to the newborn and juvenile results described above, at P120 there was a significant increase in VEGF mRNA expression in female IUGR kidneys to 169 ± 12 %* of control that was not observed in IUGR males (males: 102 ± 5 % of control values). Western blot results measuring VEGF protein levels were consistent with these findings, with a significant increase in females to 453 ± 12 %** of control, and no significant difference in IUGR males (males: 89 ± 2 % of control) (*p<0.05, **p<0.01) (Fig. 2A–B).
Figure 2.
Figure 2. A- Quantification of P120 renal VEGF mRNA and protein levels in males and females. Results are expressed as mean ± SE % relative to controls (n= 6 litters) (* p< 0.05, **p<0.01). Figure 2. B- -Theses panels are representative Western blots. GAPDH is used as internal control. C, Control; I, IUGR.
Uteroplacental insufficiency similarly affects kidney VEGFR-2 mRNA and protein levels in IUGR rats in a gender-specific manner
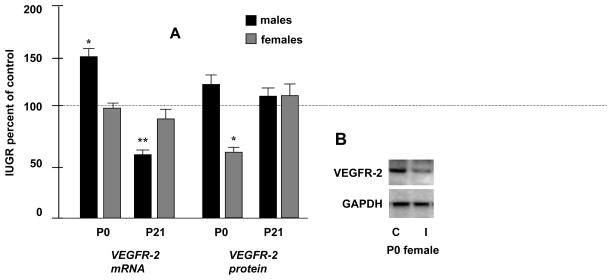
At P0, kidney VEGFR-2 mRNA levels were significantly increased in IUGR males to 150 ± 8 %* of control, with no significant difference in the IUGR females. In contrast, at P21, UPI significantly decreased VEGFR-2 mRNA levels in IUGR males to 57 ± 5 %** of control values, again with no significant difference noted for mRNA levels in females (*p<0.05; **p<0.01) (fig.3-A). Western blotting, however, demonstrated that UPI significantly decreased kidney VEGFR-2 protein levels in the IUGR females to 55 ± 7 %* of controls at P0 (*p<0.05) (fig.3A–B).
Figure 3.
Figure 3. A- Quantification of P0 and P21 renal VEGFR-2 mRNA and protein levels in males and females. Results are expressed as mean ± SE % relative to controls (n= 6 litters) (* p< 0.05, **p<0.01). Figure 3. B-To the right, panels are representative Western blots. GAPDH is used as internal control. C, Control; I, IUGR.
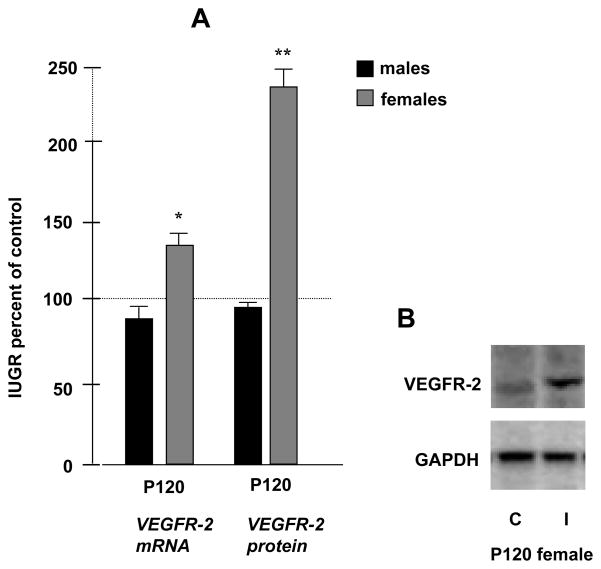
Interestingly, and parallel to the VEGF results, both kidney mRNA and protein levels of VEGFR-2 were significantly increased at P120 in IUGR females (133 ± 8 %* and 232 ± 11 %**, respectively). Also similar to the VEGF results, there was no significant difference noted in mRNA and protein levels of renal VEGFR-2 in the males at that stage (*p<0.05; **p<0.01) (Fig. 4A–B).
Figure 4.
Figure 4. A- Quantification of P120 renal VEGFR-2 mRNA and protein levels in males and females. Results are expressed as mean ± SE % relative to controls (n= 6 litters) (* p< 0.05, **p<0.01). Figure 4. B- This panel is a representative Western blot. GAPDH is used as internal control. C, Control; I, IUGR.
In contrast to VEGFR-2 results, we found no significant difference in VEGFR-1 mRNA and protein levels at P0, P21 and at P120 in males and females (data not shown).
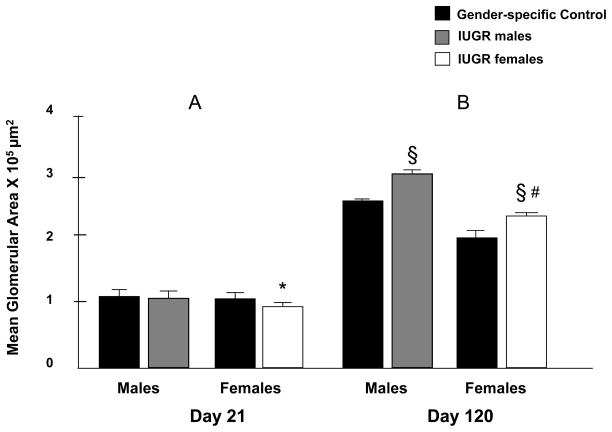
Uteroplacental insufficiency leads to increased glomerular area in adult IUGR rats
Differences in mean glomerular area were minimal at P21, with IUGR females showing a slight decrease in size compared to other groups (*p<0.05) (Fig. 5-A). However, by P120, glomerular enlargement was demonstrated in both males and females with IUGR when compared to gender-specific controls (§ p<0.001). Importantly, mean glomerular area remained greater in IUGR males than females (# p<0.001) (Figure 5-B).
Figure 5.
Effect of UPI on mean glomerular area at P21 (*p<0.05 vs all other groups) and P120 (§ p<0.001 vs. sex-matched control group, # p<0.001 vs. IUGR males). Error bars represent 1SD. Control area is mean ± 1SE (n=5)
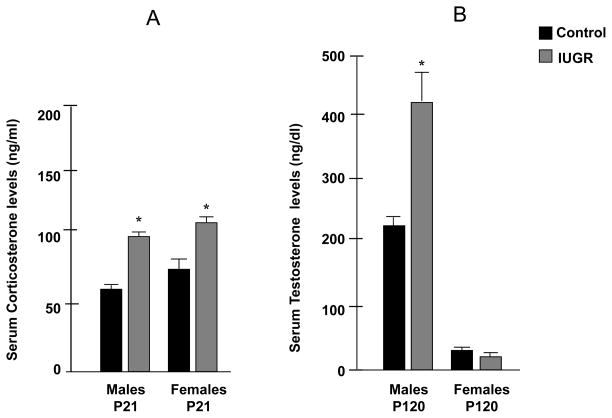
UPI increases serum corticosterone levels at P21 in IUGR animals
Serum corticosterone levels were significantly increased in both male and female IUGR juvenile rats at P21 (Male: 93 ± 3 ng/ml* in IUGR, vs. 69 ± 2 ng/ml in control; Female: 104 ± 11 ng/ml* in IUGR vs.73 ± 10 ng/ml in control, *p<0.05) (Figure 6-A).
Figure 6.
Figure 6-A. Effect of UPI on serum corticosterone levels at P21 in males and females, measured in IUGR and control offspring (n=6). Figure 6-B. Effect of UPI on serum testosterone levels at P120 in males and females, measured in IUGR and control offspring (n=6). Results are expressed as mean ± SE (*p<0.05).
UPI increases serum testosterone levels in the IUGR adult male
Serum estradiol levels were not significantly affected in the adult IUGR rat (Males: 0.5 ± 0.2 pg/ml in IUGR vs.1.1 ± 0.5 pg/ml in control; Female: 40.8 ± 13 pg/ml in IUGR vs. 40.75 ± 16 pg/ml in control) (data not shown as a graph). However, serum testosterone levels were significantly increased in the male IUGR adult rat at P120 when compared to control animals, with no significant difference in females (Male: 437 ± 81 ng/ml* in IUGR vs. 211 ± 54 ng/ml in control; Female: 7 ± 2 ng/ml in IUGR vs.10 ± 3 ng/ml in control, *p<0.05) (Figure 6-B).
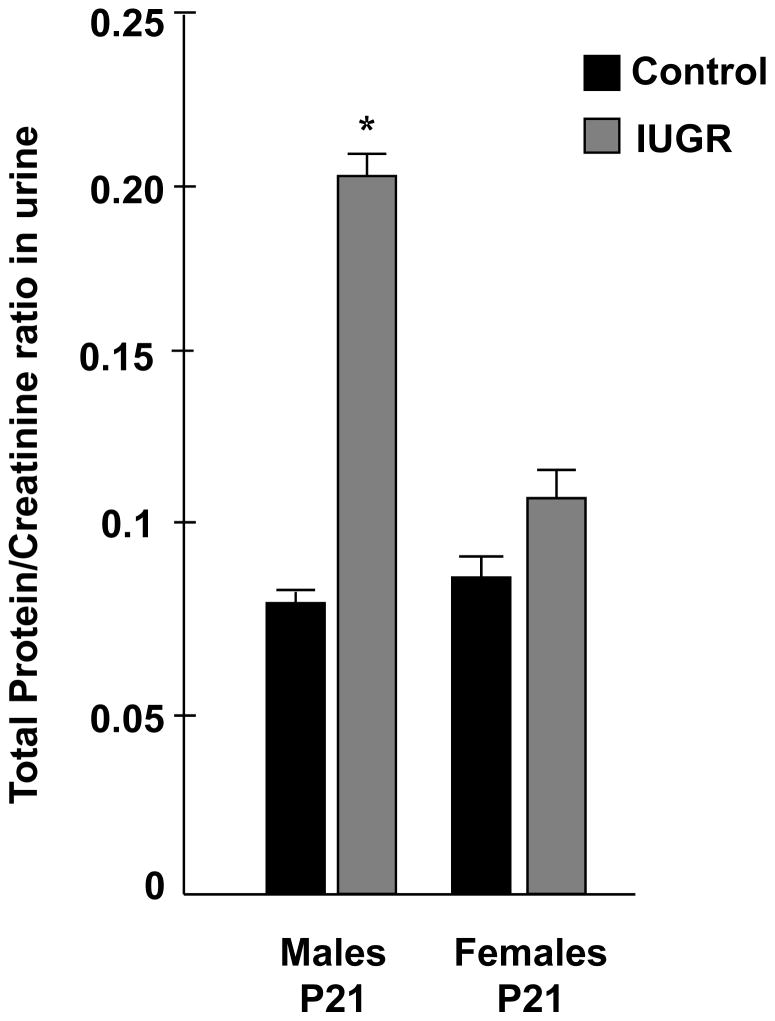
UPI significantly increases total protein/creatinine ratio in urine of IUGR rat males
Urine protein/creatinine ratio was significantly increased in adult IUGR males when compared to the control group (0.20 ± 0.02* in IUGR vs. 0.07 ± 0.01 in control group, *p<0.05). This phenomenon was not observed in the IUGR female group (0.11 ± 0.03 in IUGR vs. 0.08 ± 0.02 in control group) (Figure 7).
Figure 7.
Effect of UPI on urine total protein/creatinine ratio at P120 in males and females, measured in IUGR and control offspring (n=6). Results are expressed as mean ± SE (*p<0.05).
DISCUSSION
This study demonstrates for the first time that UPI and resultant glucocorticoid overexposure of the offspring is associated with (1) altered perinatal and postnatal kidney VEGF expression, and that (2) this phenomenon is developmentally regulated and gender-specific. In the present report, UPI decreased renal VEGF levels during a period of active nephrogenesis in the IUGR rat in both males and females. Although this observation is being reported for the first time, our most intriguing and novel finding is the significant up regulation of renal VEGF and VEGFR-2 in the adult IUGR female. This observation is important since an increasing body of literature is pointing to the impact of gender on progression of cardiovascular and renal disease. Despite the increasing evidence for a protective role of female gender in the progression of renal disease following IUGR, this study is unique in that it investigates a potential molecular mechanism that can be responsible for this phenotype.
VEGF is a potent angiogenic factor that exerts its function through induction of endothelial cell proliferation and increased vascular permeability (25). The role of the VEGF receptors, especially of VEGFR-1 is still conflicting: much of the evidence suggests that VEGFR-2 is the major mediator for developmental angiogenesis, as well as mitogenetic and permeability-enhancement effects of VEGF, whereas VEGFR-1 may perform an inhibitory role during early development, acting as a negative regulator of VEGF (25). However, targeted mutations of VEGF and both receptors, have shown all three proteins are crucial for embryonic blood vessel development (32). Furthermore, the role of VEGF and its receptors in nephrogenesis has been fairly well established.
These regulators of vascular development have been localized to the developing metanephros (33), with expression of VEGF as well as VEGFR-1 and VEGFR-2 in glomerular epithelial cells of fetal kidney. Likewise, VEGF and its receptors colocalized in the peritubular capillary endothelial cells in fetal cortex and medulla. Kitamoto et al. demonstrated that anti-VEGF neutralizing antibodies in newborn mice, in which kidneys are still developing, disrupted vessel formation in the superficial renal cortex as well as decreased nephrogenic areas and total nephron number. The authors also observed several abnormal glomeruli lacking capillary tufts, suggesting that VEGF is an essential molecule for glomerulogenesis (34).
In the present study, both VEGF and VEGFR-2 were significantly affected following UPI in the IUGR kidney at all study ages, whereas VEGFR-1 levels remained unaffected. The effect of UPI upon renal VEGFR-2 was gender specific, with significant changes in mRNA levels at P0 and P21 noted in the male but not in the females. However, kidney VEGFR-2 protein levels did not correlate with mRNA results, and IUGR females were found to have decreased VEGFR-2 protein at birth. An explanation of these findings is that VEGFR-2 protein expression heavily relies on posttranscriptional regulation such as glycosylation of the co-receptor Neuropilin-1, and phosphorylation (35). Interestingly, in the IUGR adult female rats, both mRNA and protein levels of kidney VEGFR-2 were dramatically increased, whereas we did not observe any change in the IUGR males. These findings are important since they confirm our previous observations of significantly different gender-specific gene expression in the IUGR kidney that can be long-lasting.
Significant to the present study, VEGF expression can be affected by GC levels, which are in turn highly affected by the IUGR fetal milieu (24, 36, 37). For instance, VEGF expression is down-regulated by GC in various tissues such as airway smooth muscle cells, and growth plate chondrocytes (18, 38). Similarly, dexamethasone administration inhibited placental VEGF expression and led to reduction in placental vascularization with subsequent IUGR of the fetus (39). The mechanism by which GC affect VEGF expression is still not fully understood. Most reports regarding this topic are found in the cancer literature, since the use of dexamethasone has a potential role in antiangiogenic therapy, by inhibiting VEGF production (40). In regards to possible mechanisms by which GC can affect VEGF, the 5′ promoter region of the VEGF gene does not contain a GC-response element. Alternatively, Gille et al. showed that GC significantly decreased VEGF mRNA stability in cultured keratinocytes, suggesting that enhanced VEGF mRNA turnover may in part explain the down regulation of this gene by GC (41).
Altogether, our results of decreased kidney VEGF and altered VEGF receptor message expression and protein levels during nephrogenesis, in association with the persistent increased circulating corticosterone levels following UPI, suggest that this molecular mechanism may contribute to the aberrant nephrogenesis observed in the perinatal IUGR rat.
In the adult rat, we confirmed that UPI and subsequent IUGR in our animal model is indeed associated with adult glomerular hypertrophy, both in male and female rats. Furthermore, and consistent with several reports that analyzed experimental nephron reduction, mean glomerular area was higher in IUGR males when compared to IUGR females (42). Although we describe that both IUGR male and females exhibit increased glomerular area, our data and the literature strongly suggest that the molecular mechanisms leading to compensatory glomerular hypertrophy are most likely not common to both genders.
Gender differences in compensatory glomerular hypertrophy have been mainly addressed in rat animal models. Mulroney et al. observed that there are important gender differences in renal growth and function following uninephrectomy in rats, with a significantly increased kidney growth associated with glomerular and tubular damage noted in males and a much smaller impact in the female kidney (42). The authors suggested that testosterone could play a key role in glomerular hypertrophy by driving the growth-hormone mechanism occurring after uninephrectomy. A rat model of renin mediated hypertension supports these findings, since hypertensive male rats treated with flutamide (androgen receptor antagonist) showed decreased blood pressures as well as a reversal of hypertension mediated renal damage (43). Importantly, in this study we confirmed that adult IUGR males have significant increase in serum testosterone levels when compared to control rats, in association to increased glomerular area. Elevated testosterone levels in IUGR males were also described in a previous report by Alexander et al. that uses a similar model of UPI and IUGR (15). Therefore, we suggest that high testosterone levels could stimulate growth hormone mediated molecular mechanisms and have a significant contribution to the glomerular hypertrophy observed in the IUGR males in our animal model.
Relevant to the present study, Kang and collaborators used the remnant kidney (RK) model to induce progressive renal disease and then studied the pattern and molecular mechanisms of different intrarenal vascular changes in males and females. The authors demonstrated that female RK rats developed less renal failure and glomerulosclerosis, with greater preservation of peritubular capillaries as well as increased expression of renal VEGF and VEGFR-2 when compared to the RK males. Supporting this in vivo studies, estrogens stimulated basal VEGF expression in cultured renal tubular cells in vitro (44).
These observations correlate well with the present study. In our animal model IUGR males demonstrate increased glomerular area, and exhibit increased protein/creatinine ratio in urine when compared to control rats, indicating possible glomerular injury. In contrast, we report up regulation of mRNA and protein levels of kidney VEGF and VEGFR-2, in association with a less significant increase in glomerular area and no proteinuria in the adult IUGR female when compared to IUGR males at that age. These results are intriguing in light of normal estradiol levels in adult IUGR females.
Altogether, this data suggests that the increase in VEGF in the female rat in different animal models with progressive renal disease may contribute to maintain a less affected intrarenal environment. We speculate that VEGF signaling is most likely involved in compensatory renal growth in the female, whereas high testosterone levels in the IUGR male probably underlie a different molecular mechanism in this group.
In summary, in the present study we observed a significant down regulation of kidney VEGF during nephrogenesis, a period of time that in our animal model is associated with increased serum corticosterone levels. In addition, we observed up regulation of kidney VEGF and kidney VEGFR-2 receptor in the adult IUGR female but not in the IUGR male offspring. Alternatively, the adult IUGR male exhibits increased serum testosterone levels, suggesting that mechanisms underlying renal disease are most likely regulated in a gender specific manner.
Acknowledgments
Sources of Funding: This research was supported by NICHD Grant R01HD41075, and the University of Utah PCMC Innovative Grant Award.
Footnotes
Conflict of Interest Statement
None declared.
References
- 1.Witlin AG, Sibai BM. Hypertension in pregnancy: current concepts of preeclampsia. Annu Rev Med. 1997;48:115–27. doi: 10.1146/annurev.med.48.1.115. [DOI] [PubMed] [Google Scholar]
- 2.Valdez R, Athens MA, Thompson GH, Bradshaw BS, Stern MP. Birthweight and adult health outcomes in a biethnic population in the USA. Diabetologia. 1994;37(6):624–31. doi: 10.1007/BF00403383. [DOI] [PubMed] [Google Scholar]
- 3.do Carmo Pinho Franco M, Nigro D, Fortes ZB, Tostes RC, Carvalho MH, Lucas SR, et al. Intrauterine undernutrition--renal and vascular origin of hypertension. Cardiovasc Res. 2003;60(2):228–34. doi: 10.1016/s0008-6363(03)00541-8. [DOI] [PubMed] [Google Scholar]
- 4.Mackenzie HS, Brenner BM. Fewer nephrons at birth: a missing link in the etiology of essential hypertension? Am J Kidney Dis. 1995;26(1):91–8. doi: 10.1016/0272-6386(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 5.Alexander BT. Placental insufficiency leads to development of hypertension in growth-restricted offspring. Hypertension. 2003;41(3):457–62. doi: 10.1161/01.HYP.0000053448.95913.3D. [DOI] [PubMed] [Google Scholar]
- 6.Bauer R, Walter B, Bauer K, Klupsch R, Patt S, Zwiener U. Intrauterine growth restriction reduces nephron number and renal excretory function in newborn piglets. Acta Physiol Scand. 2002;176(2):83–90. doi: 10.1046/j.1365-201X.2002.01027.x. [DOI] [PubMed] [Google Scholar]
- 7.Silver LE, Decamps PJ, Korst LM, Platt LD, Castro LC. Intrauterine growth restriction is accompanied by decreased renal volume in the human fetus. Am J Obstet Gynecol. 2003;188(5):1320–5. doi: 10.1067/mob.2003.270. [DOI] [PubMed] [Google Scholar]
- 8.Pham TD, MacLennan NK, Chiu CT, Laksana GS, Hsu JL, Lane RH. Uteroplacental insufficiency increases apoptosis and alters p53 gene methylation in the full-term IUGR rat kidney. Am J Physiol Regul Integr Comp Physiol. 2003;285(5):R962–70. doi: 10.1152/ajpregu.00201.2003. [DOI] [PubMed] [Google Scholar]
- 9.Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens. 1988;1(4 Pt 1):335–47. doi: 10.1093/ajh/1.4.335. [DOI] [PubMed] [Google Scholar]
- 10.Neuringer JR, Brenner BM. Glomerular hypertension: cause and consequence of renal injury. J Hypertens Suppl. 1992;10(7):S91–7. [PubMed] [Google Scholar]
- 11.Kaufman JM, Siegel NJ, Hayslett JP. Functional and hemodynamic adaptation to progressive renal ablation. Circ Res. 1975;36(2):286–93. doi: 10.1161/01.res.36.2.286. [DOI] [PubMed] [Google Scholar]
- 12.Merlet-Benichou C, Gilbert T, Muffat-Joly M, Lelievre-Pegorier M, Leroy B. Intrauterine growth retardation leads to a permanent nephron deficit in the rat. Pediatr Nephrol. 1994;8(2):175–80. doi: 10.1007/BF00865473. [DOI] [PubMed] [Google Scholar]
- 13.Schreuder MF, Nyengaard JR, Fodor M, van Wijk JA, Delemarre-van de Waal HA. Glomerular number and function are influenced by spontaneous and induced low birth weight in rats. J Am Soc Nephrol. 2005;16(10):2913–9. doi: 10.1681/ASN.2004100875. [DOI] [PubMed] [Google Scholar]
- 14.Li S, Chen SC, Shlipak M, Bakris G, McCullough PA, Sowers J, et al. Low birth weight is associated with chronic kidney disease only in men. Kidney Int. 2008;73(5):637–42. doi: 10.1038/sj.ki.5002747. [DOI] [PubMed] [Google Scholar]
- 15.Ojeda NB, Grigore D, Yanes LL, Iliescu R, Robertson EB, Zhang H, et al. Testosterone contributes to marked elevations in mean arterial pressure in adult male intrauterine growth restricted offspring. Am J Physiol Regul Integr Comp Physiol. 2007;292(2):R758–63. doi: 10.1152/ajpregu.00311.2006. [DOI] [PubMed] [Google Scholar]
- 16.Baserga M, Hale MA, Wang ZM, Yu X, Callaway CW, McKnight RA, et al. Uteroplacental insufficiency alters nephrogenesis and downregulates cyclooxygenase-2 expression in a model of IUGR with adult-onset hypertension. Am J Physiol Regul Integr Comp Physiol. 2007;292(5):R1943–55. doi: 10.1152/ajpregu.00558.2006. [DOI] [PubMed] [Google Scholar]
- 17.Baserga M, Hale MA, Ke X, Wang ZM, Yu X, Callaway CW, et al. Uteroplacental insufficiency increases p53 phosphorylation without triggering the p53-MDM2 functional circuit response in the IUGR rat kidney. Am J Physiol Regul Integr Comp Physiol. 2006;291(2):R412–8. doi: 10.1152/ajpregu.00880.2005. [DOI] [PubMed] [Google Scholar]
- 18.Koedam JA, Smink JJ, van Buul-Offers SC. Glucocorticoids inhibit vascular endothelial growth factor expression in growth plate chondrocytes. Mol Cell Endocrinol. 2002;197(1–2):35–44. doi: 10.1016/s0303-7207(02)00276-9. [DOI] [PubMed] [Google Scholar]
- 19.Tufro A, Norwood VF, Carey RM, Gomez RA. Vascular endothelial growth factor induces nephrogenesis and vasculogenesis. J Am Soc Nephrol. 1999;10(10):2125–34. doi: 10.1681/ASN.V10102125. [DOI] [PubMed] [Google Scholar]
- 20.Kitamoto Y, Tokunaga H, Miyamoto K, Tomita K. VEGF is an essential molecule for glomerular structuring. Nephrol Dial Transplant. 2002;17 (Suppl 9):25–7. doi: 10.1093/ndt/17.suppl_9.25. [DOI] [PubMed] [Google Scholar]
- 21.Schrijvers BF, Flyvbjerg A, Tilton RG, Lameire NH, De Vriese AS. A neutralizing VEGF antibody prevents glomerular hypertrophy in a model of obese type 2 diabetes, the Zucker diabetic fatty rat. Nephrol Dial Transplant. 2006;21(2):324–9. doi: 10.1093/ndt/gfi217. [DOI] [PubMed] [Google Scholar]
- 22.Yildiz B, Kural N, Colak O, Ak I, Akcar N. IGF-1, IGFBP-3, VEGF and MMP-9 levels and their potential relationship with renal functions in patients with compensatory renal growth. Clin Physiol Funct Imaging. 2008;28(2):107–12. doi: 10.1111/j.1475-097X.2007.00783.x. [DOI] [PubMed] [Google Scholar]
- 23.Simmons RA, Templeton LJ, Gertz SJ. Intrauterine growth retardation leads to the development of type 2 diabetes in the rat. Diabetes. 2001;50(10):2279–86. doi: 10.2337/diabetes.50.10.2279. [DOI] [PubMed] [Google Scholar]
- 24.Baserga M, Hale MA, McKnight RA, Yu X, Callaway CW, Lane RH. Uteroplacental insufficiency alters hepatic expression, phosphorylation, and activity of the glucocorticoid receptor in fetal IUGR rats. Am J Physiol Regul Integr Comp Physiol. 2005;289(5):R1348–53. doi: 10.1152/ajpregu.00211.2005. [DOI] [PubMed] [Google Scholar]
- 25.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 26.Guiding principles for research involving animals and human beings. Am J Physiol Regul Integr Comp Physiol. 2002;283(2):R281–3. doi: 10.1152/ajpregu.00279.2002. [DOI] [PubMed] [Google Scholar]
- 27.Lane RH, MacLennan NK, Hsu JL, Janke SM, Pham TD. Increased hepatic peroxisome proliferator-activated receptor-gamma coactivator-1 gene expression in a rat model of intrauterine growth retardation and subsequent insulin resistance. Endocrinology. 2002;143(7):2486–90. doi: 10.1210/endo.143.7.8898. [DOI] [PubMed] [Google Scholar]
- 28.Ogata ES, Bussey ME, Finley S. Altered gas exchange, limited glucose and branched chain amino acids, and hypoinsulinism retard fetal growth in the rat. Metabolism. 1986;35(10):970–7. doi: 10.1016/0026-0495(86)90064-8. [DOI] [PubMed] [Google Scholar]
- 29.Menon RK, Shaufl A, Yu JH, Stephan DA, Friday RP. Identification and characterization of a novel transcript of the murine growth hormone receptor gene exhibiting development- and tissue-specific expression. Mol Cell Endocrinol. 2001;172(1–2):135–46. doi: 10.1016/s0303-7207(00)00375-0. [DOI] [PubMed] [Google Scholar]
- 30.Lane PH. Determination of mean glomerular volume in nephrectomy specimens. Lab Invest. 1995;72(6):765–70. [PubMed] [Google Scholar]
- 31.Lane PH, Steffes MW, Mauer SM. Estimation of glomerular volume: a comparison of four methods. Kidney Int. 1992;41(4):1085–9. doi: 10.1038/ki.1992.165. [DOI] [PubMed] [Google Scholar]
- 32.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380(6573):435–9. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 33.Simon M, Grone HJ, Johren O, Kullmer J, Plate KH, Risau W, et al. Expression of vascular endothelial growth factor and its receptors in human renal ontogenesis and in adult kidney. Am J Physiol. 1995;268(2 Pt 2):F240–50. doi: 10.1152/ajprenal.1995.268.2.F240. [DOI] [PubMed] [Google Scholar]
- 34.Kitamoto Y, Tokunaga H, Tomita K. Vascular endothelial growth factor is an essential molecule for mouse kidney development: glomerulogenesis and nephrogenesis. J Clin Invest. 1997;99(10):2351–7. doi: 10.1172/JCI119416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shintani Y, Takashima S, Asano Y, Kato H, Liao Y, Yamazaki S, et al. Glycosaminoglycan modification of neuropilin-1 modulates VEGFR2 signaling. Embo J. 2006;25(13):3045–55. doi: 10.1038/sj.emboj.7601188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Economides DL, Nicolaides KH, Linton EA, Perry LA, Chard T. Plasma cortisol and adrenocorticotropin in appropriate and small for gestational age fetuses. Fetal Ther. 1988;3(3):158–64. doi: 10.1159/000263348. [DOI] [PubMed] [Google Scholar]
- 37.Nauck M, Karakiulakis G, Perruchoud AP, Papakonstantinou E, Roth M. Corticosteroids inhibit the expression of the vascular endothelial growth factor gene in human vascular smooth muscle cells. Eur J Pharmacol. 1998;341(2–3):309–15. doi: 10.1016/s0014-2999(97)01464-7. [DOI] [PubMed] [Google Scholar]
- 38.Alagappan VK, McKay S, Widyastuti A, Garrelds IM, Bogers AJ, Hoogsteden HC, et al. Proinflammatory cytokines upregulate mRNA expression and secretion of vascular endothelial growth factor in cultured human airway smooth muscle cells. Cell Biochem Biophys. 2005;43(1):119–29. doi: 10.1385/CBB:43:1:119. [DOI] [PubMed] [Google Scholar]
- 39.Hewitt DP, Mark PJ, Waddell BJ. Glucocorticoids prevent the normal increase in placental vascular endothelial growth factor expression and placental vascularity during late pregnancy in the rat. Endocrinology. 2006;147(12):5568–74. doi: 10.1210/en.2006-0825. [DOI] [PubMed] [Google Scholar]
- 40.Iwai A, Fujii Y, Kawakami S, Takazawa R, Kageyama Y, Yoshida MA, et al. Down-regulation of vascular endothelial growth factor in renal cell carcinoma cells by glucocorticoids. Mol Cell Endocrinol. 2004;226(1–2):11–7. doi: 10.1016/j.mce.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 41.Gille J, Reisinger K, Westphal-Varghese B, Kaufmann R. Decreased mRNA stability as a mechanism of glucocorticoid-mediated inhibition of vascular endothelial growth factor gene expression by cultured keratinocytes. J Invest Dermatol. 2001;117(6):1581–7. doi: 10.1046/j.0022-202x.2001.01573.x. [DOI] [PubMed] [Google Scholar]
- 42.Mulroney SE, Woda C, Johnson M, Pesce C. Gender differences in renal growth and function after uninephrectomy in adult rats. Kidney Int. 1999;56(3):944–53. doi: 10.1046/j.1523-1755.1999.00647.x. [DOI] [PubMed] [Google Scholar]
- 43.Baltatu O, Cayla C, Iliescu R, Andreev D, Jordan C, Bader M. Abolition of hypertension-induced end-organ damage by androgen receptor blockade in transgenic rats harboring the mouse ren-2 gene. J Am Soc Nephrol. 2002;13(11):2681–7. doi: 10.1097/01.asn.0000033327.65390.ca. [DOI] [PubMed] [Google Scholar]
- 44.Kang DH, Yu ES, Yoon KI, Johnson R. The impact of gender on progression of renal disease: potential role of estrogen-mediated vascular endothelial growth factor regulation and vascular protection. Am J Pathol. 2004;164(2):679–88. doi: 10.1016/S0002-9440(10)63155-0. [DOI] [PMC free article] [PubMed] [Google Scholar]