Abstract
Hypoxia in ischemic limbs typically initiates angiogenic and inflammatory factors to promote angiogenesis in attempt to restore perfusion, and revascularization involves multiple cell types and systems. Macrophage display phenotype plasticity, and can polarize in response to local and systemic cytokine stimuli. M2 macrophage are known to play an important role in angiogenesis and wound healing. While accepted that many pro-inflammatory cytokines induce angiogenesis, the effects of anti-inflammatory interleukins on initiation of angiogenesis are less clear. Interleukin-19 [IL-19] is a presumed anti-inflammatory cytokine, with unknown effects on macrophage polarization. In our recent study, we used several experimental approaches and determined that IL-19 regulated neovascularization in the murine hind-limb ischemia model. In addition to endothelial cells, we found that IL-19 could target and polarize macrophage to the M2 phenotype. IL-19 could induce expression of angiogenic, and reduce expression of anti-angiogenic cytokines in these cells. This is the first study to demonstrate that IL-19 could polarize macrophage, and potentially identifies IL-19 as a therapy to induce angiogenesis in ischemic tissue.
Despite an increased awareness of risk factors and a better understanding of its etiology, Peripheral vascular disease (PVD) effects millions of individuals world-wide. It is a significant medical and socioeconomic problem contributing to the mortality of multiple diseases including myocardial infarction, diabetes, and peripheral vascular disease. It will worsen with increasing co-morbidities such as metabolic syndrome and Type 2 diabetes, which are reaching epidemic proportions. Symptoms of PVD include intermittent claudication, which is pain, weakness, or other discomfort when the affected muscle are used. More severe cases are classified as critical limb ischemia, which in extreme cases results in ulceration and loss of under perfused ischemic tissue.
Neovascularization is a necessary vascular repair mechanism to preserve tissue and organ viability in response to ischemic conditions in peripheral limb vasculature and in ischemic myocardium. Hypoxia in ischemic limbs typically initiates angiogenic and inflammatory factors to promote angiogenesis in an attempt to restore perfusion. As with any complex process, ischemic revascularization involves multiple cell types and systems. While neovascularization and inflammation are independent biological processes, they are linked in response to injury and ischemia, and both inflammatory and anti-inflammatory processes participate in angiogenesis.
Macrophage have a remarkable plasticity in order to effectively respond to environmental changes. They can modify their phenotype and exert different functions depending on proximate signals. Based on the activation route macrophages can generally be divided into two major populations: classically activated or M1 macrophages induced in particular by LPS, IL-1β and Th1 cytokines, such as IFNγ. Macrophage activation by Th2 cytokines, such as IL-4, IL-13 or glucocorticoids leads to a second, or alternatively activated M2 profile [1]. M1 macrophages are associated with inflammation, while alternatively activated macrophages are thought to promote fibrosis, wound healing, neovascularization and granuloma formation [2,3]. Activated M1 macrophages which produce nitric oxide (NO) and pro-inflammatory cytokines such as TNFα, IL-6, IL-1β and IL-12 can induce a potent inflammatory response in the host tissue[4]. In contrast, alternatively activated M2 macrophages dampen or resolve local inflammation by producing IL-10, IL-1 receptor agonist and TGFβ[5]. The M2 phenotype also is characterized by the induction of Arginase 1 (ARG 1), rather than inducible nitric oxide synthase (iNOS). Arginase competes with iNOS to deplete arginine stores of macrophages and produces polyamines and proline rather than NO, both of which are important for cell differentiation and collagen production [6].
Both subtypes of macrophages are required during arteriogenesis, and the release of chemotactic factors, such as TNFα, and MCP1 by M1 macrophages enhanced recruitment of circulating leukocytes from the circulation[7,8]. Most investigations however, have demonstrated a more prominent role of M2 macrophages in arteriogenesis. M2 macrophages express a considerable number of angiogenic growth factors such as VEGF-A, TGFα, IGF-1, PDGF-β and HGF, and promote wound repair and neovascularization [9-11]. Jetten et.al demonstrated in vitro tube formation was induced by conditioned medium of M2 macrophages, in contrast with conditioned medium of M0 and M1macrophages, which actually inhibited tube formation [12]. Thus, factors which generate the M2 phenotype can be considered to be indirectly angiogenic by virtue of their ability to elicit angiogenic cytokine synthesis from macrophage.
While intuitive that the inflammatory state of ischemic tissue may dictate whether that tissue becomes neovascularized or necrotic, the role of and direct effects of anti-inflammatory interleukins on initiation of angiogenesis remain largely uncharacterized. Over the last several years, our laboratory has focused on one such anti-inflammatory cytokine, Interleukin-19 [IL-19] and the role it may play in vascular pathophysiology, including PVD. IL-19 is a unique Th2 interleukin first described in 2001[13]. IL-19 was originally placed in an IL-10 sub-family, which includes IL-20, IL-22, and IL-24, but IL-19 is functionally distinct from these sub-family members and especially IL-10. IL-19 is reported to be anti-inflammatory because in T-lymphocytes it promotes the Th2 [regulatory], rather than the Th1 (T helper) response, although the mechanism[s] behind this response have not been characterized[14-17].
In contrast to other interleukins, IL-19 targets resident vascular cells in addition to immune cells, with the potential for multiple modes of action. Our initial studies of IL-19 function outside the immune system reported that IL-19 was expressed in angiogenic tissue, and has potent pro-angiogenic effects on multiple human EC types, [umbilical vein, coronary artery, and microvascular][18]. This manuscript reported that IL-19 is chemotactic and mitogenic for EC, promotes tube-like structure formation on Matrigel, and microvessel formation in the mouse aortic ring assay. These inaugural studies, though novel, were all cell culture or ex vivo based, and lacked validation in a relevant in vivo model of angiogenesis.
We recently published a study to determine the angiogenic effects of IL-19 utilizing the hind limb ischemia model in which to mirror PVD[19]. In this manuscript, murine hind limb ischemia was induced by femoral artery ligation, and changes in blood perfusion quantitated using Laser Doppler Perfusion Imaging (LDPI). We observed that wild type mice receiving i.p. injections of rIL-19 [10 ng/g/day] showed significantly increased levels of perfusion compared to PBS controls. Using IL-19 knock out mice and femoral artery ligation, we observed significantly decreased LDPI values in IL-19−/− mice when compared to wild type mice. To draw a tighter association between IL-19 and increased blood perfusion, we utilized a “rescue” approach, in which IL-19−/− mice injected with rIL-19. In this arm of the study we found that IL-19−/− mice injected with rIL-19 had significantly increased LDPI compared with PBS control mice. Quantitative immunohistochemistry on ligated gastroc muscle determined increased capillary density in rIL-19 treated mice, and significantly less capillary density in IL-19−/− mice.
While treatment of cultured human endothelial cells induced expression of a number of angiogenic cytokines, perhaps more interesting and unexpected, were IL-19’s effects on macrophage. Several reports in the literature show that in this model of hind limb ischemia, mononuclear cell infiltration begins 1 day post-surgery and generally resolve by 7-14 days post-surgery[20]. In a second study, transcripts related to immune function were induced 1-3 days post-ligation surgery[21]. To test the hypothesis that IL-19 might modify macrophage infiltration into ischemic hind limbs, we first performed immunohistochemistry on ligated gastroc muscle using anti-CD68 antibody and detected that total macrophage infiltrate was not significantly different between IL-19 and saline-treated mice. We next performed quantitative RT-PCR using primers for several M1 and M2 markers on hind-limbs from mice ligated 5 days earlier to determine IL-19 effect on localized macrophage phenotype at the site of angiogenesis. We observed that mice treated with IL-19 had significantly increased expression of M2 markers compared with mice injected with saline. Two additional experiments were performed to further validate the M2 polarizing effects of IL-19. First, expression of macrophage phenotype markers quantitated by qRT-PCR from spleen recovered from mice subject to hind limb ischemia. Mice injected with IL-19 expressed significantly increased expression of M2 markers compared with saline-injected controls. Correspondingly, IL-19−/− mice demonstrated significantly decreased expression of M2 phenotype markers compared with wild-type controls. In a final experiment, we observed that bone marrow derived macrophage [BMDM] obtained from wild-type mice treated with IL-19 demonstrated increased expression of M2 markers compared with controls. Together, these were the first experiments to show that IL-19 could affect macrophage and polarize macrophage to the M2 phenotype.
Since M2 polarized macrophage are purported to synthesize angiogenic cytokines, we hypothesized that IL-19 could induce expression of VEGF in macrophage. VEGF-A is a primary driver of neovascularization, and its expression in macrophage presumably drives angiogenesis in a juxtacrine fashion in local EC. RNA was isolated from spleen from mice subject to hind limb ischemia, and we observed that VEGF-A mRNA expression was significantly increased in mice treated with rIL-19 compared with saline controls. Correspondingly, significantly less VEGF-A was detected in spleen from IL-19−/− mice compared with wild type controls. Both VEGF-A mRNA and protein are induced by IL-19 treatment in BMDM isolated from wild-type mice. Finally, ELISA performed on plasma recovered from mice subject to hind limb ischemia demonstrated that mice injected with rIL-19 had significantly more plasma VEGF-A compared with control mice. When taken together, these data suggest that IL-19 can induce VEGF-A expression in macrophage, and this may represent an additional angiogenic mechanism utilized by IL-19.
In addition to being pro-inflammatory, Interleukin-12 is also a potently anti-angiogenic cytokine expressed in leukocytes[23]. Angiogenesis is increased in IL-12−/− mice, and IL-12 has been considered as an anti-tumor therapy [24]. Since IL-19 is anti-inflammatory, we hypothesized that IL-19 might reduce IL-12 expression in macrophage. Using quantitative RT-PCR, we observed that IL-12p40 mRNA expression was significantly decreased in spleen from mice subject to hind limb ischemia which were injected with rIL-19. Correspondingly, IL-12p40 expression was significantly increased in spleen from IL-19−/− mice compared with wild-type controls. Finally, we observed that pretreatment of BMDM with IL-19 could significantly decrease TNFa-driven IL-12 mRNA and protein expression.
In summary, injection of IL-19 can increase, and lack of IL-19 can decrease reperfusion and angiogenesis in ischemic hind limbs. Our recent report suggest that IL-19 exerts pro-angiogenic effects by at least three mechanisms: 1- direct angiogenic effects on EC; 2-macrophage M2 polarization and VEGF-A induction, and; 3- reduction of IL-12 expression in macrophage. This work suggests that IL-19 may represent potential therapy in treatment of PVD. Future studies need to determine if IL-19 regulation of angiogenesis is primarily facilitated by direct effects on vascular cells, or by macrophage M2 polarization.
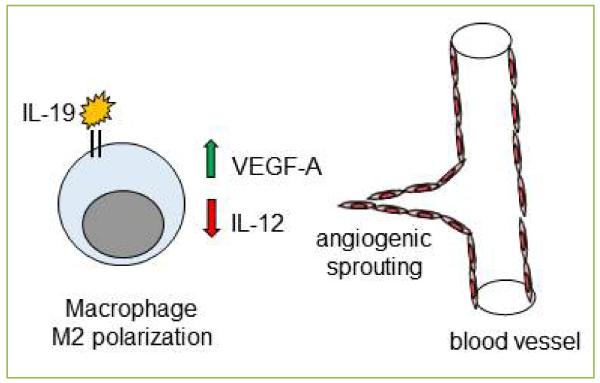
Figure 1. Interleukin-19 macrophage polarization and induction of angiogenesis.
Angiogenesis is the generation of new vessels from pre-existing vessels. One mechanism of IL-19 pro-angiogenic effects is macrophage M2 polarization, and induction of VEGF-A expression from macrophage. IL-12 as potently anti-angiogenic, and an additional mechanism is the reduction of IL-12 expression in macrophage. The combination of these events result in EC sprouting and generation of new capillaries.
Acknowledgements
This work was supported by grants HL115575 and HL117724 from the National Heart Lung, and Blood Institute of the National Institutes of Health, and Grant 13GRNT1685003 from the American Heart Association to MVA. K.G was supported by American Heart Association post-doctoral fellowship 11POST7530001.
Footnotes
Disclosures
The author(s) declare no competing financial interests or any other conflict of interest.
References
- 1.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 3.Varin A, Gordon S. Alternative activation of macrophages: Immune function and cellular biology. Immunibiology. 2009;214:630–641. doi: 10.1016/j.imbio.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 6.Troidl C, Möllmann H, Nef H, Masseli F, Voss S, Szardien S, et al. Classically and alternatively activated macrophages contribute to tissue remodelling after myocardial infarction. J. Cell. Mol. Med. 2009;13:3485–3496. doi: 10.1111/j.1582-4934.2009.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Troidl C, Jung G, Troidl K, Hoffmann J, Mollmann H, Nef H, et al. The temporal and spatial distribution of macrophage subpopulations during arteriogenesis. Curr Vasc Pharmacol. 2013;11:5–12. [PubMed] [Google Scholar]
- 8.Arras M, Ito WD, Scholz D, Winkler B, Schaper J, Schaper W. Monocyte activation in angiogenesis and collateral growth in the rabbit hindlimb. J Clin Invest. 1998;101:40–50. doi: 10.1172/JCI119877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kodelja V, Müller C, Tenorio S, Schebesch C, Orfanos CE, Goerdt S. Differences in angiogenic potential of classically vs alternatively activated macrophages. Immunobiology. 1997;197:478–493. doi: 10.1016/S0171-2985(97)80080-0. [DOI] [PubMed] [Google Scholar]
- 10.Bréchot N, Gomez E, Bignon M, Khallou-Laschet J, Dussiot M, Cazes A, et al. Modulation of macrophage activation state protects tissue from necrosis during critical limb ischemia in thrombospondin-1-deficient mice. PLoS One. 2008;3:e3950. doi: 10.1371/journal.pone.0003950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jetten N, Verbruggen S, Gijbels MJ, Post MJ, De Winther MP, Donners MM. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis. 2014;17:109–118. doi: 10.1007/s10456-013-9381-6. [DOI] [PubMed] [Google Scholar]
- 13.Gallagher G, Dickensheets H, Eskdale J, Izotova LS, Mirochnitchenko OV, Peat JD, et al. Cloning, expression and initial characterization of interleukin-19 (IL-19), a novel homologue of human interleukin-10 (IL-10) Genes Immun. 2000;1:442–450. doi: 10.1038/sj.gene.6363714. [DOI] [PubMed] [Google Scholar]
- 14.Gallagher G. Interleukin-19: Multiple roles in immune regulation and disease. Cytokine GrowthFactor Rev. 2010;21:345–352. doi: 10.1016/j.cytogfr.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Sabat R, Wallace E, Endesfelder S, Wolk K. IL-19 and IL-20: two novel cytokines with importance in inflammatory diseases. Expert Opin Ther Targets. 2007;5:601–612. doi: 10.1517/14728222.11.5.601. [DOI] [PubMed] [Google Scholar]
- 16.Oral H, Kotenko S, Yilmaz M, Mani O, Zumkehr J, Blaser K, et al. Regulation of T cells and cytokines by the interleukin-10 [IL-10]-family cytokines IL-19, IL-20, IL-22, IL-24 and IL-26. Eur J Immunol. 2006;36:380–388. doi: 10.1002/eji.200425523. [DOI] [PubMed] [Google Scholar]
- 17.Gallagher G, Eskdale E, Jordan W, Peat J, Campbell J, Boniotto M, et al. Human interleukin-19 and its receptor: a potential role in the induction of Th2 responses. International Immunopharmacology. 2004;4:615–626. doi: 10.1016/j.intimp.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Jain S, Gabunia K, Kelemen SE, Panetti TS, Autieri MV. The anti-inflammatory cytokine interleukin 19 is expressed by and angiogenic for human endothelial cells. Arterioscler Thromb Vasc Biol. 2011;31:167–175. doi: 10.1161/ATVBAHA.110.214916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richards J, Gabunia K, Kelemen SE, Kako F, Choi ET, Autieri MV. Interleukin-19 increases angiogenesis in ischemic hind limbs by direct effects on both endothelial cells and macrophage polarization. J Mol Cell Cardiol. 2014;79:21–31. doi: 10.1016/j.yjmcc.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee CW, Stabile E, Kinnaird T, Shou M, Devaney JM, Epstein SE, Burnett MS. Temporal patterns of gene expression after acute hindlimb ischemia in mice: insights into the genomic program for collateral vessel development. J Am Coll Cardiol. 2004;43:474–482. doi: 10.1016/j.jacc.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 21.Paoni NF, Peale F, Wang F, Errett-Baroncini C, Steinmetz H, Toy K, et al. Time course of skeletal muscle repair and gene expression following acute hind limb ischemia in mice. Physiol Genomics. 2002;11:263–272. doi: 10.1152/physiolgenomics.00110.2002. [DOI] [PubMed] [Google Scholar]
- 22.McLaren J, Prentice A, Charnock-Jones DS, Millican SA, Müller KH, Sharkey AM, et al. Vascular endothelial growth factor is produced by peritoneal fluid macrophages in endometriosis and is regulated by ovarian steroids. J Clin Invest. 1996;98:482–489. doi: 10.1172/JCI118815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Del Vecchio M, Bajetta E, Canova S, Lotze MT, Wesa A, Parmiani G, et al. Interleukin-12: biological properties and clinical application. Clin Cancer Res. 2007;13:4677–4685. doi: 10.1158/1078-0432.CCR-07-0776. [DOI] [PubMed] [Google Scholar]
- 24.Coughlin CM, Salhany KE, Gee MS, LaTemple DC, Kotenko S, Ma X, et al. Tumor cell responses to IFNgamma affect tumorigenicity and response to IL-12 therapy and antiangiogenesis. Immunity. 1998;9:25–34. doi: 10.1016/s1074-7613(00)80585-3. [DOI] [PubMed] [Google Scholar]