Abstract
Parvalbumin (PV)-containing neurons are functionally compromised in schizophrenia. Using double in situ hybridization in postmortem human prefrontal cortex, we found that the messenger RNA (mRNA) for the γ-aminobutyric acid transporter GAT-1 was undetectable in 22-41% of PV neurons in layers 3-4 in schizophrenia. In the remaining PV neurons with detectable GAT-1 mRNA, transcript expression was decreased by 26% in layer 3. Hence, the dysfunction of PV neurons involves the molecular dysregulation of presynaptic GABA reuptake.
Keywords: Schizophrenia, γ-aminobutyric acid transporter-1, Parvalbumin
1.INTRODUCTION
Schizophrenia is a neurodevelopmental disorder (Lewis and Murray, 1987; Weinberger, 1987) characterized by deficits in cognitive processes mediated by the circuitry of the dorsolateral prefrontal cortex (DLPFC) (Lewis and Levitt, 2002). Although no single pathological mechanism exists to explain the evolution of the symptoms associated with schizophrenia, it is considered a disease of aberrant synaptic plasticity and dysconnection stemming from deficits in key processes and structures including myelogenesis (Benes, 1989; Bartzokis et al., 2003), synaptic pruning (Feinberg, 1982; Keshavan et al., 1994; McGlashan and Hoffman 2000), perineuronal nets (Bitanihirwe and Woo, 2014) in addition to the active maturation of cortical inhibitory circuits (Berretta and Benes, 2001, Lewis et al., 2012).
Inhibitory neurons in the cerebral cortex are morphologically, neurochemically, physiologically and thereby functionally diverse, with different subsets of these neurons regulating distinct aspects of information processing (Buzsaki, 2006; Markram et al., 2004; Soltesz, 2005; Wang et al., 2004). Recent evidence suggests that disturbances of the sculpting of pyramidal circuit network activities by inhibitory neurons represent a core pathophysiologic feature of schizophrenia (Benes and Berretta, 2001; Lewis et al., 2005). Thus, the expression of the mRNA for the 67 kD isoform of the synthesizing enzyme of γ-aminobutyric acid (GABA), glutamic acid decarboxylase (GAD)67, has been consistently shown to be decreased in the cerebral cortex in schizophrenia (Akbarian et al., 1995; Costa et al., 2004; Volk et al., 2000; Woo et al., 2008; Woo et al., 2004). Furthermore, this reduction appears to occur preferentially, albeit not exclusively, in the subset of inhibitory neurons that contain parvalbumin (PV) (Hashimoto et al., 2003).
PV-containing neurons comprise two connectionally distinct populations of cells that synaptically target distinct compartments of pyramidal neurons: the perisomatically projecting basket neurons and the axoaxonally projecting chandelier neurons (DeFelipe and Farinas, 1992; Freund and Katona, 2007; Howard et al., 2005). The axon terminals of chandelier neurons form characteristic candlestick-like profiles that are termed “cartridges”. The density of these cartridges, which can be visualized immunohistochemically using an antibody against the GABA transporter GAT-1 (DeFelipe and Gonzalez-Albo, 1998), has been found to be decreased by ~40% in the DLPFC in schizophrenia (Konopaske et al., 2006; Pierri et al., 1999; Woo et al., 1998). Furthermore, the expression of the GABAA μ2 subunit protein, which is preferentially localized to synapses formed by chandelier neurons, has also been shown to be increased (Volk et al., 2002). Together these findings suggest that, in schizophrenia, chandelier neuron-mediated inhibitory neurotransmission at the axon initial segment of pyramidal neurons is disturbed at both the pre- and postsynaptic sites. In this regard, it has also been shown that the number of the GABAA μ1 receptor subunit, which is enriched in synapses formed by basket neurons, is significantly decreased in subjects with schizophrenia, suggesting that, like chandelier neurons, inhibitory regulation of pyramidal neurons by basket neurons is also aberrant (Curley et al., 2011; Curley and Lewis, 2012). Consistent with this, a recent study by Glausier and colleagues reported that while the number of PV basket cell inputs to pyramidal neurons was unaltered in schizophrenia, the amount of PV protein within PV basket cell axon terminals was decreased by 23% (Glausier et al., 2014). Likewise, the amount of GAD67 protein in PV basket cell terminals has also been shown to be decreased by as much as 50% (Curley et al., 2011). In the context of these observations, the present study aimed to evaluate whether pre-synaptic GABA reuptake in PV neurons is altered in subjects with schizophrenia.
2. METHODS and MATERIALS
2.1. Subjects
Postmortem human brains from 20 schizophrenia and 20 normal control subjects, matched for age, postmortem interval (PMI), freezer storage time, pH and sex were obtained from the Harvard Brain Tissue Resource Center (HBTRC), at McLean Hospital in Belmont, Massachusetts (Table 1). Brain collection procedures of the HBTRC, including the informed consent process, were approved by the Partners Human Research Committee. Written informed consent for use of each of the brains for research was obtained by the legal next of kin. The diagnosis of schizophrenia was made by two psychiatrists by reviewing medical records and an extensive family questionnaire that included medical, psychiatric and social history. All of the brains were also examined by a neuropathologist to rule out any neurological conditions, such as the various forms of dementia. Toxicological analysis revealed that no substances of abuse were detected in any of the schizophrenia or normal control subjects at the time of death.
Table 1.
Demographic information on subjects included in the present study
| Case | Diagnosis | Age | Sex | Race | pH | PMI | CED | Cause of Death | |
|---|---|---|---|---|---|---|---|---|---|
| Psychotropics received at the time of death | |||||||||
| 1 | CON | 49 | M | W | 6.76 | 24.6 | Myocardial | ||
| infarction | None | ||||||||
| 2 | CON | 37 | M | W | 6.68 | 18.8 | Electrocution | ||
| None | |||||||||
| 3 | CON | 54 | M | W | 6.53 | 24.2 | Cardiopulmonary | ||
| arrest | None | ||||||||
| 4 | CON | 78 | F | W | 6.22 | 14.1 | Myocardial | ||
| infarction | None | ||||||||
| 5 | CON | 53 | M | W | U | 20.2 | Cardiopulmonary | ||
| arrest | None | ||||||||
| 6 | CON | 65 | F | W | 6.40 | 24.3 | Lung cancer | ||
| None | |||||||||
| 7 | CON | 89 | M | W | 6.39 | 7.42 | Cancer | ||
| None | |||||||||
| 8 | CON | 69 | M | W | 6.88 | 15.3 | Respiratory failure | ||
| None | |||||||||
| 9 | CON | 74 | F | W | U | 12.5 | U | ||
| None | |||||||||
| 10 | CON | 66 | F | W | 6.03 | 7.4 | Cancer | ||
| None | |||||||||
| 11 | CON | 42 | M | W | 6.78 | 18.3 | Myocardial | ||
| infarction | None | ||||||||
| 12 | CON | 78 | F | W | 6.67 | 23.9 | Breast cancer | ||
| None | |||||||||
| 13 | CON | 40 | M | W | 6.24 | 16.6 | Myocardial | ||
| infarction | None | ||||||||
| 14 | CON | 67 | M | W | 6.42 | 22.3 | Cardiopulmonary | ||
| arrest | None | ||||||||
| 15 | CON | 70 | F | W | 6.26 | 22.5 | Liver cancer | ||
| None | |||||||||
| 16 | CON | 66 | M | W | 6.76 | 18.7 | Myocardial | ||
| infarction | None | ||||||||
| 17 | CON | 79 | M | W | 6.74 | 20.9 | Cancer | ||
| None | |||||||||
| 18 | CON | 38 | M | W | 6.53 | 28.8 | Myocardial | ||
| infarction | None | ||||||||
| 19 | CON | 70 | F | W | 6.59 | 15 | Cardiac arrest | ||
| None | |||||||||
| 20 | CON | 29 | M | W | U | 19 | U | ||
| None | |||||||||
| Mean (±SD) | 60.4±17.3 | 6.58±0.25 | 18.7±5.5 | ||||||
| 21 | SZ | 85 | F | W | U | 15.7 | 150 | Sepsis | |
| Risperidone, lorazepam | |||||||||
| 22 | SZ | 48 | F | W | 6.63 | 33.8 | 450 | Cardiac arrest | |
| Risperidone, divalproex | |||||||||
| 23 | SZ | 44 | M | W | 6.20 | 19 | 266 | Pneumonia | |
| Clozapine | |||||||||
| 24 | SZ | 89 | F | W | U | 13.5 | 20 | Pneumonia | |
| Trifluoperazine | |||||||||
| 25 | SZ | 78 | F | W | 6.81 | 13.4 | 750 | Sinus node disease | |
| Haloperidol, lithium, benztropine | |||||||||
| 26 | SZ | 61 | M | W | 6.68 | 19.9 | 300 | Sepsis | |
| Clozapine | |||||||||
| 27 | SZ | 61 | F | W | 6.14 | 11 | 150 | Myocardial | |
| infarction | Paroxetine, clonazepam, clozapine | ||||||||
| 28 | SZ | 84 | F | W | 6.14 | 25.8 | U | Cardiac arrest | |
| None | |||||||||
| 29 | SZ | 26 | M | W | 6.75 | 16 | 357 | Suicide by hanging | |
| Fluphenazine,decanoate | |||||||||
| 30 | SZ | 55 | F | W | 6.52 | 18 | U | Lung cancer | |
| None | |||||||||
| 31 | SZ | 47 | M | W | 6.57 | 19.2 | U | Lung cancer | |
| Clonazepam, hydroxyzine | |||||||||
| 32 | SZ | 73 | F | W | 6.08 | 24 | 600 | Lung cancer | |
| Risperidone, fluoxetine, clorazepate, midazolam | |||||||||
| 33 | SZ | 49 | M | W | 6.60 | 19 | 500 | Suicide by hanging | |
| Haloperidol decanoate, lorazepam | |||||||||
| 34 | SZ | 63 | M | W | 6.55 | 22.3 | 500 | Cardiac arrest | |
| Cloazapine, haloperidol, lorazepam, trazodone | |||||||||
| 35 | SZ | 72 | F | W | 6.65 | 21.7 | 400 | Ovarian cancer | |
| Risperidone, paroxetine | |||||||||
| 36 | SZ | 66 | M | W | 6.43 | 22.1 | 1000 | Emphysema | |
| Haloperidol | |||||||||
| 37 | SZ | 83 | F | W | 6.91 | 23.2 | 2000 | Gastrointestinal | |
| bleed | Haloperidol decanoate | ||||||||
| 38 | SZ | 46 | F | W | 6.31 | 18.5 | 200 | Sepsis | |
| Olanzapine, divalproex | |||||||||
| 39 | SZ | 42 | M | W | 6.64 | 27.1 | U | Leukemia | |
| None | |||||||||
| 40 | SZ | 31 | M | W | 6.46 | 14 | 600 | U | |
| Risperidone, olanzapine, buproprion | |||||||||
| Mean (±SD) | 60.2±16.7 | 6.65±0.28 | 19.8±5.4 | 433±468.0 | |||||
Abbreviations are as follows: PMI = postmortem interval, CED = chlorpromazine equivalent dose, U = unknown or unavailable, CON = normal control, SZ = schizophrenia, M = male, F = female, W = white
2.2. Tissue preparation and processing
Two sections (10 μm) per subject were taken from Broadmann area 9 of the DLPFC for in situ hybridization analysis. Laminae from the DLPFC were delineated and identified in adjacent sections by Nissl-counterstaining.
All of the experimental methods, including hybridization conditions (Bitanihirwe et al., 2009; Bitanihirwe et al., 2010; Woo et al., 2008; Woo et al., 2004), visualization of digoxigenin (DIG) and radiolabled probes (Bitanihirwe et al., 2009; Bitanihirwe et al., 2010; Woo et al., 2008; Woo et al., 2004) in addition to quantification procedures (Bitanihirwe et al., 2009; Bitanihirwe et al., 2010; Woo et al., 2008; Woo et al., 2004) have been described extensively in previous publications.
2.3. Riboprobe preparation
The complementary RNA (cRNA) probe for GAT-1, which was radiolabeled with Sulfur-35, was transcribed in vitro from a cDNA spanning nucleotides 1185 to 2154 within the coding region of the human SLC6A1 gene (Genbank Accession No. NM_003042). A corresponding sense probe was also generated. Hybridization of the sense probe resulted in no specific labeling. The DIG-labeled PV mRNA probe utilized here has been used in previously published studies (Bitanihirwe et al., 2009; Bitanihirwe et al., 2010).
2.4. Statistical Analysis
The densities of single (PV+) and double-labeled (PV+/GAT-1+) neurons and the amount of mRNA for GAT-1 in PV+ neurons were compared between the schizophrenia and normal control groups across layers 2 through 6 using repeated-measures analysis of variance (ANOVA) with diagnosis and layer as main effects, as previously described (Bitanihirwe et al., 2009; Bitanihirwe et al., 2010). We evaluated the effects of all of the confounding variables, such as age, PMI, pH, freezer storage time and exposure to antipsychotic medication (expressed as chloropromazine equivalent dose or CED) using analysis of covariance (ANCOVA). Because ANCOVA did not detect any statistically significant effect of any of the covariates on the dependent variables, only results from repeated-measures ANOVA are reported. In addition, Pearson's correlation was used to assess if there was any linear relationship between cell or grain densities and any of the continuous variables. All statistical tests were calculated with α < .05, and computed using JMP (SAS Institute Inc, Cary, NC).
3. RESULTS
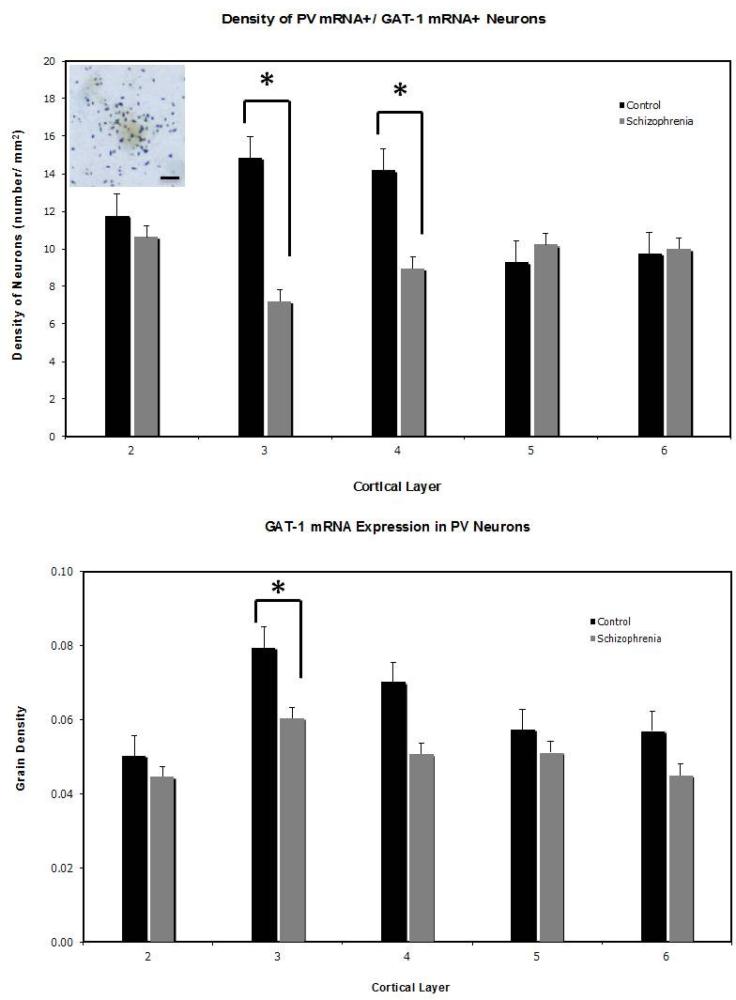
Consistent with previous observations (Hashimoto et al., 2003; Woo et al., 1997), we found that the density of cells that expressed a detectable level of PV mRNA was not altered in subjects with schizophrenia. The use of DIG, however, precludes us from addressing any possible alteration in transcript expression level per neuron. For the PV neurons that expressed GAT-1 mRNA, the effect of diagnosis was highly significant (F=20.95, P<0.0001) and this effect was layer-specific (F=5.54, P=0.02). Thus, the density of these neurons was decreased by 22% and 41% in layers 3 and 4, respectively (Figure 1). We observed a significant effect of diagnosis on the density of silver grains per neuron (F=4.49, P<0.04; Figure 1), which reflected a 26% decrease in grain density in layer 3 in the schizophrenia subjects. Hence, it appears that in the PV neurons with detectable GAT-1 mRNA, the amount of this transcript in layer 3 was decreased in the subjects with schizophrenia. Finally, we detected no statistically significant correlation between any of the potential confounding covariates and cell or grain densities. ANOVA also revealed no statistically significant interaction between sex and diagnosis.
Figure 1.
Upper panel: Mean (±SEM) density of PV+/GAT-1+ neurons is significantly decreased in layers 3 and 4 in the PFC in schizophrenia. Photomicrograph shows a double-labeled (PV+/GAT-1+) neurons. Scale bar = 10 μm. Lower panel: Mean (±SEM) density of silver grains over PV+ neurons is significantly decreased in layer 3 in the PFC in schizophrenia. Layer 1 was not included in the analyses because no PV+ neurons were found in this layer. *p<0.05, based on post-hoc group comparisons.
4. DISCUSSION
In the present study, we found that the expression of the mRNA for GAT-1 was undetectable in 22-41% of PV neurons and, among the remaining PV neurons with detectable GAT-1 mRNA, the amount of this transcript was decreased by 26%. Because basket neurons constitute the majority, possibly as much as 80-90%, of all PV neurons (Kawaguchi, 1995; Krimer et al., 2005; Markram et al., 2004; Zaitsev et al., 2004), GAT-1 mRNA reduction must occur in basket neurons in order to account for the magnitude of the reduction in neuronal densities and GAT-1 expression observed in this study. Together with the recent findings of decreased PV and GAD67 protein levels within PV basket cell axon terminals in schizophrenia (Glausier et al, 2014; Curley et al., 2011), the findings of the current study strengthen the notion that presynaptic PV basket cell neurotransmission is altered in this illness and suggest that this alteration involves the molecular dysregulation of GABA reuptake. However, the extent to which the observed decrease in mRNA expression can be translated into altered protein content is unclear.
In concert with the well-documented observation of decreased expression of the mRNA for GAD67, the finding of decreased GAT-1 mRNA expression in this study may represent a downstream consequence of some yet-to-be-defined pathophysiologic process (Curley et al., 2013). Interestingly, recent evidence suggests that oxidative stress may be a key mechanism that could contribute to PV neuronal dysfunction in schizophrenia, stemming from a combination of predisposing gene variations and environmental factors that include immune activation and stress (Behrens and Sejnowski, 2009; Bitanihirwe and Woo, 2011; Do et al., 2009; O'Donnell, 2012; O'Donnell et al., 2014). In this context, it would be of interest to investigate if the downstream consequences of oxidative stress may include decreased GAD67 and GAT-1 expression by utilizing animal models.
- Expression of GAT-1 mRNA is reduced in the DLPFC of schizophrenia subjects.
- Pre-synaptic PV neurotransmission is altered in schizophrenia.
- These findings suggest that GAT-1 reduction occurs in PV-expressing basket cells.
ACKNOWLEDGEMENTS
This study was supported by grant MH076060 from the National Institutes of Health. The authors gratefully acknowledge Dr. Clemente Garcia Rizo M.D., Ph.D. for his comments on an earlier draft of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHORS’ CONTRIBUTIONS
TUWW and BKYB performed the experiment and statistical analysis. TUWW conceptualized the study. TUWW and BKYB wrote the manuscript.
CONFLICT OF INTERESTS
The authors declare no conflict of interests.
REFERENCES
- Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr., Jones EG. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Archives of General Psychiatry. 1995;52(4):258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Nuechterlein KH, Lu PH, Gitlin M, Rogers S, Mintz J. Dysregulated brain development in adult men with schizophrenia: a magnetic resonance imaging study. Biological Psychiatry. 2003;53:412–421. doi: 10.1016/s0006-3223(02)01835-8. [DOI] [PubMed] [Google Scholar]
- Beasley CL, Reynolds GP. Parvalbumin-immunoreactive neurons are reduced in the prefrontal cortex of schizophrenics. Schizophrenia Research. 1997;24:349–355. doi: 10.1016/s0920-9964(96)00122-3. [DOI] [PubMed] [Google Scholar]
- Behrens MM, Sejnowski TJ. Does schizophrenia arise from oxidative dysregulation of parvalbumin-interneurons in the developing cortex? Neuropharmacology. 2009;57(3):193–200. doi: 10.1016/j.neuropharm.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25(1):1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Benes FM. Myelination of cortical-hippocampal relays during late adolescence. Schizophrenia Bulletin. 1989;15:585–593. doi: 10.1093/schbul/15.4.585. [DOI] [PubMed] [Google Scholar]
- Bitanihirwe BK, Lim MP, Kelley JF, Kaneko T, Woo T-UW. Glutamatergic deficits and parvalbumin-containing inhibitory neurons in the prefrontal cortex in schizophrenia. BMC Psychiatry. 2009;9:71. doi: 10.1186/1471-244X-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitanihirwe BK, Lim MP, Woo T-UW. N-methyl-D-aspartate receptor expression in parvalbumin-containing inhibitory neurons in the prefrontal cortex in bipolar disorder. Bipolar Disorders. 2010;12(1):95–101. doi: 10.1111/j.1399-5618.2009.00785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitanihirwe BK, Woo T-UW. Oxidative stress in schizophrenia: an integrated approach. Neuroscience and Biobehavioural Reviews. 2011;35(3):878–893. doi: 10.1016/j.neubiorev.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitanihirwe BK, Woo TU. Perineuronal nets and schizophrenia: The importance of neuronal nets. Neuroscience and Biobehavioral Reviews. 2014;45C:85–99. doi: 10.1016/j.neubiorev.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G. Rhythms of the brain. Oxford University Press; New York: 2006. [Google Scholar]
- Costa E, Davis JM, Dong E, Grayson DR, Guidotti A, Tremolizzo L, Veldic M. A GABAergic cortical deficit dominates schizophrenia pathophysiology. Critical Reviews in Neurobiology. 2004;16(1-2):1–23. doi: 10.1615/critrevneurobiol.v16.i12.10. [DOI] [PubMed] [Google Scholar]
- Curley AA, Arion D, Volk DW, Asafu-Adjei JK, Sampson AR, Fish KN, Lewis DA. Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: clinical, protein, and cell type-specific features. American Journal of Psychiatry. 2011;168(9):921–929. doi: 10.1176/appi.ajp.2011.11010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley AA, Eggan SM, Lazarus MS, Huang ZJ, Volk DW, Lewis DA. Role of glutamic acid decarboxylase 67 in regulating cortical parvalbumin and GABA membrane transporter 1 expression: implications for schizophrenia. Neurobiology of Disease. 2013;50:179–186. doi: 10.1016/j.nbd.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley AA, Lewis DA. Cortical basket cell dysfunction in schizophrenia. Journal of Physiol. 2012;590(Pt 4):715–724. doi: 10.1113/jphysiol.2011.224659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J, Farinas I. The pyramidal neuron of the cerebral cortex: morphological and chemical characteristics of the synaptic inputs. Progress in Neurobiology. 1992;39(6):563–607. doi: 10.1016/0301-0082(92)90015-7. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Gonzalez-Albo MC. Chandelier cell axons are immunoreactive for GAT-1 in the human neocortex. Neuroreport. 1998;9(3):467–470. doi: 10.1097/00001756-199802160-00020. [DOI] [PubMed] [Google Scholar]
- Do KQ, Cabungcal JH, Frank A, Steullet P, Cuenod M. Redox dysregulation, neurodevelopment, and schizophrenia. Current Opinion in Neurobiology. 2009;19(2):220–230. doi: 10.1016/j.conb.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? Journal of Psychiatry Research. 1982;17:319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I. Perisomatic inhibition. Neuron. 2007;56(1):33–42. doi: 10.1016/j.neuron.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, Sampson AR, Lewis DA. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. Journal of Neuroscience. 2003;23(15):6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard A, Tamas G, Soltesz I. Lighting the chandelier: new vistas for axo-axonic cells. Trends in Neuroscience. 2005;28(6):310–316. doi: 10.1016/j.tins.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Physiological subgroups of nonpyramidal cells with specific morphological characteristics in layer II/III of rat frontal cortex. Journal of Neuroscience. 1995;15(4):2638–2655. doi: 10.1523/JNEUROSCI.15-04-02638.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Anderson S, Pettegrew JW. Is schizophrenia due to excessive synaptic pruning in the prefrontal cortex? The Feinberg hypothesis revisited. Journal of Psychiatry Research. 1994;28:239–265. doi: 10.1016/0022-3956(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Konopaske GT, Sweet RA, Wu Q, Sampson A, Lewis DA. Regional specificity of chandelier neuron axon terminal alterations in schizophrenia. Neuroscience. 2006;138(1):189–196. doi: 10.1016/j.neuroscience.2005.10.070. [DOI] [PubMed] [Google Scholar]
- Krimer LS, Zaitsev AV, Czanner G, Kroner S, Gonzalez-Burgos G, Povysheva NV, Iyengar S, Barrionuevo G, Lewis DA. Cluster analysis-based physiological classification and morphological properties of inhibitory neurons in layers 2-3 of monkey dorsolateral prefrontal cortex. Journal of Neurophysiolgy. 2005;94(5):3009–3022. doi: 10.1152/jn.00156.2005. [DOI] [PubMed] [Google Scholar]
- Murray RM, Lewis SW. Res Clin., editor. Is schizophrenia a neurodevelopmental disorder? British Medical Journal. 1987;295(6600):681–2. doi: 10.1136/bmj.295.6600.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends in Neuroscience. 2012;35(1):57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nature Reviews Neuroscience. 2005;6(4):312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annual Review of Neuroscience. 2002;25:409–32. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nature Reviews Neuroscience. 2004;5(10):793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- McGlashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Archives of General Psychiatry. 2000;57(7):637–48. doi: 10.1001/archpsyc.57.7.637. [DOI] [PubMed] [Google Scholar]
- O'Donnell P. Cortical interneurons, immune factors and oxidative stress as early targets for schizophrenia. European Journal of Neuroscience. 2012;35(12):1866–70. doi: 10.1111/j.1460-9568.2012.08130.x. [DOI] [PubMed] [Google Scholar]
- O'Donnell P, Do KQ, Arango C. Oxidative/Nitrosative Stress in Psychiatric Disorders: Are We There Yet? Schizophr Bull. 2014 doi: 10.1093/schbul/sbu048. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierri JN, Chaudry AS, Woo TU, Lewis DA. Alterations in chandelier neuron axon terminals in the prefrontal cortex of schizophrenic subjects. American Journal of Psychiatry. 1999;156(11):1709–1719. doi: 10.1176/ajp.156.11.1709. [DOI] [PubMed] [Google Scholar]
- Soltesz I. Oxford University Press; New York: 2005. Diversity in the Neuronal Machine. [Google Scholar]
- Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Archives of General Psychiatry. 2000;57(3):237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- Volk DW, Pierri JN, Fritschy JM, Auh S, Sampson AR, Lewis DA. Reciprocal alterations in pre- and postsynaptic inhibitory markers at chandelier cell inputs to pyramidal neurons in schizophrenia. Cerebral Cortex. 2002;12(10):1063–1070. doi: 10.1093/cercor/12.10.1063. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Tegner J, Constantinidis C, Goldman-Rakic PS. Division of labor among distinct subtypes of inhibitory neurons in a cortical microcircuit of working memory. Proceeding in National Academy of Sciences U S A. 2004;101(5):1368–1373. doi: 10.1073/pnas.0305337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- Woo TUW, Kim AM, Viscidi E. Disease-specific alterations in glutamatergic neurotransmission on inhibitory interneurons in the prefrontal cortex in schizophrenia. Brain Research. 2008;1218:267–277. doi: 10.1016/j.brainres.2008.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo TUW, Miller JL, Lewis DA. Schizophrenia and the parvalbumin-containing class of cortical. 1997 doi: 10.1176/ajp.154.7.1013. [DOI] [PubMed] [Google Scholar]
- Woo TUW, Walsh JP, Benes FM. Density of Glutamic Acid Decarboxylase 67 Messenger RNA-Containing Neurons That Express the N-Methyl-D-Aspartate Receptor Subunit NR2A in the Anterior Cingulate Cortex in Schizophrenia and Bipolar Disorder. Archives of General Psychiatry. 2004;61(7):649–657. doi: 10.1001/archpsyc.61.7.649. [DOI] [PubMed] [Google Scholar]
- Woo TUW, Whitehead RE, Melchitzky DS, Lewis DA. A subclass of prefrontal gamma-aminobutyric acid axon terminals are selectively altered in schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(9):5341–5346. doi: 10.1073/pnas.95.9.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitsev AV, Gonzalez-Burgos G, Povysheva NV, Kroner S, Lewis DA, Krimer LS. Localization of Calcium-binding Proteins in Physiologically and Morphologically Characterized Interneurons of Monkey Dorsolateral Prefrontal Cortex. Cerebral Cortex. 2004 doi: 10.1093/cercor/bhh218. [DOI] [PubMed] [Google Scholar]