Abstract
Purpose of review
This review examines the role of actin binding proteins (ABPs) on blood-testis barrier (BTB), an androgen-dependent ultrastructure in the testis, in particular their involvement on BTB remodeling during spermatogenesis.
Recent findings
The BTB divides the seminiferous epithelium into the basal and the adluminal compartments. The BTB is constituted by coexisting actin-based tight junction (TJ), basal ectoplasmic specialization (ES) and gap junction (GJ), as well as intermediate filament-based desmosome (DS) between Sertoli cells near the basement membrane. Junctions at the BTB undergo continuous remodeling to facilitate the transport of preleptotene spermatocytes residing in the basal compartment across the immunological barrier during spermatogenesis. Thus, meiosis I/II and post-meiotic spermatid development take place in the adluminal compartment behind the BTB. BTB remodeling also regulates exchanges of biomolecules between the two compartments. Since TJ, basal ES and GJ use F-actin for attachment, actin microfilaments rapidly convert between their bundled and unbundled/branched configuration to confer BTB remodeling. The events of actin re-organization are regulated by two major classes ABPs that confer actin microfilaments into bundled versus branched/unbundled configuration.
Summary
We provide a model on how ABPs regulate BTB remodeling, shedding new lights in unexplained male infertility, such as environmental toxicant-induced reproductive dysfunction.
Keywords: Testis, blood-testis barrier, tight junction, ectoplasmic specialization, actin microfilaments, seminiferous epithelial cycle
1. Introduction
The blood-testis barrier (BTB) in the mammalian testis divides the seminiferous epithelium into two functional compartments: the basal and the adluminal (apical) compartments [1–3]. Cellular and molecular events pertinent to meiosis I/II and post-meiotic spermatid development all take place in the adluminal compartment behind the BTB. Unlike other blood-tissue barriers, such as the blood-brain barrier and the blood-retina barrier, which are conferred almost exclusively by tight junction (TJ)-barrier of endothelial cells in microvessels, the BTB is constituted by coexisting actin-based TJ/basal ectoplasmic specialization (basal ES) and basal ES/gap junction (GJ), as well as intermediate filament-based desmosome [1,4,5]. Due to the presence of an extensive network of actin microfilament bundles at the basal ES which further reinforce the structural integrity of the TJ, the BTB is one of the tightest blood-tissue barriers in mammals [1,5,6]. Since spermatogonia reside in the basal compartment, preleptotene spermatocytes derived from type B spermatogonia at stage VII-VIII must be transported across the BTB so that they can differentiate into late spermatocytes to prepare for meiosis I/II in the adluminal compartment. Thus, junctions at the BTB undergo extensive restructuring to accommodate the transport of preleptotene spermatocytes, involving rapid re-organization of actin microfilaments at the ES by efficiently converting between a “bundled” and an “unbundled/branched” configuration to confer plasticity to the BTB [7,8]. Studies during the past decade have shed new insights regarding the dynamic nature of actin microfilaments at the basal ES/BTB, which is likely mediated by two groups of actin binding proteins (ABPs): (i) branched actin polymerization inducing proteins that effectively cause branching of an existing microfilaments, and (ii) actin cross-linking and bundling proteins that organize actin microfilaments into a bundled configuration. Herein, we critically evaluate these data, we also provide a model regarding the coordinated efforts of these two groups of ABPs in regulating actin cytoskeletal re-organization pertinent to BTB remodeling.
2. Actin binding proteins (ABPs)
Actin-based cytoskeleton is working in concert with the microtubule (MT)- and intermediate filament-based cytoskeletons to maintain cell shape, structure and multiple cellular functions in mammalian cells including the Sertoli cell [7,9–13]. Actin exists in one of the two forms: globular, monomeric actin (G-actin), and filamentous polymeric actin (F-actin) [9,14]. F-actin, also known as microfilament, is formed by polymerization and assembly of G-actin into double helices. Actin microfilaments are polarized ultrastructures with the rapidly growing end known as ‘barbed end’ and the slow growing end called ‘pointed end’. Microfilaments can be cross-linked into higher order ultrastructures of actin bundles such as those found at the ES in Sertoli cells, as well as meshed or composite bundled networks. Rapid conversion between bundled and branched, unbundled or truncated network by altering the organization of actin microfilaments thus confers plasticity to cells. These changes also modulate adhesive function of protein complexes at the TJ (e.g., occludin-ZO-1) and basal ES (e.g., N-cadherin-α/β-catenin), as well as communicating function of the GJ (e.g., connexin 43/plakophilin-2), and these proteins all utilize F-actin for attachment [15–17]. Actin dynamics are regulated by >100 ABPs which modulate assembly, polymerization, cross-linking, bundling, cleavage/defragmentation, organization and localization of microfilaments [9,18,19]. ABPs can be categorized into two functional groups: (i) ABPs that regulate F-actin assembly and disassembly such as monomer-binding, nucleation, barbed end capping, cleavage and depolymerization, and (ii) ABPs that confer and regulate higher-order microfilament structures such as F-actin cross-linking, F-actin bundling, and branched actin polymerization [9,20,21]. We highlight recent findings on selected ABPs in both functional groups whereby their physiological significance in the testis has been studied, particularly their role in higher-order actin organization at the BTB, namely branched actin polymerization-inducing proteins versus actin bundling proteins (Figure 1, Table 1).
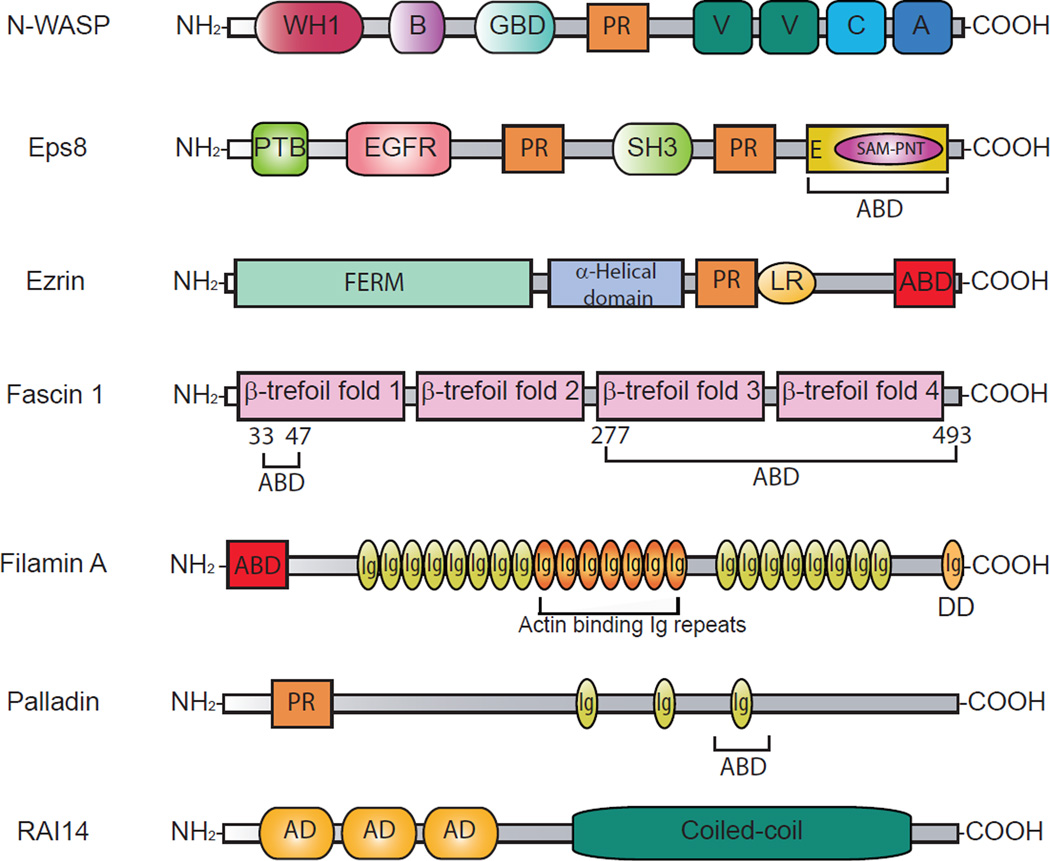
Figure 1. A schematic drawing that illustrates the various functional domains of different actin binding proteins known to regulate actin microfilaments at the ES in the mammalian testis.
Abbreviations used: A, acidic region; ABD, actin binding domain; AD, ankyrin domain; B, basic region; C, cofilin homology domain; DD, dimerizing domain; E, effector region; EGFR, epidermal growth factor receptor domain; FBR, F-actin binding region; FERM, band 4.1/ERM domain; GBD, GTPase-binding domain; Ig, immunoglobulin-like domain; LR, linker region; PR, proline-rich domain; PTB, phosphotyrosine binding domain; S, spectrin-related domain; SAM-PNT, sterile α motif/pointed domain; SH3, Src homology 3 domain; V, verprolin homology domain; WH1, WASP homology domain.
Table 1.
Function of actin-binding proteins (ABPs) based on studies of genetic models and mutation analysis
| ABP | Mr (kDa) | Phenotypes |
|---|---|---|
| Arp3 | 45 | Embryos of Arp3 deficient mice failed to develop beyond blastocysts stage [31]. |
| Eps8 | 97 | Eps8 null mice were normal and fertile [32]. Length of intestinal microvilli in Eps8 KO vs. WT mice reduced by 25%, leading to significant reduction in intestinal fat absorption [33]. Effects on ES unknown. |
| Ezrin | 85 | Ezrin mutation mouse pups died before weaning, defects in epithelial organization and villus morphogenesis were observed in the gastrointestinal tract [34,35]. |
| Fascin 1 | 54 | Fascin 1 deficient mice were viable and fertile without major developmental defects except neurons exhibited fewer and shorter filopodia vs. WT [36]. Embryonic fibroblasts lacking fascin 1 also displayed fewer and shorter filopodia and were short-lived [36]. |
| Filamin A | 280 | Filamin A-deficient mice led to embryonic leathality due to severe hemorrhage and cardiac structural defects [37]. Thus, its effects following KO on the testis remain unknown. |
| Palladin | 95 | Loss of palladin results in embryonic lethality, embryos died at E15.5 due to cranial neural tube closure defects (NTDs) and herniation of liver and intestine [38]. |
| Rai14 | 110 | Mutation of Rai14 via its deletion led to a complex neurobehavioral disorder known as Smith-Magenis syndrome (SMS) in humans [39,40], associated with schizophrenia [41] and spinocerebellar ataxia type 2 (SCA2) [42]. Its duplication led to autism [43] and Potocki-Lupski syndrome [44]. |
2.1. Branched actin-inducing proteins
2.1.a. Actin-related protein (Arp3)
The actin-related protein (Arp) 2/3 complex is a seven-subunit protein complex containing Arp2, Arp3, and also Arp2/3 complex subunit (ARPC) 1–5, known to induce branched actin polymerization at the barbed end of an existing actin microfilament, effectively converting bundled actin filaments into a branched/unbundled network [22,23]. The Arp2/3 complex, however, has to be activated by upstream activators, including Wiskott-Aldrich syndrome protein (WASP) family (e.g., neuronal WASP (N-WASP)) (Figure 1) and the cortactin family [9,24–26] proteins, before it functions as a branched actin nucleation protein. Thus, specific inactivation of N-WASP in Sertoli cells via its conditional KO that causes a failure in Arp2/3 complex function is known to induce infertility in mice [27,28] as a result of defects in: (i) spermiogenesis in which round spermatids fail to develop into elongating/elongated spermatids, and (ii) BTB function [28]. In adult rat testes, Arp3 is expressed by both Sertoli and germ cells, almost exclusively at the apical and basal ES, and its expression is spatiotemporally regulated, depending on the stage of the epithelial cycle [29]. The expression of Apr3 at the basal ES/BTB is not detectable until stage VIII [29], coinciding with BTB remodeling to facilitate the transport of preleptotene spermatocytes across the immunological barrier. The intrinsic activity of the Arp2/3 complex thus contributes to the re-organization of the actin microfilament bundles at the apical and basal ES at these stages via its spatiotemporal expression, destabilizing the ES to enabling endocytic vesicle-mediated trafficking events and BTB remodeling. Studies have shown that the activation of the Arp2/3 complex, besides N-WASP, may also involve p-FAK-Tyr407 that regulate actin filament organization at the BTB by promoting the association of Arp3 with N-WASP [30]. This action of the Arp2/3 complex, along with other ABPs, provide a unique mechanism to facilitate preleptotene spermatocyte transport at the BTB during spermatogenesis (Figure 2).
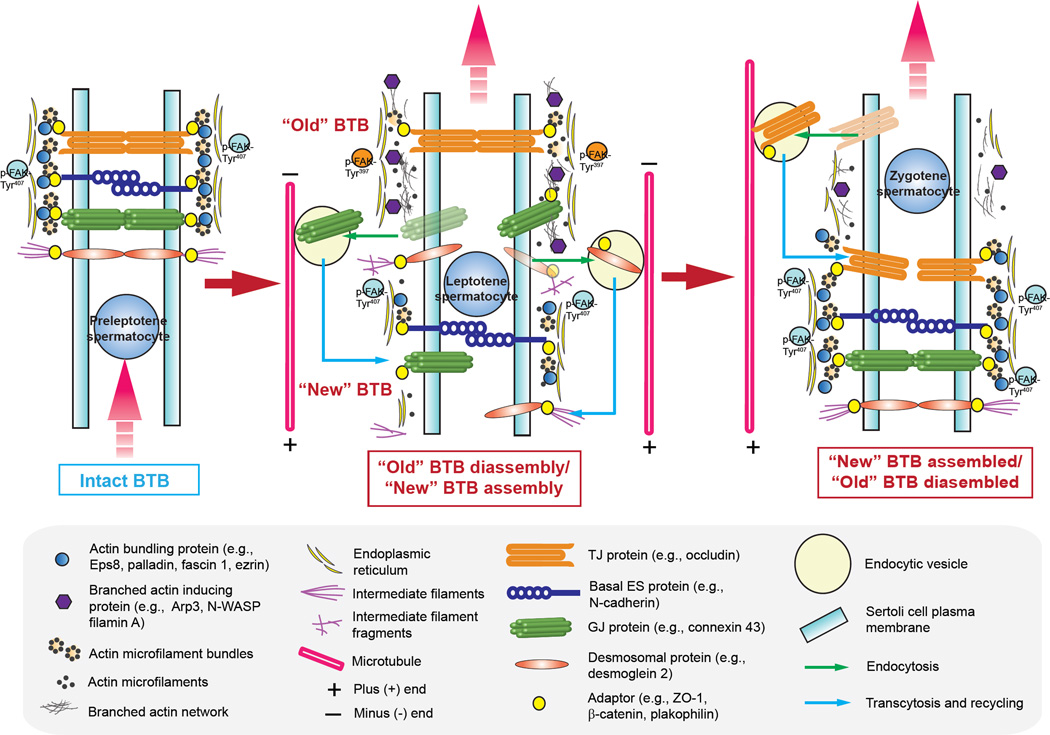
Figure 2. A hypothetical model illustrating the role of actin binding proteins (ABPs) on the transport of preleptotene spermatocytes across the BTB in the mammalian testis during the epithelial cycle of spermatogenesis.
The BTB in the mammalian testis (see left panel) is constituted by coexisting actin-based tight (TJ), basal ES and gap junction (TJ), as well as the intermediate filament-based desmosome between adjacent Sertoli cells, such as at a stage VII tubule, illustrating an intact BTB. The BTB also segregates the seminiferous epithelium into two functional compartments known as the adluminal and the basal compartment so that meiosis I/II and post-meiotic germ cell development all take place behind the BTB in the adluminal compartment. The integrity of the BTB as noted in a stage VII tubule (left panel) is maintained by adhesion protein complexes between adjacent Sertoli cells, such as integral membrane proteins of the TJ (e.g., occludin), basal ES (e.g., N-cadherin) and GJ (connexin43) which are anchored to the actin microfilament bundles via their corresponding adaptors of ZO-1, β-catenin and plakophilin-2. For desmosome, desmosomal integral membrane protein desmoglein-2 is anchored to the intermediate filament via adaptor desmocollin-2. The integrity of actin microfilament bundles are also maintained by the various actin bundling proteins such as Eps8, palladin, fascin 1, ezrin and others (see text for details) (left panel). Furthermore, it is likely that microfilaments that are maintained in their bundled configuration is also supported by p-FAK-Tyr407 at the BTB. Preleptotene spermatocytes differentiate from type B spermatogonia residing in the basal compartment, however, must be transported across the BTB at stage VIII of the epithelial cycle while developing into leptotene spermatocytes at stage IX of the cycle, so that they can prepare for meiosis I and II (see middle panel). During these stages, “old” BTB located above the preleptotene spermatocytes in transit at the BTB undergo extensive remodeling, most notably endocytic vesicle-mediated protein trafficking in which “old” BTB-associated proteins are being endocytosed, transcytosed and recycled to the basal region of preleptotene spermatocytes to assemble the “new” BTB. These changes are made possible by changes in the spatiotemporal expression of several actin binding proteins, mostly notably the up-regulation of branched actin inducing protein Arp2/3-N-WASP protein complex, concomitant with a down-regulation of actin bundling proteins (e.g., Eps8, palladin, fascin 1, and ezrin). This remodeling of BTB is also supported, at least in part, by p-FAK-Tyr397. The net result of these changes induces debundling and branching of the actin microfilaments, destabilizing adhesion protein complexes at the “old” BTB (see middle panel). The transport of spermatocytes and endocytic vesicle-mediated trafficking are also facilitated by the polarized microtubules which serve as the track for transport (see middle panel). By stage XII of the epithelial cycle, leptotene spermatocytes differentiate into zygotene spermatocytes, residing in the adluminal compartment and prepare for meiosis I/II that take place at stage XIV of the cycle, when a “new” BTB is established behind spermatocytes and the “old” BTB is degenerated (see right panel). Thus, remodeling of the BTB in response to different stages of the epithelial cycle to facilitate the transport of preleptotene spermatocytes across the BTB can be effectively regulated by changes in the spatiotemporal expression of the two classes of actin binding proteins, namely actin bundling and branched actin-inducing proteins as depicted herein (see text for details).
2.1.b. Filamin A
Filamins are a family of actin binding and cross-linking proteins with three known members: filamin A, B, and C, which are products of distinct genes found in multiple mammalian epithelia, involving in scaffolding, adhesion, signaling, and mechanical function [45–48]. Filamin A, formerly known as actin-binding protein 280 (ABP280) [49], is a nonmuscle actin filament cross-linker which induces perpendicular branching of existing F-actin microfilaments to create a network of branched filaments [50,51]. Due to the presence of a protein partner-interacting domain at the hinge region of two dimerized filamin polypeptides besides the N-terminal actin-binding domain, filamin A is known to recruit numerous proteins to create a large regulatory protein complex, with more than 90 binding partners having diversified cellular functions have been identified [50,52,53] (Figure 1). Thus, filamin A, besides creating a network of branched actin microfilaments to serve as a scaffold via its intrinsic cross-linking activity, it also recruits proteins to cell junctions to regulate cell adhesion, such as vinculin, 14-3-3, JNK, ROCK, Pak1, PKC, caspase, Smads and caveolin-1 [54–56]. In the rat testis, filamin A is expressed mostly at the basal ES/BTB [57]. Filamin A also recruits TJ- (e.g., JAM-A and ZO-1), and basal ES proteins (e.g., N-cadherin) to the developing BTB in post-natal rats at ~15–20 dpp (day postpartum) for its assembly [57]. Furthermore, a knockdown of filamin A in the testis also perturbed the organization of F-actin at the BTB [57]. In brief, while filamin A induces actin branching, unlike the Arp2/3 complex which is an barded end actin nucleation protein, the branched actin network maintained by filamin A in the testis, at least at the BTB during postnatal assembly of the barrier, is to recruit constituent proteins (e.g., JAM-A, ZO-1, N-cadherin) to the site for junction assembly [50]. Thus, while filamin A is an actin cross-linker, it creates an F-actin network composed of perpendicular branched actin microfilaments, recruiting other proteins to the site to confer cell adhesion, such as BTB assembly during post-natal development [57]. Figure 2 depicts the role of filamin A at the BTB during spermatogenesis.
2.2. Actin bundling proteins
2.2.a. Epidermal growth factor receptor pathway substrate (Eps8)
Eps8, originally identified as a substrate of epidermal growth factor receptor (EGFR), is a member of a protein family that links growth factor stimulation to actin-based cytoskeletal function [58–60] (Figure 1). Eps 8 acts either as an actin bundling, barbed end capping protein or activator of Rac GTPase to modulate actin microfilament organization depending on its association with the corresponding binding partner of IRSp53 (insulin receptor tyrosine kinase substrate p53), Abi-1 (Abelson interacting protein-1) or Sos1/Abi-1 (Son of sevenless 1/Abelson interacting protein-1), respectively [8,59]. In the rat testis, Eps8 as well as its functional partners, Abi-1, IRSp53, and Sos1 are expressed by both Sertoli and germ cells [61]. In the seminiferous epithelium, Eps8 is highly expressed at the basal ES/BTB in stage V-VI tubules, co-localizing with F-actin in the basal ES, likely to be used to maintain the integrity of the actin microfilament bundles at the basal ES [61]. Its expression at the basal ES/BTB, however, considerably diminishes in early stage VIII, virtually undetectable thereafter to facilitate BTB restructuring to accommodate the transport of preleptotene spermatocytes across the immunological barrier at late stage VIII [61] due to its intrinsic actin bundling and barbed end capping activity. At stage VIII, the expression of Eps8 at the basal ES diminishes considerably to an almost undetectable level to facilitate BTB restructuring [61]. Thus, it is through such a tightly regulated spatiotemporal expression of two ABPs that induce branched (or barbed end) nucleation (e.g., Arp3) and actin bundling (and barbed end capping) (e.g., Eps8), actin microfilaments at the basal ES/BTB can be re-organized in response to the stages of the epithelial cycle to confer junction plasticity to facilitate preleptotene spermatocyte transport. Below are several additional ABPs that are recently found in the testis, which exert similar but slightly different spatiotemporal expression pattern, and the combined action of these proteins thus provides an efficient system to modulate actin microfilament organization to support BTB functions during spermatogenesis.
2.2.b. Palladin
Palladin is a member of an actin-binding protein subfamily consisted of palladin, myotilin and myopalladin known to provide scaffolding function in mammalian cells of multiple epithelia due to its actin cross-linking and bundling activity, likely to work in concert with α-actinin to maintain the integrity of actin microfilament bundles [62–64]. Palladin possesses high binding affinity for F-actin due to the presence of three immunoglobulin (Ig)-like interacting domains near its C-terminus [63,65] (Figure 1). Studies in the rat or mouse testis and primary Sertoli cells cultured in vitro have shown that palladin, a 95 kDa protein, co-localizes with actin microfilaments in Sertoli cells, and it is an integrated component of the ES [66,67]. More important, palladin was shown to associate with Eps8, Apr3 and ARPC2 as well as c-Src, besides actin, in the rat testis [67], illustrating palladin is likely working in concert with other actin bundling (e.g., Eps8) and branched actin nucleation protein (e.g., Arp3, ARPC2) to modulate the organization of actin microfilaments at the ES. In the rat testis, palladin is highly expressed at the basal ES/BTB in all stages of the epithelial cycle but considerably down-regulated at stage VIII, coincide with BTB remodeling [67]. Since palladin structurally interacts with c-Src at the ES [67], it is likely a putative substrate of c-Src. Thus, c-Src-mediated changes in palladin phosphorylation possibly modulates its intrinsic actin bundling activity, which, in turn, affects F-actin organization. c-Src is recently shown to modulate protein endocytosis at the Sertoli cell BTB by promoting internalized proteins to endosome-mediated protein degradation [68], as these events are highly dependent on F-actin plasticity, palladin that recruits Arp3 and Eps8 to the ES site thus plays an important role in modulating the configuration of actin microfilaments in response to different stages of the epithelial cycle.
2.2.c. Rai14
Rai14 (retinoic acid induced protein 14) is a 110 kDa actin-binding protein, first identified in the liver, and subsequently found in retina, placenta, testes and other tissues including rodents and humans, with its expression induced by retinoic acid [69–71]. Rai14 is an actin cross-linker, an adaptor and a scaffolding protein, associated with cortical actin cytoskeleton and F-actin stress fibers, involving in cell adhesion [72]. Studies in the testis have shown that it is also involved in maintaining actin microfilaments at the ES, likely via palladin as its partner to modulate ES function [73] (Figure 1). In adult rat testes, Rai14 is highly expressed and limited to the ES (with a mild expression by peritubular myoid cells in the tunica propria) spatiotemporally during the epithelial cycle [74]. The expression of Rai14 at the basal ES/BTB is relatively low in all stages of the epithelial cycle except stage VIII when it is robustly expressed, coinciding with BTB remodeling [74]. In the testis, Rai14 only structurally associates with palladin but not Arp3, Eps8 and other BTB proteins (e.g., occludin, JAM-A, ZO-1, N-cadherin, and β-catenin) [74]. At the Sertoli cell BTB in vitro, Rai14 also co-localizes, almost superimposable, with actin microfilaments in Sertoli cell cytosol, and its knockdown by RNAi was found to cause actin microfilament truncation, perturbing the Sertoli cell TJ-permeability barrier function [74]. Collectively, these data illustrate the Rai14 is likely working in concert with palladin by recruiting other actin regulatory proteins, such as Arp3, to the site to modulate actin organization since palladin is known to recruit other regulatory protein partners [67].
2.2.d. Ezrin
Ezrin, an 85 kDa protein, is a member of the ezrin, radixin and moesin (ERM), and merlin (moesin/ezrin/radixin-like protein) family of structural proteins called ERM-merlin that tether integral membrane proteins (e.g., TJ and AJ proteins) and their peripheral proteins (e.g., adaptors, protein kinases) to actin-based cytoskeleton in mammalian cells, actively involved in cell movement, proliferation and survival [75–77] (Figure 1). These protein usually do not co-expressed simultaneously in a mammalian cell type. For instance, ezrin is expressed predominantly in polarized epithelial and mesothelial cells, radixin in hepatocytes, and moesin in endothelial and lymphoid cells [75–78]. Also, each of these four proteins has a unique function even they do share common functions (Table 1). Ezrin, radixin and moesin are found in the mouse testis, with ezrin associated with residual bodies, phagosomes and apical ES in the seminiferous epithelium [79]. Moreover, the expression of ezrin at the ES in the seminiferous epithelium of rat testes is highly stage-specific [80]. For instance, ezrin is expressed at the basal ES/BTB in all stages of the epithelial cycle except at stage IX when its expression is considerably diminished [80]. Ezrin knockdown in the testis in vivo was shown to impede BTB integrity [80], mediated by changes in the organization of F-actin at the ES, consistent with findings in Sertoli cells in vitro when ezrin knockdown leads to truncation and mis-organization of actin microfilaments [80]. For instance, F-actin no longer properly organized at the basal ES/BTB following ezrin knockdown in the testis in vivo, perturbing BTB function [80]. More important, ezrin was shown to be involved in the assembly of TNTs (tunneling nanotubes, also known as intercellular bridges) between distant Sertoli cells cultured in vitro since its knockdown impeded the establishment of TNT [80]. This observation is physiologically important since TNT is capable of transmitting chemical/biological signals >1.5 kDa between distant mammalian cells including miRNAs and endogenous siRNA (noted: communicating gap junctions (GJ) only limited to accommodate transporting of signaling molecules of <1–1.5 kDa) [17,81,82], thus, ezrin may be involved in coordinating signals between distant Sertoli cells across the seminiferous epithelium to support complex cellular events during spermatogenesis, such as the transport of preleptotene spermatocytes. Since ezrin is associated with Arp3, ezrin may also be working in concert with Arp3 to modulate the organization of actin microfilaments at the ES. Role of ezrin to support basal ES function is depicted in Figure 2.
2.2.e. Fascin 1
Fascins are a family actin bundling proteins composed of fascin 1, 2, and 3, which are known to cross-link actin microfilaments into tightly packed parallel bundles such as those found at the ES in Sertoli cells in the testis [83–85] (Figure 1). In mammalian cells, fascin 1 is associated with F-actin-rich ultrastructures such as stress fibers, lamellipodia and filopodia, fascin 2 most expressed by photoreceptors in retina, and fascin 3 is restricted to the testis and expressed by elongating/elongated spermatids (but not Sertoli cells) at the apical ES, and no expression of fascin 3 is detected at the basal ES/BTB [83–85]. Fascin 3 is likely involved in the assembly of F-actin ultrastructures surrounding the spermatid nucleus and the acrosome-acroplaxome-manchette complexes [86] during spermiogenesis. Fascin 1, a 54 kDa polypeptide, is robustly expressed at the BTB in all stages of the epithelial cycle except at stage VIII when BTB undergoes remodeling to facilitate the transport of preleptotene spermatocytes [87]. The function of fascin 1 in maintaining the actin microfilaments at the ES in Sertoli cells is clearly noted in a study by silencing fascin 1 by RNAi since its knockdown leads to a loss of typical paralleled actin microfilaments across the Sertoli cell cytosol, instead, these microfilaments become unbundled and truncated [87]. Fascin 1 knockdown in the testis in vivo also leads to dis-organization of F-actin at the apical and basal ES, making the ES incapable of providing proper adhesion function in which apical ES adhesion proteins (e.g., nectin-3, β1-integrin) and TJ/basal ES proteins (e.g., occludin, ZO-1) are mis-localized, this thus destabilizes adhesion function at the ES in both sites, leading to defects in spermatid polarity [87]. Similar to ezrin, fascin 1 was also shown to be a component of TNTs between distant Sertoli cells [87]. Since fascin 1 structurally associates with palladin and Arp3, it is likely that fascin 1 is working in concert with other actin bundling proteins (e.g., palladin) and actin barbed end nucleation proteins (e.g., Arp3) to modify actin microfilament organization at the ES during different stages of the epithelial cycle to confer preleptotene spermatocyte transport at the BTB.
3. A model by which actin binding proteins regulate preleptotene spermatocyte transport at the BTB
As briefly discussed above, the transport of preleptotene spermatocytes, connected in clones via intercellular bridges (or TNTs), across the BTB is regulated by rapid re-organization of actin microfilaments by converting between a bundled and an unbundled/branched configuration (Figure 2). This thus confers plasticity to the BTB. As such, integral membrane proteins at the “old” BTB above the preleptotene spermatocytes in transit can be endocytosed, transcytosed and recycled to assemble TJ-fibrils at the “new” BTB behind the spermatocytes. In short, Arp3 perhaps working in concert with filamin A to provide the necessary machineries to convert actin microfilaments from a bundled to an unbundled/branched configuration to facilitate this gradual breakdown of the “old” BTB. This can also be facilitated by the gradual down-regulation via changes in the spatiotemporal expression of Eps8, palladin, ezrin and fascin 1, possibly mediated by Rai14, at the “old” BTB. On the other hand, a gradual increase and eventually robust expression of Eps8, palladin, ezrin and fascin 1 likely involving Rai14 takes place at the “new” BTB, concomitant with a down-regulation of Arp3 (and possibly filamin A) at the site mediated by changes in their spatiotemporal expression. The combined effects of these actin bundling proteins thereby cause changes in the underlying actin-based cytoskeleton in the corresponding sites, namely the “old” and the “new” BTB. Reorganization of actin microfilaments at the “old” BTB also facilitates endocytic vesicle-mediated trafficking events as recently reported [88,89], so that “old” integral membrane proteins can be rapidly endocytosed, transcytosed and recycled to assemble TJ-fibrils at the “new” BTB. The sum of these changes thus de-stabilizes the “old” BTB, accommodating the transport of preleptotene spermatocytes across the barrier. At the “new” BTB, the newly recycled integral membrane proteins (e.g., occludin, N-cadherin) and their associated peripheral proteins (e.g., ZO-1, β-catenin) are being used to assemble the barrier behind the preleptotene spermatocytes in transit. Thus, the breakdown of the “old” BTB above the preleptotene spermatocytes in transit does not elicit any disruption of the immunological barrier due to the presence of the “new” BTB that is assembled behind these germ cells. Studies have shown that these changes are likely mediated by two activated/phosphorylated forms of FAK, namely the p-FAK-Tyr407 and p-FAK-Tyr307 [30,90], since p-FAK-Tyr407 is known to promote BTB integrity, such as the assembly of the “new” BTB; whereas p-FAK-Tyr397 promotes BTB restructuring, such as the disruption of the “old” BTB. In short, these two forms of FAK, likely working in concert with other protein kinases (e.g., c-Src and c-Yes) [68,91,92], to serve as molecular switches to induce intrinsic activities of the corresponding actin regulatory proteins via phosphorylation.
4. Clinical and therapeutic implication
Results of recent studies have shown that environmental toxicants likely exert their effects by disrupting BTB function to gain access to the testis to disrupt spermatogenesis [1,93]. This is possibly mediated by an initial disruption of cell junctions, and also actin- and/or microtubule-based cytoskeletal function at the BTB [94–96]. The model depicted in Figure 2 illustrates that there are multiple proteins (e.g., ABPs) and/or signaling molecules (e.g., FAK, MAPK) in the testis which are targets of environmental toxicants. In short, toxicant-induced male reproductive dysfunctions that cause infertility or subfertility are mediated through these molecular targets. For instance, p38 MAPK that modulates BTB function is the target of cadmium-induced BTB disruption [97]. More important, the use of specific inhibitors against MAPK or JNK was shown to modulate, such as by blocking or worsening, toxicant-induced BTB disruption [98,99]. Furthermore, recent studies using human or rat Sertoli cells as a study model of BTB function have shown that several environmental toxicants, such as cadmium, bisphenol A and PFOS exert their effects by disrupting the actin-based cytoskeleton is mediated through ABPs, such as by disrupting the spatiotemporal expression of Arp3, Eps8 and palladin in Sertoli cells [100,101]. Collectively, these findings have unequivocally demonstrated that studies on these ABPs are physiologically relevant to male reproductive health in particular unexplained or toxicant-mediated male infertility.
5. Concluding remarks and future perspectives
Figure 2 is a hypothetic model based on studies investigating the role of ABPs on BTB dynamics as discussed herein. This model, as noted above, also serves as a possible roadmap to better understand male infertility in particular unexplained or environmental toxicant-induced infertility. However, a few crucial questions remain unanswered. For instance, what is the upstream regulatory biomolecule(s) that modulate the spatiotemporal expression of these ABPs? Does this involve p-FAK-Tyr407 and p-FAK-Tyr397? Also, what triggers the spatiotemporal expression of the biomolecules at the BTB microenvironment? Is this miRNAs? However does a miRNA being transported to the appropriate micro-domain to exert its regulatory effect? Does this involve TNTs? What is the identity of these miRNAs? Due to the unprecedented advances in biotechnology, many of these questions will likely be answered in the years to come.
KEY POINTS.
The Sertoli cell blood-testis barrier (BTB) is an androgen-dependent and an F-actin-rich ultrastructure.
Unlike other blood-tissue barriers, the BTB undergoes cyclic remodeling during the epithelial cycle of spermatogenesis to facilitate the transport of preleptotene spermatocytes across the barrier. This event is regulated by the concerted efforts of actin bundling and branched actin inducing proteins.
A hypothetic model is provided herein illustrating the combined actions of ABPs confer actin-based cytoskeleton its plasticity to regulate adhesion proteins and endocytic vesicle-mediated protein trafficking at the BTB, thereby facilitating the transport of preleptotene spermatocytes at the barrier.
Acknowledgements
The authors thank the former and current members of the Cheng Laboratory, as well as other investigators in the field, many of their published findings have cited herein, which form the basis of this review. Due to limited space, many important studies could not be cited, however, every effort was made to cite recent reviews in the field which covered many of their important studies.
Financial Support and sponsorship
This work was supported by grants from the National Institutes of Health (U54 HD029990 Project 5 to C.Y.C. and R01 HD056034 to C.Y.C.)
Footnotes
Conflicts of interest
The authors have nothing to declare
References
(*, Special Interest; **, Outstanding Interest)
- 1.Cheng CY, Mruk DD. The blood-testis barrier and its implication in male contraception. Pharmacol Rev. 2012;64:16–64. doi: 10.1124/pr.110.002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franca LR, Auharek SA, Hess RA, Dufour JM, Hinton BT. Blood-tissue barriers: Morphofunctional and immunological aspects of the blood-testis and blood-epididymal barriers. Adv Exp Med Biol. 2012;763:237–259. [PubMed] [Google Scholar]
- 3.Pelletier RM. The blood-testis barrier: the junctional permeability, the proteins and the lipids. Prog Histochem Cytochem. 2011;46:49–127. doi: 10.1016/j.proghi.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Vogl AW, Vaid KS, Guttman JA. The Sertoli cell cytoskeleton. Adv Exp Med Biol. 2008;636:186–211. doi: 10.1007/978-0-387-09597-4_11. [DOI] [PubMed] [Google Scholar]
- 5.Setchell BP. Blood-testis barrier, functional and transport proteins and spermatogenesis. Adv Exp Med Biol. 2008;636:212–233. doi: 10.1007/978-0-387-09597-4_12. [DOI] [PubMed] [Google Scholar]
- 6.Russell LD, Peterson RN. Sertoli cell junctions: morphological and functional correlates. Int Rev Cytol. 1985;94:177–211. doi: 10.1016/s0074-7696(08)60397-6. [DOI] [PubMed] [Google Scholar]
- 7.Lie PPY, Mruk DD, Lee WM, Cheng CY. Cytoskeletal dynamics and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1581–1592. doi: 10.1098/rstb.2009.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng CY, Mruk DD. Regulation of spermiogenesis, spermiation and blood-testis barrier dynamics: novel insights from studies on Eps8 and Arp3. Biochem J. 2011;435:553–562. doi: 10.1042/BJ20102121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Edwards M, Zwolak A, Schafer DA, Sept D, Dominguez R, Cooper JA. Capping protein regulators fine-tune actin assembly dynamics. Nat Rev Mol Cell Biol. 2014;15:677–689. doi: 10.1038/nrm3869. **, Capping protein (CP) binds the fast growing barbed end of an actin microfilament, this thus blocks the addition of actin subunits, preventing actin microfilament elongation. This in-depth review provides insightful information on the regulators of CP that fine-tune actin dynamics in mammalian cells.
- 10. Janke C. The tubulin code: molecular components, readout mechanisms, and functions. J Cell Biol. 2014;206:461–472. doi: 10.1083/jcb.201406055. **, This review summarizes recent findings regarding the mechanisms that regulate tubulin heterogeneity such as by posttranslational modifications (PTMs). These modifications generate a "tubulin code" to modulate the complex functions of microtubules in mammalian cells.
- 11.Brouhard GJ, Rice LM. The contribution of alphabeta-tubulin curvature to microtubule dynamics. J Cell Biol. 2014;207:323–334. doi: 10.1083/jcb.201407095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leube RE, Moch M, Windoffer R. Intermediate filaments and the regulation of focal adhesion. Curr Opin Cell Biol. 2014;32C:13–20. doi: 10.1016/j.ceb.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 13. Tang EI, Mruk DD, Cheng CY. MAP/microtubule affinity-regulating kinases, microtubule dynamics, and spermatogenesis. J Endocrinol. 2013;217:R13–R23. doi: 10.1530/JOE-12-0586. *, A detailed account on the role of microtubule regulators that modulate the MT-based cytoskeleton during the epithelial cycle of spermatogenesis regarding the transport of germ cells across the BTB and the adluminal compartment.
- 14. Blanchoin L, Boujemaa-Paterski R, Sykes C, Plastino J. Actin dynamics, architecture, and mechanics in cell motility. Physiol Rev. 2014;94:235–263. doi: 10.1152/physrev.00018.2013. **, Actin microfilaments in mammalian cells are analogous to semi-flexible polymers so that actin-based cytoskeleton can rapidly re-organize in response to changes in environment or external stimuli by altering cell shape, polarity, and locomotion. This recent review provides an in-depth discussion on how the mechanical and biochemical properties of actin microfilaments are being coordinated via ABPs that organize F-actin network in mammalian cells
- 15.Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol Rev. 2002;82:825–874. doi: 10.1152/physrev.00009.2002. [DOI] [PubMed] [Google Scholar]
- 16.Morrow CMK, Mruk DD, Cheng CY, Hess RA. Claudin and occludin expression and function in the seminiferous epithelium. Philos Trans R Soc Lond B Biol Sci. 2010;365:1679–1696. doi: 10.1098/rstb.2010.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li MWM, Mruk DD, Cheng CY. Gap junctions and blood-tissue barriers. Adv Exp Med Biol. 2012;763:260–280. doi: 10.1007/978-1-4614-4711-5_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garcia-Ponce A, Citalan-Madrid Af, Velazquez-Avila M, Vargas-SRobles H, Schnoor M. The role of actin-binding proteins in the control of endothelial barrier integrity. Thromb Haemost. 2015;113:20–36. doi: 10.1160/TH14-04-0298. *, An excellent review on the role of ABPs in regulating endothelial cell contacts and tight junction-permeability, thereby modulating vascular permeability function of blood vessels.
- 19. Artman L, Dormoy-Raclet V, von Roretz C, Gallouzi IE. Planning your every move: the role of β-actin and its post-transcriptional regulation in cell motility. Semin Cell Dev Biol. 2014;34:33–43. doi: 10.1016/j.semcdb.2014.05.012. *, This reviews provides an excellent account on the mechanisms via post-transcriptional regulation of ß-actin that affects cell motility.
- 20.Winters SJ, Ayscough KR. Actin-binding proteins. J Cell Sci. 2005;118:651–654. doi: 10.1242/jcs.01670. [DOI] [PubMed] [Google Scholar]
- 21.Takeichi M. Dynamic contacts: rearranging adherens junctions to drive epithelial remodelling. Nat Rev Mol Cell Biol. 2014;15:397–410. doi: 10.1038/nrm3802. [DOI] [PubMed] [Google Scholar]
- 22.Goley ED, Welch MD. The ARP2/3 complex: an actin nucleator comes of age. Nature Rev Mol Cell Biol. 2006;7:713–726. doi: 10.1038/nrm2026. [DOI] [PubMed] [Google Scholar]
- 23.Krause M, Gautreau A. Steering cell migration: lamellipodium dynamics and the regulation of directional persistence. Nat Rev Mol Cell Biol. 2014;15:577–590. doi: 10.1038/nrm3861. [DOI] [PubMed] [Google Scholar]
- 24.Weaver AM, Young ME, Lee W, Cooper JA. Integration of signals to the Arp2/3 complex. Curr Opin Cell Biol. 2003;15:23–30. doi: 10.1016/s0955-0674(02)00015-7. [DOI] [PubMed] [Google Scholar]
- 25.Murphy DA, Coutneidge SA. The 'ins' and 'outs' of podosomes and invadopodia: chararcteristics, formation and function. Nat Rev Mol Cell Biol. 2011;12:413–426. doi: 10.1038/nrm3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fried S, Matalon O, Noy E, barda-Saad M. WIP: more than a WASp-interacting protein. J Leukoc Biol. 2014;96:713–727. doi: 10.1189/jlb.2RU0314-162R. [DOI] [PubMed] [Google Scholar]
- 27.Rotkopf S, Hamberg Y, Aigaki T, Snapper SB, Shilo BZ, Shejter ED. The WASp-based actin polymerization machinery is required in somatic support cells for spermatid maturation and release. Development. 2011;138:2729–2739. doi: 10.1242/dev.059865. [DOI] [PubMed] [Google Scholar]
- 28. Xiao X, Mruk DD, Tang EI, Massarwa R, Mok KW, Li N, Wong CK, Lee WM, Snapper SB, Shilo BZ, et al. N-WASP is required for structural integrity of the blood-testis barrier. PLoS Genet. 2014;10:e1004447. doi: 10.1371/journal.pgen.1004447. *, This study reports findings based on the use of a genetic model of deletion of N-WASP in Sertoli cells in the mouse testis, thereby disrupting the ability of barbed end nucleation of an actin microfilament. This thus eliminates the plasticity of actin-based cytoskeleton in which actin microfilaments fail to convert from a bundled to an unbundled/branched configuration. Meiosis was considerably impaired in the Sertoli cell-specific N-WASP KO mice since some round spermatids were detected, but an arrest in spermiogenesis was detected, leading to infertility in these KO mice.
- 29.Lie PPY, Chan AYN, Mruk DD, Lee WM, Cheng CY. Restricted Arp3 expression in the testis prevents blood-testis barrier disruption during junction restructuring at spermatogenesis. Proc Natl Acad Sci USA. 2010;107:11411–11416. doi: 10.1073/pnas.1001823107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lie PPY, Mruk DD, Mok KW, Su L, Lee WM, Cheng CY. Focal adhesion kinase-Tyr407 and -Tyr397 exhibit antagonistic effects on blood-testis barrier dynamics in the rat. Proc Natl Acad Sci USA. 2012;109:12562–12567. doi: 10.1073/pnas.1202316109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vauti F, Prochnow BR, Freese E, Ramasamy SK, Ruiz P, Arnold HH. Arp3 is required during preimplantation development of the mouse embryo. FEBS Lett. 2007;581:5691–5697. doi: 10.1016/j.febslet.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 32.Scita G, Nordstrom J, Carbone R, Tenca P, Giardina G, Gutkind S, Bjarnegard M, Betsholtz C, Di Fiore PP. EPS8 and E3B1 transduce signals from Ras to Rac. Nature. 1999;401:290–293. doi: 10.1038/45822. [DOI] [PubMed] [Google Scholar]
- 33.Tocchetti A, Soppo CB, Zani F, Bianchi F, Gagliani MC, Pozzi B, Rozman J, Elvert R, Ehrhardt N, Rathkolb B, et al. Loss of the actin remodeler Eps8 causes intestinal defects and improved metabolic status in mice. PLoS One. 2010;5:e9468. doi: 10.1371/journal.pone.0009468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamura A, Kikuchi S, Hata M, Katsuno T, Matsui T, Hayashi H, Suzuki Y, Noda T, Tsukita S. Achlorhydria by ezrin knockdown: defects in the formation/expansion of apical canaliculi in gastric parietal cells. J Cell Biol. 2005;169:21–28. doi: 10.1083/jcb.200410083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saotome I, Curto M, McClatchey AI. Ezrin is essential for epithelial organization and villus morphogenesis in the developing intestine. Dev Cell. 2004;6:855–864. doi: 10.1016/j.devcel.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 36.Yamakita Y, Matsumura F, Yamashiro S. Fascin1 is dispensable for mouse development but is favorable for neonatal survival. Cell Motil Cytoskeleton. 2009;66:524–534. doi: 10.1002/cm.20356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feng Y, Chen MH, Moskowitz IP, Mendonza AM, Vidali L, Nakamura F, Kwiatkowski DJ, Walsh CA. Filamin A (FLNA) is required for cell-cell contact in vascular development and cardiac morphogenesis. Proc Natl Acad Sci U S A. 2006;103:19836–19841. doi: 10.1073/pnas.0609628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo H, Liu X, Wang F, Huang Q, Shen S, Wang L, Xu G, Sun X, Kong H, Gu M, et al. Disruption of palladin results in neural tube closure defects in mice. Mol Cell Neurosci. 2005;29:507–515. doi: 10.1016/j.mcn.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Gropman AL, Elsea S, Duncan WC, Jr, Smith AC. New developments in Smith-Magenis syndrome (del 17p11.2) Curr Opin Neurol. 2007;20:125–134. doi: 10.1097/WCO.0b013e3280895dba. [DOI] [PubMed] [Google Scholar]
- 40.Elsea SH, Girirajan S. Smith-Magenis syndrome. Eur J Hum Genet. 2008;16:412–421. doi: 10.1038/sj.ejhg.5202009. [DOI] [PubMed] [Google Scholar]
- 41.Toulouse A, Rochefort D, Roussel J, Joober R, Rouleau GA. Molecular cloning and characterization of human RAI1, a gene associated with schizophrenia. Genomics. 2003;82:162–171. doi: 10.1016/s0888-7543(03)00101-0. [DOI] [PubMed] [Google Scholar]
- 42.Hayes S, Turecki G, Brisebois K, Lopes-Cendes I, Gaspar C, Riess O, Ranum LP, Pulst SM, Rouleau GA. CAG repeat length in RAI1 is associated with age at onset variability in spinocerebellar ataxia type 2 (SCA2) Hum Mol Genet. 2000;9:1753–1758. doi: 10.1093/hmg/9.12.1753. [DOI] [PubMed] [Google Scholar]
- 43.Nakamine A, Ouchanov L, Jimenez P, Manghi ER, Esquivel M, Monge S, Fallas M, Burton BK, Szomju B, Elsea SH, et al. Duplication of 17(p11.2p11.2) in a male child with autism and severe language delay. Am J Med Genet A. 2008;146A:636–643. doi: 10.1002/ajmg.a.31636. [DOI] [PubMed] [Google Scholar]
- 44.Potocki L, Bi W, Treadwell-Deering D, Carvalho CM, Eifert A, Friedman EM, Glaze D, Krull K, Lee JA, Lewis RA, et al. Characterization of Potocki-Lupski syndrome (dup(17)(p11.2p11.2)) and delineation of a dosage-sensitive critical interval that can convey an autism phenotype. Am J Hum Genet. 2007;80:633–649. doi: 10.1086/512864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Razinia Z, Makela T, Ylanne J, Calderwood DA. Filamins in mechanosensing and signaling. Annu Rev Biophys. 2012;41:227–246. doi: 10.1146/annurev-biophys-050511-102252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Modarres HP, Mofradt MR. Filamin: a structural and functional biomolecule with important roles in cell biology, signaling and mechanics. Mol Cell Biomech. 2014;11:39–65. [PubMed] [Google Scholar]
- 47. Savoy RM, Ghosh PM. The dual role of filamin A in cancer: can't live with (too much of) it, can't live without it. Endocr Relat Cancer. 2013;20:R341–R356. doi: 10.1530/ERC-13-0364. *, A detailed review on the multifunctional role of actin cross-linking protein filamin A that serves as a cancer promoting protein and a cancer suppressor depending in part on its subcellular localization in mammalian cells.
- 48.Kim H, McCulloch CA. Filamin A mediates interactions between cytoskeletal proteins that control cell adhesion. FEBS Lett. 2011;585:18–22. doi: 10.1016/j.febslet.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 49.Hartwig JH, Stossel TP. Isolation and properties of actin, myosin and a new actin binding protein in rabbit alveolar macrophages. J Biol Chem. 1975;250:5696–5705. [PubMed] [Google Scholar]
- 50.Su WH, Mruk DD, Cheng CY. Filamin A: a regulator of blood-testis barrier assembly during post-natal development. Spermatogenesis. 2012;2:73–78. doi: 10.4161/spmg.20223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakamura F, Stossel TP, Hartwig JH. The filamins. Organizers of cell structure and function. Cell Adh Migr. 2011;5:160–169. doi: 10.4161/cam.5.2.14401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stossel TP, Condeelis J, Cooley L, Hartwig JH, Noegel A, Schleicher M, Shapiro SS. Filamins as integrators of cell mechanics and signalling. Nat Rev Mol Cell Biol. 2001;2:138–145. doi: 10.1038/35052082. [DOI] [PubMed] [Google Scholar]
- 53.Zhou AX, Hartwig JH, Akyurek LM. Filamins in cell signaling, transcription and organ development. Trends Cell Biol. 2010;20:113–123. doi: 10.1016/j.tcb.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 54.Whitmarsh AJ. Filamin B: a scaffold for interferon signalling. EMBO Reports. 2009;10:349–351. doi: 10.1038/embor.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Djinovic-Carugo K, Carugo O. Structural portrait of filamin interaction mechanisms. Curr Protein Pept Sci. 2010;11:639–650. doi: 10.2174/138920310794109111. [DOI] [PubMed] [Google Scholar]
- 56.van der Flieer A, Sonnenberg A. Structural and functional aspects of filamins. Biochem Biophys Acta. 2001;1538:99–117. doi: 10.1016/s0167-4889(01)00072-6. [DOI] [PubMed] [Google Scholar]
- 57.Su WH, Mruk DD, Lie PPY, Lui WY, Cheng CY. Filamin A is a regulator of blood-testis barrier assembly during postnatal development in the rat testis. Endocrinology. 2012;153:5023–5035. doi: 10.1210/en.2012-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Di Fiore PP, Scita G. Eps8 in the midst of GTPases. Int J Biochem Cell Biol. 2002;34:1178–1183. doi: 10.1016/s1357-2725(02)00064-x. [DOI] [PubMed] [Google Scholar]
- 59.Ahmed S, Goh WI, Bu W. I-BAR domains, IRSp53 and filopodium formation. Semin Cell Dev Biol. 2010;21:350–356. doi: 10.1016/j.semcdb.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 60.Li YH, Xue TY, He YZ, Du JW. Novel oncoprotein EPS8: a new target for anticancer therapy. Future Oncol. 2013;9:1587–1594. doi: 10.2217/fon.13.104. [DOI] [PubMed] [Google Scholar]
- 61.Lie PPY, Mruk DD, Lee WM, Cheng CY. Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood-testis barrier integrity in the seminiferous epithelium. FASEB J. 2009;23:2555–2567. doi: 10.1096/fj.06-070573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mykkanen OM, Gronholm M, Ronty M, Lalowski M, Salmikangas P, Suila H, Carpen O. Characterization of human palladin, a microfilament-associated protein. Mol Biol Cell. 2001;12:3060–3073. doi: 10.1091/mbc.12.10.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Otey CA, Rachlin A, Moza M, Arneman D, Carpen O. The palladin/myotilin/myopalladin family of actin-associated scaffolds. Int Rev Cytol. 2005;246:31–58. doi: 10.1016/S0074-7696(05)46002-7. [DOI] [PubMed] [Google Scholar]
- 64.Dixon RD, Arneman DK, Rachlin AS, Sundaresan NR, Costello MJ, Campbell SL, Otey CA. Palladin is an actin cross-linking protein that uses immunoglobulin-like domains to bind filamentous actin. J Biol Chem. 2008;283:6222–6231. doi: 10.1074/jbc.M707694200. [DOI] [PubMed] [Google Scholar]
- 65.Qian X, Mruk DD, Cheng YH, Cheng CY. Actin cross-linking protein palladin and spermatogenesis. Spermatogenesis. 2013;3:e23473. doi: 10.4161/spmg.23473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Niedenberger BA, Chappell VA, Otey CA, Geyer CB. Actin dynamics regulate subcellular localization of the F-actin-binding protein PALLD in mouse Sertoli cells. Reproduction. 2014;148:333–341. doi: 10.1530/REP-14-0147. [DOI] [PubMed] [Google Scholar]
- 67.Qian X, Mruk DD, Wong EWP, Lie PPY, Cheng CY. Palladin is a regulator of actin filament bundles at the ectoplasmic specialization in the rat testis. Endocrinology. 2013;154:1907–1920. doi: 10.1210/en.2012-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Xiao X, Mruk DD, Wong EWP, Lee WM, Han D, Wong CKC, Cheng CY. Differential effects of c-Src and c-Yes on the endocytic vesicle-mediated trafficking events at the Sertoli cell blood-testis barrier: an in vitro study. Am J Physiol Endocrinol Metab. 2014;307:E553–E562. doi: 10.1152/ajpendo.00176.2014. *, This report demonstrates the contrasting roles of c-Src and c-Yes on the endocytic vescile-mediated protein trafficking events in Sertoli cells that regulate BTB dynamics during spermatogenesis.
- 69.Kutty RK, Chen S, Samuel W, Vijayasarathy C, Duncan T, Tsai JY, Fariss RN, Carper D, Jaworski C, Wiggert B. Cell density-dependent nuclear/cytoplasmic localization of NORPEG (RAI14) protein. Biochem Biophys Res Commun. 2006;345:1333–1341. doi: 10.1016/j.bbrc.2006.04.184. [DOI] [PubMed] [Google Scholar]
- 70.Kutty RK, Kutty G, Samuel W, Duncan T, Bridges CC, El-Sherbeeny A, Nagineni CN, Smith SB, Wiggert B. Molecular characterization and developmental expression of NORPEG, a novel gene induced by retinoic acid. J Biol Chem. 2001;276:2831–2840. doi: 10.1074/jbc.M007421200. [DOI] [PubMed] [Google Scholar]
- 71.Yuan W, Zheng Y, Huo R, Lu L, Huang XY, Yin LL, Li JM, Zhou ZM, Sha JH. Expression of a novel alternative transcript of the novel retinal pigment epithelial cell gene NORPEG in human testes. Asian J Androl. 2005;7:277–288. doi: 10.1111/j.1745-7262.2005.00040.x. [DOI] [PubMed] [Google Scholar]
- 72.Peng YF, Mandai K, Sakisaka T, Okabe N, Yamamoto Y, Yokoyama S, Mizoguchi A, Shiozaki H, Monden M, Takai Y. Ankycorbin: a novel actin cytoskeleton-associated protein. Genes Cells. 2000;5:1001–1008. doi: 10.1046/j.1365-2443.2000.00381.x. [DOI] [PubMed] [Google Scholar]
- 73.Qian X, Mruk DD, Cheng YH, Cheng CY. RAI14 (retinoic acid induced protein 14) is an F-actin regulator - lesson from the testis. Spermatogenesis. 2013;3:e24824. doi: 10.4161/spmg.24824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Qian X, Mruk DD, Cheng CY. Rai14 (retinoic acid induced protein 14) is involved in regulating F-actin dynamics at the ectoplasmic specialization in the rat testis. PLoS One. 2013;8:e60656. doi: 10.1371/journal.pone.0060656. *, This report demonstrates Rai14 is involved in the organization of actin microfilaments in Sertoli cells, and its knockdown also perturbs Sertoli cell tight junction permeability barrier function.
- 75.Fehon RG, McClatchey AI, Bretscher A. Organizing the cell cortex: the role of ERM proteins. Nat Rev Mol Cell Biol. 2010;11:276–287. doi: 10.1038/nrm2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McClatchey AI, Fehon RG. Merlin and the ERM proteins: regulators of receptor distribution and signaling at the cell cortrex. Trends Cell Biol. 2009;19:198–206. doi: 10.1016/j.tcb.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Adada M, Canals D, Hannun YA, Obeid LM. Sphingolipid regulation of ezrin, radixin, and moesin proteins family: implications for cell dynamics. Biochem Biophys Acta. 2014;1841:727–737. doi: 10.1016/j.bbalip.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gungor-Ordueri NE, Celik-Ozenci C, Cheng CY. Ezrin: a regulator of actin microfilaments in cell junctions of the rat testis. Asian J Androl. 2015;17 doi: 10.4103/1008-682X.146103. in press. *, A brief reviw on the likely role of actin binding protein ezrin that tethers integral membrane proteins and their peripheral adaptors to actin microfilaments in Sertoli cells during spermatogenesis.
- 79.Wakayama T, Nakata H, Kurobo M, Sai Y, Iseki S. Expression, localization, and binding activity of the ezrin/radixin/moesin proteins in the mouse testis. J Histochem Cytochem. 2009;57:351–362. doi: 10.1369/jhc.2008.952440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gungor-Ordueri NE, Tang EI, Celik-Ozenci C, Cheng CY. Ezrin is an actin binding protein that regulates Sertoli cell and spermatid adhesion during spermatogenesis. Endocrinology. 2014;155:3981–3995. doi: 10.1210/en.2014-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Loewenstein WR. Junctional intercellular communication: the cell-to-cell membrane channel. Physiol Rev. 1981;61:829–913. doi: 10.1152/physrev.1981.61.4.829. [DOI] [PubMed] [Google Scholar]
- 82. Austefjord MW, Gerdes HH, Wang X. Tunneling nanotubes: Diversity in morphology and structure. Commun Integr Biol. 2014;7:e27934. doi: 10.4161/cib.27934. *, This is an in-depth review on the role of TNTs (tunneling nanotubes, also known as intercellular bridges) that provide signaling function between distant epithelial/endothelial cells in health and in diseases such as carcinogenesis.
- 83.Hashimoto Y, Kim DJ, Adams JC. The roles of fascins in health and disease. J Pathol. 2011;224:289–300. doi: 10.1002/path.2894. [DOI] [PubMed] [Google Scholar]
- 84.Kureishy N, Sapountzi V, Prag S, Anilkumar N, Adams JC. Fascins, and their roles in cell structure and function. Bioessays. 2002;24:350–361. doi: 10.1002/bies.10070. [DOI] [PubMed] [Google Scholar]
- 85.Edwards RA, Bryan J. Fascins, a family of actin bundling proteins. Cell Motil Cytoskeleton. 1995;32:1–9. doi: 10.1002/cm.970320102. [DOI] [PubMed] [Google Scholar]
- 86.Tubb B, Mulholland DJ, Volg AW, Lan Z, Niederberger C, Cooney A, Bryan J. Testis fascin (FSCN3): a novel paralog of the actin-bundling protein fascin expressed specifically in the elongate spermatid head. Exp Cell Res. 2002;275:92–109. doi: 10.1006/excr.2002.5486. [DOI] [PubMed] [Google Scholar]
- 87. Gungor-Ordueri NE, Celik-Ozenci C, Cheng CY. Fascin 1 is an actin filament-bundling protein that regulates ectoplasmic specialization dynamics in the rat testis. Am J Physiol Endocrinol Metab. 2014;307:E738–E753. doi: 10.1152/ajpendo.00113.2014. *, A functional study that reports findings on the actin bundling protein fascin 1 in cell adhesion function in the testis via its effects on actin microfilament bundles at the ectoplasmic specialization.
- 88.Gautreau A, Oguievetskaia K, Ungermann C. Function and regulation of the endosomal fusion and fission machineries. Cold Spring Harb Perspect Biol. 2014;6.pii:a016832. doi: 10.1101/cshperspect.a016832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Collinet C, Lecuit T. Stability and dynamics of cell-cell junctions. Prog Mol Biol Transl Sci. 2013;116:25–47. doi: 10.1016/B978-0-12-394311-8.00002-9. *, An in-depth account on the interplay between cadherin-based AJs and contractile actomyosin networks that modulate epithelial funciton such as morphogenesis.
- 90.Su L, Mruk DD, Lie PPY, Silvestrini B, Cheng CY. A peptide derived from laminin-γ3 reversibly impairs spermatogenesis in rats. Nat Communs. 2012;3:1185. doi: 10.1038/ncomms2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xiao X, Mruk DD, Lee WM, Cheng CY. c-Yes regulates cell adhesion at the blood-testis barrier and the apical ectoplasmic specialization in the seminiferous epithelium of rat testes. Int J Biochem Cell Biol. 2011;43:651–665. doi: 10.1016/j.biocel.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Xiao X, Mruk DD, Cheng CY. c-Yes regulates cell adhesion at the apical ectoplasmic specialization-blood-testis barrier axis via its effects on protein recruitment and distribution. Am J Physiol Endocrinol Metab. 2013;304:E145–E159. doi: 10.1152/ajpendo.00422.2012. *, A study that illustrates c-Yes is involved in BTB dynamics by regulating protein recruitment and distribution at the basal ES.
- 93.Cheng CY, Wong EWP, Lie PPY, Li MWM, Su L, Siu ER, Yan HHN, Mannu J, Mathur PP, Bonanomi M, et al. Environmental toxicants and male reproductive function. Spermatogenesis. 2011;1:2–13. doi: 10.4161/spmg.1.1.13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cheng CY. Toxicants target cell junctions in the testis - insights from the indazole-carboxylic acid model. Spermatogenesis. 2014;4:e981485. doi: 10.4161/21565562.2014.981485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Johnson KJ. Testicular histopathology associated with disruption of the Sertoli cell cytoskeleton. Spermatogenesis. 2014;4:e979106. doi: 10.4161/21565562.2014.979106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.O'Donnell L. Mechanisms of spermatogenesis and spermiatiokn and how they are disturbed. Spermatogenesis. 2014;4:e979623. doi: 10.4161/21565562.2014.979623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lui WY, Wong CH, Mruk DD, Cheng CY. TGF-β3 regulates the blood-testis barrier dynamics via the p38 mitogen activated protein (MAP) kinase pathway: an in vivo study. Endocrinology. 2003;144:1139–1142. doi: 10.1210/en.2002-0211. [DOI] [PubMed] [Google Scholar]
- 98.Wong CH, Mruk DD, Lui WY, Cheng CY. Regulation of blood-testis barrier dynamics: an in vivo study. J Cell Sci. 2004;117:783–798. doi: 10.1242/jcs.00900. [DOI] [PubMed] [Google Scholar]
- 99.Wong CH, Mruk DD, Siu MKY, Cheng CY. Blood-testis barrier dynamics are regulated by α2-macroglobulin via the c-Jun N-terminal protein kinase pathway. Endocrinology. 2005;146:1893–1908. doi: 10.1210/en.2004-1464. [DOI] [PubMed] [Google Scholar]
- 100. Xiao X, Mruk DD, Tang EI, Wong CKC, Lee WM, John CM, Turek PJ, Silvestrini B, Cheng CY. Environmental toxicants perturb human Serotli cell adhesive function via changes in F-actin organization medicated by actin regulatory proteins. Hum Reprod. 2014;29:1279–1291. doi: 10.1093/humrep/deu011. *, This is a report using human Sertoli cells as a model to study BTB function. This study illustrates that BTB disruption induced by environmental toxicants is mediated by changes in the spatiotemporal expression of actin binding proteins, such as Arp3 and Eps8, which in turn disrupts the actin-based cytoskeleton.
- 101. Wan HT, Mruk DD, Wong CKC, Cheng CY. Perfluorooctanesulfonate (PFOS) perturbs male rat Sertoli cell blood-testis barrier function by affecting F-actin organization via p-FAK-Tyr407 - an in vitro study. Endocrinology. 2014;155:249–262. doi: 10.1210/en.2013-1657. *, This study examines the underlying molecular mechanism by which PFOS perturbs Sertoli cell BTB function through changes in the expression and/or spatiotemporal distribution of p-FAK-Tyr407 and actin binding proteins such as Eps8 and palladin.