Abstract
Background
Many observational studies have shown that exposure to fluoride in drinking water is associated with hip fracture risk. However, the findings are varied or even contradictory. In this work, we performed a meta-analysis to assess the relationship between fluoride exposure and hip fracture risk.
Methods
PubMed and EMBASE databases were searched to identify relevant observational studies from the time of inception until March 2014 without restrictions. Data from the included studies were extracted and analyzed by two authors. Summary relative risks (RRs) with corresponding 95% confidence intervals (CIs) were pooled using random- or fixed-effects models as appropriate. Sensitivity analyses and meta-regression were conducted to explore possible explanations for heterogeneity. Finally, publication bias was assessed.
Results
Fourteen observational studies involving thirteen cohort studies and one case-control study were included in the meta-analysis. Exposure to fluoride in drinking water does not significantly increase the incidence of hip fracture (RRs, 1.05; 95% CIs, 0.96–1.15). Sensitivity analyses based on adjustment for covariates, effect measure, country, sex, sample size, quality of Newcastle–Ottawa Scale scores, and follow-up period validated the strength of the results. Meta-regression showed that country, gender, quality of Newcastle–Ottawa Scale scores, adjustment for covariates and sample size were not sources of heterogeneity. Little evidence of publication bias was observed.
Conclusion
The present meta-analysis suggests that chronic fluoride exposure from drinking water does not significantly increase the risk of hip fracture. Given the potential confounding factors and exposure misclassification, further large-scale, high-quality studies are needed to evaluate the association between exposure to fluoride in drinking water and hip fracture risk.
Background
Hip fractures, or fractures of the femoral neck, are a major public health problem and are the leading cause of morbidity and mortality in people aged ≥65 years in many countries. Hip fracture is also one of the most common causes of admission to nursing homes. An estimated 1.7 million hip fractures occurred worldwide in the year 1990 [1]. The number of people sustaining a hip fracture continues to rise because of an increasing elderly population. Hip fractures also pose a substantial challenge to both patients and healthcare systems worldwide. Currently, over 320,000 hip fractures occur in North America alone each year, and this number is expected to rise to 580,000 by 2040 with healthcare costs exceeding 10 billion dollars [2, 3]. Thus, factors related to hip fracture development need better understanding.
Considering our widespread exposure to fluoride in drinking water, the effect of fluoride on hip fracture risk is an important public health issue. Fluoride in drinking water derives from natural sources or is added to protect dental health. People are exposed daily to fluoride through food and water. In the past 60 years, the possible adverse effects of fluoride on human health have been controversial. The cariostatic benefit from water fluoridation is indisputable. However, results from epidemiologic studies have been contradictory. Some studies have suggested a positive association between the concentration of fluoride in water and incidence of fractures [4–7], but others have found no association [8–15] or even an inverse relation [16, 17]. Therefore, the association between fluoride exposure in drinking water and hip fracture risk remains unclear. Therefore, a quantitative and systematic summary of the evidence should be performed using meta-analysis. In the present study, we conducted a meta-analysis to confirm the hypothesis whether fluoride exposure in drinking water has increased or decreased the risk of hip fracture.
Methods
Search strategy
We searched the PubMed and EMBASE databases (from time of inception until March 2014) to identify relevant studies that investigated the association between fluoride exposure in drinking water and hip fracture risk. The following search terms were used: “fluorides” or “fluoride” or “fluoridated” or “fluoridation” and “hip fracture”or “hip fractures” filtered by Human without language restrictions. We also reviewed the reference lists of pertinent articles and recent reviews.
Inclusion and exclusion criteria
Studies were considered acceptable for inclusion in the meta-analysis if they met the following criteria: (1) evaluating the association between fluoride exposure in drinking water and hip fracture risk with obtainable full text; (2) providing adjusted and/or unadjusted hazard ratios (HRs), odds ratios (ORs), and relative risks (RRs) with corresponding 95% confidence interval (CI) or raw data for calculating crude HRs, ORs, or RRs. Studies were excluded if they met the following criteria: letters, comments, correspondences, conference reports, or laboratory studies; or they did not contain enough data for risk estimates calculation. If duplicated data were present in several studies, only the most recent, largest, or most comprehensive study was included. Two authors (JKS and XHY) independently evaluated the eligibility of all retrieved studies, and disagreements were resolved by discussion or consultation with a third author (GLH).
Data extraction
Two authors (JKS and XHY) independently extracted data of the characteristics of the selected studies using a standardized data extraction form. Data were recorded as follows: first author’s surname, publication year, study design, country, number of subjects (cases/controls), gender, follow-up duration, period of fluoride exposure, crude and/or adjusted point estimates and corresponding 95% CIs for each category, and covariate features included in the multivariable model. Disagreements were resolved by discussion and consensus with a third author (GLH).
Quality assessment
The methodological quality of each trial was evaluated using Newcastle—Ottawa Scale (NOS). Three major components were collected: selection of study groups (0 to 4 points), ascertainment for exposure of interest in the studies (0 to 3 points), and quality of adjustment for confounding factors (0 to 2 points). A higher score represented better methodological quality. The quality of each study was graded either low (0 to 4) or high (5 to 9) level.
Statistical analysis
RRs with corresponding 95% CIs were used as common measures of association between fluoride exposure in drinking water and hip fracture risk. Given the low absolute risk of hip fracture, ORs and HRs were directly considered approximations of RRs. Six studies [7, 11, 13–15, 17] did not report overall risk estimates but separately presented results for men and women. Therefore, we combined the results using random effects and included the pooled risks estimates in the primary analysis. One study [16] reported stratified risks estimates by age and gender, and we combined these estimates using a random-effects model and then used the pooled estimates for the meta-analysis. For three studies [5, 10, 12] that reported stratified risks estimates by fluoride exposure levels, we only used the estimates for the highest versus the lowest category of fluoride exposure levels. One study [9] presented separate risks estimates for durations of exposure, and we only used the estimates for the highest versus the lowest category of fluoride exposure duration.
Statistical heterogeneity was evaluated using Cochrane Q test (significance level at <0.10). The degree of heterogeneity was quantified using I2 statistic, which represents the percentage of the total variability across studies [18]. Studies with an I2 statistic of 25% to 50% have low heterogeneity, those with 50% to 75% have moderate heterogeneity, and those with >75% have high heterogeneity. An I2 value > 50% indicates significant heterogeneity. Fixed-effects model was used as pooling method for moderate or low heterogeneity (I2 < 50%), whereas random-effects model (REM) was used for significant heterogeneity (I2 > 50%). Given that patient characteristics, study design, and other confounding factors were inconsistent among studies, we further conducted sensitivity analyses to explore possible explanations for heterogeneity and to examine the influence of various exclusion criteria on the overall pooled estimate. We also investigated the influence of individual studies on the overall risk estimate. We performed meta-regression to explore the sources of heterogeneity in the association between exposure to fluoride in drinking water and hip fracture risk as reported in individual studies, particularly the effects of five study-level characteristics (country, gender, quality of Newcastle—Ottawa Scale scores, adjustment for covariates and sample size). The presence of publication bias was assessed using Begg’s and Egger’s tests [19, 20]. A P value < 0.05 was considered statistically significant, unless otherwise specified. All statistical analyses were performed using STATA version 12.0 (Stata Corporation, College Station, Texas, USA).
Results
Study selection
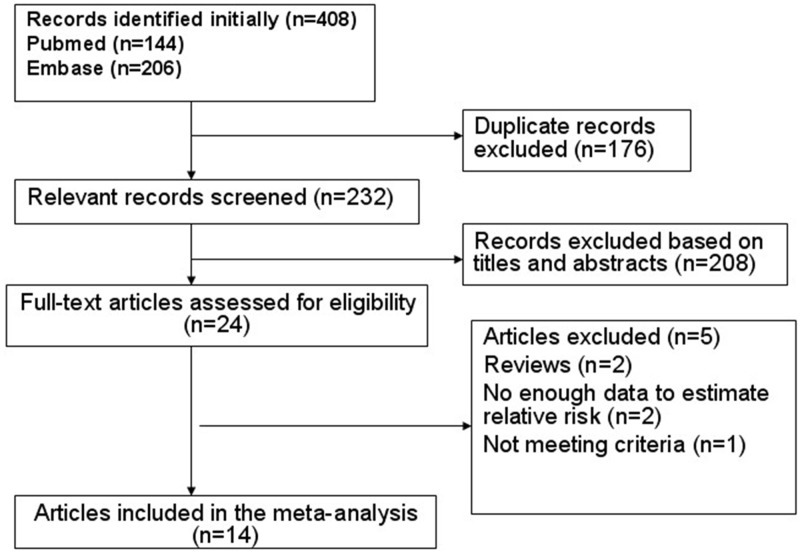
Upon search strategy, 408 records were initially retrieved. The majority of the retrieved articles were excluded after reviewing titles and abstracts, mainly because they were reviews, letters, comments, or irrelevant to our analysis. Twenty-four articles were considered of interest, and full text of each was retrieved for detailed evaluation. Ten out of these 24 articles were excluded. Finally, 14 articles were included in the meta-analysis (Fig 1).
Fig 1. Flow chart of identification of eligible studies to final inclusion.
Study characteristics
The characteristics of all included articles are presented in Tables 1 and 2. Fourteen observational articles involving more than 73,411,368 individuals and 319,608 patients were identified. Among these articles, thirteen were cohort studies [4, 5, 7–17], and one was a case-control study [6]. Seven articles were based in the United States [4, 7–9, 11, 14, 15], five in Europe [5, 6, 10, 16, 17], and two in Asia [12, 13]. The articles were published from 1983 to 2013. The sample size of the observational studies ranged from 506 to 70,000,000, with the eight largest studies recruited over 100,000 participants.
Table 1. Characteristic of studies included in the meta-analysis.
| Study | Year | Country | Study design | No. of subjects | No. of patients | Sex | Age,Median(Range),yrs | Flouride exposure (yrs) | Follow-up(yrs) | Adjustment for covariates |
|---|---|---|---|---|---|---|---|---|---|---|
| Simonen O | 1985 | Finland | Cohort study | 133,398 | 395 | F and M | NA(≥50) | 19 | 11 | Unadjust |
| Sowers MF | 1991 | United States | A prospective cohort study | 506 | 7 | F | NA(20–80) | NA | 5 | Adjusted for age and body size |
| Danielson C | 1992 | United States | Ecological cohort | 6,049 | 246 | F and M | NA(≥65) | 20 | 7 | Unadjust |
| Jacobsen SJ | 1992 | United States | Cohort study | 70,000,000 | 218,951 | F and M | NA(≥65) | NA | 4 | Adjusted for age |
| Jacobsen SJ | 1993 | United States | A retrospective-prospective cohort | NA | 751 | F and M | NA(≥50) | 20 | 10 | Adjusted for age. |
| Cauley JA | 1995 | United States | A prospective cohort study | 2,076 | 41 | F | NA(≥65) | 12.7 ± 8.0 | 6.1 | Adjusted for age, BMI, total calcium intake (diet plus supplements), history of osteoporosis, surgical menopause. history of kills in past year, drinks per week, education, current estrogen use. current thiazidc diuretic use, ever used bottled water. |
| Karagas MR | 1996 | United States | A retrospective cohort study | 444,000 | 28,194 | F and M | NA(65–89) | NA | 4 | Unadjust |
| Lehmann R | 1998 | Germany | A retrospective cohort | 623,602 | 1,198 | F and M | NA(≥35) | 7–17 | 2 | Unadjust |
| Kurttio P | 1999 | Finland | A retrospective cohort study | 144,627 | 4,449 | F and M | NA(50–80) | NA | 13 | Adjusted for age and area |
| Phipps KR | 2000 | United States | Multicentre prospective cohort study | 7,129 | 231 | F | 74.2(≥65) | 44 | 7 | Adjusted for age, weight, education, muscle strength, surgical menopause, calcium intake, drinks/week, current oestrogen use, current thiazide use, noninsulin dependent diabetes, current thyroid hormone use, walking for exercise, and smoking status |
| Hillier S | 2000 | United Kingdom | Case-control study | 1,041 | 514 | F and M | NA(≥50) | NA | NA | Adjusted for age and gender、all potential confounders |
| Li Y | 2001 | china | Cohort study | 8266 | 56 | F and M | 62.8(NA) | >25 | NA | Unadjust |
| Park EY | 2008 | Korean | Cohort study | 1,567,397 | 3,842 | F and M | NA(≥65) | NA | 7 | Unadjust |
| Näsman P | 2013 | Sweden | Cohort study | 473,277 | 60,733 | F and M | 62.8(44.0–87.0) | NA | 16.8 | Adjusted for gender, age group, county of residence, calendar group |
F, female; M, male; NA, not available.
Table 2. The effect size across included studies.
| Study | Year | exposure to waterfluoridation | RRs/HRs/ORs (95%CIs) |
|---|---|---|---|
| Simonen O | 1985 | 1.0 vs. 0–0.1mg/l | |
| female | 1.5(1.2 to 1.8) | ||
| male | 2.5(1.6 to 3.9) | ||
| Sowers MF | 1991 | 4.0±0.1 vs. 1.0 mg/l | 2.2(1.1 to 4.7) |
| Danielson C | 1992 | 1.0 vs. 0.3ppm | |
| female | 1.27(1.08 to 1.46) | ||
| male | 1.41(1.0 to 1.81) | ||
| Jacobsen SJ | 1992 | nonfluoradated vs. fluoridated | |
| female | 1.17(1.13 to 1.22) | ||
| male | 1.08(1.06 to 1.1) | ||
| Jacobsen SJ | 1993 | nonfluoradated vs. fluoridated | |
| male | 0.78(0.37 to 1.66) | ||
| female | 0.6(0.42 to 0.85) | ||
| Cauley JA | 1995 | 1.01±0.21 vs. 0.15±0.10 mg/l | |
| 0 year | 1.0 | ||
| 1–10 years | 0.89(0.42 to 1.92) | ||
| 11–20years | 0.58(0.14 to 2.48) | ||
| >20 years | 0.44(0.1 to 1.86) | ||
| Karagas MR | 1996 | nonfluoradated vs. fluoridated | |
| male | 1(0.92 to 1.09) | ||
| female | 1.01(0.96 to 1.06) | ||
| Lehmann R | 1998 | 0.77–1.20 vs. 0.08–0.36mg/l | |
| male | |||
| 60–64 years | 2.14(0.89 to 5.2) | ||
| 65–69 years | 0.55(0.22 to 1.39) | ||
| 70–74 years | 0.78(0.27 to 1.39) | ||
| 75–79 years | 1.05(0.65 to 1.69) | ||
| 80–84 years | 1.02(0.67 to 1.55) | ||
| ≥80 years | 1.92(1.07 to 3.45) | ||
| female | |||
| 60–64 years | 0.9(0.51 to 1.58) | ||
| 65–69 years | 1.56(1 to 2.44) | ||
| 70–74 years | 1.09(0.76 to 1.57) | ||
| 75–79 years | 1.38(1.06 to 1.8) | ||
| 80–84 years | 1.2(0.95 to 1.52) | ||
| ≥80 years | 1.41(1.1 to 1.81) | ||
| Kurttio P | 1999 | female | |
| ≤0.10 mg/l | 1.0 | ||
| 0.11–0.30 mg/l | 0.93(0.84 to 1.02) | ||
| 0.31–0.50 mg/l | 1.12(0.93 to 1.34) | ||
| 0.51–1.00 mg/l | 1.12(0.96 to 1.31) | ||
| 1.10–1.50 mg/l | 1.08(0.88 to 1.32) | ||
| >1.50 mg/l | 1.08(0.8 to 1.46) | ||
| male | |||
| ≤0.10 mg/l | 1.0 | ||
| 0.11–0.30 mg/l | 1.05(0.9 to 1.22) | ||
| 0.31–0.50 mg/l | 0.72(0.51 to 1.02) | ||
| 0.51–1.00 mg/l | 1.03(0.81 to 1.32) | ||
| 1.10–1.50 mg/l | 0.67(0.46 to 0.97) | ||
| >1.50 mg/l | 0.98(0.61 to 1.6) | ||
| Phipps KR | 2000 | mixed exposure | 0.73(0.49 to 1.09) |
| continuous exposure | 0.69(0.5 to 0.96) | ||
| Hillier S | 2000 | ≥0.9 vs. <0.9mg/l | 1(0.7 to 1.5) |
| Li Y | 2001 | 0.25–0.34 mg/l | 0.99(0.21 to 4.77) |
| 0.58–0.73 mg/l | 1.12(0.35 to 3.62) | ||
| 1.00–1.06 mg/l | 1.0 | ||
| 1.45–2.19 mg/l | 2.13(0.76 to 5.96) | ||
| 2.62–3.56 mg/l | 1.73(0.56 to 5.33) | ||
| 4.32–7.97 mg/l | 3.26(1.20 to 8.82) | ||
| Park EY | 2008 | fluoridated vs. nonfluoradated | |
| female | 0.88(0.77 to 1) | ||
| male | 0.91(0.84 to 0.99) | ||
| Näsman P | 2013 | Very low <0.3 | 1.0 |
| Low 0.3–0.69 | 0.97(0.94 to 0.99) | ||
| Mendium 0.7–1.49 | 0.97(0.94 to 1) | ||
| High ≥1.5 | 0.98(0.93 to 1.04) |
All 14 studies reported exposure to fluoride in drinking water. Ten studies were designed to evaluate the RRs of hip fracture [4, 5, 7–9, 11, 13–15, 17], three were designed to evaluate the ORs of hip fracture [6, 12, 16], and one was designed to evaluate the HRs of hip fracture [10]. Three studies investigated only women [4, 8, 9], whereas eleven studies investigated both women and men [5–7, 10–17]. The average follow-up ranged from 2 years to 16.8 years. Patients were followed up over five years in majority of the studies (64.3%). The association between exposure to water fluoridation and hip fracture risk was the primary outcome of interest for eight studies [5–7, 10, 13–15, 17], whereas this association was a secondary subject in six studies [4, 8, 9, 11, 12, 16]. Six studies did not adjust for confounding factors [7, 11–13, 16, 17], whereas the others controlled a group of conventional risk factors for hip fracture, such as age, gender, area, and smoking [4–6, 8–10, 14, 15].
The quality of the included studies was assessed by NOS (Table 3). The median NOS score was 6.5 (range: 4 to 9).
Table 3. Quality assessment of included studies based on Newcastle-Ottawa scale.
| Author | year | Selection | Comparability | Exposure |
|---|---|---|---|---|
| Simonen O | 1985 | 3 | 0 | 2 |
| Sowers MF | 1991 | 3 | 1 | 2 |
| Danielson C | 1992 | 3 | 1 | 3 |
| Jacobsen SJ | 1992 | 2 | 1 | 2 |
| Jacobsen SJ | 1993 | 3 | 1 | 1 |
| Cauley JA | 1995 | 3 | 2 | 2 |
| Karagas MR | 1996 | 3 | 0 | 2 |
| Lehmann R | 1998 | 3 | 0 | 1 |
| Kurttio P | 1999 | 4 | 1 | 3 |
| Phipps KR | 2000 | 3 | 2 | 2 |
| Hillier S | 2000 | 3 | 2 | 2 |
| Li Y | 2001 | 3 | 2 | 1 |
| Park EY | 2008 | 3 | 2 | 2 |
| Näsman P | 2013 | 4 | 2 | 3 |
Exposure to fluoride in drinking water and hip fracture risk
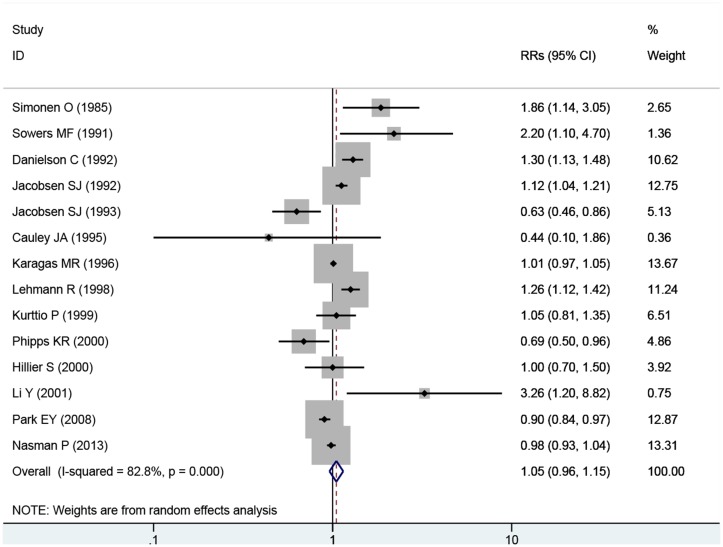
The overall RR estimates for each study were pooled to determine the total estimates of risk using random-effects model (RRs = 1.05; 95% CIs = 0.96 to 1.15, P = 0.291), and the heterogeneity was significant (P < 0.001, I2 = 82.8%). The results suggested that, exposure to fluoride in drinking water does not increase the incidence of hip fracture risk and substantial heterogeneity was observed (Fig 2).
Fig 2. Forest plot of exposure to fluoride in the drinking water and hip fracture risk.
Studies are pooled with a random-effects model.
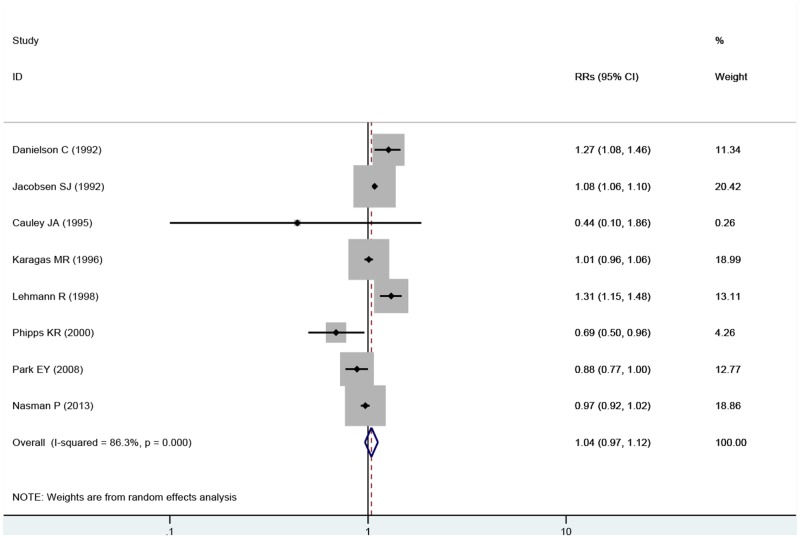
We also conducted meta-analyses based on women aged ≥65 years to explore the effect of exposure to fluoride from drinking water on hip fracture risk, and the results were relatively consistent. No association was observed in exposure to fluoride from drinking water on hip fracture risk (RRs = 1.04, 95% CIs = 0.97 to 1.12, P = 0.30) with substantial evidence of heterogeneity (P < 0.001, I2 = 86.3%) (Fig 3).
Fig 3. Forest plot of exposure to fluoride in the drinking water and hip fracture risk among women beyond 65 years.
Studies are pooled with a random-effects model.
Sensitivity analyses
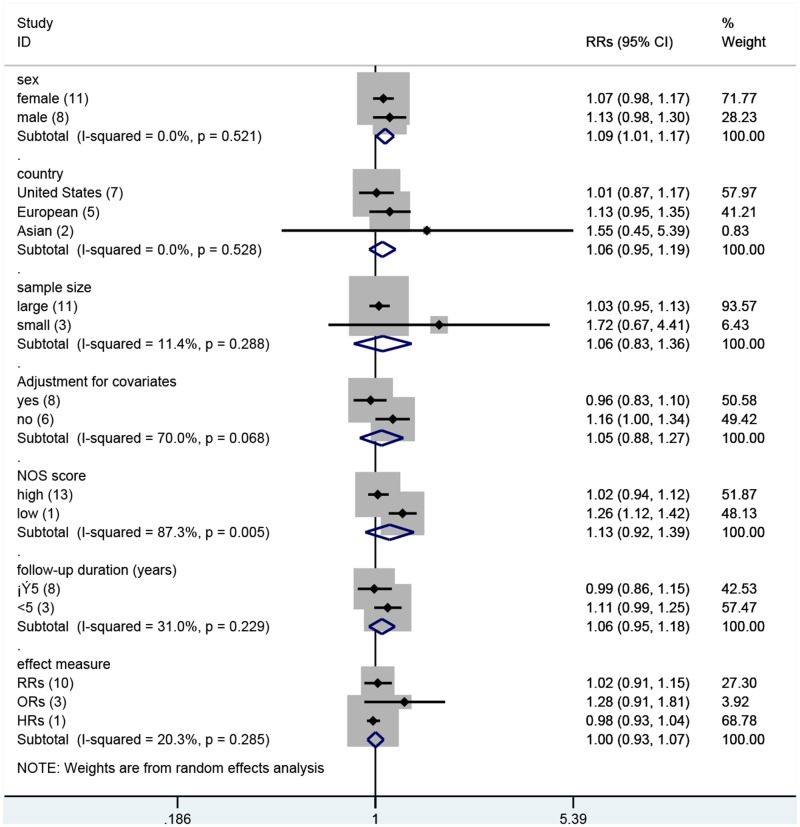
To explore potential heterogeneity, we performed sensitivity analyses to examine the effects of various exclusion criteria on combined risk estimates. Sensitivity analyses based on adjustment for covariates, effect measure, country, sex, sample size (Large, cases ≥ 100; Small, cases <100), quality of Newcastle—Ottawa Scale scores (High, NOS score ≥5, Low, NOS score, <5), and follow-up period yielded similar results with substantial evidence of heterogeneity (Table 4, Fig 4). Further exclusion of any single study did not significantly alter the combined RRs, which ranged from 1.02 (95% CI = 0.94 to 1.12) to 1.08 (95% CI = 0.99 to 1.17).
Table 4. Sensitive analyses based on various exclusion criteria.
| Studies,N | RRs,95%CIs | P value | P value for heterogeneity | I2(%) | |
|---|---|---|---|---|---|
| total | 14 | 1.05(0.96–1.15) | 0.291 | 0.000 | 82.8 |
| Country | |||||
| United States | 7 | 1.01(0.87–1.17) | 0.898 | 0.000 | 84.1 |
| Europe | 5 | 1.13(0.95–1.35) | 0.168 | 0.001 | 79.7 |
| Asia | 2 | 1.55(0.45–5.39) | 0.491 | 0.012 | 84.3 |
| Gender | |||||
| Female | 11 | 1.07(0.98–1.17) | 0.143 | 0.000 | 83.5 |
| Male | 8 | 1.13(0.98–1.30) | 0.167 | 0.000 | 86.5 |
| Effect measure | |||||
| RRs | 10 | 1.02(0.91–1.15) | 0.712 | 0.000 | 83.9 |
| ORs | 3 | 1.28(0.91–1.81) | 0.162 | 0.087 | 59.0 |
| HRs | 1 | 0.98(0.93–1.04) | 0.479 | NA | NA |
| Sample size | |||||
| Large(cases ≥ 100) | 11 | 1.03(0.95–1.13) | 0.451 | 0.000 | 84.5 |
| Small(cases <100) | 3 | 1.72(0.67–4.41) | 0.263 | 0.078 | 60.9 |
| Adjustment for covariates | |||||
| Yes | 8 | 0.96(0.83–1.10) | 0.529 | 0.000 | 75.0 |
| No | 6 | 1.16(1.00–1.34) | 0.050 | 0.000 | 89.4 |
| NOS score | |||||
| High(≥5) | 13 | 1.02(0.94–1.12) | 0.600 | 0.000 | 80.8 |
| Low(<5) | 1 | 1.26(1.12–1.42) | 0.000 | NA | NA |
| Follow-up duration(years) | |||||
| ≥5 | 8 | 0.99(0.86–1.15) | 0.978 | 0.000 | 83.0 |
| <5 | 3 | 1.11(0.99–1.25) | 0.075 | 0.000 | 87.1 |
| NA | 2 | 1.64(0.52–5.16) | 0.394 | 0.030 | 78.8 |
HRs, hazard ratios, ORs, odds ratios, RRs, relative risks, CIs, confidence intervals; NA, not available.
Fig 4. Sensitivity analyses based on various exclusion criteria.
Meta-regression
Considering the relatively high heterogeneity exhibited in the trials, a meta-regression was conducted to explore the predefined possible sources of heterogeneity. None of the regression coefficients were statistically significant (Table 5), suggesting that country, gender, quality of Newcastle—Ottawa Scale scores, adjustment for covariates and sample size were insignificant sources of heterogeneity.
Table 5. Effects of study variables by meta-regression.
| Covariant | Coefficient | P value | 95% CIs |
|---|---|---|---|
| Country(Ref = Europe) | |||
| Asia | −0.004 | 0.991 | −0.740 to 0.732 |
| United States | −0.161 | 0.475 | −0.640 to 0.318 |
| Adujusted for covariates | −0.243 | 0.180 | −0.615 to 0.129 |
| Sample size | −0.633 | 0.115 | −1.445 to 0.179 |
| Newcastle—Ottawa Scale scores | −0.051 | 0.451 | −0.193 to 0.091 |
| year of publication | -0.014 | 0.276 | −0.039 to 0.012 |
Publication bias
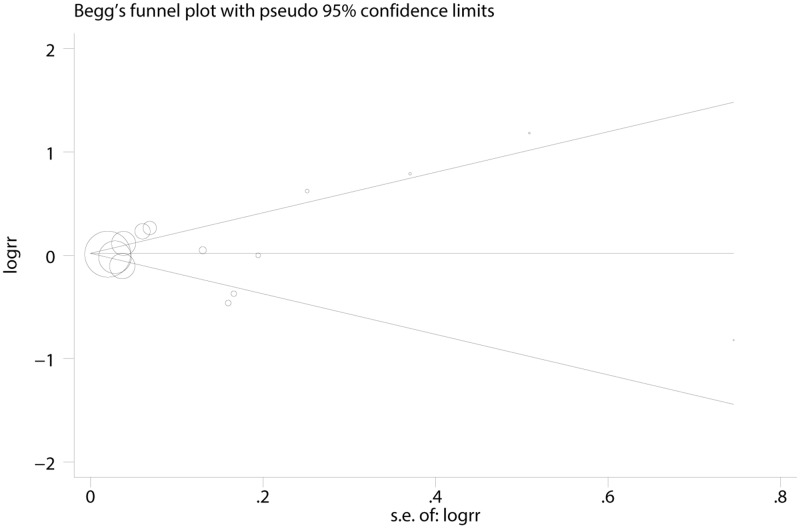
Both Begg’s (rank correlation test) and Egger’s funnel plot asymmetry test (regression method) in the meta-analysis indicated no significant publication bias (Begg’s test, P = 0.74; Egger’s test, P = 0.47; Fig 5).
Fig 5. Funnel plots of exposure to fluoride in the drinking water and hip fracture risk for assessment of publication bias.
Discussion
To our knowledge, this meta-analysis is the first to explore the effect of fluoride exposure on hip fracture patients. The pooled results from the meta-analysis of 14 observational studies using random-effects model provided evidence that chronic fluoride exposure from drinking water does not significantly increase the incidence of hip fracture. The combined estimates were robust across sensitivity and meta-regression. Few evidence of publication bias was also observed.
Several plausible mechanisms may explain the lack of association between fluoride exposure from drinking water and hip fracture risk in the present analysis. Fluoride is a bone seeker and has strong effects on bone cell function, bone structure, and bone strength [21]. Fluoride can affect bone in at least two ways. Fluoride ions can replace hydroxyl ions in the hydroxyapatite lattice, thereby increasing fluoride concentrations in plasma and osteoblastic differentiation and activity. Such changes play a crucial effect on fracture risk. This effect has only been observed when intake has been substantially higher than expected from water fluoridation. However, the implications of lower exposure for fracture risk are uncertain.
The effects of fluoride on bone have also been investigated in a randomized controlled trial of high-dose sodium fluoride (75 mg daily) as a treatment for osteoporosis. During a four-year period, the intervention led to a substantial increase in bone density in the spine [22]. Although this study indicated a hazard from high doses of fluoride in people with established osteoporosis, the risk cannot be necessarily extrapolated to much lower doses received by the general population from fluoride in water supplies.
Danielson et al. [7], Kurttio et al. [5], and Sowers et al. [4] reported that exposure to fluoride could increase hip fracture risk among older women, whereas Cauley et al. [9] and Phipps et al. [8] indicated that fluoride exposure slightly decreases hip fracture risk among older women. Jacobsen et al. [14], Karagas et al. [11], Näsman P et al. [10], and Park et al. [13] have found no association. In the present meta-analyses, no statistically significant association between fluoride exposure from drinking water and hip fracture risk was observed among older women. In older ages, several reasons account for fluoride effects. One explanation could be the effect of calcium. Calcium supplementation is usually recommended for elderly patients. However, excess dietary calcium intake may prohibit fluoride absorption, which reduces probability of fluoride effect [23]. Calcium absorption is also different in elderly patients, which decreases with age as a result of various possible mechanisms, including decreased vitamin D intake, synthesis, and metabolism [24]. Elderly persons also tend to reduce their food intake, which further decreases their opportunity for adequate nutrition. The kinetics of fluoride may be different in older ages [25]. The rate of bone formation decreases as the skeleton ages, and less fluoride is taken up by older bone [21], thereby reducing potential effects of fluoride.
The present meta-analysis has some limitations. First, our meta-analysis was based on observational studies, and half of the included studies controlled some confounding factors, such as age and gender. However, other confounding factors (estrogen level, total calcium intake, diet, and level of fluoride exposure) are difficult to control in epidemiological studies. Second, although few evidence of publication bias was observed, the statistical power for these tests was limited because of the relatively small number of included studies. Third, significant heterogeneity was detected in our meta-analysis. Heterogeneity between studies should not be ignored even if it is highly common in the meta-analysis. We also performed sensitivity analyses and meta-regression to determine the sources of heterogeneity, but heterogeneity was still observed. Fourth, because the majority of studies using different methods used to assess and categorize fluoride exposure among studies (Table 1), our findings are likely to be influenced by the misclassification of exposure. In cohort studies, this misclassification would likely be non-differential if the exposure variable was dichotomous, and thereby result in an underestimate of the true association, whereas the influence of a misclassification on the results in case-control studies is less predictable. Moreover, the potential for misclassification of exposure to fluoride may contribute to the heterogeneity for all studies in the summary analysis. Therefore, this result should be considered with caution because of exposure misclassification. Overall, these aforementioned limitations may affect our final conclusions.
In conclusion, limited evidence suggests that chronic fluoride exposure from drinking water does not significantly increase the risk of hip fracture. Although these findings are encouraging, the results of this meta-analysis should be explained with caution because of potential confounding factors, heterogeneity, and exposure misclassification. Further large-scale and well-designed trials on this topic are needed.
Supporting Information
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organization technical report series. 1994;843:1–129. . [PubMed] [Google Scholar]
- 2. Cooper C, Campion G, Melton LJ 3rd. Hip fractures in the elderly: a world-wide projection. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 1992;2(6):285–9. . [DOI] [PubMed] [Google Scholar]
- 3. Schemitsch E, Bhandari M. Femoral neck fractures: controversies and evidence. Journal of orthopaedic trauma. 2009;23(6):385 10.1097/BOT.0b013e3181acc51f . [DOI] [PubMed] [Google Scholar]
- 4. Sowers MF, Clark MK, Jannausch ML, Wallace RB. A prospective study of bone mineral content and fracture in communities with differential fluoride exposure. American journal of epidemiology. 1991;133(7):649–60. Epub 1991/04/01. . [DOI] [PubMed] [Google Scholar]
- 5. Kurttio P, Gustavsson N, Vartiainen T, Pekkanen J. Exposure to natural fluoride in well water and hip fracture: a cohort analysis in Finland. American journal of epidemiology. 1999;150(8):817–24. Epub 1999/10/16. . [DOI] [PubMed] [Google Scholar]
- 6. Hillier S, Cooper C, Kellingray S, Russell G, Hughes H, Coggon D. Fluoride in drinking water and risk of hip fracture in the UK: a case-control study. Lancet. 2000;355(9200):265–9. Epub 2000/02/16. 10.1016/s0140-6736(99)07161-5 . [DOI] [PubMed] [Google Scholar]
- 7. Danielson C, Lyon JL, Egger M, Goodenough GK. Hip fractures and fluoridation in Utah's elderly population. Jama. 1992;268(6):746–8. Epub 1992/08/12. . [PubMed] [Google Scholar]
- 8. Phipps KR, Orwoll ES, Mason JD, Cauley JA. Community water fluoridation, bone mineral density, and fractures: prospective study of effects in older women. BMJ (Clinical research ed). 2000;321(7265):860–4. Epub 2000/10/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cauley JA, Murphy PA, Riley TJ, Buhari AM. Effects of fluoridated drinking water on bone mass and fractures: the study of osteoporotic fractures. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 1995;10(7):1076–86. Epub 1995/07/01. 10.1002/jbmr.5650100712 . [DOI] [PubMed] [Google Scholar]
- 10. Nasman P, Ekstrand J, Granath F, Ekbom A, Fored CM. Estimated drinking water fluoride exposure and risk of hip fracture: a cohort study. Journal of dental research. 2013;92(11):1029–34. Epub 2013/10/03. 10.1177/0022034513506443 . [DOI] [PubMed] [Google Scholar]
- 11. Karagas MR, Baron JA, Barrett JA, Jacobsen SJ. Patterns of fracture among the United States elderly: geographic and fluoride effects. Annals of epidemiology. 1996;6(3):209–16. Epub 1996/05/01. . [DOI] [PubMed] [Google Scholar]
- 12. Li Y, Liang C, Slemenda CW, Ji R, Sun S, Cao J, et al. Effect of long-term exposure to fluoride in drinking water on risks of bone fractures. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2001;16(5):932–9. Epub 2001/05/09. 10.1359/jbmr.2001.16.5.932 . [DOI] [PubMed] [Google Scholar]
- 13. Park EY, Hwang SS, Kim JY, Cho SH. [Effects of long-term fluoride in drinking water on risks of hip fracture of the elderly: an ecologic study based on database of hospitalization episodes]. Journal of preventive medicine and public health = Yebang Uihakhoe chi. 2008;41(3):147–52. Epub 2008/06/03. . [DOI] [PubMed] [Google Scholar]
- 14. Jacobsen SJ, Goldberg J, Cooper C, Lockwood SA. The association between water fluoridation and hip fracture among white women and men aged 65 years and older. A national ecologic study. Annals of epidemiology. 1992;2(5):617–26. Epub 1992/09/01. . [DOI] [PubMed] [Google Scholar]
- 15. Jacobsen SJ, O'Fallon WM, Melton LJ 3rd. Hip fracture incidence before and after the fluoridation of the public water supply, Rochester, Minnesota. American journal of public health. 1993;83(5):743–5. Epub 1993/05/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lehmann R, Wapniarz M, Hofmann B, Pieper B, Haubitz I, Allolio B. Drinking water fluoridation: bone mineral density and hip fracture incidence. Bone. 1998;22(3):273–8. Epub 1998/03/26. . [DOI] [PubMed] [Google Scholar]
- 17. Simonen O, Laitinen O. Does fluoridation of drinking-water prevent bone fragility and osteoporosis? Lancet. 1985;2(8452):432–4. Epub 1985/08/24. . [DOI] [PubMed] [Google Scholar]
- 18. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed). 2003;327(7414):557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101. . [PubMed] [Google Scholar]
- 20. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed). 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Whitford GM. The metabolism and toxicity of fluoride. Monographs in oral science. 1996;16 Rev 2:1–153. . [PubMed] [Google Scholar]
- 22. Scrivo R, Casadei L, Valerio M, Priori R, Valesini G, Manetti C. Metabolomics approach in allergic and rheumatic diseases. Current allergy and asthma reports. 2014;14(6):445 10.1007/s11882-014-0445-5 . [DOI] [PubMed] [Google Scholar]
- 23. Ekstrand J, Ehrnebo M, Boreus LO. Fluoride bioavailability after intravenous and oral administration: importance of renal clearance and urine flow. Clinical pharmacology and therapeutics. 1978;23(3):329–37. . [DOI] [PubMed] [Google Scholar]
- 24. Riggs BL, Melton LJ 3rd. Involutional osteoporosis. The New England journal of medicine. 1986;314(26):1676–86. 10.1056/NEJM198606263142605 . [DOI] [PubMed] [Google Scholar]
- 25. Rao HV, Beliles RP, Whitford GM, Turner CH. A physiologically based pharmacokinetic model for fluoride uptake by bone. Regulatory toxicology and pharmacology: RTP. 1995;22(1):30–42. 10.1006/rtph.1995.1065 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.