Abstract
Hypotheses
Electrocochleography (ECoG) to acoustic stimuli can differentiate relative degrees of cochlear responsiveness across the population of cochlear implant recipients. The magnitude of the ongoing portion of the ECoG, which includes both hair cell and neural contributions, will correlate with speech outcomes as measured by results on CNC word score tests.
Background
Postoperative speech outcomes with cochlear implants vary from almost no benefit to near normal comprehension. A factor expected to have a high predictive value is the degree of neural survival. However, speech performance with the implant does not correlate with the number and distribution of surviving ganglion cells when measured post-mortem. We will investigate whether ECoG can provide an estimate of cochlear function that helps predict postoperative speech outcomes.
Methods
An electrode was placed at the ipsilateral round window of the ear about to be implanted during implant surgery. Tone bursts were delivered through an insert earphone. Subjects included children (N=52, 1–18 years) and postlingually hearing impaired adults (N=32). Word scores at six months were available from 21 adult subjects.
Results
Significant responses to sound were recorded from almost all subjects (80/84 or 95%). The ECoG magnitudes spanned more than 50 dB in both children and adults. The distributions of ECoG magnitudes and frequencies were similar between children and adults. The correlation between the ECoG magnitude and word score accounted for 47% of the variance.
Conclusions
ECoGs with high signal to noise ratios can be recorded from almost all implant candidates, including both adult and pediatric populations. In post-lingual adults, the ECoG magnitude is more predictive of implant outcomes than other non-surgical variables such as duration of deafness or degree of residual hearing.
Keywords: Electrocochleography, Prediction of outcome, Residual hearing, Cochlear Electrophysiology, Hearing Preservation, Intraoperative monitoring, Auditory Nerve Neurophonic, Cochlear Microphonic
INTRODUCTION
Speech outcomes with cochlear implants vary widely among adults, from almost no benefit to near normal comprehension (1–4). The reasons for this variation are in general not clear. The factor with the most consistent significant correlation is duration of deafness (4–12). The amount of variance accounted for by this factor is low, typically less than 25% in outcomes on speech tests. Even when combining additional factors such as age at implantation, degree of residual hearing, and number of active electrodes, a recent large multicenter survey could account for only 22% of the variance in speech outcomes (8; 2251 subjects).
A factor that might be expected to have a high predictive value is the degree of neuronal survival. Surprisingly however, speech performance with the implant does not correlate with the number and distribution of surviving ganglion cells (13–17). Of course, histology cannot assess the functional state of the cochlea, and histopathology at death might be different from when the behavioral measures were obtained. Other methods to assess the health of the cochlea prior to implantation include psychophysical or electrophysiological responses to electrical stimulation of the round window, promontory or eardrum. Although some studies found a good correlation using the round window as a stimulation site (6,7), in general the results from these techniques have been too variable or too invasive to be in routine use. Still, it might be expected that accurate measures of cochlear function at the time of implantation could be a useful predictor of outcomes with the implant. Here we propose that electrocochleography (ECoG) to acoustic stimuli at the round window can provide important information about cochlear health at the time of implantation, and show early data suggesting a strong correlation with outcomes.
The ECoG is a complex signal containing contributions from hair cells and auditory nerve fibers. The portion of the signals from hair cells include the cochlear microphonic (CM) and the summating potential. Signals from auditory nerve fibers include the compound action potential (CAP) at the onset of sounds, and the auditory nerve neurophonic (ANN), or evoked potential correlate of phase-locking in auditory nerve fibers. These features are well-characterized from round window recordings in animals (18–21). We have recently shown that each of these signals can be measured from the round window in patients during cochlear implant surgery (22). Because the ECoG contains contributions from hair cells as well as nerve fibers, it provides information about the physiological state of the cochlea that is different from the audiogram. A useful measure of response magnitude is one which does not readily saturate. The ongoing portion of the ECoG signal, containing the CM (to all frequencies) and ANN (to low frequencies only), has this property, and was used here to determine the relative degree of responsiveness to sounds within the cochlea across the implant population.
MATERIALS AND METHODS
The procedures were in accordance with the ethical standards of the institution’s IRB and informed consent was obtained for all subjects enrolled (Protocol number 05–2616). The procedure was to record responses from the RW of cochlear implant recipients to acoustic stimuli intraoperatively prior to electrode array insertion.
Human Subject Inclusion Criteria
All pediatric and adult subjects undergoing cochlear implantation were potential participants. The IRB for this study did not permit consent through an interpreter; so non-English speaking patients were excluded. We also excluded patients with atretic external auditory canals and those undergoing revision surgeries. Ninety-seven cases are included. Of these 45 were adults (>18 years) and 52 were children (<18 yrs, with 37 of 52 less than 3 yrs).
Surgery and Recording Set-up
After induction of anesthesia, a foam insert attached to a sound tube was placed in the external auditory canal of the ear being implanted. A sterile, stainless steel, disposable monopolar probe (Neurosign, Magstim Co., Wales, UK) served as the active input for recordings, a surface electrode on the contralateral mastoid was the return and the common was a surface electrode on the glabella. The ipsilateral ear was then prepped and draped with the foam insert in place in the ear canal. As such the auricle was folded anterior over the foam insert and special care was taken not to crimp the sound tube. A standard transmastoid facial recess approach was used to access the promontory. The monopolar probe was placed on the membranous portion of the round window and wedged against the partially removed bony overhang to hold it in place. A clinical stimulation/recording device (Biologic Navigator Pro, Natus Medical Inc., San Carlos, CA) was used to generate acoustic stimuli and to obtain all recordings using the AEP hearing diagnostics software. Impedance measurements were < 16 kOhm on all electrodes. If the impedance at the recording electrode was too high saline was added at the round window to reduce it below 16k Ohms.
Sound Stimulation and Evoked Potential Recording
Sound was delivered through Etymotic speakers (ER-3) calibrated to normal hearing level (nHL). Recordings from the RW were in response to 500 repetitions of alternating phase tone bursts. The frequencies were 250, 500, 750, 1000, 2000 and 4000 Hz. The tone bursts had rise and fall times of 1 to 4 ms shaped by a Blackman window. For tone frequencies from 250 Hz to 2 kHz the recordings started 4 ms prior to stimulus onset, the recording epoch was 32 ms and the sampling rate was 16 kHz. For the 4 kHz tone the recording started 2 ms prior to stimulus onset, the recording epoch was 12 ms and the sampling rate was 48 kHz. The series of frequencies was first tested at 90 dB nHL (87–107 dB SPL depending on the frequency). Then, a frequency with a good response (typically 500 Hz) was used for a level series decreasing from 90 dB nHL in 5 – 10 dB steps. Recordings with the sound tube clamped were taken at each frequency to estimate electrical artifact, if any. It routinely took about 10 minutes to administer the intraoperative recording protocol.
Data Analysis
The data from condensation and rarefaction phases was stored separately. The spectrum of each signal within a window that isolated the ongoing portion of the response from the CAP (either onset or offset) was obtained from the fast Fourier transform (FFT). The energy of the response was measured to the signal frequency and second harmonic. The noise and its standard deviation were determined from six bins, three on each side of the signal frequency or the second harmonic, starting nine bins away from the peak. A response was considered significant if the magnitude of the peak in question exceeded the noise plus three standard deviations. The criterion used means that less than 1% false positives are expected.
The metric for the magnitude of the cochlear response was the “total response,” which was defined as the sum of all significant first and second harmonic responses across all frequencies at the highest sound level (90 dB nHL). There were thus a maximum of 12 measurements (first and second harmonics to six frequencies) that were summed together to produce the total response. The magnitude of the total response could in principle be affected by the noise level because of the method for determining if a response was significant (see above). In practice this had little effect on the measurement because the loud stimuli used produced large responses at some frequencies which dominated the total (see Fig. 1).
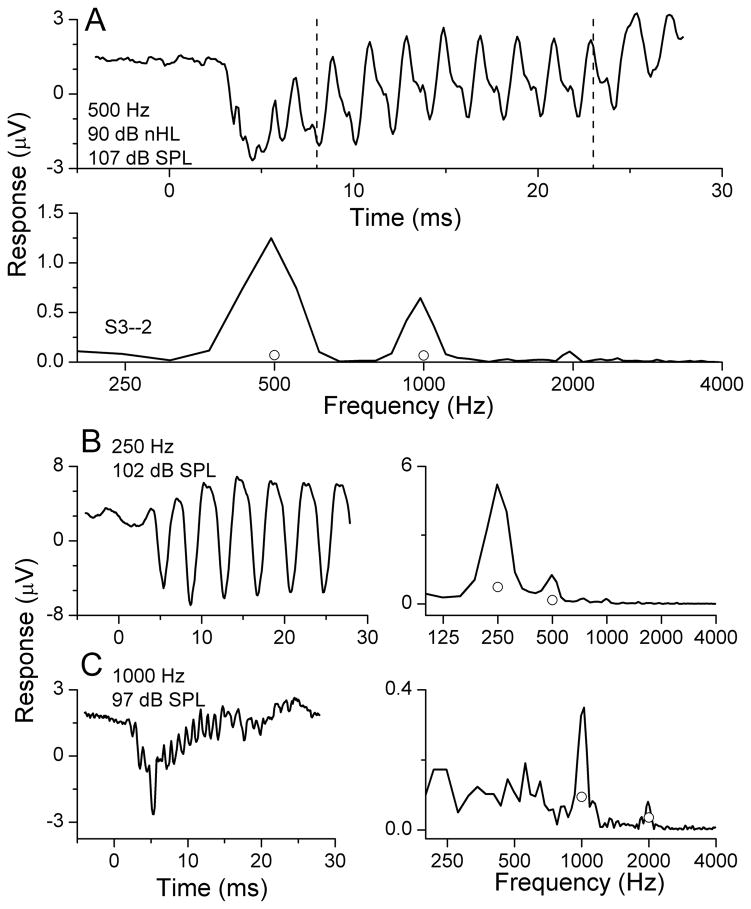
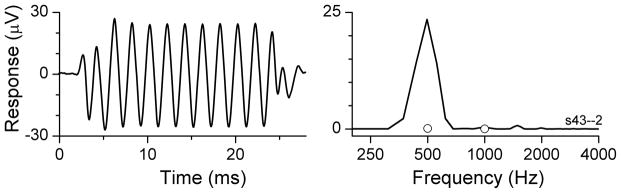
Figure 1.
Example of an ECoG recording from the round window during cochlear implantation. A: response to 500 Hz at 90 dB nHL. The top panel demonstrates the time series from the condensation starting phase. The lower panel is the spectrum of the response. Note that several harmonics are present. B: Response (time series and spectrum) to 250 Hz at 90 dB nHL. C: Response to 1000 Hz at 90 dB nHL.
Word Scores
Speech perception was assessed with Consonant-Nucleus-Consonant (CNC) words (23). The CNC words test was selected as it demonstrates the wide variability in cochlear implant recipient speech perception performance, with less limitation from ceiling effects than most other tests (24). The assessment of speech perception with monosyllabic words is used routinely in the cochlear implant field, with the CNC word list being a recommended measure as part of the Minimum Speech Test Battery since 1996 (25). The CNC test consists of 10 lists of phonetically balanced monosyllabic words. Each 50 word list is scored by the percent correct. Speech perception performance with the cochlear implant was evaluated in the sound field, with the subject facing the speaker at 0° azimuth. Lists were presented at 60 dB SPL. The results reported here are from six months after the implant procedure.
RESULTS
An example of an ECoG recording from the round window in a cochlear implant patient is shown in Fig. 1. The stimuli were tone bursts at 90 dB nHL, presented in alternating condensation and rarefaction starting phases, with 250 presentations per phase averaged together. The time waveform to 500 Hz (Fig. 1A, top panel, condensation only) is distorted compared to the tonal stimulus. The distortions are also evident in the spectrum of the ongoing response (Fig. 1A, bottom panel), taken from 8–23 ms of the recording period (dotted lines in the top panel). Most of the response is contained in the first and second harmonics, as was true in all cases. The circles indicate the noise criterion level used to judge significance (see Methods). The responses to 250 Hz and 1000 Hz show similar features, with the response at 250 Hz being the largest and the response at 1000 Hz the smallest. The response to 750 Hz is not shown, and above 1000 Hz there were no significant responses in this case.
A major result is the prevalence of auditory responses in the cochlear implant population. Of 84 successful recordings, 80 (95%) had significant responses in the ongoing response to at least one frequency. Of the four cases without such responses, one had radiation for a retrocochlear tumor, one had meningitis, one was deaf due to cytomegalovirus and one is presumably an unknown technical error, since the patient had some hearing on the audiogram. An additional 13 patients had an identified technical problem that prevented successful recording. The most common problems were a sound tube that was inadvertently crimped during draping (n=6) or a surface electrode with high impedance (n=3). Two patients had a difficult anatomy that prevented access to the round window, and two were done in a particular operating room that had an unacceptable level of power line interference.
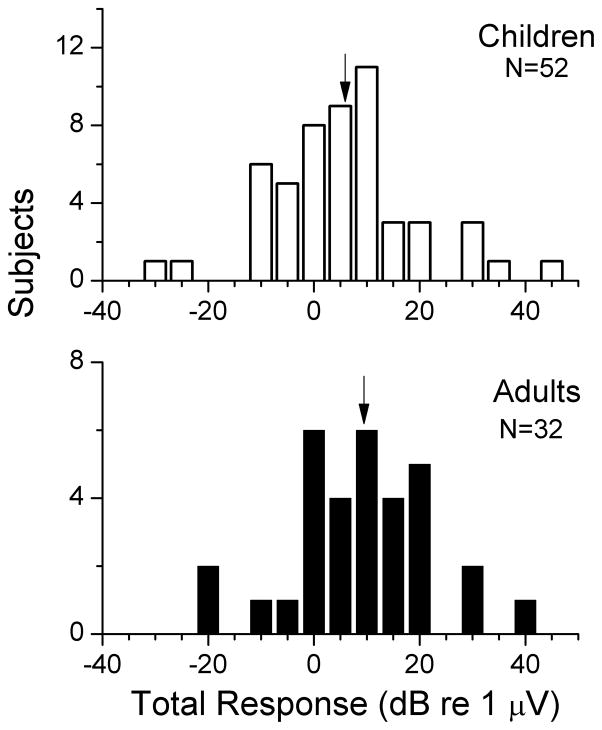
The metric used to measure the magnitude of the response from each cochlea was called the ‘total response,’ which was defined as the sum of first and second harmonics across all frequencies where the response was significant. The distributions of the total response for children and adults are shown in figure 2A. The subjects with no significant response are plotted at −20 dB (0.1 μV), which was near the noise level. Both groups have a similarly wide distribution, covering 64 dB (up to 158 μV) in children and 59 dB (up to 91 μV dB) in adults. The arrows indicate the mean of each population, which were not statistically different between children and adults (t-test, t=1.2, df=66.7, p=0.23).
Figure 2.
Distribution of total response magnitudes for pediatric and adult cochlear implant recipients. The total response is defined as the sum of first and second harmonics across all frequencies where the response was significant. Arrows are the mean for each sample.
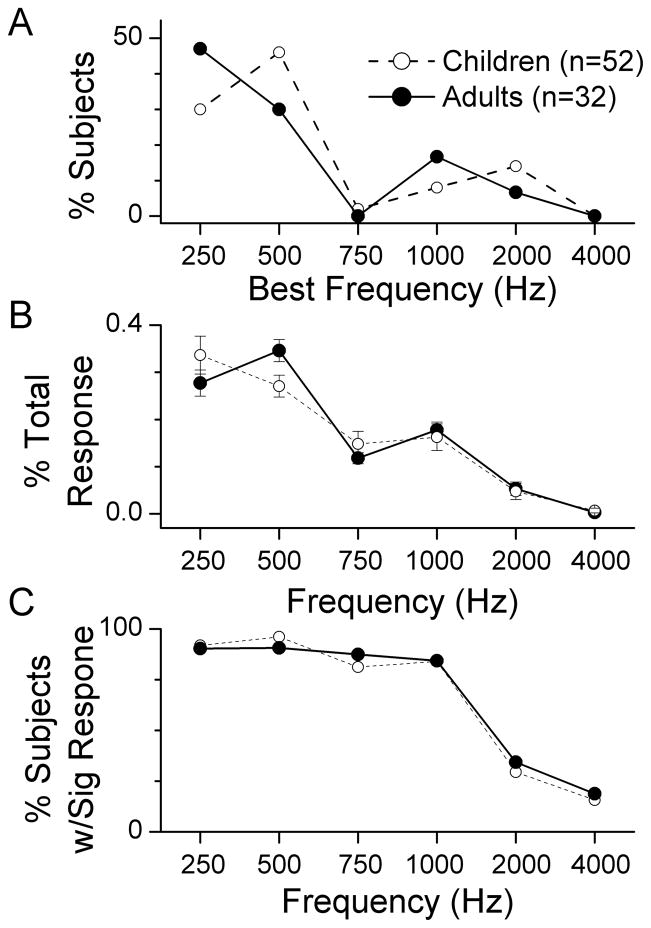
An additional similarity between adults and children was the frequency distribution of the responses. The best frequency, defined as the frequency that contributed the most to the total response, was most commonly to a low frequency (Fig. 3A). For both adults and children, 250 and 500 Hz accounted for more than 75% of the best frequencies. The same frequencies dominated in terms of the magnitudes that each frequency contributed to the total response (Fig. 3B). Described in a third way, more than 90% of cases had a significant response to each frequency from 250–1000 Hz, while a much lower proportion of cases had significant responses to 2000 and 4000 Hz (Fig. 3C). For each metric, the frequency response was similar for adults and children. A 2-way ANOVA for the data in each panel showed a significant effect of frequency (p’s <0.01) but no significant effect of group (adult or pediatric, p’s>0.5).
Figure 3.
Responses according to frequency. A. Distribution of best frequencies for children and adults. The best frequency was defined as the frequency that contributed the most to the total response. B. The proportion of the total response contributed by each frequency. Data points represent the mean and error bars are the standard error. C. Proportion of significant responses to each frequency. Note that for all cases the responses across frequency are similar for children and adults.
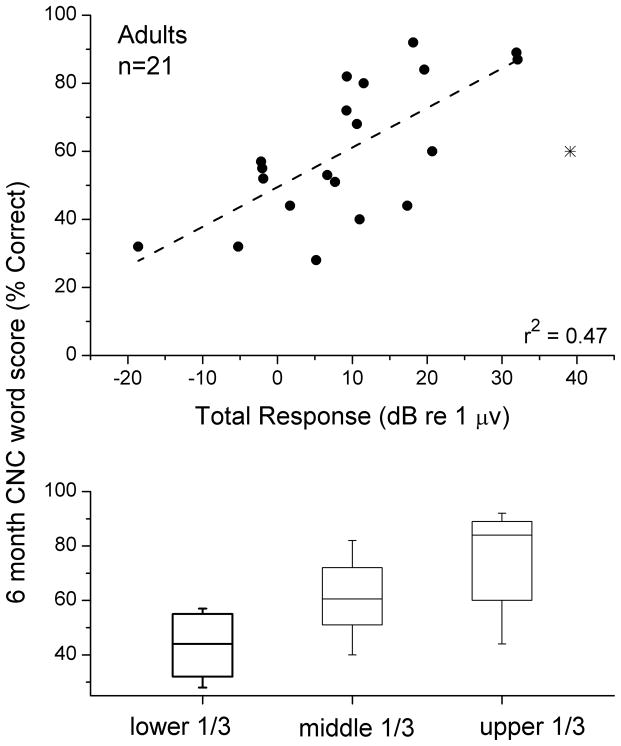
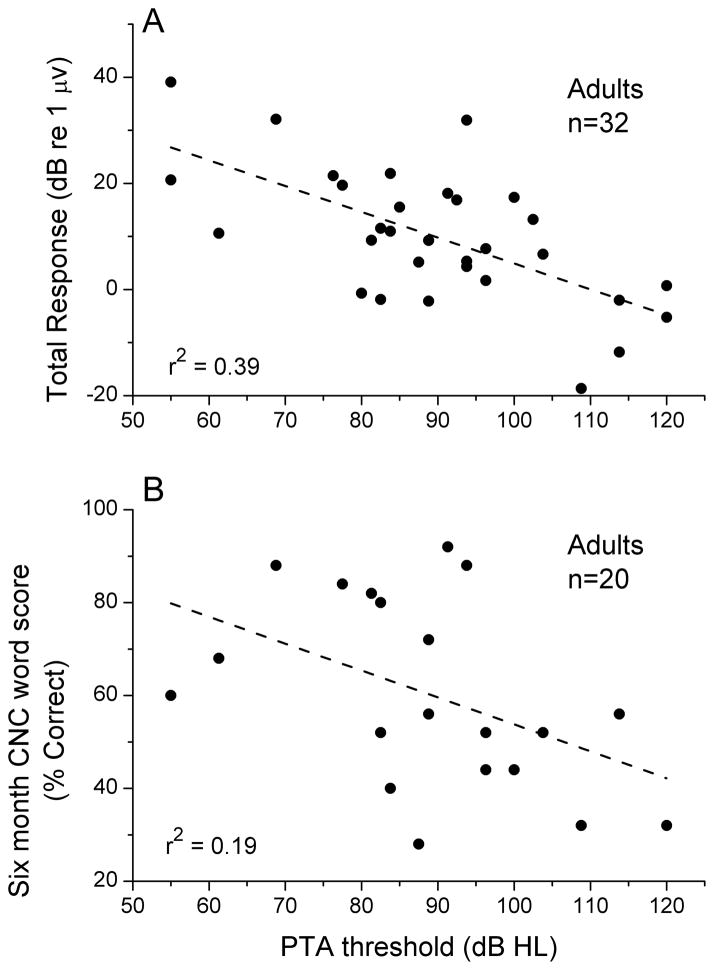
Because of the wide distribution of the total response measure across subjects, a reasonable hypothesis is that those patients with considerable residual cochlear function will have better cochlear implant outcomes compared to those with less residual cochlear function. The relationship between the total response and performance on a test of CNC words in the adult population is shown in Figure 4A. One subject for which six-month scores were available had a non-significant response at all frequencies, and all of these were summed to produce a small but non-zero total power (~1 nV). The regression line had a correlation (r) of 0.682, accounting for 47% (r2) of the variance in outcomes. The slope showed a 1.7 percentage point increase in the word recognition score for every 1 dB increase in total response. This slope was significantly different from zero (t-test, t=4.1, df=18, p<0.001, power=0.87). One subject was removed from the comparison as an outlier (asterisk). This subject was the only one of our adult sample diagnosed with auditory neuropathy spectrum disorder. In this subject, the cochlear response to a 500 Hz tone showed virtually no distortion and no CAP (Fig. 5), as was also true at other frequencies. Both of these features are consistent with the relative lack of neural compared to hair cell responses.
Figure 4.
Comparison of the ECoG magnitude (total response) with scores on the CNC words test. A. Scatterplot of results. The case with an asterisk is an outlier that was not included in the calculation of the line (see text and Fig. 5 for further consideration of this case). B. Box and whisker plot of the results for the sample divided into thirds.
Figure 5.
An outlier case where the total response magnitude was large but the subject’s speech perception performance was less than expected (see asterisk in Figure 4). The stimulus was 500 Hz at 90 dB nHL. The small amount of harmonic distortion indicates that the ongoing response was primarily a cochlear microphonic with no contribution from neural phase-locking. Note also the lack of a compound action potential. This pattern of results suggests a limited neural response.
A comparison of the sample divided into three groups also showed a significant effect of total response on word score outcomes (Fig. 4B). A one-way ANOVA of this data showed a significant effect of group (lower 1/3, middle 1/3, and upper 1/3 of the total response, f=8.5, p=0.003). Individually, the comparison between the lower and upper 1/3 was significant (t-test, p=0.002), and the difference between the lower and middle 1/3 was also significant, although barely (p=0.045) and that between the middle and upper 1/3 was not significant (p=0.12).
A correlation might be expected between the total response from the ECoG and the degree of residual hearing, i.e., those with the greatest physiological response might have the best hearing from the cochlea recorded. A variety of measures of residual hearing were tested, including the threshold to each frequency, and averages of thresholds for just the low frequencies (250–1000 Hz) or the traditional pure tone average to all frequencies (PTA). The highest correlation was with the PTA (Fig. 6A, r=0.62, t=4.47, df=29, p<0.001, power=0.95). However, the correlation with the total response (39% of variance accounted for) did not translate to comparable prediction of the CNC word scores (Fig. 6B, 19% of variance accounted for) which was significant but with little power (t=2.21, df=18, p=0.040, power =0.10).
Figure 6.
Comparisons with residual hearing. A. Pure tone average vs. total response. There was a moderate correlation between these factors. B. Pure tone average vs. CNC word score. There was a small but significant correlation between these factors.
A further correlation might be expected with duration of deafness. This was not the case. The percent of variance accounted for by this variable was <1% (t-test for slope, r=0.02, t=0.078, df=27, p=0.93). Duration of deafness also accounted for less than 4% of the variance in CNC word scores (t-test for slope, r=0.19, t=097, df=16, p=0.35).
DISCUSSION
The main results were 1) responses to auditory stimuli were recorded in 95% all cochlear implant recipients, 2) the magnitude of residual cochlear function varied by ~60 dB across subjects, 3) the range and distribution of response magnitudes was similar in adults and children, and 4) the responsiveness to acoustic stimuli in the cochlea of adults accounted for nearly half the variance in speech outcomes.
Prevalence of responses to auditory stimuli
In our recent study from the first subjects in this series, 23 of 25 subjects with successful acoustic stimulation and recording had significant ECoG potentials to at least one frequency (22). In this study the numbers have increased to 80 of 84. Other recent studies have found similar results, although with smaller numbers of subjects (26,27). Thus, ECoG can provide information about the cochlear state at the time of implantation, from virtually every cochlear implant recipient, even those with no residual hearing (PTA > 100 dB).
Two of the four cases with no responses were described in the set of 25 published previously (22). One that is new to the data set was a one-year old child with meningitis. Fibrous tissue growth that destroys cochlear tissue can be rapid with meningitis, so a negative ECoG is not unexpected. Recently, it has been reported that some patients with meningitis produce ECoGs (28), but these are apparently artifacts because with the same set-up similar results were obtained from saline-soaked drapes (29). It is not yet clear what the source of the artifact might be in those recordings, but in our recordings clamping of the sound tube virtually eliminated all responses. The fourth subject with no ECoG was a 1 year old female with CMV. Three other children with CMV had small or moderate (~1 μV) total response. One of the future benefits of additional recordings will be a population characterization of cochlear responses for different etiologies. This type of analysis could further contribute to understanding variability in cochlear implant outcomes.
The range of magnitudes of ECoG responses across the implant population is very wide
The range of ECoG magnitudes, measured as the “total response” spanned 59 dB for adults, and 64 dB for children. The distributions for the two populations were similar. This wide range shows that candidates for cochlear implants come with widely varying extents of residual cochlear function.
The measure of total response was the sum of the first and second harmonic magnitudes that were significantly above the noise floor across the frequencies that were tested. These frequencies were predominantly low, and there were no differences in the frequency response in the pediatric and adult samples (Fig. 3). Responses to low frequencies are expected for most cases as this corresponds to the progression of hearing loss with most etiologies.
Correlation between ECoG response magnitude and CNC word scores
A single, easily measured factor obtained prior to electrode insertion accounted for 47% of the variance in speech performance. This proportion is higher than is found for other pre-implantation variables such as duration of deafness or amount of residual hearing (e.g., 4,5,8,9,11,12). In our study, the residual hearing was only weakly correlated with CNC words (19% of variance accounted for), and duration of deafness was entirely uncorrelated (< 1% of variance). This latter result may be due to the fact that the modern cohort of implant patients has a shorter duration of deafness and uses hearing aids in the interim between the onset of hearing loss and the need for a cochlear implant (8). The most likely explanation for the increased information from the ongoing portion of the ECoG compared to the audiogram is that the ECoG includes responses from hair cells as well as nerve fibers. If large enough these are detectable as a CM in an ABR. However, for most patients the small residual hair cell potentials would not be detected, and any disconnection from nerve fibers means the audiogram will not reflect their presence either. It seems paradoxical that a measure of hair cell response would predict performance with the implant, which depends on auditory stimulation of nerve fibers. We suggest that the presence of responding hair cells provides information about the overall health of the cochlea that could support nerve fibers and spiral ganglion cells that have survived to be electrically stimulated. Until recently, it was thought that hair cells were the most sensitive part of the system to noise or most other insults to the cochlea. However, subjects with auditory neuropathy spectrum disorder have clear disorders of transmission between inner hair cells and nerve fibers, and maintain functioning outer hair cells (30,31). In addition, recent experiments in animals show that the part of the system most sensitive to noise trauma is the synapse between hair cells and nerve fibers rather than the hair cells themselves (32,33). As many as 50% of nerve fibers may be lost after moderate noise exposures that cause temporary threshold shift. This loss of synapses is not associated with loss of hair cell function as measured by otoacoustic emissions. The loss of synapses leads to loss of neural dendrites and spiral ganglion cells but this process is extended in time, continuing over months to years in mice and guinea pigs. Similar pathologies are evident in human temporal bone cases (34). A previous study of the EcoG from the round window of implant patients indicated greater hair cell activity than expected from the nerve responses that were apparent in the response (Choudhury et al., 2012b). Thus, it is reasonable to suggest that the information gained from the ongoing portion of the ECoG to tones consists of a mix of hair cell and neural responses to tones different from what is available in an ABR. The relative degree of hair cell survival can reflect the degree of underlying nerve fiber and spiral ganglion population available for electrical stimulation.
Combining the perioperative, round window ECoG with biographical and surgical information should increase the predictability of cochlear implant outcomes. Recently, surgical factors such as the electrode trajectory, number of elements inserted into scala vestibuli, and “wrapping factor,” or the degree to which the electrode is wrapped near the modiolis or the lateral wall, were measured using high-quality tomographic images of electrode placement (3,35). These parameters were shown to be significantly correlated with outcomes (up to ~50% of variance account for). Combined with patient factors such as duration of deafness the correlation reached 81%. Since surgical factors should be uncorrelated with the ECoG magnitude prior to implantation, they represent a significant factor contributing to outcome variability that is left out of our measurements. Similarly, however, the studies accounting for surgical variables have not had information available from the ECoG. Thus, combining both factors may account for a very large fraction of the total variance.
Another factor which in some studies showed a high correlation with speech outcomes was psychophysical testing in response to round window electrical stimulation (6,7). In principle, results from electrical stimulation are a more direct means to measure neural elements available for implant stimulation than the ECoG to auditory stimuli. However, results from other laboratories have been inconsistent enough that promontory or round window stimulation is not in common use. Similarly, CAPs evoked by electrical stimulation once the implant is in place have not produced consistent correlations with outcomes (36,37).
Because of the similarity in ECoG results between pediatric and adult cochlear implant recipients, it can be hoped that the ECoG information will prove useful in predicting outcomes in the pediatric population. Outcome measures are difficult to obtain from very young children, so these will have to wait until reliable data can be collected. In addition, social, behavioral and cognitive factors can affect the ability to obtain speech. Adults with previous speech perception present fewer challenges than in children. However, other factors being equal it might be expected that a child with good total response in the ECoG including significant nerve activity (compound action potentials and neural phase-locking), should perform well with the implant. If not, factors such as mapping and surgical factors should be considered. Thus, in both children and adults, the ECoG results can contribute information not available from other sources that could be used for counseling on likely outcomes and suggesting rehabilitative strategies.
Further Uses of The ECoG signal
Here we used a single, very simple measure of response magnitude across frequency to characterize the cochlear response. As mentioned in the introduction, the ECoG consists of the CM and summating potentials produced from hair cells, and the CAP and ANN produced by auditory nerve responses. This complexity provides a much richer source of analysis of surviving, functional cochlear elements than used here. Because the responses were predominantly to low frequencies, they should contain the ANN as well as the CM. Both of these produce energy at the stimulus frequency and 2nd harmonic distortion due to rectification. Odd harmonics can also be produced when levels are high enough for movement of hair cell stereocilia to be saturated in both directions. It is theoretically possible to separate nerve and hair cell contributions through forward masking (38), however this paradigm is time-consuming and is difficult to apply in the intraoperative setting. In at least one case, however, a lack of harmonic distortion in the signal was a strong indication that the rectified, phase-locked neural component was small or absent (see Fig. 4), leaving only the CM. This type of response should be symptomatic of auditory neuropathy, as was the diagnosis in this case. The word score from this subject was lower than the ECoG magnitude would suggest (which was the largest recorded). Thus, a more complete analysis of the different signals that comprise the ECoG may yield additional information predictive of speech outcomes.
Footnotes
Disclosures:
Douglas C. Fitzpatrick: Contractual research support from Advanced Bionics, Cochlear Corporation, and MED-EL Corporation.
Margaret T. Dillon: Contractual research support from MED-EL Corporation.
Craig A. Buchman: Contractual research support from Cochlear Corporation and MED-EL Corporation; research grant support from the NIH-NIDCD, unpaid consultant for Advanced Bionics, Cochlear Corporation, MED-EL Corporation, and Anspach Corporation.
Oliver F Adunka: Contractual research support from Advanced Bionics, Cochlear Corporation, and MED-EL Corporation.
References
- 1.Firszt JB, Holden LK, Skinner MW, et al. Recognition of speech presented at soft to loud levels by adult cochlear implant recipients of three cochlear implant systems. Ear Hear. 2004;25:375–87. doi: 10.1097/01.aud.0000134552.22205.ee. [DOI] [PubMed] [Google Scholar]
- 2.Cohen NL, Waltzman SB, Fisher SG. Prospective randomized clinical trial of advanced cochlear implants: preliminary results of a Department of Veterans Affairs Cooperative Study. Ann Otol Rhinol Laryngol. 1991;100:823–9. doi: 10.1177/000348949110001007. [DOI] [PubMed] [Google Scholar]
- 3.Holden LK, Finley CC, Firszt JB, et al. Factors Affecting Open-Set Word Recognition in Adults With Cochlear Implants. Ear Hear. 2013 doi: 10.1097/AUD.0b013e3182741aa7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gantz BJ, Woodworth GG, Knutson JF, et al. Multivariate predictors of audiological success with multichannel cochlear implants. Ann Otol Rhinol Laryngol. 1993;102:909–16. doi: 10.1177/000348949310201201. [DOI] [PubMed] [Google Scholar]
- 5.Nadol JB., Jr Patterns of neural degeneration in the human cochlea and auditory nerve: implications for cochlear implantation. Otolaryngol Head Neck Surg. 1997;117:220–8. doi: 10.1016/s0194-5998(97)70178-5. [DOI] [PubMed] [Google Scholar]
- 6.Shipp D, Nedzelski J, Chen J, et al. Prognostic indicators of speech recognition performance in postlinguistically deafened adult cochlear implant users. Adv Otorhinolaryngol. 1997;52:74–7. doi: 10.1159/000059010. [DOI] [PubMed] [Google Scholar]
- 7.Shipp DB, Nedzelski JM. Prognostic indicators of speech recognition performance in adult cochlear implant users: a prospective analysis. Ann Otol Rhinol Laryngol Suppl. 1995;166:194–6. [PubMed] [Google Scholar]
- 8.Lazard DS, Vincent C, Venail F, et al. Pre-, per- and postoperative factors affecting performance of postlinguistically deaf adults using cochlear implants: a new conceptual model over time. PLoS One. 2012;7:e48739. doi: 10.1371/journal.pone.0048739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blamey P, Arndt P, Bergeron F, et al. Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants. Audiol Neurootol. 1996;1:293–306. doi: 10.1159/000259212. [DOI] [PubMed] [Google Scholar]
- 10.Shea JJ, 3rd, Domico EH, Orchik DJ. Speech recognition ability as a function of duration of deafness in multichannel cochlear implant patients. Laryngoscope. 1990;100:223–6. doi: 10.1288/00005537-199003000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Rubinstein JT, Parkinson WS, Tyler RS, et al. Residual speech recognition and cochlear implant performance: effects of implantation criteria. Am J Otolaryngol. 1999;20:445–52. [PubMed] [Google Scholar]
- 12.Friedland DR, Venick HS, Niparko JK. Choice of ear for cochlear implantation: the effect of history and residual hearing on predicted postoperative performance. Otol Neurotol. 2003;24:582–9. doi: 10.1097/00129492-200307000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Blamey P. Are spiral ganglion cell numbers important for speech perception with a cochlear implant? Am J Otolaryngol. 1997;18:S11–2. [PubMed] [Google Scholar]
- 14.Fayad J, Linthicum FH, Jr, Otto SR, et al. Cochlear implants: Histopathologic findings related to performance in 16 human temporal bones. Ann Otol Rhinol Laryngol. 1991;100:807–11. doi: 10.1177/000348949110001004. [DOI] [PubMed] [Google Scholar]
- 15.Fayad JN, Don M, Linthicum FH., Jr Distribution of low-frequency nerve fibers in the auditory nerve: Temporal bone findings and clinical implications. Otol Neurotol. 2006;27:1074–7. doi: 10.1097/01.mao.0000235964.00109.00. [DOI] [PubMed] [Google Scholar]
- 16.Khan AM, Handzel O, Burgess BJ, et al. Is word recognition correlated with the number of surviving spiral ganglion cells and electrode insertion depth in human subjects with cochlear implants? Laryngoscope. 2005;115:672–7. doi: 10.1097/01.mlg.0000161335.62139.80. [DOI] [PubMed] [Google Scholar]
- 17.Nadol JB, Jr, Shiao JY, Burgess BJ, et al. Histopathology of cochlear implants in humans. Ann Otol Rhinol Laryngol. 2001;110:883–91. doi: 10.1177/000348940111000914. [DOI] [PubMed] [Google Scholar]
- 18.Dallos P. The Auditory Periphery: Biophysics and Physiology. New York: Academic Press; 1973. [Google Scholar]
- 19.Snyder RL, Schreiner CE. The auditory neurophonic: basic properties. Hear Res. 1984;15:261–80. doi: 10.1016/0378-5955(84)90033-9. [DOI] [PubMed] [Google Scholar]
- 20.Henry KR. Auditory nerve neurophonic recorded from the round window of the Mongolian gerbil. Hear Res. 1995;90:176–84. doi: 10.1016/0378-5955(95)00162-6. [DOI] [PubMed] [Google Scholar]
- 21.He W, Porsov E, Kemp D, et al. The group delay and suppression pattern of the cochlear microphonic potential recorded at the round window. PLoS One. 2012;7:e34356. doi: 10.1371/journal.pone.0034356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choudhury B, Fitzpatrick DC, Buchman CA, et al. Intraoperative round window recordings to acoustic stimuli from cochlear implant patients. Otol Neurotol. 2012;33:1507–15. doi: 10.1097/MAO.0b013e31826dbc80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peterson GE, Lehiste I. Revised CNC lists for auditory tests. J Speech Hear Disord. 1962;27:62–70. doi: 10.1044/jshd.2701.62. [DOI] [PubMed] [Google Scholar]
- 24.Gifford RH, Shallop JK, Peterson AM. Speech recognition materials and ceiling effects: considerations for cochlear implant programs. Audiol Neurootol. 2008;13:193–205. doi: 10.1159/000113510. [DOI] [PubMed] [Google Scholar]
- 25.Nilsson M, McCaw V, Soli S. Minimum Speech Test Battery for Adult Cochlear Implant Patients: User Manual. Los Angeles, CA: House Ear Institute; 1996. [Google Scholar]
- 26.Radeloff A, Shehata-Dieler W, Scherzed A, et al. Intraoperative monitoring using cochlear microphonics in cochlear implant patients with residual hearing. Otol Neurotol. 2012;33:348–54. doi: 10.1097/MAO.0b013e318248ea86. [DOI] [PubMed] [Google Scholar]
- 27.Mandala M, Colletti L, Tonoli G, et al. Electrocochleography during cochlear implantation for hearing preservation. Otolaryngol Head Neck Surg. 2012;146:774–81. doi: 10.1177/0194599811435895. [DOI] [PubMed] [Google Scholar]
- 28.Teschner M, Lenarz T, Battmer RD. The influence of post-meningitic obliteration and ossification of the cochlea on cochlear microphonics. Eur Arch Otorhinolaryngol. 2010;267:1547–50. doi: 10.1007/s00405-010-1294-z. [DOI] [PubMed] [Google Scholar]
- 29.Teschner M, Lenarz T, Battmer RD. Validity of cochlear microphonics at high sound pressure levels as an important clinical aspect. ORL J Otorhinolaryngol Relat Spec. 2012;74:38–41. doi: 10.1159/000334948. [DOI] [PubMed] [Google Scholar]
- 30.Starr A, Picton TW, Sininger Y, et al. Auditory neuropathy. Brain. 1996;119(Pt 3):741–53. doi: 10.1093/brain/119.3.741. [DOI] [PubMed] [Google Scholar]
- 31.Zeng FG, Oba S, Garde S, et al. Temporal and speech processing deficits in auditory neuropathy. Neuroreport. 1999;10:3429–35. doi: 10.1097/00001756-199911080-00031. [DOI] [PubMed] [Google Scholar]
- 32.Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29:14077–85. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin HW, Furman AC, Kujawa SG, et al. Primary neural degeneration in the Guinea pig cochlea after reversible noise-induced threshold shift. J Assoc Res Otolaryngol. 2011;12:605–16. doi: 10.1007/s10162-011-0277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Makary CA, Shin J, Kujawa SG, et al. Age-Related Primary Cochlear Neuronal Degeneration in Human Temporal Bones. J Assoc Res Otolaryngol. 2011 doi: 10.1007/s10162-011-0283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Finley CC, Holden TA, Holden LK, et al. Role of electrode placement as a contributor to variability in cochlear implant outcomes. Otol Neurotol. 2008;29:920–8. doi: 10.1097/MAO.0b013e318184f492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cosetti MK, Shapiro WH, Green JE, et al. Intraoperative neural response telemetry as a predictor of performance. Otol Neurotol. 2010;31:1095–9. doi: 10.1097/MAO.0b013e3181ec1b8c. [DOI] [PubMed] [Google Scholar]
- 37.McKay CM, Fewster L, Dawson P. A different approach to using neural response telemetry for automated cochlear implant processor programming. Ear Hear. 2005;26:38S–44S. doi: 10.1097/00003446-200508001-00006. [DOI] [PubMed] [Google Scholar]
- 38.Chimento TC, Schreiner CE. Selectively eliminating cochlear microphonic contamination from the frequency-following response. Electroencephalogr Clin Neurophysiol. 1990;75:88–96. doi: 10.1016/0013-4694(90)90156-e. [DOI] [PubMed] [Google Scholar]