Abstract
Background
Many breast cancer patients are plagued by the disabling complication of upper limb lymphedema after axillary surgery. Conservative treatments using massage and compression therapy do not offer a lasting relief, as they fail to address the chronic transformation of edema into excess adipose tissue. Liposuction to address the adipose nature of the lymphedema has provided an opportunity for a detailed analysis of the stromal fraction of lymphedema-associated fat to clarify the molecular mechanisms for this adipogenic transformation.
Methods
Adipose-derived stem cells were harvested from human lipoaspirate of the upper extremity from age-matched patients with lymphedema (n = 3) or subcutaneous adipose tissue from control patients undergoing cosmetic procedures (n = 3). Immediately after harvest, adipose-derived stem cells were analyzed using single-cell transcriptional profiling techniques. Osteogenic, adipogenic, and vasculogenic gene expression and differentiation were assessed by quantitative real-time polymerase chain reaction and standard in vitro differentiation assays.
Results
Differential transcriptional clusters of adipose-derived stem cells were found between lymphedema and subcutaneous fat. Interestingly, lymphedema-associated stem cells had a much higher adipogenic gene expression and enhanced ability to undergo adipogenic differentiation. Conversely, they had lower vasculogenic gene expression and diminished capability to form tubules in vitro, whereas the osteogenic differentiation capacity was not significantly altered.
Conclusions
Adipose-derived stem cells from extremities affected by lymphedema appear to exhibit transcriptional profiles similar to those of abdominal adipose-derived stem cells; however, their adipogenic differentiation potential is strongly increased and their vasculogenic capacity is compromised. These results suggest that the underlying pathophysiology of lymphedema drives adipose-derived stem cells toward adipogenic differentiation.
Breast cancer remains one of the most prevalent cancers in women, with an estimated 200,000 new cases of invasive breast cancer and over 50,000 cases of in situ breast cancer expected annually.1 Despite improved early detection and evolving strategies to minimize surgical intervention for diagnosis and treatment of axillary disease associated with breast cancer, many women are still plagued by the disabling complication of upper limb lymphedema. Conservative treatment using massage and compression therapy remains the mainstay for lymphedema; however, these treatments do not offer lasting relief of the condition because they fail to address the underlying pathologic accumulation of excess adipose tissue.2 Several investigators have reported the use of liposuction to treat upper extremity lymphedema, providing an opportunity to examine whether the stromal fraction of lymphedema-associated adipose tissue differs from nonaffected subcutaneous adipose tissue.3 We hypothesize that the stromal vascular fraction of lymphedema tissue has important differences compared with that of healthy subcutaneous fat with regard to gene expression and differentiation capacity.
The complication of lymphedema develops gradually as the lymphatic vessels are unable to drain the appropriate amount of lymph and proteins. The remaining lymphatic channels become dilated and overloaded, rendering the valves incompetent.2 Eventually, the entire extremity is affected, and even the most distal vessels become dilated. Concurrently, mononuclear phagocytotic cells and mesenchymal tissue lose their ability to transport proteins, causing these to accumulate. Excess protein creates an osmotic gradient, drawing in additional fluid. Over time, the extremity enlarges and becomes painful and weak. Traditional therapeutic approaches assumed that the enlarged extremity in lymphedema was mainly the cause of excess lymph fluid, and thus noninvasive treatments were focused on compression and enhancing lymph flow. After the first operation on an affected arm, however, surgeons realized that the majority of this excess tissue was adipose tissue and not just edematous tissue.4,5 Therefore, it appears clear that lipoaspiration is needed to address the excess adipose component associated with the chronic lymphedematous arm.
Within the stromal vascular fraction of adipose tissue, scientists have identified a group of cells known as adipose-derived stromal cells. As in other mesenchymal populations, adipose-derived stem cells have the capacity to differentiate into skeletal muscle, smooth muscle, fat, cartilage, connective tissues, tendon, and bone.6–9 The adipogenic potential of adipose-derived stem cells has been the focus of many studies, and several articles have been published on the in vitro adipogenic differentiation of adipose-derived stem cells, although we are not aware of studies assessing whether differences in adipose-derived stem cells account for the increased adiposity seen in lymphedema patients.10–12 Similarly, several studies have demonstrated the vasculogenic capacity of adipose-derived stem cells; however, it is unknown whether lymphedema-derived adipose-derived stem cells differ in their vasculogenic potential.13–17 In this study, we set out to characterize functional differences in the adipose-derived stem cells from lymphedematous and healthy adipose tissue, as this might enhance our understanding of the involved molecular mechanisms and provide further insight into the underlying abnormality.
Patients and Methods
Human Stromal Vascular Fraction Harvest and Culture
All lipoaspiration specimens used in the experiments were obtained after acquiring informed consent from the patients, in accordance with Stanford University Institutional Review Board guidelines.18 Non–ultrasound-assisted tumescent (3:1) liposuction was performed using a lactated Ringer infiltration solution containing 0.1% lidocaine and 1:1,000,000 epinephrine in all cases. Stromal vascular fraction was obtained from three female patients with lymphedema of the arm and three female control patients undergoing cosmetic liposuction procedures. Patients were matched for age (45 to 61 years) and body mass index (22 to 29 kg/m2). The stromal vascular fraction was separated as described previously.18 Briefly, the lipoaspirate was digested using 0.075% collagenase A (Roche, Indianapolis, Ind.) at 37°C for 1 hour. After centrifugation, the pellet constituting the stromal vascular fraction was filtered at 100-μm pore size and resuspended in fresh media and used for subsequent analysis.
Microfluidic-Based, High-Throughput, Single-Cell Transcriptional Profiling
Single adipose-derived stem cells were sorted using the FACSAria (BD Biosciences, San Jose, Calif.) into individual wells of 96-well plates containing reverse transcriptase/polymerase master mix (Invitrogen, Carlsbad, Calif.) and TaqMan primers (Applied Biosystems, Foster City, Calif.) for each gene target. Single-cell lysates underwent target-specific 22-cycle polymerase chain reaction preamplification and subsequent cDNA was mixed with gene expression polymerase chain reaction mix (Applied Biosystems) and loaded into the 48.48 Dynamic Array Microfluidics Chip (Fluidigm, South San Francisco, Calif.). TaqMan assays for each gene were loaded into the assay wells according to the manufacturer's instructions. The chips were primed and reagents mixed in the BioMark IFC instrument and quantitative polymerase chain reaction was performed in the BioMark Reader (Fluidigm). Expression data from all chips were normalized relative to the median expression for each gene in the pooled sample and converted to base-2 logarithms. Normalized data were used to partition cells from each population into groups based on similar expression profiles using an adaptive fuzzy c-means clustering algorithm, as described previously.19 Linear discriminate analysis was applied to the gene expression data from cells in each cluster group to determine those genes whose expression patterns differ maximally between groups, and results were evaluated using traditional receiver operating characteristic curve analysis (Table 1).
Table 1. Genes with Significantly Different Expression between Lymphedema and Control, along with Bonferroni-Corrected Kolmogorov-Smirnov p Values.
| Gene Name | p |
|---|---|
| CDKN1A | 7.52e-07 |
| NFKB1 | 6.34e-06 |
| FOXO3 | 7.42e-04 |
| THY1 | 7.42e-04 |
| LPL | 1.75e-03 |
| MYC | 1.75e-03 |
| CD44 | 8.78e-03 |
| HIF1A | 3.88e-02 |
| CDKN1A | 7.52e-07 |
| NFKB1 | 6.34e-06 |
| FOXO3 | 7.42e-04 |
Osteogenic and Adipogenic Induction and Assessments
The stromal vascular fraction from lymphedema and nonlymphedema adipose tissue were seeded onto six-well plates, 100,000 cells per well, and treated with osteogenic differentiation media containing Dulbecco's Modified Eagle Medium, 10% fetal bovine serum, 100 μg/ml ascorbic acid, and 10 mM β-glycerophosphate.18 Alkaline phosphatase staining and quantification were performed at 3 days as described previously.
Adipogenic differentiation was assessed as described previously.18 Briefly, adipose-derived stem cells were grown to confluence, and growth media was replaced with adipogenic differentiation media consisting of Dulbecco's Modified Eagle Medium, 10% fetal bovine serum, 1% penicillin/streptomycin, insulin (10 μg/ml), dexamethasone (1 μM), 3-isobutyl-1-methylxanthine (0.5 mM), and indomethacin (200 μM). At 4 days, media was exchanged for Dulbecco's Modified Eagle Medium, 10% fetal bovine serum, 1% penicillin/streptomycin, and 10 μg/ml insulin. Oil red O staining and photometric quantification were performed at 1 week of differentiation. Quantification by measurement of absorbance was performed after leaching with isopropanol.
RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction
RNA was harvested after 7 days of osteogenic or adipogenic differentiation or after 12 hours of normoxia or hypoxia for vasculogenic differentiation, to examine specific gene expression as described previously by our group.18 After reverse transcription into cDNA, quantitative real-time polymerase chain reaction was carried out using the Applied Biosystems Prism 7900HT Sequence Detection System. See Table, Supplemental Digital Content 1, which lists the primers used, http://links.lww.com/PRS/A829.
Matrigel Tubule Assay
Matrigel (BD Biosciences) was thawed and placed in four-well chamber slides at 37°C for 30 minutes to allow solidification. Then, 50,000 lymphedema-associated or healthy control adi-pose-derived stem cells were seeded onto Matrigel and incubated at 37°C under 1% hypoxia for 12 hours. Tubule formation was defined as a structure exhibiting a length four times its width. Experiments were performed with n = 6. Tubule counts were determined in 10 random fields per well using an inverted Leica DMIL light microscope (Leica Microsystems GmbH, Wetzlar, Germany) at 100× magnification as described previously.15
Statistical Analysis
All statistical analyses were performed with the expert assistance of the Stanford Department of Statistics. Means and standard deviations were calculated from numerical data, as presented in figures and figure legends. In figures, bar graphs represent means, whereas error bars represent 1 SD. Statistical analyses were performed using a two-sample t test when comparing two groups. The exact statistical analysis for each data set is described in the figure legends.
Results
Lymphedema-Associated Adipose-Derived Stem Cells Have Enhanced Adipogenic Signaling and Differentiation
Increased adiposity in the upper extremity has been shown to play a role in the pathology of chronic lymphedema.2 Thus, we set out to compare the adipose-derived stem cells from lymphedema-associated as compared with control adipose tissue. When examining global gene expression of adipogenic genes after 7 days' treatment with adipogenic differentiation medium, we found a significantly higher expression in all adipogenic genes assessed, most notably in LPL and peroxisome proliferator-activated receptor gamma (Fig. 1, above, left). Next, to determine whether this increased signaling led to increased lipid formation, oil red O staining was performed after 1 week of adipogenic differentiation. The lymphedema-associated adipose-derived stem cells demonstrated a strikingly higher adipogenic potential in vitro during differentiation correlating with higher adipogenic gene signaling (Fig. 1, below). When quantifying this staining, there were also significantly higher levels in the lymphedema-associated adipose-derived stem cells (Fig. 1, above, right), suggesting that lymphedema through an unclear mechanism (e.g., increased hydrostatic pressure) drives adipose-derived stem cells toward an adipogenic phenotype. This may in part underlie the increasingly appreciated conversion of fluid to fat observed in chronic lymphedema.
Fig. 1.
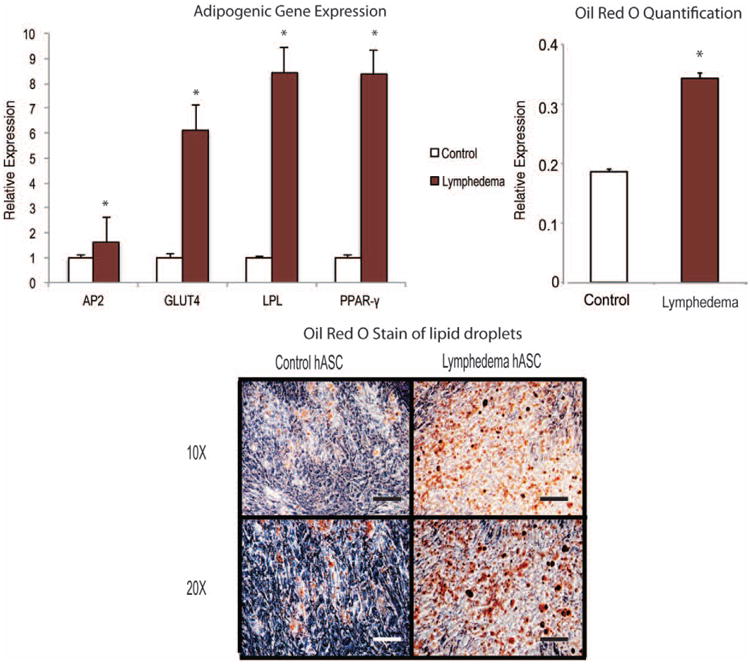
Lymphedema-associated adipose-derived stem cells demonstrate higher adipogenic signaling and differentiation compared with control adipose-derived stem cells. Gene expression analysis of adipogenic differentiation markers (above, left) and oil red O staining (below) of lymphedema-associated and control adipose-derived stem cells were performed after culturing in adipogenic differentiation medium for 7 days. (Above, right) Oil red O staining intensity was quantified after isopropanol dye extraction and photometric analysis. A two-tailed t test was used to compare groups (mean expression ± SD, *p < 0.05). hASC, human adipose-derived stromal cells.
Gene Expression Is Heterogeneous across Adipose-Derived Stem Cell Subpopulations
Using single-cell transcriptional profiling of 48 genes, we observed considerable transcriptional heterogeneity among 200 human cells from lymphedema-associated and control adipose-derived stem cells (Fig. 2). Using an information theoretic algorithm,19 both control and lymphedema-associated adipose-derived stem cells were partitioned into these four transcriptional clusters (Fig. 2). To identify those genes best able to differentiate between cells, we compared the distribution of gene expression across all three clusters using a nonparametric, two-sample, Kolmogorov-Smirnov analysis.20,21 This analysis identified nine genes (Table 1) whose distribution exhibited a statistically significant association with cluster membership (p < 0.01 following Bonferroni correction for multiple samples).22 Expression of the genes KLF4, CDKN1A, NFKB1, FOXO3, THY1, LPL, MYC, CD44, and HIF1A were the most highly correlated, with the expression of each differentially elevated in cluster 1 (i.e., expression of these genes determined to which cluster a cell would be assigned). Thus, expression levels of these genes largely determine the transcriptional profile of stromal vascular fraction cells that were assigned membership to these clusters (Fig. 2).
Fig. 2.
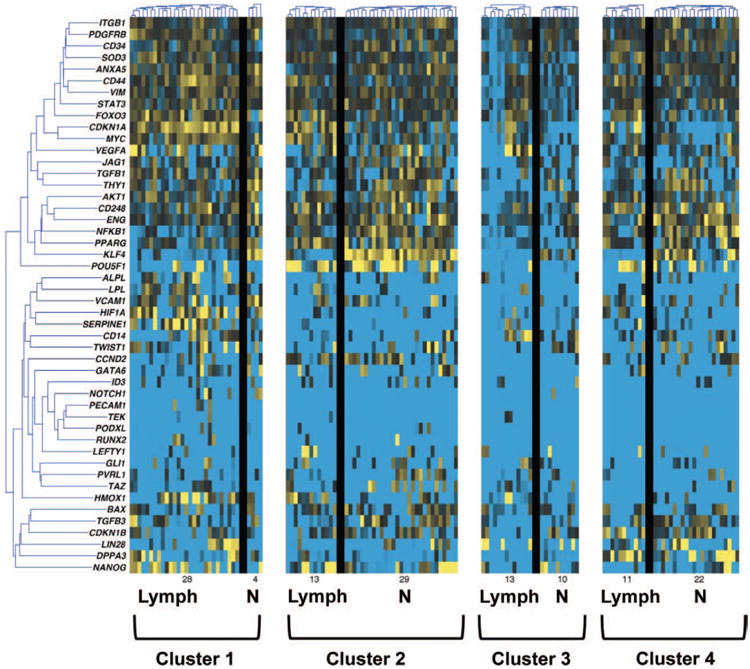
Microfluidic-based single-cell transcriptional analysis of human adipose-derived stem cells. Comparison of single-cell gene expression within human adipose-derived stem cells isolated from lymphedema-associated (Lymph) and nonaffected (N) (control) tissue. Genes are organized into rows and cells are represented in columns. Gene expression levels are shown in a color spectrum with high (yellow) and low (blue) expression. Using an information theory–based algorithm, individual cells were assigned into four distinct clusters based on common gene expression patterns. Considerable transcriptional heterogeneity is evident, and differences in cluster membership suggest meaningful differences in adipose-derived stem cell transcriptional programs.
Interestingly, KLF4 is a known stemness marker23 and appeared to have a much lower expression in the lymphedema-associated adi-pose-derived stem cells as shown in clusters 1 and 2 (Fig. 2). The proportion of cells in each of the four clusters differed widely between control and lymphedema-associated adipose-derived stem cells. Thus, significant differences appeared to exist between the adipose-derived stem cells from lymphedema-associated compared with control adipose-derived stem cells. Therefore, we set out to explore their differentiation capabilities in vitro.
Lymphedema-Associated Adipose-Derived Stem Cells Are Less Angiogenic Than Control Adipose-Derived Stem Cells In Vitro
Adipose-derived stem cells have been shown to have a vasculogenic potential in vitro and in vivo,15,24 and recent studies assessing lymphangiogenesis have suggested a role of vascular endothelial growth factor (VEGF) in lymphangio-genesis.25–27 We therefore compared angiogenic gene expression between adipose-derived stem cells harvested from lymphedema-associated stromal vascular fraction to control stromal vascular fraction. Interestingly, both VEGFA and VEGFB appeared to be more highly expressed under normoxic and hypoxic conditions in the control adi-pose-derived stem cells (Fig. 3). We observed an over twofold higher expression of both genes in control adipose-derived stem cells under hypoxic conditions.
Fig. 3.
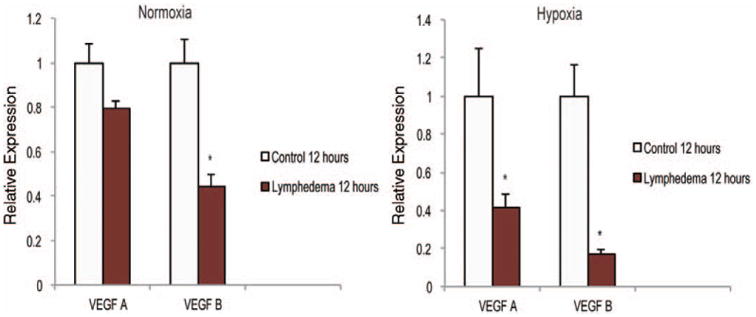
Lymphedema-associated adipose-derived stem cells are less vasculogenic than healthy control adipose-derived stem cells. Gene expression analysis of angiogenic differentiation markers in adipose-derived stem cells from lymphedema-associated or control tissue cultured under (left) normoxia or (right) hypoxia (1% oxygen) for 12 hours. Markers examined include angiogenic specific genes VEGFA and VEGFB. The two-tailed t test was used to compare groups (mean expression ± SD, *p < 0.05).
To demonstrate that this angiogenic gene expression correlated with physiologic function, we next performed a Matrigel tubule formation assay. After 12 hours in hypoxia (1% oxygen), adi-pose-derived stem cells from control tissues formed tubules, whereas there was a paucity of tubules in the lymphedema-associated adipose-derived stem cells. (Fig. 4, above, left). Tubule quantification demonstrated significantly more tubules in the control adipose-derived stem cell group (Fig. 4, above, right). We next set out to investigate the role of other factors involved in lymphangiogenesis. We analyzed the SLP-76 and Syk pathways, which are known to play a role in cell fate to either lymph vessels or blood vessels28,29 and found a trend toward higher protein expression of SLP-76 and Syk in lymphedema-associated adipose-derived stem cells (Fig. 4, center and below).
Fig. 4.
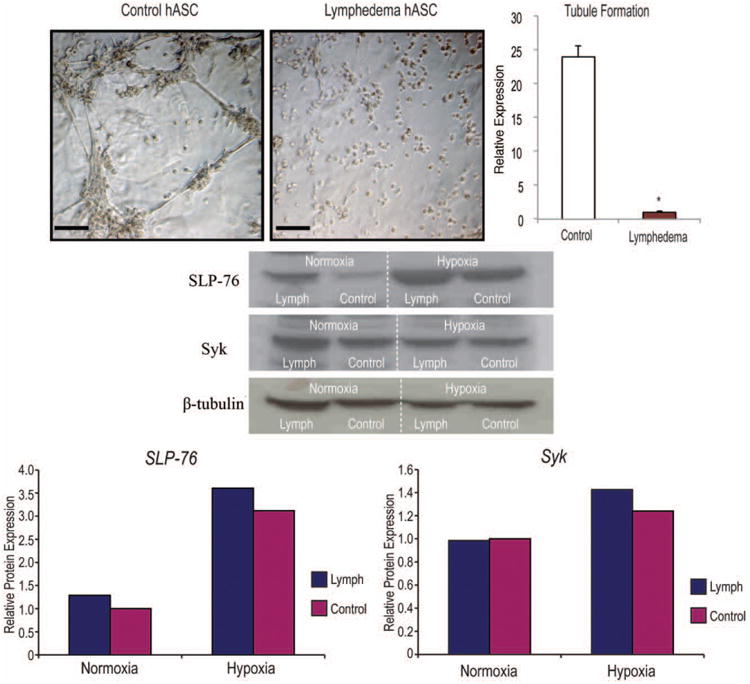
Lymphedema-associated adipose-derived stem cells are less vasculogenic than healthy control adipose-derived stem cells. (Above, left) In vitro Matrigel tubule formation assay of lymphedema-associated and control adipose-derived stem cells after 12 hours of hypoxia (1% oxygen). (Above, right) Tubule formation was quantified and counted by three blinded, independent observers. (Center) Western blot analysis of lymphedema-associated and control adipose-derived stem cells cultured either under normoxic or hypoxic (1% oxygen) conditions for 24 hours (Below). SLP-76 and Syk were analyzed and normalized to β-tubulin. Two-tailed t test was used to compare groups (mean expression ± SD, *p < 0.05). hASC, human adipose-derived stromal cells. Quantification was performed using densitometry and ImageJ (National Institutes of Health, Bethesda, Md.).
Osteogenic Differentiation Is Similar in Adipose-Derived Stem Cells from Lymphedema-Associated and Control Stromal Vascular Fraction
Adipose-derived stromal cells have been shown to be a highly osteogenic cell population both in vitro and in vivo.18,30,31 We therefore aimed to assess the effect of chronic lymphedema on the osteogenic differentiation potential of adipose-derived stem cells by comparing lymphedema-associated and control adipose-derived stem cells maintained in osteogenic differentiation media for 3 days. Gene expression analysis of early osteogenic markers alkaline phosphatase, runt-related transcription factor 2, and also the late differentiation marker osteocalcin showed no statistically significant differences; however, a slight trend toward increased expression could be observed (Fig. 5, left). Similarly, significant differences in alkaline phosphatase staining were not detected (Fig. 5, right), indicating that chronic lymphedema does not seem to affect osteogenic differentiation.
Fig. 5.
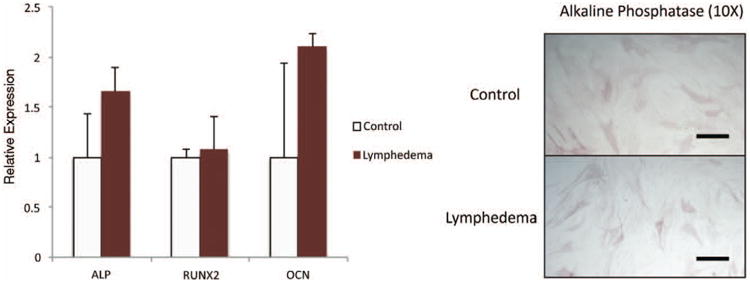
Osteogenic differentiation is not significantly affected in lymphedema-associated adipose-derived stem cells. (Left) Osteogenic gene expression analysis of lymphedema and control adipose-derived stem cells cultured in osteogenic differentiation medium for 7 days. Specific genes examined included ALP, RUNX-2, and OCN. (Right) Alkaline phosphatase staining of lymphedema and control adipose-derived stem cells after 3 days in osteogenic differentiation medium. The two-tailed t test was used to compare groups (mean expression ± SD, p < 0.05).
Discussion
Most cases of breast cancer are treated surgically with tumor extirpation followed by sentinel lymph node dissection and axillary lymph node dissection if needed. In addition, many patients will require postoperative irradiation and chemo-therapy. One of the most prevalent complications following surgery of the axillary lymph nodes is chronic upper extremity lymphedema. Early studies estimated the incidence of lymphedema as ranging from 8 to 80 percent, depending on whether the patient also underwent postoperative irradiation.32,33 More recent studies have demonstrated a significant decrease in lymphedema because of the increasing use of sentinel lymph node biopsies. A more recent study found that 5 percent of patients after sentinel lymph node biopsy alone had postoperative lymphedema compared with 16 percent of women who had biopsies followed by axillary lymph node dissection.34 Thus, lymphedema still represents a debilitating and challenging complication after axillary surgery.
There has been improved understanding of the pathology of chronic upper extremity lymphedema with improved imaging modalities and surgical approaches, as it is now evident that excess adipose tissue is a large component of the tissue seen in a chronic lymphedematous arm. A study by Brorson et al. analyzing the chronic lymphedema-affected arm demonstrated a 73 percent increase in adipose tissue compared with the unaffected arm.35 As the underlying pathology of this chronic condition is excess adipose tissue, surgeons have begun to use a methodology applied in other cases of localized excess adipose tissue, namely, liposuction.3,5,36,37 However, the actual cause of this increased adiposity remains unclear. Recent studies using a novel murine model of lymphedema have implicated lymphatic fluid stasis and inflammation in the pathophysiology of adipogenesis.38,39 Similarly, abnormalities in the Prox1 pathway, which is critical for lymphatic vasculature, have been associated with increased adipose tissue accumulation and adult-onset obesity.40
Although several groups have analyzed the adipose tissue excised from chronic lymphedema, there are limited studies investigating the role of adipose-derived stem cells in the pathogenesis of chronic lymphedema and adiposity. We therefore compared the differentiation capacity of lymphedema-associated and control adipose-derived stem cells isolated from the stromal vascular fraction. Interestingly, we found that although osteogenic differentiation was largely unaffected, adipogenic differentiation was significantly increased in lymphedema-associated adipose-derived stem cells, suggesting that lymph stasis may be a critical stimulus for this process. Mesenchymal stem cells have been shown to promote neovascularization through multiple mechanisms, including direct differentiation into endothelial cells and paracrine growth factor secretion.15,24 Interestingly, the growth of lymphatic vessels (lymphangiogenesis), which is essential for tissue homeostasis, and which may be insufficient in patients suffering from chronic lymphedema, has been shown to be directed by similar molecular mechanisms. We therefore analyzed the angiogenic capacity of lymphedema-associated adipose-derived stem cells. Strikingly, both Matrigel tubule formation and angiogenic growth factor expression were severely impaired in lymphedema-associated adipose-derived stem cells, indicating a deficiency in this process. Interestingly, the expression of SLP-76 and Syk, which are known to play a role in lymphangiogenesis, were slightly up-regulated in lymphedema-associated adipose-derived stem cells. This is consistent with previous studies that indicated Syk signaling is crucial for direct differential homing and interactions between hematopoietic cells and evolving lymphatic and blood vessels28,29 and may suggest activation of compensatory mechanisms in the progression of lymphedema.
To obtain a more comprehensive understanding of the molecular mechanisms underlying adipose-derived stem cell dysfunction in lymphedema, we performed a single-cell transcriptional analysis of several hundred cells across 48 selected gene targets related to differentiation, angiogenesis, and stemness. We previously used this technology to identify a subpopulation of human adipose-derived stem cells with a transcriptional signature for osteogenic differentiation.41 We observed significant heterogeneity in the transcriptional signatures between lymphedema-associated and control adipose-derived stem cells. These differences were further illustrated in the differential composition of cell clusters, suggesting that lymphedema has a substantial effect on multiple pathways governing adipose-derived stem cell biology, which may explain the differences observed in our functional analyses.
Conclusions
In this study, we assessed the adipose-derived stem cell population of the lymph tissue using single-cell technology and adipogenic, vasculogenic, and osteogenic differentiation assays to compare adipose-derived stem cells from lymphedema-associated and control subcutaneous adipose tissue. Our findings indicate significant functional differences between lymphedema-associated and healthy adipose-derived stem cells, suggesting that the underlying pathophysiology drives these cells toward adipogenic differentiation, which may explain the adipose tissue accumulation widely observed in chronic lymphedema. Enrollment of more patients and further studies characterizing the pathways underlying these differences will be necessary to gain a greater understanding of changes that occur during the transition from acute to chronic lymphedema and enable better treatment of this complication.
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health, National Institute of Dental and Craniofacial Research grants 1 R21 DE019274-01 and 1 RC2 DE020771-01; the National Endowment for Plastic Surgery and the Oak Foundation and Hagey Laboratory for Pediatric Regenerative Medicine (to M.T.L.); National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grant 2 RO1 DK074095-07 (to G.C.G); National Institutes of Health, National Institute of Arthritis and Musculo-skeletal and Skin Diseases grant 1F32AR057302-02 (to B.L.); and National Institutes of Health National Research Service Award F32DK088448-01 (to J.P.G.).
Footnotes
Disclosure: None of the authors has a financial interest in any of the products or devices mentioned in this article.
Supplemental digital content is available for this article. A direct URL citation appears in the text; simply type the URL address into any Web browser to access this content. A clickable link to the material is provided in the HTML text of this article on the Journal's Web site (www.PRSJournal.com).
References
- 1.American Society of Cancer. [Accessed April 18, 2013];Cancer facts & figures 2010. Available at: http://www.cancer.org/research/cancer-factsstatistics/cancerfactsfigures2010/index.
- 2.Brorson H, Ohlin K, Olsson G, Karlsson MK. Breast cancer-related chronic arm lymphedema is associated with excess adipose and muscle tissue. Lymphat Res Biol. 2009;7:3–10. doi: 10.1089/lrb.2008.1022. [DOI] [PubMed] [Google Scholar]
- 3.Brorson H. Liposuction in arm lymphedema treatment. Scand J Surg. 2003;92:287–295. doi: 10.1177/145749690309200409. [DOI] [PubMed] [Google Scholar]
- 4.Brorson H, Svensson H. Complete reduction of lymphoedema of the arm by liposuction after breast cancer. Scand J Plast Reconstr Surg Hand Surg. 1997;31:137–143. doi: 10.3109/02844319709085480. [DOI] [PubMed] [Google Scholar]
- 5.Brorson H, Svensson H. Liposuction combined with controlled compression therapy reduces arm lymphedema more effectively than controlled compression therapy alone. Plast Reconstr Surg. 1998;102:1058–1067. discussion 1068. [PubMed] [Google Scholar]
- 6.De Ugarte DA, Alfonso Z, Zuk PA, et al. Differential expression of stem cell mobilization-associated molecules on multi-lineage cells from adipose tissue and bone marrow. Immunol Lett. 2003;89:267–270. doi: 10.1016/s0165-2478(03)00108-1. [DOI] [PubMed] [Google Scholar]
- 7.De Ugarte DA, Morizono K, Elbarbary A, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101–109. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- 8.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 10.Jääger K, Neuman T. Human dermal fibroblasts exhibit delayed adipogenic differentiation compared with mesenchymal stem cells. Stem Cells Dev. 2011;20:1327–1336. doi: 10.1089/scd.2010.0258. [DOI] [PubMed] [Google Scholar]
- 11.Kim MH, Park JS, Seo MS, Jung JW, Lee YS, Kang KS. Genistein and daidzein repress adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells via Wnt/β-catenin signalling or lipolysis. Cell Prolif. 2010;43:594–605. doi: 10.1111/j.1365-2184.2010.00709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.James AW, Leucht P, Levi B, et al. Sonic Hedgehog influences the balance of osteogenesis and adipogenesis in mouse adipose-derived stromal cells. Tissue Eng Part A. 2010;16:2605–2616. doi: 10.1089/ten.tea.2010.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rasmussen JG, Frøbert O, Pilgaard L, et al. Prolonged hypoxic culture and trypsinization increase the pro-angiogenic potential of human adipose tissue-derived stem cells. Cytotherapy. 2011;13:318–328. doi: 10.3109/14653249.2010.506505. [DOI] [PubMed] [Google Scholar]
- 14.Murohara T. Autologous adipose tissue as a new source of progenitor cells for therapeutic angiogenesis. J Cardiol. 2009;53:155–163. doi: 10.1016/j.jjcc.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Thangarajah H, Vial IN, Chang E, et al. IFATS collection: Adipose stromal cells adopt a proangiogenic phenotype under the influence of hypoxia. Stem Cells. 2009;27:266–274. doi: 10.1634/stemcells.2008-0276. [DOI] [PubMed] [Google Scholar]
- 16.Verseijden F, Jahr H, Posthumus-van Sluijs SJ, et al. Angiogenic capacity of human adipose-derived stromal cells during adipogenic differentiation: An in vitro study. Tissue Eng Part A. 2009;15:445–452. doi: 10.1089/ten.tea.2007.0429. [DOI] [PubMed] [Google Scholar]
- 17.Planat-Benard V, Silvestre JS, Cousin B, et al. Plasticity of human adipose lineage cells toward endothelial cells: Physiological and therapeutic perspectives. Circulation. 2004;109:656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 18.Levi B, James AW, Glotzbach JP, Wan DC, Commons GW, Longaker MT. Depot-specific variation in the osteogenic and adipogenic potential of human adipose-derived stromal cells. Plast Reconstr Surg. 2010;126:822–834. doi: 10.1097/PRS.0b013e3181e5f892. [DOI] [PubMed] [Google Scholar]
- 19.Glotzbach JP, Januszyk M, Vial IN, et al. An information theoretic, microfluidic-based single cell analysis permits identification of subpopulations among putatively homogeneous stem cells. PLoS One. 2011;6:e21211. doi: 10.1371/journal.pone.0021211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ong LD, LeClare PC. The Kolmogorov-Smirnov test for the log-normality of sample cumulative frequency distributions. Health Phys. 1968;14:376. [PubMed] [Google Scholar]
- 21.Eadie WR, Drijard F. Statistical Methods in Experimental Physics. Amsterdam: North-Holland; 1971. [Google Scholar]
- 22.Hammer D, Romashchenko A, Shen A, Vereshchagin N. Inequalities for Shannon entropy and Kolmogorov complexity. J Comput Syst Sci. 2000;60:442–464. [Google Scholar]
- 23.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 24.Behr B, Tang C, Germann G, Longaker MT, Quarto N. Locally applied vascular endothelial growth factor A increases the osteogenic healing capacity of human adipose-derived stem cells by promoting osteogenic and endothelial differentiation. Stem Cells. 2011;29:286–296. doi: 10.1002/stem.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.An A, Rockson SG. The potential for molecular treatment strategies in lymphatic disease. Lymphat Res Biol. 2004;2:173–181. doi: 10.1089/lrb.2004.2.173. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura K, Rockson SG. Molecular targets for therapeutic lymphangiogenesis in lymphatic dysfunction and disease. Lymphat Res Biol. 2008;6:181–189. doi: 10.1089/lrb.2008.63404. [DOI] [PubMed] [Google Scholar]
- 27.Cheung L, Han J, Beilhack A, et al. An experimental model for the study of lymphedema and its response to therapeutic lymphangiogenesis. BioDrugs. 2006;20:363–370. doi: 10.2165/00063030-200620060-00007. [DOI] [PubMed] [Google Scholar]
- 28.Sebzda E, Hibbard C, Sweeney S, et al. Syk and Slp-76 mutant mice reveal a cell-autonomous hematopoietic cell contribution to vascular development. Dev Cell. 2006;11:349–361. doi: 10.1016/j.devcel.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Abtahian F, Guerriero A, Sebzda E, et al. Regulation of blood and lymphatic vascular separation by signaling proteins SLP-76 and Syk. Science. 2003;299:247–251. doi: 10.1126/science.1079477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levi B, James AW, Nelson ER, et al. Acute skeletal injury is necessary for human adipose-derived stromal cell-mediated calvarial regeneration. Plast Reconstr Surg. 2011;127:1118–1129. doi: 10.1097/PRS.0b013e318205f274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levi B, James AW, Nelson ER, et al. Human adipose derived stromal cells heal critical size mouse calvarial defects. PLoS One. 2010;5:e11177. doi: 10.1371/journal.pone.0011177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kissin MW, Querci della Rovere G, Easton D, Westbury G. Risk of lymphoedema following the treatment of breast cancer. Br J Surg. 1986;73:580–584. doi: 10.1002/bjs.1800730723. [DOI] [PubMed] [Google Scholar]
- 33.Segerström K, Bjerle P, Graffman S, Nyström A. Factors that influence the incidence of brachial oedema after treatment of breast cancer. Scand J Plast Reconstr Surg Hand Surg. 1992;26:223–227. doi: 10.3109/02844319209016016. [DOI] [PubMed] [Google Scholar]
- 34.McLaughlin SA, Wright MJ, Morris KT, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: Objective measurements. J Clin Oncol. 2008;26:5213–5219. doi: 10.1200/JCO.2008.16.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brorson H, Ohlin K, Olsson G, Nilsson M. Adipose tissue dominates chronic arm lymphedema following breast cancer: An analysis using volume rendered CT images. Lymphat Res Biol. 2006;4:199–210. doi: 10.1089/lrb.2006.4404. [DOI] [PubMed] [Google Scholar]
- 36.Brorson H. Liposuction gives complete reduction of chronic large arm lymphedema after breast cancer. Acta Oncol. 2000;39:407–420. doi: 10.1080/028418600750013195. [DOI] [PubMed] [Google Scholar]
- 37.Qi F, Gu J, Shi Y, Yang Y. Treatment of upper limb lymphedema with combination of liposuction, myocutaneous flap transfer, and lymph-fascia grafting: A preliminary study. Microsurgery. 2009;29:29–34. doi: 10.1002/micr.20567. [DOI] [PubMed] [Google Scholar]
- 38.Zampell JC, Aschen S, Weitman ES, et al. Regulation of adipogenesis by lymphatic fluid stasis: Part I. Adipogenesis, fibrosis, and inflammation. Plast Reconstr Surg. 2012;129:825–834. doi: 10.1097/PRS.0b013e3182450b2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aschen S, Zampell JC, Elhadad S, Weitman E, De Brot M, Mehrara BJ. Regulation of adipogenesis by lymphatic fluid stasis: Part II. Expression of adipose differentiation genes. Plast Reconstr Surg. 2012;129:838–847. doi: 10.1097/PRS.0b013e3182450b47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harvey NL, Srinivasan RS, Dillard ME, et al. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat Genet. 2005;37:1072–1081. doi: 10.1038/ng1642. [DOI] [PubMed] [Google Scholar]
- 41.Levi B, Wan DC, Glotzbach JP, et al. CD105 protein depletion enhances human adipose-derived stromal cell osteogenesis through reduction of transforming growth factor β1 (TGF-β1) signaling. J Biol Chem. 2011;286:39497–39509. doi: 10.1074/jbc.M111.256529. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


