Abstract
Background
Dietary vitamin D3 (D3) restriction reduces paw grip endurance and motor performance in G93A mice, and increases inflammation and apoptosis in the quadríceps of females. ALS, a neuromuscular disease, causes progressive degeneration of motor neurons in the brain and spinal cord.
Objective
We analyzed the spinal cords of G93A mice following dietary D3 restriction at 2.5% the adequate intake (AI) for oxidative damage (4-HNE, 3-NY), antioxidant enzymes (SOD2, catalase, GPx1), inflammation (TNF-α, IL-6, IL-10), apoptosis (bax/bcl-2 ratio, cleaved/pro-caspase 3 ratio), neurotrophic factor (GDNF) and neuron count (ChAT, SMI-36/SMI-32 ratio).
Methods
Beginning at age 25 d, 42 G93A mice were provided food ad libitum with either adequate (AI;1 IU D3/g feed; 12 M, 11 F) or deficient (DEF; 0.025 IU D3/g feed; 10 M, 9 F) D3. At age 113 d, the spinal cords were analyzed for protein content. Differences were considered significant at P ≤ 0.10, since this was a pilot study.
Results
DEF mice had 16% higher 4-HNE (P = 0.056), 12% higher GPx1 (P = 0.057) and 23% higher Bax/Bcl2 ratio (P = 0.076) vs. AI. DEF females had 29% higher GPx1 (P = 0.001) and 22% higher IL-6 (P = 0.077) vs. AI females. DEF males had 23% higher 4-HNE (P = 0.066) and 18% lower SOD2 (P = 0.034) vs. AI males. DEF males had 27% lower SOD2 (P = 0.004), 17% lower GPx1 (P = 0.070), 29% lower IL-6 (P = 0.023) and 22% lower ChAT (P = 0.082) vs. DEF females.
Conclusion
D3 deficiency exacerbates disease pathophysiology in the spinal cord of G93A mice, the exact mechanisms are sex-specific. This is in accord with our previous results in the quadriceps, as well as functional and disease outcomes.
Introduction
Amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig’s disease, is the most commonly occurring adult-onset motor neuron disease of unknown cause [1,2] and is typically diagnosed between 45 and 60 years of age [3,4]. It is characterized by degeneration of upper and lower motor neurons, resulting in skeletal muscle atrophy [5] and death by respiratory failure within 3–5 years of initial symptoms [6–8]. 90% of cases are of unknown etiology (sporadic ALS) [3,9], whereas the other 10% have inherited genetic mutations [3,10] (familial ALS), ~12% of these cases being a result of a mutation in the Cu2+/Zn2+ super-oxide dismutase 1 (SOD1) gene [11–14]. The most commonly used animal model of ALS is the G93A mouse model [15] that transgenically overexpresses the mutant SOD1 gene [10]. Their disease pathology and neurodegenerative patterns closely resemble that which is found in ALS patients [10]. On a cellular level, excessive stimulation of glutamate receptors [16] leads to a large influx of calcium ion into the post synaptic neuron, resulting in a destructive cascade of membrane, cytoplasmic and nuclear events [17]. These include oxidative damage [18,19], oxidative stress [20,21], inflammation [22], compromised neurotrophic factor release [22] and apoptosis [13].
Some nutrition-based interventions have shown effectiveness in mitigating ALS disease severity in animal models of ALS [23]. Vitamin D is a fat-soluble vitamin with hormone-like properties that is essential for health, growth and development [24]. Vitamin D3 and/or its metabolites [calcidiol (25(OH)D3) and calcitriol (1,25(OH)2D3)] can protect dopaminergic neurons against the neurotoxic effects of glutamate and dopaminergic toxins [25], and has anti-inflammatory and modulatory effects on CNS components such as neurotrophins and growth factors [26]. Vitamin D treatment can improve compromised functional outcomes and muscle physiology in humans and rodents, whereas vitamin D receptor (VDR) knockout mice have loss of motor function and muscle mass [27]. Vitamin D reduces the expression of biomarkers associated with oxidative stress and inflammation in diseases that share common pathophysiologies with ALS. Vitamin D deficiency has been associated with the development of inflammatory and immune diseases such as type II diabetes [28], multiple sclerosis [29], dementia and Alzheimer’s disease [30]. A deficiency in vitamin D reduces the amount of calcium buffering protein, thus leading to higher lipid peroxidation and protein damage [31]. When investigating the effects of vitamin D on biomarkers of oxidative stress in obese children aged 7–14 y, obese children with 25(OH)D insufficiency (serum calcidiol <50 nmol/L) had significantly elevated 3-nitrotyrosine (3-NY) levels, a marker of protein damage, vs. non-deficient obese children (serum calcidiol >50 nmol/L) [32]. A partial correlation analysis showed an inverse relationship between 25(OH)D and 3-NY (r = -0.424, P = 0.001).
A retrospective study in ALS patients found that those with serum calcidiol levels <25 nmol/L increased their death rate by 6 fold and their rate of decline by 4 times, and were associated with a marked shorter life expectancy compared to patients with serum calcidiol levels >75 nmol/L [33]. We have previously demonstrated the detrimental effects of vitamin D3 restriction in the G93A mouse model of ALS [34–37]. Dietary vitamin D3 at 2.5% the adequate intake (AI) resulted in lower paw grip endurance (PaGE) and motor performance [37], and in the quadriceps of female G93A resulted in increased inflammation [35] and apoptosis [36], when compared to their AI counterparts.
Does vitamin D3 restriction directly impact the CNS? And, if it does, will vitamin D3 deficiency explain the functional outcomes in our previous study [37]. Hence, the objective of this study was to investigate the effects of vitamin D deficiency via dietary restriction (0.025 IU/g feed) vs. adequate intake (1 IU/g feed) on oxidative damage, antioxidant capacity, inflammation, apoptosis, neurotrophic factor and neuron count in the spinal cord of the G93A transgenic mouse model of ALS
Methods
Ethical Statement
The experimental protocol used in this study followed the guidelines of the Canadian Council of Animal Care and was approved by York University Animal Research Ethics Board (protocol # 2007–9). All the necessary steps were taken to minimize suffering and distress to the mice in the study.
Animals
Male B6SJL-TgN(SOD1-G93A)1Gur hemizygous mice (No. 002726) were harem-bred with non-affected female B6SJL control mice (No. 100012; Jackson Laboratory, Bar Harbor, ME). We identified the presence of the human-derived G93A transgene by using polymerase chain reaction (PCR) amplification of DNA extracted from ear tissue as outlined by Sigma-Aldrich (XNAT REDExtract-N-Amp Tissue PCR Kit; XNAT-1KT). All breeding mice were housed 3 females per 1 male, and consumed Research Diet AIN-93G (1 IU D3/g feed; Research Diet, New Brunswick, NJ). All animals were housed individually at age 25 d in a 12 h light/dark cycle.
Study Design
42 (22 M, 20 F) G93A mice consumed a diet that contained an adequate intake of vitamin D3 (1 IU/g feed; Research Diet AIN-93G; Product # D10012G; Research Diets Inc, New Brunswick NJ [38]) ad libitum after weaning (21 d). At age 25 d, the mice were individually caged and divided into one of two groups: 1) adequate vitamin D3 (AI; 1 IU D3/g feed; 12 M, 11 F; Research Diet AIN-93G) or 2) deficient vitamin D3 (DEF; 1/40 IU D3/g feed; 10 M 9 F; Product #D10030801; Research Diets Inc, New Brunswick, NJ) (Table 1).
Table 1. Nutrient content of the adequate intake (AI) and deficient (DEF) vitamin D3 diets.
When the mice reached a clinical score (CS; disease severity) of 3.0, food and calorie-free gel (Harlan-Gel, Harlan Teklad, Madison WI) were placed on the floor of the cage to fulfill ethics requirements. Endpoint was determined as previously described by Solomon et al 2011 [38]. The calorie-free gel contained synthetic polymers (WATER LOCK superabsorbent polymer G-400, G-430, G-500, G-530; 95% by weight) and methanol (4.5% by weight). Two researchers who were blinded to the diets conducted all measurements.
Tissue Collection
At age 113 d, mice were sacrificed and spinal cords were harvested. The mice were placed and kept under anesthesia with gaseous isoflurane as the tissue was collected and placed in individual sterile polyethylene tubes for immediate freezing in liquid nitrogen. Samples were stored at -80°C.
Spinal Cord Homogenization
Spinal cords were weighed, and minced with a glass-Teflon Port-Evenhejm homogenizer (5% wt/vol) in radioimmunoprecipitation assay (RIPA) buffer (1:20) containing 50 mM tris HCL 8.0 (Bioshop, TRS002.500, Burlington, Ontario), 150 mM NaCl (BioBasic Canada, 7647145, Markham, Ontario), 0.1% SDS (Bioshop, SDS001.500, Burlington, Ontario), 0.5% sodium deoxycholate (Bioship, DCA333.50, Burlington, Ontario), 1% NP-40 (Thermo Scientific, 28324, Rockford, Illinois), 5 mM EDTA pH 8.0 (Bioshop, EDT001.500, Burlington, Ontario) and 1 mM PMSF (Sigma-Aldrich, 93482, St. Louis, Missouri). The protease inhibitor cocktail (Roche, 11836153001, Manheim, Germany) was added to the buffer in accordance to manufacturer’s instructions (1:100) prior to homogenization. Mouse spinal cord was homogenized for about 40 grinds using constant force to ensure consistency and homogeneity of samples. Homogenates were divided in roughly equal volumes in eppendorf tubes and were placed on a shaker at 4°C for 30 minutes. The homogenates were then centrifuged at (600 g) for 20 min at 4°C. The resulting supernatant was decanted, put into newly labeled eppendorf tubes and immediately stored at -80°C. The protein concentration was determined using the BCA Protein Assay technique [39]. The supernatant concentration was measured at 562 nm using an ultraviolet spectrophotometer (Cecil 9200 Super Aquarius, Cambridge, UK). Protein concentrations were presented as mg/ml.
Western Blot
Equal amounts of protein were size-separated by 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and were transferred to nitrocellulose membranes (#165–3322, Bio-Rad Mini-PROTEAN 2 electrophoresis system, Mississauga, ON, Canada) at 100 V for 2 h. The membranes were blocked in 3% fat free milk (SMI-36), 5% fat free milk (SOD2, catalase, TNF-α, IL-6) or 5% BSA (4-HNE, 3-NY, GPx1, IL-10, Bax, Bcl-2, pro-caspase 3, cleaved caspase 3, GDNF, ChAT, SMI-32) diluted in Tris-buffered saline with tween (1%) for 2 h at room temperature and incubated with primary antibodies in 3% fat free milk (SMI36), 5% fat free milk (catalase, TNF- α, IL-6), 1% BSA (SOD2, cleaved caspase 3, GNDF, ChAT), 3% BSA (IL-10, SMI32) or 5% BSA (4-HNE, 3-NY, GPx1, Bax, Bcl-2, pro-caspase 3) against 4-HNE (1:800; Abcam, ab46545), 3-NY (1:1000; Abcam, ab110282), SOD2 (1:8000; Abcam, ab13533), catalase (1:3500; Abcam, ab1877-10), GPx1 (1:800; Abcam, 22604), TNF- α (1:2000; Abcam, ab9739) IL-6 (1:1000; Abcam, ab6672), IL-10 (1:2000; Abcam, ab9969), Bax (1:1000; Cell Signaling Technology, 2772), Bcl-2 (1:1000; Cell Signaling Technology, 2870), pro-caspase 3 (1:1000; Millipore, 04–440), cleaved caspase 3 (1:1000; Millipore, 04–439), GDNF (1:1000; Abcam, a18956), ChAT (1:1000; Abcam, ab85609), SMI-32 (1:1000; Abcam, ab28029) and SMI-36 (1:1000; Abcam, ab24572), overnight at 4°C. Each antibody and its corresponding anti-GAPDH set were loaded on a separate gel. Equal loading was verified by ponceau staining, as well as probing for glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 1:100,000; MAB374, Millipore). The antigen-antibody complexes were detected by incubating the membranes in anti-rabbit (1: 5000; Novus Biologicals, NB730-H) or anti-mouse (1:5000; Novus Biologicals, NB7539) HRP conjugated secondary antibodies at room temperature for 2 h in 3% fat free milk (SMI36), 5% fat free milk (4-HNE, catalase, TNF- α, IL-6), 1% BSA (SOD2, cleaved caspase 3, GDNF, ChAT), 3% BSA (IL-10, SMI32) or 5% BSA (3-NY, GPx1, Bax, Bcl-2, pro-caspase 3). Immunoreactive proteins were visualized with enhanced chemiluminescence (sc-2048, Santa Cruz Biotechnology), and scanned using Kodak Imaging Station 4000MM Pro (Carestream Health, Inc. Rochester, NY, USA). Protein intensity was standardized to GAPDH and analyzed using Carestream MI (v 5.0.2.30, NY, USA). Representative western blot bands for the biomarkers are found in S1 Fig.
Calculations
Human equivalent dosage (HED) was calculated according to the US FDA [40]:
Statistical analysis
We established planned comparisons between DEF vs. AI. A one-tailed independent t-test was used to determine differences between the diets within each sex, because we hypothesized a priori that absolute and body weight-adjusted spinal cord weight, antioxidant activity, neurotrophic factors and neuronal count would be lower in DEF vs. AI; whereas oxidative stress and apoptosis would be higher in DEF vs. AI. These are based on studies conducted by us and other researchers [23,27,32,34–38,41–45]. A Student's t-test was used to determine diet and sex differences in absolute and body weight-adjusted tibialis anterior, quadriceps and brain weights. All statistical analyses were completed using GraphPad Prism 6 for Macintosh (GraphPad Software Inc, La Jolla, CA). Data were presented as means ± standard error of mean (SEM). Significance was set to P ≤ 0.10, since this was a pilot study.
Results
Oxidative Damage
4-HNE
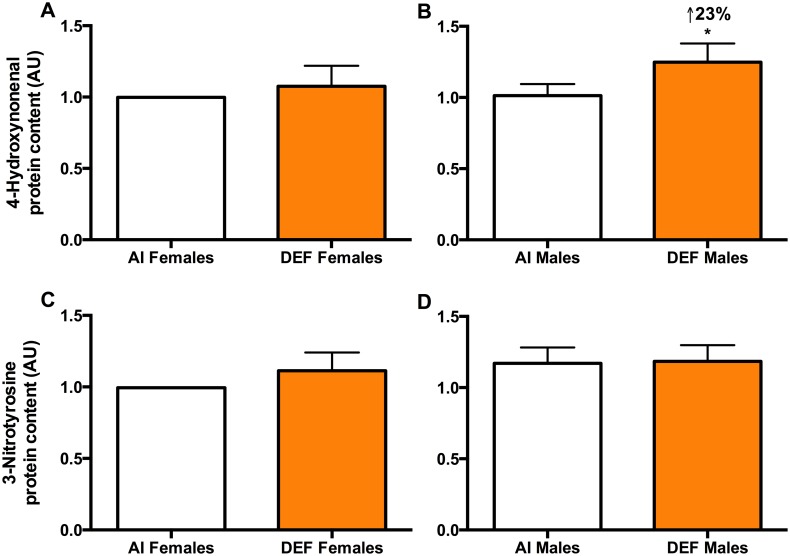
DEF mice had 16% higher 4-HNE protein content vs. AI (P = 0.056). DEF males had 23% higher 4-HNE protein content vs. AI males (P = 0.066) (Fig 1B).
Fig 1. Oxidative damage in DEF vs. AI G93A mice.
4-HNE (A and B) and 3-NY (C and D) protein content (arbitrary units; AU) in spinal cord of 42 G93A mice: 23 adequate vitamin D3 intake (AI; 1 IU D3/g feed; 12 M, 11 F) and 19 deficient vitamin D3 intake (DEF; 0.025 IU D3/g feed; 10 M, 9 F). 4-Hydroxynonenal (4-HNE, A and B): DEF mice had 16% higher 4-HNE protein content vs. AI (P = 0.056). DEF males had 23% higher 4-HNE protein content vs. AI males (P = 0.066). 3-Nitrotyrosine (3-NY, C and D): There was no significant difference in 3-NY protein content between the diets. AI males had 18% higher 3-NY protein content vs. AI females (P = 0.073). Data presented as means ± SEM.
3-NY
There was no significant difference in 3-NY protein content between the diets (Fig 1C and 1D). AI males had 18% higher 3-NY protein content vs. AI females (P = 0.073). Data presented as means ± SEM.
Antioxidant Enzymes
SOD2
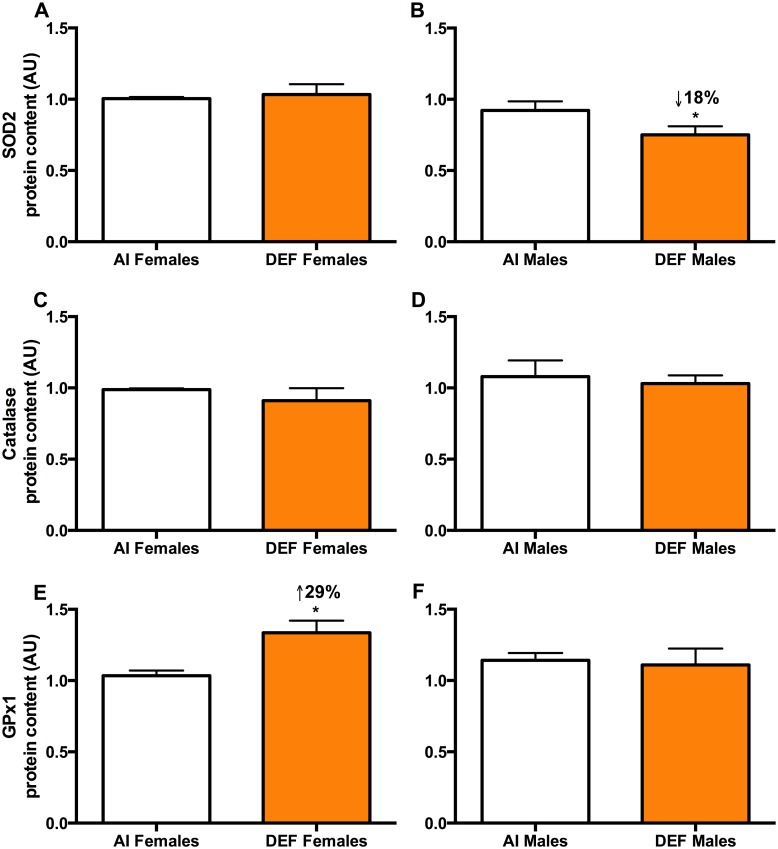
DEF males had 18% lower SOD2 protein content vs. AI males (P = 0.034) (Fig 2B). DEF males had 27% lower SOD2 protein content vs. DEF females (P = 0.004).
Fig 2. Antioxidant enzymes in DEF vs. AI G93A mice.
SOD2 (A and B), catalase (C and D) and GPx1 (E and F) protein content (arbitrary units; AU) in spinal cord of 42 G93A mice: 23 adequate vitamin D3 intake (AI; 1 IU D3/g feed; 12 M, 11 F) and 19 deficient vitamin D3 intake (DEF; 0.025 IU D3/g feed; 10 M, 9 F). SOD2 (A and B): DEF males had 18% lower SOD2 protein content vs. AI males (P = 0.034). DEF males had 27% lower SOD2 protein content vs. DEF females (P = 0.004). Catalase (C and D): There was no significant difference in catalase protein content between the diets or between the sexes. GPx1 (E and F): DEF mice had 12% higher GPx1 protein content vs. AI (P = 0.057). DEF females had 29% higher GPx1 protein content vs. AI females (P = 0.001). AI males had 10% higher GPx1 protein content vs. AI females (P = 0.054). DEF males had 17% lower GPx1 protein content vs. DEF females (P = 0.070). Data presented as means ± SEM.
Catalase
There was no significant difference in catalase protein content between the diets or between the sexes (Fig 2C and 2D)
GPx1
DEF mice had 12% higher GPx1 protein content vs. AI (P = 0.057). DEF females had 29% higher GPx1 protein content vs. AI females (P = 0.001) (Fig 2E). AI males had 10% higher GPx1 protein content vs. AI females (P = 0.054). DEF males had 17% lower GPx1 protein content vs. DEF females (P = 0.070).
Inflammation
TNF- α
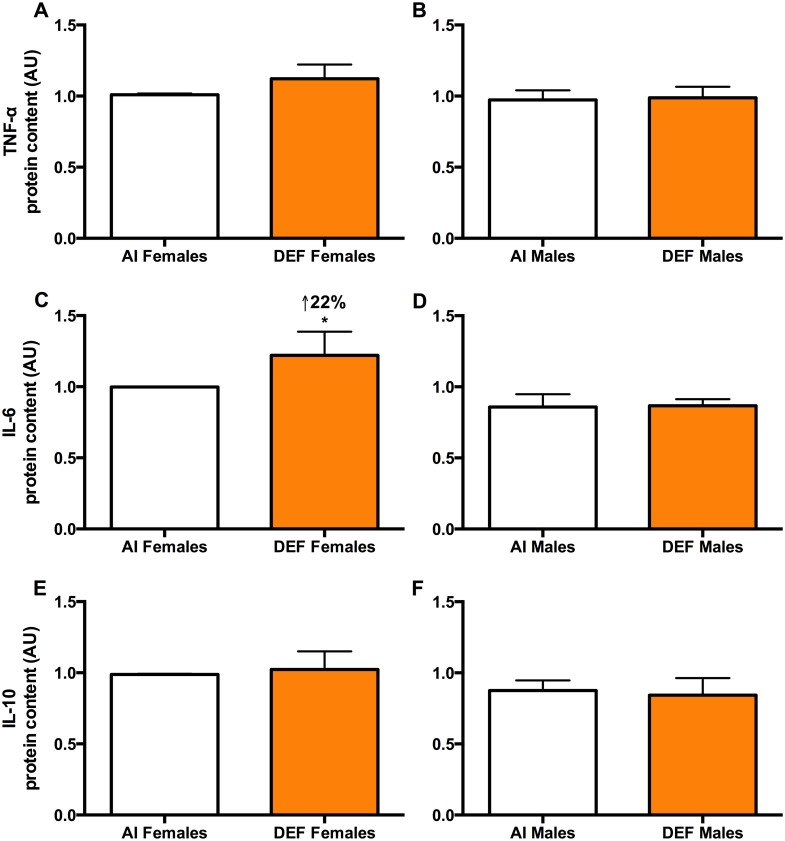
There was no significant difference in TNF-α protein content between the diets or between the sexes (Fig 3A and 3B).
Fig 3. Inflammation in DEF vs. AI G93A mice.
TNF- α (A and B), IL-6 (C and D) and IL-10 (E and F) protein content (arbitrary units; AU) in spinal cord of 42 G93A mice: 23 adequate vitamin D3 intake (AI; 1 IU D3/g feed; 12 M, 11 F) and 19 deficient vitamin D3 intake (DEF; 0.025 IU D3/g feed; 10 M, 9 F). TNF-α (A and B): There was no significant difference in TNF-α protein content between the diets or between the sexes. IL-6 (C and D): DEF females had 22% higher IL-6 protein content vs. AI females (P = 0.077). AI males had 14% lower IL-6 protein content vs. AI females (P = 0.075). DEF males had 29% lower IL-6 protein content vs. DEF females (P = 0.023). IL-10 (E and F): There was no significant difference in IL-10 protein content between the diets. AI males had 11% lower IL-10 protein content vs. AI females (P = 0.074). Data presented as means ± SEM.
Il-6
DEF females had 22% higher IL-6 protein content vs. AI females (P = 0.077) (Fig 3C). AI males had 14% lower IL-6 protein content vs. AI females (P = 0.075). DEF males had 29% lower IL-6 protein content vs. DEF females (P = 0.023).
IL-10
There was no significant difference in IL-10 protein content between the diets (Fig 3E and 3F). AI males had 11% lower IL-10 protein content vs. AI females (P = 0.074).
Apoptosis
Bax
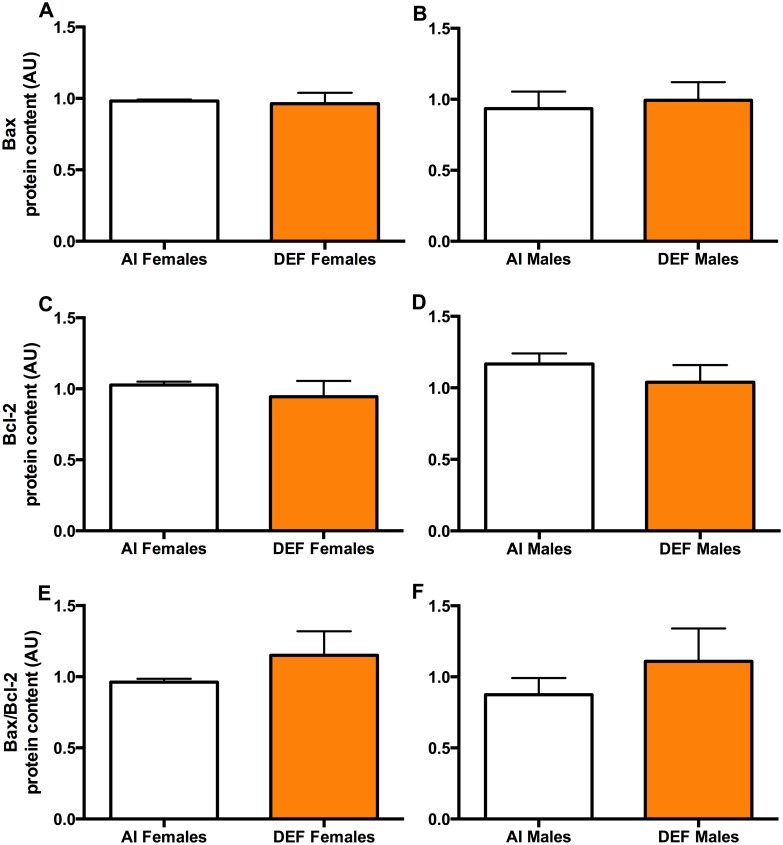
There was no significant difference in Bax protein content between the diets or between the sexes (Fig 4A and 4B).
Fig 4. Bax and Bcl-2 in DEF vs. AI G93A mice.
Bax (A and B), Bcl-2 (C and D) and Bax/Bcl-2 ratio (E and F) protein content (arbitrary units; AU) in spinal cord of 42 G93A mice: 23 adequate vitamin D3 intake (AI; 1 IU D3/g feed; 12 M, 11 F) and 19 deficient vitamin D3 intake (DEF; 0.025 IU D3/g feed; 10 M, 9 F). Bax (A and B): There was no significant difference in Bax protein content between the diets or between the sexes. Bcl-2 (C and D): There was no significant difference in Bcl-2 protein content between the diets. AI males had 14% higher Bcl-2 protein content vs. AI females (P = 0.048). Bax/Bcl-2 ratio (E and F): DEF mice had 23% higher Bax/Bcl-2 protein content vs. AI (P = 0.076). Data presented as means ± SEM.
Bcl-2
There was no significant difference in Bcl-2 protein content between the diets (Fig 4C and 4D). AI males had 14% higher Bcl-2 protein content vs. AI females (P = 0.048).
Bax/Bcl-2 ratio
DEF mice had 23% higher Bax/Bcl-2 protein content vs. AI (P = 0.076).
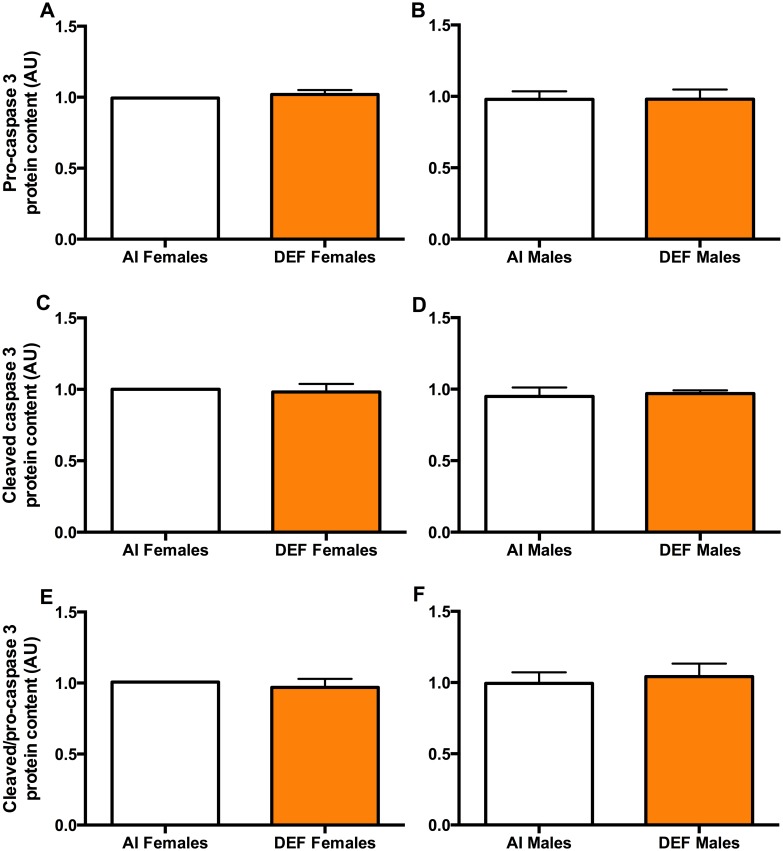
Pro-caspase 3
There was no significant difference in pro-caspase protein content between the diets or between the sexes (Fig 5A and 5B).
Fig 5. Caspase 3 in DEF vs. AI G93A mice.
Pro-caspase 3 (A and B), cleaved caspase 3 (C and D) and cleaved/pro-caspase 3 (E and F) protein content (arbitrary units; AU) in spinal cord of 42 G93A mice: 23 adequate vitamin D3 intake (AI; 1 IU D3/g feed; 12 M, 11 F) and 19 deficient vitamin D3 intake (DEF; 0.025 IU D3/g feed; 10 M, 9 F). There was no significant difference in pro-caspase 3, cleaved caspase 3 and cleaved/pro-caspase 3 protein content between the diets or between the sexes. Data presented as means ± SEM.
Cleaved caspase 3
There was no significant difference in cleaved caspase 3 protein content between the diets or between the sexes (Fig 5C and 5D).
Cleaved/pro-caspase 3
There was no significant difference in cleaved/pro-caspase 3 protein content between the diets or between the sexes (Fig 5E and 5F).
Neurotrophic Factor
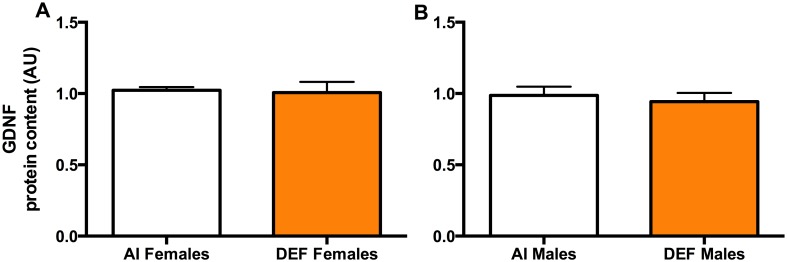
GDNF
There was no significant difference in GDNF protein content between the diets or between the sexes (Fig 6A and 6B).
Fig 6. Neurotrophic factor in DEF vs. AI G93A mice.
GDNF protein content (A and B) (arbitrary units; AU) in spinal cord of 42 G93A mice: 23 adequate vitamin D3 intake (AI; 1 IU D3/g feed; 12 M, 11 F) and 19 deficient vitamin D3 intake (DEF; 0.025 IU D3/g feed; 10 M, 9 F). There was no significant difference in GDNF protein content between the diets or between the sexes. Data presented as means ± SEM.
Neuron Count
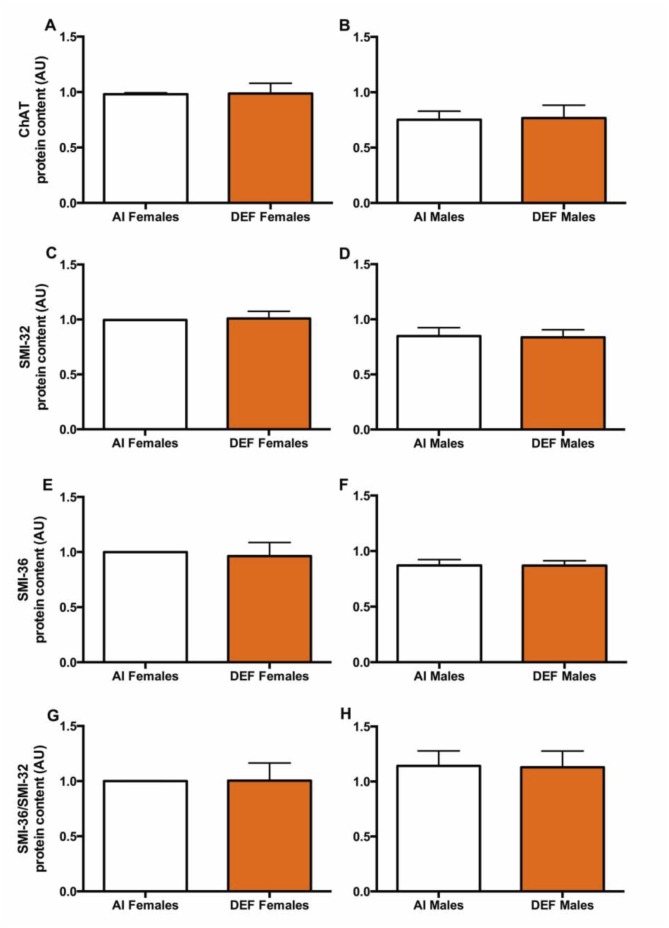
ChAT
There was no significant difference in ChAT protein content between the diets (Fig 7A and 7B). AI males had 23% lower ChAT protein content vs. AI females (P = 0.005). DEF males had 22% lower ChAT protein content vs. DEF females (P = 0.082)
Fig 7. Neuron count in DEF vs. AI G93A mice.
ChAT (A and B), SMI-32 (C and D), SMI-36 (E and F) and SMI-36/SMI-32 ratio (G and H) protein content (arbitrary units; AU) in spinal cord of 42 G93A mice: 23 adequate vitamin D3 intake (AI; 1 IU D3/g feed; 12 M, 11 F) and 19 deficient vitamin D3 intake (DEF; 0.025 IU D3/g feed; 10 M, 9 F). ChAT (A and B): There was no significant difference in ChAT protein content between the diets. AI males had 23% lower ChAT protein content vs. AI females (P = 0.005). DEF males had 22% lower ChAT protein content vs. DEF females (P = 0.082). SMI-32 (C and D): There was no significant difference in SMI-32 protein content between the diets. AI males had 15% lower SMI-32 protein content vs. AI females (P = 0.039). DEF males had 17% lower SMI-32 protein content vs. DEF females (P = 0.046). SMI-36 (E and F): There was no significant difference in SMI-36 protein content between the diets. AI males had 13% lower SMI-36 protein content vs. AI females (P = 0.016). SMI-36/SMI-32 ratio (G and H): There was no significant difference in SMI-36/SMI-32 protein content between the diets or between the sexes. Data presented as means ± SEM.
SMI-32
There was no significant difference in SMI-32 protein content between the diets (Fig 7C and 7D). AI males had 15% lower SMI-32 protein content vs. AI females (P = 0.039). DEF males had 17% lower SMI-32 protein content vs. DEF females (P = 0.046).
SMI-36
There was no significant difference in SMI-36 protein content between the diets (Fig 7E and 7F). AI males had 13% lower SMI-36 protein content vs. AI females (P = 0.016).
SMI-36/SMI-32
There was no significant difference in SMI-36/SMI-32 protein content between the diets or between the sexes (Fig 7G and 7H).
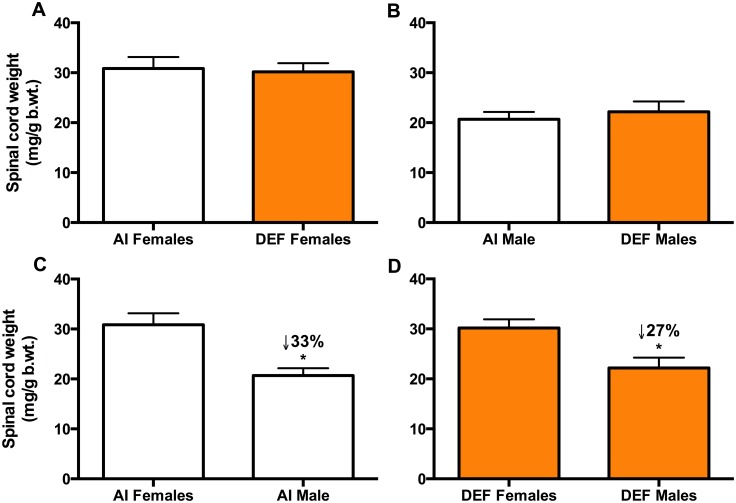
Spinal cord weights
Absolute spinal cord weight was not different between the diets (Table 2). Between the sexes, AI males had 15% lighter absolute spinal cord weight vs. AI females (P = 0.065), and DEF males had 16% lighter absolute spinal cord weight vs. DEF females (P = 0.053) (Table 2). There was no significant difference in body weight-adjusted spinal cord weights between the diets (Table 2; Fig 8A and 8B). Between the sexes, AI males had 33% lighter body weight-adjusted spinal cord weight vs. AI females (P = 0.001) (Table 2; Fig 8C), and DEF males had 27% lighter body weight-adjusted spinal cord weight vs. DEF females (P = 0.005) (Table 2; Fig 8D).
Table 2. Spinal cord weight between the diets and sexes.
| Spinal cord weights | Females | Males | Within-AI between-sex differences | Within-DEF between-sex differences | ||||
|---|---|---|---|---|---|---|---|---|
| AI | DEF | P value | AI | DEF | P value | P value | P value | |
| Absolute spinal cord weight (mg) | 583±43 | 581±33 | NS | 494±37 | 489±42 | NS | P = 0.065 | P = 0.053 |
| Body weight-adjusted spinal cord weight (mg/g b.wt.) | 31±2 | 30±5 | NS | 21±2 | 22±7 | NS | P = 0.001 | P = 0.005 |
Data are means ± SEM. AI, adequate intake; DEF, deficient vitamin D. AI Males, n = 12; AI Females, n = 11. DEF Males, n = 10; DEF Females, n = 9
Fig 8. Body weight-adjusted spinal cord weights.
Body weight-adjusted spinal cord weight (mg/g b.wt.) of 42 G93A mice: 23 adequate vitamin D3 intake (AI; 1 IU D3/g feed; 12 M, 11 F) and 19 deficient vitamin D3 intake (DEF; 0.025 IU D3/g feed; 10 M, 9 F). Between the diets (A and B): There was no significant difference in body weight-adjusted spinal cord weights between the diets. Between the sexes (C and D): AI males had 33% lighter body weight-adjusted spinal cord weight vs. AI females (P = 0.001), and DEF males had 27% lighter body weight-adjusted spinal cord weight vs. DEF females (P = 0.005). Data presented as means ± SEM.
Discussion
We investigated the effects of vitamin D deficiency, via dietary vitamin D3 restriction equivalent to 2.5% the rodent AI, on markers of oxidative damage, antioxidant enzymes, inflammation, apoptosis, growth factors and neuron count in the spinal cord of G93A mice, a rodent model of ALS. Dietary vitamin D restriction at 1/40th AI exacerbates disease pathophysiology as DEF mice displayed higher levels of lipid peroxidation and apoptosis compared to AI. DEF females had higher inflammation and a compensatory increase in the antioxidant GPx1 compared to AI females. Conversely, DEF males had reduced antioxidant capacity compared to AI males. When comparing differences between the sexes, DEF males had lower antioxidant enzymes and neuronal count compared to DEF females. The extant sexual dimorphism both in AI and DEF mice confirms that though detrimental, vitamin D deficiency negatively impacts different pathways depending on the sex, having a more deleterious effect in males compared to females.
Sexual dimorphism was observed in our current study as vitamin D deficiency caused differential results in males vs. females. Sexual dimorphism exists in a multitude of neurological and mental disorders such as multiple sclerosis (MS), Alzheimer’s disease (AD), Parkinson’s disease (PD) and ALS [27]. ALS is predominant in males, but with increasing age the ratio of male-to-female diagnoses becomes smaller. The sex difference may be a result of different aromatase activity, the enzyme that converts testosterone into estradiol. This activity is neuroprotective [46,47] and is higher in cortical female astrocytes than cortical male astrocytes [48]. The dimorphic nature of the enzyme can protect astrocytes, as well as other CNS cell types such as neurons, from damage in females. It has also been postulated that the presence of the sex hormone estrogen plays a role in this dimorphism. Recent clinical evidence has shown that estrogen treatment reduces the risk and delays the onset of many neurodegenerative diseases [49]. In primary cultures of Wistar rat spinal cord, estradiol exerts neuroprotective effects in vivo [50]. In vitro, estradiol protects cerebral neurons against glutamate excitotoxicity [50]. Administration of the phytoestrogen genistein to male mSOD1 mice reduces the difference in disease onset and mortality between the sexes (prior to genistein administration, disease onset and mortality were reached sooner in males vs. females), confirming the strong role of sex hormones [51]. Ovariectomy of G93A mice accelerates disease progression, and a high-dose of 17β-estradiol significantly slows down disease progression in these mice [52] In the presence of vitamin D3, estrogen synthesis is increased [53], allowing both estrogen and vitamin D3 to exert neuroprotective effects. A confirmed synergy exists between vitamin D3 and estrogen, which is also found in the spinal cord [54]. Estrogen causes estrogen receptor-mediated down-regulation of CYP24A1 (calcitriol deactivating enzyme) transcription to increase net calcitriol concentration, and thus enhance vitamin D function [54]. As well, estrogen up-regulates VDR to enhance vitamin D potency, and, in turn, calcitriol causes VDR-mediated up-regulation of estrogen synthase to enhance endogenous estrogen synthesis [55]. As a result, basal CNS calcitriol levels are higher in females vs. males [56].
A deficiency in vitamin D reduces the amount of calcium buffering protein, thus leading to higher lipid peroxidation [31]. This could explain why DEF males had 23% higher 4-HNE protein content, a marker of oxidative damage, vs. AI males. This difference was not observed in DEF females vs. AI. This is in agreement with estrogen’s protective role. Co-exposure of 17β-estradiol and 4-HNE in PC12 cell lines showed that estrogen was significantly effective against the cytotoxic response of 4-HNE [57]. This is because 17β-estradiol has the ability to stabilize mitochondrial potential against oxidative stress [58]. As well, estrogen works similarly to vitamin D to establish cellular calcium homeostasis. Chronic 17β-estradiol treatment represses glutamate receptor-mediated Ca2+ influx [59]. In ALS, a disruption of calcium transport can form free radicals that cause lipid peroxidation in the cell [60]. Vitamin D induces the synthesis of proteins such as parvalbumin that help maintain cellular calcium homeostasis [61], thus lowering lipid peroxidation, in diabetic rats [62]. Vitamin D also reduces malondialdehyde (MDA), a marker of lipid peroxidation, by stimulating the gene expression of calcium buffering proteins calbindin-D28k and calbindin-d9k [31]. Obese children deficient in vitamin D (serum calcidiol <50 nmol/L) had higher lipid peroxidation as marked by increased 3-NY and MDA levels compared to non-deficient obese children (serum calcidiol >50 nmol/L) [32].
The protective role of estrogen is also a factor in why AI males had 18% higher 3-NY protein content vs. AI females. 17β-estradiol’s antioxidative effect reduces 3-NY immunoreactivity [63]. Brain cell cultures of mice exposed to 17β-estradiol significantly reduced 3-NY levels regardless of whether or not they have been exposed to superoxide [64]. Estrogen can directly inhibit nitric oxide synthase (NOS) activity, thereby reducing peroxynitrite and subsequently 3-NY generation [65].
Vitamin D deficiency also had a negative impact on antioxidant capacity. SOD2 was 18% lower in DEF males vs. AI males. Compared to healthy individuals, SOD2 activity is lower in the brain and spinal cord of ALS patients [66]. It is normally expected that in response to higher oxidative damage, antioxidant capacity increases. Thus, the exact mechanism that leads to a reduction in SOD2 activity is not well understood. It is possible that the loss of activity could be a result of post-translational modification. During CNS injury, nitric oxide (NO) is released at a high rate, which reacts with superoxide and leads to the production of nitrogenous species such as peroxynitrite (ONOO-) [67]. Peroxynitrite, which is highly prevalent in ALS spinal cord, is the only known biological oxidant to inactivate enzymatic activity, nitrate important tyrosine residues and cause dityrosine formation in SOD2 [68]. Higher levels of peroxynitrite lead to increased production of 3-NY [69]. Compared to their female counterparts, AI and DEF males had 18% and 6% higher 3-NY levels, respectively, which could explain why SOD2 was lower in DEF males, but not DEF females, vs. AI. A reduction in SOD2 in DEF males may be related to vitamin D’s impact on nuclear factor-kappa B (NF-κB). In ALS, activated microglia use the NF-κB pathway to induce mitochondrial dysfunction inhibition of SOD2 and motor neuron death [70,71]. NF-κB leads to mitochondrial dysfunction inhibition of SOD2 through nitration, by activating inducible NOS (iNOS) [72]. The local conversion of calcidiol to calcitriol in the CNS is a neuroprotective response that inhibits NF-κB-related iNOS induction [73]. Without the protective effects of vitamin D, this neuroprotection diminishes, which may explain the lower levels of SOD2 in DEF males.
GPx1 was 29% higher in DEF females vs. AI females. Female hypertensive Wister rats have shown increased GPx1 activity and lower reduced glutathione (GSH) levels [74]. Under physiological conditions, vitamin D has an inverse relationship with GPx1 activity and a positive association with glutathione reductase (GR) activity [75]. This relationship is due to GSH’s role in maintaining intracellular redox balance. Increasing the activity of GR and decreasing GPx1 function allow vitamin D to enhance the GSH pool. In vitamin D deficiency however, excessive inflammation, as reflected by high IL-6 levels, increases GPx1 activity as a means of reducing oxidative protein injury [76]. This also explains why, in this study, DEF females did not have a significant increase in 3-NY vs. AI. Vitamin D deficiency increased IL-6 levels by 22% compared to AI females, thereby elevating GPx1 protein content by 29%. Thus, the adaptive increase in GPx1 may indicate heightened inflammation and cellular damage. In many cancers, including esophageal cancer, GPx1 further induces malignancy and promotes tumor progression, effects that can be reduced by vitamin D [77]. In breast cancer patients, high expression of GPx1 was associated with high rate of patient mortality and shorter overall survival [78], which may be due to NF-κB. When bound to the promoter region of GPx1, NF-κB upregulates its function and expression upstream [77]. Vitamin D and VDR inhibit NF-κB expression and thus decrease GPx1 levels [79]. In females, estrogen’s ability to convert calcidiol to calcitriol [80] can heighten the ability of the vitamin to inhibit the NF-κB pathway, thereby reducing GPx1 levels. This may explain why AI males had 10% higher GPx1 protein content than AI females.
IL-6 levels were 22% higher in DEF females vs. AI females. Damage to the CNS causes an upregulation in IL-6 and other pro-inflammatory cytokines such as TNF-α [81]. IL-6 plays a great role in astrocyte and microglia activation, microglial proliferation as well as gliosis [82]. Though it is meant to repair, gliosis can work as a double-edged sword: it can produce neurotrophic factors and protect the CNS from toxins, but it can also produce neurotoxins such as nitric oxide, an important factor in free-radical genesis [82]. Estrogen is known to regulate IL-6 expression in different cell types [83]. In biliary epithelial cells, estrogen had the ability to stimulate IL-6 production in both the neoplasmic and non-neoplasmic cells that expressed estrogen receptor alpha [83]. A study on the effects of gonadal steroids on IL-6 in peripheral blood mononuclear cells showed that 17β-estradiol promotes IL-6 production and release [84]. As well, deficiency in vitamin D after trauma puts women at a greater risk of elevated IL-6 levels [85]. This is in accord with Miller et al’s study that showed that women with serum 25(OH)D levels of <37.5 nmol/L at the time of hip fracture had higher serum IL-6 levels in the year after the hip fracture [85]. Alternatively, testosterone maintains low IL-6 levels [86–88]. This explains why we observed 14% lower IL-6 in AI males vs. AI females, and 29% lower IL-6 in DEF males vs. DEF females. Interestingly, testosterone is also negatively associated with TNF-α [88], whereas estradiol increases its expression [89]. AI and DEF males had non-significant 4% and 12% lower TNF-α levels vs. their female counterparts. Higher TNF-α levels in DEF females reflects vitamin D’s impact on this inflammatory cytokine. In females, a significant inverse association exists between 25(OH)D and TNF-α, whereby vitamin D deficiency increases levels of the inflammatory cytokine [90]. A study in endurance-trained athletes showed that circulating TNF-α does not increase linearly with decreasing 25(OH)D concentration. Instead, it is abruptly higher in those that are vitamin D deficient (lower than 80 nmol/L) [91]. In neuron and glial cells, matrix metalloproteinase-9 (MMP-9) regulates TNF-α levels and is found in high levels in damaged ALS motor neurons [92]. MMPs are largely associated with inflammation and work to remodel and break down the extracellular matrix and regulate leukocyte migration within it. Calcitriol reduces MMP-9 activity, thereby reducing TNF-α levels [93]. Within the spinal cord of G93A mice, upregulation of the p38 mitogen activated protein kinase (p38MAPK), a signaling pathway responsible for cell death, is associated with the upregulation of TNF-α receptors [94]. Calcitriol reduces p38MAPK activity, thereby reducing TNF- α levels [95,96]. In terms of anti-inflammatory cytokines, AI males had 11% lower IL-10 protein content vs. AI females. IL-10 has been shown to increase in the presence of estrogen [97]. Malaria infected female mice were shown to have higher IL-10 levels compared to males [98]. In terms of its relationship with inflammatory cytokines, higher IL-6 levels induce IL-10 production [99]. This explains why the higher IL-10 levels we observed in females were commensurate with higher IL-6 protein content.
There was 23% higher Bax/Bcl-2 in DEF mice vs AI mice. However, this may not necessarily indicate that apoptosis was prevalent. This is because no changes in activated caspase 3 and neuron count were observed between the diets. Though Bax/Bcl-2 ratio was elevated in DEF mice, the increase was not sufficient enough to activate caspase 3, the effector molecule in the apoptotic pathway. High Bax/Bcl-2 ratio increases the vulnerability of neurons to apoptosis [100], and is observed in neuromuscular disorders such as ALS [101]. What this may indicate is that apoptotic proteins can reduce neuronal viability without leading to large-scale apoptosis. This is also confirmed in our previous study in HiD female quadriceps that had reached the threshold of vitamin D3 toxicity. A 242% increase in Bax/Bcl-2 [102] only corresponded to an 87% increase in cleaved/pro-caspase 3 [43]. Thus, a deficiency in vitamin D may increase the susceptibility of motor neurons to apoptosis without necessarily leading to large-scale apoptosis. Vitamin D has been shown to reduce pro-apoptotic (Bax) and increase anti-apoptotic proteins (Bcl-2) [103]. A reduction in calcium buffering capacity brought about by vitamin D deficiency may cause the cell to exert calcium-induced excitotoxicity, which leads to elevated levels of Bax/Bcl-2.
With respect to neuron count, both AI and DEF males had lower ChAT (23% and 22%, respectively) and SMI-32 (15% and 17%, respectively) compared to their female counterparts, likely due to the protective effect of estrogen in females. In mSOD1 mice, onset, disease progression and survival are dependent on sex; males lose body weight more rapidly following disease onset and die sooner than females [104,105]. A reduction in body weight reflects muscle atrophy brought about by motor neuron degeneration. As with increased motor neuron count in females, it is possible that damaged motor neurons are also more prevalent. This explains why SMI-36 levels were 13% lower in AI males vs. AI females. This can also explain why there was no difference in SMI-36/SMI-32 ratio between AI males and AI females, as SMI-32 and SMI-36 levels were both lower in males vs. females. Ultimately, neuron count was not different between the diets, indicating that even though vitamin D deficiency may exacerbate disease pathophysiology, it does not have an impact on neuron count.
On a tissue level, there was no significant difference in body weight-adjusted spinal cord weights between the diets. However, a sexual dimorphism was confirmed as AI males and DEF males had 33% and 27% lighter body weight-adjusted spinal cord weight vs. their female counterparts. This may be due to the protective effects of estrogen in the female spinal cord. These results contrast our previous study that found no difference in body weight-adjusted brain weights between the diets and sexes [45]. Correlational analysis showed that there was no association between body weight-adjusted brain weights [45] and body weight-adjusted spinal cord weights. This confirms that ALS pathology within the CNS is mainly localized to the spinal cord.
This study outlines the detrimental effects of vitamin D deficiency and confirms the lower paw grip endurance and motor performance that was observed in our previous study in the same mouse model [37]. When compared to AI mice, DEF mice had 25% lower paw grip endurance (PaGE) AUC and 19% lower motor performance. Between the sexes, AI males had lower ability to move, PaGE and motor performance compared to AI females. AI males also had a higher clinical score, hastened disease onset, and reached hind limb paralysis and endpoint faster compared to AI females. The current study confirms that the functional outcomes observed are linked to neuronal damage in the spinal cord. Previous studies on spinal cord injury (SCI) rats have shown that motor performance disturbance following SCI is associated with the severity of spinal cord pathology [106]. Damage in the spinal cord also reflects that in the quadriceps, where DEF female, but not male, G93A mice had higher inflammation and apoptosis as compared to AI females [35,36]. Despite the fact that the spinal cords of DEF females had higher inflammation, DEF male spinal cords were more susceptible to damage as marked by lower levels of SOD2 and neuron count. We postulate that this is due to the protective effects of estrogen in females. The restricted vitamin D3 intake in this study corresponds to ~25 IU/d for an 80 kg man and ~20 IU/d for a 70 kg woman. These values may be insufficient for patients with ALS. Indeed, Karam et al’s study on ALS patients found that supplementation with 2000 IU of vitamin D3/day for 9 months improved ALS functional rating scale score [107]. A retrospective study on ALS patients found that those with serum calcidiol levels <25 nmol/L increased their death rate by 6 fold and their rate of decline by 4 times, and were associated with a marked shorter life expectancy compared to patients with serum calcidiol levels >75 nmol/L [33]. Furthermore, Guamanian Chamorros with ALS have serum calcitriol levels in the low to low-normal range [108]. Based on these studies, and given our previous studies supplementing ALS mice with 10x and 50x AI, we restate our previous hypothesis that the optimal therapeutic vitamin D dosage, both functionally and cellularly, lies between 10x and 50x AI vitamin D [27,44,45].
In conclusion, the present study demonstrates that vitamin D deficiency exacerbates disease pathophysiology in the G93A mouse model of ALS. This is marked by increased inflammation and oxidative damage and lower antioxidant capacity. However, it is important to note that sexual dimorphism exists and that the pathways that vitamin D deficiency negatively impacts differ between males and females [109].
Supporting Information
Representative immunoblots of 4-HNE, 3-NY, SOD2, catalase, GPx1, TNF-α, IL-6, IL-10, Bax, Bcl-2, pro-caspase 3, cleaved caspase 3, GDNF, ChAT, SMI-32 and SMI-36 protein expression in the spinal cord of 42 G93A mice: 23 adequate vitamin D3 intake (AI; 1 IU D3/g feed; 12 M, 11 F) and 19 deficient vitamin D3 intake (DEF; 0.025 IU D3/g feed; 10 M, 9 F). Each antibody and its corresponding anti-GAPDH set were loaded on a separate gel. Protein intensity was standardized to GAPDH.
(TIFF)
Acknowledgments
We thank Sanjeef Thampinathan, Mahshad Kolahdouzan and Safoura Sadeghimehr for assisting in lab analysis, data entry and literature search.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Natural Sciences and Engineering Research Council of Canada (http://www.nserccrsng.gc.ca/Index_eng.asp) received by MJH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Nishitoh H, Kadowaki H, Nagai A, Maruyama T, Yokota T, Fukutomi H, et al. (2008) ALS-linked mutant SOD1 induces ER stress- and ASK1-dependent motor neuron death by targeting derlin-1. Genes Dev 22: 1451–1464. 10.1101/gad.1640108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Almeida JL, Silvestre R, Pinto A, de Carvalho M. (2012) Exercise and amyotrophic lateral sclerosis. Neurological Sciences 33: 9–15. 10.1007/s10072-011-0921-9 [DOI] [PubMed] [Google Scholar]
- 3. Robberecht W, Philips T. (2013) The changing scene of amyotrophic lateral sclerosis. Nat Rev Neurosci 14: 248–264. 10.1038/nrn3430 [DOI] [PubMed] [Google Scholar]
- 4. Dadon-Nachum M, Melamed E, Offen D. (2011) The "dying-back" phenomenon of motor neurons in ALS. J Mol Neurosci 43: 470–477. 10.1007/s12031-010-9467-1 [DOI] [PubMed] [Google Scholar]
- 5. Wijesekera LC, Leigh PN. (2009) Amyotrophic lateral sclerosis. Orphanet J Rare Dis 4: 3-1172-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jennum P, Ibsen R, Pedersen SW, Kjellberg J. (2013) Mortality, health, social and economic consequences of amyotrophic lateral sclerosis: A controlled national study. J Neurol 260: 785–793. 10.1007/s00415-012-6706-0 [DOI] [PubMed] [Google Scholar]
- 7. Tripodoro VA, De Vito EL. (2008) Management of dyspnea in advanced motor neuron diseases. Curr Opin Support Palliat Care 2: 173–179. 10.1097/SPC.0b013e32830c9049 [DOI] [PubMed] [Google Scholar]
- 8. Lechtzin N. (2006) Respiratory effects of amyotrophic lateral sclerosis: Problems and solutions. Respir Care 51: 871–81; discussion 881–4. [PubMed] [Google Scholar]
- 9. Gros-Louis F, Gaspar C, Rouleau GA. (2006) Genetics of familial and sporadic amyotrophic lateral sclerosis. Biochim Biophys Acta 1762: 956–972. [DOI] [PubMed] [Google Scholar]
- 10.Berthod F, Gros-Louis F. In vivo and in vitro models to study amyotrophic lateral sclerosis.
- 11. Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, et al. (1993) Mutations in cu/zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362: 59–62. [DOI] [PubMed] [Google Scholar]
- 12. Mitchell JD. (2000) Amyotrophic lateral sclerosis: Toxins and environment. Amyotroph Lateral Scler Other Motor Neuron Disord 1: 235–250. [DOI] [PubMed] [Google Scholar]
- 13. Bruijn LI, Miller TM, Cleveland DW. (2004) Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci 27: 723–749. [DOI] [PubMed] [Google Scholar]
- 14. Renton AE, Chio A, Traynor BJ. (2014) State of play in amyotrophic lateral sclerosis genetics. Nat Neurosci 17: 17–23. 10.1038/nn.3584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD et al. (1994) Motor neuron degeneration in mice that express a human cu,zn superoxide dismutase mutation. Science 264: 1772–1775. [DOI] [PubMed] [Google Scholar]
- 16. Foran E, Trotti D. (2009) Glutamate transporters and the excitotoxic path to motor neuron degeneration in amyotrophic lateral sclerosis. Antioxid Redox Signal 11: 1587–1602. 10.1089/ars.2009.2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mark LP, Prost RW, Ulmer JL, Smith MM, Daniels DL, Strottmann JM, et al. (2001) Pictorial review of glutamate excitotoxicity: Fundamental concepts for neuroimaging. AJNR Am J Neuroradiol 22: 1813–1824. [PMC free article] [PubMed] [Google Scholar]
- 18. Trumbull KA, Beckman JS. (2009) A role for copper in the toxicity of zinc-deficient superoxide dismutase to motor neurons in amyotrophic lateral sclerosis. Antioxid Redox Signal 11: 1627–1639. 10.1089/ARS.2009.2574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pedersen WA, Fu W, Keller JN, Markesbery WR, Appel S, Smith RG et al. (1998) Protein modification by the lipid peroxidation product 4-hydroxynonenal in the spinal cords of amyotrophic lateral sclerosis patients. Ann Neurol 44: 819–824. [DOI] [PubMed] [Google Scholar]
- 20. Nguyen D, Alavi MV, Kim KY, Kang T, Scott RT, Noh YH, et al. (2011) A new vicious cycle involving glutamate excitotoxicity, oxidative stress and mitochondrial dynamics. Cell Death Dis 2: e240 10.1038/cddis.2011.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schubert D, Piasecki D. (2001) Oxidative glutamate toxicity can be a component of the excitotoxicity cascade. J Neurosci 21: 7455–7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhao L, Hart S, Cheng JG, Melenhorst JJ, Bierie B, Ernst M, et al. (2004) Mammary gland remodeling depends on gp130 signaling through Stat3 and MAPK. J Biol Chem 279: 44093–44100. [DOI] [PubMed] [Google Scholar]
- 23. Patel BP, Hamadeh MJ. Nutritional and exercise-based interventions in the treatment of amyotrophic lateral sclerosis. Clinical Nutrition 28: 604–617. 10.1016/j.clnu.2009.06.002 [DOI] [PubMed] [Google Scholar]
- 24. Summerday NM, Brown SJ, Allington DR, Rivey MP. (2012) Vitamin D and multiple sclerosis: Review of a possible association. J Pharm Pract 25: 75–84. 10.1177/0897190011421839 [DOI] [PubMed] [Google Scholar]
- 25. Ibi M, Sawada H, Nakanishi M, Kume T, Katsuki H, Kaneko S, et al. (2001) Protective effects of 1 alpha,25-(OH)(2)D(3) against the neurotoxicity of glutamate and reactive oxygen species in mesencephalic culture. Neuropharmacology 40: 761–771. [DOI] [PubMed] [Google Scholar]
- 26. Pierrot-Deseilligny C. (2009) Clinical implications of a possible role of vitamin D in multiple sclerosis. J Neurol 256: 1468–1479. 10.1007/s00415-009-5139-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gianforcaro A, Hamadeh MJ. (2014) Vitamin D as a potential therapy in amyotrophic lateral sclerosis. CNS Neurosci Ther 20: 101–111. 10.1111/cns.12204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lim S, Kim MJ, Choi SH, Shin CS, Park KS, Jang HC, et al. (2013) Association of vitamin D deficiency with incidence of type 2 diabetes in high-risk asian subjects. Am J Clin Nutr 97: 524–530. 10.3945/ajcn.112.048496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Knippenberg S, Bol Y, Damoiseaux J, Hupperts R, Smolders J. (2011) Vitamin D status in patients with MS is negatively correlated with depression, but not with fatigue. Acta Neurol Scand 124: 171–175. 10.1111/j.1600-0404.2010.01447.x [DOI] [PubMed] [Google Scholar]
- 30. Littlejohns TJ, Henley WE, Lang IA, Annweiler C, Beauchet O, Chaves PHM, et al. (2014) Vitamin D and the risk of dementia and alzheimer disease. Neurology 83: 920–928. 10.1212/WNL.0000000000000755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Halhali A, Figueras AG, Diaz L, Avila E, Barrera D, Hernandez G, et al. (2010) Effects of calcitriol on calbindins gene expression and lipid peroxidation in human placenta. J Steroid Biochem Mol Biol 121: 448–451. 10.1016/j.jsbmb.2010.03.008 [DOI] [PubMed] [Google Scholar]
- 32. Codoner-Franch P, Tavarez-Alonso S, Simo-Jorda R, Laporta-Martin P, Carratala-Calvo A, Alonso-Iglesias E. (2012) Vitamin D status is linked to biomarkers of oxidative stress, inflammation, and endothelial activation in obese children. J Pediatr 161: 848–854. 10.1016/j.jpeds.2012.04.046 [DOI] [PubMed] [Google Scholar]
- 33. Camu W, Tremblier B, Plassot C, Alphandery S, Salsac C, Pageot N, et al. (2014) Vitamin D confers protection to motoneurons and is a prognostic factor of amyotrophic lateral sclerosis. Neurobiol Aging 35: 1198–1205. 10.1016/j.neurobiolaging.2013.11.005 [DOI] [PubMed] [Google Scholar]
- 34. Milionis A, Parkhomenko E, Solomon JA, Gianforcaro A, Hamadeh MJ. (2012) Dietary vitamin D3 restriction differentially alters quadriceps contractile proteins in both sexes in the transgenic G93A mouse model of amyotrophic lateral sclerosis: A pilot study. The FASEB Journal 26: 255.8. [Google Scholar]
- 35. Parkhomenko EA, Gianforcaro A, Solomon JA, Hamadeh MJ. (2011) Vitamin D deficiency improves antioxidant capacity in males and attenuates the sexual dichotomy in the G93A mouse model of amyotrophic lateral sclerosis: Is the female sex at D3 disadvatnage? Canadian Nutrition Society 328. [Google Scholar]
- 36. Shahsavar S, Taheri-Shalmani S, Solomon JA, Gianforcaro A, Hamadeh MJ. (2013) Sexual dichotomy in calcium buffering capacity may be dependent on the severity of endoplasmic reticulum stress in the skeletal muscle of the vitamin D3 deficient transgenic G93A mouse model of amyotrophic lateral sclerosis. The FASEB Journal 27: 644.2. [Google Scholar]
- 37. Solomon JA, Gianforcaro A, Hamadeh MJ. (2011) Vitamin D3 deficiency differentially affects functional and disease outcomes in the G93A mouse model of amyotrophic lateral sclerosis. PLoS One 6: e29354 10.1371/journal.pone.0029354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Solomon JA, Tarnopolsky MA, Hamadeh MJ. (2011) One universal common endpoint in mouse models of amyotrophic lateral sclerosis. PLoS One 6: e20582 10.1371/journal.pone.0020582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sapan CV, Lundblad RL, Price NC. (1999) Colorimetric protein assay techniques. Biotechnol Appl Biochem 29 (Pt 2): 99–108. [PubMed] [Google Scholar]
- 40. Food and Drug Administration, editor. (2005) Estimating the safe starting dose in clinical trials for therapeutics in adult healthy volunteers. Rockville, Maryland, USA: U.S.: Food and Drug Administration. [Google Scholar]
- 41. Laird E, McNulty H, Ward M, Hoey L, McSorley E, Wallace JM, et al. (2014) Vitamin D deficiency is associated with inflammation in older irish adults. J Clin Endocrinol Metab 99: 1807–1815. 10.1210/jc.2013-3507 [DOI] [PubMed] [Google Scholar]
- 42. Parkhomenko EA, Gianforcaro A, Solomon JA, Hamadeh MJ. (2011) Dietary vitamin D3 at 50 fold the adequate intake increases antioxidant capacity and decreases inflammation in the G93A mouse model of ALS. Canadian Nutrition Society: 329. [Google Scholar]
- 43. Parkhomenko E, Milionis A, Gianforcaro A, Solomon JA, Hamadeh MJ. (2012) Dietary vitamin D3 at 50x the adequate intake increases apoptosis in the quadriceps of the female G93A mouse model of amyotrophic lateral sclerosis: A pilot study. The FASEB Journal 26: 255.7. [Google Scholar]
- 44. Gianforcaro A, Hamadeh MJ. (2012) Dietary vitamin D3 supplementation at 10x the adequate intake improves functional capacity in the G93A transgenic mouse model of ALS, a pilot study. CNS Neurosci Ther 18: 547–557. 10.1111/j.1755-5949.2012.00316.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gianforcaro A, Solomon JA, Hamadeh MJ. (2013) Vitamin D(3) at 50x AI attenuates the decline in paw grip endurance, but not disease outcomes, in the G93A mouse model of ALS, and is toxic in females. PLoS One 8: e30243 10.1371/journal.pone.0030243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Garcia-Segura LM, Veiga S, Sierra A, Melcangi RC, Azcoitia I. (2003) Aromatase: A neuroprotective enzyme. Prog Neurobiol 71: 31–41. [DOI] [PubMed] [Google Scholar]
- 47. Saldanha CJ, Duncan KA, Walters BJ. (2009) Neuroprotective actions of brain aromatase. Front Neuroendocrinol 30: 106–118. 10.1016/j.yfrne.2009.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liu M, Hurn PD, Roselli CE, Alkayed NJ. (2007) Role of P450 aromatase in sex-specific astrocytic cell death. J Cereb Blood Flow Metab 27: 135–141. [DOI] [PubMed] [Google Scholar]
- 49. Czlonkowska A, Ciesielska A, Gromadzka G, Kurkowska-Jastrzebska I. (2005) Estrogen and cytokines production—the possible cause of gender differences in neurological diseases. Curr Pharm Des 11: 1017–1030. [DOI] [PubMed] [Google Scholar]
- 50. Nakamizo T, Urushitani M, Inoue R, Shinohara A, Sawada H, Honda K, et al. (2000) Protection of cultured spinal motor neurons by estradiol. Neuroreport 11: 3493–3497. [DOI] [PubMed] [Google Scholar]
- 51. Trieu VN, Uckun FM. (1999) Genistein is neuroprotective in murine models of familial amyotrophic lateral sclerosis and stroke. Biochem Biophys Res Commun 258: 685–688. [DOI] [PubMed] [Google Scholar]
- 52. Groeneveld GJ, Van Muiswinkel FL, Sturkenboom JM, Wokke JH, Bar PR, Van den Berg LH. (2004) Ovariectomy and 17beta-estradiol modulate disease progression of a mouse model of ALS. Brain Res 1021: 128–131. [DOI] [PubMed] [Google Scholar]
- 53. Kinuta K, Tanaka H, Moriwake T, Aya K, Kato S, Seino Y. (2000) Vitamin D is an important factor in estrogen biosynthesis of both female and male gonads. Endocrinology 141: 1317–1324. [DOI] [PubMed] [Google Scholar]
- 54. Spach KM, Hayes CE. (2005) Vitamin D3 confers protection from autoimmune encephalomyelitis only in female mice. J Immunol 175: 4119–4126. [DOI] [PubMed] [Google Scholar]
- 55. Nashold FE, Spach KM, Spanier JA, Hayes CE. (2009) Estrogen controls vitamin D3-mediated resistance to experimental autoimmune encephalomyelitis by controlling vitamin D3 metabolism and receptor expression. J Immunol 183: 3672–3681. 10.4049/jimmunol.0901351 [DOI] [PubMed] [Google Scholar]
- 56. Spach KM, Hayes CE. (2005) Vitamin D3 confers protection from autoimmune encephalomyelitis only in female mice. J Immunol 175: 4119–4126. [DOI] [PubMed] [Google Scholar]
- 57. Siddiqui MA, Kashyap MP, Al-Khedhairy AA, Musarrat J, Khanna VK, Yadav S, et al. (2011) Protective potential of 17beta-estradiol against co-exposure of 4-hydroxynonenal and 6-hydroxydopamine in PC12 cells. Hum Exp Toxicol 30: 860–869. 10.1177/0960327110382130 [DOI] [PubMed] [Google Scholar]
- 58. Wang J, Green PS, Simpkins JW. (2001) Estradiol protects against ATP depletion, mitochondrial membrane potential decline and the generation of reactive oxygen species induced by 3-nitroproprionic acid in SK-N-SH human neuroblastoma cells. J Neurochem 77: 804–811. [DOI] [PubMed] [Google Scholar]
- 59. Numakawa T, Matsumoto T, Numakawa Y, Richards M, Yamawaki S, Kunugi H. (2011) Protective action of neurotrophic factors and estrogen against oxidative stress-mediated neurodegeneration. Journal of Toxicology 2011: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Parakh S, Spencer DM, Halloran MA, Soo KY, Atkin JD. (2013) Redox regulation in amyotrophic lateral sclerosis. Oxid Med Cell Longev 2013: 408681 10.1155/2013/408681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wrzosek M, Lukaszkiewicz J, Wrzosek M, Jakubczyk A, Matsumoto H, Piatkiewicz P, et al. (2013) Vitamin D and the central nervous system. Pharmacol Rep 65: 271–278. [DOI] [PubMed] [Google Scholar]
- 62. Hamden K, Carreau S, Jamoussi K, Miladi S, Lajmi S, Aloulou D, et al. (2009) 1Alpha,25 dihydroxyvitamin D3: Therapeutic and preventive effects against oxidative stress, hepatic, pancreatic and renal injury in alloxan-induced diabetes in rats. J Nutr Sci Vitaminol (Tokyo) 55: 215–222. [DOI] [PubMed] [Google Scholar]
- 63. Tripanichkul W, Sripanichkulchai K, Duce JA, Finkelstein DI. (2007) 17Beta-estradiol reduces nitrotyrosine immunoreactivity and increases SOD1 and SOD2 immunoreactivity in nigral neurons in male mice following MPTP insult. Brain Res 1164: 24–31. [DOI] [PubMed] [Google Scholar]
- 64. Rao AK, Dietrich AK, Ziegler YS, Nardulli AM. (2011) 17beta-estradiol-mediated increase in cu/zn superoxide dismutase expression in the brain: A mechanism to protect neurons from ischemia. J Steroid Biochem Mol Biol 127: 382–389. 10.1016/j.jsbmb.2011.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chakrabarti S, Cheung CC, Davidge ST. (2012) Estradiol attenuates high glucose-induced endothelial nitrotyrosine: Role for neuronal nitric oxide synthase. Am J Physiol Cell Physiol 302: C666–75. 10.1152/ajpcell.00181.2011 [DOI] [PubMed] [Google Scholar]
- 66. Uchino M, Ando Y, Tanaka Y, Nakamura T, Uyama E, Mita S, et al. (1994) Decrease in cu/zn- and mn-superoxide dismutase activities in brain and spinal cord of patients with amyotrophic lateral sclerosis. J Neurol Sci 127: 61–67. [DOI] [PubMed] [Google Scholar]
- 67. Beckman JS, Carson M, Smith CD, Koppenol WH. (1993) ALS, SOD and peroxynitrite. Nature 364: 584 [DOI] [PubMed] [Google Scholar]
- 68. Macmillan-Crow LA, Cruthirds DL. (2001) Invited review: Manganese superoxide dismutase in disease. Free Radic Res 34: 325–336. [DOI] [PubMed] [Google Scholar]
- 69. Bishop A, Gooch R, Eguchi A, Jeffrey S, Smallwood L, Anderson J, et al. (2009) Mitigation of peroxynitrite-mediated nitric oxide (NO) toxicity as a mechanism of induced adaptive NO resistance in the CNS. J Neurochem 109: 74–84. 10.1111/j.1471-4159.2009.05884.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Frakes AE, Ferraiuolo L, Haidet-Phillips AM, Schmelzer L, Braun L, Miranda CJ, et al. (2014) Microglia induce motor neuron death via the classical NF-kappaB pathway in amyotrophic lateral sclerosis. Neuron 81: 1009–1023. 10.1016/j.neuron.2014.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Keeney JT, Forster S, Sultana R, Brewer LD, Latimer CS, Cai J et al. (2013) Dietary vitamin D deficiency in rats from middle to old age leads to elevated tyrosine nitration and proteomics changes in levels of key proteins in brain: Implications for low vitamin D-dependent age-related cognitive decline. Free Radic Biol Med 65: 324–334. 10.1016/j.freeradbiomed.2013.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tangpong J, Cole MP, Sultana R, Estus S, Vore M, St Clair DK, et al. (2007) Adriamycin-mediated nitration of manganese superoxide dismutase in the central nervous system: Insight into the mechanism of chemobrain. J Neurochem 100: 191–201. [DOI] [PubMed] [Google Scholar]
- 73. Garcion E, Sindji L, Montero-Menei C, Andre C, Brachet P, Darcy F. (1998) Expression of inducible nitric oxide synthase during rat brain inflammation: Regulation by 1,25-dihydroxyvitamin D3. Glia 22: 282–294. [PubMed] [Google Scholar]
- 74. Barp J, Sartorio CL, Campos C, Llesuy SF, Araujo AS, Bello-Klein A. (2012) Influence of ovariectomy on cardiac oxidative stress in a renovascular hypertension model. Can J Physiol Pharmacol 90: 1229–1234. 10.1139/y2012-078 [DOI] [PubMed] [Google Scholar]
- 75. Saedisomeolia A, Taheri E, Djalali M, Djazayeri A, Qorbani M, Rajab A, et al. (2013) Vitamin D status and its association with antioxidant profiles in diabetic patients: A cross-sectional study in iran. Indian J Med Sci 67: 29–37. 10.4103/0019-5359.120695 [DOI] [PubMed] [Google Scholar]
- 76. Jin X, Zhang Z, Beer-Stolz D, Zimmers TA, Koniaris LG. (2007) Interleukin-6 inhibits oxidative injury and necrosis after extreme liver resection. Hepatology 46: 802–812. [DOI] [PubMed] [Google Scholar]
- 77. Gan X, Chen B, Shen Z, Liu Y, Li H, Xie X, et al. (2014) High GPX1 expression promotes esophageal squamous cell carcinoma invasion, migration, proliferation and cisplatin-resistance but can be reduced by vitamin D. Int J Clin Exp Med 7: 2530–2540. [PMC free article] [PubMed] [Google Scholar]
- 78. Jardim BV, Moschetta MG, Leonel C, Gelaleti GB, Regiani VR, Ferreira LC, et al. (2013) Glutathione and glutathione peroxidase expression in breast cancer: An immunohistochemical and molecular study. Oncol Rep 30: 1119–1128. 10.3892/or.2013.2540 [DOI] [PubMed] [Google Scholar]
- 79. Wu S, Sun J. (2011) Vitamin D, vitamin D receptor, and macroautophagy in inflammation and infection. Discovery Medicine 11: 325–335. [PMC free article] [PubMed] [Google Scholar]
- 80. Gallagher JC, Riggs BL, DeLuca HF. (1980) Effect of estrogen on calcium absorption and serum vitamin D metabolites in postmenopausal osteoporosis. J Clin Endocrinol Metab 51: 1359–1364. [DOI] [PubMed] [Google Scholar]
- 81. Woodroofe MN. (1995) Cytokine production in the central nervous system. Neurology 45: S6–10. [DOI] [PubMed] [Google Scholar]
- 82. Swartz KR, Liu F, Sewell D, Schochet T, Campbell I, Sandor M, et al. (2001) Interleukin-6 promotes post-traumatic healing in the central nervous system. Brain Res 896: 86–95. [DOI] [PubMed] [Google Scholar]
- 83. Isse K, Specht SM, Lunz JG 3rd, Kang LI, Mizuguchi Y, Demetris AJ. (2010) Estrogen stimulates female biliary epithelial cell interleukin-6 expression in mice and humans. Hepatology 51: 869–880. 10.1002/hep.23386 [DOI] [PubMed] [Google Scholar]
- 84. Li ZG, Danis VA, Brooks PM. (1993) Effect of gonadal steroids on the production of IL-1 and IL-6 by blood mononuclear cells in vitro. Clin Exp Rheumatol 11: 157–162. [PubMed] [Google Scholar]
- 85. Miller RR, Hicks GE, Shardell MD, Cappola AR, Hawkes WG, Yu-Yahiro JA, et al. (2007) Association of serum vitamin D levels with inflammatory response following hip fracture: The baltimore hip studies. J Gerontol A Biol Sci Med Sci 62: 1402–1406. [DOI] [PubMed] [Google Scholar]
- 86. Maggio M, Basaria S, Ble A, Lauretani F, Bandinelli S, Ceda GP, et al. (2006) Correlation between testosterone and the inflammatory marker soluble interleukin-6 receptor in older men. J Clin Endocrinol Metab 91: 345–347. [DOI] [PubMed] [Google Scholar]
- 87. Coletta RD, Reynolds MA, Martelli-Junior H, Graner E, Almeida OP, Sauk JJ. (2002) Testosterone stimulates proliferation and inhibits interleukin-6 production of normal and hereditary gingival fibromatosis fibroblasts. Oral Microbiol Immunol 17: 186–192. [DOI] [PubMed] [Google Scholar]
- 88. Bobjer J, Katrinaki M, Tsatsanis C, Lundberg Giwercman Y, Giwercman A. (2013) Negative association between testosterone concentration and inflammatory markers in young men: A nested cross-sectional study. PLoS One 8: e61466 10.1371/journal.pone.0061466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zaldivar V, Magri ML, Zarate S, Jaita G, Eijo G, Radl D, et al. (2011) Estradiol increases the expression of TNF-alpha and TNF receptor 1 in lactotropes. Neuroendocrinology 93: 106–113. 10.1159/000323760 [DOI] [PubMed] [Google Scholar]
- 90. Peterson CA, Heffernan ME. (2008) Serum tumor necrosis factor-alpha concentrations are negatively correlated with serum 25(OH)D concentrations in healthy women. J Inflamm (Lond) 5: 10-9255-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Willis KS, Smith DT, Broughton KS, Larson-Meyer DE. (2012) Vitamin D status and biomarkers of inflammation in runners. Open access journal of sports medicine 3: 35 10.2147/OAJSM.S31022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kiaei M, Kipiani K, Calingasan NY, Wille E, Chen J, Heissig B, et al. (2007) Matrix metalloproteinase-9 regulates TNF-alpha and FasL expression in neuronal, glial cells and its absence extends life in a transgenic mouse model of amyotrophic lateral sclerosis. Exp Neurol 205: 74–81. [DOI] [PubMed] [Google Scholar]
- 93. Long K, Nguyen LT. (2013) Roles of vitamin D in amyotrophic lateral sclerosis: Possible genetic and cellular signaling mechanisms. Mol Brain 6: 16-6606-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Veglianese P, Lo Coco D, Bao Cutrona M, Magnoni R, Pennacchini D, Pozzi B, et al. (2006) Activation of the p38MAPK cascade is associated with upregulation of TNF alpha receptors in the spinal motor neurons of mouse models of familial ALS. Mol Cell Neurosci 31: 218–231. [DOI] [PubMed] [Google Scholar]
- 95. Ravid A, Rubinstein E, Gamady A, Rotem C, Liberman UA, Koren R. (2002) Vitamin D inhibits the activation of stress-activated protein kinases by physiological and environmental stresses in keratinocytes. J Endocrinol 173: 525–532. [DOI] [PubMed] [Google Scholar]
- 96. Zhang Y, Leung DY, Richers BN, Liu Y, Remigio LK, Riches DW, et al. (2012) Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol 188: 2127–2135. 10.4049/jimmunol.1102412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Whitacre CC. (2001) Sex differences in autoimmune disease. Nat Immunol 2: 777–780. [DOI] [PubMed] [Google Scholar]
- 98. Cernetich A, Garver LS, Jedlicka AE, Klein PW, Kumar N, Scott AL, et al. (2006) Involvement of gonadal steroids and gamma interferon in sex differences in response to blood-stage malaria infection. Infect Immun 74: 3190–3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Steensberg A, Fischer CP, Keller C, Moller K, Pedersen BK. (2003) IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab 285: E433–7. [DOI] [PubMed] [Google Scholar]
- 100. Oltvai ZN, Milliman CL, Korsmeyer SJ. (1993) Bcl-2 heterodimerizes in vivo with a conserved homolog, bax, that accelerates programmed cell death. Cell 74: 609–619. [DOI] [PubMed] [Google Scholar]
- 101. Gonzalez de Aguilar JL, Gordon JW, Rene F, de Tapia M, Lutz-Bucher B, Gaiddon C, et al. (2000) Alteration of the bcl-x/bax ratio in a transgenic mouse model of amyotrophic lateral sclerosis: Evidence for the implication of the p53 signaling pathway. Neurobiol Dis 7: 406–415. [DOI] [PubMed] [Google Scholar]
- 102. Taheri-Shalmani S, Shahsavar S, Gianforcaro A, Solomon JA, Hamadeh MJ. (2013) Dietary vitamin D3 supplementation at 50x the adequate intake decreases calbindin d28k and endoplasmic reticulum stress and increases apoptosis, suggesting toxicity, in the female transgenic G93A mouse model of amyotrophic lateral sclerosis. The FASEB Journal 27: 644.1. [Google Scholar]
- 103. Samuel S, Sitrin MD. (2008) Vitamin D's role in cell proliferation and differentiation. Nutr Rev 66: S116–24. 10.1111/j.1753-4887.2008.00094.x [DOI] [PubMed] [Google Scholar]
- 104. Cervetto C, Frattaroli D, Maura G, Marcoli M. (2013) Motor neuron dysfunction in a mouse model of ALS: Gender-dependent effect of P2X7 antagonism. Toxicology 311: 69–77. 10.1016/j.tox.2013.04.004 [DOI] [PubMed] [Google Scholar]
- 105. Suzuki M, Tork C, Shelley B, McHugh J, Wallace K, Kleim SM, et al. (2007) Sexual dimorphism in disease onset and progression of a rat model of ALS. Amyotroph Lateral Scler 8: 20–25. [DOI] [PubMed] [Google Scholar]
- 106. Sharma HS, Badgaiyan RD, Alm P, Mohanty S, Wiklund L. (2005) Neuroprotective effects of nitric oxide synthase inhibitors in spinal cord injury-induced pathophysiology and motor functions: An experimental study in the rat. Ann N Y Acad Sci 1053: 422–434. [DOI] [PubMed] [Google Scholar]
- 107. Karam C, Barrett MJ, Imperato T, Macgowan DJ, Scelsa S. (2013) Vitamin D deficiency and its supplementation in patients with amyotrophic lateral sclerosis. J Clin Neurosci. [DOI] [PubMed] [Google Scholar]
- 108. Yanagihara R, Garruto RM, Gajdusek DC, Tomita A, Uchikawa T, Konagaya Y, et al. (1984) Calcium and vitamin D metabolism in guamanian chamorros with amyotrophic lateral sclerosis and parkinsonism-dementia. Ann Neurol 15: 42–48. [DOI] [PubMed] [Google Scholar]
- 109. Moghimi E, Gianforcaro A, Solomon J, Hamadeh M. (2015. (Submitted) Vitamin D3 at 50X the adequate intake attenuates disease pathophysiology in the spinal cord of the male, but is toxic in female, G93A mouse model of amyotrophic lateral sclerosis. PloS one. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Reeves PG, Nielsen FH, Fahey GC Jr. (1993) AIN-93 purified diets for laboratory rodents: Final report of the american institute of nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123: 1939–1951. [DOI] [PubMed] [Google Scholar]
- 111. Bieri J,Stoewsand G., Briggs G,S., Phillips G,M., Woodard R,W., Knapka J,C., J J. (1977) Report of the american institute of nurtition ad hoc committee on standards for nutritional studies. J Nutr 107: 1340–1348. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative immunoblots of 4-HNE, 3-NY, SOD2, catalase, GPx1, TNF-α, IL-6, IL-10, Bax, Bcl-2, pro-caspase 3, cleaved caspase 3, GDNF, ChAT, SMI-32 and SMI-36 protein expression in the spinal cord of 42 G93A mice: 23 adequate vitamin D3 intake (AI; 1 IU D3/g feed; 12 M, 11 F) and 19 deficient vitamin D3 intake (DEF; 0.025 IU D3/g feed; 10 M, 9 F). Each antibody and its corresponding anti-GAPDH set were loaded on a separate gel. Protein intensity was standardized to GAPDH.
(TIFF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.