Abstract
Dermatologic diseases are common in the HIV-infected population. Many of the cutaneous diseases are not unique to this group, but the presentation can be more severe. Although the introduction of antiretroviral therapy has been followed by a decline in many of the skin diseases associated with HIV, drug reactions and other non-infectious skin conditions have increased. This article reviews the current spectrum of HIV-associated skin conditions, focusing on common complaints, infections, drug-associated toxicity and malignancies.
Keywords: HIV, Cutaneous disease, MRSA, Polyomavirus, Skin cancer, SJS/TEN
Introduction
Worldwide about 34 million people are living with HIV infection according to the most recent data from the Joint United Nations Programme on HIV/AIDS (UNAIDS) in 2011 [1]. The Centers for Disease Control and Prevention estimates the prevalence of HIV infection to be 1.1 million people in the USA with an incidence of about 50,000 new infections per year [2, 3].
HIV-associated dermatoses are very common [4–6]. Skin disease can be uniquely associated with HIV disease, but more often represents common disorders, which may be more severe and recalcitrant to treatment. The spectrum of skin conditions includes skin findings associated with primary HIV infection and a broad range of skin problems related to the immune deficiency of advanced AIDS [5]. Recognition of characteristic eruptions can facilitate early diagnosis of HIV. A broad variety of neoplastic, infectious and non-infectious diseases can manifest in the skin and may alert the clinician to decline of the immune system [7].
In this review, we describe a general clinical approach to the HIV-infected patient presenting with common skin complaints, providing a differential diagnosis for commonly encountered syndromes and a review of recently published literature relevant to this area.
Overview/Approach to Physical Findings
Diagnosis of cutaneous disease can be challenging. While some conditions reliably present with stereotyped lesions, other diseases may have highly variable manifestations, leading to diagnostic uncertainty that may necessitate specialist consultation and skin biopsy. The approach to diagnosis of skin lesions includes the assessment of location, extent, primary lesions, and secondary changes. The extent and severity of lesions can be helpful diagnostic clues and can provide insight regarding the severity of immunosuppression.
Categories of discrete skin lesions, brief descriptions of physical findings, and the associated differential diagnoses are shown in Table 1. Papules and plaques are defined as elevated, circumscribed skin lesions involving the epidermis and dermis, which are less than 1 cm and greater than 1 cm in diameter, respectively. Nodules involve deeper tissues and are greater than 2 cm in size [8]. Papules, plaques, and nodules can be caused by infectious, inflammatory, as well as neoplastic disease. Infectious etiologies include diseases like warts, molluscum contagiosum, staphylococcal and streptococcal as well as bacillary angiomatosis, and atypical mycobacterial as well as deep fungal infections. Frequent external rubbing and scratching can lead to lichenification as well as the development of prurigo nodularis. Neoplasms, especially skin cancer, can also present as papules with various secondary changes, often noted in sun-exposed skin. Kaposi sarcoma, an AIDS-defining cancer, presents with red to brown papules and nodules.
Table 1.
Approach to cutaneous lesions associated with HIV
| Localized vs generalized | Lesion description | Differential diagnosis | Clinical findings |
|---|---|---|---|
| Localized skin findings *can be generalized in immunosuppressed patients |
Papules and Nodules | Molluscum Ecthyma | 2–3-mm skin-colored umbilicated papules Eroded, ulcerated papules with overlying crust |
| Furuncle/carbuncle | Inflammatory papules and nodules, tender | ||
| Bacillary angiomatosis | Friable, red, purple, or flesh-colored papules and nodules | ||
| Verruca vulgaris | Verruciform hyperkeratotic papules | ||
| Condylomata | Skin-colored often pedunculated verruciform papules | ||
| Prurigo nodularis | Excoriated, often hyperkeratotic papules and nodules | ||
| Non-melanoma skin cancer: SCC | Erythematous papules with variable hyperkeratosis, crusting, and ulceration | ||
| BCC | Pearly papules with overlying telangiectasias | ||
| Kaposi sarcoma | Red-to-brown macules, papules and plaques | ||
| Plaques | Cellulitis | Erythematous plaques; increased warmth, tenderness | |
| Intertrigo | Erythematous thin papules and plaques with superficial erosions and fine scale; skin folds | ||
| Thrush | White plaque of the oral mucosa, can be removed by scraping | ||
| Oral hairy leukoplakia | Non-painful white plaque along the lateral tongue | ||
| Other fungal or bacterial infections | Variable cutaneous presentation | ||
| Erythematous plaques with scale: Seborrheic dermatitis | Erythematous plaques with greasy scale in a seborrheic distribution | ||
| Psoriasis | Well-demarcated erythematous plaques with silvery scale, often on extensor surfaces | ||
| Vesicles and Bullae | Bullous impetigo | Superficial vesicles, erosions, honey-colored crust | |
| Folliculitis | Follicular pustules and papules | ||
| Herpes simplex | Grouped vesicles on erythematous base | ||
| Herpes zoster | Grouped vesicles in dermatomal distribution | ||
| Bullous tinea or candida | Predominantly erosions and few superficial vesicles on erythematous background | ||
| Exanthem± enanthem | Viral diseases, acute HIVexanthem | Morbilliform macular eruptions, often associated with systemic symptoms | |
| Generalized skin findings | Exanthem± enanthem | Drug reaction | Often generalized morbilliform macular eruptions |
| Erythema with desquamation | SJS, TEN | erythematous to dusky macules and patches; check for mucosal involvement | |
| Papules, plaques | Pruritic papular eruption | Widespread skin-colored to erythematous papule with signs of excoriation | |
| Scabies | Widespread hyperkeratotic, scaly papules; severe pruritus | ||
| Erythema with scaling | Eczema/dermatitis, xerosis | Erythematous scaly papules and plaques, often worse in the winter |
Plaques can be associated with infectious diseases like cellulitis or intertrigo, as well as non-infectious causes. Inflammatory diseases like papular eczema can present with localized as well as widespread papules coalescing to plaques which are often associated with scaling and pruritus. Other common rashes associated with HIV include seborrheic dermatitis, which presents as erythematous papules and plaques with greasy scale, usually in a seborrheic distribution (oily and hair-bearing skin). There can be an overlap with psoriasis which typically shows well-demarcated plaques with silvery scale on the extensor surfaces; nail findings, including pitting and oil spots, can be helpful in distinguishing the two entities.
Vesicles and bullae are elevated lesions filled with clear fluid, also distinguished by size of less or greater than 1 cm, respectively [8]. Their presentation should raise concern for underlying infectious disease. Grouped vesicles on an erythematous base are a common presentation for herpes simplex, while herpes zoster often presents similarly in a dermatomal distribution. Both diseases can be widespread or generalized in case of severe immunosuppression. A honey-colored crust is a common finding in bullous impetigo, while bullous tinea or candida will present with very superficial erosions. Of course other causes including contact dermatitis, edema, or pressure bullae need to be ruled out. Follicular inflammatory papules and pustules are suggestive of folliculitis.
Macules and patches are defined as flat, circumscribed skin lesions, which can present in localized as well as widespread patterns [8]. Macular and morbilliform erythematous eruptions are often associated with drug reactions, but can also represent a viral infection. If widespread erythema is associated with bullous lesions, desquamation, and mucosal involvement, then severe drug reactions like Stevens-Johnson syndrome (SJS) or toxic epidermal necrolysis (TEN) need to be excluded.
In the era of antiretroviral therapy (ART), the spectrum of cutaneous manifestations in HIV-infected patients has changed, and clinicians are faced with different skin complaints today.
Pruritus
In the post-antiretroviral era, pruritus has become the most common skin-related symptom reported by patients with HIV [4, 9•]. Kaushik et al. reported a high prevalence of chronic pruritus in a cross-sectional study on patients in the USA. The majority (91 %) of the participants were receiving antiretroviral therapy. Forty-five percent of the surveyed patients reported pruritus with negative effects on their quality of life. Overall, no significant association between the reported pruritus and CD4 or eosinophil counts was reported. The most common dermatoses found were xerosis (23 %), fungal infections (12 %), seborrheic dermatitis (9 %), and eczema (7 %) [9•].
Similarly, pruritic papular dermatitis was reported as the most common skin manifestation in 61 Indian HIV patients with skin lesions, not on ART [10]. The most common infectious manifestation found was molluscum. The prospective study also compared histopathologic evaluations with the patient’s CD4:CD8 ratios. They demonstrated an inverse relationship between skin lesions and CD4 counts. The majority of skin lesions were associated with CD4 counts of <220/ul and all patients with skin lesions demonstrated a CD4: CD8 ratio of <0.5. Since most patients with skin lesions presented with stage 3 and 4 HIV infection, specific cutaneous manifestations were considered a good clinical indicator for the patient’s immune status [10].
Bacterial Infections
Despite improved disease control, infections continue to be a relevant morbidity in the HIV infected population. They include fungal, bacterial, viral, as well as parasitic infections and can be an indicator of the degree of immunosuppression. Many infections common in the general population appear to be more recalcitrant in HIV. With improved immune function on ART, a growing incidence of methicillin-resistant Staphylococcus aureus (MRSA) has been noted. Especially, community-associated MRSA (CA-MRSA) has been increasing in the community as well as the nosocomial setting [11]. Colonization is thought to be an important factor for infection, and decolonization attempts have shown little success [12, 13]. CA-MRSA infections are more prevalent in HIV-infected individuals compared to the HIV-negative population, which might be attributed to a higher colonization burden [14]. Popovich et al. showed that 20 % of HIVand only 11 % of HIV-negative patients were colonized with CA-MRSA [15••]. HIV patients showed a higher prevalence of nasal and extranasal colonization. Perirectal and inguinal sites represent the most common extranasal sites. Additionally, the colonization burden was found to be higher in the HIV-infected patients. Positive predictive factors for higher colonization burden were HIV infection, male sex, illicit drug use, younger age, African-American race, and temporary housing. The presence of chronic skin conditions or wounds was also associated with a higher colonization burden. Compared with HIV-negative patients, the HIV population had a higher proportion of chronic skin disease. CD4 count and viral load was not found to influence the colonization burden [15••].
Similar findings were made in a meta-analysis by Zervou et al. [16]. The prevalence of MRSA colonization among HIV-infected individuals was estimated to be 6.9 % worldwide and 8.8 % in North America. Extranasal screening increased the yield of the testing by 31.6 % and USA300 was the most frequent strain. Risk factors included hospitalization within the past 12 months, as well as previous and current incarceration. Antibiotic or antiretroviral treatment was not found to influence the risk.
Vyas et al. evaluated initial and recurrent infections with MRSA retrospectively in a cohort of mainly HIV-infected men, with 80 % on ART [17]. Eight percent developed a primary infection; associated risk factors included a CD4 count <500 cells/ml, HIV RNA levels >400 copies/ml, and injection drug use. Abscesses were the most common type of infection, often noted on the lower extremity, buttocks, and scrotum. Twenty-seven percent of patients developed recurrence, which was associated with risk factors including hospital admission, and a lower CD4 count at the initial infection. Interestingly, treatment of the initial infection with minocycline was linked to an 80 % decrease in odds ratio for recurrent infections.
Viral Infections
Viral infections are also more prevalent in immunosuppressed patients and can be associated with malignancies. In the era of ART, the focus of attention has shifted from long-known viral infections like herpes, molluscum, and virus associated with AIDS-defining cancers to the discovery of new viral disease. Recently, trichodysplasia spinulosa-associated polyomavirus (TSPyV) has been detected in patients with immunosuppression [18] (Fig. 1). Furthermore, several new human polyomaviruses (HPyVs) have been discovered [19]. Polyomavirus-associated infections are commonly asymptomatic, but in the immunosuppressed population, reactivation can lead to serious disease [20]. Wieland et al. found a higher rate of HPyVs, in particular HPyV 6, V7, V10, and TSPyV in HIV-infected men compared to the healthy male controls [21]. Also, the presence of multiple viruses was more common in HIV-positive persons. Viral loads, CD4 count, and ART did not seem to have an impact on the HPV status, though there was a tendency of higher HPyV6 loads in poorly controlled patients. Further studies and long-term follow-up will be necessary to determine the significance of the infections.
Fig. 1.
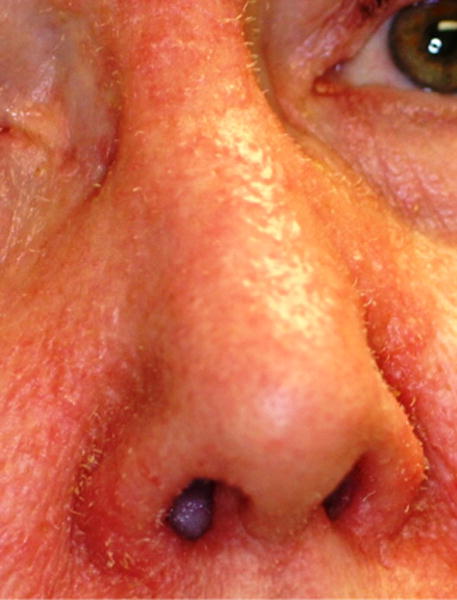
Clinical characteristics of trichodysplasia spinulosa, presenting with small hyperkeratotic spicules on the nose
HIV-associated immunosuppression was proposed to play an important but reduced role compared to transplant patients. Infectious causes, in particular viral disease, were suggested to possibly account for the association of squamous cell carcinomas (SCCs) in patients with a decreased CD4 count. Acquired epidermodysplasia verruciformis (EV) presents as extensive verruciform cutaneous lesions in patients with compromised immunity. The lesions are HPV-associated and rare cases have been reported [22]. Vicente et al. identified 5 cases of acquired EVamong 240 HIV-infected pediatric patients [23]. Three out of the five were found to carry high-risk HPV types [23]. Similar to previous reports, immunologic recovery and viral load suppression upon ART did not lead to improvement of the cutaneous lesions [24]. Long-term follow-up is necessary since the risk of cutaneous malignancy is unknown.
Medication Toxicity
Skin reactions to drugs complicate in 2–3 % of all hospital-based treatments [25]. These reactions can range from morbilliform eruptions to life-threatening forms like TEN. HIV infection has been associated with an increased risk for SJS and TEN [26]. Increased exposures to medications or decreased immunity have been proposed as possible etiologies. A majority of the recent data on SJS and TEN has been generated in the African HIV population. Antiretroviral drugs appear to pose an additional risk of disease-related toxicity, and a high frequency of antiretroviral drugs as the cause of SJS has been reported. Nevirapine, a non-nucleoside reverse transcriptase inhibitor was associated with 84 % of the 19 SJS cases in a pediatric population in a small series by Dziuban et al. [27]. Most of the patients (84 %) had advanced immunosuppression. Sulfonamides were the most commonly implicated drug (38.4 %) closely followed by nevirapine (19.8 %) in a study of patients with SJS/TEN by Saka et al. [28]. More than half (54.8 %) of the patients were HIV-positive. There was also a trend to more severe reactions in the HIV-infected population. In European and Western studies, anticonvulsants, allopurinol, and antibiotics have been associated with the largest increase in risk of SJS, which can be explained by the lower prevalence of HIV infection in those countries. HIV patients are common consumers of all prior mentioned medications, but this alone might not explain the up to 1000-fold higher risk of severe skin reactions [26]. T cells have been proposed a role in mediating keratinocyte cytotoxicity [29]. Especially, regulatory T cells might play a protective role in the skin [30]. An inverse correlation of the serum CD4 count and the incidence of cutaneous drug reactions have been reported [31]. Yang et al. demonstrated an eightfold increase in CD8/CD4 ration in the dermis of HIV patients while the dermal CD4 count was decreased [32]. Furthermore, they noted a trend to a decreased number of CD25 to CD4-positive T cells in the epidermis. The decrease in CD25+ regulatory T cells (T-regs) could explain the increased risk of drug reactions. HIV could represent a predisposition for severe drug reaction by depletion of CD4 cells and loss of T-regs in the skin, which subsequently leads to an upregulation of cytotoxic CD8 T cells.
Skin Cancer
Age-related cancers are a comorbidity that affects the HIV-infected population earlier than non-infected individuals [33, 34]. Furthermore, compared to the general population, HIV infection has been associated with a higher risk of certain cancers, especially AIDS-defining cancers [35]. Since the introduction of ART, decreasing incidences of many cancers have been noted [36–38]. Hleyhel et al. evaluated long-term trends for the incidence of Kaposi sarcoma (KS), non-Hodgkin lymphoma (NHL), and cervical cancer, which are all thought to be associated with immunosuppression [39]. They noted an overall reduction for all those cancers, but the incidences remained higher than the general population. Relative risks in patients with restored immunity stayed elevated for KS (SIR 35.4 % confidence interval (CI) 18.3–61.9) and were comparable to the general population for NHL. HIV-infected patients were found to be diagnosed at a younger age, particularly for NHL, which was diagnosed about 11 years prior to the control group [39].
Non-melanoma skin cancers are the most common cancer in the USA [40]. Immunosuppression has been associated with an increased risk, which is well established in solid organ transplant patients [41, 42]. HIV patients have been found to have an overall twofold increased risk for skin cancer [43•]. The adjusted rate ratio for SCC was 2.6 (95 % CI 2.1–3.2) for HIV-positive versus HIV-negative subjects and 2.1 (95 % CI 1.8–2.3) for basal cell carcinoma (BCC). Higher rate ratios for SCCs were noted in patients with lower CD4 counts. Similar associations could not be shown for BCCs. Silverberg et al. also reported a difference in the clinical presentation for BCCs, which was found to be less invasive and more commonly on the extremities in HIV-positive subjects [43•].
Conclusions
Cutaneous diseases continue to be an important aspect of HIV infections. The introduction of antiretroviral therapy has led to improved immune status and a change in the spectrum of presentations. Physicians in the Western world are mostly faced with dermatoses seen in the general public, though often in a more recalcitrant or earlier presentation. Immunosuppression continues to represent a risk factor for infections and increased risk of malignancy. Risk for CA-MRSA infection and skin cancer is increased in HIV-infected persons compared to the general population.
Disease-associated immunosuppression also constitutes a risk for severe adverse reactions in combination with disease-directed therapy. The risk for SJS/TEN is significantly increased in HIV-infected patients and might be associated with the decrease in cutaneous regulatory T cells. Though significant progress has been made in the treatment of HIV and associated manifestations, cutaneous disease continues to be an important comorbidity and can affect the quality of life.
Acknowledgments
This work is supported by NIH grant K23DA032306.
Footnotes
Conflict of Interest We declare that we have no conflicts of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by the author.
This article is part of the Topical Collection on Skin, Soft Tissue, Bone and Joint Infectious Diseases
Contributor Information
Kirstin Altman, Department of Dermatology, University of Wisconsin School of Medicine & Public Health, Madison, WI, USA.
Erin Vanness, Department of Dermatology, University of Wisconsin School of Medicine & Public Health, Madison, WI, USA.
Ryan P. Westergaard, Email: rpw@medicine.wisc.edu, Departments of Medicine and Population Health Sciences, University of Wisconsin School of Medicine & Public Health, Madison, WI, USA; Division of Infectious Diseases, 1685 Highland Ave, MFCB 5220, Madison, WI 53705, USA.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.UNAIDS. Global Report: UNAIDS Report on the Global AIDS Epidemic 2012. 2012 http://www.avert.org/worldwide-hiv-aids-statistics.htm.
- 2.CDC. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 U.S. dependent areas—2011. HIV Surveillance Supplemental Report. 2013;18(5) [Google Scholar]
- 3.CDC. Estimated HIV incidence in the United States, 2007–2010. HIV Surveillance Supplemental Report. 2012;17(4) [Google Scholar]
- 4.Mankahla A, Mosam A. Common skin conditions in children with HIV/AIDS. Am J Clin Dermatol. 2012;13(3):153–66. doi: 10.2165/11593900-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Cedeno-Laurent F, Gómez-Flores M, Mendez N, Ancer-Rodríguez J, Bryant JL, Gaspari AA, et al. New insights into HIV-1-primary skin disorders. J Int AIDS Soc. 2011;14:5. doi: 10.1186/1758-2652-14-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith KJ, Skelton HG, Yeager J, Ledsky R, McCarthy W, Baxter D, et al. Cutaneous findings in HIV-1-positive patients: a 42-month prospective study. Military Medical Consortium for the Advancement of Retroviral Research (MMCARR) J Am Acad Dermatol. 1994;31(5 Pt 1):746–54. doi: 10.1016/s0190-9622(94)70236-5. [DOI] [PubMed] [Google Scholar]
- 7.Rodgers S, Leslie KS. Skin infections in HIV-infected individuals in the era of HAART. Curr Opin Infect Dis. 2011;24(2):124–9. doi: 10.1097/QCO.0b013e328342cb31. [DOI] [PubMed] [Google Scholar]
- 8.Bolognia J, Jorizzo J, Schaffer J. Dermatology. 3. Elsevier; 2012. [Google Scholar]
- 9•.Kaushik SB, Cerci FB, Miracle J, Pokharel A, Chen SC, Chan YH, et al. Chronic pruritus in HIV-positive patients in the southeastern United States: its prevalence and effect on quality of life. J Am Acad Dermatol. 2014;70(4):659–64. doi: 10.1016/j.jaad.2013.12.015. This cross sectional study evaluates the most common skin complaints in patients in the US and their effect on the quality of life. [DOI] [PubMed] [Google Scholar]
- 10.Rane SR, Agrawal PB, Kadgi NV, Jadhav MV, Puranik SC. Histopathological study of cutaneous manifestations in HIV and AIDS. J Eur Acad Dermatol Venereol. 2013 Feb;27(2) doi: 10.1111/ijd.12298. [DOI] [PubMed] [Google Scholar]
- 11.Fridkin SK, Hageman JC, Morrison M, Sanza LT, Como-Sabetti K, Jernigan JA, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352(14):1436–44. doi: 10.1056/NEJMoa043252. [DOI] [PubMed] [Google Scholar]
- 12.Von Eiff C, Becker K, Machka K, Stammer H, Peters G. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study group. N Engl J Med. 2001;344(1):11–6. doi: 10.1056/NEJM200101043440102. [DOI] [PubMed] [Google Scholar]
- 13.Buehlmann M, Frei R, Fenner L, Dangel M, Fluckiger U, Widmer AF. Highly effective regimen for decolonization of methicillin-resistant Staphylococcus aureus carriers. Infect Control Hosp Epidemiol. 2008;29(6):510–6. doi: 10.1086/588201. [DOI] [PubMed] [Google Scholar]
- 14.Popovich KJ, Weinstein RA, Aroutcheva A, Rice T, Hota B. Community-associated methicillin-resistant Staphylococcus aureus and HIV: intersecting epidemics. Clin Infect Dis. 2010;50(7):979–87. doi: 10.1086/651076. [DOI] [PubMed] [Google Scholar]
- 15••.Popovich KJ, Hota B, Aroutcheva A, Kurien L, Patel J, Lyles-Banks R, et al. Community-associated methicillin-resistant Staphylococcus aureus colonization burden in HIV-infected patients. Clin Infect Dis. 2013;56(8):1067–74. doi: 10.1093/cid/cit010. This study shows increased MRSA colonization burden in HIV patients and highlights associated risk factors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zervou FN, Zacharioudakis IM, Ziakas PD, Rich JD, Mylonakis E. Prevalence of and risk factors for methicillin-resistant Staphylococcus aureus colonization in HIV infection: a meta-analysis. Clin Infect Dis. 2014;59(9):1302–11. doi: 10.1093/cid/ciu559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vyas KJ, Shadyab AH, Lin CD, Crum-Cianflone NF. Trends and factors associated with initial and recurrent methicillin-resistant Staphylococcus aureus (MRSA) skin and soft-tissue infections among HIV-infected persons: an 18-year study. J Int Assoc Provid AIDS Care. 2014;13(3):206–13. doi: 10.1177/2325957412473780. [DOI] [PubMed] [Google Scholar]
- 18.Kazem S, van der Meijden E, Feltkamp MC. The trichodysplasia spinulosa-associated polyomavirus: virological background and clinical implications. APMIS. 2013;121(8):770–82. doi: 10.1111/apm.12092. [DOI] [PubMed] [Google Scholar]
- 19.Ehlers B, Wieland U. The novel human polyomaviruses HPyV6, 7, 9 and beyond. APMIS. 2013;121(8):783–95. doi: 10.1111/apm.12104. [DOI] [PubMed] [Google Scholar]
- 20.Dalianis T, Hirsch HH. Human polyomaviruses in disease and cancer. Virology. 2013;437(2):63–72. doi: 10.1016/j.virol.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 21.Wieland U, Silling S, Hellmich M, Potthoff A, Pfister H, Kreuter A. Human polyomaviruses 6, 7, 9, 10 and Trichodysplasia spinulosa-associated polyomavirus in HIV-infected men. J Gen Virol. 2014;95(Pt 4):928–32. doi: 10.1099/vir.0.061259-0. [DOI] [PubMed] [Google Scholar]
- 22.Rogers HD, Macgregor JL, Nord KM, Tyring S, Rady P, Engler DE, et al. Acquired epidermodysplasia verruciformis. J Am Acad Dermatol. 2009;60(2):315–20. doi: 10.1016/j.jaad.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 23.Vicente A, Pau-Charles I, González-Enseñat MA, Muñoz-Almagro C, Cañadas MP, Noguera-Julian A, et al. High-risk alpha-human papillomavirus types: detection in HIV-infected children with acquired epidermodysplasia verruciformis. J Am Acad Dermatol. 2013;68(2):343–5. doi: 10.1016/j.jaad.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 24.Jacobelli S, Laude H, Carlotti A, Rozenberg F, Deleuze J, Morini JP, et al. Epidermodysplasia verruciformis in human immunodeficiency virus-infected patients: a marker of human papillomavirus-related disorders not affected by antiretroviral therapy. Arch Dermatol. 2011;147(5):590–6. doi: 10.1001/archdermatol.2010.399. [DOI] [PubMed] [Google Scholar]
- 25.Valeyrie-Allanore L, Sassolas B, Roujeau JC. Drug-induced skin, nail and hair disorders. Drug Saf. 2007;30(11):1011–30. doi: 10.2165/00002018-200730110-00003. [DOI] [PubMed] [Google Scholar]
- 26.Rzany B, Mockenhaupt M, Stocker U, Hamouda O, Schöpf E. Incidence of Stevens-Johnson syndrome and toxic epidermal necrolysis in patients with the acquired immunodeficiency syndrome in Germany. Arch Dermatol. 1993;129(8):1059. [PubMed] [Google Scholar]
- 27.Dziuban EJ, Hughey AB, Stewart DA, Blank DA, Kochelani D, Draper HR, et al. Stevens-Johnson syndrome and HIV in children in Swaziland. Pediatr Infect Dis J. 2013;32(12):1354–8. doi: 10.1097/INF.0b013e31829ec8e5. [DOI] [PubMed] [Google Scholar]
- 28.Saka B, Barro-Traoré F, Atadokpédé FA, Kobangue L, Niamba PA, Adégbidi H, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis in sub-Saharan Africa: a multicentric study in four countries. Int J Dermatol. 2013;52(5):5. doi: 10.1111/j.1365-4632.2012.05743.x. [DOI] [PubMed] [Google Scholar]
- 29.Pereira FA, Mudgil AV, Rosmarin DM. Toxic epidermal necrolysis. J Am Acad Dermatol. 2007 doi: 10.1016/j.jaad.2006.04.048. [DOI] [PubMed] [Google Scholar]
- 30.Azukizawa H, Sano S, Kosaka H, Sumikawa Y, Itami S. Prevention of toxic epidermal necrolysis by regulatory T cells. Eur J Immunol. 2005;35(6):1722–30. doi: 10.1002/eji.200425773. [DOI] [PubMed] [Google Scholar]
- 31.Smith KJ, Skelton HG, Yeager J, Ledsky R, Ng TH, Wagner KF. Increased drug reactions in HIV-1-positive patients: a possible explanation based on patterns of immune dysregulation seen in HIV-1 disease. The Military Medical Consortium for the Advancement of Retroviral Research (MMCARR) Clin Exp Dermatol. 1997;22(3):118–23. [PubMed] [Google Scholar]
- 32.Yang C, Mosam A, Mankahla A, Dlova N, Saavedra A. HIV infection predisposes skin to toxic epidermal necrolysis via depletion of skin-directed CD4+ T cells. J Am Acad Dermatol. 2014;70(6):1096–102. doi: 10.1016/j.jaad.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 33.Brock MV, Hooker CM, Engels EA, Moore RD, Gillison ML, Alberg AJ, et al. Delayed diagnosis and elevated mortality in an urban population with HIVand lung cancer: implications for patient care. J Acquir Immune Defic Syndr. 2006;43(1):47–55. doi: 10.1097/01.qai.0000232260.95288.93. [DOI] [PubMed] [Google Scholar]
- 34.Puoti M, Bruno R, Soriano V, Donato F, Gaeta GB, Quinzan GP, et al. Hepatocellular carcinoma in HIV-infected patients: epidemiological features, clinical presentation and outcome. AIDS. 2004;18(17):2285–93. doi: 10.1097/00002030-200411190-00009. [DOI] [PubMed] [Google Scholar]
- 35.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosup-pressed transplant recipients: a meta-analysis. Lancet. 2007;370(9581):59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 36.Grabar S, Abraham B, Mahamat A, Del Giudice P, Rosenthal E, Costagliola D. Differential impact of combination antiretroviral therapy in preventing Kaposi’s sarcoma with and without visceral involvement. J Clin Oncol. 2006;24(21):3408–14. doi: 10.1200/JCO.2005.05.4072. [DOI] [PubMed] [Google Scholar]
- 37.Polesel J, Franceschi S, Suligoi B, et al. Cancer incidence in people with AIDS in Italy. Int J Cancer. 2010;127(6):1437–45. doi: 10.1002/ijc.25153. [DOI] [PubMed] [Google Scholar]
- 38.Franceschi S, Lise M, Clifford GM, Rickenbach M, Levi F, Maspoli M, et al. Swiss HIV Cohort Study. Changing patterns of cancer incidence in the early- and late-HAART periods: the Swiss HIV Cohort Study. Br J Cancer. 2010;103(3):416–22. doi: 10.1038/sj.bjc.6605756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hleyhel M, Belot A, Bouvier AM, et al. Risk of AIDS-defining cancers among HIV-1-infected patients in France between 1992 and 2009: results from the FHDH-ANRS CO4 cohort. Clin Infect Dis. 2013;57(11):1638–47. doi: 10.1093/cid/cit497. [DOI] [PubMed] [Google Scholar]
- 40.Rogers HW, Weinstock MA, Harris AR, Hinckley MR, Feldman SR, Fleischer AB, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146(3):283–7. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- 41.Webb MC, Compton F, Andrews PA, Koffman CG. Skin tumours posttransplantation: a retrospective analysis of 28 years’ experience at a single centre. Transplant Proc. 1997;29(1–2):828–30. doi: 10.1016/s0041-1345(96)00152-2. [DOI] [PubMed] [Google Scholar]
- 42.Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med. 2003;348(17):1681–91. doi: 10.1056/NEJMra022137. [DOI] [PubMed] [Google Scholar]
- 43•.Silverberg MJ, Leyden W, Warton EM, Quesenberry CP, Jr, Engels EA, Asgari MM. HIV infection status, immunodeficiency, and the incidence of non-melanoma skin cancer. J Natl Cancer Inst. 2013;105(5):350–60. doi: 10.1093/jnci/djs529. This is a cohort study showing a higher risk of non-melanoma skin cancer in HIV patients. [DOI] [PMC free article] [PubMed] [Google Scholar]


