Abstract
How sleep helps learning and memory remains unknown. We report in mouse motor cortex that sleep after motor learning promotes the formation of postsynaptic dendritic spines on a subset of branches of individual layer V pyramidal neurons. New spines are formed on different sets of dendritic branches in response to different learning tasks and are protected from being eliminated when multiple tasks are learned. Neurons activated during learning of a motor task are reactivated during subsequent non-rapid eye movement sleep, and disrupting this neuronal reactivation prevents branch-specific spine formation. These findings indicate that sleep has a key role in promoting learning-dependent synapse formation and maintenance on selected dendritic branches, which contribute to memory storage.
Sleep has an important role in learning and memory consolidation (1–5). During sleep, neurons involved in wakeful experiences are reactivated in multiple brain regions (6–12), and neuronal networks exhibit various patterns of rhythmic activity (13, 14). Given the crucial function of neuronal activity in synaptic plasticity, sleep likely modulates synaptic connections that are important for long-term memory formation (15–18). Nevertheless, the role of sleep in experience-dependent changes of synaptic connections remains controversial (19–22). Overall synaptic strength and numerous synaptic proteins are up-regulated during wakefulness and down-regulated during slow-wave sleep (23, 24). A net loss of synapses is found during sleep in the developing mouse cortex (25, 26) and in the invertebrate nervous system (27, 28). These observations support the hypothesis that sleep is important for the downscaling of synaptic connectivity that has been potentiated during wakefulness (29). However, ocular dominance plasticity and cortical-evoked local field potential increase rather than decrease after a slow-wave sleep episode (30, 31). The expression of several proteins required for synaptic plasticity increases during the early hours of sleep (32, 33). Furthermore, the number of synapses increases during early development when animals sleep the most (34, 35). Together, these studies support the opposing view that sleep promotes, rather than down-regulates, synaptic plasticity related to learning and memory.
We examined how sleep affects the remodeling of postsynaptic dendritic spines induced by motor learning in the mouse primary motor cortex. Rotarod motor learning increases dendritic spine formation on apical tuft dendrites of layer V pyramidal neurons in the motor cortex within 2 days (18, 36). To investigate whether sleep is involved in this process, we first determined the time course of spine remodeling in mice that were trained to run forward on an accelerated rotating rod. Yellow fluorescent protein (YFP)–labeled dendrites in the hind limb region of the motor cortex were imaged in awake head-restrained mice before and in the hours after training with transcranial two-photon microscopy (18, 37). The formation rate of new spines in trained mice was significantly higher within 6 hours after training and continued to increase within the first day when compared to that in untrained controls (P < 0.05) (Fig. 1, A and B). In contrast, rotarod training had no significant effect on the elimination rate of existing spines within 6 to 48 hours (Fig. 1C).
Fig. 1. Motor learning induces branch-specific spine formation.
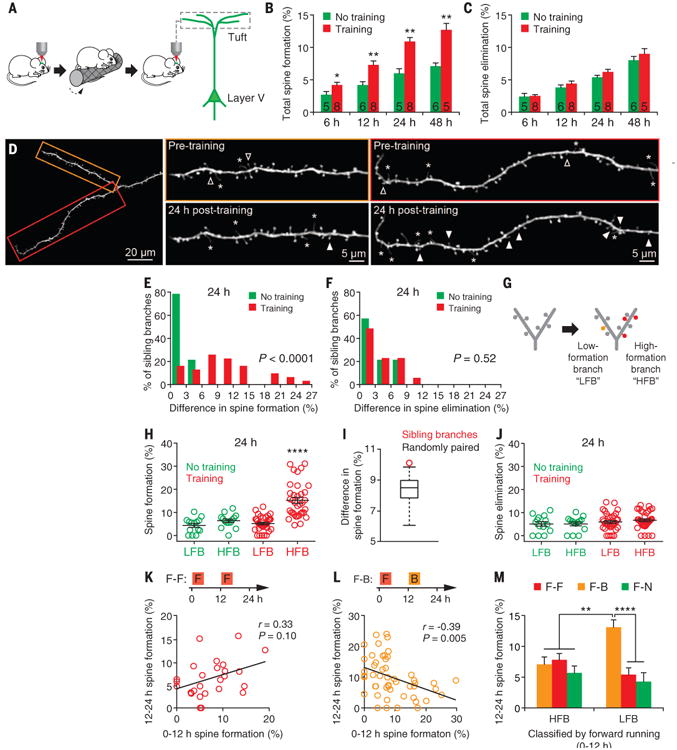
(A) Transcranial two-photon imaging in the primary motor cortex of awake, head-restrained mice before and after rotarod motor training. (B and C) The percentage of dendritic spines formed (B) and eliminated (C) over time after one session of rotarod training (20 trials). Motor training progressively increased new spine formation over the course of 6 to 48 hours. No significant difference in the rate of spine elimination was observed within 48 hours after training. The number of animals is indicated on each column. (D) An example of two sibling apical tuft branches with different degrees of spine formation 24 hours after training. Filled arrowheads indicate newly formed dendritic spines and open ones indicate eliminated spines over a 24-hour interval. Asterisks indicate dendritic filopodia. (E) Motor training–induced spine formation was significantly different between sibling branches (15 trained mice and 8 control mice). (F) No significant difference in spine elimination between sibling branches. (G) Classification of sibling dendritic branches to HFBs and LFBs on the basis of the spine formation rate relative to each other. (H) Motor training significantly increased the rate of spine formation on HFBs 24 hours after training. (I) The average of measured difference in spine formation between HFBs and LFBs was statistically larger (P < 0.0001) for sibling branches (red circle) than for randomly paired branches (box plot of results from 100 simulations of random pairing). The simulation was performed to test the null hypothesis that learning-induced spine changes are distributed randomly across all branches. (J) There was no significant difference in spine elimination between HFBs and LFBs 24 hours after training. (K and L) Mice were first trained to run forward on an accelerating rotarod and, 12 hours later, to run either forward (F-F) or backward (F-B). Correlation of spine formation rate on individual branches between 0–12 hours and 12–24 hours. The correlation was positive when animals were subjected to the same forward training [(K) n = 6 mice] and negative when the animals were trained with a backward running task [(L) n = 8 mice]. (M) Experimental designs are shown in (K) and (L). Sibling branches were classified as HFBs and LFBs on the basis of the degree of spine formation induced by the initial forward training from 0 to 12 hours. There is a significant increase in spine formation on LFBs than on HFBs after backward training, not after forward running or no training, from 12 to 24 hours. Data are presented as means ± SEM. *P < 0.05. **P < 0.01. ****P < 0.0001, nonparametric test.
We observed that, 24 hours after motor training, only a fraction (∼30%) of apical tuft branches (average branch length: 62.7 ± 1.3 μm) in trained mice showed a higher rate of spine formation than the branches in untrained mice (Fig. 1D and fig. S1). When spine formation on two sibling branches sharing the same parent branch was compared, the difference in spine formation, but not spine elimination, between sibling branches was also significantly larger in trained mice than in untrained controls (Fig. 1, D to F) (P < 0.0001 for spine formation; P = 0.52 for spine elimination) (fig. S2). To investigate this branch-specific spine formation further, we classified the sibling branch with higher spine formation as a “high-formation branch” (HFB) and the other as a “low-formation branch” (LFB) (Fig. 1G). Twenty-four hours after training, the average rate of spine formation on HFBs in trained mice (15.3 ± 1.3%) was 2.4 to 3.5 times that of HFBs (6.4 ± 0.8%) or LFBs (4.4 ± 0.9%) in untrained control mice (P < 0.0001) (Fig. 1H). The difference in spine formation between HFBs and LFBs was statistically larger for sibling branches than for randomly paired branches (P < 0.0001) (Fig. 1I). However, spine formation on LFBs in trained mice (5.2 ± 0.5%) was not significantly different from that on either HFBs (P = 0.19) or LFBs (P = 0.49) in untrained controls. There was also no significant difference in spine elimination between HFBs and LFBs in both trained (P = 0.15) and untrained animals (P > 0.9) (Fig. 1J).
Different motor learning tasks often activate the same neurons in the motor cortex (38). We wondered whether different learning tasks lead to spine formation on the same or different dendritic branches. To address this question, we trained mice to run forward and, 12 hours later, to run either forward or backward (Fig. 1, K and L). When mice were subjected to the second session of forward running 12 hours after the initial forward-running session, new spines formed during 0 to 12 hours and 12 to 24 hours tended to occur on the same set of branches, although the effect was not statistically significant (Fig. 1K). In contrast, running backwards induced spine formation on a set of branches that showed little formation of new spines in response to the previous forward running (Fig. 1L). Furthermore, when sibling branches were classified as HFBs and LFBs based on the degree of spine formation induced by the initial forward training, we found that backward running, not forward running or no training, induced spine formation mainly on the LFBs but not on the HFBs during the second 12 hours (Fig. 1M).
Our results thus far have revealed task- and branch-specific spine formation over the course of 24 hours after motor skill learning. To test a potential role of sleep in this process, we examined spine formation in mice that were subjected to rotarod training (one 40-trial session of forward running, ∼1 hour) and then sleep deprived (SD) for 7 hours by gentle handling (Fig. 2A). Electro-encephalography (EEG) monitoring over 7 hours showed that SD mice were awake 97.0 ± 2.1% of the time, whereas mice with undisturbed sleep (non-SD) were awake only 26.4 ± 2.9% of the time (P < 0.05) (Fig. 2, B and C). There was a significant reduction in learning-induced spine formation over the entire 8 hours in SD mice when compared to non-SD mice (Fig. 2D). Sleep deprivation specifically reduced spine formation on HFBs (4.9 ± 0.7% versus 9.3 ± 0.7%; P < 0.0001), but not on LFBs (2.4 ± 0.4% versus 1.8 ± 0.4%; P = 0.16). To investigate whether the effect of sleep deprivation on spine formation might be stress-related, we administered the stress hormone corticosterone (2.5 mg/kg) to non-SD mice after motor training (fig. S3). Corticosterone administration had no significant effects on spine formation on either HFBs or LFBs in the course of 8 hours (Fig. 2D), which suggested that the elevation of stress hormones associated with sleep deprivation is not important for the reduction in spine formation after learning.
Fig. 2. Postlearning sleep promotes branch-specific spine formation.

(A) Schematic of experimental paradigm. After imaging and training (40 trials per session), the animals were either subjected to sleep deprivation or left undisturbed to assess the effect of sleep deprivation. (B) Examples of the EEG and EMG traces. (C) Sleep structure in undisturbed control and SD animals. (D) Percentage of spine formation on the sibling branches over the course of 8 hours under various conditions. Sleep deprivation significantly reduced the rate of spine formation on HFBs, but not LFBs, after training. Corticosterone injection (2.5 mg/kg; n = 4 mice) into non-SD mice had no significant effect on spine formation on HFBs or LFBs during 8 hours. Spine formation on HFBs was significantly higher in SD mice with intensive training (5 mice) than with regular training (9 mice) or no training (4 mice), but significantly lower than that in non-SD mice with regular training (9 mice). (E) Over the course of 16 hours after sleep deprivation, new spine formation on HFBs or LFBs was significantly lower in SD mice than in non-SD mice. Data are presented as means ± SEM. *P < 0.05. **P < 0.01. ***P < 0.001. ****P < 0.0001, nonparametric test.
To better understand the importance of sleep in dendritic spine formation, we tested whether the reduced spine formation after sleep deprivation could be compensated for by additional training. Although spine formation on HFBs was significantly higher with intensive training (two 40-trial sessions) than with regular training (one 40-trial session) or no training in SD mice (P < 0.05) (Fig. 2, A and D), it remained significantly lower than in non-SD mice with regular training (P < 0.05). There was no significant difference in spine formation on LFBs among all five groups [P = 0.35, one-way analysis of variance (ANOVA)] (Fig. 2D). We also tested whether the reduction in spine formation could be compensated for by subsequent sleep by allowing animals to sleep during the next 16 hours after the initial 7-hour sleep deprivation (Fig. 2A). Over the subsequent 16 hours, the rate of spine formation on either HFBs or LFBs was found to be significantly lower in SD mice than non-SD mice (P < 0.05) (Fig. 2E and fig. S4). Thus, the reduction in spine formation after the 7-hour sleep deprivation could not be rescued by either an additional training session or subsequent sleep.
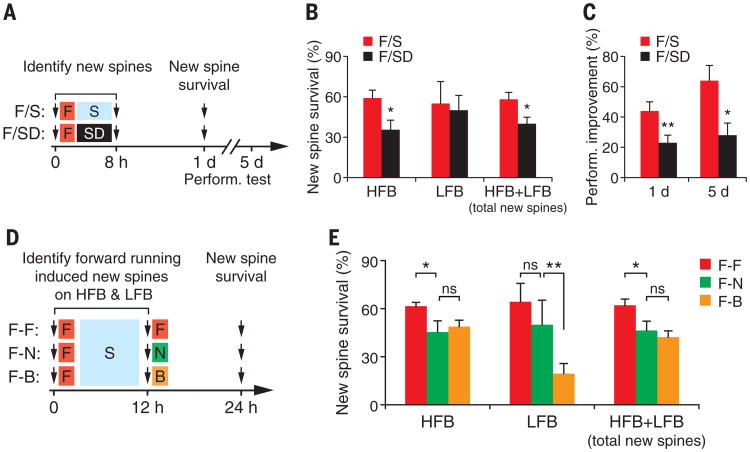
A fraction of learning-induced new spines persists over time, and the number of persisting new spines correlates with long-term retention of motor skills (18, 36). We followed the fate of all new spines that were formed during 8 hours with or without posttraining sleep (Fig. 3A). The survival of new spines on HFBs was significantly higher during the next day in mice with sleep after learning than without (P < 0.05) (Fig. 3B). In contrast, the survival of new spines on LFBs was not significantly different between mice with and without sleep (P = 0.97) (Fig. 3B). The performance improvement in mice with post-training sleep, when tested 1 or 5 days after the initial training, was significantly larger when compared to that of SD mice (P < 0.05) (Fig. 3C and fig. S5). These results suggest that sleep contributes significantly to the formation of persistent new spines on HFBs, as well as motor skill retention.
Fig. 3. New spines formed during postlearning sleep persist.
(A) Schematic of experimental paradigm. (B) More new spines formed on HFBs during hours 0 to 8 persist at 24 hours in non-SD mice (n = 7) than in SD mice (n = 8). (C) Performance improvement is significantly larger in non-SD mice than in SD mice 1 or 5 days after training. (D) New spines formed within 12 hours after forward running were followed over the next 12 hours when the animals were either not trained (n = 5), trained again with the same task (n = 6), or trained with a new task (backward running) (n = 8). (E) Continued training with the same forward-running task facilitates the maintenance of new spines that are formed previously on HFBs. Training with a different task (backward running) significantly reduced the survival of new spines that are formed on LFBs. Data are presented as means ± SEM. *P < 0.05. **P < 0.01, nonparametric test.
Previous studies have shown that the survival of new spines is modulated by subsequent experiences (18, 36). To better understand the persistence of new spines formed during post- learning sleep, we examined how new spines induced by forward running are affected by subsequent motor learning experiences (Fig. 3D). The survival rate of new spines on HFBs was significantly higher when animals were trained again with the forward-running task than when animals were not trained or were subjected to backward running (Fig. 3E). Notably, the survival rate of new spines on LFBs was significantly lower in mice subjected to backward running when compared with mice subjected to either forward training or no training (P < 0.01) (Fig. 3, D and E). This reduction in new dendritic spine survival on LFBs could be related to the fact that backward training tended to promote new spine formation on LFBs (Fig. 1M). Because the majority (78%) of total new spines were formed on HFBs after forward running, the persistence of the total new spines induced by forward running was not significantly affected after backward running (Fig. 3E). The persistence of new spines formed during postlearning sleep may underlie a well-known feature of motor skill learning that, once a skill is learned, it persists for long periods of time with minimum interference by other learning tasks.
How does sleep promote branch-specific spine formation after learning? Sleep consists of two basic states, rapid eye movement (REM) sleep and non-REM (NREM) sleep. To explore the mechanisms underlying sleep-dependent spine formation, we first examined whether REM sleep is required for spine formation after rotarod learning. Mice were subjected to rotarod training (40 trials, ∼1 hour) and deprived of REM sleep (REMD) for 7 hours (Fig. 4A). REM sleep was monitored continuously by EEG and electromyography (EMG) recordings and disrupted by gentle touching upon detection. EEG and EMG monitoring in the course of 7 hours showed that REM sleep in REMD mice was significantly reduced when compared to control mice (6.9 ± 1.1 min versus 32.1 ± 4.0 min; P < 0.01) (Figs. 2C and 4A). REM deprivation during 7 hours did not disrupt branch-specific spine formation induced by learning (Fig. 4B). Similar to mice with undisturbed sleep, spine formation during 8 hours after training was ∼3.1 times as much on HFBs as on LFBs in REMD mice.
Fig. 4. Branch-specific spine formation involves neuronal reactivation during NREM sleep.

(A) Mice were deprived of REM sleep (REMD) over the course of 7 hours after rotarod training. (B) Learning-induced branch-specific spine formation was not affected by REMD (n = 5 mice). (C) Two-photon calcium imaging of layer V neurons from mice expressing GCaMP6 during quiet awake state, prerunning NREM sleep, running, and postrunning NREM sleep. Red arrow points to a soma activated during forward running, and blue arrow points to the same soma reactivated during NREM sleep. (D) Calcium fluorescence traces of three neurons under various states. Examples of 5-min traces are shown. (E) Frequency distribution of cells with somatic Ca2+ change during forward running (617 cells, 17 mice). About 41% of cells show a large increase (>50%) of Ca2+ level in somata during forward running (>1.5 relative to the quiet awake state). (F) Cells (either inactive or active during prerunning sleep) show a large increase (>50%) in somatic Ca2+ level both during running and during postrunning NREM sleep. MK801 administration after running reduced somatic Ca2+ level during NREM sleep. (G) Experimental design to reduce reactivation of forward-running neurons during sleep. Three groups of mice were trained to run forward and allowed to sleep for 4 hours. Subsequently, each group was either subjected to no training (F-N) or trained to run backward (F-B) or forward (F-F), then allowed to sleep for another 4 hours. Reactivation of forward running-specific cells (ΔFforw. running/ΔFquiet > 1.5 and ΔFbackw. running/ΔFquiet < 1.5) was significantly reduced during the second 4-hour sleep after mice were trained with a backward-running task (F-B). (H) Experimental design is the same as in (G). The rate of spine formation on HFBs was significantly reduced either after MK801 administration or in the F-B group as compared to the F-F or F-N group. Data are presented as means ± SEM. *P < 0.05. **P < 0.01. ***P < 0.001. ****P < 0.0001, non-parametric test.
Neurons associated with wakeful experience are reactivated in multiple brain regions during subsequent NREM sleep, and this sleep reactivation occurs after the prior wakeful experience (6–11). Because neuronal activity is critical for regulating synaptic plasticity, neuronal reactivation during NREM sleep could be involved in promoting spine formation. We therefore examined whether motor task-related neurons are reactivated in the primary motor cortex during NREM sleep by performing calcium imaging of layer V pyramidal neurons expressing the genetically encoded calcium indicator GCaMP6 (39) (Fig. 4, C and D) (see methods). In this experiment, head-restrained mice were trained to run on a custom-built treadmill under a two-photon microscope. We found that, similar to rotarod motor learning, forward and backward running on the treadmill induced branch-specific spine formation in the course of 8 hours (fig. S6). Many layer V pyramidal neurons showed increased activity, as indicated by elevated levels of Ca2+ in cell somata, during forward running on the treadmill as compared to a state of quiet wakefulness (Fig. 4E). Over the 5-min recording period, ∼41% (250 out of 617) of neurons showed a large increase (>50%) in somatic Ca2+ level (ΔFrunning/ΔFquiet > 1.5) and ∼39% (242 out of 617) of neurons showed no or moderate increase (ΔFrunning/ΔFquiet = 1.0–1.5). When the same neurons were followed over the next 8 hours, neurons with >50% increase in somatic Ca2+ during running (ΔFrunning/ΔFquiet > 1.5, defined as task-related neurons) also showed higher levels of somatic Ca2+ during NREM sleep when compared to that under the quiet awake state (P < 0.0001) (Fig. 4F). To rule out the possibility that certain neurons active during postrunning sleep were not task-related, we removed neurons highly active during prerunning sleep from the analysis of sleep reactivation during postrunning sleep (fig. S7). We found that neurons highly activated during forward running but not during prerunning sleep (ΔFrunning/ΔFquiet > 15; ΔFprerun sleep/ΔFquiet < 15) were reactivated during the postrunning sleep episode (Fig. 4F). In contrast, neurons with no or moderate increase (<50%) in somatic Ca2+ level during running did not show a significant increase of Ca2+ activity during NREM sleep. These observations are consistent with previous electrophysiological studies of sleep replay in several brain regions (6–11) and suggest that neuronal reactivation of prior motor experience also occurs in the motor cortex during extended periods of time (>4 hours).
To test whether neuronal reactivation might be involved in branch-specific spine formation, we first blocked N-methyl-d-aspartate (NMDA) receptors with MK801 and examined branch-specific spine formation. MK801 (0.25 mg/kg) injection after training significantly reduced the activity of forward running-related neurons during NREM sleep within 8 hours after training (P < 0.001) (Fig. 4F). MK801 administration also blocked branch-specific spine formation after training (P < 0.0001) (Fig. 4H).
MK801 not only reduces neuronal activity during sleep but also alters the animals' locomotion behavior in the first few hours after drug administration (40). Therefore, the effect of MK801 on spine formation may not be specifically related to altered neuronal activity during sleep. To manipulate the extent of neuronal reactivation more specifically, we took advantage of the findings that sleep reactivation is related to prior wakeful experience. We trained mice to run forward and allowed them to sleep for 4 hours. Subsequently, mice either received no further training (F-N) or were trained to run forward (F-F) or backward (F-B) (Fig. 4G). During the second 4-hour sleep period, the reactivation of neurons specific to forward running in the F-B group was significantly reduced when compared to neurons specific to backward-running or neurons activated during both forward and backward running in the same F-B group (P < 0.01) (Fig. 4G). The reactivation of neurons specific to forward running in the F-B group was also significantly less than neurons activated during forward running in the F-F and F-N groups (P < 0.01) (Fig. 4G). Notably, when spine formation on sibling branches was examined over the course of 8 hours, the rate of spine formation on HFBs was significantly reduced in the F-B group when compared to the F-F or F-N group (P < 0.05) (Fig. 4H). The ratio of spine formation rates between HFBs and LFBs was 1.8 in the F-B group, substantially lower than 3.5 and 5.6 in the F-F and F-N groups, respectively. Because all three experimental groups experienced a similar amount of sleep but differed in the extent of neuronal reactivation associated with forward training, these results provide further evidence for the role of sleep reactivation in branch-specific spine formation.
Sleep is widely believed to be important for memory consolidation, but the underlying processes remain elusive. There are conflicting views as to whether non-REM sleep contributes to memory consolidation by either promoting or down-regulating synaptic plasticity (19–22, 29). By directly imaging postsynaptic dendritic spines over time in the mouse cortex, our results indicate that sleep after learning promotes new spine formation on different sets of apical tuft branches of individual layer V pyramidal neurons. Furthermore, this sleep-dependent, branch-specific spine formation facilitates new spine survival when animals learn different tasks. These findings suggest that sleep promotes learning-induced synapse formation to aid long-term memory storage.
Different motor learning tasks cause spine formation on different sets of dendritic branches. Furthermore, additional training without sleep could promote branch-specific formation (Fig. 2D). Thus, it appears that which set of dendritic branches forms new spines is determined by specific features (input or activity patterns) of a learning task, rather than by sleep. Our data suggest that reactivation of task-specific neurons during NREM sleep is involved in forming new synapses after learning, although definitive proof that reactivation causes synaptic formation would require simultaneous imaging of both reactivation and synapses in the same neurons over time. Neuronal reactivation during sleep may promote branch-specific spine formation in a manner similar to awake learning experiences (Fig. 2D), and its effectiveness in promoting spine formation may vary at different times of the day (fig. S8). Sleep reactivation could also allow the expression of specific genes critical for the growth of new synaptic connections (32, 33). Future studies are needed to address these questions in order to better understand how sleep contributes to memory storage in the brain.
Supplementary Material
Acknowledgments
We thank all the members in the Gan laboratory and T. Franke for comments on the manuscript. This work was supported by NIH R01 NS047325 and P01 NS074972 to W.-B.G. and by a Whitehall Foundation research grant and an American Federation for Aging Research grant to G.Y.
Footnotes
References and Notes
- 1.Maquet P. Science. 2001;294:1048–1052. doi: 10.1126/science.1062856. [DOI] [PubMed] [Google Scholar]
- 2.Siegel JM. Nature. 2005;437:1264–1271. doi: 10.1038/nature04285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stickgold R. Nature. 2005;437:1272–1278. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- 4.Diekelmann S, Born J. Nat Rev Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 5.Benington JH, Frank MG. Prog Neurobiol. 2003;69:71–101. doi: 10.1016/s0301-0082(03)00018-2. [DOI] [PubMed] [Google Scholar]
- 6.Pavlides C, Winson J. J Neurosci. 1989;9:2907–2918. doi: 10.1523/JNEUROSCI.09-08-02907.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skaggs WE, McNaughton BL. Science. 1996;271:1870–1873. doi: 10.1126/science.271.5257.1870. [DOI] [PubMed] [Google Scholar]
- 8.Dave AS, Margoliash D. Science. 2000;290:812–816. doi: 10.1126/science.290.5492.812. [DOI] [PubMed] [Google Scholar]
- 9.Wilson MA, McNaughton BL. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 10.Ji D, Wilson MA. Nat Neurosci. 2007;10:100–107. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- 11.Ribeiro S, et al. PLOS Biol. 2004;2:E24. doi: 10.1371/journal.pbio.0020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dave AS, Yu AC, Margoliash D. Science. 1998;282:2250–2254. doi: 10.1126/science.282.5397.2250. [DOI] [PubMed] [Google Scholar]
- 13.Llinás RR, Steriade M. J Neurophysiol. 2006;95:3297–3308. doi: 10.1152/jn.00166.2006. [DOI] [PubMed] [Google Scholar]
- 14.Crunelli V, Hughes SW. Nat Neurosci. 2010;13:9–17. doi: 10.1038/nn.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bailey CH, Kandel ER. Annu Rev Physiol. 1993;55:397–426. doi: 10.1146/annurev.ph.55.030193.002145. [DOI] [PubMed] [Google Scholar]
- 16.Lichtman JW, Colman H. Neuron. 2000;25:269–278. doi: 10.1016/s0896-6273(00)80893-4. [DOI] [PubMed] [Google Scholar]
- 17.Bhatt DH, Zhang S, Gan WB. Annu Rev Physiol. 2009;71:261–282. doi: 10.1146/annurev.physiol.010908.163140. [DOI] [PubMed] [Google Scholar]
- 18.Yang G, Pan F, Gan WB. Nature. 2009;462:920–924. doi: 10.1038/nature08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Timofeev I. Prog Brain Res. 2011;193:121–144. doi: 10.1016/B978-0-444-53839-0.00009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang G, Grone B, Colas D, Appelbaum L, Mourrain P. Trends Neurosci. 2011;34:452–463. doi: 10.1016/j.tins.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frank MG. Neural Plast. 2012;2012:264378. doi: 10.1155/2012/264378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Born J, Feld GB. Neuron. 2012;75:933–935. doi: 10.1016/j.neuron.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Cirelli C, Tononi G. Ann Med. 1999;31:117–124. doi: 10.3109/07853899908998787. [DOI] [PubMed] [Google Scholar]
- 24.Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Nat Neurosci. 2008;11:200–208. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- 25.Maret S, Faraguna U, Nelson AB, Cirelli C, Tononi G. Nat Neurosci. 2011;14:1418–1420. doi: 10.1038/nn.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang G, Gan WB. Dev Neurobiol. 2012;72:1391–1398. doi: 10.1002/dneu.20996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donlea JM, Ramanan N, Shaw PJ. Science. 2009;324:105–108. doi: 10.1126/science.1166657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bushey D, Tononi G, Cirelli C. Science. 2011;332:1576–1581. doi: 10.1126/science.1202839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tononi G, Cirelli C. Brain Res Bull. 2003;62:143–150. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Frank MG, Issa NP, Stryker MP. Neuron. 2001;30:275–287. doi: 10.1016/s0896-6273(01)00279-3. [DOI] [PubMed] [Google Scholar]
- 31.Chauvette S, Seigneur J, Timofeev I. Neuron. 2012;75:1105–1113. doi: 10.1016/j.neuron.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aton SJ, et al. Neuron. 2009;61:454–466. doi: 10.1016/j.neuron.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seibt J, et al. Curr Biol. 2012;22:676–682. doi: 10.1016/j.cub.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roffwarg HP, Muzio JN, Dement WC. Science. 1966;152:604–619. doi: 10.1126/science.152.3722.604. [DOI] [PubMed] [Google Scholar]
- 35.Jouvet-Mounier D, Astic L, Lacote D. Dev Psychobiol. 1969;2:216–239. doi: 10.1002/dev.420020407. [DOI] [PubMed] [Google Scholar]
- 36.Liston C, et al. Nat Neurosci. 2013;16:698–705. doi: 10.1038/nn.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang G, Pan F, Chang PC, Gooden F, Gan WB. Methods Mol Biol. 2013;1010:35–43. doi: 10.1007/978-1-62703-411-1_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zelenin PV, et al. J Neurophysiol. 2011;105:2698–2714. doi: 10.1152/jn.00120.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen TW, et al. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campbell IG, Feinberg I. J Neurophysiol. 1996;76:3714–3720. doi: 10.1152/jn.1996.76.6.3714. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.