Abstract
The efficacy of pharmacotherapy for PTSD, anxiety, and depression among combat veterans is not well-established.
Objectives
To estimate the effect of pharmacotherapy on PTSD, anxiety, and depression among combat veterans; to determine whether the effects varied according to patient and intervention characteristics; and to examine differential effects of pharmacotherapy on outcomes.
Materials and Methods
Google Scholar, PILOTS, PsycINFO, PubMed, and Web of Science databases were searched through November 2014. Searches resulted in eighteen double-blind, placebo controlled trials of 773 combat veterans diagnosed with PTSD and included only validated pre- and post-intervention PTSD and anxiety or depression measures. Authors extracted data on effect sizes, moderators, and study quality. Hedges’ d effect sizes were computed and random effects models estimated sampling error and population variance. The Johnson-Neyman procedure identified the critical points in significant interactions to define regions of significance.
Results
Pharmacotherapy significantly reduced (Δ, 95%CI) PTSD (0.38, 0.23-0.52), anxiety (0.42, 0.30-0.54), and depressive symptoms (0.52, 0.35-0.70). The effects of SSRIs and tricyclic antidepressants on PTSD were greater than other medications independent of treatment duration. The effect of SSRIs and tricyclic antidepressants were greater than other medications up to 5.2 and 13.6 weeks for anxiety and depression, respectively. The magnitude of the effect of pharmacotherapy on concurrently-measured PTSD, anxiety, and depression did not significantly differ.
Conclusions
Pharmacotherapy reduced PTSD, anxiety, and depressive symptoms in combat veterans. The effects of SSRIs and tricyclic antidepressants were greater for PTSD and occurred quicker for anxiety and depression than other medications.
Introduction
Posttraumatic stress disorder (PTSD) is a debilitating trauma-related disorder resulting from exposure to a traumatic event or events [1]. PTSD is a pervasive problem among military personnel who have experienced combat [2]. The lifetime prevalence of combat-related PTSD in US combat veterans ranges from approximately 6% to 31% [3]. With over 21.2 million military veterans in the US population, approximately 1.3 to 6.6 million veterans will experience PTSD during their lifetime [4].
Since September 11, 2001, over 2.4 million American service members have served in Iraq or Afghanistan with over 1 million service members deployed twice or more to war zones [5]. Consequently, the Veterans Health Administration and military healthcare systems have seen dramatic increases in cases of combat-related PTSD and depressive and anxiety disorders. Over 54% of the approximately 934,000 OEF/OIF/OND veterans utilizing Veterans Health Administration facilities since 2001 have received diagnosis for a mental health disorder. PTSD (29.4%), depressive disorders (23.2%), and anxiety disorders (20.7%) were the most frequent diagnoses [6].
The 2010 National Defense Authorization Act requested that the Institute of Medicine (IOM) examine the effectiveness of the growing number of PTSD programs and services available to service members and veterans in DoD and VA, respectively. The IOM committee’s report [7] indicated that, although there is a wealth of information on PTSD, there are also substantial gaps in our knowledge of how best to manage PTSD in service members and veterans diagnosed with PTSD [7].
Pharmacotherapy is a common method of treating combat-related PTSD [8]. Several pharmacological approaches have been investigated in the treatment of PTSD (e.g., antidepressants, adrenoreceptor antagonists, anticonvulsants, atypical antipsychotics, benzodiazepines), but the efficacy of pharmacotherapy for PTSD has not been well-established [9]. The aforementioned IOM committee report specifically identified several gaps in PTSD-treatment research in combat-veterans relative to pharmacotherapy to include: (i) further examination of pharmacotherapy for PTSD comorbid with other disorders, and, (ii) concern that although polypharmacy may result in improvement in PTSD symptoms, it may also result in more side effects and contribute to noncompliance to treatment [7]. These issues may be related to the high comorbidity of PTSD with symptoms of other psychological disorders like depression and anxiety or the treatment of specific symptoms (e.g., insomnia, flashbacks) rather than diagnosed psychological disorders [8]. Thus, there is a need to identify which drug classes best manage PTSD symptoms in conjunction with other comorbid psychological symptoms among veterans. These issues are both examined in the current review.
Selective serotonin re-uptake inhibitors (SSRIs) have shown efficacy as a first-line pharmacotherapy, but less than 60% of patients respond to treatment [10]. Other pharmacotherapies have shown similar efficacy to SSRIs, but are less well tolerated and therefore have not become first line therapies [9]. Although the efficacy of different classes of drugs remains uncertain, treating co-occurring disorders and symptoms, such as depression and anxiety, is essential in maximizing treatment outcomes in combat-related PTSD [8]. For example, the National Vietnam Veterans Readjustment Study indicated that 98.9% of veterans with PTSD met criteria for a lifetime comorbid psychiatric diagnosis [11]. The high comorbidity of anxiety and depressive symptoms likely exacerbates the chronic, debilitating effects of PTSD and the resistance to treatment. Thus, there also is a need to identify whether different pharmacological approaches differentially affect PTSD and other comorbid psychological conditions like anxiety and depression.
Although the majority of empirical research has focused on SSRIs, prior reviews have supported the efficacy of numerous short- and long-term pharmacotherapies for PTSD [12, 13], including PTSD diagnoses with comorbid anxiety and depressive symptoms [14]. A prior systematic review which focused on combat-related PTSD supported the efficacy of pharmacotherapy for PTSD among combat veterans [14], but did not focus on the best available evidence (e.g., randomized controlled trials (RCTs) of pharmacotherapy) and was limited by the use of inadequate statistical models. Moreover, no systematic review has examined the potential differential effects of pharmacotherapy across concurrently-measured PTSD, anxiety, and depressive symptoms among combat veterans with PTSD.
Thus, the primary aims of this systematic review were: (1) to estimate the effect size for pharmacotherapy on combat-related PTSD, anxiety, and depressive symptom severity among combat veterans; (2) to determine whether the effects varied according to patient characteristics and modifiable features of pharmacotherapy; and, (3) to examine potential differential effects of pharmacotherapy on concurrently-measured PTSD, anxiety, and depressive symptoms.
Materials and Methods
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines [15]. As detailed in the authors’ previously published systematic reviews, standard methods were used for data extraction and quality assessment [16–19], data synthesis and analysis [16–19], meta-regression analysis [16–19], and differential effects analysis [18].
Data Sources and Searches
Electronic searches of databases were conducted via Google Scholar, PILOTS, PsycINFO, PubMed, and Web of Science from database inception to November 2014 using the search strategy: (Posttraumatic Stress Disorder or PTSD) and (pharmacotherapy or pharmacological treatment) and (combat or combat veteran or military or military personnel or war or war veteran or veteran) and (anxiety or depression). Searches were restricted to randomized controlled trials. Reference lists from retrieved articles were manually searched.
Study Selection
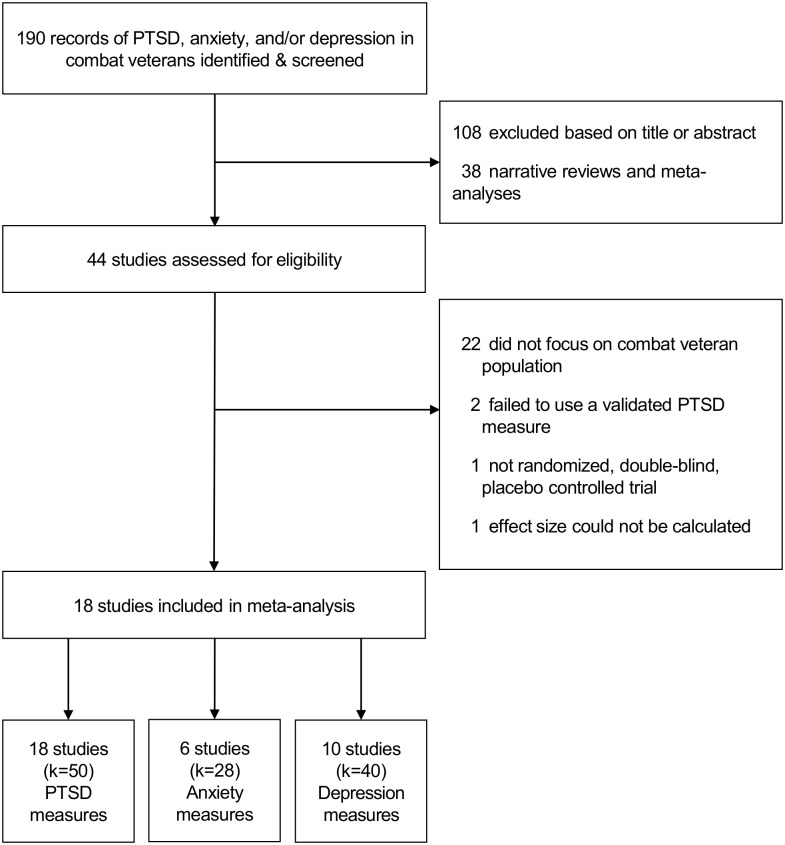
Inclusion criteria were: (1) a sample that included only combat veterans diagnosed with PTSD, (2) randomized, double-blind allocation to either pharmacotherapy or placebo condition, and (3) a PTSD symptom severity outcome measured at baseline and during and/or post-intervention. Exclusion criteria were: (1) use of nonrandomized, uncontrolled, or open trial designs; (2) failure to include or specify the inclusion of combat veterans with PTSD; (3) lack of data necessary for the calculation of effect size for PTSD; or (4) failure to use a validated PTSD outcome measure [20]. Fig 1 presents a flowchart of study selection.
Fig 1. Flow Chart of Study Selection.
Data Extraction and Quality Assessment
Data were independently extracted by the authors and discrepancies were resolved by consensus judgment. Effect sizes were calculated by subtracting the mean change in the comparison condition from the mean change in the treatment condition and dividing the difference by the pooled standard deviation of baseline scores [21]. Effect sizes were adjusted for small sample size bias and calculated so that decreases in PTSD, anxiety, and depression resulted in positive effect sizes [21]. When a standard deviation was not reported (k = 1) [22] it was estimated [23] from the largest study of combat-related PTSD using the same PTSD symptom severity measure [24]. Two-way (Effects x Raters) intraclass correlation coefficients (ICC) for absolute agreement were calculated to examine inter-rater reliability for symptom effect sizes and moderators. The initial ICCs, based on 10 effects, were ≥0.90.
Authors independently assessed study quality using a widely recognized method that addressed randomization, sample selection, quality of outcome measures, and statistical analysis [25]. Quality scores were reported for each study for descriptive purposes, but were not used as weights or moderators in the analysis because of the potential disparity in results that depends on the specific quality scale employed [26].
Data Synthesis and Analysis
Separate statistical analyses were performed for effects of pharmacotherapy on PTSD, anxiety, and depressive symptom severity. Meta-regression was used as the primary analysis of moderator effects in each of these models in order to reduce the probability of type I error by computing simultaneous estimates of independent effects by multiple moderator variables on the variation in effect size across trials.
An SPSS macro (i.e., MeanES; SPSS version 22.0, SPSS Inc., Chicago, IL) was used to calculate the aggregated mean effect size delta (Δ), associated 95% confidence interval, and the sampling error variance according to a random effects model [27]. Random effects models were used to account for between-studies heterogeneity associated with both study-level sampling error and population variance [27]. Each effect was weighted by the inverse of its variance and re-estimated after the random effects variance component was added [21]. Heterogeneity and consistency were evaluated with the Q statistic and the I2 statistic, respectively [28]. Heterogeneity also was examined relative to observed variance and was indicated if the sampling error accounted for less than 75% of the observed variance [21]. Publication bias (i.e., smaller studies showing larger effects) was addressed by inspection of a funnel plot [29] and quantified with rank correlation and regression methods [29, 30].
Primary Moderators
Three primary moderators were selected based on logical, theoretical, or empirical relations to PTSD, anxiety, depression, and/or pharmacotherapy: type of pharmacotherapy, treatment duration, and pharmacotherapy x duration interaction. These variables were tested in each model that met criteria for heterogeneity of effects. Definitions of these variables can be found in S1 Table.
Primary Moderator Analysis
An SPSS macro (MetaReg; SPSS version 22.0, SPSS Inc., Chicago, IL) was used to conduct separate moderator analyses for PTSD, anxiety, and depression symptom severity models [27]. For each model, primary moderator variables were included in a random-effects multiple linear regression analysis with maximum-likelihood estimation [21, 27] adjusted both for non-independence of multiple effects contributed by single studies [31] and for age because of its univariate association with outcomes. Tests of the regression model (Q R ) and its residual error (Q E ) are reported for each model. Significant categorical moderators in the regression analyses were decomposed using a random effects model to compute mean effect sizes and 95% confidence intervals [27]. The Johnson-Neyman procedure was conducted to identify the critical point in significant interactions of categorical and continuous variables in order to define regions of significance [32, 33].
Secondary Moderators and Analysis
Secondary moderators were selected for descriptive, univariate analyses for PTSD, anxiety, and depressive symptom severity models. These variables were grouped into patient characteristics (i.e., age, sex, combat sample, baseline symptom score), intervention characteristics (i.e., pharmacotherapy type, program duration, concomitant medication), and study design characteristics (i.e., adherence, time period, outcome measure). Definitions for these variables can be found in S1 Table. Random effects models were used to calculate mean effect sizes (Δ) and 95% confidence intervals for continuous and categorical variables [27].
Direct Comparison of Concurrent Effects on PTSD, Anxiety, and Depression
Pharmacotherapy investigations that concurrently measured PTSD, anxiety, and depressive symptom severity were used to directly compare the magnitude of the effects among the three mental health outcomes in combat veterans with PTSD. PTSD, anxiety, and depression effect sizes were then dummy coded. Using a SPSS macro (MetaF; SPSS version 22.0, SPSS Inc., Chicago, IL), mean effect sizes (Δ) and 95% confidence intervals were computed and the significance of coded effect size variables was tested [27]. Differences among the effects for PTSD, anxiety, and depressive symptoms were determined using the Q B statistic [21]. For significant tests, pairwise contrasts were tested at p<0.05. Random effects models were used for all analyses [27].
Results
Eighteen trials of 773 combat veterans were included in the meta-analysis and are presented in S1 References. Characteristics of included trials and study quality assessment are present in Table 1. An annotated table of descriptors of the unweighted Hedge’s d effects is presented in Table 2. Funnel plots for all analysis models were inspected and found to be roughly symmetrical (S1, S2, and S3 Figs). The Begg’s rank correlation and Egger’s regression analyses were not statistically significant for any of the models suggesting absence of publication bias (S2 Table).
Table 1. Characteristics of Included Trials.
| PTSD | Anxiety | Depression | ||||
|---|---|---|---|---|---|---|
| Study Characteristics: | ||||||
| Studies (n) | 18 | 6 | 10 | |||
| Effects (k) | 50 | 28 | 40 | |||
| Total Sample (N) | 773 | 365 | 550 | |||
| Patient Characteristics: | ||||||
| Age (mean, [SD]) | 47.3 (7.8) | 47.7 (8.7) | 47.1 (8.8) | |||
| Male (%) | 98.3 | 98.6 | 98.2 | |||
| Time Since PTSD Diagnosis (years [SD]) | 19.0 (9.9) | 16.4 (9.2) | 19.7 (9.8) | |||
| Number of Studies Reporting Time Since PTSD Diagnosis (n [%]) | 5 (27.8) | 2 (33.3) | 3 (30.0) | |||
| Baseline Score (T-Score [SD]) | 61.5 (11.1) | 63.8 (7.4) | 72.1 (11.8) | |||
| Intervention Characteristics: | ||||||
| Type of Medication (%) | ||||||
| Anticonvulsant | 22.0 | 35.7 | 25.0 | |||
| Antipsychotic | 6.0 | 3.6 | 2.5 | |||
| Novel Class | 2.0 | 0.0 | 0.0 | |||
| SSRI | 24.0 | 28.6 | 20.0 | |||
| Tricyclic | 28.0 | 25.0 | 35.0 | |||
| Other | 18.0 | 7.1 | 17.5 | |||
| Treatment Duration (weeks [SD]) | 9.8 (5.3) | 10.0 (6.6) | 9.8 (5.9) | |||
| Treatment Adherence (% [SD]) | 80.1 (13.9) | 83.5 (10.5) | 78.6 (13.9) | |||
| Number of Studies Reporting Treatment Adherence (n [%]) | 12 (66.7) | 5 (83.3) | 8 (80.0) | |||
| Most Frequently Used Outcome Measures (k [%]) | CAPS: | 16 (32.0) | HAM-A: | 26 (92.9) | MADRS: | 18 (42.5) |
| CGI-S: | 9 (18.0) | BAI: | 2 (7.1) | HAM-D: | 17 (45.0) | |
| Study Quality: | ||||||
| Study Quality (mean rating [SD]) | 11.6 (0.9) | 12.4 (0.9) | 12.1 (0.9) | |||
Abbreviations: SSRI, Selective Serotonin Reuptake Inhibitor; CAPS, Clinician Administered PTSD Scale; CGI-S, Clinical Global Impression-Severity; HAM-A, Hamilton Anxiety Rating Scale; BAI, Beck Anxiety Inventory; MADRS, Montgomery–Åsberg Depression Rating Scale; HAM-D, Hamilton Depression Rating Scale.
Table 2. Annotated Descriptors of Unweighted Hedges’ d Effect Sizes.
| Source | Total N | Age (years) | Sample | Drug Class | Drug Name | Duration (weeks) | Adherence (%) | PTSD Scale | Hedges’ d (95%CI) |
|---|---|---|---|---|---|---|---|---|---|
| Bartzokis et al., 2004 | 48 | 51.6 | US Vietnam Veterans | Antipsychotic | Risperidone | 16 | NR | CAPS | 0.67 (0.09 to 1.26) |
| Batki et al., 2014 | 30 | 50 | US Mixed Conflict Veterans | Anticonvulsant | Topiramate | 12 | 90 | PCL-M | 0.49 (-0.24 to 1.21) |
| Davidson et al., 1990 | 40 | 53.6 | US Mixed Conflict Veterans | TCA | Amitriptyline | 8 | 71 | CGI-S | 0.98 (0.33 to 1.64) |
| Davidson et al., 1990 | 40 | 53.6 | US Mixed Conflict Veterans | TCA | Amitriptyline | 8 | 71 | SIP | 0.07 (-0.55 to 0.70) |
| Davidson et al., 1990 | 40 | 53.6 | US Mixed Conflict Veterans | TCA | Amitriptyline | 8 | 71 | IES | 0.41 (-0.22to 1.04) |
| Davidson et al., 1990 | 33 | 53.6 | US Mixed Conflict Veterans | TCA | Amitriptyline | 8 | 71 | CGI-S | 0.81 (0.10 to 1.52) |
| Davidson et al., 1990 | 33 | 53.6 | US Mixed Conflict Veterans | TCA | Amitriptyline | 8 | 71 | SIP | 0.26 (-0.43 to 0.94) |
| Davidson et al., 1990 | 33 | 53.6 | US Mixed Conflict Veterans | TCA | Amitriptyline | 8 | 71 | IES | 0.53 (-0.16 to 1.23) |
| Davis et al., 2004 | 41 | 53.8 | US Vietnam Veterans | TCA | Nefazodone | 4 | 56 | CAPS | 0.90 (0.23 to 1.56) |
| Davis et al., 2004 | 41 | 53.8 | US Vietnam Veterans | TCA | Nefazodone | 8 | 56 | CAPS | 0.99 (0.32 to 1.66) |
| Davis et al., 2004 | 41 | 53.8 | US Vietnam Veterans | TCA | Nefazodone | 12 | 56 | CAPS | 0.29 (-0.35 to 0.93) |
| Davis et al., 2004 | 41 | 53.8 | US Vietnam Veterans | TCA | Nefazodone | 4 | 56 | PCL-M | 0.29 (-0.35 to 0.92) |
| Davis et al., 2004 | 41 | 53.8 | US Vietnam Veterans | TCA | Nefazodone | 8 | 56 | PCL-M | 0.26 (-0.38 to 0.90) |
| Davis et al., 2004 | 41 | 53.8 | US Vietnam Veterans | TCA | Nefazodone | 12 | 56 | PCL-M | 0.38 (-0.26 to 1.02) |
| Davis et al., 2008 | 82 | 55.2 | US Mixed Conflict Veterans | Anticonvulsant | Divalproex | 4 | 83 | CAPS | -0.05 (-0.48 to 0.38) |
| Davis et al., 2008 | 82 | 55.2 | US Mixed Conflict Veterans | Anticonvulsant | Divalproex | 8 | 83 | CAPS | -0.08 (-0.51 to 0.35) |
| Davis et al., 2008 | 82 | 55.2 | US Mixed Conflict Veterans | Anticonvulsant | Divalproex | 2 | 83 | TOP-8 | -0.04 (-0.47 to 0.39) |
| Davis et al., 2008 | 82 | 55.2 | US Mixed Conflict Veterans | Anticonvulsant | Divalproex | 4 | 83 | TOP-8 | -0.02 (-0.45 to 0.41) |
| Davis et al., 2008 | 82 | 55.2 | US Mixed Conflict Veterans | Anticonvulsant | Divalproex | 6 | 83 | TOP-8 | -0.04 (-0.47 to 0.39) |
| Davis et al., 2008 | 82 | 55.2 | US Mixed Conflict Veterans | Anticonvulsant | Divalproex | 8 | 83 | TOP-8 | 0.02 (-0.41 to 0.45) |
| Davis et al., 2008 | 82 | 55.2 | US Mixed Conflict Veterans | Anticonvulsant | Divalproex | 2 | 83 | CGI-S | -0.10 (-0.54 to 0.33) |
| Davis et al., 2008 | 82 | 55.2 | US Mixed Conflict Veterans | Anticonvulsant | Divalproex | 4 | 83 | CGI-S | -0.31 (-0.75 to 0.12) |
| Davis et al., 2008 | 82 | 55.2 | US Mixed Conflict Veterans | Anticonvulsant | Divalproex | 6 | 83 | CGI-S | 0.00 (-0.43 to 0.43) |
| Davis et al., 2008 | 82 | 55.2 | US Mixed Conflict Veterans | Anticonvulsant | Divalproex | 8 | 83 | CGI-S | -0.21 (-0.64 to 0.23) |
| Frank et al., 1988 | 23 | 38 | US Vietnam Veterans | TCA | Imipramine | 8 | NR | IES | 0.80 (-0.05 to 1.65) |
| Frank et al., 1988 | 22 | 38 | US Vietnam Veterans | MAOI | Phenelzine | 8 | NR | IES | 1.68 (0.71 to 2.65) |
| Germain et al., 2012 | 33 | 40.9 | US Mixed Conflict Veterans | Antihypertensive | Prazosin | 8 | 85 | PCL-M | 0.66 (-0.05 to 1.36) |
| Germain et al., 2012 | 33 | 40.9 | US Mixed Conflict Veterans | Antihypertensive | Prazosin | 8 | 70 | PCL-M | 0.77 (0.06 to 1.47) |
| Hamner et al., 2003 | 37 | 52 | US Vietnam Veterans | Antipsychotic | Risperidone | 5 | NR | CAPS | -0.06 (-0.70 to 0.59) |
| Hertzberg et al., 2000 | 12 | 46 | US Vietnam Veterans | SSRI | Fluoxetine | 12 | 92 | DTS | -0.27 (-1.40 to 0.87) |
| Hertzberg et al., 2000 | 12 | 46 | US Vietnam Veterans | SSRI | Fluoxetine | 12 | 92 | SIP | 0.00 (-1.13 to 1.13) |
| Martenyi et al., 2006 | 144 | 36.2 | European Veterans | SSRI | Fluoxetine | 12 | 97 | TOP-8 | 0.95 (0.55 to 1.35) |
| Martenyi et al., 2006 | 144 | 36.2 | European Veterans | SSRI | Fluoxetine | 12 | 97 | CAPS | 0.97 (0.57 to 1.37) |
| Martenyi et al., 2006 | 144 | 36.2 | European Veterans | SSRI | Fluoxetine | 12 | 97 | DTS | 0.60 (0.21 to 1.00) |
| Martenyi et al., 2006 | 144 | 36.2 | European Veterans | SSRI | Fluoxetine | 12 | 97 | CGI-S | 1.36 (0.95 to 1.78) |
| Martenyi et al., 2006 | 144 | 36.2 | European Veterans | SSRI | Fluoxetine | 24 | 97 | TOP-8 | 0.63 (0.12 to 1.13) |
| Martenyi et al., 2006 | 144 | 36.2 | European Veterans | SSRI | Fluoxetine | 24 | 97 | CAPS | 0.42 (-0.09to 0.92) |
| Martenyi et al., 2006 | 144 | 36.2 | European Veterans | SSRI | Fluoxetine | 24 | 97 | DTS | 0.31 (-0.19to 0.81) |
| Martenyi et al., 2006 | 144 | 36.2 | European Veterans | SSRI | Fluoxetine | 24 | 97 | CGI-S | 1.63 (1.05 to 2.20) |
| Monnelly et al., 2003 | 15 | 51.2 | US Mixed Conflict Veterans | Antipsychotic | Risperidone | 6 | NR | PCL-M | 0.75 (-0.30 to 1.79) |
| Neylan et al., 2006 | 54 | 44 | US Mixed Conflict Veterans | Antihypertensive | Guanfacine | 8 | 90 | CAPS | -0.08 (-0.61 to 0.45) |
| Neylan et al., 2006 | 54 | 44 | US Mixed Conflict Veterans | Antihypertensive | Guanfacine | 8 | 90 | IES | -0.30 (-0.84 to 0.23) |
| Petrakis et al., 2006 | 43 | 46.3 | US Mixed Conflict Veterans | ADI | Disulfiram | 12 | NR | CAPS | 0.20 (-0.50 to 0.90) |
| Petrakis et al., 2006 | 43 | 46.3 | US Mixed Conflict Veterans | Opioid Antagonist | Naltrexone | 12 | NR | CAPS | -0.06 (-0.76 to 0.64) |
| Raskind et al., 2003 | 10 | 53 | US Vietnam Veterans | Antihypertensive | Prazosin | 10 | 100 | CAPS | 1.29 (-0.07 to 2.65) |
| Raskind et al., 2013 | 67 | 30.4 | US Mixed Conflict Veterans | Antihypertensive | Prazosin | 15 | 69 | CAPS | 0.48 (-0.01 to 0.97) |
| Reist et al., 1989 | 18 | 38.4 | US Vietnam Veterans | TCA | Desipramine | 4 | 67 | IES | 0.10 (-0.83 to 1.02) |
| Rothbaum et al., 2008 | 77 | 42 | US Mixed Conflict Veterans | SNRI | Venlafaxine | 12 | 73 | CAPS | -0.11 (-0.84 to 0.62) |
| Zohar et al., 2002 | 42 | 39.5 | Israeli Veterans | SSRI | Sertaline | 10 | NR | CAPS | 0.40 (-0.21 to 1.02) |
| Zohar et al., 2002 | 42 | 39.5 | Israeli Veterans | SSRI | Sertaline | 10 | NR | CGI-S | 0.41 (-0.20 to 1.03) |
Abbreviations: ADI, Aldehyde Dehydogenase Inhibitor; MAOI, Monoamine Oxidase Inhibitor; SNRI, Seratonin-Norepinephrine Reuptake Inhibitor; SSRI, Selective Serotonin Reuptake Inhibitor; TCA, Tricyclic Antidepressant; NR, Not Reported; CAPS, Clinician Administered PTSD Scale; PCL-M, PTSD Checklist-Military; TOP-8, Treatment Outcome PTSD Scale; CGI-S, Clinical Global Impression-Severity; SIP, Structured Interview for PTSD; IES, Impact of Events Scale; DTS, Davidson Trauma Scale.
PTSD Symptom Severity
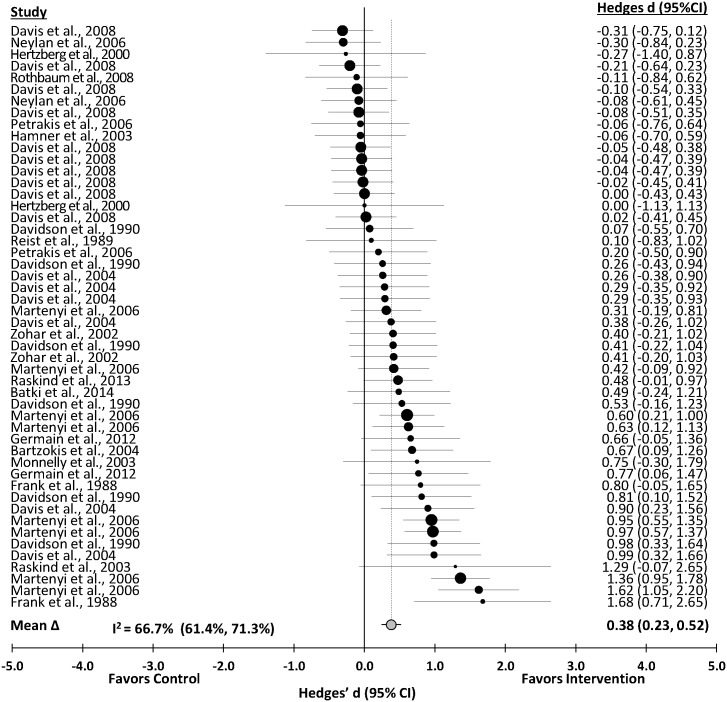
PTSD symptom severity was significantly reduced after pharmacotherapy interventions (Δ = 0.38 (0.23 to 0.52); z = 5.24, p<0.001). The distribution of the effects is presented in Fig 2. The effect was heterogeneous (Q T(49) = 144.23, p<0.001). Sampling error accounted for 38.2% of the observed variance. The effect was not consistent across studies (I2 = 66.7%; 95% CI, 61.4% to 71.3%).
Fig 2. Forest Plot of the Unweighted Distribution of Pharmacotherapy Effects on PTSD.
Moderator analysis
The overall multiple regression model for PTSD was significantly related to effect size (Q R(5) = 58.14; p< 0.001, R2 = 0.50; Q E(44) = 59.01, P = 0.065). Only type of pharmacotherapy (β = 0.77, z = 3.15, P = 0.002) was independently related to effect size. Significantly larger effects were found in SSRIs and Tricyclic anti-depressants (Δ = 0.63, [95% CI, 0.48, 0.78]) compared to the average of effect of all other drug therapies (Δ = 0.10, [95% CI, -0.05, 0.25]; QB(1) = 23.37, p<0.001).
Anxiety Symptom Severity
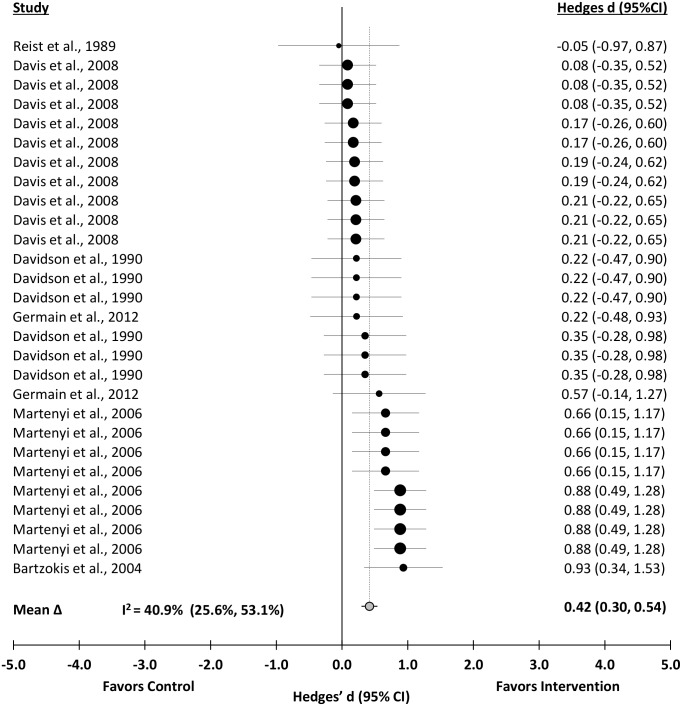
Anxiety was significantly reduced after pharmacotherapy interventions (Δ = 0.42 (0.30 to 0.54); z = 6.75, p<0.001). A distribution of effects is presented in Fig 3. The effect was heterogeneous (Q T(27) = 44.01, p = 0.0206). Sampling error accounted for 62.7% of the observed variance. The effect was moderately consistent across studies (I2 = 40.9, 95% CI, 25.6% to 53.1%).
Fig 3. Forest Plot of the Unweighted Distribution of Pharmacotherapy Effects on Anxiety.
Moderator analysis
The overall multiple regression model for anxiety was significantly related to effect size (Q R(5) = 36.99; p< 0.001, R2 = 0.84; Q E(22) = 7.02, P = 0.999). The pharmacotherapy x duration interaction (β = 0.06, z = 1.94, P = 0.050) was independently related to effect size. The Johnson-Neyman procedure yielded critical points for treatment duration at 5.2 (t = -2.07, p = 0.050) and 11.0 weeks (t = 2.07, p = 0.050) when comparing the effects of pharmacotherapy treatment using SSRIs and Tricyclic antidepressants and other drug classes.
Depressive Symptom Severity
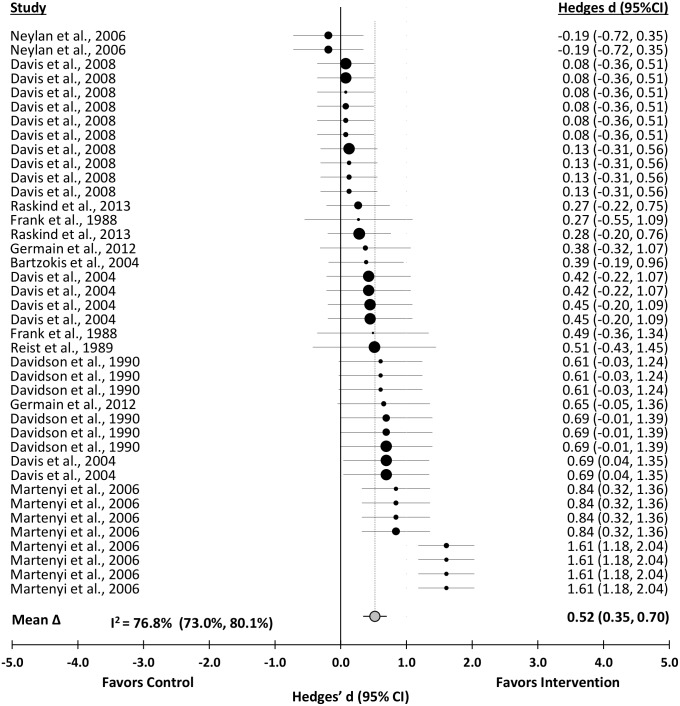
Depression was significantly reduced after pharmacotherapy interventions (Δ = 0.52 (0.35 to 0.70); z = 5.81, p< 0.001). A distribution of effects is presented in Fig 4. The effect was heterogeneous (Q T(38) = 159.58, p<0.001). Sampling error accounted for 42.6% of the observed variance. The effect was not consistent across studies (I2 = 76.8%; 95% CI, 73.0% to 80.1%).
Fig 4. Forest Plot of the Unweighted Distribution of Pharmacotherapy Effects on Depression.
Moderator analysis
The overall multiple regression model for depression was significantly related to effect size (Q R(5) = 136.57; p< 0.001, R2 = 0.85; Q E(34) = 23.96, P = 0.900). The pharmacotherapy x duration interaction (β = 0.06, z = 2.22, P = 0.026) was independently related to effect size. The Johnson-Neyman procedure yielded a critical point for treatment duration at 13.6 weeks (t = -2.03, p = 0.050) when comparing the effects of pharmacotherapy treatment using SSRIs and Tricyclic antidepressants and other drug classes.
Secondary Moderator Analyses
The number of effects (k), mean effect size (Δ), 95% CI, p value, and I2 for each level of each moderator for PTSD, anxiety, and depression models are presented in S3 Table, S4 Table and S5 Table, respectively.
Differential Effects Analysis
A total of 28 effects concurrently measured PTSD, anxiety, and depressive symptom severity in combat veterans with PTSD. These effects were derived from 6 studies (N = 365) which had an average sample size of 61 (range = 18 to 144). Direct comparisons indicated no significant difference in the magnitude of the effect of pharmacotherapy on PTSD, anxiety, and depressive symptom severity (Q B (2) = 3.36, p = 0.186). These effects were further decomposed by type of medication for descriptive purposes (S4 Fig).
Discussion
The cumulative evidence summarized in this review indicates that pharmacotherapy significantly reduces PTSD, anxiety, and depressive symptom severity among combat veterans with PTSD. The magnitude of the overall effects of pharmacotherapy on PTSD (Δ = 0.38), anxiety (Δ = 0.42), and depressive symptoms (Δ = 0.52) were moderate and similar to effects seen in previous reviews of pharmacotherapeutic effects on PTSD, anxiety, and depressive symptoms in non-veteran group [12–14, 34–36]. The reduction in PTSD, anxiety, and depressive symptoms found among combat veterans using pharmacotherapy is equivalent to a number needed to treat [37] of approximately 6 (4.0 to 8.8), 5 (3.8 to 7.0), and 4 (3.0 to 5.8), respectively. Differential analyses showed that pharmacotherapy does not elicit significantly different effects on PTSD, anxiety, and depressive symptoms. These findings support the use of pharmacotherapy as a concurrent treatment for PTSD, anxiety, and depression among combat veterans.
Heterogeneous main effects of pharmacotherapy for PTSD, anxiety, and depression required further examination of the specific treatment characteristics moderating the relationship. The type of medication and the duration of the treatment were especially prominent factors, and are discussed in greater detail in the following sections.
PTSD Symptom Severity
Reductions in PTSD symptom severity in response to pharmacotherapy among combat veterans were greater for SSRI and Tricyclic antidepressants (Δ = 0.63) compared with other medications (Δ = 0.10) regardless of treatment duration. These findings support the involvement of the serotonergic (5-HT) and noradrenergic (NE) systems in the etiology of PTSD [38]. For example, evidence of the plausible role of the 5-HT system includes associated genetic variation in a polymorphism of the 5-HT transporter and increased 5-HT neurotransmission in key brain areas (e.g., hippocampus, amygdala) following traumatic events [38]. The present findings also are consistent with previous evidence supporting the efficacy of both SSRI and tricyclic antidepressants in treating symptoms of PTSD despite some literature suggesting resistance to these medication classes in combat-related PTSD [10, 39]. However, it is important to examine these findings in the context of the IOM report which acknowledged the concern that, although polypharmacy may result in improvement in PTSD symptoms, it may also increase side effects and contribute to noncompliance to treatment [7]. Indeed, some evidence has suggested that SSRI and Tricyclic antidepressant treatment may not be optimal for individuals with anxiety symptoms [40], reiterating the call within the IOM report for further examination of pharmacotherapy for PTSD comorbid with other psychological symptoms and disorders [7]. The following sections discuss the present findings for comorbid symptoms of depression and anxiety within this context.
PTSD and Depressive Symptom Severity
Pharmacotherapy significantly improved comorbid depressive symptoms among combat veterans with PTSD. Improvement in depressive symptom severity among combat veterans with PTSD undergoing pharmacotherapy treatment varied according to an interaction between the type of medication and duration of treatment. The effects of SSRI and Tricyclic antidepressants on depressive symptoms were significantly greater than other medications up to a treatment period of approximately 14 weeks, after which there was no longer significant difference between SSRI and Tricyclic antidepressants and other classes of medication. These findings support previous evidence suggesting that PTSD and depression are highly correlated, but independent, responses to trauma, plausibly resulting in differences in treatment response [40–42]. Clinicians may benefit from addressing these differences throughout the course of pharmacotherapy.
PTSD and depression occur frequently following traumatic exposure both concurrently and as separate disorders [40, 42]. While PTSD and comorbid PTSD/depression are often indistinguishable, previous studies support the existence of depression as a separate construct in the acute aftermath of trauma with its own unique characteristics and its own unique course of recovery [40]. PTSD symptoms are strongly predictive of later depression [40, 42]. For example, non-cognitive factors such as hyperarousal have reliably preceded symptoms of depression [42]. Cognitive factors such as intrusive memories also can begin to differentiate comorbid PTSD/depression from depression alone as soon as three months post-trauma. These cognitive factors may act as a mediator between PTSD and depression [40].
Our findings support the symptom-specific time course linking the bidirectional relation between PTSD and depression. SSRIs and tricyclic antidepressants had a greater effect than other drug classes in the management of PTSD symptoms regardless of treatment duration; whereas, the differential therapeutic effects of these medications were most effective for depressive symptoms until about three and a half months into treatment. In addition to alleviating the core symptoms of PTSD, some SSRIs are also effective in treating common comorbidities, such as depression and anxiety [43]. Thus, SSRIs can address depression symptoms directly and also indirectly through non-cognitive factors (i.e., hyperarousal) which may facilitate prevention of future depressive episodes [40, 42]. The slower onset of therapeutic effects found in other drug classes, such as antipsychotics, may be associated with a mechanism of action related to cognitive factors that can mediate the PTSD and depression relationship [42]. This mediated response may be especially important for those patients that do not respond to short-term treatment with SSRI or tricyclic antidepressants.
Although the IOM has questioned the merit of polypharmacy [7], monotherapy with traditional antidepressants may not be sufficient in patients with combat-related PTSD. For example, atypical antipsychotics are an emerging class of drugs that may help alleviate PTSD symptoms along cognitive symptom dimensions [12, 44]. Future studies should investigate new combinations of pharmacotherapy that may provide improvement in both cognitive and non-cognitive PTSD symptoms and aid the prevention of PTSD/depression comorbidity.
PTSD and Anxiety Symptom Severity
Pharmacotherapy significantly improved comorbid anxiety symptoms among combat veterans with PTSD. Improvement in anxiety symptom severity among combat veterans with PTSD undergoing pharmacotherapy treatment similarly varied according to an interaction between the type of medication and duration of treatment. The effects of SSRI and Tricyclic antidepressants on PTSD symptoms were significantly greater than other medications up to a treatment period of approximately 5 weeks. However, following 11 weeks of treatment the effects of other medication classes were significantly greater than SSRIs and Tricyclic antidepressants.
Nearly 60% of veterans with PTSD report anxiety symptoms, and 20% have reported a panic attack in the previous month [38]. The results reported here support other findings that antidepressant medications, particularly SSRIs, have been effective in the treatment of not only core symptoms of PTSD but also comorbid conditions including panic disorder, social anxiety disorder, and generalized anxiety disorder [43]. These positive effects are likely related to neural circuits and substrates underlying acute and chronic stress responses and to trauma memory encoding and retrieval [9], and underscore the critical need to further examine effects of pharmacotherapy on comorbid symptoms of anxiety among individuals with PTSD [7].
Comorbid PTSD/anxiety also is important to consider during PTSD treatment. For example, higher depressive symptom severity was found among patients with PTSD and comorbid panic disorder compared to patients without the comorbidity [45]. Thus, comorbid anxiety may complicate not only treatment of PTSD, but also depression based-treatment, as it is related to non-cognitive factors such as hyperarousal. Future studies should investigate how to maximize the use of antidepressant agents in the treatment of not only PTSD and depression following trauma, but also in the context of comorbid anxiety disorders.
Limitations
Limitations in the adequacy of reporting and the methodological rigor of included trials are of note. Several trials did not provide adequate information about features of the intervention, particularly regarding concomitant medication use and adherence/compliance to the prescribed pharmacotherapy, while others did not utilize the most well-validated outcome measures available. Moreover, the limited number of effects derived from studies that examined novel class treatments such as atypical antipsychotics or novel class antidepressants preclude meaningful interpretations of findings for these drug classes and warrant future research. Finally, given both the prevalence of comorbid anxiety and depressive symptoms in PTSD and the present findings which suggest that pharmacotherapy can concurrently attenuate these symptoms among combat veterans with PTSD, future research should prioritize concurrent assessment of related symptoms.
Conclusions
PTSD is a pervasive problem among combat veterans. Concurrent anxiety and depressive symptoms are frequently reported among veterans with PTSD, and likely exacerbate the chronic, debilitating effects of PTSD and the resistance to treatment. PTSD currently has few proven pharmacotherapies. However, the evidence reviewed here suggests that pharmacotherapy has a positive, but modest, therapeutic effect on PTSD, anxiety, and depressive symptom severity, and it also successfully acts as a concurrent treatment for these symptoms among combat veterans. This is especially evident for SSRI and Tricyclic antidepressants. The therapeutic effects of SSRI and tricyclic antidepressant medications were greater for PTSD and occurred more quickly for anxiety and depression than with other commonly prescribed medications. While the pathophysiology of PTSD implicates many different neurotransmitter and neuroanatomical pathways, the delineation of the abnormalities in these chemical, structural, and neural systems will require time to fully understand. Until that time, the available evidence suggests that SSRIs and Tricyclic antidepressants should be considered a first-line treatment while making an allowance for other emerging classes of medication that may further alleviate symptoms in refractory PTSD relative to cognitive dimensions such as avoidance and intrusive memories.
Supporting Information
(TIF)
(TIF)
(TIF)
(TIF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
Funding for this work was provided by the National Institutes of Health under grant R01 HL095799. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
All relevant data included in the meta-analysis are presented within the paper and its supporting information files. Additional raw data extracted from the included trials on which the aggregated data presented in the meta-analysis are available within the original manuscripts of included trials as well as in spreadsheet format by request from the authors.
Funding Statement
Funding for this work was provided by the National Institutes of Health under grant R01 HL095799. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (2013). 5th ed. Arlington, VA: American Psychiatric Press. [Google Scholar]
- 2. Smith T, Ryan MA, Wingard DL, Slymen DJ, Sallis JF, Kritz-Silverstein D, et al. (2008) New onset and persistent symptoms of post-traumatic stress disorder self reported after deployment and combat exposures: Prospective population based US military cohort study. BMJ. 2008;336(7640): 366–371. 10.1136/bmj.39430.638241.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Richardson LK, Frueh BC, Acierno R. Prevalence estimates of combat-related PTSD: A critical review. Aust N Z J Psychiatry. 2010;44(1): 4–19. 10.3109/00048670903393597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.United States Census Bureau. Veteran Status: 2012 American Community Survey 1-Year Estimates. 2012 American Community Survey. U.S. Census Bureau website. Available: http://factfinder2.census.gov. Accessed 31 December 2013.
- 5.Defense Manpower Data Center, Department of Defense. Contingency Tracking System: Deployment File Baseline Report, as of July 11, 2012.
- 6.Epidemiology Program, Post-Deployment Health Group, Office of Public Health, Veterans Health Administration, Department of Veterans Affairs. Analysis of VA Health Care Utilization among Operation Enduring Freedom, Operation Iraqi Freedom, and Operation New Dawn Veterans, from 1st Qtr FY 2002 through 2nd Qtr FY 2013. Washington, DC: Author; 2013.
- 7. IOM (Institute of Medicine). Treatment for posttraumatic stress disorder in military and veteran populations: Final assessment. Washington, DC: The National Academies Press; 2014. [PubMed] [Google Scholar]
- 8. Mohamed S, Rosenheck RA. Pharmacotherapy of PTSD in the U.S. Department of Veterans Affairs: diagnostic- and symptom-guided drug selection. J Clin Psychiatry. 2008;69(6): 959–65. [DOI] [PubMed] [Google Scholar]
- 9. Steckler T, Risbrough V. Pharmacological treatment of PTSD—Established and new approaches. Neuropharmacol. 2012;62(2): 617–627. 10.1016/j.neuropharm.2011.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stein MB, Kline NA, Matloff JL. Adjunctive olanzapine for SSRI-resistant combat-related PTSD: A double-blind, placebo-controlled study. Am J Psychiatry. 2002;159(10): 1777–1779. [DOI] [PubMed] [Google Scholar]
- 11. Kulka RA, Schelenger WE, Fairbank JA, Hough RL, Jordan BK, Marmar CR, et al. Trauma and the Vietnam War generation: Report of findings from the National Vietnam Veterans Readjustment Study. New York, NY: Brunner/Mazel; 1990. [Google Scholar]
- 12. Ahearn EP, Juergens T, Cordes T, Becker T, Krahn D. A review of atypical antipsychotic medications for posttraumatic stress disorder. Int Clin Psychopharmacol. 2011;26(4): 193–200. 10.1097/YIC.0b013e3283473738 [DOI] [PubMed] [Google Scholar]
- 13. Berger W, Mendlowicz MV, Marques-Portella C, Kinrys G, Fontenelle LF, Marmar CR, et al. Pharmacologic alternatives to antidepressants in posttraumatic stress disorder: A systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(2): 169–180. 10.1016/j.pnpbp.2008.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stewart CL, Wrobel TA. Evaluation of the efficacy of pharmacotherapy and psychotherapy in treatment of combat-related post-traumatic stress disorder: A meta-analytic review of outcome studies. Mil Med. 2009;174(5): 460–469. [DOI] [PubMed] [Google Scholar]
- 15. Moher D, Liberati A, Tetzlaff J, Altman DG, Antes G, Atkins D, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Internal Med. 2009;151(4): 264–269. [DOI] [PubMed] [Google Scholar]
- 16. Herring MP, O’Connor PJ, Dishman RK. The effect of exercise training on anxiety symptoms among patients. A systematic review. Arch Intern Med. 2010;170(4): 321–331. 10.1001/archinternmed.2009.530 [DOI] [PubMed] [Google Scholar]
- 17. Herring MP, Puetz TW, O’Connor PJ, Dishman RK. Effect of exercise training on depressive symptoms among patients with a chronic illness. A systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2012;172(2): 101–111. 10.1001/archinternmed.2011.696 [DOI] [PubMed] [Google Scholar]
- 18. Puetz TW, Herring MP. Differential effects of exercise on cancer-related fatigue during and following treatment. A meta-analysis. Am J Prev Med. 2012;43(2): e1–e24. 10.1016/j.amepre.2012.04.027 [DOI] [PubMed] [Google Scholar]
- 19. Puetz TW, Morley CA, Herring MP. Effects of creative arts therapies on psychological symptoms and quality of life in patients with cancer. JAMA Intern Med. 2013173(11): 960–969. [DOI] [PubMed] [Google Scholar]
- 20. Betthauser LM, Bahraini N, Krengel MH, Brenner LA. Self-report measures to identify post-traumatic stress disorder and/or mild traumatic brain injury and associated symptoms in military veterans of Operation Enduring Freedom (OEF)/Operation Iraqi Freedom (OIF). Neuropsychol Rev. 2012;22(1): 35–53. 10.1007/s11065-012-9191-4 [DOI] [PubMed] [Google Scholar]
- 21. Hedges LV, Olkin I. Statistical Methods for Meta-analysis. New York, NY: Academic Press; 1985. [Google Scholar]
- 22. Monnelly DP, Ciraul DA, Knapp C, Keane T. Low-dose risperidone as adjunctive therapy for irritable aggression in Posttraumatic Stress Disorder. J Clin Psychopharmacol. 2003;23(2): 193–196. [DOI] [PubMed] [Google Scholar]
- 23. Rosenthal R. Meta-analytic Procedures for Social Research. London, England: Sage Publications; 1991. [Google Scholar]
- 24. Schnurr PP, Friedman MJ, Foy DW, Shea MT, Hsieh FY, Lavori PW, et al. Randomized trial of trauma-focused group therapy for Posttraumatic Stress Disorder. Results from a department of veterans affairs cooperative study. Arch Gen Psychiatry. 2003;60: 481–489. [DOI] [PubMed] [Google Scholar]
- 25. Detsky AS, Naylor CD, O’Rourke K, McGeer AJ, L’Abbe KA. Incorporating variations in the quality of individual randomized trials into meta-analysis. J Clin Epidemiol. 1992;43: 255–265. [DOI] [PubMed] [Google Scholar]
- 26. Jüni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA. 1999;282(11): 1054–60. [DOI] [PubMed] [Google Scholar]
- 27. Lipsey MW, Wilson DB. Practical Meta-analysis. Newbury Park, CA: Sage; 2001. [Google Scholar]
- 28. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414): 557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Egger M, Davey-Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109): 629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4): 1088–1101. [PubMed] [Google Scholar]
- 31. Gleser LJ, Olkin I. Stochastically dependent effect sizes In: Cooper H, Hedges LV, eds. The Handbook of Research Synthesis. New York, NY: Sage; pp. 339–55; 1994. [Google Scholar]
- 32. Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. J Educ Behav Stat. 2006;31(4): 437–48. [Google Scholar]
- 33. Lazar AA, Zerbe GO. Solution for determining the significance region using the Johnson-Neyman type procedure in generalized linear (mixed) models. J Educ Behav Stat. 2001;36(6): 699–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stein DJ, Ipser J, McAnda N. Pharmacotherapy of posttraumatic stress disorder: A review of meta-analyses and treatment guidelines. CNS Spectr. 2009;14:1(Suppl 1): 25–31. [PubMed] [Google Scholar]
- 35. Ipser JC, Stein DJ. Evidence-based pharmacotherapy of post-traumatic stress disorder (PTSD). Int J Neuropsychopharmacol. 2012;15: 825–840. 10.1017/S1461145711001209 [DOI] [PubMed] [Google Scholar]
- 36. Baldwin D, Woods R, Lawson R, Taylor D. Efficacy of drug treatments for generalised anxiety disorder: Systematic review and meta-analysis. BMJ. 2001;342: d1199. [DOI] [PubMed] [Google Scholar]
- 37. Cook RJ, Sackett DL. The number needed to treat: A clinically useful measure of treatment effect. BMJ. 1995;310(6977): 452–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Krystal JH, Neumeister A. Noradrenergic and serotonergic mechanisms in the neurobiology of posttraumatic stress disorder and resilience. Brain Res. 2009;1293: 13–23. 10.1016/j.brainres.2009.03.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Celik C, Ozdemir B, Ozmenler KN, Yelboga Z, Balikci A, Doruk A, et al. Efficacy of paroxetine and amitriptyline in combat related posttraumatic stress disorder: An open-label comparative study. Bull Clin Psychopharmacol. 2011;21(3): 179–85. [Google Scholar]
- 40. O’Donnell ML, Creamer M, Pattison P. Posttraumatic stress disorder and depression following trauma: Understanding Comorbidity. Am J Psychiatry. 2004;161(8): 1390–1396. [DOI] [PubMed] [Google Scholar]
- 41. Blanchard EB, Buckley TC, Hickling EJ, Taylor AE. Posttraumatic stress disorder and comorbid major depression: Is the correlation an illusion? J Anxiety Disord. 1998;12(1): 21–37. [DOI] [PubMed] [Google Scholar]
- 42. Erickson DJ, Wolfe J, King DW, King LA, Sharkansky EJ. Posttraumatic stress disorder and depression symptomatology in a sample of Gulf War veterans: a prospective analysis. J Consult Clin Psychol. 2001;69(1): 41–9. [DOI] [PubMed] [Google Scholar]
- 43. Hidalgo RB, Davidson JRT. Selective serotonin reuptake inhibitors in post-traumatic stress disorder. J Psychopharmacol. 2000;14(1): 70–76. [DOI] [PubMed] [Google Scholar]
- 44. Asnis GM, Kohn SR, Henderson M, Brown NL. SSRIs versus non-SSRIs in post-traumatic stress disorder. An update with recommendations. Drugs. 2004;64(4): 383–404. [DOI] [PubMed] [Google Scholar]
- 45. Campbell DG, Felker BL, Liu CF, Yano EM, Kirchner JE, Chan D, et al. Prevalence of depression-PTSD comorbidity: Implications for clinical practice guidelines and primary care-based interventions. J Gen Intern Med. 2007;22(6): 711–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
(TIF)
(TIF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data included in the meta-analysis are presented within the paper and its supporting information files. Additional raw data extracted from the included trials on which the aggregated data presented in the meta-analysis are available within the original manuscripts of included trials as well as in spreadsheet format by request from the authors.