Abstract
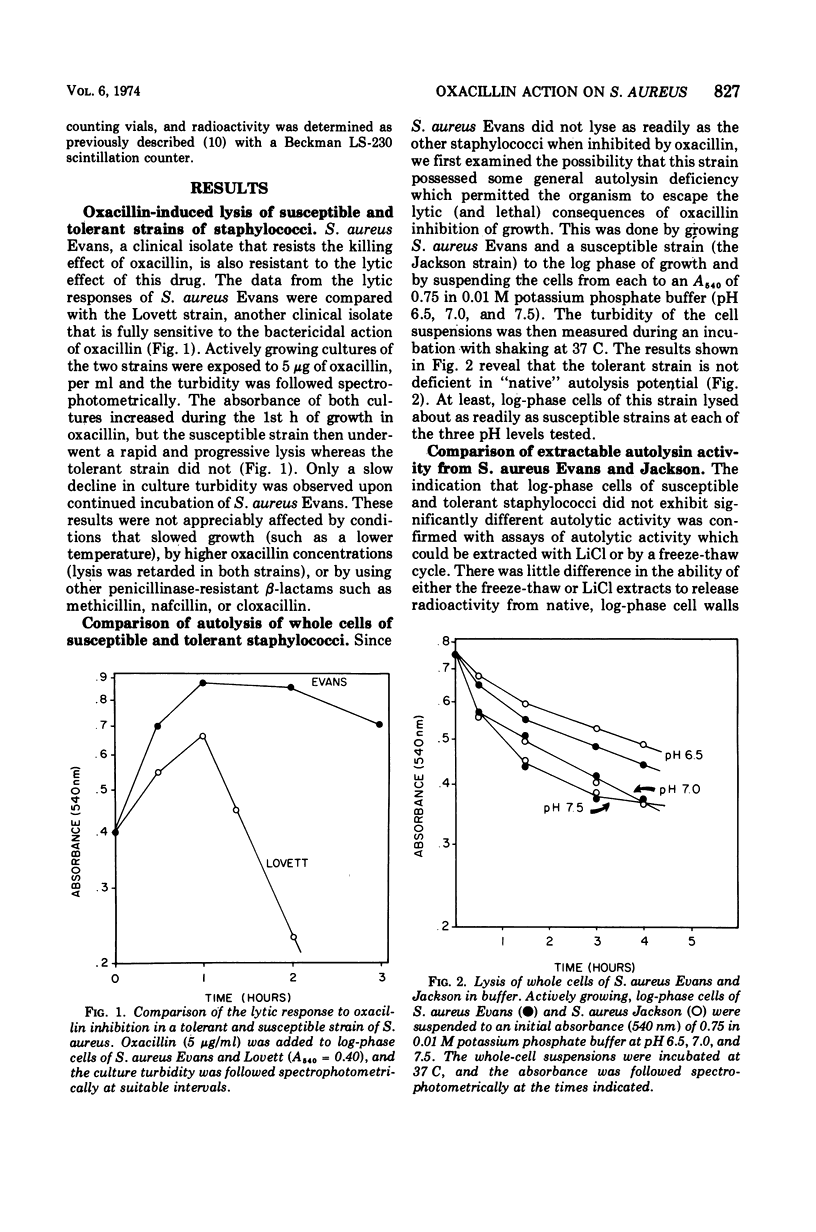
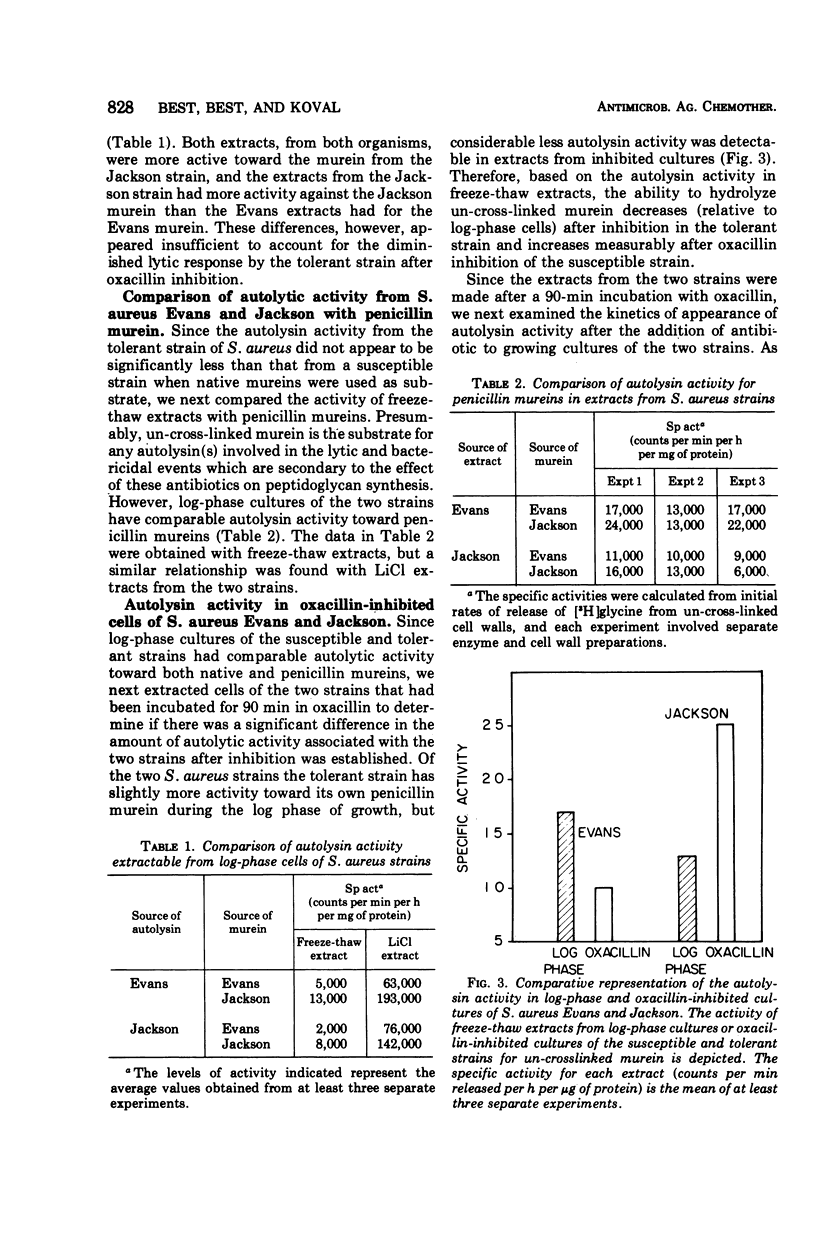
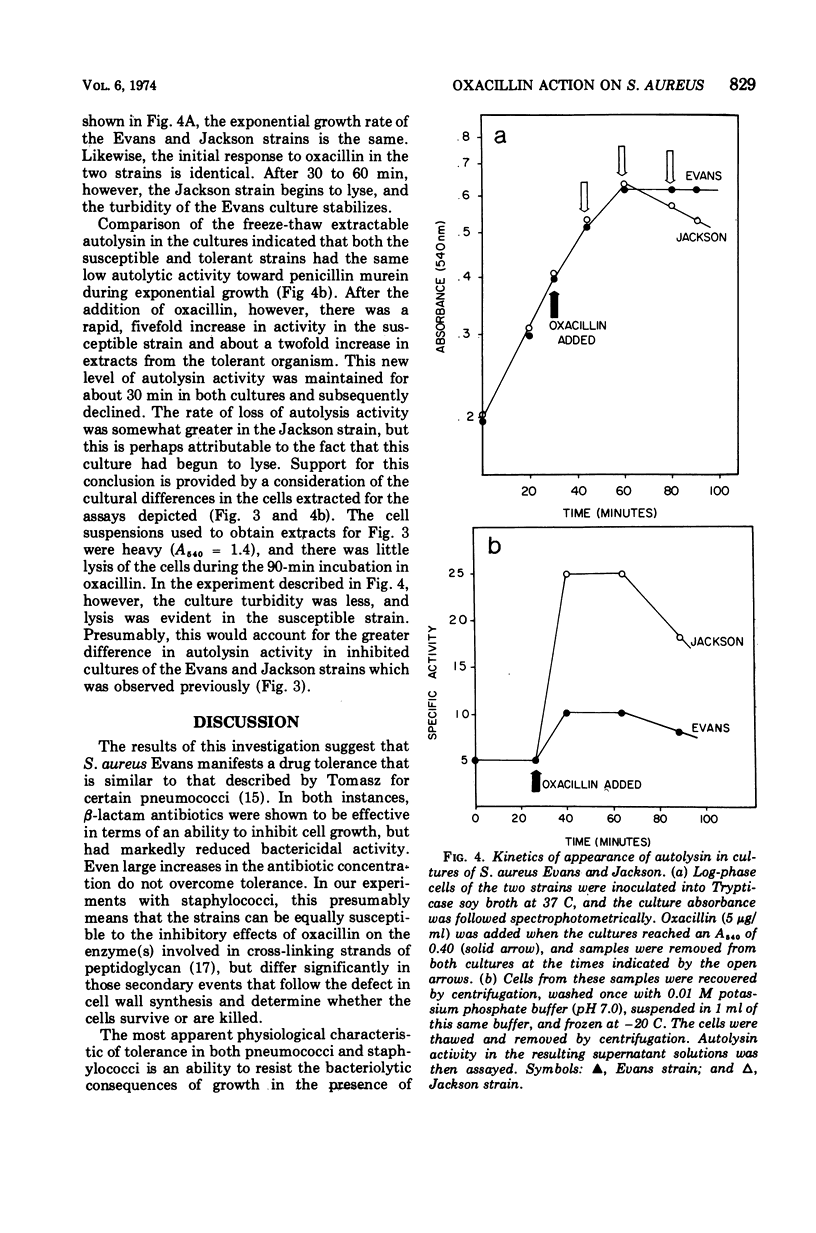
A comparison of the autolytic enzyme activity in Staphylococcus aureus strains that differ markedly in their rates of lysis and killing after exposure to oxacillin has been made. Log-phase cells of the clinical isolate that is tolerant to oxacillin inhibition were found to contain a level of autolytic enzyme activity comparable to that in a sensitive strain. This autolysin from log-phase cells was recovered after a single freeze-thaw cycle and assayed by using both native and penicillin (un-cross-linked) mureins. These same assays, however, revealed a significant difference in autolysin activity extractable from the two strains if the cells were inhibited by oxacillin. Under these conditions, the S. aureus strain that is susceptible to the killing and lytic effects of oxacillin had considerably more activity on penicillin murein than did the tolerant organism. These results provide evidence that hydrolytic enzymes on the cell surface are required to augment the wall damage initiated by oxacillin and other β-lactam antibotics to produce a bactericidal effect.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett H. J. D-alanine carboxypeptidases of Bacillus stearothermophilus: solubilisation of particulate enzymes and mechanism of action of penicillin. Biochim Biophys Acta. 1973 Apr 28;304(2):332–352. doi: 10.1016/0304-4165(73)90252-3. [DOI] [PubMed] [Google Scholar]
- Forsberg C., Rogers H. J. Autolytic enzymes in growth of bacteria. Nature. 1971 Jan 22;229(5282):272–273. doi: 10.1038/229272a0. [DOI] [PubMed] [Google Scholar]
- Ghuysen J. M., Leyh-Bouille M., Frère J. M., Dusart J., Marquet A. The penicillin receptor in Streptomyces. Ann N Y Acad Sci. 1974 May 10;235(0):236–268. doi: 10.1111/j.1749-6632.1974.tb43269.x. [DOI] [PubMed] [Google Scholar]
- Huff E., Silverman C. S., Adams N. J., Awkard W. S. Extracellular cell wall lytic enzyme from Staphylococcus aureus: purification and partial characterization. J Bacteriol. 1970 Sep;103(3):761–769. doi: 10.1128/jb.103.3.761-769.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff E., Silverman C. S. Lysis of Staphylococcus aureus cell walls by a soluble staphylococcal enzyme. J Bacteriol. 1968 Jan;95(1):99–106. doi: 10.1128/jb.95.1.99-106.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mattingly S. J., Best G. K. Effect of temperature on the integrity of Bacillus psychrophilus cell walls. J Bacteriol. 1972 Feb;109(2):645–651. doi: 10.1128/jb.109.2.645-651.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moellering R. C., Jr, Medoff G., Leech I., Wennersten C., Kunz L. J. Antibiotic synergism against Listeria monocytogenes. Antimicrob Agents Chemother. 1972 Jan;1(1):30–34. doi: 10.1128/aac.1.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto M., Perkins H. R., Frère J. M., Ghuysen J. M. Fluorescence and circular dichroism studies on the Streptomyces R61 DD-carboxypeptidase-transpeptidase. Penicillin binding by the enzyme. Biochem J. 1973 Nov;135(3):493–505. doi: 10.1042/bj1350493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Rogers H. J., Forsberg C. W. Role of autolysins in the killing of bacteria by some bactericidal antibiotics. J Bacteriol. 1971 Dec;108(3):1235–1243. doi: 10.1128/jb.108.3.1235-1243.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972 Dec;36(4):407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer H. J., Wise E. M., Jr, Park J. T. Properties and purification of N-acetylmuramyl-L-alanine amidase from Staphylococcus aureus H. J Bacteriol. 1972 Nov;112(2):932–939. doi: 10.1128/jb.112.2.932-939.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strominger J. L., Willoughby E., Kamiryo T., Blumberg P. M., Yocum R. R. Penicillin-sensitive enzymes and penicillin-binding components in bacterial cells. Ann N Y Acad Sci. 1974 May 10;235(0):210–224. doi: 10.1111/j.1749-6632.1974.tb43267.x. [DOI] [PubMed] [Google Scholar]
- Tipper D. J. Mechanism of autolysis of isolated cell walls of Staphylococcus aureus. J Bacteriol. 1969 Feb;97(2):837–847. doi: 10.1128/jb.97.2.837-847.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper D. J., Strominger J. L. Mechanism of action of penicillins: a proposal based on their structural similarity to acyl-D-alanyl-D-alanine. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1133–1141. doi: 10.1073/pnas.54.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. The role of autolysins in cell death. Ann N Y Acad Sci. 1974 May 10;235(0):439–447. doi: 10.1111/j.1749-6632.1974.tb43282.x. [DOI] [PubMed] [Google Scholar]
- Umbreit J. N., Strominger J. L. D-alanine carboxypeptidase from Bacillus subtilis membranes. II. Interaction with penicillins and cephalosporins. J Biol Chem. 1973 Oct 10;248(19):6767–6771. [PubMed] [Google Scholar]



