Abstract
Background
We conducted a cross-sectional study of primary total joint replacement (TJR) patients to determine predictors for prolonged length of stay (LOS) in hospital to identify patient characteristics that may inform resource allocation, accounting for patient complexity.
Methods
Preoperative demographics, medical comorbidities and acute hospital LOS from a consecutive series of primary TJR patients from an academic arthroplasty centre were abstracted. We categorized patients as LOS of 3 or fewer days, 4 days, or 5 or more days to align results with varying LOS benchmarks. To identify predictors for LOS, we used a generalized logistic regression model fitted on an LOS ternary outcome, using LOS of 3 or fewer days as a reference category.
Results
The sample included 1459 patients: 61.7% total knee and 38.3% total hip. Male sex was predictive of an LOS of 3 or fewer days (4 d: odds ratio [OR] 0.48, 95% confidence interval [CI] 0.364–0.631; ≥ 5 d: OR 0.57, 95% CI 0.435–0.758), as was current smoking status (4 d: OR 0.425, 95% CI 0.274–0.659; ≥ 5 d: OR 0.489, 95% CI 0.314–0.762). Strong predictors of prolonged LOS included total hip versus total knee arthroplasty, age 75 years or older, American Society of Anesthesiologists classification of 3 and 4 and number of cardiovascular comorbidities.
Conclusion
Not all patients undergoing TJR are equal. The goal should be individual patient-focused care rather than a predetermined LOS that is not achievable for all patients. Hospital resource planning must account for patient complexity when planning future bed management.
Abstract
Contexte
Nous avons réalisé une étude transversale auprès de patients soumis à une chirurgie pour prothèse articulaire totale (PAT) afin de déterminer les facteurs prédictifs d’une durée du séjour hospitalier (DSH) prolongée (en établissement de soins de courte durée) et de dégager les caractéristiques des patients qui permettraient d’orienter l’allocation des ressources en tenant compte de la complexité des cas.
Méthodes
Nous avons extrait les données démographiques préopératoires, les comorbidités médicales et la DSH pour une série de cas consécutifs de PAT primaire dans un centre d’arthroplastie universitaire. Nous avons classé les patients par catégorie de DSH, soit 3 jours ou moins, 4 jours, ou 5 jours et plus, de manière à répartir les résultats selon les diverses cibles de DSH. Pour dégager les facteurs prédictifs de la DSH, nous avons utilisé un modèle de régression logistique généralisé intégré à un paramètre ternaire de DSH, en utilisant la DSH de 3 jours ou moins comme catégorie de référence.
Résultats
L’échantillon regroupait 1459 patients : 61,7 % recevant une prothèse totale du genou (PTG) et 38,3 % recevant une prothèse totale de la hanche (PTH). Le fait d’être de sexe masculin était prédictif d’une DSH de 3 jours ou moins (4 j : rapport des cotes [RC] 0,48, intervalle de confiance [IC] à 95 % 0,364–0,631; ≥ 5 j : RC 0,57, IC à 95 % 0,435–0,758), tout comme le statut à l’égard du tabagisme (4 j : RC 0,425, IC à 95 % 0,274–0,659; ≥ 5 j : RC 0,489, IC à 95 % 0,314–0,762). Les facteurs prédictifs fiables d’une DSH prolongée incluaient la PTH c. PTG, l’âge de 75 ans ou plus, une classification de 3 ou 4 selon l’American Society of Anesthesiologists et le nombre de comorbidités cardiovasculaires.
Conclusion
Les patients soumis à une PAT ne s’équivalent pas tous. L’objectif devrait être d’administrer des soins centrés sur le patient plutôt que sur une DSH prédéterminée qui, dans les faits, ne s’applique pas à tous patients. La planification des ressources hospitalières devra à l’avenir tenir compte de la complexité des cas dans la planification de la gestion des lits.
The demand for total joint replacement (TJR) surgery is steadily increasing.1 In 2010–11, a total of 93 446 hip and knee replacements were performed in Canada, representing a 10-year increase of 118% (from 42 917 in 2000–01).2,3 Despite ample support for TJR being a cost-effective intervention,4–6 the large burden of care and the measurable costs associated with joint replacement surgery has led payers (Medicare and Medicaid in the United States) to target this procedure for cost containment.7 Canadian government health care agencies have targeted this surgery as one of the first to be funded outside the global hospital budget as a quality-based procedure (QBP).8
Owing to the recently reduced funding allocation per total joint procedure in Ontario, Canada, administrators and physicians have had to explore creative ways to continue to provide improved patient care at a lower cost. Standardization of materials and implants has been one method of reducing cost that many centres have used. Reducing length of stay (LOS) in hospital is the other primary method being targeted to reduce costs. The current benchmark in Ontario is a mean LOS of 4 days, and some centres have reduced the LOS to 2–3 days. The TJR population typically represents an aged population with multiple comorbidities and sometimes limited social supports. Having multiple comorbidities has been linked to a longer LOS and higher in-hospital costs.9 The current funding model for joint replacement in our region and others does not account for variation in patient complexity.10,11 Moreover, the current literature is scant with regard to risk stratification and risk reduction strategies in the TJR population.12
The purpose of this cross-sectional study was to determine predictors for prolonged LOS to identify patient characteristics that may inform resource allocation, specifically in terms of accounting for patient complexity.
Methods
We conducted a cross-sectional study of a consecutive series of primary TJR patients to determine patient demographic and comorbidity profiles predictive of acute LOS. The study sample comprised all patients who underwent elective primary total hip or knee surgery and attended the preoperative clinic for medical history and assessment by the anesthesiology department between Apr. 1, 2011, and Mar. 31, 2012. All surgeries were performed among 10 orthopedic surgeons at 1 high-volume academic arthroplasty centre in Ontario, Canada. Patients with an incomplete or nonretrievable preoperative anesthetic record or who underwent nonelective or revision TJR were not considered for inclusion.
Once Research Ethics Board approval was obtained, we retrieved preoperative anesthetic records from the patient medical record for all primary TJR cases performed in fiscal year 2011–12. Data abstracted included operative joint and patient demographic characteristics (i.e., sex, age and body mass index [BMI]). We further classified BMI according to the Canadian Weight Classification System, which categorizes BMI ranges associated with health risk.13 The American Society of Anesthesiologists (ASA) classification rating of physical status,14 as a measure of preoperative comorbidity determined by the attending anesthesiologist, was also captured. We abstracted individual preoperative comorbidities as per the following review of body systems: cardiovascular; respiratory; endocrine; neurologic; renal; gastrointestinal; hematologic; other musculoskeletal conditions, including chronic pain syndrome; and psychiatric diagnoses and substance abuse, as assessed by the anesthesiologist at the preoperative visit approximately 2–3 weeks before surgery. Existing comorbidities were also summed to provide the number of comorbidities per system and the total number overall.
Additional variables included patient receipt of workplace safety insurance board (WSIB) benefits for associated hip/knee injury as well as the type and extent of social support and living environment postdischarge, as determined by the intake physiotherapist/occupational therapist on admission to the acute orthopedic ward. In-hospital data included acute LOS; admission to a special care unit; and discharge disposition, including discharge to a rehabilitation unit.
Statistical analysis
Statistical analysis included descriptive statistics and regression modelling. In the descriptive analysis, we summarized categorical variables as counts and proportions, whereas normally distributed continuous variables were summarized as means ± standard deviations. Where the normality assumptions were violated, we report medians and interquartile ranges (IQR). We categorized LOS as 3 or fewer days, 4 days, or 5 or more days to align results with varying LOS benchmarks. To identify predictors for LOS, we used a generalized logistic regression model fitted on an LOS ternary outcome, using an LOS of 3 or fewer days as a reference category. We systematically evaluated 102 preoperative covariates in the model using a stepwise selection procedure (see the Appendix, available at canjsurg.ca). The selection level of entry (or retaining) in the model was set at p ≤ 0.2. We used a receiver operating characteristic (ROC) curve to assess the classification power of the model. An area under the ROC curve (AUC) equal to or exceeding a value of 0.60 was considered adequate for classifying LOS patients. We considered results to be significant at p < 0.05. Odds ratios (OR) and 95% confidence intervals (CI) for the final model are reported. Also reported are the predictive equations for LOS derived from the final logistic model. Data were analyzed using SAS software version 9.3 (SAS institute), R software version 3.0.3 (R Development Core Team) and SPSS version 20 (IBM Corp.).
Results
A total of 1541 patients who underwent elective primary unilateral TJR in fiscal year 2011–12 were identified. Of these, 82 (5.3%) patients had an incomplete or nonretrievable preoperative anesthetic record and were thus excluded from the analysis. The final study sample included 1459 patients of whom 900 (61.7%) underwent primary total knee (TKA) and 559 (38.3%) underwent primary total hip arthroplasty (THA). The study cohort comprised 57.6% female and 42.4% male patients. The median age of patients was 67 (IQR 52–82) years, with 390 (26.7%) patients aged 75 years or older. The median BMI at the time of preoperative assessment was 30.4 (IQR 22.4–38.4); 778 (53.3%) patients were classified as obese, presenting with a BMI of 30 or higher.
Preoperative demographic characteristics and medical comorbidities are outlined in Table 1. Patients presented with a median of 3 (IQR 0–6) comorbidities; 62% of patients had an ASA classification of 3. The median acute LOS was 4 (IQR 2–6) days for TJR, and likewise for both THA and TKA. Overall, 512 (35.1%) patients had an LOS of 3 or fewer days, 431 (29.5%) had an LOS of 4 days, and 516 (35.4%) had an LOS of 5 or more days. Of the patients with an LOS of 5 or more days, 44% were aged 75 years or older.
Table 1.
Preoperative demographics and comorbidities stratified by acute length of stay in hospital
| Variables | Group, no. (%) or median [IQR] | |||
|---|---|---|---|---|
| All cases (n = 1459) | ≤ 3 d (n = 512) | 4 d (n = 431) | ≥ 5 d (n = 516) | |
| Joint replacement | ||||
| Total hip replacement | 559 (38.3) | 146 (28.5) | 174 (40.4) | 239 (46.3) |
| Total knee replacement | 900 (61.7) | 366 (71.5) | 257 (59.6) | 277 (53.7) |
| Sex | ||||
| Male | 618 (42.4) | 262 (51.2) | 151 (35) | 205 (39.7) |
| Female | 841 (57.6) | 250 (48.8) | 280 (65) | 311 (60.3) |
| Age, yr | 67 [52–82] | 63 [50–76] | 67 [53–81] | 73 [59–87] |
| Male | 66 [50–81] | 62 [49–75] | 66 [51–81] | 73 [61–85] |
| Female | 68 [53–83] | 64 [50–78] | 68 [54–82] | 73 [58–88] |
| Age ≥ 75 yr | 390 (26.7) | 65 (12.7) | 98 (22.7) | 227 (44) |
| BMI | 30.4 [22.4–38.4] | 30.7 [22.3–39.1] | 30.7 [22.6–38.8] | 30 [22–38] |
| Male | 30.1 [23.6–36.6] | 30.3 [23.7–36.9] | 30.2 [23.4–37] | 29.8 [23.4–36.2] |
| Female | 30.8 [21.2–40.4] | 31.6 [21.1–42.1] | 31 [21.8–40.2] | 30.1 [20.6–39.6] |
| BMI class | ||||
| Underweight (< 18.5) | 9 (0.6) | 6 (1.2) | 2 (0.5) | 1 (0.2) |
| Normal (18.5–24.9) | 191 (13.1) | 63 (12.3) | 46 (10.7) | 82 (15.9) |
| Overweight (25.0–29.9) | 481 (33.0) | 158 (30.9) | 146 (33.9) | 177 (34.3) |
| Obese ( ≥ 30.0) | 778 (53.3) | 285 (55.7) | 237 (55.0) | 256 (49.6) |
| Obesity class | ||||
| I (BMI 30.0–34.9) | 417 (28.6) | 150 (29.3) | 126 (29.2) | 417 (28.6) |
| II (BMI 35.0–39.9) | 205 (14.1) | 79 (15.4) | 56 (13.0) | 70 (13.6) |
| III (BMI ≥ 40.0) | 156 (10.7) | 56 (10.9) | 55 (12.8) | 45 (8.7) |
| Primary TJR diagnosis | ||||
| Osteoarthritis | 1418 (97.2) | 500 (97.7) | 414 (96.1) | 504 (97.7) |
| Rheumatoid arthritis | 37 (2.5) | 10 (2.0) | 15 (3.5) | 12 (2.3) |
| Avascular necrosis | 4 (0.3) | 2 (0.4) | 2 (0.5) | 0 (0) |
| ASA classification | ||||
| 1 | 11 (0.8) | 7 (1.4) | 4 (0.9) | 0 (0) |
| 2 | 344 (23.6) | 163 (31.8) | 103 (23.9) | 78 (15.1) |
| 3 | 905 (62) | 303 (59.2) | 276 (64.0) | 326 (63.2) |
| 4 | 199 (13.6) | 39 (7.6) | 48 (11.1) | 112 (21.7) |
| Total no. comorbidities | 3 [0–6] | 3 [0–6] | 3 [0–6] | 3 [0–6] |
| No. patients with comorbidities | ||||
| Cardiovascular diagnosis | 971 (66.6) | 291 (56.8) | 292 (67.7) | 388 (75.2) |
| Respiratory diagnosis | 472 (32.4) | 183 (35.7) | 126 (29.2) | 163 (31.6) |
| Endocrine diagnosis | 431 (29.5) | 125 (24.4) | 136 (31.6) | 170 (32.9) |
| Neurologic diagnosis | 109 (7.5) | 19 (3.7) | 39 (9.0) | 51 (9.9) |
| Renal diagnosis | 44 (3.0) | 4 (0.8) | 8 (1.9) | 32 (6.2) |
| Gastrointestinal diagnosis | 472 (32.4) | 152 (29.7) | 155 (36.0) | 165 (32.0) |
| Hematologic diagnosis | 93 (6.4) | 21 (4.1) | 25 (5.8) | 47 (9.1) |
| Other MSK diagnosis | 213 (14.6) | 67 (13.1) | 59 (13.7) | 87 (16.9) |
| Psychiatric/substance abuse | 312 (21.4) | 130 (25.4) | 81 (18.8) | 101 (19.5) |
| Other — HIV or hepatitis | 9 (0.6) | 5 (1.0) | 1 (0.2) | 3 (0.6) |
ASA = American Society of Anesthesiologists; BMI = body mass index; HIV = human immunodeficiency virus; IQR = interquartile range; MSK = musculoskeletal; TJR = total joint replacement.
During the hospital stay 159 (11%) patients were admitted to a special care unit (intensive care or critical care) for postoperative cardiac monitoring or management of an event; 1326 (90.9%) were discharged home or to a respite facility and 133 (9.1%) were discharged to a continuing care rehabilitation unit.
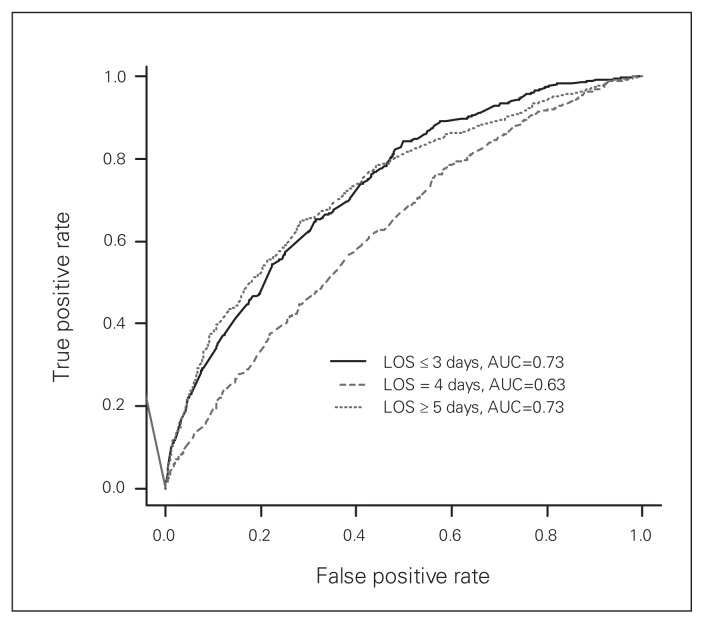
As per the stepwise procedure in the generalized logistic regression model, the following covariates were selected as predictors for LOS: sex, operative joint, age 75 years or older, ASA classification of 3 or 4, number of cardiovascular comorbidities, number of renal comorbidities, diabetes treated orally or with insulin, previous stroke or transient ischemic attack (TIA), other musculoskeletal diagnoses and current smoking status (Table 2). Male sex was predictive of an acute LOS of 3 or fewer days (4 d: OR 0.48, 95% CI 0.364–0.631; ≥ 5 d: OR 0.57, 95% CI 0.435–0.758), as was current smoking status (4 d: OR 0.425, 95% CI 0.274–0.659; ≥ 5 d: OR 0.489, 95% CI 0.314–0.762). Strong predictors of prolonged LOS included THA versus TKA (4 d: OR 2.04, 95% CI 1.53–2.72; ≥ 5 d: OR 2.63, 95% CI 1.97–3.51), age 75 years or older (4 d: OR 1.64, 95% CI 1.14–2.36; ≥ 5 d: OR 4.13, 95% CI 2.94–5.79), ASA classification of 3 (4 d: OR 1.35, 95% CI 0.977–1.85; ≥ 5 d: OR 1.72, 95% CI 1.22–2.43), ASA classification of 4 (4 d: OR 1.47, 95% CI 0.839–2.58; ≥ 5 d: OR 3.03, 95% CI 1.76–5.22) and number of cardiovascular comorbidities (4 d: OR 1.13, 95% CI 0.958–1.32; ≥ 5 d: OR 1.32, 95% CI 1.13–1.54). The model performance was good, with the LOS of 3 or fewer days, 4 days and 5 or more days having AUCs of 0.73, 0.63 and 0.73, respectively (Fig. 1). The model is provided in the Appendix.
Table 2.
Generalized logistic regression model for acute length of stay in hospital
| Variable | LOS category | Adjusted OR (95% CI) | p value |
|---|---|---|---|
| Male sex | 4 d | 0.479 (0.364–0.631) | < 0.001 |
| ≥ 5 d | 0.574 (0.435–0.758) | < 0.001 | |
| ≤ 3 d | Ref | Ref | |
| Hip replacement | 4 d | 2.044 (1.533–2.724) | < 0.001 |
| ≥ 5 d | 2.632 (1.972–3.513) | < 0.001 | |
| ≤ 3 d | Ref | Ref | |
| Age ≥ 75 yr | 4 d | 1.638 (1.140–2.355) | 0.007 |
| ≥ 5 d | 4.127 (2.943–5.789) | < 0.001 | |
| ≤ 3 d | Ref | Ref | |
| ASA 3 | 4 d | 1.346 (0.977–1.853) | 0.07 |
| ≥ 5 d | 1.722 (1.220–2.432) | 0.002 | |
| ≤ 3 d | Ref | Ref | |
| ASA 4 | 4 d | 1.471 (0.839–2.580) | 0.18 |
| ≥ 5 d | 3.027 (1.757–5.217) | < 0.001 | |
| ≤ 3 d | Ref | Ref | |
| No. cardiovascular comorbidities | 4 d | 1.126 (0.958–1.323) | 0.15 |
| ≥ 5 d | 1.319 (1.130–1.540) | < 0.001 | |
| ≤ 3 d | Ref | Ref | |
| Number of renal comorbidities | 4 d | 1.675 (0.501–5.606) | 0.40 |
| ≥ 5 d | 3.236 (1.125–9.307) | 0.029 | |
| ≤ 3 d | Ref | Ref | |
| Diabetes, oral treatment | 4 d | 2.051 (1.328–3.167) | 0.001 |
| ≥ 5 d | 1.276 (0.814–2.000) | 0.29 | |
| ≤ 3 d | Ref | Ref | |
| Diabetes, insulin | 4 d | 0.553* (0.244–1.253) | 0.16 |
| ≥ 5 d | 1.501 (0.757–2.977) | 0.25 | |
| ≤ 3 d | Ref | Ref | |
| Previous stroke or TIA | 4 d | 1.363 (0.712–2.611) | 0.35 |
| ≥ 5 d | 1.087 (0.572–2.066) | 0.80 | |
| ≤ 3 d | Ref | Ref | |
| Other musculoskeletal conditions | 4 d | 1.129 (0.759–1.679) | 0.55 |
| ≥ 5 d | 1.588 (1.080–2.335) | 0.019 | |
| ≤ 3 d | Ref | Ref | |
| Current smoker | 4 d | 0.425 (0.274–0.659) | < 0.001 |
| ≥ 5 d | 0.489 (0.314–0.762) | 0.002 | |
| ≤ 3 d | Ref | Ref |
ASA = American Society of Anesthesiologists; CI = confidence interval; LOS = length of stay in hospital; OR = odds ratio; Ref = reference category; TIA = transient ischemic attack.
OR should be interpreted with caution owing to small sample (n = 11).
Fig. 1.
Receiver operating characteristic (ROC) curve for the performance of the generalized logistic regression model (maximum rescaled R2 = 0.2475). AUC = area under the ROC curve; LOS = length of stay in hospital.
Further analysis of current smoking status revealed that it was significantly more prevalent among men than women (13.8% v. 9.3%, p = 0.009), and a significantly greater proportion of men than women had a spousal/family caregiver at home (90% v. 79.7%, p < 0.001). While current smoking status was predictive of a shortened LOS, it was also correlated with an increased number of preoperative medical comorbidities (r = 0.174, p < 0.001). However, we could not show any statistical difference in current smoking status and admission to a special care unit between smokers and nonsmokers (15.3% v. 10.3%, p = 0.05).
Discussion
Given the multitude of advancements in TJR in recent years, much of the older literature is difficult to apply to current orthopedic practice. It is well accepted that multimodal anesthesia, surgical technique and accelerated postoperative care maps have all shortened the recovery time following TJR. While patients may have previously stayed in hospital for 1–2 weeks, in 2010–11 the median LOS was 4 days for TKA and 5 days for THA in Canada.3 Although these rates have been steady over the preceding 5 years, we have seen a recent push to a lower LOS benchmark with the advent of QBP, where our median LOS was 4 days with 35.1% of patients being discharged home within 3 or fewer days. Based on current funding formulas, the difference of 1 day in hospital may critically affect the ability of hospitals to come in under budget.
In our study, male sex was a strong predictor of an acute LOS of 3 or fewer days, as was current smoking status. We hypothesize that men may be more motivated than women to leave hospital, as our data have shown that a significantly greater proportion of men than women have a caregiver at home to assist in their recovery (90% v. 79.7%, p < 0.001). Similarly, smokers may be more motivated than nonsmokers to leave the hospital setting in order to resume smoking. The finding that smoking was predictive of a reduced LOS in our study highlights the fact that funding agencies must be aware that a reduced LOS does not necessarily equate to a healthier patient, as further analysis revealed that smoking correlated significantly with an increased number of preoperative comorbidities (r = 0.174, p < 0.001). Furthermore, smoking is known to have detrimental effects on the immune system and wound healing.15 The literature shows that preoperative smoking cessation is associated with a relative risk reduction of 41% (95% CI 15–59, p = 0.01) for the prevention of postoperative complications.16 In a recently published study from our institution, the readmission rate to hospital did not increase with shorter LOS, which at least in part suggests that we are not missing postdischarge complications.17 However, as we approach the benchmark LOS of 3 or fewer days, the readmission rate will require re-evaluation with a focus on the current smoker cohort.
Advanced age (≥ 75 yr) in and of itself was an independent predictor of a prolonged LOS; 44% of this cohort had an acute LOS of 5 or more days. This is a key point of concern considering that this age group accounted for 26.7% of our sample population and 33.2% of all TJR patients in Canada in 2010–11.3 Advanced age is associated with increased rates of medical complications and death following TJR.18,19 Certainly these patients may have more clinically important medical comorbidities, as evidenced by the rates of ASA 3 and 4 classifications, and require special care postoperatively, but other reasons may also exist. Advanced age is also associated with poorer strength, balance and agility, vision and hearing loss, bowel and bladder retention and incontinence issues, all of which can affect safe mobilization and prolong LOS.
It is recognized that THA patients generally require a longer LOS than TKA patients.2 In our study, THA was a factor identified with an acute LOS of 4 days or more. This suggests that it may be difficult to target THA patients for discharge within 3 days, as they generally require a longer LOS than TKA patients for pain control and learning safe mobilization.
In our study, the presence of multiple pre-existing cardiac comorbidities was associated with a prolonged LOS, specifically an acute LOS of 5 days or more (OR 1.319, 95% CI 1.13–1.54). It stands to reason that these patients will require more investigations and treatment in hospital and may mobilize at a slower rate owing to their comorbidities. Cardiac complications account for the majority of major systemic events following TJR20,21 and are a leading cause for readmission within 30 days of discharge, second only to surgical site infections.17 The in-hospital prevalence of myocardial infarction following TJR is 1.8% and occurs at a mean of 3 days postoperatively.22 This suggests that earlier discharge of patients with cardiac disease may actually be detrimental.
Pre-existing renal pathology was also predictive of an LOS of 5 days or more in our study (OR 3.24, 95% CI 1.13–9.31). Chronic kidney disease is common in adults older than 65 years, who are the fastest growing subset of patients with end-stage renal disease.23 While studies have shown that THA and TKA can be performed safely in this patient cohort,24,25 close attention must be paid to mitigate the perioperative risks associated with chronic renal disease.
Diabetes affects up to 10% of patients undergoing TJR,26 and in our study, diabetic patients treated orally and those treated with insulin had a greater likelihood of an LOS of 4 days or more. Patients with poorly controlled diabetes have been reported to be at higher risk of stroke, urinary tract infection, ileus, bleeding, wound infection and death than patients with well-controlled diabetes.27 The American Diabetes Association recommends a target hemoglobin A1C level of less than 7%.28 Elective TJR should ideally be delayed until good diabetic control is demonstrated; however, to our knowledge, this is not current standard practice. We did not have the ability to separate well- versus poorly controlled diabetes in the present study, but the finding of a prolonged LOS suggests that some of these patients did require more care and potentially experienced more problems while in hospital.
The TJR patients with musculoskeletal conditions other than their primary arthritis diagnosis (e.g., chronic low back pain, fibromyalgia) also had a greater likelihood of an acute LOS of 5 days or more (OR 1.59, 95% CI 1.08–2.3). Certainly these conditions may impede early mobility and rehabilitation following TJR, leading to a prolonged LOS. Likewise, while not a strong predictor, a history of previous stroke or TIA was associated with an acute LOS of 4 days or more, which may be explained by associated functional deficits that may compromise early mobility and rehabilitation, ultimately delaying hospital discharge.
In a meta-analysis by Olthof and colleagues9 that evaluated the association between comorbidity and LOS in THA patients, 9 studies with a primary outcome of LOS were identified. Of note are the varied methods for measuring comorbidity; the Charlson Comorbidity Index (CCI), ASA classification and the number of medical comorbidities are all recognized as valid tools to assess patient complexity/comorbidity. Each measure of comorbidity has its merits and pitfalls, as outlined by Olthof and colleagues.9 It is important to note that the LOS in the studies reviewed in their meta-analysis varied from a mean of 3.9 to 13 days and may not fully reflect current orthopedic practice for TJR patients. Our study used both the number of comorbidities and the ASA classification to evaluate patient complexity. While the number of medical comorbidities in our analysis did not predict increased LOS, ASA classifications of 3 and 4, indicating severe systemic disease, were predictive of increased LOS. While it can be argued that ASA classification has shortcomings, as it does not detail specific medical conditions, it has the advantage of focusing on complexity rather than just the sheer number of comorbidities, which was clearly a factor associated with prolonged LOS in our study population.
Ng and colleagues12 outlined a detailed review of perioperative strategies for risk stratification and risk reduction in TJR that may be effective in reducing LOS. Although many of the risk factors and medical illnesses reviewed in our study (e.g., obesity, chronic pain, lung disease, rheumatoid arthritis, sleep apnea, alcohol abuse) were not independently predictive of a prolonged LOS, we believe that they are in part reflected in the ASA 3 and 4 classifications, which were predictive of an acute LOS of 4 days or more, and we advocate treating these conditions before proceeding with TJR when possible.
Limitations
We acknowledge that there are occasional social circumstances that may delay discharge and affect LOS, such as family member availability for travel, but these events were not captured in our study. We note that the majority of our patient population resided locally, and a rigorous preoperative protocol to facilitate discharge planning was in place for the duration of the study.
In-hospital complications in the present study were not evaluated and certainly do contribute to prolonged LOS. Our study, however, was designed to elucidate preoperative predictors for a prolonged LOS. Future work should include evaluating in-hospital complications that could have been linked to modifiable preoperative risk factors to improve overall cost-effectiveness of patient care delivery.
Conclusion
Given the current culture to provide more care, albeit with fewer resources, there is a constant pressure to discharge patients quickly from hospital. Certainly, most would agree that there is a limit to how aggressively we should discharge patients, as this must be balanced against the risk of complications and readmissions to hospital. As our data have shown, not all patients presenting for TJR are “equal,” and the ultimate goal should be individual patient-focused care rather than a predetermined LOS that is not achievable for all patients. The bottom line is that hospital resource planning must account for patient complexity when planning future bed management.
Acknowledgements
The authors thank Liz Piccirillo, RN, and Stephanie Kniss, PTA, for their contributions toward project completion.
Footnotes
Competing interests: None declared.
Contributors: M. Winemaker, D. Petruccelli and J. de Beer designed the study. D. Petruccelli acquired the data, which all authors analyzed. All authors wrote and reviewed the article and approved the final version for publication.
References
- 1.Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–5. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 2.Canadian Joint Replacement Registry 2003 report. Ottawa: CIHI; 2003. [accessed 2014 Mar 7]. Total hip and total knee replacements in Canada. Available: https://secure.cihi.ca/free_products/CJRR2003Report.pdf. [Google Scholar]
- 3.Hip and knee replacements in Canada. Canadian Joint Replacement Registry 2013 annual report. Ottawa: CIHI; 2013. [accessed 2014 Mar 7]. Available: https://secure.cihi.ca/free_products/CJRR_2013_Annual_Report_EN.pdf. [Google Scholar]
- 4.Jenkins PJ, Clement ND, Hamilton DF, et al. Predicting the cost-effectiveness of total hip and knee replacement: a health economic analysis. Bone Joint J. 2013;95-B:115–21. doi: 10.1302/0301-620X.95B1.29835. [DOI] [PubMed] [Google Scholar]
- 5.Daigle ME, Weinstein AM, Katz JN, et al. The cost-effectiveness of total joint arthroplasty: a systematic review of published literature. Best Pract Res Clin Rheumatol. 2012;26:649–58. doi: 10.1016/j.berh.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hawker GA, Badley EM, Croxford R, et al. A population-based nested case-control study of the costs of hip and knee replacement surgery. Med Care. 2009;47:732–41. doi: 10.1097/MLR.0b013e3181934553. [DOI] [PubMed] [Google Scholar]
- 7.Scott WN, Booth RE, Jr, Dalury DF, et al. Efficiency and economics in joint arthroplasty. J Bone Joint Surg Am. 2009;91(Suppl 5):33–6. doi: 10.2106/JBJS.I.00365. [DOI] [PubMed] [Google Scholar]
- 8.Health Quality Ontario; Ministry of Health and Long-Term Care. Quality-based procedures: clinical handbook for primary hip and knee replacement. Toronto: Health Quality Ontario; 2013. Nov, [accessed 2014 Mar 7]. p. 95. Available: www.hqontario.ca/evidence/publications-and-ohtac-recommendations/clinical-handbooks. [Google Scholar]
- 9.Olthof M, Stevens M, Bulstra SK, et al. The association between comorbidity and length of hospital stay and costs in total hip arthroplasty patients: a systematic review. J Arthroplasty. 2014;29:1009–14. doi: 10.1016/j.arth.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Geissler A, Scheller-Kreinsen D, Quentin W EuroDRG group. Do diagnosis-related groups appropriately explain variations in costs and length of stay of hip replacement? A comparative assessment of DRG systems across 10 European countries. Health Econ. 2012;21(Suppl 2):103–15. doi: 10.1002/hec.2848. [DOI] [PubMed] [Google Scholar]
- 11.Ellis RP. Creaming, skimping and dumping: provider competition on the intensive and extensive margins. J Health Econ. 1998;17:537–55. doi: 10.1016/s0167-6296(97)00042-8. [DOI] [PubMed] [Google Scholar]
- 12.Ng VY, Lustenberger D, Hoang K, et al. Preoperative risk stratification and risk reduction for total joint reconstruction: AAOS exhibit selection. J Bone Joint Surg Am. 2013;20:191–15. doi: 10.2106/JBJS.L.00603. [DOI] [PubMed] [Google Scholar]
- 13.Health Canada. Canadian Guidelines for Body Weight Classification in Adults. Ottawa: Public Works and Government Services Canada; 2003. [accessed 2014 Mar 7]. Available: www.hc-sc.gc.ca/fn-an/nutrition/weights-poids/guide-ld-adult/index-eng.php. [Google Scholar]
- 14.Owens WD, Felts JA, Spitznagel EL. ASA physical status classifications: a study of consistency of ratings. Anesthesiology. 1978;49:239–43. doi: 10.1097/00000542-197810000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Sopori ML, Kozak W. Immunomodulatory effects of cigarette smoke. J Neuroimmunol. 1998;83:148–56. doi: 10.1016/s0165-5728(97)00231-2. [DOI] [PubMed] [Google Scholar]
- 16.Mills E, Eyawo O, Lockhart I, et al. Smoking cessation reduces postoperative complications: a systematic review and meta-analysis. Am J Med. 2011;124:144–154.e8. doi: 10.1016/j.amjmed.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Avram V, Petruccelli D, Winemaker M, et al. Total joint arthroplasty readmission rates and reasons for 30-day hospital readmission. J Arthroplasty. 2014;29:465–8. doi: 10.1016/j.arth.2013.07.039. [DOI] [PubMed] [Google Scholar]
- 18.Memtsoudis SG, Ma Y, Gonzalez Della Valle A, et al. Demographics, outcomes, and risk factors for adverse events associated with primary and revision total hip arthroplasties in the United States. Am J Orthop. 2010;39:E72–7. [PubMed] [Google Scholar]
- 19.Mantilla CB, Horlocker TT, Schroeder DR, et al. Frequency of myocardial infarction, pulmonary embolism, deep venous thrombosis, and death following primary hip or knee arthroplasty. [Erratum in: Anesthesiology 2002 Aug;97]. [2].:531. Anesthesiology. 2002;96:1140–6. doi: 10.1097/00000542-200205000-00017. [DOI] [PubMed] [Google Scholar]
- 20.Pulido L, Parvizi J, Macgibeny M, et al. In hospital complications after total joint arthroplasty. J Arthroplasty. 2008;23(Suppl 1):139–45. doi: 10.1016/j.arth.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 21.Aynardi M, Pulido L, Parvizi J, et al. Early mortality after modern total hip arthroplasty. Clin Orthop Relat Res. 2009;467:213–8. doi: 10.1007/s11999-008-0528-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gandhi R, Petruccelli D, Devereaux PJ, et al. Incidence and timing of myocardial infarction after total joint arthroplasty. J Arthroplasty. 2006;21:874–7. doi: 10.1016/j.arth.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Dousdampanis P, Trigka K, Fourtounas C. Diagnosis and management of chronic kidney disease in the elderly: a field of ongoing debate. Aging Dis. 2012;3:360–72. [PMC free article] [PubMed] [Google Scholar]
- 24.Miric A, Inacio MC, Namba RS. Can total knee arthroplasty be safely performed in patients with chronic renal disease? Acta Orthop. 2014;85:71–8. doi: 10.3109/17453674.2013.878829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miric A, Inacio MC, Namba RS. The effect of chronic kidney disease on total hip arthroplasty. J Arthroplasty. 2014;29:1225–30. doi: 10.1016/j.arth.2013.12.031. [DOI] [PubMed] [Google Scholar]
- 26.Bolognesi MP, Marchant MH, Jr, Viens NA, et al. The impact of diabetes on perioperative patient outcomes after total hip and total knee arthroplasty in the United States. J Arthroplasty. 2008;23(Suppl 1):92–8. doi: 10.1016/j.arth.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 27.Marchant MH, Jr, Viens NA, Cook C, et al. The impact of glycemic control and diabetes mellitus on perioperative outcomes after total joint arthroplasty. J Bone Joint Surg Am. 2009;91:1621–9. doi: 10.2106/JBJS.H.00116. [DOI] [PubMed] [Google Scholar]
- 28.Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists, American Diabetes Association. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract. 2009;15:353–69. doi: 10.4158/EP09102.RA. [DOI] [PubMed] [Google Scholar]