Abstract
Aims
To explore the relationship between chronic pain and characteristics, behaviors, and psychological status of suburban Connecticut injection drug users.
Methods
Cross-sectional study with quantitative interview and serological testing for HIV and hepatitis B and C in 456 individuals who injected drugs in the past month were dichotomized into those reporting current chronic pain of at least six months duration and all others. The interview covered (i) sociodemographics, (ii) injection drug use, (iii) interactions with drug treatment, criminal justice, and harm reduction, (iv) screening for alcohol use, chronic pain, anxiety, and depression, and (v) knowledge regarding HIV, hepatitis B (HBV) and C (HCV), and opioid overdose. Serological testing for HIV, HBV, and HCV was conducted.
Results
One-third (n=143) reported chronic pain. These individuals differed significantly from those not reporting chronic pain on characteristics that included older age, lower educational achievement, and injection of pharmaceutical opioids. They also reported experiencing more psychological and family problems on the ASI and higher levels of depression and anxiety. Four of five individuals with chronic pain (117 of 148 providing chronology data) reported non-medical opioid use prior to the onset of chronic pain.
Conclusions
Chronic pain is common among drug injectors in our study population although it was unusual for chronic pain to have preceded non-medical opioid use. Psychological problems in injectors with co-occurring chronic pain are likely pose significant complications to successful treatment for substance abuse, pain, or infectious disease treatment.
Keywords: Injection drug use, chronic pain, suburbs, opioids, depression, anxiety
1. INTRODUCTION
The relationship between chronic pain and addictive disorders has received increasing attention as both the use and the abuse of pharmaceutical opioids have increased. While rates of addiction among those with chronic pain seem to be no different than those in the general population (Weaver and Schnoll, 2002), it also appears that chronic pain may be more prevalent in those with substance abuse history (Jamison et al., 2000; Rosenblum et al., 2003; Warner, 2012). The relationship between pain and addiction is remarkably complex, but most research has focused on clinical populations seeking treatment for pain (Cicero et al., 2008; Krashin et al., 2012; Sehgal et al., 2012). A more modest corpus of research has addressed the unique challenges introduced when treating chronic pain among patients receiving addiction treatment (for review see Pohl and Smith, 2012). In sum, most of what we have learned about the relationship between chronic pain and addiction has been among those who have already assumed a patient role. What little is known about non-clinical populations who have untreated pain and substance use disorders come from urban and rural populations (Davis and Johnson, 2008; Havens et al., 2009; Wunsch et al., 2008), but nothing about suburban people who inject drugs (PWID) who differ from their urban counterparts in that none is homeless or resides in neighborhoods that are deemed most disadvantaged, the vast majority is white, they are a decade younger on average than sample drawn from urban centers (Heimer et al., 2014).
Toward reducing this gap, we explored the intersection of chronic pain and ongoing injection drug use as part of a longitudinal study in a sample of individuals who reside in suburban communities in southwestern Connecticut and who inject drugs. The vast majority injected heroin, and one-third injected more than one substance in the thirty days prior to their entry into our study (Akselrod et al., 2014; Heimer et al., 2014). Unlike urban areas, individuals reported little contact with harm reduction services, but like their urban counterparts the majority had experience both in the criminal justice system and with substance abuse treatment programs. Previous findings (Heimer et al., 2012) led us to include questions concerning chronic pain in our baseline survey of suburban residents who inject drugs. In this report, we use the data from baseline interviews to focus on four subjects: the rates of chronic pain among suburban injectors, characteristics that distinguish those with and without chronic pain, the relationships between chronic pain and psychological problems, and the temporal relationship between the onsets of opioid misuse and chronic pain.
2. METHODS
The study was designed and conducted as a multiple-methods, longitudinal study of adults who inject drugs and reside in the suburbs of Fairfield and New Haven Counties in Connecticut. The study, all associated materials, and the informed consent procedures were approved by the Yale Human Investigations Committee. Participants were required to meet the following inclusion criteria before providing informed consent: (1) self-reported injection drug use within the past 30 days with evidence of injection stigmata when possible, (2) ≥18 years of age, (3) proof of residence for at least six months prior to date of enrolment in a Fairfield or New Haven County town, excluding the urban centers of Bridgeport, Danbury, New Haven, Norwalk, Stamford, or Waterbury, (4) willingness to participate in a longitudinal study that involved completing a survey and providing a blood specimen for serological testing, and (5) competence to provide informed consent. Participants were interviewed semi-annually, and serological testing occurred annually. The current manuscript reports on the procedures and preliminary analytic results from the baseline assessment. Participants received $50 for completing the entire baseline assessment. More information on methods can be found in published reports (Akselrod et al., 2014; Heimer et al., 2014).
2.1. Study Sample
Between November 1, 2008 and January 31, 2012 a total of 462 participants were enrolled in the study. To our knowledge, this is the largest sample of active injectors residing in suburban locales. Respondent-driven sampling (RDS) procedures (Heckathorn, 2002; Salganik and Heckathorn, 2004) were used as the recruitment strategy; 82 seeds were recruited from social service agencies or among those who newly entering a local substance abuse treatment program; 46 seeds (56%) were non-productive (i.e., did not generate referral chains). This analysis includes data from the 456 individuals who answered the question about chronic pain.
2.2. Data Collection, Measures, and Laboratory Procedures
Participants completed a two-part baseline interview beginning with a face-to-face, semi-structured interview to collect information more easily obtained with some interviewer assistance followed by a self-administered, structured survey using Audio-Computer Assisted Self Interview (A-CASI) software (NOVA Research Company, Bethesda, MD). As described in detail in previous publications, the interview covered sociodemographics, social support, substance abuse and general medical history, current injection behaviors, clinical screening instruments, assessment of HIV, hepatitis, and overdose knowledge, and interactions with medical, substance abuse treatment, harm reduction, and criminal justice systems (Akselrod et al., 2014; Heimer et al., 2014).
Several clinical screening measures and their relationship to each other were explored in this report. The Brief Pain Inventory (BPI) was used to assess the extent to which pain impaired daily functioning (interference subscale) and the perceived level of pain (severity subscale) in the month prior to being interviewed (Cleeland, 1989; Keller et al., 2004). Both subscales use a 10-point Likert scale response format. The Addiction Severity Index (ASI) was used to assess the impact of drug use on seven domains (McLellan et al., 1992, 1980; Stoffelmayr et al., 1994). We also screened for depression, anxiety, and problem alcohol use using the Center for Epidemiologic Studies Depression (CESD) Scale, the Beck Anxiety Index (BAI), and the Alcohol Use Disorders Identification Test (AUDIT-C; Beck et al., 1988; Bush et al., 1998; Radloff, 1977). The CESD is a 20-item inventory with a 4-point Likert scale response format and an overall score range of 0–60; a score of 16 or greater indicates the presence of depression (Eaton et al., 2004; Radloff, 1977). The BAI is a 21-item inventory with a 4-point Likert scale response format and an overall score range of 0–63; clinical cutoff scores are 10–18 for minimal anxiety, 19–29 for moderate anxiety, and ≥30 for severe anxiety (Beck et al., 1988). The AUDIT-C is a 3-item inventory with a 5-point response format and an overall score range of 0–12; a score of ≥4 for men or ≥3 for women suggests problematic alcohol consumption (Bush et al., 1998; Saunders et al., 1993).
After completing both sections of the baseline interview, a 4–6 ml blood sample was collected by a trained phlebotomist. Study participants provided a blood sample for serological testing for HIV, HBV, and HCV as previously described (Akselrod et al., 2014). Participants received their results in a face-to-face post-test counseling session, and, if positive for antibodies to HIV or HCV or for HBV surface antigen, were referred for confirmatory testing and counseled on strategies to prevent further transmission. Those who tested negative for all three HBV tests were informed that they were susceptible and advised to receive vaccination.
2.3 Data Analysis
Our primary outcome is chronic pain and includes separate analysis of four variables collected in the baseline survey. Ongoing chronic pain was determined by a yes answer to the question: “Are you experiencing recurrent pain that interferes with your daily functioning?” combined with a duration of six months or more. Of the 152 people reporting current chronic pain, 143 (94%) reported more than six months duration. These individuals were asked to report pain severity and interference with daily living using the BPI to assess the current extent of the problem.
We applied bivariate analysis to compare those in the sample who reported current chronic pain of at least six months to those who did not for demographic, social network, somatic health and healthcare access, drug use, and the negative consequences of drug use including injection risk behaviors, syringe-borne virus seroprevalence, overdose experience, and the depression, anxiety, and problematic alcohol use screens. Significance for all bivariates was set at α ≥ 0.05. Multiple logistic regression was then conducted to examine the characteristics that were associated with reporting chronic pain. This analysis did not include the depression, anxiety, and problematic alcohol use assessments since we were interested in identifying sociodemographic and behavioral characteristics that differentiated between those with and without chronic pain. We excluded the physical health subscale of the ASI because of the obvious collinearity with the BPI.
To further explore the associations among the clinical assessments among those reporting chronic pain, we performed linear regression analysis to look at the relationship of BPI pain severity or interference subscale scores with the scores on two domains of the ASI – psychological and family problems – and on the BAI and the CESD. Pain interference was determined using an average of the scores on the BPI that specifically asked about interference in seven domains: general activity, mood, walking, normal work routine, interpersonal relationships, sleep, and enjoyment of life. Pain severity was determined combining the scores for average severity over the prior month and severity during the interview.
Finally, again among those reporting chronic pain, we analyzed the data on the duration of chronic pain and the initiation of opioid abuse. For both items on the questionnaire, individuals were asked to indicate when they began abusing opioids and when they first experienced chronic pain that continued up to the date of the interview. We compared these reports to determine the first year in which these were reported to ascertain which, if either, occurred first.
All statistical analyses were conducted using SAS software version 9.1 (SAS Institute Inc., 2003).
3. RESULTS
To summarize the demographic profile of the 456 injectors who answered questions on chronic pain, more than 80% were white, more than 60% were male, and two-thirds had never been married. One-fifth had less than a high school education, two-thirds were not employed, and one-quarter lacked health insurance. We explored the extent of chronic pain in our study sample (Table 1). Analysis of the baseline data revealed that 31.4% (143/456) of the study participants reported experiencing chronic pain that interferes with daily activities. We investigated five features among the members of this group. The median duration of chronic reported by these individuals was nearly 10 years (Table 1) with a distribution skewed towards individuals with longer duration. The reported mean levels of pain severity and interference with daily activities are presented in Table 1. Thirty percent reported receiving pain medication, and of those most (76%) reported taking pain medication as prescribed.
TABLE 1.
Chronic pain among suburban injectors in Connecticut, 2008–2012
| Variables | Percentage (n/N) /Mean ± s.d. |
|---|---|
| Percent experiencing | 31.4% (143/456) |
| Duration of symptoms (among those reporting chronic pain) -- mean ± s.d. (months) | 118.1 ± 102.5 |
| Intensity – average past month (among those reporting chronic pain) -- mean ± s.d. | 6.5 ± 2.0 |
| Interference (among those reporting chronic pain) -- mean ± s.d. | 6.9 ± 1.7 |
| Prescribed medication(s) for pain (among those reporting chronic pain) | 31.5% (45/143) |
| Taking Medication(s) as prescribed for chronic pain | 75.6% (34/45) |
We then compared the characteristics for those reporting chronic pain to those not reporting (Table 2). Among those positively associated with chronic pain were older age, lower educational achievement, having been previously married, reporting poorer health, having a regular medical provider, receiving healthcare services somewhere other than an emergency room, and current use of methadone as part of opioid substitution therapy. Social support was significantly lower among those with chronic pain. Also associated were four variables regarding drug use including longer duration of injection, injection of pharmaceutical opioids, having ever experienced an opioid overdose, and not being vaccinated against hepatitis B virus infection (Table 3).
TABLE 2.
Characteristics of suburban injectors by status of chronic pain in Connecticut, 2008–2012
| Characteristics | Chronic Pain (n = 143) | No Chronic Pain (n = 313) | p-value1 |
|---|---|---|---|
| Demographic Variables | |||
| Sex -- % male | 56.6% | 64.5% | 0.11 |
| Ethnicity -- % white | 86.0% | 82.4% | 0.34 |
| Age – mean ± s.d., median, IQR | 39.8 ± 10.6, 42.0, 18.0 | 33.5 ± 10.6, 30.0, 17.0 | <.0001 |
| Education – less than high school graduation | 25.0% | 16.3% | 0.03 |
| Marital Status | 0.0004 | ||
| Never married | 54.6% | 72.8% | |
| Married | 15.4% | 10.9% | |
| Separated/widowed/divorced | 30.1% | 16.3% | |
| Currently employed | 25.2% | 30.8% | 0.22 |
| Social Network Variables2 | |||
| Injection Network – mean ± s.d., median & IQR3 | 1.7 ± 1.9, 1.0, 1.0 | 1.9 ± 2.3, 1.0, 3.0 | 0.64 |
| RDS Network – mean ± s.d., median & IQR3 | 9.9 ± 14.3, 6.0, 7.0 | 9.9 ± 13.8, 6.0, 6.0 | 0.53 |
| Social Support– mean ± s.d., median & IQR | 48.1 ± 19.8, 45.0, 28.0 | 52.2 ± 22.1, 52.0, 35.0 | 0.06 |
| Somatic Health Status | |||
| Self-reported poor or fair overall health | 49.0% | 19.8% | <.0001 |
| Use of Medical Services | |||
| Location of usual care | 0.03 | ||
| Don't ever get medical care | 6.4% | 13.6% | |
| Emergency room | 35.0% | 38.7% | |
| Others | 58.6% | 47.7% | |
| Regular Provider | 51.1% | 36.7% | 0.004 |
| Use of Substance Abuse Treatment Services | |||
| Any use ever of a substance abuse treatment service | 82.5% | 75.1% | 0.08 |
| Currently in methadone maintenance | 48.3% | 34.2% | 0.01 |
| Currently in buprenorphine maintenance | 2.1% | 1.9% | 1.00 |
| Currently in a 12-step program | 15.4% | 13.5% | 0.59 |
| Health Insurance | 0.09 | ||
| None | 16.8% | 26.7% | |
| Government | 72.5% | 63.9% | |
| Private | 10.7% | 9.4% |
Without specific notes, p-value was obtained using chi-square test for categorical variables and t-test for continuous variables.
- Injection Network is the number of different people who were together with the participant when both were injecting drugs in the 30 days prior to interview.
- RDS network is the number of people who the participant knew drug injectors and whom the participants had had contact with (and could therefore have given that person a recruitment coupon) in the 30 days prior to interview.
- Social support scale comes from Zimet et al, 1988 and Dahlen et al., 1991.
P-value was obtained using t-test on transformed variable using log (1 + variable).
TABLE 3.
Drug use history and current injection practices among suburban injectors by status of chronic pain in Connecticut, 2008–2012
| Drug use | Chronic Pain (n = 143) | No Chronic Pain (n = 313) | p-value1 |
|---|---|---|---|
| Duration of Injection, mean ± s.d., median & IQR | 14.7 ± 12.2, 10.0, 21.0 | 9.4 ± 8.8, 6.0, 11.0 | <.0001 |
| Frequency of Injection, last 30 days, mean ± s.d., median & IQR2 | 87.9 ± 223.4, 29.0, 57.0 | 63.2 ± 107.3, 34.0, 68.0 | 0.18 |
| Heroin as drug of choice | 88.8% | 91.0% | 0.46 |
| Heroin as drug most commonly injected, last 30 days | 82.1% | 86.8% | 0.31 |
| Injection of Pharmaceutical Opioids | |||
| Ever | 48.3% | 36.7% | 0.02 |
| Last 30 days | 16.8% | 13.7% | 0.39 |
| Injection Risk | |||
| Syringe Sharing | 25.2% | 20.5% | 0.26 |
| Paraphernalia Sharing | 43.4% | 36.7% | 0.18 |
| Sharing of Dissolved Drugs | 22.6% | 20.5% | 0.70 |
| Overdose | |||
| Ever | 37.1% | 27.6% | 0.04 |
| Number of ODs (lifetime)2 | 1.0 ± 1.9, 0.0, 2.0 | 0.7 ± 1.8, 0.0, 1.0 | 0.03 |
| OD last Year | 21.7% | 23.5% | 0.82 |
| OD Knowledge | 58.0 ± 15.8, 57.1, 14.3 | 60.4 ± 17.8, 64.3, 21.4 | 0.16 |
| Serology | |||
| HIV3 | 1.4% | 1.7% | 1.00 |
| HCV | 44.3% | 39.2% | 0.31 |
| HBV | 0.03 | ||
| Never infected | 45.7% | 43.7% | |
| Vaccinated | 23.6% | 34.7% | |
| Infected | 30.7% | 21.7% |
Without specific notes, p-value was obtained using chi-square test for categorical variables and t-test for continuous variables.
P-value was obtained using t-test on log-transformed variable.
P-value was obtained using Fisher’s exact test.
We explored correlations between reporting chronic pain and the clinical screening instruments (Table 4). Those reporting chronic pain also reported more problems on the medical, family, psychological domains of the ASI and were more likely to exceed clinical cut-offs on the CESD (indicating moderate or severe depression) and the BAI (indicating moderate or severe anxiety). There was no difference in mean AUDIT-C scores even taking into account the different cut-off for men and women.
TABLE 4.
Psychological problems among suburban injectors by status of chronic pain in Connecticut, 2008–2012.
| Psychological Problems | Chronic Pain (n = 143) | No Chronic Pain (n = 313) | p-value |
|---|---|---|---|
| ASI Subscales | |||
| Psychological | 0.3 ± 0.2, 0.3, 0.4 | 0.1 ± 0.2, 0.0, 0.3 | <.0001 |
| Family | 0.4 ± 0.2, 0.3, 0.4 | 0.3 ± 0.2, 0.2, 0.3 | <.0001 |
| ASI Health | 0.5 ± 0.3, 0.5, 0.6 | 0.1 ± 0.2, 0, 0.2 | <.0001 |
| CES-D mean score | 24.3 ± 13.0, 24.0, 21.0 | 15.0 ± 11.5, 12.0, 15.0 | <.0001 |
| Beck’s Anxiety Index mean score | 18.9 ± 13.6, 17.0, 21.5 | 8.3 ± 9.5, 5.0, 13.0 | <.0001 |
| AUDIT-C --# (%) with problematic drinking | 71 (51.1%) | 172 (57.7%) | 0.20 |
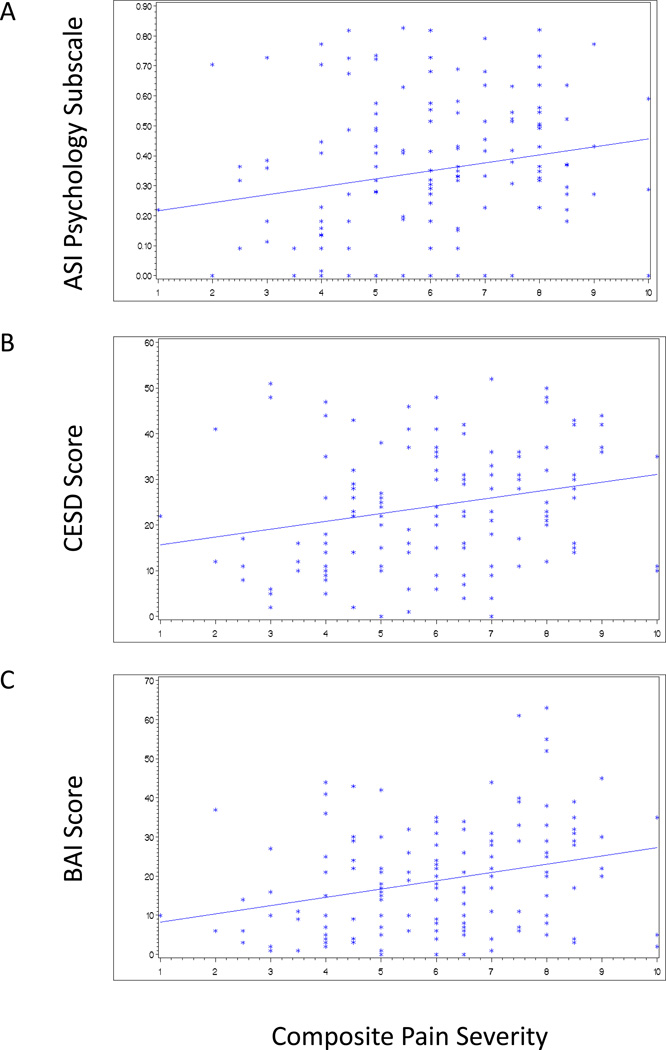
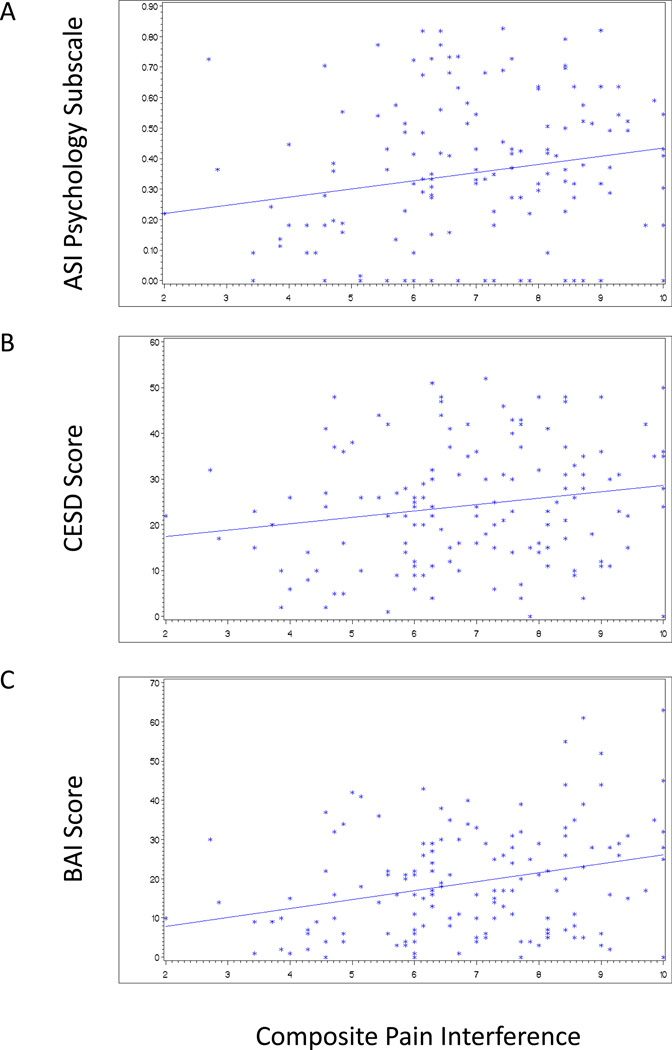
Restricting the analysis to those reporting chronic pain, we compared scores on pain severity and interference subscales to scores on the ASI family and psychological subscales, the CESD and the Beck’s Anxiety Index scores using linear regression analysis. Significant linear relationships with both pain severity (Figure 1A–C) and interference (Figure 2A–C) were found for the ASI psychological problems subscale, depression, and anxiety, but not for family problems.
Figure 1.
Associations of pain severity with the ASI psychology subscale, the CESD Depression Index score, and Beck Anxiety Index score among suburban injectors reporting chronic pain (N = 143). Cut-offs for moderate to severe depression on the CESD and moderate to severe anxiety on the BAI were 16 and 19, respectively. (A) ASI psychology subscale (r2=0.04; p=0.02); (B) CESD (r2=0.06; p=0.003) and (C) BAI (r2=0.08; p=0.0005).
Figure 2.
Associations of pain interference with the ASI psychology subscale, the CESD Depression Index score, and Beck Anxiety Index score among suburban injectors reporting chronic pain (N = 143). Cut-offs for moderate to severe depression on the CESD and moderate to severe anxiety on the BAI were 16 and 19, respectively. (A) ASI psychology subscale (r2=0.04; p=0.02); (B) CESD (r2=0.03; p=0.02) and (C) BAI (r2=0.09; p=0.0003).
We constructed a multivariate model to ascertain the factors that were most strongly associated with reporting chronic pain. Only three participant characteristics distinguished those with chronic pain from other suburban injectors. They were being older, higher BAI score, and higher ASI psychology subscale score (Table 5).
TABLE 5.
Logistic regression analysis of factors associated with chronic pain among suburban injectors in Connecticut, 2008–2012
| Factors | Unadjusted Odds Ratio (95% CI) | Adjusted Odds Ratio (95% CI) |
|---|---|---|
| Age | 1.05 (1.04–1.07) | 1.05 (1.03–1.07) |
| Less than high school education | 0.59 (0.36–0.96) | – |
| Marital Status | – | |
| Never married | 1.00 | |
| Married | 1.89 (1.04–3.43) | |
| Separated/widowed/divorced | 2.47 (1.53–3.99) | |
| Social support | 0.99 (0.98–1.00) | – |
| Location of usual care | – | |
| Don't ever get medical care | 0.52 (0.24–1.16) | |
| Emergency room | 1.00 | |
| Others | 1.36 (0.89–2.09) | |
| Regular provider | 1.80 (1.20–2.68) | – |
| Currently in methadone maintenance | 1.80 (1.15–2.82) | – |
| Health insurance | – | |
| None | 1.00 | |
| Government | 1.81 (1.06–3.09) | |
| Private | 1.81 (0.81–4.05) | |
| Duration of injection | 1.05 (1.03–1.07) | – |
| Ever injected pharmaceutical opioids | 1.61 (1.08–2.40) | – |
| Ever overdose | 1.55 (1.02–2.36) | – |
| HBV | – | |
| Never infected | 1.00 | |
| Vaccinated | 0.65 (0.40–1.06) | |
| Infected | 1.35 (0.83–2.21) | |
| AUDIT-C (problematic drinking) | 0.77 (0.51–1.15) | – |
| Psychological ASI subscale | 51.73 (19.97–34.00) | 9.23 (2.62–32.54) |
| Family ASI subscale | 10.61 (3.76–29.95) | – |
| CES-D mean score | 1.06 (1.04–1.08) | – |
| Beck’s Anxiety Index mean | 1.08 (1.06–1.10) | 1.05 (1.02–1.08) |
Finally, we investigated the variable about duration of pain to determine if the onset of pain came before, after, or in the same year as initiation of non-medical opioid use (Table 6). For the vast majority (nearly 80%), non-medical opioid use preceded the onset of chronic pain. Only 6 of 143 individuals (4.2%) reported onsets of non-medical opioid use and chronic pain within the same year. We hypothesized that those who initiated non-medical opioid use prior to chronic pain would be older than those with onset of chronic pain preceding opioid abuse. This hypothesis was based on the assumption that since most persons in the study began non-medical use of opioids at a relatively young age and were injecting by their mid-20s, their likelihood of experiencing chronic pain would increase with age. Logically, then, older individuals would have more time to experience chronic pain after initiating abuse. Conversely, those experiencing chronic pain at a younger age might have turned to illegal opioids once experiencing difficulties in obtaining pain medication by prescription and then initiating injection to reduce costs. However, when we tested our hypothesis, we found that there were no significant age differences associated with the sequence.
TABLE 6.
Comparing Duration of Chronic Pain and Initiation of Non-medical Opioid Use
| Order of Use (n = 143) | |||
|---|---|---|---|
| Chronic Pain before Opioid Use | At the Same Age | Opioid Use before Chronic Pain | |
| Number | 25 | 6 | 112 |
| Percent | 17.5% | 4.2% | 78.3% |
| Age, mean ± s.d. | 40.0 ± 9.6 | 34.7 ± 9.6 | 40.1 ± 11.0 |
4. DISCUSSION
To our knowledge, this is the largest study to date of the relationship between chronic pain and injection drug abuse in a population neither seeking treatment for chronic pain nor drawn from those in substance abuse treatment. Our sample differs substantially from samples drawn from general medical populations (Banta-Green et al., 2009a, 2009b; Barry et al., 2010). In the clinical population of people prescribed opioids studied by Banta-Green et al. (2009b), three classes of individuals with chronic pain were identified. The predominant class had persistent, moderate mental health and pain symptoms. Two atypical classes were also identified: one reported elevated mental health symptoms and opioid problems, but pain similar to the predominant class, and one reported significantly higher pain interference as well as elevated mental health and opioid problems. In our sample, one-third reported both addictive behavior and high levels of pain comparable to this last group reported by Banta-Green, which covered only 10% of the clinical sample, suggesting that our non-clinical sample of PWID had higher levels of concomitant pain and opioid abuse problems.
Our results are consistent with our previous findings from Cumberland County, Maine (Heimer et al., 2012). Both studies recruited among substance abusing populations using respondent driven sampling, but the sampling frames differed. In Maine, participant eligibility criteria included any non-prescribed opioid use in the month prior to recruitment regardless of the route of administration; in Connecticut, eligibility was based upon injection in the month prior to recruitment of any psychoactive drug. The vast majority of the Connecticut sample preferred heroin and injected it most often; in the Maine sample, pharmaceutical opioids were consumed most often, generally by mouth (Grau et al., 2007). But in both samples there was a similar prevalence of chronic pain, similar strong correlations between pain and the ASI medical, psychological, and family problems subscales, and a similar predominance of opioid abuse preceding the onset of chronic pain. This, in itself, suggests some generalizability of our findings, and we hope that this research can be used as a springboard to assess if they are indeed generalizable among populations in which opioid abuse and chronic pain intersect.
This study also reveals some key differences between people who inject drugs with and without concomitant chronic pain. The most salient of these, identified through multivariate modeling, are that those with chronic pain were older, less educated, more likely to have ever injected pharmaceutical opioids, and more likely to suffer from depression and anxiety. Given the likelihood that those experiencing chronic pain may have easier access to pharmaceutical opioids, it is not surprising that they are more likely to have ever injected them. It is interesting to note, however, that both groups preferred heroin and injected it most frequently. It is also not surprising that those with chronic pain reported more somatic and psychological health problems on the ASI and greater levels of depression and anxiety.
We observed a bivariate association between greater family problems on the ASI subscale and reporting chronic pain that was not included in the final multivariate. The existence of family problems was consistent with the finding of lower social support. As Evans and de Souza note in one of the few studies on the influence of chronic pain on pain dynamics, “Despite the substantial monetary, personal, and social cost of chronic pain, research into the family life of sufferers is wanting “ (Evans and De Souza, 2008). Their qualitative study found both positive and negative influences; in contrast, our study suggested the combination of chronic pain and addiction produces more perceived family problems than addiction alone. While it is accepted that both addiction and chronic pain have familial patterns (Compton et al., 2002; Coviello et al., 2004; Jamison and Walker, 1992; Kendler et al., 1997; Luthar et al., 1992), more research is needed to explore intergenerational associations, influences, and interactions among those experiencing both addiction and chronic pain. This is especially necessary since most family studies have focused on families with adolescent children and the impact of parental pain on children as opposed to looking at both parents and children of individuals with chronic pain.
This study has several limitations. There are limitations associated with cross-sectional data that rely on participant self-report. We do not know the extent to which response biases influenced answers. It is felt that using computer-assisted interviewing reduces under-reporting of risky or stigmatized behaviors, especially among drug using samples (Newman et al., 2002; Turner et al., 1998). For that reason we chose this method for collecting data. However, we cannot rule out inaccurate self-reporting or recall bias. There are three limitations related to the process of accruing the sample. First, there is no known sampling frame for active suburban injectors, which led us to employ RDS. Second, we relied on a substance abuse treatment program in our recruitment of some of our RDS seeds. This might have resulted in oversampling from treatment-seeking segments of the population of interest. However, these individuals were eligible because they were still, by our definition, active injectors, having injected within 30 days prior to enrollment. Third, we recruited few participants either of high socio-economic status or who resided in the wealthier towns, which might have skewed the sample towards more indigent individuals, even though the counties themselves are among the richest in the U.S. For all these reasons, the assumptions of RDS that are necessary to calculate population estimates are violated, and we have characterized our sample as one of convenience and not necessarily representative of the underlying population. However, in a previous report, we have detailed the four analyses we undertook to compare our sample to other estimates of that population that led us to believe that the results from our sample may be broadly representative of the underlying population and hence generalizable to some degree (Heimer et al., 2014). More research is needed on populations of suburban injectors to confirm our belief.
Despite the limitations discussed in the previous paragraph, this report does constitute the first quantitative presentation on the interrelationships among addiction, drug injection, and chronic pain, not only in a suburban population, but as far as we can ascertain from the published literature, any population not drawn exclusively from patients seeking medical care for chronic pain or from individuals in substance abuse treatment at the time of the baseline interview. In conclusion, the major findings were that in this sample, selected on the criterion of having injected drugs in the past 30 days, chronic pain was accompanied by psychological problems, especially anxiety, to a greater degree than that found in active injectors free of chronic pain. This suggests that treatment of these individuals for substance abuse, pain, and infectious diseases will be more complicated and will require substantial attention and resources to manage the co-occurring conditions.
Highlights.
In this unique sample of suburban drug injectors, chronic pain was reported by one-third of participants.
Those with chronic pain were more likely to inject pharmaceutical opioids and to have more family problems, and higher levels of depression and anxiety compared to those without chronic pain.
In three-quarters of the cases, opioid abuse preceded the onset of chronic pain.
Acknowledgements
The authors would especially like to thank John Hamilton, Joann Montgomery, and the staff at the Center for Human Services, part of the Recovery Network of Programs, who allowed us access to their facility and their newly entering patients as seeds for sample recruitment. The authors also wish to thank members of the study team, Lisa G. Nichols and Christina White whose efforts organizing and collecting data were a vital part of the study.
Role of Funding Source
The funders had no role in the design of the study, the analysis of study data, or in the preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Dr. Heimer designed the study and led in the preparation of the manuscript. Dr. Zhan conducted much of the analysis, especially the multivariate analysis. Dr. Grau led the collection of the data and the preparation of the study database prior to analysis and conducted parts of the analysis.
Conflict of Interest
No conflict declared
REFERENCES
- Akselrod H, Grau LE, Barbour R, Heimer R. Seroprevalence of HIV, HBV, and HCV among injection drug users in Connecticut: understanding infection and co-infection risks in a suburban population. Am. J. Public Health. 2014;109:1713–1721. doi: 10.2105/AJPH.2013.301357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banta-Green CJ, Merrill JO, Doyle SR, Boudreau DM, Calsyn DA. Measurement of opioid problems among chronic pain patients in a general medical population. Drug Alcohol Depend. 2009a;104:43–49. doi: 10.1016/j.drugalcdep.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banta-Green CJ, Merrill JO, Doyle SR, Boudreau DM, Calsyn DA. Opioid use behaviors, mental health, and pain -- development of a typology of chronic pain patients. Drug Alcohol Depend. 2009b;104:34–42. doi: 10.1016/j.drugalcdep.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry DT, Irwin KS, Jones ES, Becker WC, M TJ, Sullivan LE, Hansen H, O'Connor PG, Schottenfeld RS, Fiellin DA. Opioids, chronic pain, and addiction in primary care. J. Pain. 2010;11:1442–1450. doi: 10.1016/j.jpain.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring anxiety: psychometric properties. J. Consult. Clin. Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Bush K, Kivlahan DR, McDonnell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): An effective brief screening test for problem drinking. Arch. Intern. Med. 1998;158:1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Lynskey M, Todorov A, Inciardi JA, Surratt HL. Co-morbid pain and psychopathology in males and females admitted to treatment for opioid analgesic abuse. Pain. 2008;139:127–135. doi: 10.1016/j.pain.2008.03.021. [DOI] [PubMed] [Google Scholar]
- Cleeland CS. Measurement of pain by subjective report. In: Chapman CR, Loeser JD, editors. Advances In Pain Research And Therapy: Issues In Pain Management. Vol. 12. New York: Raven Press; 1989. pp. 391–403. [Google Scholar]
- Compton WM, Cottler LB, Ridenour T, Ben-Abdallah A, Spitznagel EL. The specificity of family history of alcohol and drug abuse in cocaine abusers. Am. J. Addict. 2002;11:85–94. doi: 10.1080/10550490290087866. [DOI] [PubMed] [Google Scholar]
- Coviello DM, Altermann AI, Cacciola JS, Rutherford MJ, Zanis DA. The role of family history in addiction severity and treatment response. J. Subst. Abuse Treat. 2004;26:303–313. doi: 10.1016/s0740-5472(03)00143-0. [DOI] [PubMed] [Google Scholar]
- Dahlem NW, Zimet GD, Walker RR. The multidimensional scale of perceived social support: a confirmation study. J Clin. Psych. 1991;47:756–761. doi: 10.1002/1097-4679(199111)47:6<756::aid-jclp2270470605>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Davis WR, Johnson BD. Prescription opioid use, misuse, and diversion among street drug users in New York City. Drug Alcohol Depend. 2008;92:267–276. doi: 10.1016/j.drugalcdep.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton WW, Mutaner C, Smith C, Tien A, Ybarra M. Center for Epidemiologic Studies Depression Scale: review and revision (CESD and CESD-R) In: Maruish ME, editor. The Use of Psychological Testing for Treatment Planning and Outcomes Assessment. Mahwah: Lawrence Erlbaum; 2004. pp. 363–377. [Google Scholar]
- Evans S, De Souza L. Dealing with chronic pain: giving voice to the experiences of mothers with chronic pain and their children. Qual. Health Res. 2008;18:489–500. doi: 10.1177/1049732308315433. [DOI] [PubMed] [Google Scholar]
- Grau LE, Dasgupta N, Irwin KS, Kinzly ML, Givens A, Phinney A, Heimer R. Illicit use of opiates: is Oxycontin a “gateway drug”? Am. J. Addict. 2007;16:166–173. doi: 10.1080/10550490701375293. [DOI] [PubMed] [Google Scholar]
- Havens JR, Stoops WW, Leukefeld CG, Garrity TF, Carlson RG, Falck R, Wang J, Booth BM. Prescription opiate misuse among rural stimulant users in a multistate community-based study. Am. J. Drug Alcohol Abuse. 2009;35:18–23. doi: 10.1080/00952990802326298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckathorn D. Respondent driven sampling II: deriving valid population estimates from chain-referral samples of hidden populations. Soc. Probl. 2002;49:11–34. [Google Scholar]
- Heimer R, Barbour R, Palacios WR, Nichols LG, Grau LE. Associations between injection risk and community disadvantage among suburban injection durug sers in southwestern Connecticut, USA. AIDS Behav. 2014;18:452–463. doi: 10.1007/s10461-013-0572-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer R, Dasgupta N, Irwin KS, Phinney Harvey A, Givens A, Grau LE. Chronic pain, addiction severity, and misuse of opioids in Cumberland County, Maine. Addict. Behav. 2012;37:346–349. doi: 10.1016/j.addbeh.2011.11.017. [DOI] [PubMed] [Google Scholar]
- Jamison RN, Kauffman J, Katz NP. Characteristics of methadone maintenance patients with chronic pain. J. Pain Symptom Manage. 2000;19:53–62. doi: 10.1016/s0885-3924(99)00144-x. [DOI] [PubMed] [Google Scholar]
- Jamison RN, Walker LS. Illness behavior in children of chronic pain patients. Int. J. Psychiatry Med. 1992;22:329–342. doi: 10.2190/AMAN-GJ29-4N1C-6JR2. [DOI] [PubMed] [Google Scholar]
- Keller S, Bann CM, Didd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the Brief Pain Inventory for use in documenting the outcomes of patients with noncancer pain. Clin. J. Pain. 2004;20:309–318. doi: 10.1097/00002508-200409000-00005. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Davis CG, Kessler RC. The familial aggregation of common psychiatric and substance use disorders in the National Comorbidity Survey: a family history study. Br. J. Psychiatry. 1997;170:541–548. doi: 10.1192/bjp.170.6.541. [DOI] [PubMed] [Google Scholar]
- Krashin D, Murinova N, Ballantyne J. Management of pain with comorbid substance abuse. Curr. Psychiatry Rep. 2012;14:462–468. doi: 10.1007/s11920-012-0298-3. [DOI] [PubMed] [Google Scholar]
- Luthar SS, Anton SF, Merikangas KR, Roundsaville BJ. Vulnerability to drug abuse among opioid addicts' siblings: individual, familial, and peer influences. Compr. Psychiatry. 1992;33:190–196. doi: 10.1016/0010-440x(92)90029-p. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The fifth edition of the Addiction Severity Index. J Subst. Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, O'Brien CP, Woody GE. An improved diagnostic instrument for substance abuse patients, the Addiction Severity Index. J. Nerv. Ment. Dis. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- Newman JC, Des Jarlais DC, Turner CF, Gribble J, Cooley P, Paone D. The differential effects of face-to-face and computer interview modes. Am. J. Public Health. 2002;92:294–297. doi: 10.2105/ajph.92.2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl M, Smith L. Chronic pain and addiction: challenging co-occurring disorders. J. Psychoactive Drugs. 2012;44:119–124. doi: 10.1080/02791072.2012.684621. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977;1:385–401. [Google Scholar]
- Rosenblum A, Joseph H, Fong C, Kipnis S, Cleland C, Portenoy RK. Prevalence and characteristics of chronic pain among chemically dependent patients in methadone maintenance and residential treatment facilities. JAMA. 2003;289:2370–2378. doi: 10.1001/jama.289.18.2370. [DOI] [PubMed] [Google Scholar]
- Salganik MJ, Heckathorn DD. Sampling and estimation in hidden populations using respondent-driven sampling. Sociol. Methodol. 2004;34:193–240. [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, De La Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption. II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Sehgal N, Mancikanti L, Smith HS. Prescription opioid abuse in chronic pain: a review of opioid abuse predictors and strategies to curb opioid abuse. Pain Physician. 2012;15(3 Suppl):ES67–ES92. [PubMed] [Google Scholar]
- Stoffelmayr BE, Mavis BE, Kasim RM. The longitudinal stability of the Addiction Severity Index. J. Subst. Abuse Treat. 1994;11:373–378. doi: 10.1016/0740-5472(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Turner CF, Ku L, Rogers SM, Lindberg LD, Pleck JH, Sonenstein FL. Adolescent sexual behavior, drug use, violence: increased reporting with computer survey technology. Science. 1998;280:867–873. doi: 10.1126/science.280.5365.867. [DOI] [PubMed] [Google Scholar]
- Warner EA. Opioids for the treatment of chronic noncancer pain. Am. J. Med. 2012;125:1155–1161. doi: 10.1016/j.amjmed.2012.04.032. [DOI] [PubMed] [Google Scholar]
- Weaver M, Schnoll S. Abuse liability in opioid therapy for pain treatment in patients with an addiction history. Clin. J. Pain. 2002;18(Suppl 4):S61–S69. doi: 10.1097/00002508-200207001-00007. [DOI] [PubMed] [Google Scholar]
- Wunsch MJ, Cropsey KL, Campbell ED, Knisely JS. OxyContin use and misuse in three populations: substance abuse patients, pain patients, and criminal justice participants. J. Opioid Manage. 2008;4:73–79. doi: 10.5055/jom.2008.0011. [DOI] [PubMed] [Google Scholar]
- Zimet GD, Dahlem NW, Zimet SG, Farley GK. The multidimensional scale of perceived social support. J. Pers. Assess. 1988;52:30–41. doi: 10.1080/00223891.1990.9674095. [DOI] [PubMed] [Google Scholar]