Abstract
Background
Nontraumatic osteonecrosis of the femoral head (ONFH) is a progressive disease in young adults producing substantial morbidity and frequently resulting in total hip arthroplasty. Although hip-preserving surgical procedures represent the current mainstay of treatment for early disease, medical therapies targeting specific pathways in the ONFH pathogenesis could help prevent disease progression while producing less morbidity. Acetylsalicylic acid (ASA) is a promising alternative to other therapies for ONFH owing to its anti-inflammatory and antithrombotic mechanisms of action and its relatively benign side effect profile.
Methods
We followed a prospective cohort of 10 patients (12 hips) with precollapse ONFH who were given ASA to prevent disease progression. Their outcomes were compared with those of a historic control group taken from the literature.
Results
Progression occurred in 1 of 12 (8%) patients taking ASA compared with 30 of 45 (66.6%) controls (p = 0.002) at a mean follow-up of 3.7 years. Patients taking ASA also tended to exhibit decreased femoral head involvement at the end of therapy.
Conclusion
This hypothesis-generating study leads us to believe that ASA may be a simple and effective treatment option for delaying disease progression in patients with early-stage ONFH.
Abstract
Contexte
L’ostéonécrose non traumatique de la tête fémorale (ONTF) est une maladie progressive qui affecte les adultes jeunes, s’accompagne d’une morbidité substantielle et mène souvent à une arthroplastie totale de la hanche. Même si les interventions chirurgicales visant à préserver la hanche représentent la pierre angulaire actuelle du traitement pour la maladie au stade précoce, les traitements médicamenteux qui ciblent les voies spécifiques de la pathogenèse de l’ONTF pourraient contribuer à prévenir la progression de la maladie tout en atténuant la morbidité. L’acide acétylsalicylique (AAS) est une solution de rechange prometteuse aux autres traitements indiqués pour l’ONTF en raison de ses propriétés anti-inflammatoires et antithrombotiques et de son profil d’innocuité relativement bénin.
Méthodes
Nous avons suivi une cohorte prospective de 10 patients (12 hanches) présentant une ONTF au stade précollapsus qui ont reçu de l’AAS pour prévenir la progression de la maladie. Leurs résultats ont été comparés à ceux d’un groupe témoin historique de patients décrits dans la littérature.
Résultats
La progression a affecté 1 patient sur 12 (8 %) traités par AAS, contre 30 témoins sur 45 (66,6 %) (p = 0,002) après un suivi moyen de 3,7 ans. Les patients sous AAS avaient tendance à présenter une atteinte moins prononcée de la tête fémorale à la fin du traitement.
Conclusion
Cette étude exploratoire nous amène à croire que l’AAS pourrait être une option thérapeutique simple et efficace pour retarder la progression de la maladie chez les patients au stade précoce d’une ONTF.
Nontraumatic osteonecrosis of the femoral head (ONFH) has an incidence of up to 20 000 cases per year in the United States.1 This debilitating skeletal disorder of young adults leads to femoral head collapse, and up to 85% of all patients will eventually require total hip arthroplasty (THA).2 The disorder is a complication observed in patients with illnesses, such as chronic autoimmune diseases, acute lymphoblastic leukemia and chronic obstructive pulmonary disease, that require long-term treatment with corticosteroids. In addition to corticosteroids, sickle cell disease and alcohol abuse are also well-documented causes of nontraumatic ONFH.3,4 The pathogenesis of ONFH is complex and is often represented by a “multiple hit theory.” Specific genetic mutations and abnormalities of the coagulation cascade, angiogenesis and bone formation are coupled with exogenous agents, such as medications, and disease states that interfere with the vascular and endothelial lining of the microvessels within the femoral head.5,6 This combination of exogenous insults and an inherent imbalance between thrombotic factors and natural anticoagulants leads to a direct ischemic phenomenon resulting in endothelial injury.7
Patients with nontraumatic ONFH often have a poor surgical prognosis; therefore, interest is emerging in developing medical therapies that target endothelial–coagulation cascade interactions. Owing to limitations of available options, there is currently no consensus regarding effective medical treatment for nontraumatic ONFH at the precollapse stage. Although several theoretically appealing therapies, including bisphosphonates, nonsteroidal anti-inflammatories and a limited weight-bearing protocol, have been clinically evaluated, they have failed to prevent or halt disease progression.8,9 Low molecular-weight heparin (LMWH) has been shown to delay progression of early ONFH in 1 study;8 however, concerns regarding the risk of bleeding along with a growing body of evidence regarding heparin-induced osteoporosis and associated fractures, limit the appeal of prolonged LMWH therapy for ONFH prevention.10,11
Acetylsalicylic acid (ASA) is an appealing alternative to LMWH for the early treatment of ONFH owing to its fairly benign side effect profile and an impressive range of mechanisms acting upon the known endothelial disease process causing ONFH. Salicylates are some of the most commonly used anti-inflammatory agents.12 For more than 3 decades, the anti-inflammatory properties of ASA have been almost exclusively attributed to blockade of prostaglandin synthesis via inhibition of cyclooxygenase-1 (COX-1) activity and subsequent inhibition of thromboxane A2.13 Recent studies have been directed at identifying additional effects of these drugs that could help explain their pharmacologic properties. As such, ASA has been found to inhibit the activation of nuclear factor κ-light-chain-enhancer of activated B cells (NF-kB) without affecting other transcription factors.14 This finding could help explain the antithrombotic effects of ASA-like drugs on endothelial cells, for instance, the blockade of the expression of COX-2 induced by interleukin (IL)-1,15 the blunting of the expression of vascular cell adhesion molecule-1 (VCAM-1) and E-selectin in response to tumour-necrosis factor α (TNF-α) and the inhibition of the adhesion of monocytes to endothelial cells.16 Other effects include a potential enhancing effect on fibrinolytic activity by decreasing plasminogen activator inhibitor-1 (PAI-1) concentration (especially if ASA is administered chronically at low doses),17 blockade of the activation of the extracellular signal-regulated kinase18 subgroup of mitogen-activated protein kinases in response to TNF-α19 and the transcriptional inhibition of endothelial nitric oxide synthase (eNOS).20
More recently, ASA has also been shown to modulate the vascular endothelium via the eicosanoid lipoxin A4 pathway.21 Lipoxin A4 is derived from endothelium-produced arachidonic acid, which binds to polymorphonuclear leukocytes (PMN) and platelets at the location where lipoxins are stored. In addition, ASA has been shown to regulate several pathways involved in vascular hemostasis and, as such, has a vasoprotective effect by inhibiting reactive oxygen species on the endothelium. In addition, ASA regulates the nitric oxide (NO) release pathway, which has indirectly been linked to platelet aggregation and adhesion,11,21,22 and inhibits mammalian target of rapamycin (mTor),21 a potent regulator of platelet activation and aggregation.23 Finally, ASA has a role in the structure and function of the fibrin clot (cross-linked fibrin) via a protein acetylation mechanism.24–26 Taken together, these findings indicate that the mechanism of action of salicylates in both the inflammatory response and the progression of microthrombotic processes is not straightforward. In addition to its effect on cyclo-oxygenase and eicosanoid metabolism, other mechanisms involving the vascular endothelium contribute to the anti-inflammatory and antithrombotic effects of ASA.
Owing to an expanded understanding of the effects of ASA on the coagulation cascade and the endothelium as well as lack of safe or effective medical alternatives, we designed a study to evaluate the effect of prolonged administration of ASA on the clinical and radiological progression of nontraumatic ONFH.
Methods
In our institution, all patients with suspected ONFH are referred to a dedicated ONFH clinic that has been run by the senior authors (E.J.H. and C.S.) since 2004. All patients presenting with symptomatic precollapse ONFH were consented to daily oral consumption of 81 mg of ASA. The rationale for using ASA was its established antiplatelet and anticoagulant effects as well as its added anti-inflammatory benefit.
Patient follow-up took place at the ONFH clinic in either 6-month or 1-year intervals. Radiographs and/or magnetic resonance images (MRIs) were obtained at each follow-up visit, and compliance with ASA was documented.
We compared the study cohort with historical ONFH controls in the literature. The primary outcome was the radiologic progression of ONFH on either radiographs or MRIs. A successful outcome was defined as no difference in ONFH staging, according to the Ficat & Arlet classification,27 at last patient follow-up compared with initial radiological staging. The secondary outcome was the percentage involvement of the femoral head on radiographs and/or MRI.
Patient selection
We acquired institutional review board approval before data collection. Only patients presenting to our ONFH clinic with nontraumatic precollapse ONFH who consented to receive daily doses of 81 mg ASA were included in our analysis. Patients with traumatic cases of ONFH were excluded as they are inherently variable in terms of the type of trauma sustained, fracture pattern, delay to operative treatment and amount of articular cartilage damage. Conversely, generalized consensus exists regarding the final pathological processes in nontraumatic ONFH as well as its natural history. Therefore, to establish an internally consistent group of patients, we followed only those with nontraumatic ONFH.
Exclusion criteria were history of hip trauma or previous hip surgery, previous use of anticoagulants or bisphosphonates for any reason, noncompliance with ASA treatment and immediate referral for surgical consultation owing to end-stage joint arthritis. It should be noted that evaluation against these criteria as well as radiography and/or MRI of the affected hip occur in our ONFH clinic for every patient visit. For the patients who fulfilled inclusion criteria, we recorded the following characteristics for analysis: age, sex and risk factors for ONFH. Although nontraumatic ONFH has been associated with more than 50 medical conditions,3,4 the 6 most common etiologies were specifically noted: history of corticosteroid use, alcohol use, sickle cell anemia, diabetes mellitus, previous hip trauma and hypercholesterolemia/hypertriglyceridemia.
We retrieved radiographs and, when available, MRIs for each patient, and we analyzed 2 specific image time points. We retained images taken at initial presentation and either the MRI taken at the most recent follow-up visit or the last MRI before surgical intervention. An independent radiologist blinded to the study purpose reviewed all images. For each time point, we recorded ONFH stage using the Ficat & Arlet27 classification for radiographs and the Steinberg classification28 for MRIs. In addition to classification, the percentage of femoral head involvement and location of the ONFH lesion(s) were quantified using previously described methods.29,30 We determined interval change in the percentage of femoral head involvement from the most recent MRI. Owing to fundamental similarities in disease classification and variability in MRI or radiograph availability for every patient, as per literature standards, we considered Steinberg stage 0–1 and Ficat & Arlet stage I to be equivalent and Steinberg stage 2 and Ficat & Arlet stage II to be equivalent.
Control group
We compared the treatment group with an established group of historic controls taken from 2 peer-reviewed publications (n = 45)1,31 that have been used for comparison of ONFH survivorship in at least 1 other study.8 The publication by Stulberg and colleagues31 prospectively compared conservative treatment with core decompression in 36 patients (52 hips) for ONFH. Glucocorticoid use (35%) and ethanol (19%) were the most common etiologic factors in the cohort. The conservatively treated group was composed of 26 patients: 3 with Ficat & Arlet stage 0 hips, 5 with stage I hips, 7 with stage II hips, 10 with stage III hips and 1 with a stage IV hip. The mean patient age was 38.6 years and the mean follow-up was 26.8 months. The publication by Koo and colleagues1 prospectively compared conservative treatment with core decompression in 33 patients (37 hips). Ethanol (89%) and glucocorticoids (11%) represented the etiologic causes in this cohort. The conservatively treated group was composed of 19 patients: 12 with Ficat & Arlet stage I hips, 4 with stage II hips and 3 with stage III hips. The mean patient age was 47 years, and the reported follow-up was a minimum of 24 months. When the 2 control groups are considered together, the average patient age is 42.1 years, and the 2 most common etiological factors of ONFH are alcohol abuse (22 of 46; 47.8%) or corticosteroid use (10 of 46; 21.7%). At last patient follow-up, 15 of 26 (57.7%) patients in the study by Stulberg and colleagues31 and 15 of 19 (78.9%) patients in the study by Koo and colleagues1 showed evidence of radiographic progression, resulting in 30 of 45 (66.6%) patients having an unsuccessful radiographic outcome with conservative treatment.
Statistical analysis
We compared nominal and ordinal demographic values using the Pearson χ2 test. Patient demographic characteristics are reported as means with standard deviations. The percent femoral head involvement at first and last study follow-up were recorded as continuous variables and assessed for normality using a Shapiro–Wilk test. We compared femoral head involvement between the groups using a paired Student t test. The presence or absence of radiological progression was recorded as a binary variable. Comparison of progression bewteen the 2 groups was compared using a Fisher exact test. For all statistical tests, we considered results to be significant at p < 0.05.
Results
From 2004 to 2012, 10 patients (6 men, 4 women, 12 painful hips) with nontraumatic ONFH received ASA therapy, met the study criteria and were included in our analysis (Table 1). The average patient age in the study group was 52.9 ± 15.3 years, and the average follow-up was 44.4 ± 21.6 (range 18–84) months. Patient age did not significantly differ between the study and control groups (t = 2.253; p = 0.08), but the ASA group demonstrated a trend toward older patients.
Table 1.
Demographic characteristics, ONFH etiology, disease classification and femoral head involvement for ASA-treated patients
| Patient # | Age, yr; sex* | ONFH etiology | Laterality | ONFH lesion location | Pre-ASA Ficat stage | Most recent Ficat stage | Pre-ASA %femoral head involvement† | Most recent %femoral head involvement‡ |
|---|---|---|---|---|---|---|---|---|
| 1 | 65 M | Corticosteroids | Right | B | 2 | 2 | 30 | 25 |
| 2 | 61 M | Alcohol | Left | C2 | 2 | 4 | 90 | 100 |
| 3 | 76 F | Corticosteroids | Right | B | 2 | 2 | 20 | 20 |
| Left | B | 2 | 2 | 30 | 20 | |||
| 4 | 57 M | Unknown | Left | B | 2 | 2 | 30 | 20 |
| 5 | 52 M | Corticosteroids | Left | C1 | 2 | 2 | 30 | 20 |
| 6 | 67 F | Corticosteroids | Left | C1 | 2 | 2 | 30 | 30 |
| 7 | 62 M | Unknown | Right | C1 | 2 | 2 | 30 | 30 |
| 8 | 28 F | Sickle cell | Right | C1 | 2 | 2 | 60 | 60 |
| 9 | 37 M | Sickle cell | Right | C1 | 2 | 2 | 50 | 25 |
| Left | B | 2 | 2 | 25 | 25 | |||
| 10 | 43 F | Sickle cell | Right | C1 | 2 | 2 | 30 | 30 |
ASA = acetylsalicylic acid; F = female; M = male; ONFH = osteonecrosis of the femoral head.
Mean 54.8 ± 11 yr.
Mean 38% ± 20%.
Mean 34% ± 24%.
In the ASA group, 2 hips were diagnosed as primary ONFH and 10 were secondary. The most frequently identified causes of secondary ONFH were prior use of corticosteroids (5 of 10), sickle cell disease (4 of 10) and elevated alcohol consumption (1 of 10). All patients reported appropriate compliance with their daily oral dose of 81 mg of ASA. Treatment with ASA did not lead to any bleeding episodes or other adverse effects.
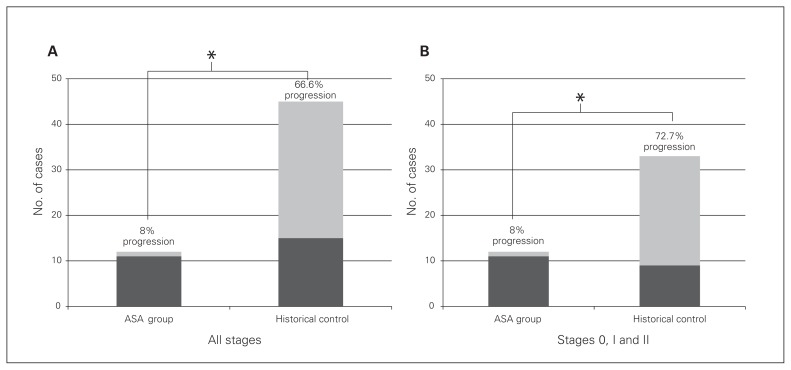
At the time of initial diagnosis, all patients taking ASA had Ficat & Arlet/Steinberg stage II disease on imaging with an average area of femoral head involvement of 38% ± 20% (range 20%–90%). With regard to the study’s primary outcome, at an average follow-up of 3.7 years, 11 of 12 patients taking ASA experienced a successful outcome, with no progression of ONFH staging on radiographs or MRI. Compared with the control group, patients taking ASA exhibited a significantly decreased rate of radiographic progression (30 of 45 patients, 66.6% rate of progression v. 1 of 12, 8% rate of progression, p = 0.002) despite older age and a longer average follow-up (Fig. 1a). When matching groups for the presenting ONFH stage (all patients taking ASA presented with Stage 2 ONFH, and 33 of 45 historical controls presented with ONHF stage 0, 1 or 2), patients taking ASA still exhibited a significantly decreased rate of radiographic progression (1 of 12 patients taking ASA v. 24 of 33 controls, p = 0.001; Fig. 1b).
Fig. 1.
(A) Patients taking acetylsalicylic acid (ASA) and historical controls with any presenting Ficat & Arlet stage osteonecrosis of the femoral head (ONFH) after more than 2 years of follow-up. The ASA group exhibited a significantly lower percentage of cases with radiographic progression of ONFH. (B) Patients taking ASA and historical controls initially presenting with Ficat & Arlet stage 0–2 ONFH after more than 2 years of follow-up. Even when controlling for presenting ONFH stage, the ASA group exhibited a significantly lower percentage of cases with radiographic progression of ONFH. *p < 0.05.
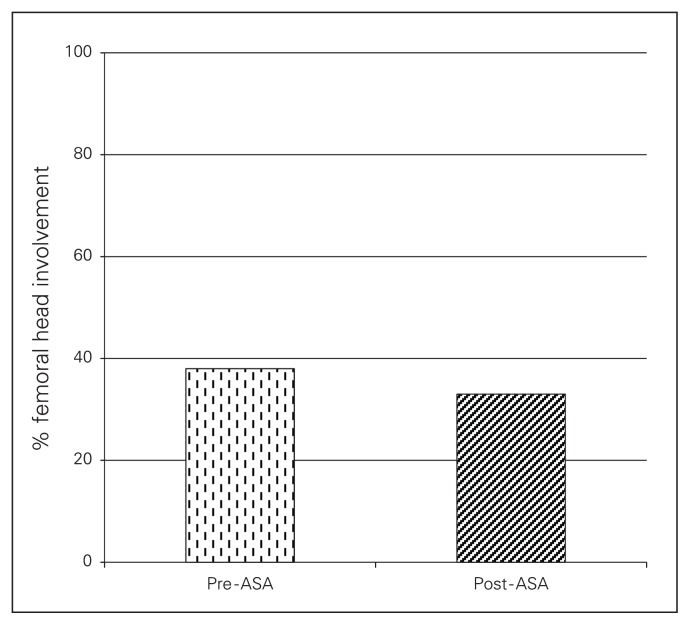
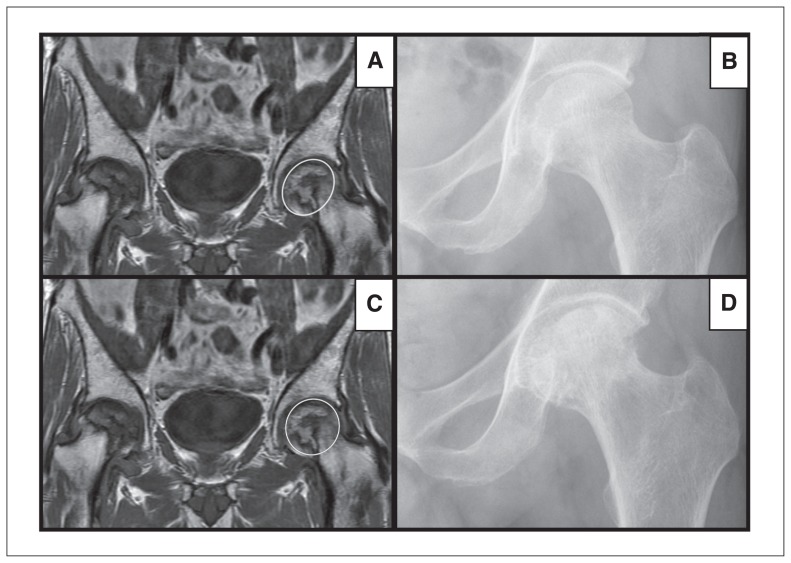
With regard to femoral head involvement, the average area of involvement within the group decreased over time (34% ± 24%, p = 0.13; Fig. 2). The 1 exception in whom femoral involvement increased despite ASA therapy was a 61-year-old man with a history of heavy ethanol use. He continued his high alcohol intake during the course of his treatment. This patient exhibited substantially higher femoral head involvement (90%) on initial index MRI than the other patients in the ASA cohort (pre-ASA mean of 38%) and rapidly progressed to 100% involvement and Steinberg stage 4 disease over the course of 1 year, requiring referral for total hip arthroplasty (Fig. 3). When excluding this patient from our analysis, the average femoral head ONFH involvement in the remaining patients taking ASA significantly decreased compared with involvement at the initial diagnosis (27.7%, p = 0.045).
Fig. 2.
Percent osteonecrosis of the femoral head (ONFH) involvement of the femoral head in patients prior to and following an average of 2 years of acetylsalicylic acid (ASA) administration.
Fig. 3.
(A–B) Section of T1-weighted coronal MRI and plain radiograph of a 61-year-old patient presenting with Steinberg stage II osteonecrosis of the femoral head (ONFH) in the left hip and 90% femoral head involvement (circle in panel A). (C–D) Follow-up section of T1-weighted coronal MRI and plain radiograph of the same patient after treatment with oral acetylsalicylic acid (ASA) for < 12 months. The ONFH involvement of the femoral head increased, with evidence of subchondral collapse (circle in panel C) and progression to Steinberg stage IV ONFH.
Discussion
Recent breakthroughs in the understanding of molecular mechanisms involved in ONFH have spurred interest in evaluating pre-existing medical therapies that could theoretically halt and potentially reverse disease progression. Medical therapies as well as nonpharmacological therapies, including modified weight bearing, hyperbaric oxygen and extracorporeal shock wave therapy, have been the subject of multiple investigations, including several prospective studies.18,32–47 Bisphosphonates have received specific attention owing to their known effect of decreasing osteoclast-mediated bone turnover that theoretically could prevent subchondral bone collapse. A randomized controlled trial assessing 54 hips reported significantly lower rates of disease progression and need for arthroplasty in patients treated with bisphosphonates for 2 months.34 However, such positive results are offset by a more recent multicentre, double-blind, prospective, controlled study of alendronate versus placebo that showed no statistically significant difference in the rates of THA, disease progression or quality of life at 2 year follow-up.35 Statin therapy provides theoretical benefits in decreasing ONFH progression through a reduction in bone marrow adipocyte size that could reduce intraosseous pressure.48 Unfortunately, clinical studies performed to date have not shown any difference in ONFH progression.39
One potential explanation for the failure of these medical therapies is that they target the end-effects of ONFH and do not target the underlying insult. Recent evidence points to a vascular hypothesis for the etiology of ONFH, whereby local endothelial dysfunction leads to clot and fatty emboli formation.7,49,50 It is for this reason that we explored the administration of ASA, a known antiplatelet and modulator of the vascular endothelium, to determine if the medication affects the progression of nontraumatic ONFH.
We found that most patients treated with daily ASA tended not to progress in radiological staging of ONFH over a medium-term follow-up. With the exception of 1 patient with near 100% head involvement, patients taking ASA exhibited a significant decline in femoral head involvement on MRI, indicating a reversal in the disease process. The rate of progression in these patients was markedly improved compared with historical controls despite the control group comprising younger patients followed for a shorter amount of time. To our knowledge, our study represents the first clinical examination of ASA therapy for the treatment of ONFH and the first clinical investigation to show a reversal in the ONFH process in the majority of patients receiving medical therapy.
Limitations
We acknowledge several limitations to our study. First, the small number of patients in the cohort limits the potential conclusions on the effect of ASA to only patients who present with a round head (no subchondral step). However, it should be noted that the study’s sample size is comparable to that of other pilot studies for preventative therapies, including enoxaparin8 and hyperbaric oxygen,43,44 for ONFH. Second, the use of historical controls for comparison raises concerns for selection bias or equivalence of populations. We attempted to diminish any potential for bias by having minimal selection criteria for patients started on ASA in the clinic and by using historical controls who have been used in other recent investigations when comparing other treatments for ONFH.1,8 It would have been impossible to recruit a control group from the population in the ONFH clinic because all patients were started on ASA if possible. Third, we acknowledge that the follow-ups of both study groups are only short- to mid-term and that a longer, prospective follow-up period is required to fully understand the effect of ASA on disease progression and whether ASA therapy will lead to a substantial delay to the need for THA. Also, it is not specified within the historical control population if the use of anticoagulants or antiplatelet agents was part of the study’s exclusion criteria. However, given the relatively young average age of this population (43 yr), the regular use of these agents to prevent heart disease or stroke is unlikely or could be limited to only a minority of these patients.
Conclusion
Findings from this observational study suggest that ASA administration for patients in early to intermediate stages of nontraumatic ONFH halts radiographic progression and may actually reverse the disease process in the short-term. Such effects are surmised to arise from ASA’s known anticoagulant properties and its positive modulatory and protective action on the vascular endothelium. Although our study was limited by its small sample size, it is important to note that this size is comparable to that in recent literature examining other pharmacological candidates for ONFH treatment. We have used these preliminary findings as a basis to develop a larger, multicentre prospective study to further elucidate the potential benefit of ASA treatment for precollapse ONFH.
Acknowledgments
The authors gratefully acknowledge Dr. Shereen Ghali for assistance with the initial literature review on Aspirin and preparation of documents for the initial research ethics board review at the start of the study in 2004 and Dr. Ali Bessissow, who reviewed the radiographs and magnetic resonance images.
Footnotes
Funding: This work was supported by awards to C. Séguin from the Canadian Leukemia & Lymphoma Society, the Fonds de la Recherche en Santé du Québec (FRQ-S) and the Yvon Boulanger Foundation, and to E.J Harvey by the Canadian Institutes of Health Research (CIHR) and the FRQ-S-sponsored Réseau de Recherche en Santé Buccodentaire et Osseuse (RSBO). Graduate students were supported by awards from the McGill Surgical Scientist program (AC). C. Séguin is a Chercheur-Boursier Clinicien of the FRQ-S and the RI-MUHC is an FRQ-S-sponsored Centre de Recherche.
Competing interests: None declared.
Contributors: A. Albers, A. Carli, E. Harvey and C. Séguin designed the study. A. Albers, B. Routy, E. Harvey and C. Séguin acquired the data, which A. Albers, A. Carli, E. Harvey and C. Séguin analyzed. All authors wrote and reviewed the article and approved the final version for publication.
References
- 1.Koo KH, Kim R, Ko GH, et al. Preventing collapse in early osteonecrosis of the femoral head. A randomised clinical trial of core decompression. J Bone Joint Surg Br. 1995;77:870–4. [PubMed] [Google Scholar]
- 2.Mont MA, Zywiel MG, Marker DR, et al. The natural history of untreated asymptomatic osteonecrosis of the femoral head. A systematic literature review. J Bone Joint Surg Am. 2010;92:2165–70. doi: 10.2106/JBJS.I.00575. [DOI] [PubMed] [Google Scholar]
- 3.Assouline-Dayan Y, Chang C, Greenspan A, et al. Pathogenesis and natural history of osteonecrosis. Semin Arthritis Rheum. 2002;32:94–124. [PubMed] [Google Scholar]
- 4.Akinyoola AL, Adediran IA, Asaleye CM, et al. Risk factors for osteonecrosis of the femoral head in patients with sickle cell disease. Int Orthop. 2009;33:923–6. doi: 10.1007/s00264-008-0584-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinstein RS. Glucocorticoid-induced osteonecrosis. Endocrine. 2012;41:183–90. doi: 10.1007/s12020-011-9580-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mankin HJ. Nontraumatic necrosis of bone (osteonecrosis) N Engl J Med. 1992;326:1473–9. doi: 10.1056/NEJM199205283262206. [DOI] [PubMed] [Google Scholar]
- 7.Kerachian MA, Seguin C, Harvey EJ. Glucocorticoids in osteonecrosis of the femoral head: a new understanding of the mechanisms of action. J Steroid Biochem Mol Biol. 2009;114:121–8. doi: 10.1016/j.jsbmb.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glueck CJ, Freiberg RA, Sieve L, et al. Enoxaparin prevents progression of stages I and II osteonecrosis of the hip. Clin Orthop Relat Res. 2005;(435):164–70. doi: 10.1097/01.blo.0000157539.67567.03. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Yin L, Li Y, et al. Preventive effects of puerarin on alcohol-induced osteonecrosis. Clin Orthop Relat Res. 2008;466:1059–67. doi: 10.1007/s11999-008-0178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lefkou E, Khamashta M, Hampson G, et al. Review: Low-molecular-weight heparin-induced osteoporosis and osteoporotic fractures: a myth or an existing entity? Lupus. 2010;19:3–12. doi: 10.1177/0961203309353171. [DOI] [PubMed] [Google Scholar]
- 11.Brancaleone V, Gobbetti T, Cenac N, et al. A vasculo-protective circuit centered on lipoxin A4 and aspirin-triggered 15-epi-lipoxin A4 operative in murine microcirculation. Blood. 2013;122:608–17. doi: 10.1182/blood-2013-04-496661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bayón Y, Alonso A, Crespo MS. 4-trifluoromethyl derivatives of salicylate, triflusal and its main metabolite 2-hydroxy-4-trifluoromethylbenzoic acid, are potent inhibitors of nuclear factor kB activation. Br J Pharmacol. 1999;126:1359–66. doi: 10.1038/sj.bjp.0702441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231:232–5. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 14.Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994;265:956–9. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 15.Wu KK, Sanduja R, Tsai A-L, et al. Aspirin inhibits interleukin 1-induced prostaglandin H synthase expression in cultured endothelial cells. Proc Natl Acad Sci U S A. 1991;88:2384–7. doi: 10.1073/pnas.88.6.2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weber C, Erl W, Pietsch A, et al. Aspirin inhibits nuclear factor–kb mobilization and monocyte adhesion in stimulated human endothelial cells. Circulation. 1995;91:1914–7. doi: 10.1161/01.cir.91.7.1914. [DOI] [PubMed] [Google Scholar]
- 17.Buczko W, Mogielnicki A, Kramkowski K, et al. Aspirin and the fibrinolytic response. Thromb Res. 2003;110:331–4. doi: 10.1016/j.thromres.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Disch AC, Matziolis G, Perka C. The management of necrosis-associated and idiopathic bone-marrow oedema of the proximal femur by intravenous iloprost. J Bone Joint Surg Br. 2005;87:560–4. doi: 10.1302/0301-620X.87B4.15658. [DOI] [PubMed] [Google Scholar]
- 19.Schwenger P, Skolnik EY, Vilcek J. Inhibition of tumor necrosis factor-induced p42/p44 mitogen-activated protein kinase activation by sodium salicylate. J Biol Chem. 1996;271:8089–94. doi: 10.1074/jbc.271.14.8089. [DOI] [PubMed] [Google Scholar]
- 20.Kepka-Lenhart D, Chen LC, Morris SM., Jr Novel actions of aspirin and sodium salicylate: discordant effects on nitric oxide synthesis and induction of nitric oxide synthase mRNA in a murine macrophage cell line. J Leukoc Biol. 1996;59:840–6. doi: 10.1002/jlb.59.6.840. [DOI] [PubMed] [Google Scholar]
- 21.Guertin DA, Sabatini DM. The pharmacology of mTOR inhibition. Sci Signal. 2009;2:pe24. doi: 10.1126/scisignal.267pe24. [DOI] [PubMed] [Google Scholar]
- 22.Bauer V, Sotníková R. Nitric oxide — the endothelium-derived relaxing factor and its role in endothelial functions. Gen Physiol Biophys. 2010;29:319. [PubMed] [Google Scholar]
- 23.Aslan JE, Tormoen GW, Loren CP, et al. S6K1 and mTOR regulate Rac1-driven platelet activation and aggregation. Blood. 2011;118:3129–36. doi: 10.1182/blood-2011-02-331579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams S, Fatah K, Hjemdahl P, et al. Better increase in fibrin gel porosity by low dose than intermediate dose acetylsalicylic acid. Eur Heart J. 1998;19:1666–72. doi: 10.1053/euhj.1998.1088. [DOI] [PubMed] [Google Scholar]
- 25.Ajjan RA, Standeven KF, Khanbhai M, et al. Effects of aspirin on clot structure and fibrinolysis using a novel in vitro cellular system. Arterioscler Thromb Vasc Biol. 2009;29:712–7. doi: 10.1161/ATVBAHA.109.183707. [DOI] [PubMed] [Google Scholar]
- 26.Svensson J, Bergman AC, Adamson U, et al. Acetylation and glycation of fibrinogen in vitro occur at specific lysine residues in a concentration dependent manner: a mass spectrometric and isotope labeling study. Biochem Biophys Res Commun. 2012;421:335–42. doi: 10.1016/j.bbrc.2012.03.154. [DOI] [PubMed] [Google Scholar]
- 27.Ficat RP. Idiopathic bone necrosis of the femoral head. [Br] J Bone Joint Surg. 1985;67:3–9. doi: 10.1302/0301-620X.67B1.3155745. [DOI] [PubMed] [Google Scholar]
- 28.Steinberg ME, Hayken GD, Steinberg DR. A quantitative system for staging avascular necrosis. J Bone Joint Surg Br. 1995;77:34–41. [PubMed] [Google Scholar]
- 29.Koo KH, Kim R. Quantifying the extent of osteonecrosis of the femoral head. A new method using MRI. J Bone Joint Surg Br. 1995;77:875–80. [PubMed] [Google Scholar]
- 30.Min B-W, Song K-S, Cho C-H, et al. Untreated asymptomatic hips in patients with osteonecrosis of the femoral head. Clin Orthop Relat Res. 2008;466:1087–92. doi: 10.1007/s11999-008-0191-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stulberg BN, Davis AW, Bauer TW, et al. Osteonecrosis of the femoral head. A prospective randomized treatment protocol. Clin Orthop Relat Res. 1991;(268):140–51. [PubMed] [Google Scholar]
- 32.Agarwala S, Jain D, Joshi VR, et al. Efficacy of alendronate, a bisphosphonate, in the treatment of AVN of the hip. A prospective open-label study. Rheumatology (Oxford) 2005;44:352–9. doi: 10.1093/rheumatology/keh481. [DOI] [PubMed] [Google Scholar]
- 33.Agarwala S, Shah S, Joshi VR. The use of alendronate in the treatment of avascular necrosis of the femoral head: follow-up to eight years. J Bone Joint Surg Br. 2009;91:1013–8. doi: 10.1302/0301-620X.91B8.21518. [DOI] [PubMed] [Google Scholar]
- 34.Lai KA, Shen WJ, Yang CY, et al. The use of alendronate to prevent early collapse of the femoral head in patients with nontraumatic osteonecrosis. A randomized clinical study. J Bone Joint Surg Am. 2005;87:2155–9. doi: 10.2106/JBJS.D.02959. [DOI] [PubMed] [Google Scholar]
- 35.Chen CH, Chang JK, Lai KA, et al. Alendronate in the prevention of collapse of the femoral head in nontraumatic osteonecrosis: a two-year multicenter, prospective, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2012;64:1572–8. doi: 10.1002/art.33498. [DOI] [PubMed] [Google Scholar]
- 36.Hsu SL, Wang CJ, Lee MS, et al. Cocktail therapy for femoral head necrosis of the hip. Arch Orthop Trauma Surg. 2010;130:23–9. doi: 10.1007/s00402-009-0918-5. [DOI] [PubMed] [Google Scholar]
- 37.Wang CJ, Wang FS, Yang KD, et al. Treatment of osteonecrosis of the hip: comparison of extracorporeal shockwave with shockwave and alendronate. Arch Orthop Trauma Surg. 2008;128:901–8. doi: 10.1007/s00402-007-0530-5. [DOI] [PubMed] [Google Scholar]
- 38.Pritchett JW. Statin therapy decreases the risk of osteonecrosis in patients receiving steroids. Clin Orthop Relat Res. 2001;(386):173–8. doi: 10.1097/00003086-200105000-00022. [DOI] [PubMed] [Google Scholar]
- 39.Ajmal M, Matas AJ, Kuskowski M, et al. Does statin usage reduce the risk of corticosteroid-related osteonecrosis in renal transplant population? Orthop Clin North Am. 2009;40:235–9. doi: 10.1016/j.ocl.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang GJ, Cui Q, Balian G. The Nicolas Andry award. The pathogenesis and prevention of steroid-induced osteonecrosis. Clin Orthop Relat Res. 2000;(370):295–310. doi: 10.1097/00003086-200001000-00030. [DOI] [PubMed] [Google Scholar]
- 41.Norman D, Miller Y, Sabo E, et al. The effects of enoxaparin on the reparative processes in experimental osteonecrosis of the femoral head of the rat. APMIS. 2002;110:221–8. doi: 10.1034/j.1600-0463.2002.100304.x. [DOI] [PubMed] [Google Scholar]
- 42.Jäger M, Zilkens C, Bittersohl B, et al. Efficiency of iloprost treatment for osseous malperfusion. Int Orthop. 2011;35:761–5. doi: 10.1007/s00264-010-0998-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Camporesi EM, Vezzani G, Bosco G, et al. Hyperbaric oxygen therapy in femoral head necrosis. J Arthroplasty. 2010;25(Suppl):118–23. doi: 10.1016/j.arth.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 44.Reis ND, Schwartz O, Militianu D, et al. Hyperbaric oxygen therapy as a treatment for stage-I avascular necrosis of the femoral head. J Bone Joint Surg Br. 2003;85:371–5. doi: 10.1302/0301-620x.85b3.13237. [DOI] [PubMed] [Google Scholar]
- 45.Wang CJ. Extracorporeal shockwave therapy in musculoskeletal disorders. J Orthop Surg Res. 2012;7:11. doi: 10.1186/1749-799X-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang CJ, Wang FS, Huang CC, et al. Treatment for osteonecrosis of the femoral head: comparison of extracorporeal shock waves with core decompression and bone-grafting. J Bone Joint Surg Am. 2005;87:2380–7. doi: 10.2106/JBJS.E.00174. [DOI] [PubMed] [Google Scholar]
- 47.Massari L, Fini M, Cadossi R, et al. Biophysical stimulation with pulsed electromagnetic fields in osteonecrosis of the femoral head. J Bone Joint Surg Am. 2006;88(Suppl 3):56–60. doi: 10.2106/JBJS.F.00536. [DOI] [PubMed] [Google Scholar]
- 48.Kang P, Gao H, Pei F, et al. Effects of an anticoagulant and a lipid-lowering agent on the prevention of steroid-induced osteonecrosis in rabbits. Int J Exp Pathol. 2010;91:235–43. doi: 10.1111/j.1365-2613.2010.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kerachian MA, Cournoyer D, Harvey EJ, et al. Isolation and characterization of human bone-derived endothelial cells. Endothelium. 2007;14:115–21. doi: 10.1080/10623320701347062. [DOI] [PubMed] [Google Scholar]
- 50.Kerachian MA, Harvey EJ, Cournoyer D, et al. Avascular necrosis of the femoral head: vascular hypotheses. Endothelium. 2006;13:237–44. doi: 10.1080/10623320600904211. [DOI] [PubMed] [Google Scholar]