Abstract
Background
Veliparib is a potent small molecule inhibitor of PARP-1/2, which is cytotoxic in tumor cells with deficiencies in BRCA1 or BRCA2. We studied the clinical activity and toxicity of veliparib in ovarian cancer patients carrying a germline BRCA1 or BRCA2 mutation (gBRCA).
Methods
Eligibility included three or fewer prior chemotherapy regimens, measurable disease and no prior use of a PARP inhibitor. Veliparib was administered at 400 mg orally BID with one cycle being 28 days. The two-stage Simon design was capable of detecting a 25% response probability with 90% power while controlling alpha=10% (at a 10% assumed null response probability).
Results
The median age of the 50 eligible patients was 57 years (range 37–94) and 14, 18, and 18 patients had 1, 2, and 3 prior therapies respectively. Thirty patients (60%) were platinum-resistant. The median number of cycles administered was 6 (1–27). There was one grade 4 thrombocytopenia. Grade 3 adverse events were: fatigue (n=3), nausea (2), leukopenia (1), neutropenia (1), dehydration (1), and ALT (1). Grade 2 events >10% were: nausea (46%), fatigue (26%), vomiting (18%), and anemia (14%). The proportion responding was 26% (90% CI: 16%–38%, CR:2, PR:11); for platinum-resistant and platinum-sensitive patients the proportion responding was 20% and 35%, respectively. The most common reason for treatment discontinuation was progression (62%). Twenty-nine patients are alive; two with SD remain on veliparib. The median PFS is 8.18 months.
Conclusions
The single agent efficacy and tolerability of veliparib for BRCA mutation-associated recurrent ovarian cancer warrants further investigation.
Keywords: veliparib, ovarian cancer, PARP inhibitor, toxicity, Phase II trial, BRCA1, BRCA2 mutation
Introduction
Synthetic lethality was first described by the American geneticist Calvin Bridges in 1922 who noted when crossing fruit flies that certain non-allelic genes were lethal only in combination1. His colleague Theodore Dobzhansky coined the term 20 years later,2 and in 1997 Hartwell et al proposed exploiting this phenomenon as an anti-cancer strategy3. Clinically, one of the more developed synthetic lethality programs has been the administration of poly-(ADP-ribose) polymerase (PARP) inhibitors in patients carrying a mutation in the tumor suppressor genes, BRCA1 or BRCA24. The BRCA1 and BRCA2 proteins both function in the performance of error-free repair of double-strand DNA breaks through homologous recombination5. Loss of functional protein via germline or somatic mutation leads to increased reliance on more error prone DNA repair mechanisms, promoting carcinogenesis. The loss of homologous recombination DNA repair in ovarian carcinomas associated with BRCA1 or BRCA2 mutations leads to increased sensitivity to platinum-based agents and longer survival6. Preclinically, it was observed that cells lacking functional BRCA1 or BRCA2, were up to 1000 fold more sensitive to PARP inhibition than wild type cells7,8. The exact mechanism by which this synthetic lethality is leveraged is not completely understood, but likely occurs due to the functionality of PARP in repairing single strand defects as well as, release of governance over error-prone non-homologous end joining (NHEJ) pathways leading to more frequent mitotic catastrophe and cellular death9.
Clinically, evidence of tumor response has been documented in several clinical settings among germline BRCA mutation carriers, including treatment of measurable breast or ovarian metastases as well as, secondary maintenance in patients with ovarian carcinoma responding to platinum10–15. Veliparib (ABT-888) is a novel small molecule agent that inhibits PARP-1 and PARP-2 at nanomolar concentrations16. It has good oral bioavailability and crosses the blood-brain barrier. In syngeneic and xenograft tumor models, veliparib potentiates temozolomide, platinum compounds, cyclophosphamide, and radiation16.
In the clinical arena veliparib has been predominantly studied in combination with cytotoxic chemotherapy. In the I-SPY2 breast cancer trial, the combination of veliparib and carboplatin graduated with the triple-negative signature17. As documented for other PARP inhibitors, objective responses were observed and indicated further clinical investigation. However, limited information exists regarding the efficacy of single agent veliparib. A single-agent phase I study demonstrated the maximum tolerated dose to be 400 mg BID18–20. In light of these findings and the strong preclinical and clinical rationale, we conducted an open label, phase II, multi-centered clinical trial to evaluate veliparib in a population of BRCA mutation-carrying women with recurrent ovarian cancer. Herein, we demonstrate that veliparib met pre-specified efficacy parameters warranting further clinical investigation.
Methods
Patients
Eligible patients had histologic documentation of primary ovarian, fallopian tube, or primary peritoneal cancer by central pathology review [Gynecologic Oncology Group(GOG) Pathology Committee] and carried a deleterious mutation in BRCA1 or BRCA2 (confirmation was required via clinical report, BRCAnalysis, Myriad Genetics, Salt Lake City, UT). Up to 3 prior cytotoxic regimens were allowed. GOG performance status 0–2 was allowed for one previous regimen; 0–1, for 2–3 regimens. Prior biological therapy was allowed. All patients were required to have measurable disease by Response Evaluation Criteria in Solid Tumors (RECIST 1.1), have discontinued prior chemotherapy (≥3 weeks) and hormonal therapy (≥1 week) before registration, and recovered from effects of recent surgery, radiotherapy, or chemotherapy21. Other eligibility and ineligibility are presented in the Supplemental Methods. All patients signed approved informed consent in accordance with federal, state, and local requirements and provided authorization, permitting release of personal health information.
Treatment
Enrolled patients received veliparib 400 mg orally BID until progression or intolerance. One cycle equaled 28 days. Dose modifications were allowed (300 mg BID and 200 mg BID) for toxicity. Patients were to take veliparib 12 hours apart; dosing delays of ≥4 hours were skipped. Veliparib could be taken with or without food but patients were cautioned about agents inhibiting CYP1A2 or CYP3A4. A pill calendar was kept by the patient and reviewed at each visit, as were concomitant medications. As nausea was an anticipated side effect, patients were instructed on the use of anti-emetics.
Toxicity
Toxicity was monitored before each treatment cycle, with adverse events defined and graded according to Common Terminology Criteria for Adverse Events (version-4). Veliparib was held up to a maximum of 3 weeks for grade 3–4 hematological or non-hematological toxicity. Continuation with dose reduction was allowed if there was recovery to grade 0–1. Grade 2 or greater peripheral neuropathy required reduction of one dose level and delay of subsequent therapy until resolution to grade 0–1 for a maximum of 3 weeks. In addition, veliparib could be held and/or reduced for grade 2 toxicity not adequately controlled by concomitant medication and/or supportive care. It was anticipated patients could have nausea and diarrhea with veliparib limiting dose compliance. As such, investigators were allowed to reduce the dose of veliparib within a treatment cycle for persistent grade 1–2 toxicity. Dose reduction was preferred to dose delay. However, patients experiencing a treatment-related dose delay of ≥ 3 weeks or intolerable toxicity at the lowest dose (200 mg PO BID) were removed from study. No dose escalations were allowed. Treatment was planned until disease progression or adverse events prohibited further therapy.
Evaluation Criteria
All patients had measurable disease and were evaluated for clinical efficacy using Response Evaluation Criteria in Solid Tumors (RECIST) guidelines version 1.121. Target lesions were to be ≥1cm in longest diameter by computed tomography or magnetic resonance imaging, ≥2cm by chest X-Ray, or ≥1 cm by physical exam using calipers, except lymph nodes, which were to be ≥1.5cm on the short axis.22 CA-125 information was collected, but was not used as a criterion for progression. However, patients achieving a complete clinical response of measurable disease had to additionally have a normalized CA-125, if it was elevated upon study entry. Assessment was performed at baseline, every other cycle for the first six months, and every three months thereafter until documentation of disease progression was obtained or as clinically indicated.
Statistical Methods
The primary endpoint of this trial was objective tumor response as assessed by the investigator. The null hypothesis relating to uninteresting levels of activity was determined from results of a study evaluating a PARP agent, previously reported in the literature and an analysis of a historical control of recurrent ovarian cancer patients with high grade serous cell type23. The null hypothesis specified the probability of a patient experiencing a tumor response to be ≤10%. Interesting levels of the proportion responding under the alternative hypothesis was ≥25%. To evaluate these hypotheses in a two-stage design, a method provided by Chen and Ng was used to determine if there were sufficient objective responses to continue study into the second stage and deem the drug worthy of further investigation22. The targeted accrual for stage 1 was 23 (allowed to deviate from 19–26 patients24) and at least three responses were required before the study would open to the second stage. If met, then 48 patients was the targeted accrual (allowed to deviate 44–51 patients) requiring at least 8 responses before declaring the regimen worthy of further investigation. This study had a 45.9% probability of early termination under the null hypothesis. The study had a level of significance of 10.2% with 92.1% power under the alternative with true probability of response equal to 25%.
Secondary objectives were progression-free survival (PFS), event-free survival (EFS) and overall survival (OS), the proportion of patients who survived progression-free/event-free for at least six months (PFS6/EFS6), and the frequency and severity of treatment-related adverse events. PFS was defined as the time from study enrollment until progression or death; EFS was defined as the time until progression, death, or subsequent therapy, and OS was defined as the time from registration until death or last visit. Kaplan-Meier estimates of the survival function were provided for both PFS and OS. Estimated proportions between groups were compared by Fisher’s exact test.
Exploration of response modifiers, such as single nucleotide polymorphisms (SNPs) in DNA repair genes, PARP1 expression levels, and P-glycoprotein transporter regulation will be reported subsequently.
RESULTS
Patient’s characteristics
Fifty-two patients were enrolled from April 2012 through November 2012; two were excluded, one for inadequate pathology and one for a clerical error. This left 50 evaluable patients for toxicity and response. Table 1 presents patient characteristics. Of note, 72% of patients received two or three prior regimens of therapy and 60% were platinum-resistant. As expected, the majority of patients had serous epithelial cancer and aged younger (median 57 years, range:37–94) than typical recurrent ovarian cancer patients without BRCA mutations.
Table 1.
Patient Demographics (N=50)
| Characteristic | Category | No. of Cases | % of Cases |
|---|---|---|---|
| Age | 30–39 | 2 | 4·0 |
| 40–49 | 15 | 30·0 | |
| 50–59 | 14 | 28·0 | |
| 60–69 | 15 | 30·0 | |
| 70–79 | 2 | 4·0 | |
| 80–89 | 1 | 2·0 | |
| 90–99 | 1 | 2·0 | |
| Race | Unspecified | 2 | 4·0 |
| Asian | 1 | 2·0 | |
| African American | 2 | 4·0 | |
| Hispanic | 2 | 4·0 | |
| White | 43 | 86·0 | |
| Performance Status | 0 | 33 | 66·0 |
| 1 | 17 | 34·0 | |
| Site of Disease | Ovary | 44 | 88·0 |
| Fallopian tube | 1 | 2·0 | |
| Peritoneum | 5 | 10·0 | |
| Cell Type | Serous | 41 | 82·0 |
| Mixed Epithelial | 2 | 4·0 | |
| Undifferentiated | 2 | 4·0 | |
| Adenocarcinoma, NOS | 4 | 8·0 | |
| Other | 1 | 2·0 | |
| Prior Chemotherapy | 1 Prior Regimen | 14 | 28·0 |
| 2 Prior Regimens | 18 | 36·0 | |
| 3 Prior Regimens | 18 | 36·0 | |
| Prior Radiation | No | 46 | 92·0 |
| Yes | 4 | 8·0 | |
| Prior Immunotherapy | No | 47 | 94·0 |
| Yes | 3 | 6·0 | |
| Prior Surgery | No | 1 | 2·0 |
| Yes | 49 | 98·0 | |
| Platinum Sensitivity* | Platinum Resistant | 30 | 60·0 |
| Overall Platinum Sensitive | 20 | 40·0 | |
| GOG Platinum Sensitive | 13 | 26·0 | |
| Platinum Sensitive | 7 | 14·0 | |
| Prior Platinum Regimens | 1 Prior Regimen | 16 | 32·0 |
| 2 Prior Regimens | 28 | 56·0 | |
| 3 Prior Regimens | 6 | 12·0 | |
| BRCA Mutation | BRCA1 | 39 | 78·0 |
| BRCA2 | 11 | 22.0 |
”Platinum-resistant” patients are those in whom disease has progressed within 6 months of completing platinum-based therapy. “Overall Platinum Sensitive” is the sum of “GOG Platinum-sensitive” patients, which comprises those in whom disease recurrence was documented between 6 and 12 months following completion of the last platinum-based therapy and “Platinum-Sensitive” patients are those in whom disease recurrence was documented more than 12 months from completion of the last platinum-based therapy.
Adverse Events
The median dose intensity over all patients across all cycles was 17525 mg/cycle (first and second quartiles were 12000 and 22239 mg/cycle, respectively. This translates into a median of 78.2% of the targeted dose (Q1 and Q3 are 54 and 99%, respectively). A plot of the empirical cumulative distribution function is provided in Supplemental Figure 1 and 2. Table 2 lists hematological and non-hematological toxicities. There were no fatal events observed related to the study agent. The most common hematological toxicity was anemia and leukopenia, but was predominantly grade 1–2. There was one grade 3 neutropenia and one grade 4 thrombocytopenia, both of which resolved with dose delay and dose reduction. As expected, the most common non-hematological event was gastrointestinal-related. While there were no grade 4 events, 25 (50%) of the cohort reported grade 2 (N=23), or grade 3 (N=2) nausea and nine (18%) had grade 2 vomiting. These were most problematic in the first cycle (nausea, N=41; vomiting, N=24) and easily controlled with brief dose interruption and/or dose reduction. However, 32 (78%) and 9 (38%) of these patients had nausea or vomiting, respectively, in subsequent cycles. Antiemetic use was not captured in this cohort. In addition, 16 patients (grade 2, N=13, grade 3, N=3) reported fatigue. In all, 11 evaluable patients (22%) were removed from study due to toxicity after a median 2 cycles (range 1–6, Table 3). Overall, there were 11 patients who experienced dose delays over 22 total cycles. Most of these delays were due to scheduling issues, however, 2 cycles were delayed due to adverse events. Thirty-one patients (N=95 total events) underwent dose reductions, predominately for GI toxicity (N=33 events) and hepatic toxicity (N=2 events).
Table 2.
Maximum Grade Adverse Events Observed on Trial (N=50)
| AE Category | 0 | 1 | 2 | 3 | 4 | 5 | Total |
|---|---|---|---|---|---|---|---|
| Leukopenia | 30 | 14 | 5 | 1 | 0 | 0 | 50 |
| Thrombocytopenia | 41 | 8 | 0 | 0 | 1 | 0 | 50 |
| Neutropenia | 35 | 9 | 5 | 1 | 0 | 0 | 50 |
| Anemia | 26 | 17 | 7 | 0 | 0 | 0 | 50 |
| Other Investigations | 34 | 11 | 2 | 3 | 0 | 0 | 50 |
| Ear and labyrinth | 45 | 4 | 1 | 0 | 0 | 0 | 50 |
| Eye | 46 | 4 | 0 | 0 | 0 | 0 | 50 |
| Nausea | 7 | 18 | 23 | 2 | 0 | 0 | 50 |
| Vomiting | 21 | 20 | 9 | 0 | 0 | 0 | 50 |
| Other Gastrointestinal | 17 | 24 | 9 | 0 | 0 | 0 | 50 |
| General and administration site | 16 | 18 | 13 | 3 | 0 | 0 | 50 |
| Infections/infestations | 48 | 0 | 2 | 0 | 0 | 0 | 50 |
| Metabolism/nutrition | 26 | 18 | 5 | 1 | 0 | 0 | 50 |
| Musculoskeletal/connective tissue | 38 | 12 | 0 | 0 | 0 | 0 | 50 |
| Peripheral sensory neuropathy | 48 | 2 | 0 | 0 | 0 | 0 | 50 |
| Nervous system | 27 | 17 | 6 | 0 | 0 | 0 | 50 |
| Psychiatric | 35 | 11 | 4 | 0 | 0 | 0 | 50 |
| Renal/urinary | 49 | 0 | 1 | 0 | 0 | 0 | 50 |
| Reproductive/breast | 49 | 1 | 0 | 0 | 0 | 0 | 50 |
| Respiratory/thoracic/mediastinal | 45 | 4 | 1 | 0 | 0 | 0 | 50 |
| Skin/subcutaneous | 42 | 7 | 1 | 0 | 0 | 0 | 50 |
| Vascular disorders | 45 | 4 | 1 | 0 | 0 | 0 | 50 |
Other investigations included laboratory parameters such as increased alanine aminotransferase (N=1 grade 3, N=4 grade 1) and aspartate aminotransferase (N=4 grade 1, N=1 grade 2), and weight loss (N=2, grade 1)
Table 3.
Treatment Outcome Parameters (N=50)
| Characteristic | Category | No. | % |
|---|---|---|---|
| Response | Complete response | 2 | 4·0 |
| Partial response | 11 | 22·0 | |
| Stable disease1 | 24 | 48·0 | |
| Increase disease | 7 | 14·0 | |
| Indeterminate | 6 | 12·0 | |
| PFS > 6 Months | No | 23 | 46·0 |
| Yes | 27 | 54·0 | |
| EFS > 6 Months | No | 28 | 56·0 |
| Yes | 22 | 44·0 | |
| Cycles of Treatment | 1 | 8 | 16·0 |
| 2 | 6 | 12·0 | |
| 4 | 8 | 16·0 | |
| 5 | 2 | 4·0 | |
| 6 | 7 | 14·0 | |
| 9+ | 19 | 38·0 | |
| Off Study | No1 | 4 | 8·0 |
| Yes | 46 | 92·0 | |
| Why Off Study | On Study/Unspecified | 4 | 8·0 |
| Disease progression | 31 | 62·0 | |
| Refused further treatment | 4 | 8·0 | |
| Toxicity | 11 | 22·0 | |
| Alive | 29 | 58·0 | |
| Dead | From disease | 19 | 38·0 |
| Undetermined | 2 | 4·0 | |
Two patients with stable disease are still on study therapy and could respond. However, all of these patients have been on study for at least 6 months.
Clinical Activity
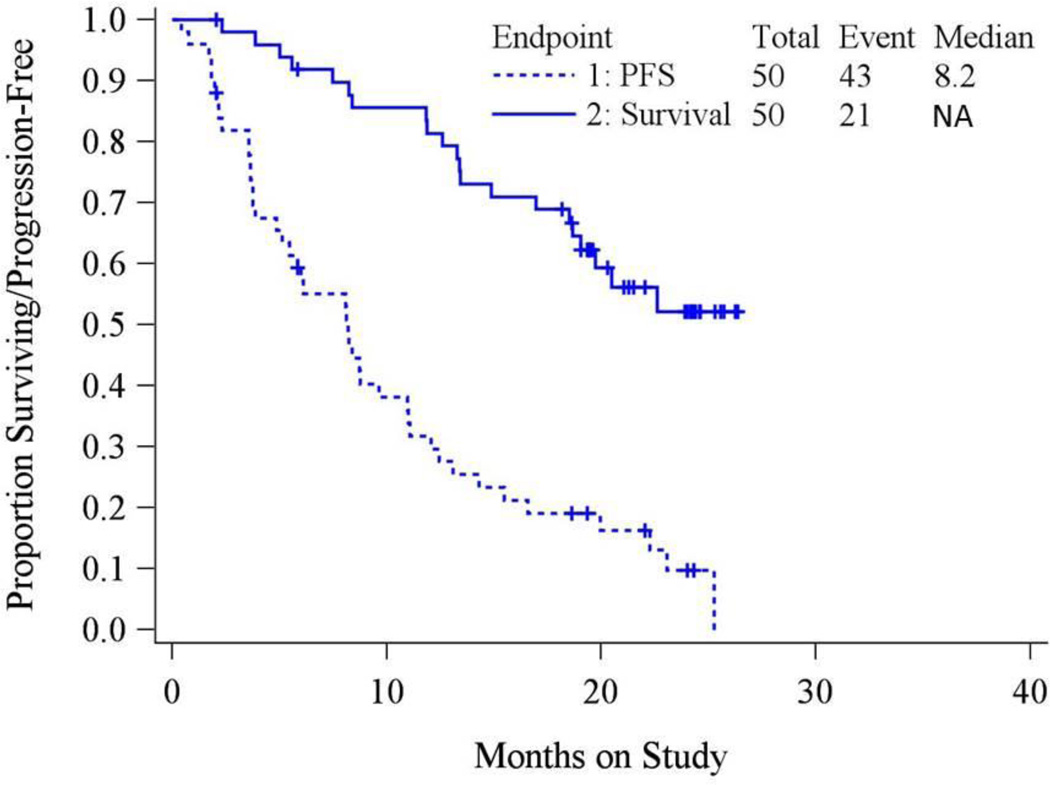
Table 3 details further clinical efficacy parameters. More than 424 monthly doses of veliparib were administered to this cohort with the median number of cycles being 6 (Inner Quartile Range (IQR):2–12 cycles). There were two complete and 11 partial responses producing an overall response rate of 26% (90% Confidence Interval (CI):16%–38%). Stable disease was observed in 24 other patients (48%), and includes 2 patients still on study treatment for more than 6 months. The most common reason for treatment discontinuation was disease progression, occurring in 31 (62%) of participants. Twenty-seven patients were progression-free at six months (PFS6:54%; 90% CI:41%–66%). Figure 1 presents the Kaplan-Meier survival PFS and OS curves for evaluable patients; median PFS is 8.18 months and median OS is not estimable at this time (likely >26 months). PFS based on CA125 (GCIG) criteria is presented in Supplementary Figure 3. The median PFS was 23.4 months, but this analysis is considered unreliable due to the low number of events (n=14) and the potential for non-random censoring.
Figure 1.
Progression-Free Survival and Overall Survival are presented for the evaluable cohort (N=50). Median PFS is 8.11 months and ranges from 0.43 to 19.55 months; Median OS is 19.7 and ranges (to date) from 2.3 to 19.7 months.
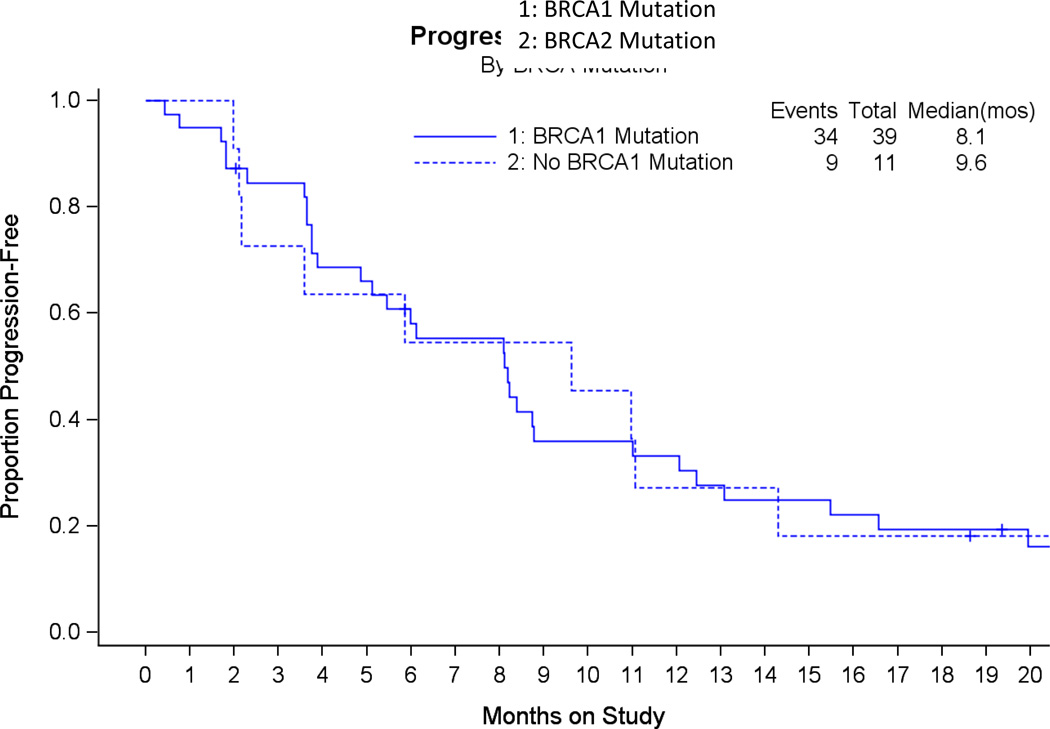
Since PARP inhibitor clinical efficacy has been proportionally associated with platinum-sensitivity we analyzed this variable (defined as progression on or within 6 months of completion of the last platinum-based regimen) relative to veliparib response. As can be appreciated in Table 4, veliparib demonstrated objective responses in both patients with platinum-resistant (N=6/30, 20%; 90% CI:9%–36%) and platinum-sensitive (N=7/20, 35%; 90%CI:18%–56%) recurrent disease; this difference was not significantly different (Fisher’s Exact P=0.33). Similarly, the proportion responding between BRCA1 and BRCA2 mutation carriers was similar (26% and 27%, respectively) as was the PFS. (Figure 2). Finally, since up to 3 prior lines of therapy were allowed, we analyzed the frequency of cases responding by the number of prior regimens. The proportion responding was 43%, 22% and 17% for 1, 2, and 3 prior lines of therapy respectively. The Cochran-Armitage trend test was borderline suggestive (0.05 < exact onesided p-value < 0.10). Spearman’s correlation coefficient was −0.23 (asymptotic 95% CI −0.50 ~ 0.04).
Table 4.
Best Clinical Response and Progression-Free Survival by Platinum-Sensitivity Status
| Platinum Sensitivity | ||
|---|---|---|
| Response | Resistant N (% of category) |
Sensitive N (% of category) |
| Complete | 0 | 2 (10) |
| Partial | 6 (20) | 5 (25) |
| Stable Disease | 16 (53) | 8 (40) |
| Progression | 6 (20) | 1 (5) |
| Indeterminate | 2 (7) | 4 (20) |
| PFS median | 5.8 mos | 11.0 mos |
| Total | 30 | 20 |
PFI: Platinum-Free Interval. PFS: Progression-Free Survival.
Figure 2.
Progression-Free Survival is illustrated by genotype in the evaluable cohort (N=50). There was no significant difference in PFS between these cohorts with the median PFS in BRCA1 and BRCA2 patients being 8.1 and 5.8 months, respectively.
DISCUSSION
This multicenter, open-label phase II clinical trial demonstrated single agent activity of veliparib among women with BRCA-mutation positive recurrent epithelial ovarian, fallopian tube or primary peritoneal cancer and represents the first such study with veliparib. The trial was conducted in this setting to provide rationale for further clinical development. The observed objective response rate of 26% met our primary endpoint, which was established by outlining a sufficient level of clinical activity to distinguish veliparib from other ineffective therapies evaluated in recurrent ovarian cancer patients. Of interest, veliparib produced a 20% response rate in patients with platinum-resistant disease, and despite a trend toward lower response rates following each line of subsequent therapy, veliparib was associated with responses across a broad spectrum of patients with recurrent disease. This may be due to disassociation of mechanisms inducing platinum resistance that do not similarly define PARP inhibitor activity. In addition, an induced platinum-resistance phenotype may revert under stem cell clonal expansion with subsequent lines of non-platinum therapy26,27. Previous studies have also suggested that PFS and OS may be better for gBRCA2 carriers reflecting greater vulnerability to platinum-based chemotherapy. Although a small sample, we observed similar progression-free survival hazard rates in gBRCA1 and gBRCA2 carriers. Based on these observations, neither selection parameter appears definitive to dissuade use of veliparib in these settings28.
Veliparib use produced several adverse events, particularly gastrointestinal. However, its toxicity profile is similar to other PARP inhibitors, including olaparib, the most extensively studied agent in this class10,12. Compared to olaparib, veliparib appears to have similar rates of nausea but less hematologic toxicity (e.g., 2% grade 3 or 4 neutropenia versus 9% with olaparib in a similar setting)23. As noted, the median dose intensity was 17525 mg/cycle and 78% of patients had dose modification, predominately for nausea and vomiting. As an orally administered agent, these adverse events can be troubling in maintaining compliance with dose administration. We sought to aggressively institute preemptive nausea control interventions, as well as, intra-cycle dose reduction. This appears to have significantly improved tolerance without significant additional treatment delay. In addition, while there was frequent dose modification in this trial due to nausea, it appeared to abate after the first two weeks of treatment. Although, no dose re-escalations were allowed in this trial, we would recommend future trials of single agent veliparib initiate therapy at 400 mg p.o. b.i.d. but allow later dose re-escalation for a nauseainduced reduction.
PARP inhibitors have been of great interest in patients who carry a germline mutation in the BRCA genes (gBRCA) based on the preclinical synthetic lethality seen in this setting7,8. Subsequent reports have identified that somatic deletion in BRCA (sBRCA) also confers sensitivity to this class of agent11,29. A recent update of the randomized phase II olaparib maintenance trial provided an expanded analysis (79% of randomized population) of gBRCA or sBRCA status. In this analysis 136 of 265 patients carried either gBRCA or sBRCA. Relative to control, those patients receiving olaparib maintenance had a reduction in the hazard of progression by 82% (HR:0.18, 95% CI:0.1–0.31). Since only approximately 15% of ovarian cancer patients carry gBRCA, the expansion of the potential target audience by demonstrating efficacy among those with sBRCA, as well as somatic events impairing other genes governing homologous recombination (HR) could greatly expand the target population. It is estimated that an additional 35% of primary ovarian cancer patients develop such somatic events and could achieve objective benefit from this class of therapy.30,31
Given the strong connection between HR deficiency and response due to PARP inhibitors, it is unclear why patients, particularly gBRCA carriers, are either primarily resistant to PARP inhibition or develop resistance on therapy. In our study of gBRCA carriers, 14% were primarily resistant to veliparib and 28% ultimately progressed on therapy. Previous preclinical work has suggested that relative to primary ovarian tumors, metastatic cells may be less sensitive PARP inhibition32. Understanding these processes would help predict those most likely to benefit from this line of therapy. At least 4 different mechanisms of innate or acquired PARP resistance have been postulated33. The best defined has been discovery of a secondary mutation in the BRCA gene that either restores it to wild-type status or restores BRCA gene functionality via alterations in its open reading frame (ORF)34–36. This aberration in the ORF nearly always encodes the C-terminal RAD51 binding domain thus promoting protein translation. The frequency to which mutational restoration of the ORF occurs is not well known, but may be related in part to primary platinum-refractory disease and those who develop secondary platinum-resistance35. A second resistance mechanism related to BRCA1 lies in the loss of 53BP1, a gene that regulates and promotes non-homologous end joining (NHEJ)37. Under normal circumstances, PARP inhibition would promote this error-prone repair mechanism leading to cancer cell cytotoxicity. However, it has been recently shown that loss of 53BP1 expression promotes HR competency in gBRCA1-mutated cells. Of interest, this loss promoted sensitivity to DNA crosslinking agents, such as platinum and if confirmed could be used as a new treatment paradigm37,38. Third, cellular transport mechanisms that impact intracellular drug accumulation may also act to export PARP inhibitors before initiating cytotoxicity. It has been shown that PARP inhibitor responses are altered by ATP-binding cassette (ABC) transporters, such as P-glycoprotein (P-gp)39. Preclinical studies pharmacologically inhibiting of P-gp (e.g. verapamil) restored PARP inhibitor response. These observations support clinical investigation of concomitant administration or P-gp and PARP inhibitors, as well as, pharmaceutical development of PARP inhibitors that are not P-gp substrates. Finally, levels of PARP expression may influence activity of these agents and may implicate differential activity among PARP inhibitors. While both single strand breaks and DNA-PARP complexes are cytotoxic, the later may be more so. Trapping PARP-DNA complexes at the site of DNA damage appears to confer greater cytotoxicity, thus less enzymatic activity from decreased intrinsic or acquired PARP expression may impact an agent’s cytotoxicity40. The overall impact in exploring each of these mechanisms is establishing better patientdrug matching and developing new avenues of treatment based on acquired events from treatment. Since there is potential for prolonged and repeated therapy with this class of agent alone and in combination with cytotoxic therapy, careful assessment of attendant and emergent toxicity will need to be conducted to fully understand their therapeutic ratio.
In summary, veliparib demonstrated significant treatment effects (objective response and delay in progression) with an acceptable toxicity profile in women with gBRCA mutant epithelial ovarian cancer. We acknowledge that an open-label single arm trial has limitations in assessing magnitude of effect and toxicity against control comparators41, and can be subject to investigator bias. However, our intent was exploratory in a well-defined genotyped population and we established parameters of desired clinical activity from similarly treated patients on other GOG trials. In this regard, the trial met its pre-specified level of clinical activity to warrant further investigation, which is already underway as veliparib now joins several other PARP inhibitors (e.g. olaparib NCT01844986, NCT01874353), niraparib NCT 01847274, rucaparib NCT01968213) being studied in the phase III setting among patients with ovarian cancer.
Future analysis of response characteristics relative to alterations in the genes governing homologous recombination will provide additional support to further hone patient selection in coming clinical investigations.
Supplementary Material
RESEARCH HIGHLIGHTS.
Veliparib demonstrated single agent activity among recurrent ovarian cancer patients carrying a germline BRCA1/2 mutation
Adverse events were observed but generally mild and managed conservatively
Clinical responses were observed among enrolled with platinum-sensitive and -resistant recurrent disease
ACKNOWLEDGEMENTS
This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group Administrative Office (CA 27469) and the Gynecologic Oncology Group Statistical and Data Center (CA 37517). This was also supported, in part, by NIH grant P50 CA098258.
Dr. Coleman is supported in part by the Ann Rife Cox Chair in Gynecology. We also acknowledge Abbvie (North Chicago, Ill) for providing the investigational drug.
Dr. Coleman reports that he has received funding from Clovis as well as non-financial support from AstraZeneca and Merck. Dr. Coleman also reports funding from Merck, Janssen, Amgen, Novartis, Merrimack, Millennium, OncoMed, Array, and EMD Serono, Inc. Dr. Carol Aghajanian received an honorarium as a one-time ad board member in addition to travel expenses. Additionally, Dr. Aghajanian received funding for travel from Abbvie for clinical trial planning meetings. Dr. Thomas Rutherford reports that he is a member of GOG#280 clinical trial at Yale University.
Appendix
The following Gynecologic Oncology Group member institutions participated in this study: Duke University Medical Center, Florida Hospital Cancer Institute Protocol Office, Johns Hopkins University, Cancer Care Northwest - Spokane South, Tacoma General Hospital, Pacific Gynecology Specialists, Providence Regional Cancer Partnership, Seattle Cancer Care Alliance, Northwest Hospital, Abramson Cancer Center of The University of Pennsylvania, Norton Health Care Pavilion – Downtown, Hope Women's Cancer Centers-Ashville, Stanford University Hospitals and Clinics, Cleveland Clinic Cancer Center/Fairview Hospital, Washington University School of Medicine, Memorial Sloan Kettering Cancer Center, Ohio State University Medical Center, M D Anderson Cancer Center, University of Oklahoma Health Sciences Center, Baylor All Saints Medical Center at Fort Worth, University of Chicago, Loyola University Medical Center, Summa Akron City Hospital/Cooper Cancer Center, Yale University, John Muir Medical Center-Concord Campus, Northside Hospital, UCSF-Mount Zion, Mercy Hospital - Coon Rapids, Memorial Medical Center, Christiana Care Health System-Christiana Hospital, McFarland Clinic PC-William R Bliss Cancer Center
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This trial was registered at clinicaltrials.gov (NCT01540565)
Conflict of Interest
The authors wish to disclose that there are no conflicts of interest with the exception of Dr. Robert Coleman, Dr. Carol Aghajanian and Dr. Thomas Rutherford as detailed below.
REFERENCES
- 1.Bridges CB. The origin of variation. Amer Nat. 1922;56:51–63. [Google Scholar]
- 2.Dobzhansky T. Genetics of natural populations. XIII. Recombination and variability in populations of Drosophila pseudoobscura. Genetics. 1946;31:269–290. doi: 10.1093/genetics/31.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartwell LH, Szankasi P, Roberts CJ, et al. Integrating genetic approaches into the discovery of anticancer drugs. Science. 1997;278:1064–1068. doi: 10.1126/science.278.5340.1064. [DOI] [PubMed] [Google Scholar]
- 4.Banerjee S, Kaye SB, Ashworth A. Making the best of PARP inhibitors in ovarian cancer. Nat Rev Clin Oncol. 2010;7:508–519. doi: 10.1038/nrclinonc.2010.116. [DOI] [PubMed] [Google Scholar]
- 5.Bajrami I, Frankum JR, Konde A, et al. Genome-wide Profiling of Genetic Synthetic Lethality Identifies CDK12 as a Novel Determinant of PARP1/2 Inhibitor Sensitivity. Cancer Res. 2014;74:287–297. doi: 10.1158/0008-5472.CAN-13-2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jervis S, Song H, Lee A, et al. Ovarian cancer familial relative risks by tumour subtypes and by known ovarian cancer genetic susceptibility variants. J Med Genet. 2014;51:108–113. doi: 10.1136/jmedgenet-2013-102015. [DOI] [PubMed] [Google Scholar]
- 7.Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 8.Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 9.Patel AG, Sarkaria JN, Kaufmann SH. Nonhomologous end joining drives poly(ADP-ribose) polymerase (PARP) inhibitor lethality in homologous recombination-deficient cells. Proc Natl Acad Sci U S A. 2011;108:3406–3411. doi: 10.1073/pnas.1013715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 11.Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366:1382–1392. doi: 10.1056/NEJMoa1105535. [DOI] [PubMed] [Google Scholar]
- 12.Kaye SB, Lubinski J, Matulonis U, et al. Phase II, open-label, randomized, multicenter study comparing the efficacy and safety of olaparib, a poly (ADP-ribose) polymerase inhibitor, and pegylated liposomal doxorubicin in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer. J Clin Oncol. 2012;30:372–379. doi: 10.1200/JCO.2011.36.9215. [DOI] [PubMed] [Google Scholar]
- 13.Oza A, Cibula D, Benzaquen A, et al. Olaparib plus paclitaxel and carboplatin followed by olaparib maintenance treatment in patients with platinum-sensitive recurrent serous ovarian cancer: A randomized, open-label Phase II study. J Clin Oncol. 2012;30(15 suppl):5001. [Google Scholar]
- 14.Gelmon KA, Tischkowitz M, Mackay H, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011;12:852–861. doi: 10.1016/S1470-2045(11)70214-5. [DOI] [PubMed] [Google Scholar]
- 15.Sandhu SK, Schelman WR, Wilding G, et al. The poly (ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2013;14:882–892. doi: 10.1016/S1470-2045(13)70240-7. [DOI] [PubMed] [Google Scholar]
- 16.Donawho CK, Luo Y, Luo Y, et al. ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin Cancer Res. 2007;13:2728–2737. doi: 10.1158/1078-0432.CCR-06-3039. [DOI] [PubMed] [Google Scholar]
- 17.Positive results for drug combo veliparib in I-SPY 2 trial. Cancer Discov. 2014;4(2):OF2. doi: 10.1158/2159-8290.CD-NB2013-182. Epub 2014 Jan 9. (No authors listed) [DOI] [PubMed] [Google Scholar]
- 18.Kummar S, Ji J, Morgan R, et al. A phase I study of veliparib in combination with metronomic cyclophosphamide in adults with refractory solid tumors and lymphomas. Clin Cancer Res. 2012;18:1726–1734. doi: 10.1158/1078-0432.CCR-11-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kummar S, Chen A, Ji J, et al. Phase I study of PARP inhibitor ABT-888 in combination with topotecan in adults with refractory solid tumors and lymphomas. Cancer Res. 2011;71:5626–5634. doi: 10.1158/0008-5472.CAN-11-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veliparib plus temozolomide in metastatic melanoma trends toward increased PFS but results are not statistically significant. Oncology (Williston Park) 2011;25:1213–1232. [PubMed] [Google Scholar]
- 21.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 22.Chen TT, Ng TH. Optimal flexible designs in phase II clinical trials. Stat Med. 1998;17:2301–2312. doi: 10.1002/(sici)1097-0258(19981030)17:20<2301::aid-sim927>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 23.Audeh MW, Carmichael J, Penson RT, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010;376:245–251. doi: 10.1016/S0140-6736(10)60893-8. [DOI] [PubMed] [Google Scholar]
- 24.Sill M. Technical Report 04-01. University at Buffalo; 2001. Adapting rejection boundaries when the targeted accrual is not attained in 2-stage phase II clinical trials. [Google Scholar]
- 25.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 26.Bryant CS, Kumar S, Spannuth W, et al. Feasibility of extension of platinum-free interval with weekly bolus topotecan and subsequent platinum retreatment outcomes in recurrent ovarian cancer. Arch Gynecol Obstet. 2011;283:361–367. doi: 10.1007/s00404-010-1462-9. [DOI] [PubMed] [Google Scholar]
- 27.Ledermann JA. Benefits of enhancing the platinum-free interval in the treatment of relapsed ovarian cancer: more than just a hypothesis? Int J Gynecol Cancer. 2011;21:S9–S11. doi: 10.1097/IGC.0b013e318217b30b. [DOI] [PubMed] [Google Scholar]
- 28.Yang D, Khan S, Sun Y, et al. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA. 2011;306:1557–1565. doi: 10.1001/jama.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JM, Ledermann JA, Kohn EC. PARP Inhibitors for BRCA1/2 mutation-associated and BRCAlike malignancies. Ann Oncol. 2014;25:32–40. doi: 10.1093/annonc/mdt384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hennessy BT, Timms KM, Carey MS, et al. Somatic mutations in BRCA1 and BRCA2 could expand the number of patients that benefit from poly (ADP ribose) polymerase inhibitors in ovarian cancer. J Clin Oncol. 2010;28:3570–3576. doi: 10.1200/JCO.2009.27.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pennington KP, Walsh T, Harrell MI, et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014;20:764–775. doi: 10.1158/1078-0432.CCR-13-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stordal B, Timms K, Farrelly A, et al. BRCA1/2 mutation analysis in 41 ovarian cell lines reveals only one functionally deleterious BRCA1 mutation. Mol Oncol. 2013;7:567–579. doi: 10.1016/j.molonc.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lord CJ, Ashworth A. Mechanisms of resistance to therapies targeting BRCA-mutant cancers. Nat Med. 2013;19:1381–1388. doi: 10.1038/nm.3369. [DOI] [PubMed] [Google Scholar]
- 34.Edwards SL, Brough R, Lord CJ, et al. Resistance to therapy caused by intragenic deletion in BRCA2. Nature. 2008;451:1111–1115. doi: 10.1038/nature06548. [DOI] [PubMed] [Google Scholar]
- 35.Norquist B, Wurz KA, Pennil CC, et al. Secondary somatic mutations restoring BRCA1/2 predict chemotherapy resistance in hereditary ovarian carcinomas. J Clin Oncol. 2011;29:3008–3015. doi: 10.1200/JCO.2010.34.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakai W, Swisher EM, Karlan BY, et al. Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature. 2008;451:1116–1120. doi: 10.1038/nature06633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bunting SF, Callen E, Wong N, et al. 53BP1 inhibits homologous recombination in Brca1-deficient cells by blocking resection of DNA breaks. Cell. 2010;141:243–254. doi: 10.1016/j.cell.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bunting SF, Callen E, Kozak ML, et al. BRCA1 functions independently of homologous recombination in DNA interstrand crosslink repair. Mol Cell. 2012;46:125–135. doi: 10.1016/j.molcel.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rottenberg S, Jaspers JE, Kersbergen A, et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc Natl Acad Sci U S A. 2008;105:17079–17084. doi: 10.1073/pnas.0806092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murai J, Huang SY, Das BB, et al. Trapping of PARP1 and PARP2 by Clinical PARP Inhibitors. Cancer Res. 2012;72:5588–5599. doi: 10.1158/0008-5472.CAN-12-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2014 Nov 3; doi: 10.1200/JCO.2014.56.2728. Pii:JCO.2014.56.2728. [ePub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.