Abstract
Background
The muscular dystrophies target muscle groups differentially. In mouse models of muscular dystrophy, notably the mdx model of Duchenne Muscular Dystrophy, the diaphragm muscle shows marked fibrosis and at an earlier age than other muscle groups, more reflective of the histopathology seen in human muscular dystrophy.
Methods
Using a mouse model of limb girdle muscular dystrophy, the Sgcg mouse, we compared muscle pathology across different muscle groups and heart. A cohort of nearly 200 Sgcg mice were studied using multiple measures of pathology including echocardiography, Evans blue dye uptake and hydroxyproline content in multiple muscle groups. Spearman rank correlations were determined among echocardiographic and pathological parameters.
Findings
The abdominal muscles were found to have more fibrosis than other muscle groups, including the diaphragm muscle. The abdominal muscles also had more Evans blue dye uptake than other muscle groups. The amount of diaphragm fibrosis was found to correlate positively with fibrosis in the left ventricle, and abdominal muscle fibrosis correlated with impaired left ventricular function. Fibrosis in the abdominal muscles negatively correlated with fibrosis in the diaphragm and right ventricles. Together these data reflect the recruitment of abdominal muscles as respiratory muscles in muscular dystrophy, a finding consistent with data from human patients.
Keywords: muscular dystrophy, heart, abdominal muscle, diaphragm muscle, fibrosis, membrane damage
INTRODUCTION
The muscular dystrophies (MD) are a heterogeneous set of muscular disorders often linked to mutations in proteins within the dystrophin-glycoprotein complex (DGC). The DGC is a large protein complex localized to the sarcolemma of skeletal, smooth, and cardiac muscle fibers. The dystrophin complex physically links the extracellular matrix to intracellular cytoskeletal elements. Dystrophin and its associated proteins prevent sarcolemmal damage by mechanically stabilizing the lipid membrane, while additionally localizing important membrane repair mechanisms to their site of action [1]. Mouse models of muscular dystrophy are highly useful as preclinical models because they are small, reproduce easily, and can be genetically manipulated. The most studied mouse model of muscular dystrophy, the mdx mouse, recapitulates many of the pathologic hallmarks seen in Duchenne Muscular Dystrophy (DMD), and the mdx diaphragm muscle displays profound features of disease with severe replacement fibrosis that can be seen grossly on inspection [2–4]. Mouse models of sarcoglycan gene mutations similarly reflect the pathology seen in humans with Limb Girdle Muscular Dystrophies (LGMDs), and like the mdx mouse have marked pathology in the diaphragm muscle compared to other muscle groups [5].
In heart failure, diaphragm muscle and cardiac function are interdependent [6, 7]. This interdependence is especially evident in muscular dystrophy, where primary deficits in respiratory muscles and the myocardium may accelerate pathology and dysfunction. Deletion of the skeletal muscle specific transcription factor MyoD in the mdx mouse, which specifically impairs skeletal muscle regeneration, accelerates cardiomyopathy [8]. Correspondingly, rescue of diaphragm muscle with utrophin overexpression in the mdx/utrophin double null mice improved right-ventricular (RV) and left-ventricular (LV) ejection fraction (EF) [9]. In another study, cardiomyopathy was evident in older mdx mice whose skeletal muscle, including diaphragm muscle, had been rescued by micro-dystrophin transgene expression [10]. These findings emphasize the importance of correcting multiple muscle groups, including the respiratory and cardiac muscles.
The diaphragm muscle is responsible for approximately 50% of respiratory force, but this contribution varies with body position and exercise demands [11, 12]. Other muscle groups contribute significantly to respiration, especially when the primary breathing muscle is diseased, as it is in muscular dystrophy. The abdominal muscle group is considered an accessory of respiration, aiding mostly in expiration [13]. Increasing evidence suggests that the abdominal muscles adapt for additional function in patients with muscular dystrophy by contributing to both expiration and inspiration [14–16]. The progression of disease in the abdominal musculature is not as well characterized as the diaphragm muscle in human patients or animal models of MD, and it still remains largely unknown what relationship, if any, the abdominal muscles have with in vivo measures of cardiopulmonary function. We now used a mouse model of muscular dystrophy to assess the relationship between markers of abdominal muscle pathology and measures of cardiopulmonary dysfunction. We took advantage of a large well characterized cohort of mice lacking the dystrophin associated protein γ-sarcoglycan, as these mice are a model for severe childhood autosomal recessive muscular dystrophy, also known as LGMD 2C [17]. This subtype of muscular dystrophy is notable for its similar phenotype to DMD, and this model was selected since it displays an earlier cardiac phenotype more reminiscent of what is seen in human DMD and LGMD 2C. The rectus abdominus muscle is the primary abdominal muscle, and abdominal muscles are important in maintaining posture for proper respiration, in addition to being the primary muscle of expiration. We studied mice at an early time-point in disease, 8 weeks, and found that abdominal muscle pathology correlated with in vivo and postmortem markers of cardiopulmonary dysfunction. These findings demonstrate that the abdominal muscles are useful and accessible muscles to study in preclinical models and reflect cardiopulmonary pathology.
MATERIALS AND METHODS
Animals
Sgcg mice, a model for limb girdle muscular dystrophy, were described previously [3, 18]. Sgcg on the DBA/2J (D2) background and the 129T2/SvEmsJ (129) background were intercrossed to create homozygous Sgcg mice on a mixed D2/129 genetic background. Male (n=95) and female (n=101) SgcgD2/129 mice were studied (total cohort 196). Animals were housed in a single pathogen-free barrier facility under the approval of the University of Chicago Animal Care and Use Committee. At 8 weeks of age, animals were sacrificed and muscle groups removed and isolated for further processing. Hydroxyproline (HOP) content was measured in the whole diaphragm, the right ventricular free wall (right ventricle), left ventricular free wall and septum (left ventricle), abdominal muscle, and one half of each quadriceps muscle. The abdominal muscle was taken from two 1cm square dissections adjacent to, but not including, the non-muscular linea alba. The abdominal muscle taken did not include lateral portions of the abdominal wall, and therefore, the abdominal rectus muscle is likely most represented in our dissection. Evans blue dye (EBD) content was measured in whole triceps, gluteus group/hamstrings, gastrocnemius/soleus and abdominal muscles, and one half of each quadriceps muscle. Together, this generated 35 different data points measured for each animal, and all 35 measurements were available from 126 animals. The remaining 70 animals had more than 30 data points available. The measurements of HOP and EBD utilized the entire muscle groups. Histopathology was performed on separate animals.
Echocardiography
Echocardiographic studies were conducted on animals following standard protocols [19] and as described previously [20]. Animals were anesthetized using isoflurane, 2% in O2, and then placed on a heated pad. Imaging studies were typically completed in less than 15 minutes of total anesthesia time. During imaging, isoflurane was titrated to avoid heart rates less than 350 bpm. Imaging utilized a Vevo 770 (Visualsonics, Toronto, Ontario, Canada) ultrasound machine with 30Mhz probe to acquire all measurements and images. Left-ventricular fractional shortening was acquired by collecting M-mode images in both the parasternal long-axis and parasternal short-axis views.
Hydroxyproline measurement
Hydroxyproline (HOP) assay was performed as described previously [18]. Muscles were removed, minced, weighed and hydrolyzed in 2ml of 6 M hydrochloric acid at 110°C overnight. After incubation, Ehrlich’s reagent was added and absorbance was measured at 558 nm. Heart ventricles were assayed after removal of atria and great vessels. Results were reported per milligram of tissue. HOP measurements were performed on half the abdominal and quadriceps muscles, and on the entire diaphragm and cardiac ventricles where the right and left ventricle were assayed separately.
Dye uptake
Evans blue dye (EBD) uptake into muscle was measured as described previously [18, 21]. Forty hours prior to sacrifice, animals were injected intraperitoneally with 5μl/g body mass EBD (Sigma, E-2129) in phosphate-buffered saline at 10 mg/ml. The entire gastrocnemius/soleus, gluteus/hamstrings, and triceps muscles were harvested for the dye uptake assay. Half of each abdominal and quadriceps muscle was used, while the other half was used for HOP measurement (see above). Muscles were removed, minced, weighed and incubated in 1 ml of formamide for 2 h at 55°C. Absorbance was measured at 620 nm, and results reported as the Z score of absorbance per milligram of tissue.
Histology
Hematoxylin and eosin staining or Mason Trichrome was performed on Sgcg (DBA/2J) mice or mdx (C57Bl10) mice as described [3] (n= 2–5 mice of each genotype).
Statistical Analysis
Statistical analysis was performed using Prism 6 software (GraphPad Software, San Diego California, USA, www.graphpad.com) and R (R Foundation for Statistical Computing, Vienna, Austria). For 3 images (LV fibrosis vs abdominal fibrosis, LV fibrosis vs diaphragm fibrosis, and RV fibrosis vs diaphragm fibrosis), 3 data points were removed for the purposes of presentation. The 3 data points were removed only from the visual depiction of the data because these points were so elevated as to compress the remaining data visualization. All data was included for statistical calculations, including those three points that were removed from each of the three above referenced images. All parameters were evaluated for the normality of their distribution using a D’Agostino and Pearson omnibus test. Non-parametric Spearman rank correlation coefficients were used to examine pairwise relationships among muscle groups due to the non-normal distribution of multiple parameters. Additionally, non-parametric methods (Dunn’s multiple comparison test and Mann-Whitney U test) were used to test for significant differences in parameters across muscle groups. P values < 0.05 were accepted as significant.
RESULTS
A large, genetically heterogeneous cohort of mice reflects variability seen in human muscular dystrophy
Sgcg mice lack the dystrophin-associated protein, γ-sarcoglycan, and are a model for limb girdle muscular dystrophy (LGMD) type 2C [3]. Like LGMD 2C and DMD patients, Sgcg mice characteristically develop a progressive muscle disease marked by inflammation, necrosis and muscle weakness [22]. LGMD 2C is phenotypically heterogeneous, even with an identical primary gene mutation [23]. To model this phenotypic variability in mice, we generated a cohort of Sgcg mice on a mixed genetic background taking advantage of the observation that the muscular dystrophy phenotype is enhanced in the DBA/2J (D2) background and repressed in the 129T2/SvEmsJ (129) background [18]. The D2 strain background harbors a number of genetic loci that enhance muscular dystrophy, and the genetic locus that contributes is the chromosome 7 encoded latent TGFβ binding protein 4 (Ltbp4) gene [24]. A cohort of 196 Sgcg mice was assessed for cardiac function at 8 weeks of age using echocardiography followed by post mortem measurements of fibrosis and muscle damage. Although increased fibrosis can be detected in the heart at this stage, severe cardiac dysfunction is not yet present. This cohort showed a range of disease severity affecting multiple muscle groups and the heart (Figure 1).
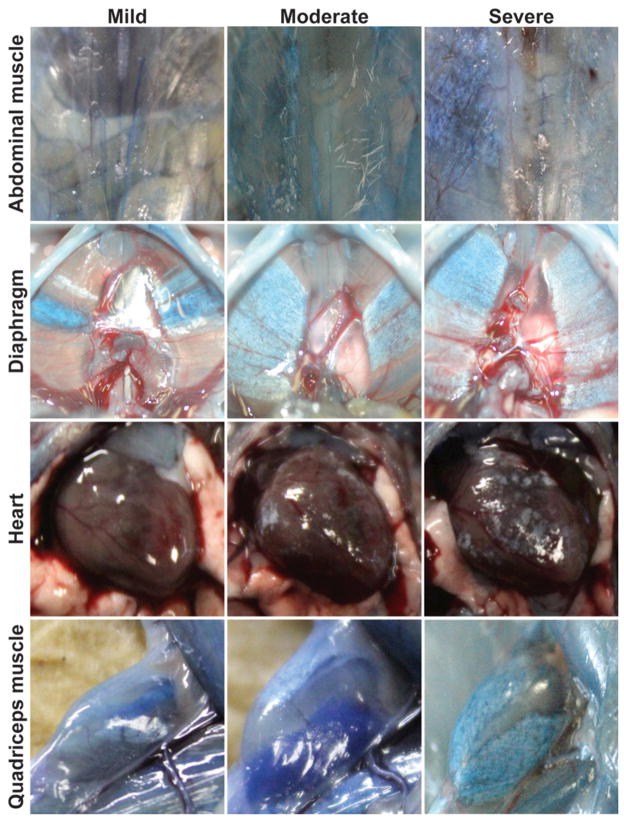
Figure 1. SgcgD2/129 mice replicate a range of severity and muscle group involvement similar to human muscular dystrophy.
Sgcg mice, a model for limb girdle muscular dystrophy, were studied on a mixed genetic background. At eight weeks of age, a relatively early time point in disease, SgcgD2/129 mice displayed significant variability in severity of membrane leak and fibrosis. Gross images are shown from the abdominal, diaphragm and quadriceps muscles, as well as the heart. Animals were injected with Evans blue dye to mark muscle damage, and this is seen as blue areas of muscles. Fibrosis appears as white patches or streaks, and turns otherwise translucent muscle opaque. Dye uptake and fibrosis often occur together, and individual animals can vary in how severely individual muscle groups are affected.
The abdominal muscle group experiences the most severe membrane leak
Evans blue dye (EBD) is an azol dye with high binding affinity for serum albumin. EBD does not accumulate in healthy muscle tissue; rather, it characteristically accumulates in muscles where the sarcolemma has been disrupted. Dye uptake, as a reflection of abnormal muscle membrane permeability, can be quantified as a temporal marker of tissue damage present at the time of analysis [25, 26]. Dye uptake was determined for the abdominal, triceps, quadriceps, gastrocnemius/soleus and gluteus/hamstring muscle groups (n=124 mice) that are subject to heavy use, even in cage-bound animals. Compared to the limb skeletal muscles, the abdominal muscle group had a significantly higher mean dye uptake (6.67 ± 2.59 OD/mg) (Figure 2A). This mean was 34.9% higher than mean for gluteus/hamstring, the second most affected muscle group. Mean abdominal muscle dye uptake was significantly higher than all other muscle groups assayed (p<0.0001 for all pairwise comparisons with the abdominal muscle). No significant differences were found when considering dye uptake between other muscles groups. The distributions of values for dye uptake in the abdominal muscles and gastrocnemius/soleus muscles were non-normal (p=0.003 for abdominal, and p<0.0001 for gastrocnemius/soleus) (Figure 2B). No other significant departures from normality were observed in any of the other muscles assayed for dye uptake.
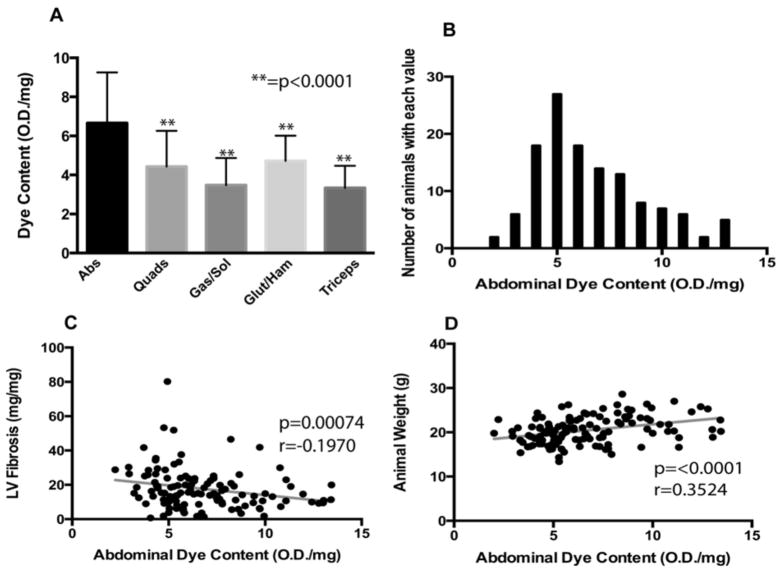
Figure 2. Mean dye uptake, as marker of muscle damage, was highest in the abdominal muscles.
A large cohort (n=196) of SgcgD2/129 mice was assayed for dye uptake in multiple muscle groups. A) Mean dye uptake was found to be highest in the abdominal muscles (m=6.67 ± 2.59 OD/mg), This value was found to be significantly higher than other muscles assayed for EBD when compared by Dunn’s multiple comparison tests (p<0.0001). Error bars represent standard deviation. B) A histogram representing the distribution of abdominal muscle dye uptake. This distribution was found to be non-normal, and right-skewed. C) Abdominal dye uptake was significantly, negatively correlated with fibrosis in the left ventricle of the heart. D) Furthermore, abdominal dye uptake was significantly, positively correlated with animal weight. Taken together, these data support a model in which abdominal muscles are recruited to support cardiopulmonary output.
It has previously been reported that mdx mice may display an asymmetry that was observed to affect both the quadriceps muscle and gastrocnemius muscles [27]. The lateral preference was seen to affect performance on a treadmill and correlate with increased muscle damage on the side bearing more of the load. The Sgcg model similarly displayed asymmetry in two muscle groups: the gastrocnemius/soleus muscle groups and the quadriceps muscles. A Wilcoxon signed rank test found that mean dye uptake was significantly higher in the left gastrocnemius/soleus (p=0.0495), while mean fibrosis, measured as hydroxyproline content, was significantly higher in the left quadriceps (p<0.0001). Additionally, we found two traits to exhibit sexual dimorphism. As expected, male mice had greater body mass than female mice (male: 21.96 g ± 2.95, female: 19.50 g ± 2.58, p<0.007). Male mice were also found to have significantly higher dye uptake in the abdominal muscles than female mice (male: 7.63 ± 2.49, female: 5.86 ± 2.33, p<0.0001). However, these sex-based differences did not alter any of the reported correlations.
Abdominal muscle dye uptake correlates with reduced left ventricular fibrosis and increased animal mass
Spearman based correlations were used to analyze relationships between abdominal dye uptake and all other functional and pathologic markers. Abdominal dye content correlated negatively with left-ventricular fibrosis, as measured by hydroxyproline (HOP) content (Figure 2C). Abdominal muscle dye uptake was found to correlate significantly and positively with animal weight (Figure 2D). No other studied muscle group was found to correlate significantly with animal weight. Interestingly, abdominal muscle dye uptake showed a weak correlation with the measurement of mean velocity of blood across the pulmonary artery (r =0.1898, p=0.0502), which is a reflection of right ventricular function. Taken together, these data suggest that increased dye uptake in the abdominal muscles was seen in larger animals with less left ventricular fibrosis. An animal’s mass may be large in part due to edema, which would be reflected as dye uptake. We infer the increased abdominal muscle damage is consistent with these muscles being recruited as accessory muscles of respiration, which in the shorter term, may be protective for the heart by improving oxygenation.
The abdominal muscles have more fibrosis than other muscles
Hydroxyproline (HOP) content was used as reflection of fibrosis because this modified amino acid is found in matrix deposited collagen. The abdominal muscles had a mean HOP measurement of 43.79 ± 22.16 mg/mg, which was 47.5% higher than the diaphragm muscle and 131% higher than what was seen in the left ventricle. Fibrosis in the abdominal muscles was significantly higher than all other groups assayed (Figure 3A). Much like abdominal dye uptake, the distribution of values was right-skewed (p=0.0002). Total body mass was found to correlate negatively with abdominal muscle fibrosis (r = −0.206, p=0.0071), which could be influenced by sexual dimorphic differences in body mass.
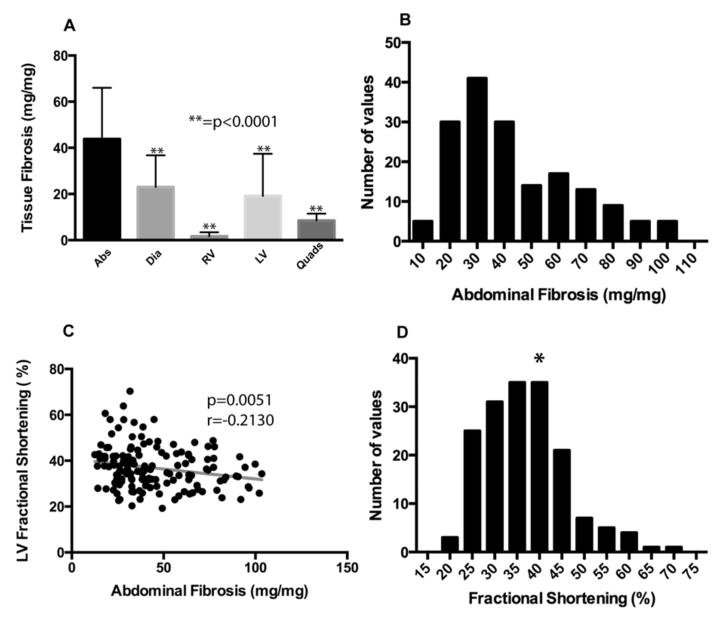
Figure 3. Mean tissue fibrosis was highest in the abdominal muscles.
SgcgD2/129 mice from the same cohort (n=196) were assayed for hydroxyproline (HOP) content as a direct measure of fibrosis. A) Mean fibrosis, measured by HOP content, was highest in the abdominal muscles (43.79 ± 22.16 mg/mg). This value was found to be significantly higher than other muscles when compared by Dunn’s multiple comparison tests (p<0.0001). Error bars represent standard deviation. B) A histogram representing the distribution of abdominal muscle fibrosis (HOP content.) This is a non-normal distribution and is right-skewed. C) Abdominal muscle fibrosis was significantly negatively correlated with left-ventricular fractional shortening (LVFS) using Spearman based calculations. D) Distribution of LVFS in the Sgcg cohort. The asterisk indicates normal LVFS.
Left ventricular fractional shortening (LVFS) is a commonly used measure of heart function applicable to both human and murine hearts. A normal LVFS for mice under isoflurane anesthesia, as used here, is 39% [28]. The mean LVFS for Sgcg mice was 36.93% (SD ± 9.1), and more than 30 animals had LVFS < 30% reflecting cardiomyopathy. Those animals with the least abdominal muscle fibrosis had the best cardiac function measured by LVFS (Figure 3C). The distribution of LVFS in the cohort is shown in Figure 3D. Abdominal muscle fibrosis did not correlate with LV fibrosis (r = −0.0975, p=0.2124). However, cardiac function measured by LVFS also did not correlate significantly with LV fibrosis (r = −0.1300, p=0.1165), indicating that cardiac dysfunction at this stage is not arising from fibrotic infiltration of the heart. These findings are consistent with the view that respiratory dysfunction contributes to cardiac dysfunction.
Diaphragm fibrosis correlates positively with left-ventricular fibrosis, and negatively with right-ventricular fibrosis
The diaphragm, in both large and small animals, is thought to be the major respiratory muscle and is known to be particularly affected in muscle dystrophy in humans and mouse models [2]. Diaphragm muscle fibrosis, as measured by HOP content, was found to correlate positively with left-ventricular fibrosis (Figure 4A). These data suggest that the left-ventricle and diaphragm become fibrotic relatively early in this model of muscular dystrophy. Conversely, diaphragm muscle fibrosis correlated negatively with right-ventricular fibrosis (Figure 4B, r=0.1420, p=0.016). It is important to note that, on average, the right-ventricle experienced the least fibrosis of all muscles sampled by the HOP assay (1.771 ± 1.72 OD/mg). This was 79% lower than the next most fibrotic organ, the quadriceps muscle (mean quadriceps HOP 8.48 ± 3.03 OD/mg).
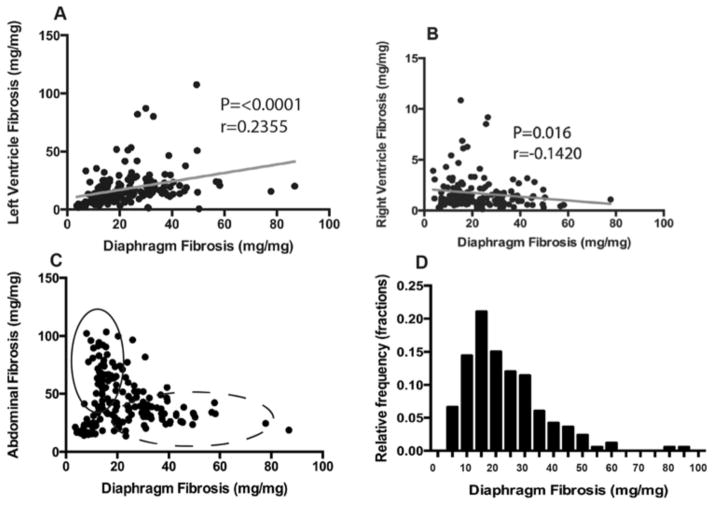
Figure 4. Diaphragm fibrosis associates with fibrosis in the heart.
A) Diaphragm fibrosis correlated positively with LV fibrosis using Spearman based methods, further supporting the concept that scarring in the respiratory muscles may predict scarring in the LV. B) Interestingly, diaphragm fibrosis correlated negatively with RV fibrosis, indicating that the diaphragm correlation with LV fibrosis is independent of RV pathology. C) Scatter plot representing abdominal and diaphragm fibrosis for each animal. Although abdominal fibrosis correlates negatively with diaphragm fibrosis, the distribution suggests a more complex relationship between the two organs. Specifically, there are very few animals with high level abdominal and diaphragm fibrosis (intersection point). Rather, the cohort can be divided into two virtual populations: those that preferentially developed diaphragm fibrosis with little abdominal fibrosis, and those that preferentially developed abdominal fibrosis with little diaphragm fibrosis. D) Frequency distribution of fibrosis found within the diaphragm of the full cohort.
Very few animals have severe diaphragm fibrosis and abdominal fibrosis
Figure 4C is a paired scatter plot correlating diaphragm muscle fibrosis and abdominal muscle fibrosis. These data are plotted without transformation and without a best-fit line in order to better visualize the data. Although there are independent populations of mice that have severe diaphragm fibrosis (dashed line) or abdominal fibrosis (solid line), there are few to no animals that have both severely scarred abdominal and diaphragm muscles. The distribution for diaphragm HOP content is right skewed (p=<0.0001) (Figure 4D).
Comparable histopathology between abdominal muscles and diaphragm muscles in mouse models of MD
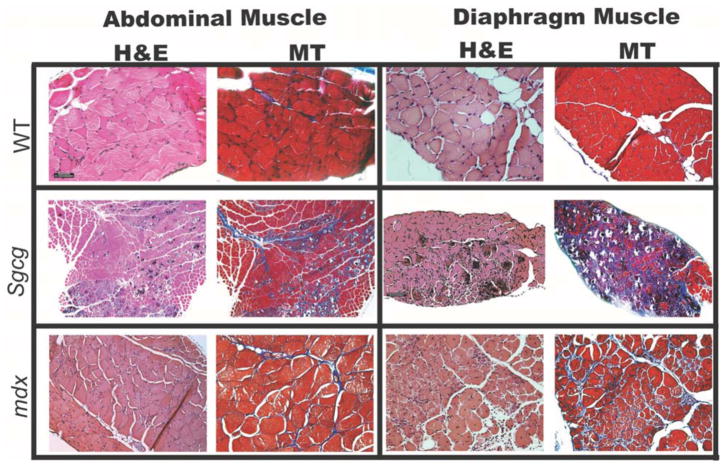
To correlate the quantitative measures of pathology used above, histological sections were examined from Sgcg mice to evaluate abdominal and diaphragm muscle pathology (Figure 5). The abdominal muscle displayed the same range of pathology seen in the diaphragm muscle with notable calcification, necrosis, and inflammation in both muscles. Mason Trichrome staining highlighted significant fibrosis in the abdominal muscles, consistent with the measurements made using hydroxyproline. Abdominal muscles from mdx mice were examined and were similarly found to display qualitatively comparably dystrophic features as the diaphragm muscle. Together these data complement the findings in the large Sgcg cohort.
Figure 5. Abdominal muscle and diaphragm muscle histopathology is comparable in mouse models of MD.
Representative histopathologic sections taken from the diaphragm and abdominal muscles of normal wild-type (WT), Sgcg, and mdx and Sgcg mice at an early time point in disease. All mice were less than 12 weeks old. Hematoxylin and eosin (HE) staining highlights necrosis, calcification, and inflammation. Mason’s trichrome (MT) stained sections from Sgcg and mdx mice both revealed a more dense intercellular fibrosis throughout the abdominal and diaphragm muscles. This degree of histopathology is greater than what is seen in limb-based skeletal muscles (not shown).
DISCUSSION
Abdominal muscles are severely affected in a mouse model of muscular dystrophy
At 8 weeks of age, a time-point that reflects established muscle pathology and emerging cardiac dysfunction, we found the abdominal muscles had significantly elevated Evans blue dye uptake as well as fibrosis. Evans blue dye is a measure of sarcolemmal disruption and directly reflects myofiber injury and damage [29, 30]. What triggers such notable pathology in the abdominal muscles at such an early time point is not known, but it is likely that these muscles are being recruited to participate in respiratory function. In this model of muscular dystrophy, like the mdx model of muscular dystrophy, the diaphragm muscle displays profound histopathological changes. At 8 weeks of age, the diaphragm muscle is nearly completely replaced by fibrosis, which is visible both grossly and histologically, which is anticipated to alter compliance. With the loss of the diaphragm muscle, other muscle groups are expected to contribute to the work of breathing. The pathological findings in the abdominal muscles in Sgcg mice are consistent with these muscles being recruited for respiration.
The lack of correlation between diaphragm muscle and abdominal muscle fibrosis is striking. Specifically, there were virtually no animals that displayed high levels of fibrosis in both the diaphragm muscle and abdominal muscle. Many of the animals collected at this time point had severe diaphragm fibrosis or severe abdominal fibrosis, but rarely both. From this, we infer that animals with severe fibrosis in both muscle groups may have a survival disadvantage. For example, if breathing were severely compromised in the first four weeks of life, such animals may not survive weaning and would not be included in our analysis. The animals in this study were evaluated at 8 weeks of age, post puberty, and an equivalent age of the late second decade of human life.
Respiratory muscle pathology correlates with cardiac measures of pathology and dysfunction
Markers of abdominal and diaphragm muscle pathology correlated significantly with cardiac functional measures in this mouse model of muscular dystrophy. The strongest correlates were between abdominal muscle damage and the mean velocity of blood across the aortic valve, a measure of heart function. Fibrosis in the abdominal muscles also correlated negatively with fractional shortening in the LV. Those animals with the greatest abdominal muscle fibrosis had the most preserved LV function. We interpret these findings as consistent with a model whereby cardiac function can be maintained at the cost of increased breathing, reflected in the use and damage in the abdominal muscles. It is notable that diaphragm muscle fibrosis and LV fibrosis were correlated. This progression is supported by the correlation between diaphragm muscle fibrosis and left ventricular fibrosis, furthering the notion that respiratory muscle fibrosis has a negative impact on cardiac fibrosis (Figure 6).
Figure 6. The abdominal muscle group may compensate for early diaphragm failure in the Sgcg model of MD.
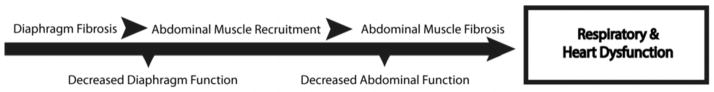
Above is a simplified timeline representing a progression for respiratory and heart dysfunction in muscular dystrophy based on data taken at 8 weeks of age in mouse model of muscular dystrophy. This model parallels studies in human DMD and myotonic dystrophy suggesting that the abdominal muscles are “paradoxically” recruited during inspiration to compensate for diaphragm paralysis and maintain function [16, 37]. Mice with poor heart function had higher levels of fibrosis in the abdominal muscles, suggesting a relationship between abdominal muscle health and heart function. Furthermore, animals with the highest levels of abdominal fibrosis had relatively low levels of diaphragm fibrosis, and vice versa. This relationship is supportive of a model in which these two organs are compensatory to one another.
Implications for human muscular dystrophy
The diaphragm and abdominal muscles oppose one another biomechanically, the former serving as the primary muscle of inspiration while the latter serves as the primary muscle of expiration [16]. Advanced plethsymography methods have been used to characterize respiratory mechanics in DMD patients [14, 15, 31–33]. With disease progression, DMD patients demonstrated less abdominal wall distention during normal inspiration in the supine position. It was suggested that these low abdominal volume changes during inspiration correlate strongly with nocturnal hypoxemia, serving as a predictor of nocturnal desaturation. These data are congruent with other observations that the paradoxical activation of the abdominal muscles during inspiration may help expand the chest wall and thoracic cavity during inspiration [16]. Abdominal muscle activation may also enhance venous return to the heart by further lowering thoracic pressures. Clinically, supporting the abdominal muscles may increase both heart and lung function. Indeed, abdominal binding has been shown to improve cardiac function in a handful of patients with spinal cord injury [34].
Supporting and maintaining proper cardiopulmonary function in neuromuscular disease is a mainstay of therapy. Sufficient ventilation is important for preventing hypoxia and acidosis; two conditions that exacerbate cardiomyocyte dysfunction [35, 36]. Maintaining diaphragm health has been the focus of many studies in both humans and mice with muscular dystrophy, but few studies have focused on supporting and evaluating the accessory muscles of respiration such as the abdominal muscles. Therapies that spare the muscles of respiration in muscular dystrophy have been shown to slow down overall disease progression and prolong life [37, 38]. The effects of nocturnal noninvasive ventilation on the accessory muscles of respiration are relatively unknown. The accessory muscles of respiration, whether in human patients or animal models, may prove a viable target especially for therapy directed at specific muscle groups. The abdominal muscles are easily accessible and dissectible in mice, making them readily available for study.
Acknowledgments
Supported by: NIH HL61322, NIH NS047726, NIH AR052646
Abbreviations
- EBD
Evans Blue dye
- EF
ejection fraction
- HOP
hydroxyproline
- LV
left-ventricle
- LVFS
left-ventricle fractional shortening
- RV
right-ventricle
- MD
muscular dystrophy
- DMD
Duchenne muscular dystrophy
- HE
hematoxylin and eosin
- MT
Mason trichrome
Footnotes
CONFLICTS OF INTERESTM
None
References
- 1.Rybakova IN, Patel JR, Ervasti JM. The dystrophin complex forms a mechanically strong link between the sarcolemma and costameric actin. J Cell Biol. 2000;150:1209–1214. doi: 10.1083/jcb.150.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stedman HH, Sweeney HL, Shrager JB, Maguire HC, Panettieri RA, Petrof B, Narusawa M, Leferovich JM, Sladky JT, Kelly AM. The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature. 1991;352:536–539. doi: 10.1038/352536a0. [DOI] [PubMed] [Google Scholar]
- 3.Hack AA, Ly CT, Jiang F, Clendenin CJ, Sigrist KS, Wollmann RL, McNally EM. Gamma-sarcoglycan deficiency leads to muscle membrane defects and apoptosis independent of dystrophin. J Cell Biol. 1998;142:1279–1287. doi: 10.1083/jcb.142.5.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishizaki M, Suga T, Kimura E, Shiota T, Kawano R, Uchida Y, Uchino K, Yamashita S, Maeda Y, Uchino M. Mdx respiratory impairment following fibrosis of the diaphragm. Neuromuscul Disord. 2008;18:342–348. doi: 10.1016/j.nmd.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Vainzof M, Ayub-Guerrieri D, Onofre PC, Martins PC, Lopes VF, Zilberztajn D, Maia LS, Sell K, Yamamoto LU. Animal models for genetic neuromuscular diseases. J Mol Neurosci. 2008;34:241–248. doi: 10.1007/s12031-007-9023-9. [DOI] [PubMed] [Google Scholar]
- 6.van Hees HW, Ottenheijm CA, Granzier HL, Dekhuijzen PN, Heunks LM. Heart failure decreases passive tension generation of rat diaphragm fibers. Int J Cardiol. 2010;141:275–283. doi: 10.1016/j.ijcard.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 7.Habedank D, Meyer FJ, Hetzer R, Anker SD, Ewert R. Relation of respiratory muscle strength, cachexia and survival in severe chronic heart failure. J Cachexia Sarcopenia Muscle. 2013 doi: 10.1007/s13539-013-0109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Megeney LA, Kablar B, Perry RL, Ying C, May L, Rudnicki MA. Severe cardiomyopathy in mice lacking dystrophin and MyoD. Proc Natl Acad Sci U S A. 1999;96:220–225. doi: 10.1073/pnas.96.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crisp A, Yin H, Goyenvalle A, Betts C, Moulton HM, Seow Y, Babbs A, Merritt T, Saleh AF, Gait MJ, Stuckey DJ, Clarke K, Davies KE, Wood MJ. Diaphragm rescue alone prevents heart dysfunction in dystrophic mice. Hum Mol Genet. 2011;20:413–421. doi: 10.1093/hmg/ddq477. [DOI] [PubMed] [Google Scholar]
- 10.Wasala NB, Bostick B, Yue Y, Duan D. Exclusive skeletal muscle correction does not modulate dystrophic heart disease in the aged mdx model of Duchenne cardiomyopathy. Hum Mol Genet. 2013;22:2634–2641. doi: 10.1093/hmg/ddt112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sant’Ambrogio G, Decandia M, Provini L. Diaphragmatic contribution to respiration in the rabbit. J Appl Physiol. 1966;21:843–847. doi: 10.1152/jappl.1966.21.3.843. [DOI] [PubMed] [Google Scholar]
- 12.Wang CS, Josenhans WT. Contribution of diaphragmatic-abdominal displacement to ventilation in supine man. J Appl Physiol. 1971;31:576–580. doi: 10.1152/jappl.1971.31.4.576. [DOI] [PubMed] [Google Scholar]
- 13.Kaneko H, Horie J. Breathing Movements of the Chest and Abdominal Wall in Healthy Subjects. Respiratory Care. 2012;57:1442–1451. doi: 10.4187/respcare.01655. [DOI] [PubMed] [Google Scholar]
- 14.Mauro AL, D’Angelo MG, Romei M, Motta F, Colombo D, Comi GP, Pedotti A, Marchi E, Turconi A, Bresolin N. Abdominal volume contribution to tidal volume as an early indicator of respiratory impairment in Duchenne Muscular Dystrophy. European respiratory journal. 2009;35:1118–1125. doi: 10.1183/09031936.00037209. [DOI] [PubMed] [Google Scholar]
- 15.Romei M, D’Angelo MG, LoMauro A, Gandossini S, Bonato S, Brighina E, Marchi E, Comi GP, Turconi AC, Pedotti A. Low abdominal contribution to breathing as daytime predictor of nocturnal desaturation in adolescents and young adults with Duchenne Muscular Dystrophy. Respiratory medicine. 2012;106:276–283. doi: 10.1016/j.rmed.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Ugalde V, Walsh S, Abresch RT, Bonekat HW, Breslin E. Respiratory abdominal muscle recruitment and chest wall motion in myotonic muscular dystrophy. Journal of Applied Physiology. 2001;91:395–407. doi: 10.1152/jappl.2001.91.1.395. [DOI] [PubMed] [Google Scholar]
- 17.Azibi K, Bachner L, Beckmann JS, Matsumura K, Hamouda E, Chaouch M, Chaouch A, Ait-Ouarab R, Vignal A, Weissenbach J, et al. Severe childhood autosomal recessive muscular dystrophy with the deficiency of the 50 kDa dystrophin-associated glycoprotein maps to chromosome 13q12. Hum Mol Genet. 1993;2:1423–1428. doi: 10.1093/hmg/2.9.1423. [DOI] [PubMed] [Google Scholar]
- 18.Heydemann A, Huber JM, Demonbreun A, Hadhazy M, McNally EM. Genetic background influences muscular dystrophy. Neuromuscular Disorders. 2005;15:601–609. doi: 10.1016/j.nmd.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Spurney CF, Knoblach S, Pistilli EE, Nagaraju K, Martin GR, Hoffman EP. Dystrophin-deficient cardiomyopathy in mouse: expression of Nox4 and Lox are associated with fibrosis and altered functional parameters in the heart. Neuromuscul Disord. 2008;18:371–381. doi: 10.1016/j.nmd.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wheeler MT, Korcarz CE, Collins KA, Lapidos KA, Hack AA, Lyons MR, Zarnegar S, Earley JU, Lang RM, McNally EM. Secondary coronary artery vasospasm promotes cardiomyopathy progression. Am J Pathol. 2004;164:1063–1071. doi: 10.1016/S0002-9440(10)63193-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- 22.Griffin MA, Feng H, Tewari M, Acosta P, Kawana M, Sweeney HL, Discher DE. γ-Sarcoglycan deficiency increases cell contractility, apoptosis and MAPK pathway activation but does not affect adhesion. Journal of Cell Science. 2005;118:1405–1416. doi: 10.1242/jcs.01717. [DOI] [PubMed] [Google Scholar]
- 23.Kefi M, Amouri R, Driss A, Ben Hamida C, Ben Hamida M, Kunkel LM, Hentati F. Phenotype and sarcoglycan expression in Tunisian LGMD 2C patients sharing the same del521-T mutation. Neuromuscul Disord. 2003;13:779–787. doi: 10.1016/s0960-8966(03)00136-6. [DOI] [PubMed] [Google Scholar]
- 24.Swaggart KA, Heydemann A, Palmer AA, McNally EM. Distinct genetic regions modify specific muscle groups in muscular dystrophy. Physiological Genomics. 2011;43:24–31. doi: 10.1152/physiolgenomics.00172.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamer PW, McGeachie JM, Davies MJ, Grounds MD. Evans Blue Dye as an in vivo marker of myofibre damage: optimising parameters for detecting initial myofibre membrane permeability. J Anat. 2002;200:69–79. doi: 10.1046/j.0021-8782.2001.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wooddell CI, Zhang G, Griffin JB, Hegge JO, Huss T, Wolff JA. Use of Evans blue dye to compare limb muscles in exercised young and old mdx mice. Muscle Nerve. 2010;41:487–499. doi: 10.1002/mus.21527. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi YM, Rader EP, Crawford RW, Campbell KP. Endpoint measures in the mdx mouse relevant for muscular dystrophy pre-clinical studies. Neuromuscul Disord. 2012;22:34–42. doi: 10.1016/j.nmd.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao S, Ho D, Vatner DE, Vatner SF. Echocardiography in Mice. Curr Protoc Mouse Biol. 2011;1:71–83. doi: 10.1002/9780470942390.mo100130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuda R, Nishikawa A, Tanaka H. Visualization of dystrophic muscle fibers in mdx mouse by vital staining with Evans blue: evidence of apoptosis in dystrophin-deficient muscle. J Biochem. 1995;118:959–964. doi: 10.1093/jb/118.5.959. [DOI] [PubMed] [Google Scholar]
- 30.Straub V, Rafael JA, Chamberlain JS, Campbell KP. Animal models for muscular dystrophy show different patterns of sarcolemmal disruption. J Cell Biol. 1997;139:375–385. doi: 10.1083/jcb.139.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lo Mauro A, D’Angelo MG, Romei M, Motta F, Colombo D, Comi GP, Pedotti A, Marchi E, Turconi AC, Bresolin N, Aliverti A. Abdominal volume contribution to tidal volume as an early indicator of respiratory impairment in Duchenne muscular dystrophy. Eur Respir J. 2010;35:1118–1125. doi: 10.1183/09031936.00037209. [DOI] [PubMed] [Google Scholar]
- 32.Lomauro A, Romei M, D’Angelo MG, Aliverti A. Determinants of cough efficiency in Duchenne muscular dystrophy. Pediatr Pulmonol. 2013 doi: 10.1002/ppul.22836. [DOI] [PubMed] [Google Scholar]
- 33.D’Angelo MG, Romei M, Lo Mauro A, Marchi E, Gandossini S, Bonato S, Comi GP, Magri F, Turconi AC, Pedotti A, Bresolin N, Aliverti A. Respiratory pattern in an adult population of dystrophic patients. Journal of the Neurological Sciences. 2011;306:54–61. doi: 10.1016/j.jns.2011.03.045. [DOI] [PubMed] [Google Scholar]
- 34.West CR, Campbell IG, Shave RE, Romer LM. Effects of abdominal binding on cardiorespiratory function in cervical spinal cord injury. Respiratory Physiology & Neurobiology. 2012;180:275–282. doi: 10.1016/j.resp.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka M, Ito H, Adachi S, Akimoto H, Nishikawa T, Kasajima T, Marumo F, Hiroe M. Hypoxia induces apoptosis with enhanced expression of Fas antigen messenger RNA in cultured neonatal rat cardiomyocytes. Circ Res. 1994;75:426–433. doi: 10.1161/01.res.75.3.426. [DOI] [PubMed] [Google Scholar]
- 36.Portal L, Martin V, Assaly R, d’Anglemont de Tassigny A, Michineau S, Berdeaux A, Ghaleh B, Pons S. A model of hypoxia-reoxygenation on isolated adult mouse cardiomyocytes: characterization, comparison with ischemia-reperfusion, and application to the cardioprotective effect of regular treadmill exercise. J Cardiovasc Pharmacol Ther. 2013;18:367–375. doi: 10.1177/1074248412475158. [DOI] [PubMed] [Google Scholar]
- 37.Smith PE, Edwards RH, Calverley PM. Ventilation and breathing pattern during sleep in Duchenne muscular dystrophy. Chest. 1989;96:1346–1351. doi: 10.1378/chest.96.6.1346. [DOI] [PubMed] [Google Scholar]
- 38.Kohler M, Clarenbach CF, Bahler C, Brack T, Russi EW, Bloch KE. Disability and survival in Duchenne muscular dystrophy. J Neurol Neurosurg Psychiatry. 2009;80:320–325. doi: 10.1136/jnnp.2007.141721. [DOI] [PubMed] [Google Scholar]